Abstract
Intermittent hypoxia (IH) during sleep, such as occurs in sleep apnea (SA), induces increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation and deficits in hippocampal learning and memory. Similar to IH, high fat-refined carbohydrate diet (HFD), a frequent occurrence in patients with SA, can also induce similar oxidative stress and cognitive deficits under normoxic conditions, suggesting that excessive NADPH oxidase activity may underlie central nervous system (CNS) dysfunction in both conditions. The effect of HFD and IH during the light period on two forms of spatial learning in the water maze as well as on markers of oxidative stress was assessed in male mice lacking NADPH oxidase activity (gp91phox-/Y) and wild-type littermates fed HFD. On a standard place training task, gp91phox_/Y displayed normal learning, and was protected from the spatial learning deficits observed in wild-type littermates exposed to IH. Moreover, anxiety levels were increased in wild-type mice exposed to HFD and IH as compared to controls, while no changes emerged in gp91phox_/Y mice. Additionally, wild-type mice, but not gp91phox_/Y mice, had significantly elevated levels of malondialdehyde (MDA) and 8-hydroxydeoxyguanosine (8-OHdG) in hippocampal lysates following IH-HFD exposures. The cognitive deficits of obesity and westernized diets and those of sleep disorders that are characterized by IH during sleep are both mediated, at least in part, by excessive NADPH oxidase activity.
Keywords: Intermittent hypoxia, NADPH oxidase, high fat diet, sleep apnea, oxidative stress, cognitive impairment
Sleep apnea (SA) is a clinical syndrome characterized by repeated episodes of upper airway obstruction during sleep, which is now recognized as a significant and highly prevalent health problem, not only because of its association with substantial cardiovascular and metabolic morbidity, but also because of the prominent cognitive and behavioral deficits that occur in this condition. The neuropsychological impairments are accompanied by increased levels of systemic markers of oxidative stress and inflammation in addition to gray matter losses in neural sites contributing to cognitive function (Beebe and Gozal, 2002; Carpagnano et al., 2002; Gozal et al., 2007; Macey et al., 2002; Montplaisir et al., 1992).
Diet is a major factor in maintaining neural and cognitive health throughout the lifespan, and changes in diet and lifestyle have promoted an epidemic of obesity and related health problems all over the world, and particularly in the United States (Mokdad et al., 1999). For example, high fat diet (HFD), that is rich in saturated fat and refined sugar, contributes to accelerated cognitive decline in aging, and in the course of dementia in Alzheimer disease (Kalmijn et al., 1997; Thirumangalakudi et al., 2008). Carbohydrate-enriched HFD also aggravates impairments of cognitive functions following traumatic brain injury (Wu et al., 2003), cerebral ischemia/reperfusion injury (Li et al., 2007a; Li et al., 2007b) and intermittent hypoxia (Goldbart et al., 2006). It is now well established that one of the primary causes of the current epidemic of obesity in developed countries is the increased consumption of a diet rich in saturated fat and simple sugars, such as refined carbohydrates (Hill and Peters, 1998). Although such diets have been linked to a host of health risks such as oxidative stress, hypertension, diabetes mellitus, and obstructive sleep apnea, the degree to which such dietary factors affect cognitive function has only been more recently examined (Elias et al., 2003). Even in healthy animals, carbohydrate-enriched HFD impairs learning and memory (Molteni et al., 2004; Pathan et al., 2008; Wainwright et al., 1999; Zhao et al., 2004) and synaptic plasticity (Stranahan et al., 2008), by affecting brain-derived neurotrophic factor and cyclic AMP-response element-binding protein (Molteni et al., 2004; Wu et al., 2004). HFD will also reduce hippocampal neurogenesis (Hwang et al., 2008; Lindqvist et al., 2006; Tozuka et al., 2009), and lead to oxidative stress by inducing lipid peroxidation, including increased 4-hydroxy-2Enonenal levels in the hippocampus (Hwang et al., 2009; Wu et al., 2004). On the other hand, ketogenic diets, i.e., low carbohydrate/high fat diets exert a neuroprotective effect in Alzheimer’s disease, Parkinson’s disease, traumatic brain injury, epilepsy and stroke (Gasior et al., 2006; Noh et al., 2008). In older individuals, diets rich in monounsaturated fatty acids and in fruits and fibers are associated with better memory scores and protection against cognitive decline (Capurso et al., 1997; Floel et al., 2008; Solfrizzi et al., 1999).
The enzyme nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase was initially identified and studied in the context of its role in phagocyte oxidative burst (Quinn and Gauss, 2004). This enzyme has however emerged as a major source of reactive oxygen species (ROS) generation in mammalian cells, including the central nervous system (CNS; (Bedard and Krause, 2007; Dringen, 2005; Serrano et al., 2003; Vallet et al., 2005) where it it plays major functional roles in astrocytes (Abramov et al., 2005), and neurons (Tejada-Simon et al., 2005; Vallet et al., 2005), in the latter being primarily localized at the synapse level (Tejada-Simon et al., 2005). Mutations in the gp91phox and p47phox genes are the most common mutations that cause chronic granulomatous disease (Winkelstein et al., 2000). These mutations disable the NADPH oxidase complex, thereby preventing the oxidation of NADPH and the subsequent production of superoxide (Lomax et al., 1989; Royer-Pokora, 1987), which is required for pathogen destruction as well as most superoxide-dependent signal transduction in nonphagocytic cells (Jackson et al., 2004; Kishida et al., 2005; Sumimoto et al., 2004). gp91phox (Pollock et al., 1995) and p47phox (Jackson et al., 1995) mutant mice have been generated and can therefore be used to explore the putative role of NADPH oxidase in disease models such as HFD or IH.
