Abstract
We have identified a human cytomegalovirus cell-death suppressor, denoted vICA, encoded by the viral UL36 gene. vICA inhibits Fas-mediated apoptosis by binding to the pro-domain of caspase-8 and preventing its activation. vICA does not share significant sequence homology with FLIPs or other known suppressors of apoptosis, suggesting that this protein represents a new class of cell-death suppressors. Notably, resistance to Fas-mediated apoptosis is delayed in fibroblasts infected with viruses that encode mutant vICA, suggesting that vICA suppresses death-receptor-induced cell death in the context of viral infection. Although vICA is dispensable for viral replication in vitro, the common targeting of caspase-8 activation by diverse herpesviruses argues for an important role for this antiapoptotic mechanism in the pathogenesis of viral infection in the host, most likely in avoiding immune clearance by cytotoxic lymphocytes and natural killer cells.
Apoptosis is an antiviral defense mechanism by which the host can eliminate infected cells and restrict viral propagation. To overcome this response, many viruses encode proteins that prevent or attenuate apoptosis in infected cells (1). We are interested in the role that viral anti-apoptotic genes may play in the pathogenesis of human cytomegalovirus (CMV) infections. CMV, a betaherpesvirus with a widespread distribution, is a major cause of morbidity and mortality in immunocompromised individuals such as organ transplant recipients and patients with AIDS. During pregnancy, CMV is a major cause of congenital disease. Productive CMV infection confers resistance to apoptosis induced by a variety of stimuli such as ligation of the death receptors Fas or TNFR-1 (2), an E1B19K-deficient adenovirus (3), serum withdrawal (4), or chemical inducers such as doxorubicin (2). This pattern of resistance led to an effort to discover viral genes responsible for blocking apoptosis despite the absence of any overt sequence similarity of the more than two hundred CMV proteins (5–7) to other known viral or cellular cell-death suppressors.
To identify candidate antiapoptotic genes encoded by CMV, we have used an expression library-screening strategy that is based on biological activity. We recently identified a viral mitochondria-localized inhibitor of apoptosis (vMIA) encoded by the CMV immediate-early UL37 gene that protects cells from apoptosis induced by a variety of stimuli (2). Here we report that another CMV-encoded protein, pUL36, a product of the immediate-early gene UL36, functions as a potent cell-death suppressor via a mechanism that is distinct from that of vMIA. The UL36 gene product inhibits a more proximal event in Fas-mediated apoptosis by complexing with pro-caspase-8 in a way that suppresses its proteolytic activation, prompting its designation as viral inhibitor of caspase-8-induced apoptosis (vICA). By interfering with caspase-8 activation, vICA functions in a manner similar to the viral and cellular FLIPs (8), but lack of any sequence homology suggests that this CMV death suppressor may represent a new class of viral and potentially cellular antiapoptotic proteins.
Materials and Methods
Cells and Viruses.
Human MRC-5 fibroblasts, HeLa cells, and CMV AD169varATCC were purchased from ATCC. CMV TownevarATCC was provided by A. Colberg-Poley (George Washington University). CMV TownevarRIT was a subclone (selected by three subsequent plaque purifications) of Towne (vaccine lot 131) obtained from S. Plotkin from RIT (now SmithKline Beecham Biologicals; ref. 9). CMVvarDE, a 1980 passage of AD169 used to prepare the cosmid clones described in 1982 and was provided by M. Mach (Institute für Virologie, Erlangen, Germany). The 293T cells were a gift from G. Nolan (Stanford University). Human B cell line BJAB (10) was a gift from E. Kieff (Harvard Medical School). HeLa and BJAB cells were cultured in DMEM or RPMI medium 1640, respectively, and each was supplemented with 10% (vol/vol) FBS. HeLa and BJAB cells, constitutively expressing either vMIAmyc (2) or pUL36myc, were generated by transfection with UL37exon1myc/pcDNA3 or UL36myc/pcDNA3, respectively, with subsequent selection with G418. Expression of vMIA or vICA in these cells was confirmed by immunoblot analysis with the 9E10 anti-myc antibody. Control HeLa/pcDNA3 and BJAB/pcDNA3 cells were generated by transfection with the empty vector and subsequent selection with G418.
Construction and Screening of a CMV Genomic DNA Library.
A DNA library in a mammalian expression vector was constructed from cosmids containing segments of the CMV strain AD169varDE genome plus a cosmid containing sequences unique to CMV strain Toledo (2). Functional screening of this library has been described (2). Briefly, pools of ≈500 colonies per pool were generated from the library. Plasmid DNA from each pool was mixed with a lacZ expression plasmid, and HeLa cells were transfected with the mixtures exposed to anti-Fas + cycloheximide (CHX), and cell survival was detected by a β-galactosidase (β-gal) ELISA. Individual plasmids with antiapoptotic activity were isolated from pools by repeated subdivision and β-gal transfection assays.
Plasmids and Transfections.
