Abstract
West Nile virus (family Flaviviridae, genus Flavivirus, WNV) is now endemic in California across a variety of ecological regions that support a wide diversity of potential avian and mammalian host species. Because different avian hosts have varying competence for WNV, determining the blood-feeding patterns of Culex (Diptera: Culicidae) vectors is a key component in understanding the maintenance and amplification of the virus as well as tangential transmission to humans and horses. We investigated the blood-feeding patterns of Culex tarsalis Coquillett and members of the Culex pipiens L. complex from southern to northern California. Nearly 100 different host species were identified from 1,487 bloodmeals, by using the mitochondrial gene cytochrome c oxidase I (COI). Cx. tarsalis fed on a higher diversity of hosts and more frequently on nonhuman mammals than did the Cx. pipiens complex. Several WNV-competent host species, including house finch and house sparrow, were common bloodmeal sources for both vector species across several biomes and could account for WNV maintenance and amplification in these areas. Highly competent American crow, western scrub-jay and yellow-billed magpie also were fed upon often when available and are likely important as amplifying hosts for WNV in some areas. Neither species fed frequently on humans (Cx. pipiens complex [0.4%], Cx. tarsalis [0.2%]), but with high abundance, both species could serve as both enzootic and bridge vectors for WNV.
Keywords: Culex tarsalis, Culex pipiens, bloodmeal, host identification, host diversity
Arthropod blood-feeding behavior is a crucial component in the transmission of vectorborne pathogens because it defines the frequency of host–vector contact. Understanding vector blood-feeding patterns can elucidate when and where particular vertebrate hosts are at risk of infection as well as what competent hosts contribute to maintenance and amplification transmission. Although important for all vectorborne diseases, blood-feeding patterns are especially of interest for a zoonosis such as West Nile virus (family Flaviviridae, genus Flavivirus, WNV), where literally hundreds of vertebrate species have been found to be infected in the field (Kramer et al. 2008), but relatively few taxa develop sufficient viremias to infect blood-feeding mosquitoes (Komar et al. 2003, Kilpatrick et al. 2007). Because the primary Culex vectors of WNV feed on a wide variety of avian and mammalian hosts with varying levels of WNV competence, insight into spatial variation in the blood-feeding patterns of these mosquitoes may be crucial in understanding why the transmission dynamics of WNV varies in different areas.
Culex tarsalis Coquillett and members of the Culex pipiens L. complex are the primary vectors of WNV (Goddard et al. 2002, Reisen et al. 2008a) and St. Louis encephalitis viruses (Reeves et al. 1990) in California. Cx. tarsalisis also the primary vector of western equine encephalomyelitis virus (Reeves et al. 1990). Cx. tarsalis is historically a rural mosquito (Reisen and Reeves 1990), although recent housing foreclosures and associated neglected swimming pools have led to an increased abundance in urban areas (Reisen et al. 2008c, 2009). The Cx. pipiens complex, typically a more urban vector, is composed of at least two species in California. Traditionally, members of the complex found north of 39°N have been considered Cx. pipiens and those found south of 36°N have been called Culex quinquefasciatus, whereas members found between these latitudes have been identified as either species or as interspecific hybrids (Iltis 1966, Barr 1967, Tabachnick and Powell 1983). There is evidence, however, that hybridization of these two species is not limited to the area between these latitudes and may be extensive throughout California, especially in the Central Valley (Urbanelli et al. 1997, Cornel et al. 2003). With no reliable markers currently available for morphological or molecular identification, distinguishing these species and their hybrids can be difficult, if not impossible. For this study, members were simply referred to as the Cx. pipiens complex regardless of collection location.
The blood-feeding patterns of Cx. tarsalis and the Cx. pipiens complex have been assessed in several locations in the United States since the introduction of WNV and the advent of new molecular methods for host species identification (Kent 2009). These species seem to be generalist feeders, with host use patterns that have differed among species and collection locations (Kilpatrick et al. 2006a; Molaei et al. 2006, 2007; Savage et al. 2007; Hamer et al. 2009; Kent et al. 2009). In California, Culex feeding patterns were explored >40 yr ago in rural areas of the Central Valley by using serological methods (Tempelis and Reeves 1964; Tempelis et al. 1965, 1976; Tempelis and Washino 1967) and recently in more residential areas of southern California (Orange County and western portions of San Bernardino and Riverside counties) and rural Sacramento and Yolo counties by using molecular methods (Molaei et al. 2010, Montgomery et al. 2011, Thiemann et al. 2011). The current study explored the blood-feeding patterns of Cx. tarsalis and Cx. pipiens complex mosquitoes collected from five areas within four diverse ecological regions, all of which have supported WNV enzootic and epidemic transmission.
Materials and Methods
Study Areas
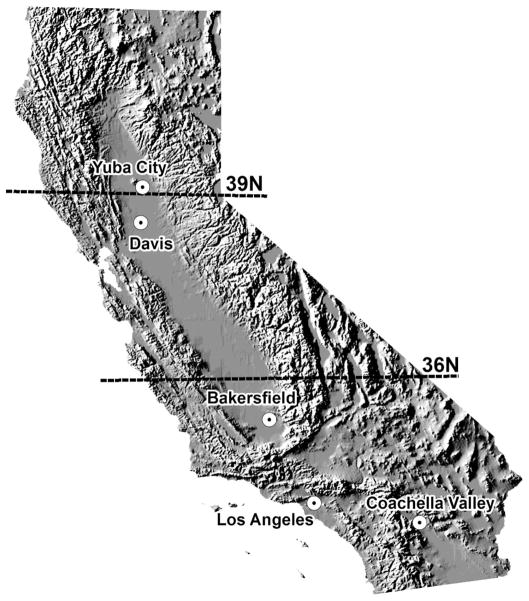
Blood-feeding patterns were determined for Culex mosquitoes collected in five areas throughout California, namely, the southeastern deserts of Coachella Valley, maritime coastal and highly urbanized Los Angeles, the southern San Joaquin Valley near Bakersfield, and the Davis and Yuba City areas of the Sacramento Valley (Fig. 1). These sampling sites span over 850 km from southern to northern California and offer a diversity of climates, ranges of urbanization, and a wide variety of potential host species.
