Abstract
AIM: To compare results of liver stiffness measurements by transient elastography (TE) obtained in our patients population with that used in a recently published meta-analysis.
METHODS: This was a single center cross-sectional study. Consecutive patients with chronic viral hepatitis scheduled for liver biopsy at the outpatient ward of our Infectious Diseases Department were enrolled. TE was carried out by using FibroScan™ (Echosens, Paris, France). Liver biopsy was performed on the same day as TE, as day case procedure. Fibrosis was staged according to the Metavir scoring system. The diagnostic performance of TE was assessed by using receiver operating characteristic (ROC) curves and the area under the ROC curve analysis.
RESULTS: Two hundred and fifty-two patients met the inclusion criteria. Six (2%) patients were excluded due to unreliable TE measurements. Thus, 246 (171 men and 75 women) patients were analyzed. One hundred and ninety-five (79.3%) patients had chronic hepatitis C, 41 (16.7%) had chronic hepatitis B, and 10 (4.0%) were coinfected with human immunodeficiency virus. ROC curve analysis identified optimal cut-off value of TE as high as 6.9 kPa for F ≥ 2; 7.9 kPa for F ≥ 3; 9.6 kPa for F = 4 in all patients (n = 246), and as high as 6.9 kPa for F ≥ 2; 7.3 kPa for F ≥ 3; 9.3 kPa for F = 4 in patients with hepatitis C (n = 195). Cut-off values of TE obtained by maximizing only the specificity were as high as 6.9 kPa for F ≥ 2; 9.6 kPa for F ≥ 3; 12.2 kPa for F = 4 in all patients (n = 246), and as high as 7.0 kPa for F ≥ 2; 9.3 kPa for F ≥ 3; 12.3 kPa for F = 4 in patients with hepatitis C (n = 195).
CONCLUSION: The cut-off values of TE obtained in this single center study are comparable to that obtained in a recently published meta-analysis that included up to 40 studies.
Keywords: Chronic viral hepatitis, Hepatitis C, Liver, Liver biopsy, Liver fibrosis, Elastography, Transient elastography, FibroScan, Ultrasound
INTRODUCTION
In chronic viral hepatitis, the degree of liver fibrosis affects both prognosis and treatment. Biopsy is still considered the reference standard for the assessment of liver fibrosis, even though it evaluates 1/50 000 only of the liver parenchyma and is subject to intra and inter-observer variability[1,2]. Moreover, it is an invasive procedure not well accepted by patients that is burdened by complications even though at a low rate[3,4]. For these reasons, in recent years imaging[5-8] or biochemical methods[9-13] for a noninvasive assessment of liver fibrosis have been tested. The imaging methods estimate elasticity of liver parenchyma, which is directly related to the amount of fibrosis. The method most widely used and validated is transient elastography (TE) (FibroScan Echosens, Paris)[9,14-17], which allows to evaluate the elasticity of a portion of liver parenchyma that is around 100 times greater than that examined by biopsy[18].
Several studies have shown significant positive correlation between TE and the stage of liver fibrosis[14-20]. The performance of TE is optimal for advanced stages of fibrosis[15,21-27].
A limitation of all the studies performed so far is that reference cut-off values of TE for each fibrosis stage were calculated from their own cohort of patients after data collection, and they are different across studies[8]. Few metanalysis have been carried out that have analysed the heterogeneity between studies and the overall performance of TE[5-8]. In the most recent one the cut-off values of 7, 9.5, and 12 kPa in stages F2, F3, and F4, respectively, were used[8].
The aim of this study was to compare the cut-offs of TE obtained by receiver operating characteristic (ROC) curve analysis in our series of consecutive patients, with chronic viral hepatitis referring to our center for liver biopsy, to those used in the recently published meta-analysis[8].
MATERIALS AND METHODS
Patients
This was a single center cross-sectional study. From June, 2009 through February, 2012, all consecutive patients with chronic viral hepatitis scheduled for liver biopsy at the outpatient ward of our Infectious Diseases Department were enrolled into the study.