Mammalian expression vector pcDNA3myc, which contains a DNA sequence encoding three tandem copies of the human c-myc epitope for fusion at the C terminus of proteins, and UL37 exon 1myc/pcDNA3 were described (2). Genomic copies of the UL36-coding region were generated by PCR, using viral stocks of various CMV strains as a template, and cloned into the expression vector pcR3.1 or pcDNA3 (Invitrogen). A full-length human pro-caspase-8 (MACH α1) as well as its truncated version encoding amino acids 1–220, both HA-tagged on its N terminus, were cloned into pcDNA3. The truncated clone encodes an amino acid sequence containing both of its death effector domains but not the amino acid sequence of the active enzyme. Transfections were performed by a standard SuperFect protocol (Qiagen, Chatsworth, CA).
Cell Death/Apoptosis Assays.
Fas-mediated apoptosis in BJAB cells was induced by exposure to the 7C11 anti-Fas antibody (0.2 μg/ml; Coulter). A death-receptor-mediated apoptosis in HeLa cells was induced by exposure to a death-receptor ligand [7C11 anti-Fas antibody (0.2 μg/ml), recombinant human tumor necrosis factor α (TNFα), T 6674 (25 ng/ml), Sigma; or TNF-related apoptosis inducing ligand (0.12 μg/ml), Cell Sciences, Norwood, MA] either in the presence of CHX (10 μg/ml; Sigma) or in serum-free DMEM. Cell death was examined by visual observation of cells under a phase microscope (2) or in a clonogenic assay (11). Cell death induced in HeLa cells by doxorubicin or E1B19k-deficient adenovirus was examined as described (2).
Immunoprecipitation.
Cells were lysed in 150 mM NaCl/5 mM EDTA/50 mM Tris⋅HCl, pH 8.0/1% Triton-X100 in the presence of protease inhibitors, and were centrifuged at 10,000 × g at 4°C for 10 min. The supernatants were precleared first with ethanolamine-treated Affi-Prep 10 beads (Bio-Rad), then incubated with 9E10 antibody covalently linked to Affi-Prep 10 beads, and washed with the lysis buffer. Protein samples were separated under reduced conditions by SDS/PAGE after being loaded at equal protein amounts per well, and were analyzed by a standard immunoblot protocol using the enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia) and secondary HRP-conjugated antisera. Antibodies 5F7 anti-human caspase-8 (Upstate Biotechnology, Lake Placid, NY), anti-FADD (PharMingen), anti-human Bid C-20 (Santa Cruz Biotechnology), C210 anti-PARP (Biomol, Plymouth Meeting, PA), and anti-HA Y-11 (Santa Cruz Biotechnology) were used.
Results
Identification of pUL36 as a Death Suppressor.
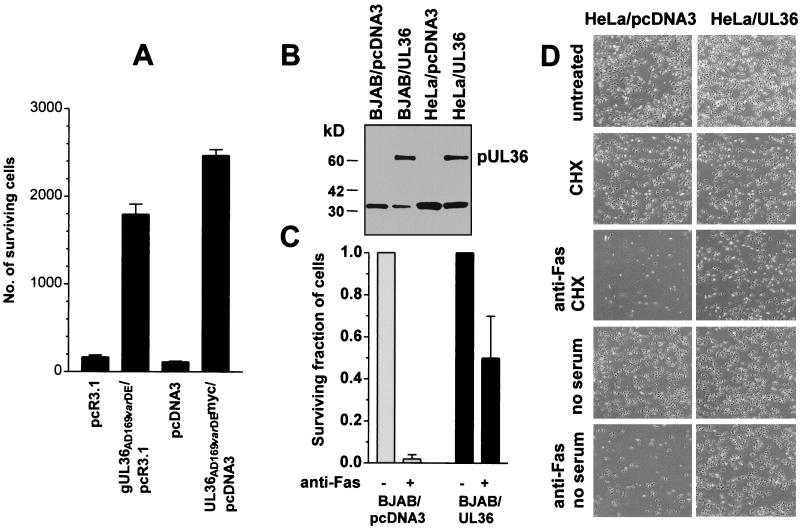
To identify CMV genes that suppress Fas-mediated apoptosis, we used a cell-survival assay that allowed the viability of HeLa cells treated with anti-Fas plus CHX to be assessed rapidly by the level of expression of a transfected lacZ [encoding β-galactosidase (β-gal)] indicator plasmid (2). Cotransfection of known cell death-suppressor genes such as Bcl-2 or CMV vMIA significantly enhances β-gal expression as an indicator of sustained cell viability that may be ascribed to antiapoptotic functions (2). An expression library prepared from fragmented CMV cosmids containing DNA of a pre-1982 German stock of CMVAD169 (AD169varDE; ref. 12) was divided into pools, and these pools were screened for their ability to block apoptosis in this assay. Five active pools were identified, and a plasmid with strong antiapoptotic activity was isolated from each pool. Previously, we reported that the sequence of the CMV DNA inserts in three of these plasmids contained the coding region of vMIA (CMV immediate-early gene UL37 exon 1; ref. 2). The two remaining plasmids with strong antiapoptotic activity spanned a region of the CMV genome (AD169, nucleotides 47,532–49,917; and AD169, nucleotides 48,241–49,917, respectively) that in each case covers the coding region of the previously described protein product of the CMV immediate-early gene UL36 (13, 14). To assign definitively the antiapoptotic activity detected in these two plasmids to pUL36, a genomic DNA segment spanning just the UL36-coding region of AD169varDE was generated by PCR and cloned into the mammalian expression vector pcR3.1 (denoted gUL36/pcR3.1). To isolate a cDNA clone of the UL36-coding region, 293T cells were transfected with gUL36/pcR3.1. UL36 cDNA was generated by RT-PCR and cloned into pcDNA3myc (denoted UL36myc/pcDNA3), which introduced a myc-tag epitope at the C terminus of pUL36. Both gUL36/pcR3.1 and UL36myc/pcDNA3 protected HeLa cells against Fas-mediated apoptosis in transient transfection assays (Fig. 1A), demonstrating that the UL36 gene was responsible for the antiapoptotic function of the plasmids isolated from the CMV genomic DNA library and that a C terminal epitope tag did not disrupt this function.
