Abstract
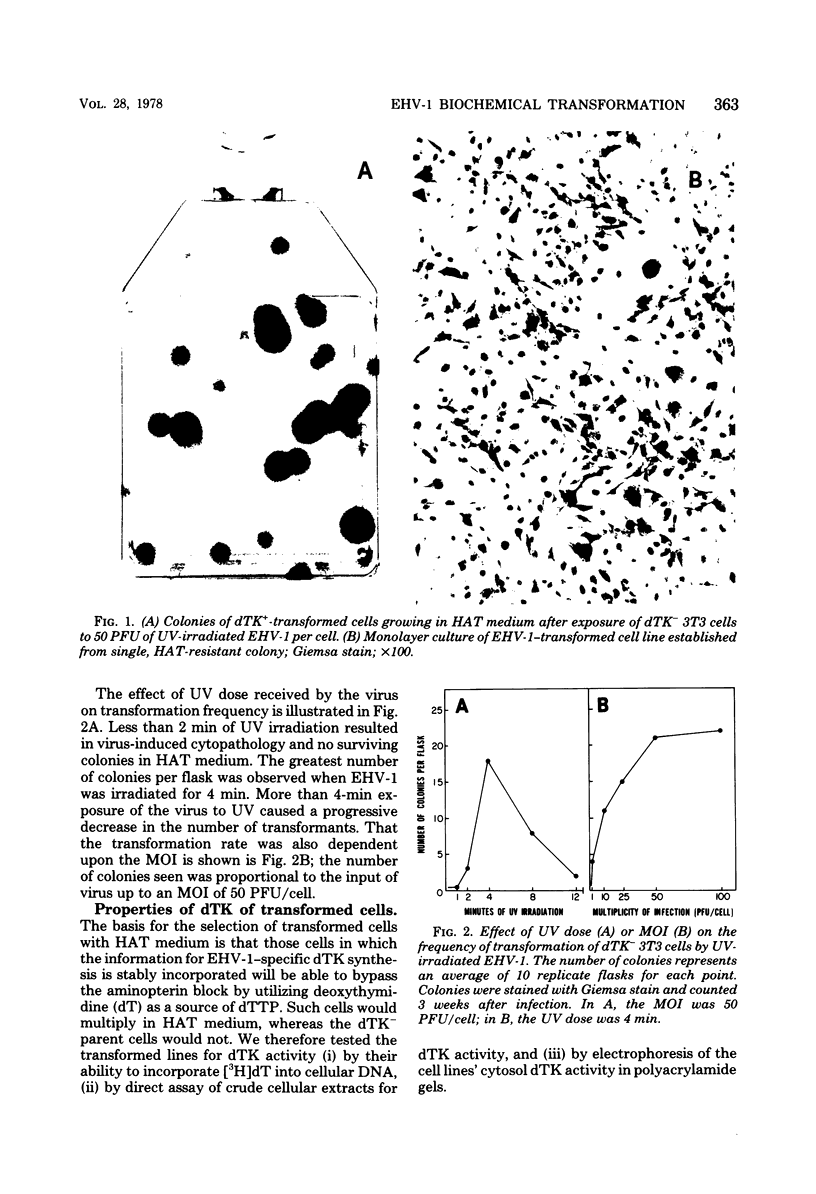
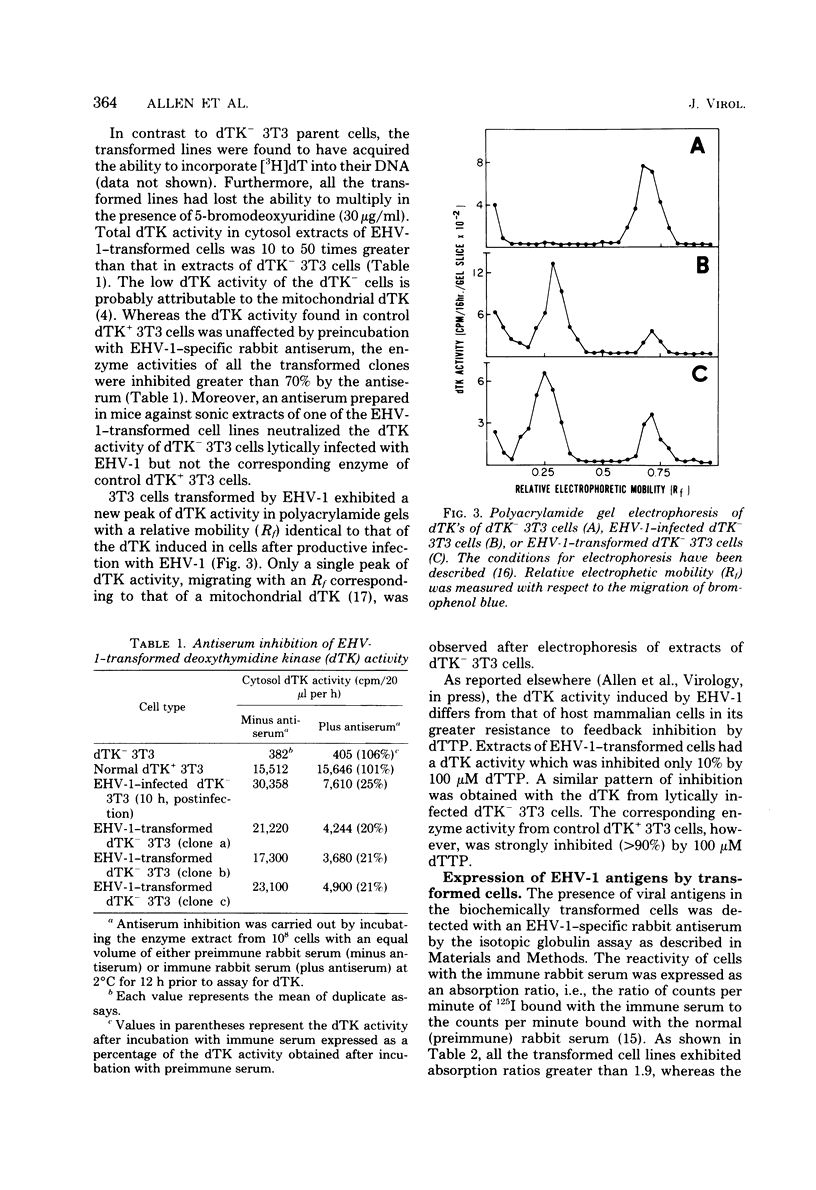
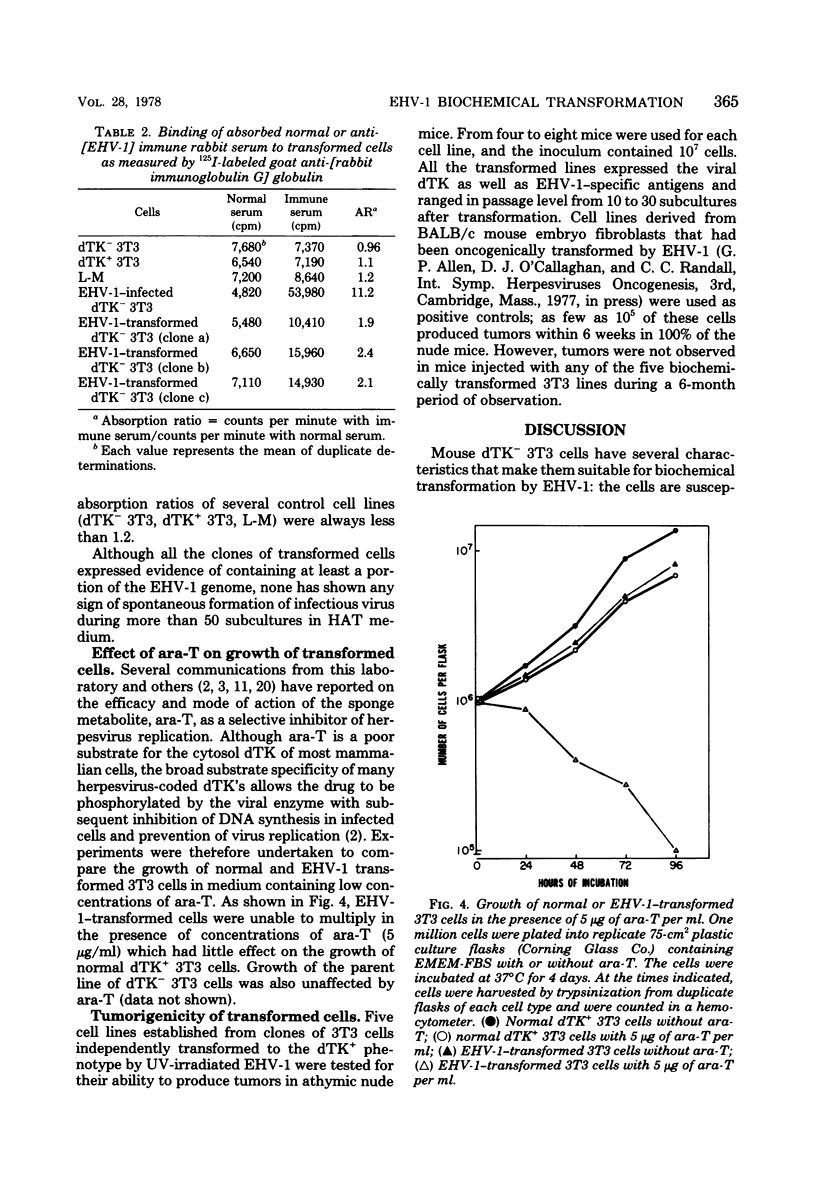
A line of 3T3 mouse cells lacking deoxythymidine kinase (dTK-) was stably transformed to the dTK+ phenotype after exposure to UV-irradiated equine herpesvirus type 1 (EHV-1). Biochemical transformants were isolated in a system selective for the dTK+ phenotype (Eagle minimal essential medium containing 10(-4) M hypoxanthine, 6 X 10(-7) M aminopterin, and 2 X 10(-5) M deoxythymidine). Transformation was accompanied by the acquisition of a dTK activity with immunological, electrophoretic, and biochemical characteristics identical to those of the dTK induced by EHV-1 during productive infection. The transformed cells have been maintained in selective culture medium for more than 50 passages and have retained the capacity to express EHV-1--specific antigens. Spontaneous release of infectious virus has not been detected in the transformed lines, and the the cells were not oncogenic for athymic nude mice. In contrast to normal dTk+ 3T3 cells, EHV-1 transformants were unable to grow in the presence of arabinosylthymine, a drug selectively phosphorylated by herpesvirus-coded dTK's. These results indicate that a portion of the EHV-1 genome is able to persist in the transformed cells for many generations and be expressed as an enzymatically active viral gene product.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., Cohen J. C., Randall C. C., O'Callaghan D. J. Replication of equine herpesvirus type 1 and type 3: resistance to hydroxyurea and thymidine. Intervirology. 1978;9(5):276–285. doi: 10.1159/000148945. [DOI] [PubMed] [Google Scholar]
- Aswell J. F., Allen G. P., Jamieson A. T., Campbell D. E., Gentry G. A. Antiviral activity of arabinosylthymine in herpesviral replication: mechanism of action in vivo and in vitro. Antimicrob Agents Chemother. 1977 Aug;12(2):243–254. doi: 10.1128/aac.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswell J. F., Gentry G. A. Cell-dependent antiherpesviral activity of 5-methylarabinosylcytosine, an intracellular ara-T donor. Ann N Y Acad Sci. 1977 Mar 4;284:342–350. doi: 10.1111/j.1749-6632.1977.tb21969.x. [DOI] [PubMed] [Google Scholar]
- Attardi B., Attardi G. Persistence of thymidine kinase activity in mitochondria of a thymidine kinase-deficient derivative of mouse L cells. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2874–2878. doi: 10.1073/pnas.69.10.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha K. C., Munyon W. Presence of herpes simplex virus-related antigens in transformed L cells. J Virol. 1975 Jun;15(6):1475–1486. doi: 10.1128/jvi.15.6.1475-1486.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Chadha K. C., Hughes R. G., Jr Biochemical and immunological characterization of deoxythymidine kinase of thymidine kinaseless HeLa cells biochemically transformed by herpes simplex virus type. Infect Immun. 1977 May;16(2):486–492. doi: 10.1128/iai.16.2.486-492.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Ostrander M. Deoxythymidine kinase induced in HeLa TK- cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976 May 10;251(9):2605–2610. [PubMed] [Google Scholar]
- Davidson R. L., Adelstein S. J., Oxman M. N. Herpes simplex virus as a source of thymidine kinase for thymidine kinase-deficient mouse cells: suppression and reactivation of the viral enzyme. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1912–1916. doi: 10.1073/pnas.70.7.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. B., Kingsbury D. T. Quantitation of the viral DNA present in cells transformed by UV-irradiated herpes simplex virus. J Virol. 1976 Mar;17(3):788–793. doi: 10.1128/jvi.17.3.788-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. B., Munyon W., Buchsbaum R., Chawda R. Virus type-specific thymidine kinase in cells biochemically transformed by herpes simplex virus types 1 and 2. J Virol. 1974 Jan;13(1):140–145. doi: 10.1128/jvi.13.1.140-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry G. A., Aswell J. F. Inhibition of herpes simplex virus replication by araT. Virology. 1975 May;65(1):294–296. doi: 10.1016/0042-6822(75)90034-3. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Macnab J. C., Perbal B., Clements J. B. Virus specified enzyme activity and RNA species in herpes simplex virus type 1 transformed mouse cells. J Gen Virol. 1976 Sep;32(3):493–508. doi: 10.1099/0022-1317-32-3-493. [DOI] [PubMed] [Google Scholar]
- Kraiselburd E., Gage L. P., Weissbach A. Presence of a herpes simplex virus DNA fragment in an L cell clone obtained after infection with irradiated herpes simplex virus I. J Mol Biol. 1975 Oct 5;97(4):533–542. doi: 10.1016/s0022-2836(75)80057-x. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. STUDIES ON THYMIDINE KINASE IN CULTURED MOUSE FIBROBLASTS. Biochim Biophys Acta. 1965 Jan 11;95:14–22. doi: 10.1016/0005-2787(65)90206-6. [DOI] [PubMed] [Google Scholar]
- Lausch R. N., Murasko D. M., Albrecht T., Rapp F. Detection of specific surface antigens on cells transformed by cytomegalovirus with the techniques of mixed hemagglutination and 125I-labeled antiglobulin. J Immunol. 1974 May;112(5):1680–1684. [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976 May 10;251(9):2600–2604. [PubMed] [Google Scholar]
- Leung W. C., Dubbs D. R., Trkula D., Kit S. Mitochondrial and herpesvirus-specific deoxypyrimidine kinases. J Virol. 1975 Sep;16(3):486–497. doi: 10.1128/jvi.16.3.486-497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuya Y., Green H. Somatic cell hybrid between the established human line D98 (presumptive HeLa) and 3T3. Science. 1969 Feb 14;163(3868):697–698. doi: 10.1126/science.163.3868.697. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Iltis J. P., Rapp F. Differential effect of arabinofuranosylthymine of the replication of human herpesviruses. J Virol. 1977 Sep;23(3):679–684. doi: 10.1128/jvi.23.3.679-684.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D. J., Hyde J. M., Gentry G. A., Randall C. C. Kinetics of viral deoxyribonucleic acid, protein, and infectious particle production and alterations in host macromolecular syntheses in equine abortion (herpes) virus-infected cells. J Virol. 1968 Aug;2(8):793–804. doi: 10.1128/jvi.2.8.793-804.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M. L., Kemp M. C., Randall C. C., O'Callaghan D. J. Studies of the molecular anatomy of the L-M cell strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974 May;59(1):201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- RANDALL C. C., LAWSON L. A. Adaptation of equine abortion virus to Earle's L cells in serum-free medium with plaque formation. Proc Soc Exp Biol Med. 1962 Jul;110:487–489. doi: 10.3181/00379727-110-27558. [DOI] [PubMed] [Google Scholar]
- Rapp F., Buss E. R. Comparison of herpes simplex virus isolates using a quantitative selection assay for transformation. Intervirology. 1975;6(2):72–82. doi: 10.1159/000149458. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Chadha K. C., Munyon W. H. Serological specificity of thymidine kinase activity in herpes simplex virus-transformed L cells. Virology. 1976 Jan;69(1):350–351. doi: 10.1016/0042-6822(76)90225-7. [DOI] [PubMed] [Google Scholar]