Diagnosis of chronic hepatitis was based on the presence, at the time of the first observation, of active viral replication or detectable hepatitis C virus (HCV)-RNA in hepatitis B surface antigen or anti-HCV positive carriers, respectively, and, at least transiently, elevated serum alanine aminotransferase level. Patients characteristics, epidemiological data, and biochemical tests were recorded. Liver biopsy was performed on the same day as TE, as day case procedure.
The study protocol was approved by the institutional Ethics Committee. Participants gave their informed written consent.
Transient elastography
TE was carried out by using FibroScan™ (Echosens, Paris, France), a dedicated medical device which provides a quantifiable estimate of liver stiffness in kilopascal (kPa). Two physicians (Ferraioli G and Zicchetti M), with experience of at least 50 TE procedures as previously recommended[9], performed all the examinations. Measurements of liver stiffness were performed on the right lobe through intercostal spaces on patients lying in the dorsal decubitus position with the right arm in maximal abduction, following the examination procedure previously described[14]. Only patients with 10 validated measurements, a success rate of at least 60%, and interquartile range (IQR) of less than 30% of the median liver stiffness value were included. Values were expressed in kPa.
Liver biopsy and histology
Ultrasound-assisted percutaneous liver biopsy (LB) was performed by three experienced physicians (Filice C, Brunetti E, Michelone G) by using an intercostal approach. A disposable 1.4 mm-diameter modified Menghini needle (Hepafix; Braun, Melsungen, Germany) was used. All biopsy specimens were fixed in formalin and embedded in paraffin. The length of each LB specimen (in centimeters) was recorded.
The specimens were read on site by a single expert liver pathologist (Dal Bello B), blind to the TE results, but not to the patient’s clinical and biochemical data. Fibrosis was evaluated semiquantitatively and staged according to the Metavir scoring system (F0, absent; F1, enlarged fibrotic portal tract; F2, periportal or initial portal-portal septa but intact architecture; F3, architectural distortion but no obvious cirrhosis; and F4, cirrhosis)[28]. For the purpose of this study, F0 and F1 scores were grouped in the subsequent statistical analysis. Necro-inflammatory activity was evaluated using the histologic activity index (HAI) described by Knodell et al and classified as follows for statistical analysis: HAI score, 1-5 = mild; HAI score, 6-9 = moderate; HAI score, ≥ 10 = severe[29]. Steatosis was expressed as a percentage of fat in the hepatocytes and classified as absent (S0), < 33% (S1), between 33% and 66% (S2), and > 66% (S3)[30].
Statistical analysis
Descriptive statistics were produced for demographic, clinical and laboratory characteristics for this study sample of patients. The Shapiro-Wilk test was used to test the normal distribution of quantitative variables. When quantitative variables were normally distributed, the results were expressed as mean values and SD, otherwise median and IQR (25th-75th percentile) were reported; qualitative variables were summarized as counts and percentages. Spearman rank coefficient was used to test correlation between two study variables. Quantile regression was used for multivariate model to assess the association between TE and fibrosis, HAI, steatosis, and biochemical tests. A frequency distribution was obtained for choosing optimal cut-off values of TE to maximize the sum of sensitivity and specificity for different fibrosis thresholds: F0-F1 vs F2- F4 (F ≥ 2), F0-F2 vs F3-F4 (F ≥ 3), F0-F3 vs F4 (F = 4). The diagnostic performance of TE was assessed by using ROC curves and the area under the ROC (AUC) curve analysis.
Data analysis was performed with STATA statistical package (release 11, 1, 2010, Stata Corporation, College Station, Texas, United States) and Medcalc (Version 11.2, 2011 MedCalc Software bvba , Be).