Fig. 1.
Map of California study areas.
Coachella Valley
Mosquitoes were collected within the Coachella Valley Mosquito and Vector Control District (MVCD) that is situated within the arid Colorado Desert, encompasses 6,200 km2 of Riverside County, and has a human population of 332,000. Mosquitoes were collected at >50 sites throughout the valley, including wetlands near the Salton Sea, agricultural areas with citrus and date orchards, and in residential areas in the upper valley near Palm Springs. Although WNV has been enzootically active each year in this area, few human cases have been reported (Reisen et al. 2008b).
Los Angeles
Blood-fed mosquitoes were collected within the Greater Los Angeles County Vector Control District (VCD) that encompasses a 3,400 km2 area of Los Angeles County, with a human population of >6 million residents. Sampling was conducted at >100 sites distributed in both residential areas and embedded parklands. Two WNV outbreaks and intervening enzootic WNV transmission were documented previously (Kwan et al. 2010).
Kern County (Near Bakersfield)
Samples were collected in Kern County near Bakersfield within the boundaries of the Kern MVCD. The district covers 4,300 km2 in the southern San Joaquin Valley and includes a population of >800,000 residents. Mosquitoes were collected from rural and urban sites, including residential, golf course, and agricultural and riparian habitats. West Nile virus transmission achieved epidemic levels during most years since its introduction, with the number of human cases peaking in 2007 (Reisen et al. 2009).
Yolo County (Within and Surrounding Davis)
Blood-fed Culex mosquitoes were collected in Yolo County from several residential neighborhoods and parks within the city of Davis (human population, 60,000). Additional samples were collected from the Yolo Bypass Wildlife Area, a 65-km2 area consisting of rice (Oryza sativa L.) fields and managed wetlands. The wildlife area is home to >300 avian and mammalian species at various times of the year. A detailed investigation of Cx. tarsalis feeding patterns at a farmstead near Davis supporting a large nesting colony of herons and egrets was described separately (Thiemann et al. 2011). Enzootic WNV activity has been consistently detected in Davis, with a single outbreak documented during 2006 (Nielsen et al. 2008).
Sutter County (Near Yuba City)
Blood-fed mosquitoes were collected in Sutter County, around Yuba City, within the Sutter-Yuba MVCD that covers 1,800 km2 and serves ≈ 160,000 people. This rural area of the northern Sacramento Valley is primarily agricultural habitat with extensive rice culture. WNV has been repeatedly active enzootically, but relatively few human cases have been reported (http://westnile.ca.gov/reports.php).
Mosquito Collection
Initially, walk-in red boxes (Meyer 1987) were deployed within urban, suburban, and rural locations in each of the study areas in an effort to collect fully blood-fed Culex mosquitoes. Red boxes were highly successful in Sutter County, where this method has been used for surveillance for >20 yr and productive collection sites have been located by trial and error. In contrast, few of the red boxes in other areas were sufficiently productive for our study. Although it was unclear why some red box locations were successful and others were not, it seemed that plentiful natural resting sites were detrimental to productive red box collections. In addition to red box collections, blood-fed mosquitoes were retained from traps used for WNV surveillance, including dry ice (CO2)–baited CDC-style traps (Sudia and Chamberlain 1962, Newhouse et al. 1966) and up-draft gravid female traps baited with an alfalfa (Medicago sativa L.) infusion (Cummings 1992). Suction traps, operated without bait, were used in Los Angeles and the Coachella Valley. Because sampling methods may bias host selection (Thiemann and Reisen 2012), multiple trapping methods were used whenever feasible.
Culex mosquitoes were collected weekly or biweekly from April to October 2007–2009 from multiple sites (ranging from five to >100) in each study area. Mosquitoes were anesthetized and enumerated by species. Blood-fed females were stored individually at −80°C pending host identification.
Bloodmeal Identification
Bloodmeals were identified using methods described previously (Thiemann et al. 2012). In brief, DNA was extracted from the blood-fed Culex abdomens by using the DNeasy 96 Blood & Tissue kit (QIAGEN, Valencia, CA). The 658-bp “barcoding” region of the mitochondrial gene cytochrome c oxidase I (COI) was amplified using a nested polymerase chain reaction (PCR). First, primers for the tRNA-coding regions flanking COI were used to amplify ≈ 1,900 bp. Then, the barcoding region of COI was amplified using vertebrate-specific primers (Ivanova et al. 2006, Cooper et al. 2007). Host DNA was identified either using a microsphere assay for COI with species-specific probes (Thiemann et al. 2012) for common hosts in each study area or by sequencing the amplicon and using the Identify Specimen feature of the Barcode of Life Data Systems (BOLD; www.boldsystems.org) (Ratnasingham and Hebert 2007, Kent et al. 2009). Mixed bloodmeals were identified exclusively with the microsphere assay (Thiemann et al. 2012).
Statistical Analysis
To compare host species richness across study areas, rarefaction was used to generate the expected number of host species from a 50 bloodmeal subsample, by using the rarefy function in the vegan package version 1.17–8 of R (RDC 2011). Bloodmeal percentages within and between study areas were compared by pairwise chi-square analysis.
Bird Population Estimates
The weekly frequency of avian species from May to September 2007–2009 was downloaded from eBird (Sullivan et al. 2009), a project developed by Cornell Laboratory of Ornithology and the National Audubon Society to monitor avian prevalence from citizen-based reporting. The frequency of a particular species represents the proportion of citizen-submitted checklists reporting that species in a given area.