Figure 1.
Antiapoptotic activity of pUL36 in HeLa and BJAB cells. (A) HeLa cells (4 × 104 cells per sample) were transfected transiently with an expression vector containing genomic UL36AD169varDE, cDNA UL36AD169varDEmyc, or one of the respective empty vectors. Twenty-four hours after transfection, the cells were exposed to anti-Fas + CHX for an additional 24 h, and surviving cells were scored under a microscope. The data are presented as means ± SEM (n = 4). (B) Expression of pUL36 in stably transfected HeLa and BJAB cells. Lysates from HeLa and BJAB cells stably transfected with either UL36AD169varDEmyc/pcDNA3 or an empty vector (pcDNA3myc) were immunoprecipitated with the anti-myc antibody 9E10 covalently linked to Affi-Prep-10 beads. Bound pUL36 was detected by immunoblot analysis with anti-myc 9E10 antibody. (C) Resistance of BJAB/pcDNA3 cells and BJAB/UL36 cells to Fas-mediated apoptosis. Cells were exposed to anti-Fas antibody or to medium alone (untreated) for 24 h, and then the surviving fractions of cells were determined in a clonogenic assay as described (11). The data are presented as the means ± SEM (n = 2). (D) Resistance of HeLa/UL36 cells to Fas-mediated apoptosis. Cells were incubated for 24 h either in DMEM containing 10% (vol/vol) FBS or in serum-free DMEM. The following reagents were added to the medium: anti-Fas antibody + CHX, CHX, or none (untreated). Then the cells were photographed under a phase-contrast microscope. The cells in cultures exposed to CHX alone ceased to proliferate, whereas the control cells nearly doubled in number during the 24-h incubation.
To examine further the antiapoptotic function of pUL36, we transfected HeLa and BJAB cells with UL36myc/pcDNA3 and isolated clones that constitutively express pUL36myc (Fig. 1B). Both HeLa/UL36 and BJAB/UL36 cells were resistant to Fas-mediated apoptosis. A 24-h exposure to anti-Fas antibody killed most of the control BJAB/pcDNA3 cells, whereas about 50% BJAB/UL36 cells survived the treatment (Fig. 1C). Similarly, a 24-h exposure to anti-Fas antibody, either in the presence of CHX or in the absence of serum, killed a vast majority of the control HeLa/pcDNA3 cells, whereas most of HeLa/UL36 cells survived either treatment (Fig. 1D). The resistance of pUL36-expressing HeLa cells to Fas-mediated apoptosis was not the result of a lack of Fas expression, because these cells expressed as much Fas on their surfaces as did control cells by flow cytometric analysis (as described in ref. 2 and data not shown). Of ≈700 HeLa colonies generated by transfection with UL36myc/pcDNA3 and selection with G418, 71 were resistant to Fas-mediated apoptosis (consistent with our observations that about 10% of stably transfected colonies express the gene), whereas all cells in a similar pool of HeLa colonies stably transfected with the empty vector died after this treatment.
HeLa cells expressing pUL36 also were resistant to apoptosis triggered by tumor necrosis factor-α and TRAIL (tested either in the presence of CHX or in the absence of serum), whereas all control HeLa/pcDNA3 cells were killed (data not shown). HeLa/UL36 cells also displayed modestly delayed cell death (compared with the control cells) when exposed to the anti-cancer drug doxorubicin or infected with the E1B19K-deficient adenovirus mutant Ad2dl250 (these experiments were performed as described in ref. 2).
CMV Strains AD169varATCC and TownevarRIT Encode Inactive Mutants of pUL36.
We identified the antiapoptotic function of the UL36 gene by using DNA and cDNA prepared from an early (pre-1982) passage of the CMV strain AD169 (denoted AD169varDE). A recent study revealed strain-specific variation in the predicted amino acid sequences of pUL36 (14). We therefore examined whether amino acid sequence variations affected the antiapoptotic activity of pUL36. First, UL36-coding regions of several CMV strains were sequenced. AD169varDE, which was used to prepare the initial library screen; AD169, obtained from ATCC (denoted below as AD169varATCC); TownevarATCC; and TownevarRIT, which was used in our previous experiments simply as strain Towne (2), all were compared. The predicted amino acid sequences of pUL36 from AD169varATCC and TownevarATCC are identical to the published sequences for these strains (5, 14). An alignment of the pUL36 sequences encoded by AD169varDE, AD169varATCC, TownevarATCC, TownevarRIT, and the clinical isolate Toledo (14) are depicted in Fig. 2. pUL36 encoded by AD169varATCC differs from pUL36 encoded by AD169varDE by a single amino acid substitution changing Cys131 to Arg131. The three other sequenced strains of human CMV, TownevarATCC, TownevarRIT, and Toledo also encode Cys131, indicating that the Cys131 ↛ Arg131 substitution in pUL36 from AD169varATCC is a mutation that occurred during propagation of this virus in culture. The sequences of pUL36 encoded by TownevarATCC, AD169varDE, and Toledo are nearly identical, whereas pUL36 from the TownevarRIT has a large deletion/rearrangement between Leu156 and His295, apparently also a result of extensive in vitro propagation to attenuate this virus for use as a candidate vaccine (15).
Figure 2.
Sequence alignment of ORF translations of pUL36 encoded by several laboratory CMV strains. The predicted amino acid sequences for the UL36-coding region obtained from the cosmid pCM1017 containing a segment of the genome of AD169varDE and those from gUL36AD169varDE/pcR3.1 and from UL36AD169varDEmyc/pcDNA3 were identical, and their translation is shown here as AD169varDE. The sequences for the UL36-coding region obtained from a cosmid containing the genomic DNA for TownevarRIT and from gUL36TownevarRIT/pcDNA3 were identical. The sequence obtained from UL36AD169varATCCmyc/pcDNA3 was identical to that published previously (5). The amino acid sequence for the translation from gUL36TownevarATCC/pcDNA3 was identical to that of pUL36TownevarATCC published previously (14). The sequences for the pUL36 translations obtained from TownevarATCC (denoted as Towne) and Toledo were taken from ref. 14.
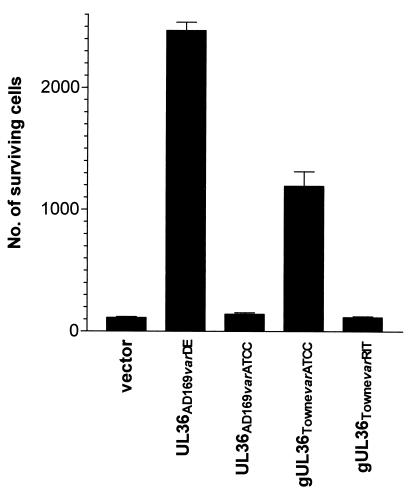
Second, we sought to test the function of pUL36 variants by isolating genomic DNA segments spanning the UL36-coding regions of CMV strains AD169varATCC, TownevarATCC, and TownevarRIT by PCR, cloning them into an expression vector, and comparing antiapoptotic activity in transient transfection assays (Fig. 3). pUL36 from AD169varDE and TownevarATCC were active as death suppressors in these experiments, whereas other variants from AD169varATCC or TownevarRIT completely lacked antiapoptotic activity. These findings indicate that the single-point mutation in AD169varATCC (Cys131 ↛ Arg131) and the large deletion in TownevarRIT are sufficient to abrogate the antiapoptotic function of pUL36.
Figure 3.
Comparison of antiapoptotic activities of pUL36 proteins encoded by CMVAD169varDE, CMVAD169varATCC, CMVTownevarATCC, and CMVTownevarRIT. Cells (4 × 104 cells per sample) were transfected transiently with an expression vector that was either empty or contained an ORF for one of these genes, and 24 h later the cells were exposed to anti-Fas + CHX for an additional 24 h. Surviving cells were scored under a microscope. The data are presented as means ± SEM (n = 4).
pUL36 Prevents Caspase-8 Activation.
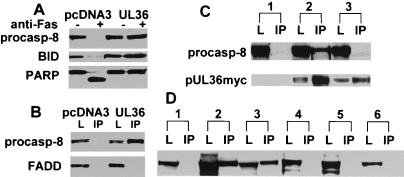
Fas-mediated signaling results in caspase-8 being recruited to the Fas complex through an adapter death-domain-containing protein called FADD that leads to caspase-8 activation by means of proteolytic processing (16). To determine whether pUL36 prevents any of these events, we examined the effect of AD169varDE pUL36 on caspase-8 activation after ligation of Fas. The proteolytic processing and activation of caspase-8 was inhibited in both BJAB/UL36 cells and HeLa/UL36 but not in the respective control cell lines, HeLa/pcDNA3 and BJAB/pcDNA3 (Fig. 4A and data not shown). This result indicates that pUL36 functions to interrupt Fas-mediated apoptotic signaling at, or upstream of caspase-8 activation, and as such is designated vICA. Consistent with this finding, two biochemical events that occur downstream of caspase-8 activation, processing of BID (17, 18) and cleavage of PARP (19), also were inhibited in vICA-expressing cells but not in controls (Fig. 4A).
Figure 4.
pUL36 interrupts Fas-mediated apoptosis at the step of caspase-8 activation. (A) Effects of pUL36 expression on biochemical events associated with Fas-mediated apoptosis. BJAB/pcDNA3 cells and BJAB/UL36 cells were treated for 24 h with anti-Fas or were left untreated. Cell lysates were prepared, separated by SDS/PAGE, and blotted onto nitrocellulose. Then procaspase-8, Bid, and PARP were detected by immunoblot analysis. (B) Association of pUL36 with pro-caspase-8. Lysates from BJAB/UL36 cells or from control BJAB/pcDNA3 cells were immunoprecipitated (IP) with the anti-myc antibody 9E10 covalently linked to Affi-Prep-10 beads. Lanes designated IP are proteins bound with anti-myc antibody. Pro-caspase-8 and FADD were detected by immunoblot analysis. Lanes designated L are samples of the transfected cell lysate taken before immunoprecipitation. (C) Pro-caspase-8 fails to coimmunoprecipitate with pUL36AD169varATCC in transiently transfected 293T cells. Cells were transfected with pcDNA3 (lane 1), UL36AD169varDEmyc/pcDNA3 (lane 2), or UL36AD169varATCCmyc/pcDNA3 (lane 3). The cells were lysed 24 h later, and pUL36 immunoprecipitated with anti-myc antibody. Cell lysates and immunoprecipitates were probed with either anti-pro-caspase-8 antibody or anti-myc antibody. Pro-caspase-8 was easily detectable in an IP sample of pUL36AD169varDE diluted to the concentration that gave a similar pUL36 signal to that of the pUL36AD169varATCC sample, but no pro-caspase-8 signal (data not shown). (D) pUL36AD169varDE, but not pUL36AD169varATCC, coimmunoprecipitates with either HA-tagged pro-caspase-8 or the HA-tagged pro-domain fragment of pro-caspase-8 in transiently transfected 293T cells. Cells were transfected with the following pairs of plasmids: UL36AD169varDEmyc plus either vector (lane 1), HA-caspase-8-pro-domain (lane 2), or HA-pro-caspase-8 (lane 3); UL36AD169varATCCmyc plus either vector (lane 4); HA-caspase-8-pro-domain (lane 5); or HA-pro-caspase-8 (lane 6). The cells were lysed 24 h later, immunoprecipitated with anti-HA antibody, and coprecipitated pUL36myc was detected by immunoblot with anti-myc antibody.
vICA Forms a Complex with Pro-Caspase-8.
Given evidence that vICA prevented the activation of caspase-8 by proteolytic cleavage in a Fas-induced apoptosis cascade, we tested whether this protein exhibited the ability to interact physically with either the pro-caspase-8 or FADD. pUL36myc was immunoprecipitated from lysates of stably transfected BJAB cells, protein complexes were separated by electrophoresis on SDS polyacrylamide gels, and immunoblot analysis was performed with anti-caspase-8 and anti-FADD antibodies (Fig. 4B). Activated caspase-8 was not detected in these samples in immunoblot analysis with antibodies recognizing active caspase-8 (not shown), indicating that pUL36 had affinity to pro-caspase-8 but not to active caspase-8. Pro-caspase-8 was detected in anti-myc immunoprecipitates from pUL36myc-expressing BJAB cells but not from control cells lacking pUL36myc, indicating that vICA formed a complex with pro-caspase-8 in BJAB/UL36 cells. FADD was not detected in pUL36myc immunoprecipitates, indicating that FADD did not form a complex with pUL36myc in this assay. Lysates from both pUL36myc-expressing and control cells contained equivalent levels of FADD and pro-caspase-8 (Fig. 4B). Similar results were obtained with pUL36myc-expressing HeLa cell lysates; pro-caspase-8 but not FADD coprecipitated with pUL36myc (data not shown).
To examine whether the interaction with pro-caspase-8 correlates with the antiapoptotic function of vICA, we tested the ability of this protein from AD169varDE, which inhibits Fas-mediated apoptosis, and an inactive mutant form of pUL36 from AD169varATCC to bind to pro-caspase-8 in transiently transfected 293T cells. Both pUL36 proteins were expressed in the transfected cells (Fig. 4C) but differed in their ability to form a complex with pro-caspase-8. AD169varDE pUL36 coprecipitated pro-caspase-8, whereas the mutant pUL36 did not (Fig. 4C). This result, taken together with the experiment shown in Fig. 3, indicates that the Cys131 ↛ Arg131 mutation in the AD1659varATCC pUL36 disrupts both the antiapoptotic and caspase-8-binding functions of vICA.
The pro-domain of caspase-8 mediates its interaction with the adapter molecule FADD and with FLIPs, viral proteins that likewise prevent activation of caspase-8 (8). To determine whether the pro-domain of caspase-8 interacts with vICA, 293T cells were cotransfected with pUL36myc and with a truncated pro-caspase-8, containing only its HA-tagged pro-domain region. The pro-domain was then immunoprecipitated with anti-HA antibody, and associated pUL36myc was detected by immunoblot analysis with the anti-myc antibody. As controls, pUL36myc was cotransfected with either the empty vector (negative control) or with HA-tagged full-length pro-caspase-8 (positive control). As shown in Fig. 4D, functional pUL36myc coprecipitated with the caspase-8 pro-domain as well as with the full-length pro-caspase-8 but not with the empty vector. A nonfunctional mutant pUL36myc from AD169varATCC failed to coprecipitate with either the HA-pro-domain or HA-pro-caspase-8 (Fig. 4D). Thus, pUL36 from AD169varDE inhibits Fas-mediated apoptosis, associates with the pro-domain of pro-caspase-8, and inhibits its processing, whereas the Cys131 ↛ Arg point mutant pUL36 from AD169varATCC fails to exhibit any antiapoptotic activity or association. Taken together, these results indicate that vICA interrupts the Fas-apoptotic signaling pathway at the step of activation of caspase-8.
Human Fibroblasts Infected with pUL36-Deficient CMV Acquire Resistance to Fas-Mediated Apoptosis with Delayed Kinetics.
Previously we reported (2) that human fibroblasts infected with CMV acquire resistance to Fas-mediated apoptosis. Cells infected with CMV strain TownevarRIT did not become resistant until 2 days after infection, which correlated with the onset of mitochondrial accumulation of vMIA (2). These experiments were performed with a strain of CMV that we now know carries an inactive mutant, pUL36 (see above). Although both UL37 and UL36 are immediate-early (α) genes, abundant expression of pUL36/vICA occurs earlier than pUL37 exon 1/vMIA expression. pUL36 accumulation was detectable readily within a few hours after virus adsorption (14). To determine whether this abundant early expression had an impact on resistance to cell death, we compared the time course of the development of resistance to Fas-mediated apoptosis in cells infected with CMV strains that differ in vICA function but that all encode functional vMIA, AD169varDE, AD169varATCC, TownevarATCC, and TownevarRIT. Comparable levels of vICA were produced in cells 24 h after infection with AD169varATCC, AD169varDE, or TownevarRIT, whereas TownevarATCC-infected cells accumulated more vICA (immunoblot analysis; data not shown), consistent with the data of Patterson and Shenk (14). Cells infected with any of the four viruses produced comparable levels of vMIA at this time point (immunoblot; data not shown).
Human fibroblasts were infected separately with each of these four CMV strains, and then exposed to anti-Fas antibody plus CHX immediately, or at 1, 2, or 3 days postinfection. The results of these experiments are summarized in Table 1. Consistent with previous results (2), cells infected with either of the two pUL36-deficient CMV strains, AD169varATCC or TownevarRIT, acquired resistance to apoptosis after only 2 days of infection. In contrast, cells infected with either of the two CMV strains encoding active vICA, AD169varDE or TownevarATCC, became resistant to apoptosis within 24 h after infection, consistent with the early abundant accumulation of pUL36 in CMV-infected cells (14). These results suggest that CMV-infected cells acquire resistance to Fas-mediated apoptosis initially by means of expression of vICA, with later protection against a broad range of apoptotic stimuli acquired by means of expression and mitochondrial localization of vMIA.
Table 1.
Resistance of MRC5 human fibroblasts infected with CMV to Fas-mediated apoptosis
| CMV strain | Time after infection*
|
|||
|---|---|---|---|---|
| 0 days | 1 day | 2 days | 3 days | |
| AD169varDE | 0 ± 0 | 47 ± 4 | 73 ± 5 | 81 ± 1 |
| AD169varATCC | 0 ± 0 | 0.13 ± 0.03 | 72 ± 6 | 72 ± 2 |
| TownevarATCC | 0 ± 0 | 40 ± 6 | 74 ± 8 | 80 ± 7 |
| TownevarRIT | 0 ± 0 | 0.10 ± 0.06 | 72 ± 9 | 73 ± 5 |
The data represent three independent experiments (means ± SEM). Cells were infected with CMV (approximately 3 plaque-forming units per cell). At the indicated times after infection, cells were exposed for an additional 12 h to anti-Fas antibody plus CHX or to medium alone. Viable cells remaining after these treatments were quantified by counting representative fields under a phase-contrast microscope. Cultures exposed to anti-Fas plus CHX were compared to those exposed to medium alone.
Cells surviving a 12-h exposure to anti-Fas plus CHX (% control).
Discussion
We have shown that pUL36/vICA, a protein product of the CMV immediate-early gene UL36, functions as a potent cell-death suppressor by binding to pro-caspase-8 and inhibiting its activation after ligation of Fas to induce apoptosis. The mode of action by which vICA inhibits apoptosis is reminiscent of FLIPs (20). This protein, however, does not share any amino acid sequence homology with cellular or viral FLIPs and, most notably, does not contain death-effector domains, which are conserved in FLIPs, FADD, and certain caspase pro-domains (20). Therefore, vICA seems to represent a separate class of antiapoptotic proteins.
vICA is the second antiapoptotic CMV gene discovered through a rapid and straightforward functional screening strategy initially described in the characterization of vMIA (2). vMIA, a product of the viral UL37 exon 1 gene, was identified previously by this approach and acts as a mitochondria-localized inhibitor of apoptosis that blocks the release of cytochrome c during Fas-mediated apoptosis (2). These functional properties of vMIA resemble those ascribed to Bcl-2 and its antiapoptotic homologs. However, the lack of any homology to domains conserved in Bcl-2 family members, and subsequent structure/function studies (21), indicates that vMIA represents a distinct class of mitochondrial antiapoptotic proteins. Similarly, although vICA resembles FLIPs at a functional level by blocking caspase-8 activation, the lack of sequence homology argues that this protein functions in a distinct manner. Viral Bcl-2 homologs and FLIPs have been discovered by the presence of conserved amino acid motifs [e.g., Bcl-2 homology (BH) and death-effector domains] that define members of these families. The identification of vICA and vMIA, CMV-encoded proteins that lack overt homology to any known cell-death suppressors, shows the value of a functional approach for identifying novel classes of proteins that regulate apoptosis. Potentially, similar approaches could be used to reveal additional novel viral or cellular antiapoptotic genes.
Cells infected with pUL36-deficient strains of CMV exhibit a delay in their acquired resistance to Fas-mediated apoptosis. Apart from this phenotype, the antiapoptotic function of vICA clearly is dispensable for replication of CMV in cultured fibroblasts, because laboratory strains of CMV can acquire readily inactivating mutations in the UL36 gene (e.g., AD169varATCC and TownevarRIT), and complete deletion of the UL36-coding region (in AD169varATCC) has no impact on propagation of CMV on in vitro-cultured human fibroblasts (14). We infer from these findings that there is little selective pressure to maintain vICA function during the propagation of laboratory CMV strains, at least in cultured fibroblasts. This mutability of UL36 may reflect in part a redundant antiapoptotic function provided by vMIA/pUL37, which is conserved highly in clinical isolates and laboratory strains (21). The conditions used to culture fibroblasts and propagate CMV are unlikely to present the range of apoptotic stimuli that the virus might encounter during infections in the host, particularly those that arise in a cell-type-specific fashion or that are imposed by cytotoxic immune-effector mechanisms.
Why does CMV encode multiple antiapoptotic proteins? There are a number of host-cell types infected by CMV during the course of a natural infection (6), and vMIA and pUL36 differ in both their antiapoptotic mechanisms and their onsets of expression in infected cells. These two cell-death suppressors, therefore, may have distinct and important antiapoptotic functions during CMV infection in vivo. vICA and vMIA may have evolved to protect different infected cell populations of the host; provide protection from apoptosis at different times of CMV infection; protect cells from apoptosis induced by different stimuli of apoptosis; and/or protect cells from a variety of cytotoxic clearance mechanisms. vICA provides an efficient block to apoptosis mediated by death receptors given its intervention at a proximal point (caspase-8 activation) in this pathway. By contrast, vMIA (and Bcl-2) cannot inhibit apoptosis triggered by Fas in certain cell types (so-called type I cells; refs. 22–24) that do not require mitochondrial events downstream of caspase-8 activation for Fas-mediated cell death. For example, although vICA functions well in both fibroblasts (type II) and lymphoid BJAB (type I) cells (see Results), we found that vMIA protects normal human fibroblasts and HeLa cells but not type I (22, 25) BJAB cells from Fas-mediated apoptosis (data not shown). This finding is in accord with a previously published result that Bcl-2 protected MCF7-Fas cells but failed to protect BJAB cells from Fas-mediated apoptosis (22, 25). vMIA may provide efficient protection in infected cells against other apoptotic signals, such as DNA damage induced by viral replication, that are mediated mainly by mitochondrial events rather than caspase-8 activation.
In a broader context, our findings add CMV to an expanding group of viruses that encodes two or more antiapoptotic genes with differing modes of action (20, 26) and suggests that intervening in cell-death pathways at multiple points may be a general strategy of viruses. Most strikingly, a number of herpesviruses (herpesvirus saimiri, Kaposi sarcoma-associated herpesvirus, equine herpesvirus-2, bovine herpesvirus-4, and herpesvirus ateles) encode both a FLIP homolog and a Bcl-2 homolog (20). Instead of encoding such proteins, CMV encodes vICA and vMIA, proteins that seem to differ structurally and functionally from FLIP and Bcl-2, respectively, but nonetheless interrupt Fas-mediated apoptosis at similar steps. We infer from these similarities among CMV and a number of other herpesviruses that the two targeted apoptotic signals, caspase-8 activation and cytochrome c release, play important roles in restricting propagation of herpesviruses. Several herpesviruses, Epstein–Barr virus, alcelaphine herpesvirus-1, and murine gammaherpesvirus-68, are known to encode one or more Bcl-2 homologs (20, 27). Arguing by analogy with other herpesviruses, we predict that these viruses are likely to encode an inhibitor of caspase-8 activation as well. It may be that all herpesviruses must retain the ability to interfere with multiple types of apoptosis to be successful pathogens.
Acknowledgments
We thank our colleagues at ImmunoGen for support, reagents, and plasmids. We especially acknowledge the contribution of Dr. Carol Vater who prepared the CMV genomic library used in this study. We thank Dr. Michael Mach for AD169varDE, Dr. Colberg-Poley for AD169varATCC, and Dr. George Kemble (Aviron) for sharing UL36 sequencing results.
Abbreviations
- CHX
cycloheximide
- CMV
human cytomegalovirus
- vICA
viral inhibitor of caspase-8-induced apoptosis
- vMIA
viral mitochondria-localized inhibitor of apoptosis
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.O'Brien V. J Gen Virol. 1998;79:1833–1845. doi: 10.1099/0022-1317-79-8-1833. [DOI] [PubMed] [Google Scholar]
- 2.Goldmacher V S, Bartle L M, Skaletskaya A, Dionne C A, Kedersha N L, Vater C A, Han J, Lutz R J, Watanabe S, McFarland E D, et al. Proc Natl Acad Sci USA. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu H, Shen Y, Shenk T. J Virol. 1995;69:7960–7970. doi: 10.1128/jvi.69.12.7960-7970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovacs A, Weber M L, Burns L J, Jacob H S, Vercellotti G M. Am J Pathol. 1996;149:1531–1539. [PMC free article] [PubMed] [Google Scholar]
- 5.Chee M S, Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchison C A d, Kouzarides T, Martignetti J A, et al. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 6.Mocarski E S, Courcelle C T. In: Fields Virology. Knipe D M, Howley P M, editors. New York: Lippincott–Raven; 2001. pp. 2629–2673. [Google Scholar]
- 7.Cha T A, Tom E, Kemble G W, Duke G M, Mocarski E S, Spaete R R. J Virol. 1996;70:78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tschopp J, Irmler M, Thome M. Curr Opin Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- 9.Plotkin S A, Higgins R, Kurtz J B, Morris P J, Campbell D A, Jr, Shope T C, Spector S A, Dankner W M. Transplantation. 1994;58:1176–1178. [PubMed] [Google Scholar]
- 10.Menezes J, Leibold W, Klein G, Clements G. Biomedicine. 1975;22:276–284. [PubMed] [Google Scholar]
- 11.Han J W, Dionne C A, Kedersha N L, Goldmacher V S. Cancer Res. 1997;57:176–182. [PubMed] [Google Scholar]
- 12.Fleckenstein B, Muller I, Collins J. Gene. 1982;18:39–46. doi: 10.1016/0378-1119(82)90054-3. [DOI] [PubMed] [Google Scholar]
- 13.Colberg-Poley A M. Intervirology. 1996;39:350–360. doi: 10.1159/000150506. [DOI] [PubMed] [Google Scholar]
- 14.Patterson C E, Shenk T. J Virol. 1999;73:7126–7131. doi: 10.1128/jvi.73.9.7126-7131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plotkin S A, Farquhar J, Horberger E. J Infect Dis. 1976;134:470–475. doi: 10.1093/infdis/134.5.470. [DOI] [PubMed] [Google Scholar]
- 16.Ashkenazi A, Dixit V M. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Zhu H, Xu C J, Yuan J. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 18.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 19.Salvesen G S, Dixit V M. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 20.Tschopp J, Thome M, Hofmann K, Meinl E. Curr Opin Genet Dev. 1998;8:82–87. doi: 10.1016/s0959-437x(98)80066-x. [DOI] [PubMed] [Google Scholar]
- 21.Hayajneh W A, Colberg-Poley A M, Skaletskaya A, Bartle L M, Lesperance M M, Contopoulos-Ioannidis D G, Kedersha N L, Goldmacher V S. Virology. 2001;279:233–240. doi: 10.1006/viro.2000.0726. [DOI] [PubMed] [Google Scholar]
- 22.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scaffidi C, Schmitz I, Zha J, Korsmeyer S J, Krammer P H, Peter M E. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz I, Walczak H, Krammer P H, Peter M E. Cell Death Differ. 1999;6:821–822. doi: 10.1038/sj.cdd.4400569. [DOI] [PubMed] [Google Scholar]
- 25.Foghsgaard L, Jaattela M. J Virol. 1997;71:7509–7517. doi: 10.1128/jvi.71.10.7509-7517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teodoro J G, Branton P E. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall W L, Yim C, Gustafson E, Graf T, Sage D R, Hanify K, Williams L, Fingeroth J, Finberg R W. J Virol. 1999;73:5181–5185. doi: 10.1128/jvi.73.6.5181-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]