RESULTS
Patients
Two hundred and fifty-two patients met the inclusion criteria. Six (2%) patients were excluded due to unreliable TE measurements (no successful acquisitions in four patients; IQR > 30% in two patients). Thus, 246 patients were analyzed. Their characteristics are summarized in Table 1. There were 171 men and 75 women. One hundred and ninety-five (79.3%) patients had chronic hepatitis C, 41 (16.7%) had chronic hepatitis B, and 10 (4.0%) were coinfected with HIV. The mean length of the LB samples was 2.7 cm (± 0.8 cm). The fibrosis grade distribution was as follows: F0-F1, 129 patients; F2, 54 patients; F3, 24 patients; F4, 39 patients.
Table 1.
Patients demographics, and their biochemical and histological data at liver biopsy examination (%)
| Characteristics | All patients (n = 246) | HCV patients (n = 195) | HBV patients (n = 41) |
| Sex (men) | 171 (69.5) | 139 (71.3) | 30 (73.2) |
| Age (yr) | 44.7 (11.8)1 | 45.3 (11.5)1 | 41.2 (13.7)1 |
| BMI (kg/m2) | 25.2 (3.9)1 | 25.3 (3.6)1 | 25.2 (5.3)1 |
| AST (IU/L) | 39 (24-78)2 | 40 (26-80)2 | 28 (21-48)2 |
| ALT (IU/L) | 65 (32-116)2 | 70 (35-120)2 | 50 (23-109)2 |
| Platelets count (103/mm3) | 209 (171-262)2 | 212 (176-270)2 | 203 (172-253)2 |
| Fibrosis score (Metavir) | |||
| F0-F1 | 129 (52.3) | 98 (50.3) | 30 (73.2) |
| F2 | 54 (22.0) | 46 (23.6) | 6 (14.6) |
| F3 | 24 (9.8) | 19 (9.7) | 3 (7.3) |
| F4 | 39 (15.9) | 32 (16.4) | 2 (4.9) |
| Steatosis | |||
| S0 (0%) | 157 (63.8) | 126 (64.6) | 26 (63.4) |
| S1 (< 33%) | 56 (22.8) | 44 (22.6) | 10 (24.4) |
| S2 (33%-66%) | 19 (7.7) | 15 (7.7) | 4 (9.8) |
| S3 (> 66%) | 14 (5.7) | 10 (5.1) | 1 (2.4) |
| HAI | |||
| Mild | 109 (44.3) | 80 (41.0) | 28 (68.3) |
| Moderate | 102 (41.5) | 89 (45.7) | 7 (17.1) |
| Severe | 35 (14.2) | 26 (13.3) | 6 (14.6) |
mean ± SD
Median value and interquartile range (IQR). BMI: Body mass index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; HAI: Histological activity index; HCV: Hepatitis C virus; HBV: Hepatitis B virus.
Liver stiffness measurements
All patients (n = 246): Medians and IQR of TE values for each fibrosis stage were 5.2 kPa (4.4-6.4) for F0-F1, 6.4 kPa (5.6-8.6) for F2, 9.0 kPa (7.8-14.8) for F3, and 17.8 kPa (12.9-23.7) for F4. Multiple comparison between groups provided an adjusted P value for significance of 0.004 and showed that there was a statistical significant difference for F0-F1 vs F2 (P = 0.000 026), and F2 vs F3 (P = 0.001), but not for F3 vs F4 (P = 0.02).
In univariate analysis, TE showed a good correlation with the degree of fibrosis (r = 0.72, P < 0.00 001), a moderate correlation with the degree of histological activity index (r = 0.49, P < 0.00 001) and the aspartate aminotransferase (AST) value (r = 0.40, P < 0.00 001), and a poor correlation with the degree of steatosis despite being statistically significant (r = 0.19, P = 0.002). TE also showed a moderate negative correlation with platelet counts (r = -0.41, P < 0.00 001). Multivariate regression analysis including fibrosis stage, histological activity index, steatosis, AST, platelet counts confirmed the correlation with fibrosis stage (P = 0.0001) but not with all other variables.
ROC curve analysis identified optimal cut-off values of liver stiffness measurements as high as 6.9 kPa for F ≥ 2; 7.9 kPa for F ≥ 3; 9.6 kPa for F = 4 (Figure 1). Corresponding values of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR), negative LR and AUCs are shown in Table 2.