We hypothesized that both HFD and IH-induced deleterious effects on learning and memory, mood, and anxiety would be reduced in NADPH oxidase mutant mice (gp91phox-/Y) (Akashiba et al., 2002; Bardwell et al., 2001; Borak et al., 1996; El-Sheikh et al., 2010; Kaplan, 1992; Sanchez et al., 2001).
Experimental procedures
Animals
Eight-week-old male hemizygous gp91phox-/Y (B6.129S-Cybbtm1Din/J) mice (20–22 grams) and C57BL/6J mice (20–22 grams) were purchased from Jackson Laboratories (Bar Harbor, Maine), housed in a 12 hr light/dark cycle (lights on from 7:00 am to 7:00 pm) at a constant temperature (26 ±1°C). Mice were housed in groups of four in a standard clear polycarbonate cages, and were allowed access to food (see below) and water ad libitum. All behavioral experiments were performed during the light period (between 9:00 am and 12:30 pm). Mice were randomly assigned to either IH or room air (RA) exposures. The experimental protocols were approved by the Institutional Animal Use and Care Committee and were in close agreement with the National Institutes of Health Guide in the Care and Use of Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Intermittent Hypoxia Exposures
Animals were maintained in 4 identical commercially-designed chambers (30"×20" ×20"; Oxycycler model A44XO, BioSpherix, Redfield, NY) operated under a 12 hour light-dark cycle (7:00 am–7:00 pm) for 14 days prior to behavioral testing. Oxygen concentration was continuously measured by an O2 analyzer, and was changed by a computerized system controlling gas outlets, as previously described (Gozal et al., 2001; Kheirandish et al., 2005; Row et al., 2004), such as to generate oxyhemoglobin nadir values in the 65–72% range every 180 seconds. Ambient temperature was kept at 22–24°C.
High fat/refined carbohydrate (HFD) diet
The HFD diet used was high in fat and refined sugar (42% kcal from fat, 42.7% kcal from carbohydrate), and contained a standard vitamin and mineral mix with all essential nutrients (Harlan Laboratories Product # TD 88137; Madison, WI) and was available to all mice ad libitum.
Behavioral Testing
Spatial reference learning and memory as well as working memory was assessed using the standard Morris water maze, consisting of a circular tank (diameter, 1.4m and 0.6m in height) filled to a level of 35cm with water maintained at a temperature of 21°C. The pool water was made opaque by addition of 150 ml of non-toxic white tempera paint. A Plexiglas escape platform (10 cm in diameter) was positioned 1 cm below the water surface and placed randomly at any of the four arbitrarily defined quadrants of the pool. Visual cues were placed in each of the quadrants of the tank. Maze performance was recorded by a video camera suspended above the maze and interfaced with a video tracking system (HVS Imaging, Hampton, UK).
Briefly, standard place-training reference memory task was conducted on mice in the water maze following exposure to 14 days of IH or RA. The mice were habituated to the water maze during a free swim one day prior to the place learning. Each training session consisted of three trials separated by a 10 minute inter-trial interval. On a given daily session, each mouse was placed into the pool from 1 of 4 quasirandom start points (N, S, E or W) and their order of use was changed daily. The mice were given 90-second training trials per day to escape to the platform where the mice were allowed to stay for 15 sec. Mice that failed to escape were led to the platform. To obtain measures of spatial bias following 24 h of the final training session, the platform was removed for a probe trial. The performance in the water maze was assessed by analyzing the mean escape latencies and swim distance as previously described (Nair et al., 2011).
Reference memory
After acquisition of the task, the mice were kept in their home cage under respective experimental conditions (RA and IH) for 14 days. Following this, the retention tests were carried out. In the retention test, performance in a single session (two trials) was assessed, and the mean average performance of the two trials was calculated as previously described (Nair et al., 2011).
Forced swimming test (FST)
To assess the level of depression, mice were individually forced to swim in an open cylindrical container (diameter 14 cm, height 20 cm), with a depth of 15 cm of water at 25 ± 1 °C. During the 6 min of total test time, the immobility time, defined as the absence of escape-oriented behaviors, was scored as previously described (Nair et al., 2011). Each mouse was judged to be immobile when it ceased struggling, and remained floating motionless in the water, making only those movements necessary to keep its head above water. The average percentage immobility was calculated by a blinded experimenter during the last 4 min of the test.
Elevated plus maze (EPM)
The elevated plus maze (EPM) was used to assess anxiety. Mice were placed in the center of the maze and animal behavior was recorded on videotape for 10 minutes, followed by offline analysis. The following parameters were scored: (a) Percent time spent in open and closed arms; (b) number of entries to closed arms. An arm entry was defined as the entry of all four feet into either one of the closed arm as previously described (Nair et al., 2011). Of note, the maze was cleaned with 30 % ethanol between trials to remove any odor cues. A 60 w light was placed above the apparatus and the test was recorded by an overhead camera using ethovision software from Noldus.
Biochemical studies
After the completion of all the behavioral experiments (Nair et al., 2011), the mice were sacrificed at 7:00 p.m. by cervical dislocation and brains were immediately dissected under dry-ice and hippocampal tissues were extracted. The tissues were flash frozen in liquid nitrogen and stored in −80 °C until assayed.
Lipid Peroxidation Assay
The levels of malondialdehyde (MDA) production, a commonly used indicator of lipid peroxidation from the hippocampus were measured by the MDA-586 kits (OxisResearch, Portland OR) according to the manufacturer's instruction as previously described (Nair et al., 2011).