Results
Vertebrate host species were successfully identified from 515 (81%) blood-fed Cx. tarsalis and 972 (81%) blood-fed members of the Cx. pipiens complex across all study areas. In total, 99 host species (80 birds, 18 mammals, and one reptile) were identified from these 1,487 bloodmeals. Unidentified bloodmeals either did not produce a visible amplification product, probably due to small bloodmeal size or digestion of the blood-meal in half-gravid (Sella’s stage III–IV) and subgravid (Sella’s stage V) individuals (Sella 1920, WHO 1975), or could not be conclusively identified to host species.
Coachella Valley
In total, 399 blood-fed mosquitoes from the Cx. pipiens complex and 94 blood-fed Cx. tarsalis were collected from >50 sites in the Coachella Valley (Table 1). Overall, chickens comprised 47.6% of the Cx. pipiens complex bloodmeals and 16% of the bloodmeals from Cx. tarsalis. At collection sites ≤30 m from sentinel chicken flocks, >70% of the Cx. pipiens complex bloodmeals were from chickens. Because of this bias, collections within 50 m of sentinel chicken flocks were excluded from further analysis.
Table 1.
Number and percentage of avian- and mammalian-derived bloodmeals from the Cx. pipiens complex and Cx. tarsalis in the Coachella Valley, Riverside County, 2007–2009
| Host |
Cx. pipiens complex
|
Cx. tarsalis
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2a | GRb | Total | % total | % chickc,d | CO2a | GRb | STe | Total | % total | % chickc,d | |
| Avian | |||||||||||
| Chicken, Gallus gallus | 167 | 23 | 190 | 47.6 | 4.2bcd | 12 | 3 | 15 | 16.0 | 1.4a | |
| House finch, Carpodacus mexicanus | 6 | 19 | 25 | 6.3 | 13.3a | 1 | 1 | 1.1 | 1.4a | ||
| Mourning dove, Zenaida macroura | 8 | 16 | 24 | 6.0 | 12.6a | 4 | 4 | 4.3 | 5.8a | ||
| House sparrow, Passer domesticus | 13 | 7 | 20 | 5.0 | 7.0abc | 3 | 3 | 3.2 | 1.4a | ||
| Greater roadrunner, Geococcyx californianus | 1 | 18 | 19 | 4.8 | 10.5ab | ||||||
| Northern mockingbird, Mimus polyglottos | 2 | 11 | 13 | 3.3 | 7.0abc | 2 | 2 | 2.1 | 2.9a | ||
| Common raven, Corvus corax | 5 | 5 | 1.3 | 3.5cd | |||||||
| Great-tailed grackle, Quiscalus mexicanus | 5 | 5 | 1.3 | 2 | 2 | 2.1 | 2.9a | ||||
| Verdin, Auriparus flaviceps | 2 | 3 | 5 | 1.3 | 2.1cd | ||||||
| Barn owl, Tyto alba | 1 | 3 | 4 | 1.0 | 0.7d | 2 | 1 | 1 | 4 | 4.3 | 4.3a |
| Gambel’s quail, Callipepla gambelii | 1 | 3 | 4 | 1.0 | 2.8cd | 1 | 2 | 3 | 3.2 | 4.3a | |
| Abert’s towhee, Pipilo aberti | 3 | 3 | 0.8 | 1.4d | |||||||
| Brewer’s blackbird, Euphagus cyanocephalus | 3 | 3 | 0.8 | 1.4d | 2 | 2 | 2.1 | 2.9a | |||
| Burrowing owl, Athene cunicularia | 3 | 3 | 0.8 | 2.1cd | |||||||
| Eurasian collared-dove, Streptopelia decaocto | 2 | 1 | 3 | 0.8 | 2.1cd | 1 | 1 | 1.1 | 1.4a | ||
| European starling, Sturnus vulgaris | 1 | 2 | 3 | 0.8 | 1 | 1 | 1.1 | ||||
| Great Horned owl, Bubo virginianus | 1 | 2 | 3 | 0.8 | |||||||
| Western scrub-jay, Aphelocoma californica | 3 | 3 | 0.8 | 2.1cd | 1 | 1 | 1.1 | 1.4a | |||
| Wild turkey, Meleagris gallopavo | 3 | 3 | 0.8 | 1 | 1 | 1.1 | |||||
| Brown-headed cowbird, Molothrus ater | 1 | 1 | 2 | 0.5 | 0.7d | ||||||
| Cactus wren, Campylorhynchus brunneicapillus | 1 | 1 | 2 | 0.5 | 1.4d | ||||||
| Common ground-dove, Columbina passerina | 1 | 1 | 2 | 0.5 | 0.7d | 2 | 1 | 3 | 3.2 | 4.3a | |
| Prarie falcon, Falco mexicanus | 2 | 2 | 0.5 | 1.4d | |||||||
| Western kingbird, Tyrannus verticalis | 1 | 1 | 2 | 0.5 | 0.7d | ||||||
| White-winged dove, Zenaida asiatica | 2 | 2 | 0.5 | 1.4d | |||||||
| American robin, Turdus migratorius | 1 | 1 | 0.3 | ||||||||
| Black-tailed gnatcatcher, Polioptila melanura | 1 | 1 | 0.3 | 0.7d | 1 | 1 | 1.1 | 1.4a | |||
| Brewer’s sparrow, Spizella breweri | 1 | 1 | 0.3 | 0.7d | |||||||
| Green heron, Butorides virescens | 1 | 1 | 0.3 | 0.7d | 1 | 1 | 1.1 | 1.4a | |||
| Inca dove, Columbina inca | 1 | 1 | 0.3 | 0.7d | 2 | 2 | 2.1 | 2.9a | |||
| Indian peafowl, Pavo cristatus | 1 | 1 | 0.3 | 0.7d | 1 | 1 | 1.1 | 1.4a | |||
| Peregrine falcon, Falco peregrinus | 1 | 1 | 0.3 | 0.7d | |||||||
| Rock pigeon, Columba livia | 1 | 1 | 0.3 | 0.7d | |||||||
| Ruddy duck, Oxyura jamaicensis | 1 | 1 | 0.3 | 0.7d | |||||||
| Western bluebird, Sialia mexicana | 1 | 1 | 0.3 | 0.7d | |||||||
| Western tanager, Piranga ludoviciana | 1 | 1 | 0.3 | 0.7d | |||||||
| Yellow warbler, Dendroica petechia | 1 | 1 | 0.3 | 0.7d | |||||||
| Yellow-rumped warbler, Dendroica coronata | 1 | 1 | 0.3 | ||||||||
| American kestrel, Falco sparverius | 1 | 2 | 3 | 3.2 | 4.3a | ||||||
| American white pelican, Pelecanus erythrorhynchos | 1 | 1 | 1.1 | ||||||||
| Barn swallow, Hirundo rustica | 1 | 1 | 1.1 | 1.4a | |||||||
| Black-crowned night-heron, Nycticorax nycticorax | 1 | 1 | 2 | 2.1 | 2.9a | ||||||
| Caspian tern, Sterna caspia | 1 | 1 | 1.1 | ||||||||
| Cooper’s hawk, Accipiter cooperii | 2 | 2 | 2.1 | 2.9a | |||||||
| Great blue heron, Ardea herodias | 2 | 2 | 2.1 | 2.9a | |||||||
| Least bittern, Ixobrychus exilis | 1 | 2 | 3 | 3.2 | 4.3a | ||||||
| Marsh wren, Cistothorus palustris | 1 | 1 | 1.1 | ||||||||
| Pied-billed grebe, Podilymbus podiceps | 2 | 2 | 2.1 | 2.9a | |||||||
| Ruby-crowned kinglet, Regulus calendula | 1 | 1 | 1.1 | 1.4a | |||||||
| Song sparrow, Melospiza melodia | 1 | 1 | 1.1 | 1.4a | |||||||
| Undetermined duck, Anas sp. | 1 | 1 | 1.1 | ||||||||
| Mammalian | |||||||||||
| Domestic dog, Canis familiaris | 13 | 9 | 22 | 5.5 | 11.9a | 4 | 4 | 4.3 | 5.8a | ||
| Black rat, Rattus rattusf | 7 | 7 | 1.8 | 0.7d | 1 | 1 | 1.1 | 1.4a | |||
| Domestic cat, Felis catus | 1 | 2 | 3 | 0.8 | 0.7d | ||||||
| Coyote, Canis latrans | 1 | 1 | 0.3 | ||||||||
| Desert cottontail, Sylvilagus audubonii | 1 | 1 | 0.3 | 1 | 1 | 1.1 | 1.4a | ||||
| Black-tailed jackrabbit, Lepus californicus | 2 | 2 | 2.1 | 2.9a | |||||||
| Domestic cow, Bos taurus | 5 | 5 | 5.3 | 7.2a | |||||||
| Domestic horse, Equus caballus | 7 | 7 | 7.4 | 5.8a | |||||||
| House mouse, Mus musculus | 1 | 1 | 1.1 | 1.4a | |||||||
| Human, Homo sapiens | 1 | 1 | 1.1 | 1.4a | |||||||
| Raccoon, Procyon lotor | 1 | 1 | 1.1 | 1.4a | |||||||
| White-throated woodrat, Neotoma albigula | 1 | 1 | 1.1 | 1.4a | |||||||
| Mixed bloodmeals | |||||||||||
| Chicken/common ground-dove | 1 | 1 | 0.3 | 1 | 1 | 1.1 | 1.4a | ||||
| Chicken/house sparrow | 1 | 1 | 0.3 | ||||||||
| Total 63 species | 243 | 156 | 399 | n = 143 | 75 | 9 | 10 | 94 | n = 69 | ||
Dry ice–baited CDC trap.
Gravid trap.
Percentages exclude sites with sentinel chicken flocks.
Percentages with different lowercase letters indicate that they are significantly different by chi-square test (P < 0.05).
Suction trap (no bait).
These samples were identified in BOLD as Rattus tanezumi, but they are probably Rattus rattus Lineage II (Aplin et al. 2011) per personal communication with C. Conroy (Museum of Vertebrate Zoology, University of California–Berkeley).
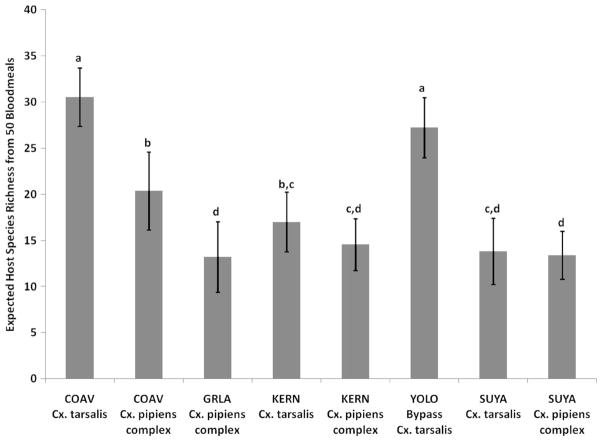
Away from sentinel chicken sites, house finch (13.3%), mourning dove (12.6%), domestic dog (11.5%), greater roadrunner (10.5%), house sparrow (7.0%), and northern mockingbird (7.0%) were the most frequent hosts of the Cx. pipiens complex. In total, 32 avian host species and three mammalian species were identified. For Cx. tarsalis, no host species accounted for >8% of the total bloodmeals. Three mammalian species, domestic cow (7.2%), domestic horse (5.8%), and domestic dog (5.8%) were repeated hosts, and 10 mammalian species, including human, comprised >30% of the total bloodmeals. There was no significant difference in the bloodmeal percentage of any host (Table 1), and 36 host species in total were identified from 69 bloodmeals. Overall, host species richness was greater for Cx. tarsalis (30.5 host species/50 bloodmeals) than the Cx. pipiens complex (20.4 host species/50 bloodmeals) (Fig. 2).
Fig. 2.
Expected host species richness (number of host species), as determined by rarefaction, in a random 50 bloodmeal subsample of Cx. tarsalis and the Cx. pipiens complex at each study site: Coachella Valley (COAV), Los Angeles (GRLA), Kern County (KERN), Yolo Bypass Wildlife Area (YOLO Bypass), and Sutter County (SUYA) Residential Yolo County was omitted because of small sample size. Error bars depict the 95% confidence intervals, and letters denote statistical similarity.
Los Angeles
Walk-in red boxes were not productive, and most of the blood-fed Culex were collected in gravid and CO2 traps from >100 sites dispersed throughout the Greater Los Angeles County VCD. Few Cx. tarsalis were collected over the 3-yr study period (10 females), so only bloodmeals from the 275 Cx. pipiens complex were identified and reported (Table 2). As in the Coachella Valley, chickens were the dominant host from mosquitoes collected near sentinel flocks, so bloodmeal host composition was presented with and without sentinel chicken sites. Contrary to Coachella, where no host species away from sentinel chicken flocks comprised >14% of the total bloodmeals (Table 1), members of the Cx. pipiens complex in Los Angeles County acquired >60% of their bloodmeals from two host species, house finch (39.4%) and house sparrow (21.6%) (Table 2). Mourning dove (6.9%), northern mockingbird (4.1%), and domestic dog (3.7%) were the next most frequent hosts. Mammalian bloodmeals comprised just 6% of the total, and only two human bloodmeals (0.9%) were identified, despite the dense human population in this area. With a majority of Cx. pipiens complex feeding on house finch and house sparrow, and 27 hosts in total identified away from sentinel chicken flocks, host species richness was 13.2 for 50 bloodmeals, significantly less than the more rural Coachella Valley (Fig. 2).
Table 2.
Number and percentage of avian- and mammalian-derived bloodmeals from the Cx. pipiens complex in Los Angeles County, 2007–2009
| Host |
Cx. pipiens complex
|
||||||
|---|---|---|---|---|---|---|---|
| CO2a | GRb | RBc | USDSd | Total | % total | % chicke,f | |
| Avian | |||||||
| House finch, Carpodacus mexicanus | 15 | 69 | 8 | 92 | 33.5 | 39.4a | |
| Chicken, Gallus gallus | 22 | 30 | 1 | 53 | 19.3 | 3.2de | |
| House sparrow, Passer domesticus | 5 | 42 | 1 | 48 | 17.5 | 21.6b | |
| Mourning dove, Zenaida macroura | 2 | 9 | 3 | 2 | 16 | 5.8 | 6.9c |
| Northern mockingbird, Mimus polyglottos | 3 | 7 | 10 | 3.6 | 4.1cd | ||
| Western scrub-jay, Aphelocoma californica | 4 | 1 | 2 | 7 | 2.5 | ||
| American robin, Turdus migratorius | 1 | 4 | 5 | 1.8 | 1.8def | ||
| California towhee, Pipilo crissalis | 5 | 5 | 1.8 | 2.3def | |||
| Bushtit, Psaltriparus minimus | 1 | 2 | 3 | 1.1 | 1.4def | ||
| Western bluebird, Sialia mexicana | 3 | 3 | 1.1 | 1.4def | |||
| American kestrel, Falco sparverius | 1 | 1 | 2 | 0.7 | 0.9ef | ||
| Brewer’s blackbird, Euphagus cyanocephalus | 2 | 2 | 0.7 | 0.9ef | |||
| Common raven, Corvus corax | 1 | 1 | 2 | 0.7 | 0.9ef | ||
| European starling, Sturnus vulgaris | 1 | 1 | 2 | 0.7 | 0.9ef | ||
| Oak titmouse, Baeolophus inornatus | 2 | 2 | 0.7 | 0.9ef | |||
| Cedar waxwing, Bombycilla cedrorum | 1 | 1 | 0.4 | 0.5f | |||
| Cooper’s hawk, Accipiter cooperii | 1 | 1 | 0.4 | 0.5f | |||
| Double-crested cormorant, Phalacrocorax auritus | 1 | 1 | 0.4 | 0.5f | |||
| Green heron, Butorides virescens | 1 | 1 | 0.4 | 0.5f | |||
| House wren, Troglodytes aedon | 1 | 1 | 0.4 | 0.5f | |||
| Rock pigeon, Columba livia | 1 | 1 | 0.4 | 0.5f | |||
| Swainson’s thrush, Catharus ustulatus | 1 | 1 | 0.4 | 0.5f | |||
| Yellow-rumped warbler, Dendroica coronata | 1 | 1 | 0.4 | ||||
| Mammalian | |||||||
| Domestic dog, Canis familiaris | 5 | 3 | 8 | 2.9 | 3.7cde | ||
| Human, Homo sapiens | 1 | 1 | 2 | 0.7 | 0.9ef | ||
| Domestic cat, Felis catus | 1 | 1 | 0.4 | 0.5f | |||
| Domestic horse, Equus caballus | 1 | 1 | 0.4 | 0.5f | |||
| Raccoon, Procyon lotor | 1 | 1 | 0.4 | 0.5f | |||
| Mixed bloodmeals | |||||||
| House sparrow/house finch | 2 | 2 | 0.7 | 0.5f | |||
| Total 29 species | 62 | 191 | 15 | 7 | 275 | ||
Dry ice–baited CDC trap.
Gravid trap.
Walk-in red box.
Underground storm drain system trap (no bait).
Percentages exclude sites with sentinel chicken flocks.
Percentages with different lowercases letter(s) indicate that they are significantly different by chi-square test (P < 0.05).
Underground Storm Drain System traps were operated without CO2 or light as bait in the underground system of Los Angeles. Although few blood-fed mosquitoes were collected from these traps, it is interesting to note that these bloodmeals were derived from a variety of avian hosts, indicating that these mosquitoes fed above ground and then entered the underground to rest rather than remaining underground and feeding on small mammals such as rats that were abundant in the system.
Kern County (Near Bakersfield)
Blood-fed Cx. pipiens complex (n = 162) and Cx. tarsalis (n = 101) were collected from 15 sites, with the majority collected from a golf course, a riparian area, and various residential areas in and around Bakersfield (Table 3). European starling (19.1%), predominantly identified from an urban golf course, and chicken (12.3%) were the most frequently fed-upon host of the Cx. pipiens complex, whereas Cx. tarsalis most frequently fed on mourning dove (18.8%), western scrub-jay (11.9%), northern mockingbird (10.9%), and chicken (8.9%). Despite this difference in dominant host species, blood-feeding patterns were quite similar between the Culex species in this area. Eight host species, European starling, chicken, black-headed grosbeak, western scrub-jay, house finch, mourning dove, house sparrow, and northern mockingbird, were shared as the eight most fed-upon hosts by both mosquito species. It should be noted that although chickens were a frequent host for both mosquito species, these samples were not collected near sentinel chicken flocks, but rather from residential areas where chickens were kept in backyard coops. Domestic dog (5.6%) was the only nonavian host identified from the Cx. pipiens complex, whereas Cx. tarsalis took bloodmeals from domestic dog (2.0%), domestic cow (1.0%), and western fence lizard (1.0%). The host species richness for the Cx. pipiens complex near Bakersfield (14.6 host species/50 bloodmeals) was similar to that found in Greater Los Angeles (13.2 host species/50 blood-meals). Cx. tarsalis host richness (17.0 host species/50 bloodmeals) in this region was slightly but not significantly higher than that of the Cx. pipiens complex (Fig. 2).
Table 3.
Number and percentage of avian-, reptilian-, and mammalian-derived bloodmeals from the Cx. pipiens complex and Cx. tarsalis in Kern County, near Bakersfield, 2007–2009
| Host |
Cx. pipiens complex
|
Cx. tarsalis
|
||||||
|---|---|---|---|---|---|---|---|---|
| CO2a | RBb | Total | %c | CO2a | RBb | Total | %c | |
| Avian | ||||||||
| European starling, Sturnus vulgaris | 1 | 30 | 31 | 19.1a | 6 | 6 | 5.9bcde | |
| Chicken, Gallus gallus | 2 | 18 | 20 | 12.3ab | 3 | 6 | 9 | 8.9abcd |
| Black-headed grosbeak, Pheucticus melanocephalus | 15 | 15 | 9.3b | 6 | 6 | 5.9bcde | ||
| Western scrub-jay, Aphelocoma californica | 1 | 14 | 15 | 9.3b | 12 | 12 | 11.9ab | |
| House finch, Carpodacus mexicanus | 13 | 13 | 8.0b | 6 | 6 | 5.9bcde | ||
| Mourning dove, Zenaida macroura | 14 | 14 | 8.6b | 1 | 18 | 19 | 18.8a | |
| House sparrow, Passer domesticus | 11 | 11 | 6.8bc | 1 | 6 | 7 | 6.9bcde | |
| Northern mockingbird, Mimus polyglottos | 10 | 10 | 6.2bc | 11 | 11 | 10.9abc | ||
| American robin, Turdus migratorius | 4 | 4 | 2.5cd | 1 | 1 | 1.0e | ||
| Cockatiel, Nymphicus hollandicus | 3 | 3 | 1.9cd | 1 | 1 | 1.0e | ||
| Greater roadrunner, Geococcyx californianus | 3 | 3 | 1.9cd | 2 | 2 | 2.0de | ||
| Western tanager, Piranga ludoviciana | 3 | 3 | 1.9cd | |||||
| Mallard, Anas platyrhynchos | 3 | 3 | 1.9cd | |||||
| American kestrel, Falco sparverius | 2 | 2 | 1.2d | 6 | 6 | 5.9cde | ||
| Bullock’s oriole, Icterus bullockii | 2 | 2 | 1.2d | 1 | 1 | 1.0e | ||
| Swainson’s thrush, Catharus ustulatus | 2 | 2 | 1.2d | |||||
| Brewer’s blackbird, Euphagus cyanocephalus | 1 | 1 | 0.6d | |||||
| Cedar waxwing, Bombycilla cedrorum | 1 | 1 | 0.6d | 1 | 1 | 1.0e | ||
| American crow, Corvus brachyrhynchos | 1 | 1 | 1.0e | |||||
| Barn owl, Tyto alba | 3 | 3 | 3.0cde | |||||
| Black phoebe, Sayornis nigricans | 2 | 2 | 2.0de | |||||
| Black-crowned night-heron, Nycticorax nycticorax | 1 | 1 | 1.0e | |||||
| Bushtit, Psaltriparus minimus | 1 | 1 | 1.0e | |||||
| Yellow warbler, Dendroica petechia | 1 | 1 | 1.0e | |||||
| Reptilian | ||||||||
| Western fence lizard, Sceloporus occidentalis | 1 | 1 | 1.0e | |||||
| Mammalian | ||||||||
| Domestic dog, Canis familiaris | 1 | 8 | 9 | 5.6bc | 2 | 2 | 2.0de | |
| Domestic cow, Bos taurus | 1 | 1 | 1.0e | |||||
| Total 27 species | 5 | 157 | 162 | 11 | 90 | 101 | ||
Dry-ice baited CDC trap.
Walk-in red box.
Percentages with different lowercase letters indicate that they are significantly different by chi-square test (P < 0.05).
Yolo County (Within and Surrounding Davis)
Despite the use of CO2 traps, gravid traps, walk-in red boxes, and backpack aspiration from backyard vegetation during over 3 yr of sampling, only 29 Cx. pipiens complex and 17 Cx. tarsalis engorged females were collected from residential areas in Davis, CA (Table 4). Although few definitive conclusions can be drawn from this small sample size, the host feeding patterns were generally similar to those in other areas of the Central Valley. Western scrub-jay was frequently fed upon host by both mosquito species, and house finch was used frequently by members of the Cx. pipiens complex. American robin, a species considered a preferred host in several previous Culex bloodmeal studies (Kilpatrick et al. 2006b, Hamer et al. 2009, Molaei et al. 2010, Montgomery et al. 2011), comprised >13% of the Cx. pipiens complex bloodmeals and nearly 30% of bloodmeals by Cx. tarsalis. Two unique galliform birds, helmeted guineafowl and wild turkey, were identified from bloodmeals collected at the Davis Cemetery, an area where these birds were frequently found in the wild. Only one mammalian bloodmeal from a black rat was identified from the residential samples. Because of the small sample size, host species richness was not calculated.
Table 4.
Number and percentage of avian- and mammalian-derived bloodmeals from the Cx. pipiens complex and Cx. tarsalis in Davis, Yolo County, 2007–2009
| Host |
Cx. pipiens complex
|
Cx. tarsalis
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BKPKa | CO2b | RBc | Total | %d | BKPKa | CO2b | RBc | Total | %d | |
| Avian | ||||||||||
| Western scrub-jay, Aphelocoma californica | 2 | 7 | 9 | 31.0a | 4 | 4 | 23.5a | |||
| House finch, Carpodacus mexicanus | 1 | 1 | 4 | 6 | 20.7ab | |||||
| American robin, Turdus migratorius | 1 | 3 | 4 | 13.8ab | 1 | 4 | 5 | 29.4a | ||
| Helmeted guineafowl, Numida meleagris | 4 | 4 | 13.8ab | |||||||
| Northern mockingbird, Mimus polyglottos | 4 | 4 | 13.8ab | 3 | 3 | 17.6a | ||||
| Chicken, Gallus gallus | 1 | 1 | 3.4b | 1 | 1 | 5.9a | ||||
| Yellow-billed magpie, Pica nuttalli | 1 | 1 | 3.4b | |||||||
| Barn owl, Tyto alba | 1 | 1 | 5.9a | |||||||
| Mourning dove, Zenaida macroura | 1 | 1 | 5.9a | |||||||
| Wild turkey, Meleagris gallopavo | 1 | 1 | 5.9a | |||||||
| Mammalian | ||||||||||
| Black rat, Rattus rattus | 1 | 1 | 5.9a | |||||||
| Total 11 Species | 6 | 1 | 22 | 29 | 1 | 3 | 13 | 17 | ||
Backpack aspirator.
Dry ice–baited CDC trap.
Walk-in red box.
Percentages with different lowercase letters indicate that they are significantly different by chi-square test (P < 0.05).
Engorged Cx. tarsalis collected at the Yolo Bypass Wildlife Area exhibited a remarkable diversity of bloodmeal hosts from a small collection area and relatively small number of engorged females. Thirty-three host species were identified from 72 bloodmeals (Table 5), resulting in a host richness of 27.2 host species/50 bloodmeals (Fig. 2) as calculated by rarefaction. Twenty-five avian species, including a variety of owls, hawks, and waterfowl, were identified, and the diverse mammalian bloodmeals included North American river otter, California vole, North American beaver, and common muskrat.
Table 5.
Number and percentage of avian- and mammalian-derived bloodmeals from Cx. tarsalis at the Yolo Bypass Wildlife Area, Yolo County, 2007–2009
| Host |
Cx. tarsalis
|
|||
|---|---|---|---|---|
| CO2a | RBb | Total | %c | |
| Avian | ||||
| House finch, Carpodacus mexicanus | 1 | 9 | 10 | 13.9a |
| Red-winged blackbird, Agelaius phoeniceus | 3 | 4 | 7 | 9.7ab |
| Black-crowned night-heron, Nycticorax nycticorax | 2 | 2 | 4 | 5.6ab |
| Savannah sparrow, Passerculus sandwichensis | 2 | 2 | 4 | 5.6ab |
| Ring-necked pheasant, Phasianus colchicus | 1 | 2 | 3 | 4.2ab |
| Great egret, Ardea alba | 1 | 2 | 3 | 4.2ab |
| Mallard, Anas platyrhynchos | 2 | 1 | 3 | 4.2ab |
| Red-tailed hawk, Buteo jamaicensis | 3 | 3 | 4.2ab | |
| Brewer’s blackbird, Euphagus cyanocephalus | 1 | 1 | 2 | 2.8b |
| Brown-headed cowbird, Molothrus ater | 1 | 1 | 2 | 2.8b |
| Mourning dove, Zenaida macroura | 1 | 1 | 2 | 2.8b |
| White-faced ibis, Plegadis chihi | 2 | 2 | 2.8b | |
| Barn owl, Tyto alba | 1 | 1 | 1.4b | |
| Cooper’s hawk, Accipiter cooperii | 1 | 1 | 1.4b | |
| European starling, Sturnus vulgaris | 1 | 1 | 1.4b | |
| Gadwall, Anas strepera | 1 | 1 | 1.4b | |
| Great horned owl, Bubo virginianus | 1 | 1 | 1.4b | |
| Marsh wren, Cistothorus palustris | 1 | 1 | 1.4b | |
| Northern pintail, Anas acuta | 1 | 1 | 1.4b | |
| Northern shoveler, Anas clypeata | 1 | 1 | 1.4b | |
| Pied-billed grebe, Podilymbus podiceps | 1 | 1 | 1.4b | |
| Swainson’s hawk, Buteo swainsoni | 1 | 1 | 1.4b | |
| Undetermined goose, Chen sp. | 1 | 1 | 1.4b | |
| Western meadowlark, Sturnella neglecta | 1 | 1 | 1.4b | |
| Yellow warbler, Dendroica petechia | 1 | 1 | 1.4b | |
| Mammalian | ||||
| North American river otter, Lontra canadensis | 3 | 3 | 4.2ab | |
| California vole, Microtus californicus | 2 | 2 | 2.8b | |
| Mule deer, Odocoileus hemionus | 2 | 2 | 2.8b | |
| North American beaver, Castor canadensis | 1 | 1 | 2 | 2.8b |
| Raccoon, Procyon lotor | 2 | 2 | 2.8b | |
| Black-tailed jackrabbit, Lepus californicus | 1 | 1 | 1.4b | |
| Common muskrat, Ondatra zibethicus | 1 | 1 | 1.4b | |
| Domestic cow, Bos taurus | 1 | 1 | 1.4b | |
| Total 33 species | 33 | 39 | 72 | |
Dry ice–baited CDC trap.
Walk-in red box.
Percentages with different lowercase letters indicate that they are significantly different by chi-square test (P < 0.05).
Sutter County (Near Yuba City)
Blood-fed Culex were collected resting in walk-in red boxes at five rural and semirural residential sites in Sutter County. Bloodmeals from 107 members of the Cx. pipiens complex and 231 Cx. tarsalis were identified (Table 6). Cx. pipiens complex mosquitoes fed almost exclusively on avian hosts, with American crow (26.2%), American robin (17.8%), and chicken (11.2%) most frequent. Yellow-billed magpie and western scrub-jay also were fed upon in this area, so corvid bloodmeals comprised >35% of the total. Cx. tarsalis fed predominantly on American robin (27.3%), domestic cow (20.3%), American crow (13.4%), and yellow-billed magpie (8.2%). Although both mosquito species were collected concurrently at the same red box sites, Cx. tarsalis fed on six mammalian species (27.3% of total), whereas human (1.9%) was the only mammalian bloodmeal identified from the Cx. pipiens complex. The Cx. pipiens complex did not feed on cattle or other domestic mammals, although these hosts were abundant. Despite differences in mammalian feeding, the host richness calculated by rarefaction of the two Culex species was similar in this area (13.8 host species/50 bloodmeals for Cx. tarsalis and 13.4 host species/50 bloodmeals for the Cx. pipiens complex) (Fig. 2).
Table 6.
Number and percentage of avian- and mammalian-derived bloodmeals from the Cx. pipiens complex and Cx. tarsalis in Sutter County, near Yuba City, 2007–2009
| Host |
Cx. pipiens complex
|
Cx. tarsalis
|
||
|---|---|---|---|---|
| RBa | %b | RBa | %b | |
| Avian | ||||
| American robin, Turdus migratorius | 19 | 17.8ab | 63 | 27.3a |
| American crow, Corvus brachyrhynchos | 28 | 26.2ab | 31 | 13.4b |
| Yellow-billed magpie, Pica nuttalli | 7 | 6.5cde | 19 | 8.2bc |
| Chicken, Gallus gallus | 12 | 11.2bc | 13 | 5.6cd |
| Western scrub-jay, Aphelocoma californica | 3 | 2.8de | 9 | 3.9cde |
| House sparrow, Passer domesticus | 8 | 7.5cd | 7 | 3.0def |
| Brewer’s blackbird, Euphagus cyanocephalus | 5 | 4.7cde | 6 | 2.6def |
| House finch, Carpodacus mexicanus | 6 | 5.6cde | 5 | 2.2def |
| Green heron, Butorides virescens | 3 | 2.8de | 3 | 1.3ef |
| European starling, Sturnus vulgaris | 7 | 6.5cde | 2 | 0.9ef |
| Mourning dove, Zenaida macroura | 2 | 0.9ef | ||
| American kestrel, Falco sparverius | 2 | 1.9e | 1 | 0.4f |
| Brown-headed cowbird, Molothrus ater | 1 | 0.9e | 1 | 0.4f |
| Red-winged blackbird, Agelaius phoeniceus | 1 | 0.4f | ||
| Ring-necked pheasant, Phasianus colchicus | 1 | 0.4f | ||
| Rock pigeon, Columba livia | 1 | 0.4f | ||
| Western kingbird, Tyrannus verticalis | 1 | 0.4f | ||
| White-faced ibis, Plegadis chihi | 1 | 0.4f | ||
| Wild turkey, Meleagris gallopavo | 1 | 0.4f | ||
| American bittern, Botaurus lentiginosus | 1 | 0.9e | ||
| Northern mockingbird, Mimus polyglottos | 1 | 0.9e | ||
| Tree swallow, Tachycineta bicolor | 1 | 0.9e | ||
| Undetermined duck, Anas sp. | 1 | 0.9e | ||
| Mammalian | ||||
| Domestic cow, Bos taurus | 47 | 20.3ab | ||
| Domestic horse, Equus caballus | 5 | 2.2def | ||
| Black-tailed jackrabbit, Lepus californicus | 3 | 1.3ef | ||
| Raccoon, