Abstract
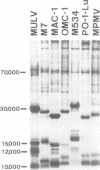
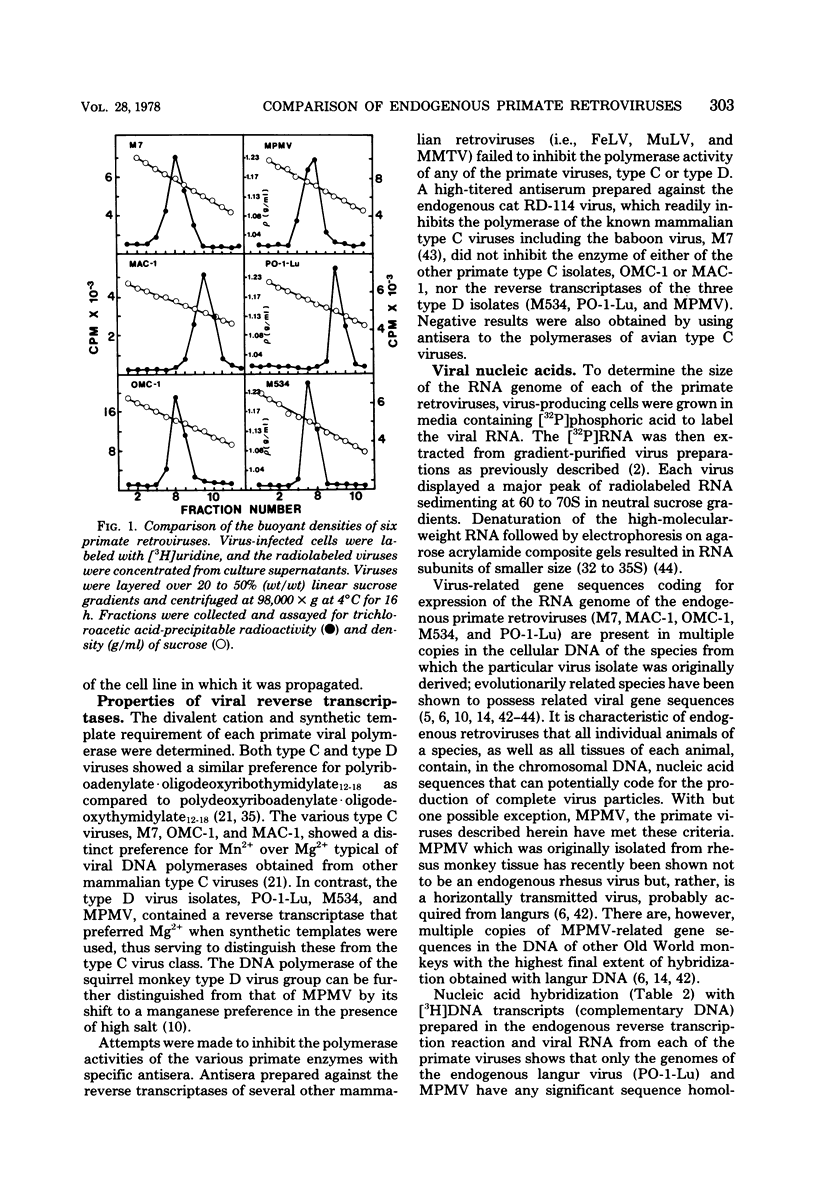
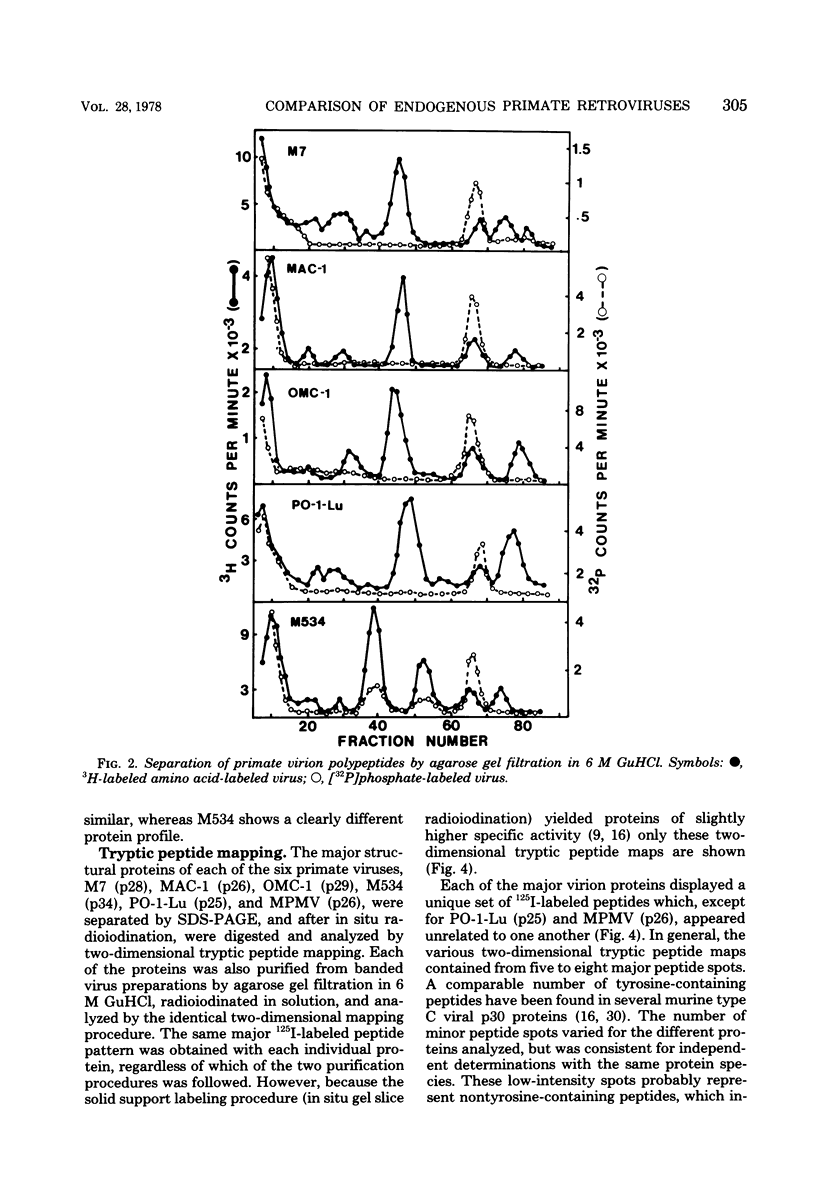
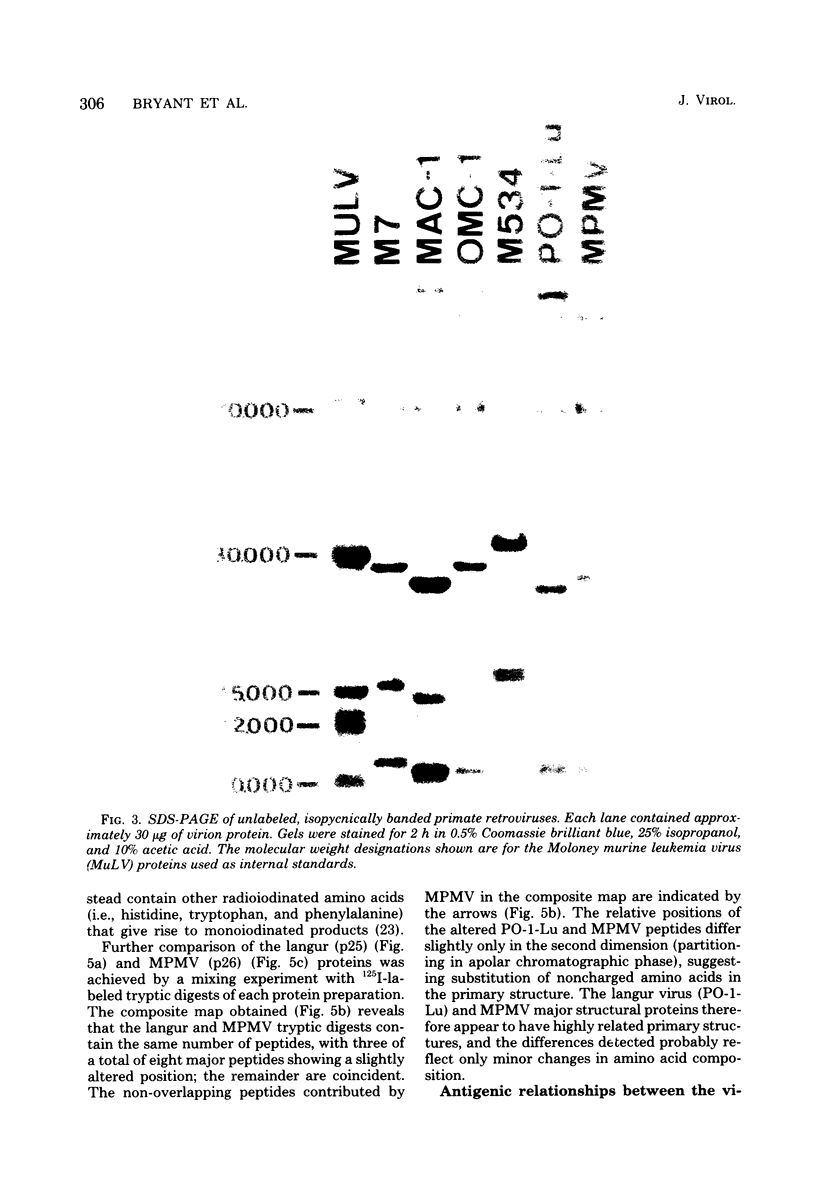
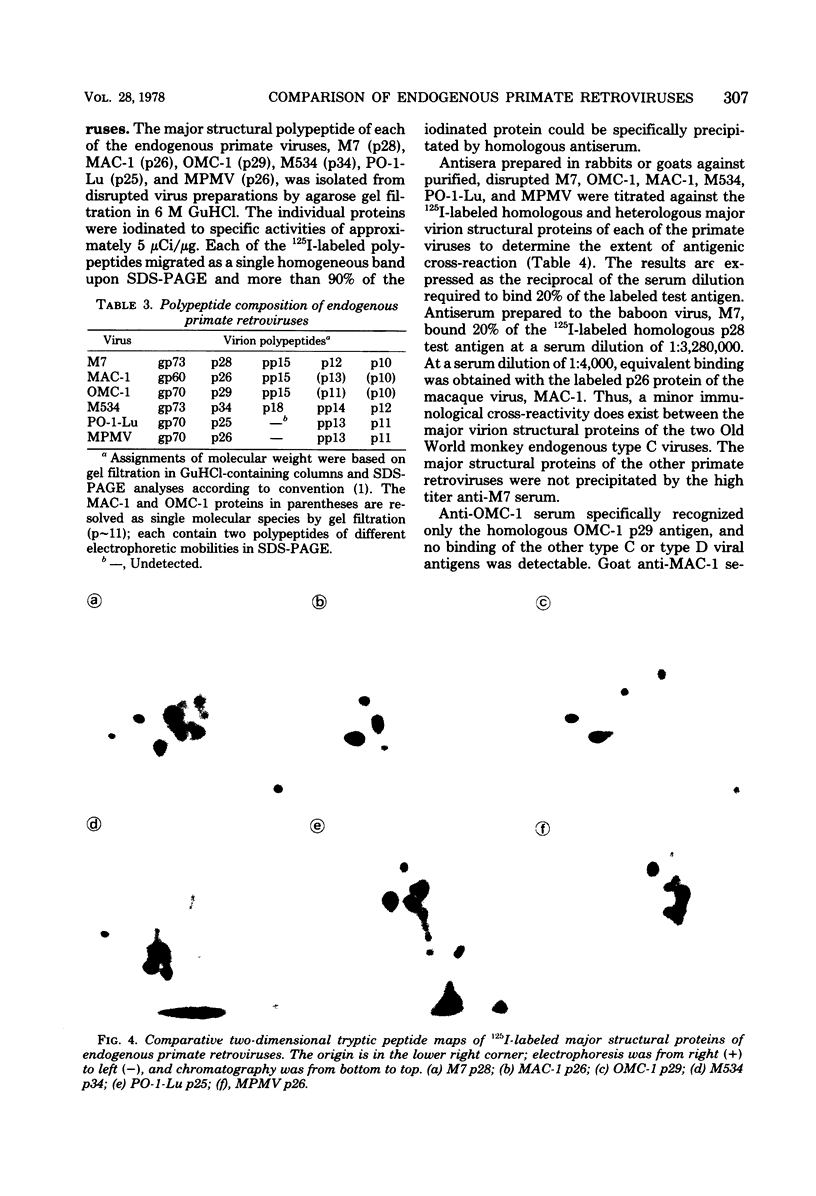
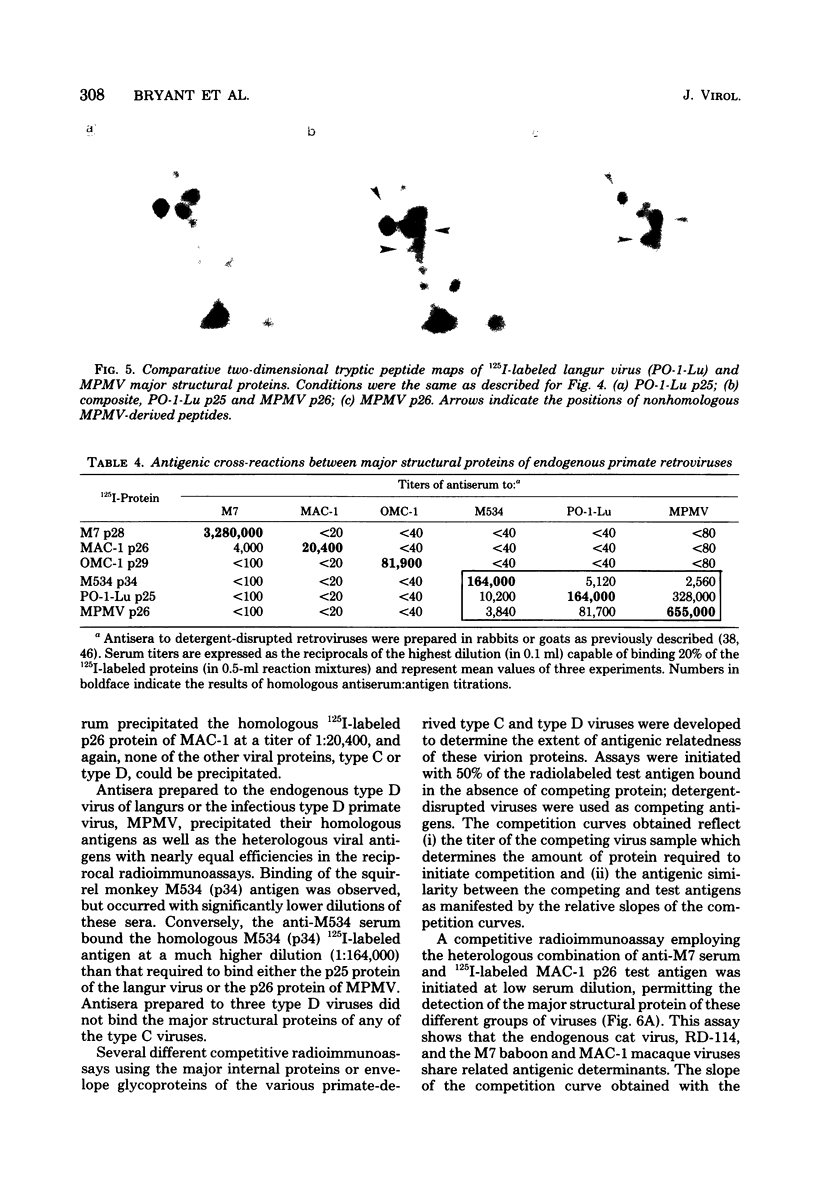
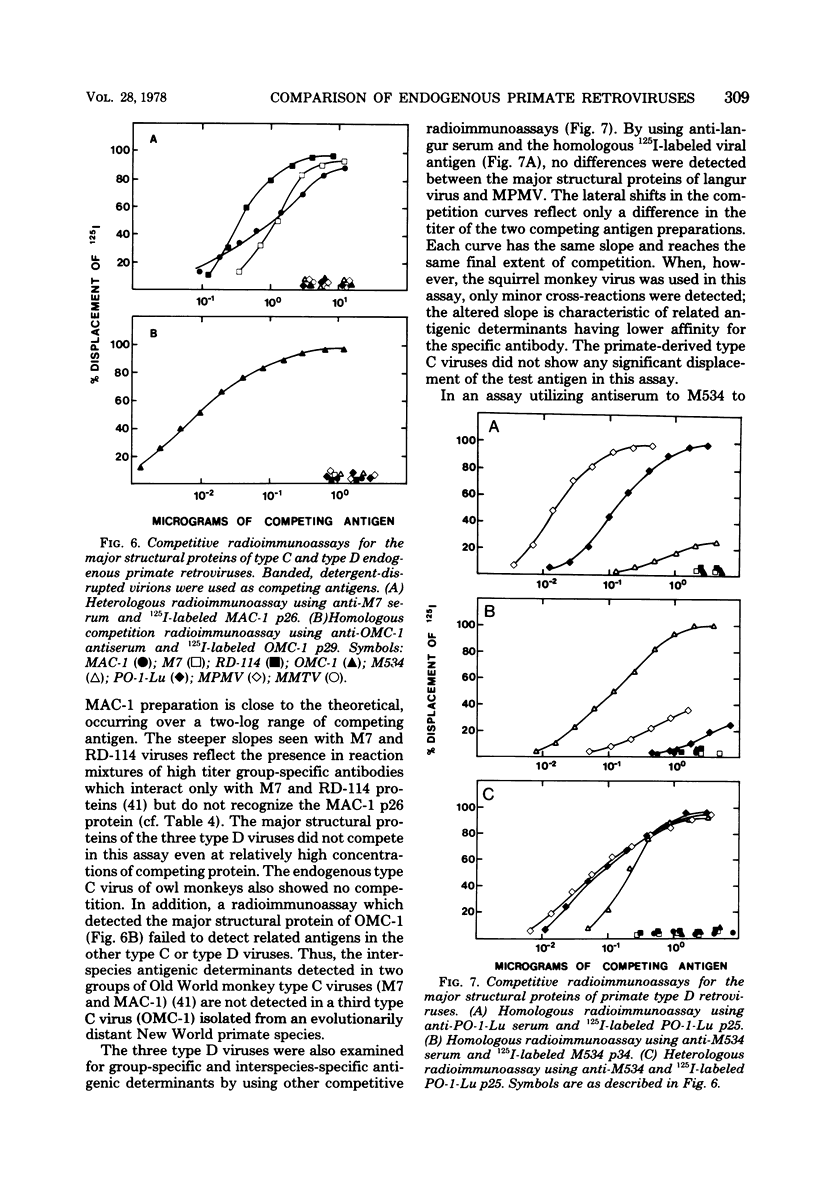
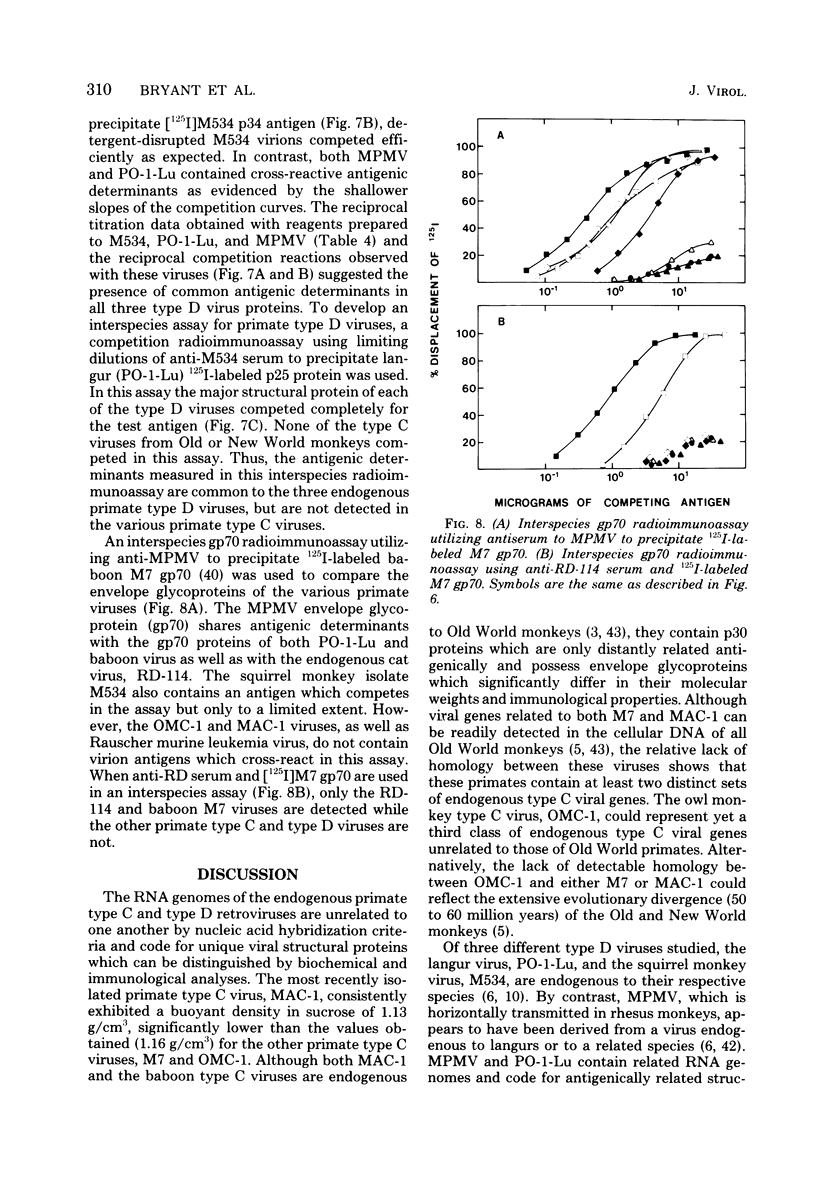
Genetically transmitted retroviruses of Old and New World monkeys include type C viruses isolated from baboons (M7), macaque (MAC-1), and owl monkeys (OMC-1) and type D viruses from langurs (PO-1-Lu) and squirrel monkeys (SMRV, M534). Each of these isolates is unrelated to the others by nucleic acid hybridization criteria and contains a unique array of virion-associated proteins which can be resolved by agarose gel filtration and polyacrylamide gel electrophoresis under denaturing conditions. The major structural protein of each virus has a distinct primary structure, as determined by two-dimensional tryptic peptide analysis, and is antigenically different from the others. The major virion phosphoproteins of endogenous primate type C viruses (pp15) are also different from those of type D viruses (pp13-pp14). Immunological and structural analyses show that the endogenous langur virus and the horizontally transmitted Mason-Pfizer virus of rhesus monkeys are closely related to one another, consistent with the sequence homology detected in their RNA genomes. Although certain radioimmunoassays detect interspecies antigenic determinants common to either the p30 or gp70 proteins of some of these viruses, no one assay has yet been designed which can detect all groups of endogenous primate retroviridae. The data lead to the conclusion that primates contain a minimum of three different sets of genetically transmitted type C and type D retroviral genes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Livingston D. M., Sherr C. J., Todaro G. J., Kalter S. S. Infectious C-type virus isolated from a baboon placenta. Nature. 1974 Mar 1;248(5443):17–20. doi: 10.1038/248017a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of primate oncornaviruses: An endogenous virus from langurs (Presbytis spp.) with related virogene sequences in other Old World monkeys. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4557–4561. doi: 10.1073/pnas.74.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: evidence for an Asian origin of man. Nature. 1976 May 13;261(5556):101–108. doi: 10.1038/261101a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Multiple divergent copies of endogenous C-type virogenes in mammalian cells. Nature. 1974 Nov 8;252(5479):170–173. doi: 10.1038/252170a0. [DOI] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Colcher D., Heberling R. L., Kalter S. S., Schlom J. Squirrel monkey retrovirus: an endogenous virus of a new world primate. J Virol. 1977 Aug;23(2):294–301. doi: 10.1128/jvi.23.2.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcher D., Teramoto Y. A., Schlom J. Immunological and structural relationships between langur virus and other primate type-D retroviruses. Virology. 1978 Jul 15;88(2):384–388. doi: 10.1016/0042-6822(78)90295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcher D., Teramoto Y. A., Schlom J. Interspecies radioimmunoassay for the major structural proteins of primate type-D retroviruses. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5739–5743. doi: 10.1073/pnas.74.12.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Arthur L. O., Fine D. L., Stephenson J. R. Primate retroviruses: immunological cross-reactivity between major structural proteins of new and old world primate virus isolates. J Virol. 1978 Mar;25(3):797–805. doi: 10.1128/jvi.25.3.797-805.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Hanson R. E., Jr, Stephenson J. R. Primate retroviruses: envelope glycoproteins of endogenous type C and type D viruses possess common interspecies antigenic determinants. J Virol. 1978 May;26(2):316–324. doi: 10.1128/jvi.26.2.316-324.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drohan W., Colcher D., Schochetman G., Schlom J. Distribution of Mason-Pfizer virus-specific sequences in the DNA of primates. J Virol. 1977 Jul;23(1):36–43. doi: 10.1128/jvi.23.1.36-43.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Heberling R. L., Barker S. T., Kalter S. S., Smith G. C., Helmke R. J. Oncornavirus: isolation from a squirrel monkey (Saimiri sciureus) lung culture. Science. 1977 Jan 21;195(4275):289–292. doi: 10.1126/science.63993. [DOI] [PubMed] [Google Scholar]
- Hino S., Tronick S. R., Heberling R. L., Kalter S. S., Hellman A., Aaronson S. A. Endogenous New World primate retrovirus: interspecies antigenic determinants shared with the major structural protein of type-D RNA viruses of Old World monkeys. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5734–5738. doi: 10.1073/pnas.74.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lieber M., Smith B., Szakal A., Nelson-Rees W., Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976 Jan 15;17(1):62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- Mayer R. J., Smith R. G., Gallo R. C. Reverse transcriptase in normal rhesus monkey placenta. Science. 1974 Sep 6;185(4154):864–867. doi: 10.1126/science.185.4154.864. [DOI] [PubMed] [Google Scholar]
- Nelson-Rees W. A., Owens R. B., Arnstein P., Kniazeff A. J. Source, alterations, characteristics and use of a new dog cell line (Cf2Th). In Vitro. 1976 Oct;12(10):665–669. doi: 10.1007/BF02797468. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Fleissner E., Sarkar N. H., Aoki T. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. II. Mammalian leukemia-sarcoma viruses. J Virol. 1972 Feb;9(2):359–366. doi: 10.1128/jvi.9.2.359-366.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Summers M. R., Foreman C., Gilden R. V. Murine type-C virus group-specific antigens: interstrain immunochemical, biophysical, and amino acid sequence differences. J Virol. 1974 Dec;14(6):1559–1574. doi: 10.1128/jvi.14.6.1559-1574.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal B. K., Wright M., Officer J. E., Gardner M. B., Roy-Burman P. Subviral components of a wild mouse embryo-derived type C oncornavirus. Virology. 1973 Nov;56(1):189–197. doi: 10.1016/0042-6822(73)90298-5. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Radioimmunoassay of mammalian type-C viral proteins: interspecies antigenic reactivities of the major internal polypeptide. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1766–1770. doi: 10.1073/pnas.69.7.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Kortright K., Schlom J. Mason-Pfizer monkey virus: analysis and localization of virion proteins and glycoproteins. J Virol. 1975 Nov;16(5):1208–1219. doi: 10.1128/jvi.16.5.1208-1219.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Todaro G. J., Aaronson S. A. Immunological characterization of primate C-type virus reverse transcriptases. Nat New Biol. 1972 Jan 12;235(54):35–40. doi: 10.1038/newbio235035a0. [DOI] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Specific binding of the type C viral core protein p12 with purified viral RNA. Cell. 1976 Jan;7(1):21–32. doi: 10.1016/0092-8674(76)90251-8. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Benveniste R. E., Todaro G. J. Type C viral expression in primate tissues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3721–3725. doi: 10.1073/pnas.71.9.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Todaro G. J. Radioimmunoassay of the major group specific protein of endogenous baboon type C viruses: relation to the RD-114-CCC group and detection of antigen in normal baboon tissues. Virology. 1974 Sep;61(1):168–181. doi: 10.1016/0042-6822(74)90252-9. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Endogenous C-type viral expression in primates. Nature. 1977 Mar 31;266(5601):469–472. doi: 10.1038/266469a0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Hino S., Garrett E. W., Aaronson S. A. Immunological cross reactivity of Mason-Pfizer monkey virus with type C RNA viruses endogenous to primates. Nature. 1976 Jun 17;261(5561):609–611. doi: 10.1038/261609a0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Reynolds R. K., Aaronson S. A. Comparisons of the immunological properties of two structural polypeptides of type C RNA viruses endogenous to old world monkeys. J Virol. 1976 Feb;17(2):374–384. doi: 10.1128/jvi.17.2.374-384.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Sherr C. J., Schlom J., Schidlovsky G., Stephenson J. R. Isolation and characterization of a new type D retrovirus from the asian primate, Presbytis obscurus (spectacled langur). Virology. 1978 Jan;84(1):189–194. doi: 10.1016/0042-6822(78)90231-3. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Sherwin S. A., Sherr C. J. MAC-1, a new genetically transmitted type C virus of primates: "low frequency" activation from stumptail monkey cell cultures. Cell. 1978 Apr;13(4):775–782. doi: 10.1016/0092-8674(78)90227-1. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E. Baboons and their close relatives are unusual among primates in their ability to release nondefective endogenous type C viruses. Virology. 1976 Jul 1;72(1):278–282. doi: 10.1016/0042-6822(76)90331-7. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Sen A., King N., Daniel M. D., Fleckenstein B. Endogenous New World primate type C viruses isolated from owl monkey (Aotus trivirgatus) kidney cell line. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1004–1008. doi: 10.1073/pnas.75.2.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick S. R., Stephenson J. R., Aaronson S. A. Immunological characterization of a low molecular weight polypeptide of murine leukemia virus. Virology. 1973 Jul;54(1):199–206. doi: 10.1016/0042-6822(73)90129-3. [DOI] [PubMed] [Google Scholar]
- Wallace R. E., Vasington P. J., Petricciani J. C., Hopps H. E., Lorenz D. E., Kadanka Z. Development and characterization of cell lines from subhuman primates. In Vitro. 1973 Mar-Apr;8(5):333–341. doi: 10.1007/BF02619057. [DOI] [PubMed] [Google Scholar]