Figure 1.
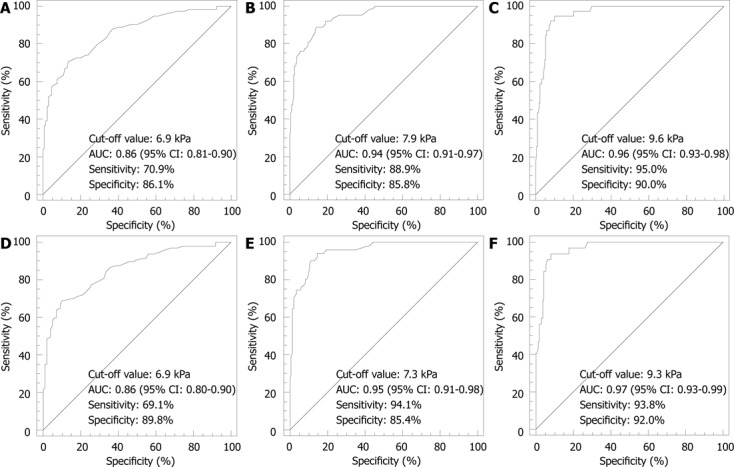
Receiver operating characteristic curve. A: F0-F1 vs F2- F4 (F > 2) in all patients (n = 246); B: F0-F2 vs F3- F4 (F > 3) in all patients (n = 246); C: F0-F3 vs F4 (F = 4) in all patients (n = 246); D: F0-F1 vs F2- F4 (F > 2) in patients with chronic hepatitis C (n = 195); E: F0-F2 vs F3- F4 (F > 3) in patients with chronic hepatitis C (n = 195); F: F0-F3 vs F4 (F = 4) in patients with chronic hepatitis C (n = 195).
Table 2.
Cut-off values of transient elastography obtained by receiver operating characteristic curve analysis for each Metavir fibrosis stage
| Sensitivity% | Specificity% | PPV% | NPV% | LR+ | LR- | AUC | |
| F ≥ 21 | |||||||
| 7.0 kPa | 70 (66-75) | 81 (77-85) | |||||
| F ≥ 22 | |||||||
| 6.9 kPa | 70.9 (61.8-79.0) | 86.1 (78.8-91.5) | 82.2 (73.3-89.1) | 76.6 (68.8-83.2) | 5.1 (4.4-5.8) | 0.34 (0.2-0.6) | 0.86 (0.81-0.90) |
| F ≥ 23 | |||||||
| 6.9 kPa | 69.1 (58.9-78.1) | 89.8 (82.0-95.0) | 87.0 (77.4-93.6) | 74.6 (65.7-82.1) | 6.8 (5.8-7.9) | 0.34 (0.2-0.7) | 0.86 (0.80-0.90) |
| F ≥ 31 | |||||||
| 9.5 kPa | 80 (72-85) | 85 (79-90) | |||||
| F ≥ 32 | |||||||
| 7.9 kPa | 88.9 (78.4-95.4) | 85.8 (79.9-90.5) | 68.3 (57.1-78.1) | 95.7 (91.4-98.3) | 6.3 (5.6-7.0) | 0.13 (0.06-0.3) | 0.94 (0.91-0.97) |
| 9.6 kPa4 | 76.3 (73.8-86.9) | 94.5 (90.2-97.3) | 82.8 (70.6-91.4) | 92.0 (87.2-95.5) | 13.9 (12.1-16.1) | 0.25 (0.1-0.5) | |
| F ≥ 33 | |||||||
| 7.3 kPa | 94.1 (83.8-98.8) | 85.4 (70.6-90.7) | 69.6 (57.3-80.1) | 97.6 (93.2-99.4) | 6.5 (5.9-7.1) | 0.07 (0.02-0.2) | 0.95 (0.91-0.98) |
| 9.3 kPa4 | 74.5 (60.4-85.7) | 96.5 (92.1-98.9) | 88.4 (74.9-96.1) | 91.4 (85.8-95.4) | 21.5 (18.2-25.3) | 0.3 (0.1-0.7) | |
| F = 41 | |||||||
| 12 kPa | 86 (80-91) | 88 (82-91) | |||||
| F = 42 | |||||||
| 9.6 kPa | 95 (83-99) | 90 (85-94) | 64 (50-76) | 99 (96-100) | 9.4 (8.6-10.2) | 0.06 (0.01-0.2) | 0.96 (0.93-0.98) |
| 12.2 kPa4 | 84.6 (69.5-94.1) | 94.7 (90.7-97.3) | 75.0 (59.7-86.8) | 97.0 (93.6-98.9) | 15.9 (13.9-18.3) | 0.16 (0.06-0.4) | |
| F = 43 | |||||||
| 9.3 kPa | 93.8 (79.2-99.2) | 92.0 (86.7-95.7) | 69.0 (53.9-82.8) | 98.7 (93.3-99.8) | 11.8 (10.6-13.0) | 0.07 (0.02-0.3) | 0.97 (0.93-0.99) |
| 12.3 kPa4 | 81.3 (63.6-92.8) | 95.7 (91.4-98.3) | 78.8 (61.1-91.0) | 96.3 (92.1-98.6) | 18.9 (16.0-22.4) | 0.2 (0.07-0.5) |
Cut-off values of transient elastography from the paper of Tsochatzis et al[8];
Cut-off values of transient elastography in all patients (n = 246);
Cut-off values of transient elastography in patients with chronic hepatitis C (n = 195);
Cut-off values obtained by maximizing specificity. PPV: Positive predictive value; NPV: Negative predictive value; LR+: Positive likelihood ratio; LR-: Negative likelihood ratio; AUC: The area under the receiver operating characteristic.
Cut-off values of liver stiffness measurements obtained maximizing only the specificity were as high as 6.9 kPa for F ≥ 2; 9.6 kPa for F ≥ 3; 12.2 kPa for F = 4.
Patients with hepatitis C (n = 195): Medians and IQR of TE values for each fibrosis stage were 5.1 kPa (4.2-6.3) for F0-F1, 6.4 kPa (5.4-7.9) for F2, 8.9 kPa (8.0-13.3) for F3, and 17.4 kPa (12.8-22.2) for F4.
Multiple comparison between groups gave an adjusted P-value for significance of 0.004 and showed that there was a statistical significant difference for F0-F1 vs F2 (P = 0.0002), and F2 vs F3 (P = 0.001), but not for F3 vs F4 (P = 0.04).
In univariate analysis, TE showed a good correlation with the degree of fibrosis (r = 0.72, P < 0.00 001), a moderate correlation with the degree of histological activity index (r = 0.43, P < 0.00 001) and the AST value (r = 0.36, P < 0.00 001), and a poor correlation with the degree of steatosis despite being statistically significant (r = 0.19, P = 0.002). TE also showed a moderate negative correlation with platelet counts (r = -0.39, P = 0.002). Multivariate regression analysis including fibrosis stage, histological activity index, steatosis, AST, platelet counts confirmed the correlation with fibrosis stage (P < 0.0001) but not with all other variables.
ROC curve analysis identified optimal cut-off values of liver stiffness measurements as high as 6.9 kPa for F ≥ 2; 7.3 kPa for F ≥ 3; 9.3 kPa for F = 4 (Figure 1). Corresponding values of sensitivity, specificity, PPV, NPV, positive LR, negative LR and AUCs are shown in Table 2.
Cut-off values of liver stiffness measurements obtained maximizing only the specificity were as high as 7.0 kPa for F ≥ 2; 9.3 kPa for F ≥ 3; 12.3 kPa for F = 4.
Patients with hepatitis B (n = 41): Due to the small number of patients, only patients with F0-F1 stages vs F2-F4 stages were considered for statistical analysis. There was a statistical significant difference for F0-F1 vs F2-F4 (P = 0.0006).
In univariate analysis, TE showed a good correlation with the degree of fibrosis (r = 0.60, P < 0.00 001), a high correlation with the degree of histological activity index (r = 0.70, P < 0.00 001) and the AST value (r = 0.76, P < 0.00 001); no correlation was found with steatosis. Due to the small number of patients, multivariate regression analysis was not performed.
DISCUSSION
The results of this study show that the cut-off values of TE obtained for each fibrosis stage in a population of consecutive patients with chronic viral hepatitis from a single center are comparable to that obtained in the recent meta-analysis by Tsochatzis et al[8] that included up to 40 studies. In their study, the meta-analysis was conducted for cut-offs 7.0, 9.5 and 12 kPa in stages F2, F3, and F4, respectively.
In our series, when maximizing the sum of sensitivity and specificity for different fibrosis thresholds, the optimal cut-offs of TE were similar to those of the meta-analysis for the diagnosis of significant fibrosis (F ≥ 2), and were lower for the diagnosis of advanced fibrosis (F ≥ 3) and cirrhosis (F = 4). However, when maximizing specificity, the cut-offs were similar to those used by Tsochatzis et al[8], and showed specificity even higher with respect to those of the meta-analysis for all fibrosis stages.
In our series the results obtained in all patients with chronic viral hepatitis were almost similar to that obtained excluding patients with chronic hepatitis B. On the other hand, it should be underlined that our series included only 41/246 (16.7%) patients with chronic hepatitis B. Due to the small number, the statistical analysis of the data obtained in this group of patients was not performed.
An uneven distribution of patients over fibrosis stage, as observed in our study, is commonly found when consecutive patients undergoing liver biopsy are enrolled. Indeed, in our series the distribution of patients over fibrosis stages is almost similar to that observed in other studies[17,20,31,32], or when large series - as in the study of Degos et al[17] were enrolled.
Unlike to that reported by other authors[33,34], who found that the prevalence of liver steatosis in patients with hepatitis C was more than 50%, in our series the majority of patients had no to mild steatosis. On the other hand, it should be noted that, in our study, the mean value of BMI was within the normal range. This patient’s characteristic could also explain the lower rate of TE unreliable results with respect to that reported in the literature[35,36].
In the seminal work of Sandrin et al[14] 93% of patients with TE values ≤ 5.1 kPa were F0-F1, whereas 94% of patients with TE values ≥ 7.6 kPa were F2 or more. On the other hand, patients with cirrhosis presented a very large range (from 14 kPa to 69 kPa). A limitation of that study is that liver biopsy and TE measurements were not performed in the same day; indeed, an inclusion criterion was liver biopsy performed less than one year before TE assessment. Since then , several studies have been published[15,31,37,38]. A wide range of TE cut-off value for F4 stage - from 11.8 kPa to 26.5 kPa - is found when comparing the results of several studies[5-9,15,17,31,35,37-39]. The different optimal TE cut-offs for F4 stage found in these studies by ROC curve analysis depend also on the fact that the characteristics of the patient population are not similar. Harada et al[37], that found a cut-off value of 26.5 kPa, enrolled patients with HCV recurrence after liver transplantation. In the study of Masuzaki et al[38], in which a cut-off of 15.9 kPa was found for F4 stage, there was a prevalence of patients with cirrhosis or pre-cirrhosis. In the study of Ziol et al[31], in which the optimal cut-off value for F4 stage was 14.5 kPa, liver biopsy was performed by the transjugular route in 63/251 (25.1%) of patients. It could be inferred that these patients presented already with clinical signs of cirrhosis, thus higher values of TE in the F4 stage - respect to patients without a clinical diagnosis of liver cirrhosis - could be expected[15,40]. Indeed, it has been demonstrated that there is an increase of TE values with liver disease progression beyond the F4 stage[15,19], and a highly positive correlation between TE values and portal pressure has been found[24,26]. Results similar to that obtained in our series were found in studies in which consecutive patients without clinical signs of liver cirrhosis were enrolled[39,41]. Cirrhosis should be regarded as a dynamic process, thus it is likely that patients in the initial stage of liver cirrhosis show lower values of TE respect to patients with compensated disease. Moreover, in the study of Foucher et al[15] liver stiffness was significantly correlated with Child-Pugh score. In their study, which included a large patient population with liver disease of different etiologies and also patients with decompensated cirrhosis, the optimal TE cut-off for F4 stage 17.6 kPa.
In conclusion, the results of our study show that the TE values used in the metanalysis of Tsochatzis et al[8] - namely 7.0, 9.5, and 12.0 kPa in stages F2, F3, and F4, respectively - could be used as given reference values in future studies aimed at evaluating the performance of TE in the assessment of liver fibrosis.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Livia Astroni, Ms. Natali Calabrese, Mr. Filippo Cuda, Mr. Lorenzo Guioli, Ms. Maura Marchisoni, Ms. Giampiera Nava, Ms. Loredana Pavesi, Ms. Barbara Ricci, nurses in the outpatient ward of the Infectious Diseases Department, for their valuable help in complying with the study protocol.
COMMENTS
Background
Several studies have shown significant positive correlation between transient elastography (TE) and the stage of liver fibrosis. A limitation of all the studies is that the cut-off values for each fibrosis stage were calculated from their own cohort of patients after data collection, and they are different across studies.
Research frontiers
The aim of this study was to compare the cut-offs of TE obtained by receiver operating characteristic (ROC) curve analysis in their series of consecutive patients with chronic viral hepatitis to that used in a recently published meta-analysis.
Innovations and breakthroughs
The results of this study show that the cut-off values of TE obtained for each fibrosis stage in a population of consecutive patients with chronic viral hepatitis from a single center are comparable to that obtained in a recent meta-analysis that included up to 40 studies.
Applications
The values of TE obtained in the recently published meta-analysis could be used as reference values in future studies aimed at evaluating the performance of the technique in the assessment of liver fibrosis.
Terminology
Transient elastography is a technique that noninvasively quantify liver stiffness by estimating the velocity of propagation of a shear wave along an ultrasonic A-line. The harder the tissue, the faster the shear wave travels.
Peer review
The authors conducted this study to compare the cut-offs of TE obtained by ROC curve analysis in their series of consecutive patients, with chronic viral hepatitis referred to their center for liver biopsy, to that used in a recently published meta-analysis.
Footnotes
P- Reviewer Atta HM S- Editor Gou SX L- Editor A E- Editor Xiong L
References
- 1.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 2.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 3.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 4.Castéra L, Nègre I, Samii K, Buffet C. Pain experienced during percutaneous liver biopsy. Hepatology. 1999;30:1529–1530. doi: 10.1002/hep.510300624. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102:2589–2600. doi: 10.1111/j.1572-0241.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 6.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 7.Stebbing J, Farouk L, Panos G, Anderson M, Jiao LR, Mandalia S, Bower M, Gazzard B, Nelson M. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroenterol. 2010;44:214–219. doi: 10.1097/MCG.0b013e3181b4af1f. [DOI] [PubMed] [Google Scholar]
- 8.Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–659. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 9.Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert-Bismut F, Naveau S, Thabut D, Lebrec D, Zoulim F, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol. 2007;7:40. doi: 10.1186/1471-230X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin Chim Acta. 2007;381:107–113. doi: 10.1016/j.cca.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912–921. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 13.Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95–106. doi: 10.1038/ncpgasthep1025. [DOI] [PubMed] [Google Scholar]
- 14.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda T, Yasuda T, Nakayama Y, Nakaya M, Kimura M, Yamashita M, Sawada A, Abo K, Takeda S, Sakaguchi H, et al. Usefulness of noninvasive transient elastography for assessment of liver fibrosis stage in chronic hepatitis C. World J Gastroenterol. 2006;12:7768–7773. doi: 10.3748/wjg.v12.i48.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study) J Hepatol. 2010;53:1013–1021. doi: 10.1016/j.jhep.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 18.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Carrión JA, Navasa M, Bosch J, Bruguera M, Gilabert R, Forns X. Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl. 2006;12:1791–1798. doi: 10.1002/lt.20857. [DOI] [PubMed] [Google Scholar]
- 20.Fraquelli M, Rigamonti C, Casazza G, Donato MF, Ronchi G, Conte D, Rumi M, Lampertico P, Colombo M. Etiology-related determinants of liver stiffness values in chronic viral hepatitis B or C. J Hepatol. 2011;54:621–628. doi: 10.1016/j.jhep.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 21.de Lédinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, Marcellin P, Dhumeaux D, Beaugrand M. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–179. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- 22.Ganne-Carrié N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, Dhumeaux D, Trinchet JC, Beaugrand M. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511–1517. doi: 10.1002/hep.21420. [DOI] [PubMed] [Google Scholar]
- 23.Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, Milani S, Lorefice E, Petrarca A, Romanelli RG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57:1288–1293. doi: 10.1136/gut.2008.149708. [DOI] [PubMed] [Google Scholar]
- 24.Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290–1297. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- 25.Rigamonti C, Donato MF, Fraquelli M, Agnelli F, Ronchi G, Casazza G, Rossi G, Colombo M. Transient elastography predicts fibrosis progression in patients with recurrent hepatitis C after liver transplantation. Gut. 2008;57:821–827. doi: 10.1136/gut.2007.135046. [DOI] [PubMed] [Google Scholar]
- 26.Kazemi F, Kettaneh A, N’kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, Beaugrand M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230–235. doi: 10.1016/j.jhep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Rockey DC. Noninvasive assessment of liver fibrosis and portal hypertension with transient elastography. Gastroenterology. 2008;134:8–14. doi: 10.1053/j.gastro.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 28.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 29.Desmet VJ. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis [Hepatology 1981; 1: 431-435] J Hepatol. 2003;38:382–386. doi: 10.1016/s0168-8278(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 30.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 31.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 32.Karlas T, Pfrepper C, Wiegand J, Wittekind C, Neuschulz M, Mössner J, Berg T, Tröltzsch M, Keim V. Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease. Scand J Gastroenterol. 2011;46:1458–1467. doi: 10.3109/00365521.2011.610004. [DOI] [PubMed] [Google Scholar]
- 33.Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636–1642. doi: 10.1053/j.gastro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Asselah T, Rubbia-Brandt L, Marcellin P, Negro F. Steatosis in chronic hepatitis C: why does it really matter? Gut. 2006;55:123–130. doi: 10.1136/gut.2005.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–973. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 37.Harada N, Soejima Y, Taketomi A, Yoshizumi T, Ikegami T, Yamashita Y, Itoh S, Kuroda Y, Maehara Y. Assessment of graft fibrosis by transient elastography in patients with recurrent hepatitis C after living donor liver transplantation. Transplantation. 2008;85:69–74. doi: 10.1097/01.tp.0000297248.18483.16. [DOI] [PubMed] [Google Scholar]
- 38.Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Goto T, Yoshida H, Kanai F, Sugioka Y, et al. Comparison of liver biopsy and transient elastography based on clinical relevance. Can J Gastroenterol. 2008;22:753–757. doi: 10.1155/2008/306726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lupşor M, Badea R, Stefănescu H, Grigorescu M, Sparchez Z, Serban A, Branda H, Iancu S, Maniu A. Analysis of histopathological changes that influence liver stiffness in chronic hepatitis C. Results from a cohort of 324 patients. J Gastrointestin Liver Dis. 2008;17:155–163. [PubMed] [Google Scholar]
- 40.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 41.Rizzo L, Calvaruso V, Cacopardo B, Alessi N, Attanasio M, Petta S, Fatuzzo F, Montineri A, Mazzola A, L’abbate L, et al. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:2112–2120. doi: 10.1038/ajg.2011.341. [DOI] [PubMed] [Google Scholar]