8-hydroxydeoxyguanosine (8-OHdG)
Levels of 8-OHgG was measured in the hippocampus using a commercially available assay (Cell Biolabs, San Diego, CA). Briefly, hippocampal samples or 8-OHdG standards were first added to an 8-OHdG/BSA conjugate preabsorbed enzyme immunoassay plate. After a brief incubation, an anti–8-OHdG mAb was added, followed by an horseradish peroxidase-conjugated secondary antibody. The 8-OHdG content in the hippocampal samples was then determined by comparison with the 8-OHdG standard curve as previously described (Nair et al., 2011).
Data Analysis
To elucidate the nature of interactions between conditions and mouse groupings, all data were initially analyzed by one way ANOVA. In addition, we included in some of the analyses comparisons with previously published experiments in mice exposed to IH and RA but fed normal chow (Nair et al., 2011). First, overall statistical significance was determined for the entire training period between the treatment groups. In addition, two-way repeated measures ANOVA was used to analyze each trial block, followed by post-hoc Tukey tests. Finally, data were divided into 6 blocks (containing 3 trials/day). We used multivariate MANOVA model (SPSS 17.0, Chicago, IL) to allow full assessment whether different conditions such as RA or IH on the different strains were present. The MANOVA model had latency, pathlength and swim speed and two between factors: (1) groups (four levels): RA C57BL/6J, IH C57BL/6J, RA gp91phox_/Y and IH gp91phox_/Y (2) condition (two levels): RA or IH. All F statistics are reported using Pillai’s Trace. Further, to compare between normal chow and HFD, we used previously published data using normal chow (Nair et al., 2011) and a multivariate MANOVA model. The MANOVA model had latency and pathlength and two-between factors: (1) groups (eight levels): RA C57BL/6J (normal diet), RA C57BL/6J (HFD), RA gp91phox_/Y(normal diet), RA gp91phox_/Y (HFD diet), IH C57BL/6J (normal diet), IH C57BL/6J (HFD), IH gp91phox_/Y (normal diet), IH gp91phox_/Y (HFD diet), (2) condition (two levels): RA or IH. All F statistics are reported using Pillai’s Trace The interaction of three different factors, i.e., time, condition and group were determined using this mixed model repeated measures MANOVA. Similar statistical approaches were used to compare probe trial, reference memory, EPM and FST. For all comparisons, a p value <0.05 was considered to achieve statistical significance.
Results
Spatial Learning Performance
On a standard place discrimination task, wild type mice exposed to 14 days of IH (IH-C57BL6/J) exhibited longer latencies and pathlengths to locate the hidden platform when compared to room air controls RA-C57BL6/J, RA- gp91phox_/Y and gp91phox_/Y mice exposed to 14 days IH (IH-gp91phox_/Y) animals (n=12 per experimental condition; Figures 1A and B). Overall latency analysis for the entire trial blocks revealed significant changes between the different treatment groups, [F(11,23) =25.44; p<0.001] and pathlength, [F(11,23) =16.35; p<0.001] indicating that IH adversely affected task performance in C57BL6/J mice fed with HFD. Significant differences in latencies were observed during block 1 [F(3,11) =6.76; p<0.001], blocks 2 [F(3,11) =4.95; p<0.006], 3 [F(3,11) =8.38; p<0.001], 4 [F(3,11) =3.35; p<0.03], 5 [F(3,11) =7.06; p<0.001] and 6 [F(3,11) = 4.457; p<0.01]. Repeated measures ANOVA revealed significant differences in pathlengths during blocks 3 [F(3,11) =5.25; p<0.004], 4 [F(3,11) =4.36; p<0.01], 5 [F(3,11) =6.73; p<0.001] and 6 [F(3,11) =2.99; p<0.04], with no significant differences in blocks 1 and 2 when compared with C57BL6/J mice fed with HFD. In the probe-trial test, one-way ANOVA revealed a significant effect of treatment [IH vs. RA: F(3,11) =12.78; p<0.001]. The magnitude of impairment was greatest in IH-C57BL6/J mice fed with HFD (Figure 1C). In the reference memory tests, IH-C57BL6/J mice exhibited significant deficits in memory retention in both latency [F(3,11) =19.61; p<0.001] and pathlength [F(3,11) =12.84; p<0.001]. However, the IH-gp91phox_/Y mice fed with HFD performed similar to normoxic controls (Figure 2A and B).
Figure 1. gp91phox_/Y mice exposed to IH and HFD do not exhibit any deficits in learning and memory functions.
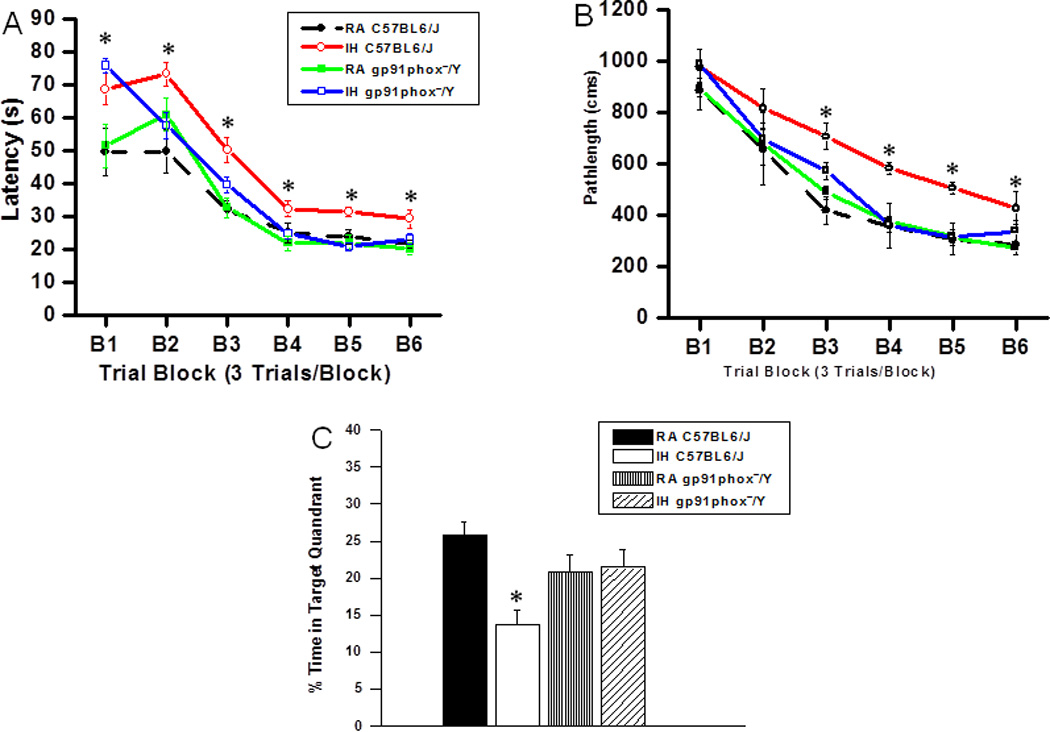
(A) Mean latencies and (B) pathlengths to locate the target platform during place training in C57BL6/J and gp91phox_/Y fed HFD, and exposed to either intermittent hypoxia (IH) or maintained in room air (RA) (n = 12 per group. (C) Mean percentage time in the target quadrant during probe trial after completion of water maze testing in either C57BL6/J and gp91phox_/Y exposed to IH or maintained in RA. (All values shown are mean ± SEM; n=12/experimental group;*p < 0.05).
Figure 2. gp91phox_/Y mice exposed to IH and HFD do not exhibit any deficits in retention.
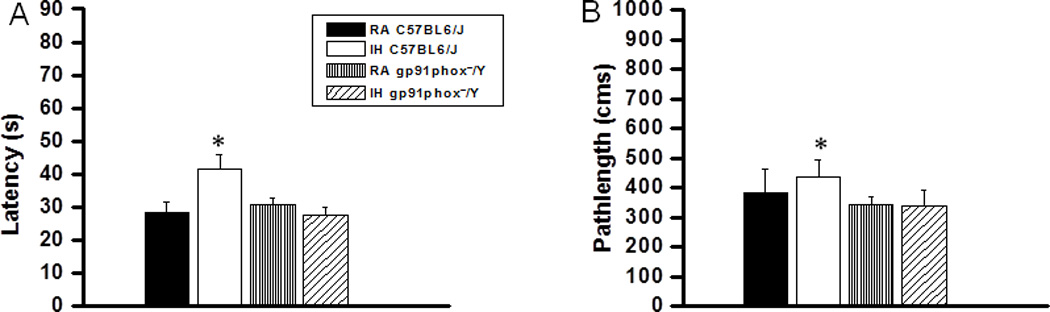
(A) Mean latencies (s) and (B) pathlengths (cm) to locate the target platform during retention in C57BL6/J and gp91phox_/Y fed HFD, and exposed to either intermittent hypoxia (IH) or maintained in room air (RA) during retention of the Morris water maze task. (All values shown are mean ± SEM; n=12/experimental group;*p < 0.05).
Comparison between normal diet and high fat diet
Multivariate analysis showed that latency was found to vary with blocks and groups, as reflected in a significant two-way interaction of blocks×groups (F(30,440)=1.757, p<0.009) and also a significantly increased latency in HFD when compared to normal diet (F(36,528)=1.92, p<0.001). However, there was no difference in pathlength as reflected in a significant two-way interaction of blocks×groups (F(30,440)=0.902, p<0.619).
Elevated Plus Maze
IH-C57BL6/J mice fed with HFD showed significant differences in the percentage of time spent in the open arm [F(3,11) =16.63; p<0.001] and in the number of entries into the closed arm [F(3,11) =6.17; p<0.002] (Figure 3A and B).
Figure 3. gp91phox_/Y mice exposed to IH and HFD do not exihibit anxiety and depression.
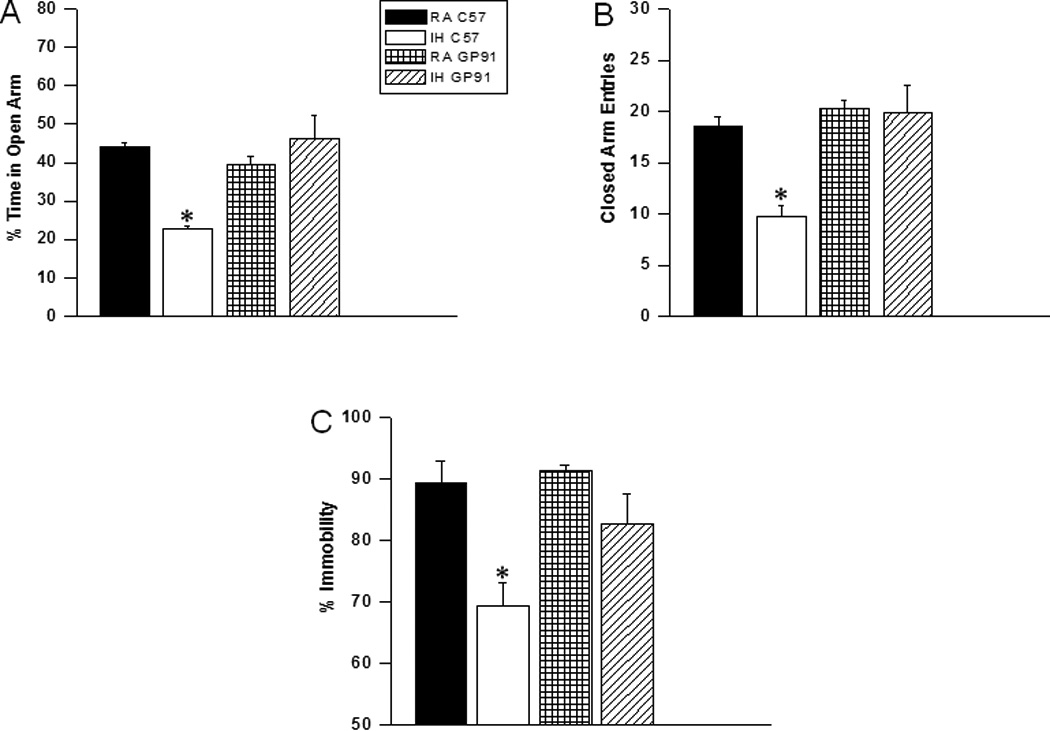
Top Panels: C57BL6/J mice fed HFD, and exposed to either intermittent hypoxia (IH) spend significantly less time in the open arm of the elevated plus maze compared to RA C57BL6/J, or gp91phox_/Y mice exposed to either RA or IH (A). A reduced number of closed--arm entries emerged in C57BL6/J mice exposed to IH (B). Bottom Panel: gp91phox_/Y exposed to IH and HFD show less immobility as compared to C57BL6/J mice fed HFD, and exposed to either intermittent hypoxia or C57BL6/J mice exposed to RA and HFD during a forced swim test. See text for more details. (All values shown are mean ± SEM; n=12/experimental group;*p < 0.05).
Forced Swim Test
IH-C57BL6/J mice fed with HFD had significantly higher immobility durations during the last 4 min of the FST [F(3,7) =7.84; p<0.001] when compared to all other treatment groups, including IH-gp91phox_/Y fed with HFD (Figure 3C).
Lipid Peroxidation
After the behavioral experiments, hippocampal tissues were harvested and processed for assessment of lipid peroxidation as indicated by MDA levels. Figure 4A shows MDA concentrations in homogenates of hippocampi from all treatment groups. A significant increase in MDA levels was observed in IH-C57BL6/J mice fed with HFD [F(3,7) =35.41; p<0.001] when compared to all other groups fed with HFD.
Figure 4. Lipid peroxidation and oxidative DNA damage are reduced in the hippocampus of gp91phox_/Y exposed to IH and HFD.
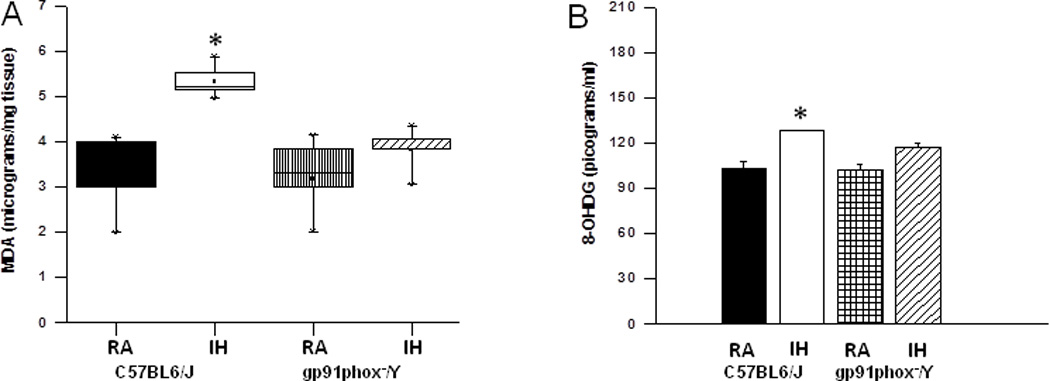
Left Panel - MDA tissue levels in hippocampus of and gp91phox_/Y and C57BL6/J mice exposed to either room air (RA) or intermittent hypoxia for 14 days (IH) while consuming HFD. (n = 6 per experimental group; *P < 0.05).
Right Panel- 8-OHdG levels in hippocampus of gp91phox_/Y and C57BL6/J mice exposed to either room air (RA) or intermittent hypoxia for 14 days (IH) while consuming HFD. (All values shown are mean ± SEM; n = 6/ experimental group; *p < 0.05).
8-OHdG Levels
The levels of 8-OHdG in homogenates of hippocampi were significantly higher in IH-C57BL6/J mice fed with HFD [F(3,5) =12.80; p<0.001] when compared to all other groups fed with HFD (Figure 5B). Multivariate analysis on comparison between normal diet and high fat diet also showed that a significant difference in 8-OHdG between groups (F(1,7)=10.592, p<0.0001).
Discussion
Chronic intermittent hypoxia and high fat diet are conditions that adversely affect cognitive functions. Furthermore, we have previously shown that HFD exacerbates IH-induced cognitive dysfunction in rats (Goldbart et al., 2006). In this study, we found that young mice exposed to both IH and HFD were affected and displayed impaired performances on a place-training reference memory task and that increased activity of NADPH oxidase-mediated oxidative stress pathways appears to underlie both HFD and IH-induced effects. HFD increases plasma free fatty acids and causes oxidative stress followed by the accumulation of peroxidized lipids (Yamato et al., 2007). HFD with intermittent hypoxia increased MDA levels and 8-OHdG levels in the hippocampus, indicating higher lipid peroxidation and DNA oxidation. Oxidative stress induced by HFD elevates the expression and release of inflammatory molecules in the brain and impairs cognitive function (White et al., 2009; Wu et al., 2004). Although ROS can be generated from various subcellular compartments, including mitochondria, the cellular membrane, lysosomes, peroxisomes, and the endoplasmic reticulum (Akki et al., 2009; Bedard and Krause, 2007; Droge, 2002; Kubota et al., 2010; Santos et al., 2009), we here show that NADPH oxidase, which is expressed in nonphagocytic cells such as sympathetic ganglion neurons and cortical neurons (Noh and Koh, 2000; Sorce and Krause, 2009; Tammariello et al., 2000), appears to mediate substantial components of HFD and IH-induced neuronal dysfunction. A large body of evidence has shown that NADPH oxidase is implicated in conditions that remotely resemble hypoxia-reoxygenation (Baran and Richert, 2003; Brennan et al., 2009; Bruce-Keller et al., 2010; Girouard et al., 2009; Groeger et al., 2009; Ha et al., 2010; Suh et al., 2008). While we cannot infer from our current findings on the cellular source of NADPH oxidase contribution to HFD-IH-induced cognitive and behavioral deficits, the near complete abrogation of such deficits in the gp91phox_/Y mice is highly suggestive of the critical role played by NADPH oxidase in this context. Of note, we have previously shown that IH-induced cognitive and behavioral deficits are attenuated by reductions in oxidative stress and inflammatory signaling cascades through pharmacological interventions (Li et al., 2003; Row et al., 2003), as well as through attenuation of oxidative stress via targeted genetic manipulations of manganese superoxide dismutase, platelet-activating factor receptor, nitric oxide synthase or NADPH oxidase system (Li et al., 2004; Row et al., 2004; Shan et al., 2007). However, there was little if any evidence to suggest that both HFD and IH shared similar pathogenetic pathways, since in rats HFD exacerbated IH-induced cognitive effects (Goldbart et al., 2006). Our current findings suggest that that NADPH oxidase plays a critical role in both HFD and IH-mediated effects. Previous work from our lab has shown that normal diet does not induce cognitive impairments in either C57BL6/J or gp91phox_/Y in RA conditions. However when the C57BL6/J mice are exposed to IH significant behavioral deficits emerge in the C57BL6/J mice but are conspicuously absent in the gp91phox_/Y mice (Nair et al., 2011). Taken together, these data suggest that HFD consumption and IH individually affect brain function and homeostasis, and may interact when concomitantly present to exacerbate the deleterious effects that each of them imposes. More specifically, the present data implicate the presence of increased hippocampal oxidative stress as derived from NADPH oxidase type 2 activity as a critically important mechanism underlying HFD-induced declines in cognitive performance in the brain. We should remark that the slight differences in performance during the probe trial between the C57BL6/J mice and the gp91phox_/Y mice could be due to differences in strain.
Patients with SA exhibit higher levels of anxiety and are also more likely to exhibit depressive mood (Akashiba et al., 2002; File, 1992; Ohayon, 2003), the latter also accounting for the sense of fatigue reported by many of the patients (Bardwell et al., 2001). The FST model employed in the present study is based on the observation that when mice are forced to swim in a situation from which there is no escape, they will after an initial period of vigorous activity eventually cease to move altogether making only those movements necessary to keep the head above water. This type of behavior indicates that immobility during such conditions reflects mood, and indeed the level of immobility in this test is modified by anti-depressant pharmacological treatments. Because little if any immobility occurs during the initial 2 min of this test, only the immobility occurring during the last 4 min was counted (Porsolt et al., 1977; Eckeli et al., 2000) . The elevated plus-maze is the most frequently utilized animal model for assessing anxiety-like behaviors (Hui-guo et al., 2010; Pellow et al., 1985) since it enables researchers to observe the conflict between two innate rodent behaviors, namely the avoidance of open space exposure as countering the tendency to explore novel environments (Hui-guo et al., 2010). This study provides initial evidence that IH and HFD can induce anxiety-like behaviors in wild type mice, but that such disturbances were absent in gp91phox_/Y mice, suggesting that regions underlying these anxiety-related patterns are susceptible to oxidant stress mediated by activation of NADPH oxidase in both HFD and IH (Goldbart et al., 2003; Veasey et al., 2004; Zhan G et al., 2005).
In the present study, we demonstrate the explicit mechanistic involvement of NADPH oxidase in the CNS end-organ injury induced by HFD and IH as evidenced by increases in oxidative stress markers. We have previously shown that dietary manipulations such as inclusion of green tea polyphenols in drinking water attenuates the increase in NADPH oxidase gene expression under IH conditions, and reduces cognitive deficits in rats(Burckhardt et al., 2008). Given the serious negative health consequences of combined obesity and SA in inducing cognitive deficits even in children (Spruyt and Gozal, 2012), and the effect of NADPH oxidase genomic variation on the cognitive susceptibility to SA (Gozal et al., 2012), our current findings would support early and sustained implementation of healthier modifications in diet along with potential targeting of NADPH oxidase excessive activation in an effort to prevent or palliate the deleterious CNS effects of sleep apnea.
In conclusion, combined exposure to HFD and IH adversely increases lipid peroxidation and DNA oxidation in the hippocampus, and induce cognitive dysfunction and learning and memory impairments associated via excessive activation of NADPH oxidase.
Highlights.
HFD and SA-induced learning deficits are absent in NADPH oxidase null mice.
Oxidative stress in hippocampus of NADPH oxidase null mice is absent.
Western diet-induced cognitive deficits are mediated by NADPH oxidase activity.
Acknowledgments
Financial Support: This study was supported by National Institutes of Health grant HL-086662 to DG.
Abbreviations
- 8-OHdG
8-hydroxydeoxyguanosine
- ANOVA
analysis of variance
- CNS
central nervous system
- EPM
elevated plus maze
- FST
forced swimming test
- HFD
high fat-refined carbohydrate diet
- IH
intermittent hypoxia
- MANOVA
multivariate analysis of variance
- MDA
malondialdehyde
- NADPH
nicotinamide adenine dinucleotide phosphate
- RA
room air
- ROS
reactive oxygen species
- SA
sleep apnea
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
DN performed all experiments, analyzed the data and drafted the initial version of the manuscript. VR performed data and statistical analysis, interpretation of the data and drafted the initial version of the manuscript. DG provided the initial conceptual framework, coordinated the experimental planning, assisted with data analysis and interpretation, and edited and approved the final version of the manuscript.
References
- 1.Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akashiba T, Kawahara S, Akahoshi T, Omori C, Saito O, Majima T, Horie T. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest. 2002;122:861–865. doi: 10.1378/chest.122.3.861. [DOI] [PubMed] [Google Scholar]
- 3.Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15–22. doi: 10.1016/j.yjmcc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Baran AS, Richert AC. Obstructive sleep apnea and depression. CNS Spectr. 2003;8:128–134. doi: 10.1017/s1092852900018356. [DOI] [PubMed] [Google Scholar]
- 5.Bardwell WA, Ancoli-Israel S, Dimsdale JE. Types of coping strategies are associated with increased depressive symptoms in patients with obstructive sleep apnea. Sleep. 2001;24:905–909. doi: 10.1093/sleep/24.8.905. [DOI] [PubMed] [Google Scholar]
- 6.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 7.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 8.Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, Weidner AM, LeVine H, 3rd, Keller JN, Markesbery WR. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010;12:1371–1382. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burckhardt IC, Gozal D, Dayyat E, Cheng Y, Li RC, Goldbart AD, Row BW. Green tea catechin polyphenols attenuate behavioral and oxidative responses to intermittent hypoxia. Am J Respir Crit Care Med. 2008;177:1135–1141. doi: 10.1164/rccm.200701-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capurso A, Solfrizzi V, Panza F, Torres F, Mastroianni F, Grassi A, Del Parigi A, Capurso C, Pirozzi MR, Centonze S, et al. Dietary patterns and cognitive functions in elderly subjects. Aging (Milano) 1997;9:45–47. doi: 10.1007/BF03339702. [DOI] [PubMed] [Google Scholar]
- 11.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122:1162–1167. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 12.Eckeli AL, Dach F, Rodrigues ALS. Acute treatment with GMP produces antidepressant-like effects in mice. Neuro Rep. 2000;11:1839–1843. doi: 10.1097/00001756-200006260-00008. [DOI] [PubMed] [Google Scholar]
- 13.El-Sheikh M, Kelly RJ, Buckhalt JA, Benjamin Hinnant J. Children's sleep and adjustment over time: the role of socioeconomic context. Child Dev. 2010;81:870–883. doi: 10.1111/j.1467-8624.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- 14.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 15.Floel A, Witte AV, Lohmann H, Wersching H, Ringelstein EB, Berger K, Knecht S. Lifestyle and memory in the elderly. Neuroepidemiology. 2008;31:39–47. doi: 10.1159/000137378. [DOI] [PubMed] [Google Scholar]
- 16.Gasior M, Rogawski MA, Hartman AL. Neuroprotective and diseasemodifying effects of the ketogenic diet. Behav Pharmacol. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldbart A, Row BW, Kheirandish L, Schurr A, Gozal E, Guo SZ, Payne RS, Cheng Z, Brittian KR, Gozal D. Intermittent hypoxic exposure during light phase induces changes in cAMP response element binding protein activity in the rat CA1 hippocampal region: water maze performance correlates. Neuroscience. 2003;122:585–590. doi: 10.1016/j.neuroscience.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 18.Goldbart AD, Row BW, Kheirandish-Gozal L, Cheng Y, Brittian KR, Gozal D. High fat/refined carbohydrate diet enhances the susceptibility to spatial learning deficits in rats exposed to intermittent hypoxia. Brain Res. 2006;1090:190–196. doi: 10.1016/j.brainres.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 19.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176:188–193. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gozal D, Khalyfa A, Capdevila OS, Kheirandish-Gozal L, Khalyfa AA, Kim J. Cognitive function in prepubertal children with obstructive sleep apnea: a modifying role for NADPH oxidase p22 subunit gene polymorphisms? Antioxid Redox Signal. 2012;16:171–177. doi: 10.1089/ars.2011.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ha JS, Lee JE, Lee JR, Lee CS, Maeng JS, Bae YS, Kwon KS, Park SS. Nox4-dependent H2O2 production contributes to chronic glutamate toxicity in primary cortical neurons. Exp Cell Res. 2010;316:1651–1661. doi: 10.1016/j.yexcr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 24.Hui-guo L, Kui L, Yan-ning Z, Yong-jian X. Apocynin attenuate spatial learning deficits and oxidative responses to intermittent hypoxia. Sleep Med. 2010;11:205–212. doi: 10.1016/j.sleep.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Hwang IK, Kim IY, Kim DW, Yoo KY, Kim YN, Yi SS, Won MH, Lee IS, Yoon YS, Seong JK. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res. 2008;1241:1–6. doi: 10.1016/j.brainres.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Hwang IK, Kim IY, Kim YN, Yi SS, Park IS, Min BH, Doo HK, Ahn SY, Kim YS, Lee IS, et al. Comparative study on high fat diet-induced 4- hydroxyl-2E-nonenal adducts in the hippocampal CA1 region of C57BL/6N and C3H/HeN mice. Neurochem Res. 2009;34:964–972. doi: 10.1007/s11064-008-9846-y. [DOI] [PubMed] [Google Scholar]
- 27.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 28.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 30.Kheirandish L, Row BW, Li RC, Brittian KR, Gozal D. Apolipoprotein E-deficient mice exhibit increased vulnerability to intermittent hypoxia-induced spatial learning deficits. Sleep. 2005;28:1412–1417. doi: 10.1093/sleep/28.11.1412. [DOI] [PubMed] [Google Scholar]
- 31.Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D, Du CY, Tang XJ, Jin YX, Lei T, Yao Y, Yang Z, Zhang T. [Changes of heart rate variability and impairment of learning and memory induced by cerebral ischemia/reperfusion in rats] Sheng Li Xue Bao. 2007a;59:35–41. [PubMed] [Google Scholar]
- 33.Li J, Savransky V, Nanayakkara A, Smith PL, O'Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol. 2007b;102:557–563. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 34.Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, Brittian KR, Guo SZ, Sachleben LR, Jr, Gozal D. Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med. 2003;168:469–475. doi: 10.1164/rccm.200211-1264OC. [DOI] [PubMed] [Google Scholar]
- 35.Li RC, Row BW, Kheirandish L, Brittian KR, Gozal E, Guo SZ, Sachleben LR, Jr, Gozal D. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis. 2004;17:44–53. doi: 10.1016/j.nbd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- 37.Lomax KJ, Leto TL, Nunoi H, Gallin JI, Malech HL. Recombinant 47-kilodalton cytosol factor restores NADPH oxidase in chronic granulomatous disease. Science. 1989;245:409–412. doi: 10.1126/science.2547247. [DOI] [PubMed] [Google Scholar]
- 38.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 39.Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One. 2011;6:e19847. doi: 10.1371/journal.pone.0019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noh HS, Kim YS, Choi WS. Neuroprotective effects of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):120–123. doi: 10.1111/j.1528-1167.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 42.Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci. 2000;20:RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64:1195–1200. doi: 10.4088/jcp.v64n1009. quiz, 1274-1196. [DOI] [PubMed] [Google Scholar]
- 44.Pathan AR, Gaikwad AB, Viswanad B, Ramarao P. Rosiglitazone attenuates the cognitive deficits induced by high fat diet feeding in rats. Eur J Pharmacol. 2008;589:176–179. doi: 10.1016/j.ejphar.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 46.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 47.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 48.Row BW, Kheirandish L, Li RC, Guo SZ, Brittian KR, Hardy M, Bazan NG, Gozal D. Platelet-activating factor receptor-deficient mice are protected from experimental sleep apnea-induced learning deficits. J Neurochem. 2004;89:189–196. doi: 10.1111/j.1471-4159.2004.02352.x. [DOI] [PubMed] [Google Scholar]
- 49.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med. 2003;167:1548–1553. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- 50.Royer-Pokora B. Novel approaches for cloning human genes: the chronic granulomatous disease (CGD) Dis Markers. 1987;5:57–58. [PubMed] [Google Scholar]
- 51.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 52.Solfrizzi V, Panza F, Torres F, Mastroianni F, Del Parigi A, Venezia A, Capurso A. High monounsaturated fatty acids intake protects against agerelated cognitive decline. Neurology. 1999;52:1563–1569. doi: 10.1212/wnl.52.8.1563. [DOI] [PubMed] [Google Scholar]
- 53.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 54.Spruyt K, Gozal D. A mediation model linking body weight, cognition, and sleep-disordered breathing. Am J Respir Crit Care Med. 2012;185:199–205. doi: 10.1164/rccm.201104-0721OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci. 2000;20:RC53. doi: 10.1523/JNEUROSCI.20-01-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, Bhat NR. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem. 2008;106:475–485. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tozuka Y, Wada E, Wada K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 2009;23:1920–1934. doi: 10.1096/fj.08-124784. [DOI] [PubMed] [Google Scholar]
- 60.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 61.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 62.Wainwright PE, Xing HC, Ward GR, Huang YS, Bobik E, Auestad N, Montalto M. Water maze performance is unaffected in artificially reared rats fed diets supplemented with arachidonic acid and docosahexaenoic acid. J Nutr. 1999;129:1079–1089. doi: 10.1093/jn/129.5.1079. [DOI] [PubMed] [Google Scholar]
- 63.White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Wu A, Molteni R, Ying Z, Gomez-Pinilla F. A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience. 2003;119:365–375. doi: 10.1016/s0306-4522(03)00154-4. [DOI] [PubMed] [Google Scholar]
- 66.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 67.Yamato M, Shiba T, Yoshida M, Ide T, Seri N, Kudou W, Kinugawa S, Tsutsui H. Fatty acids increase the circulating levels of oxidative stress factors in mice with diet-induced obesity via redox changes of albumin. FEBS J. 2007;274:3855–3863. doi: 10.1111/j.1742-4658.2007.05914.x. [DOI] [PubMed] [Google Scholar]
- 68.Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E, SC V. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med. 2005;172:921–929. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr Res. 2004;55:498–506. doi: 10.1203/01.PDR.0000112032.47575.D1. [DOI] [PubMed] [Google Scholar]


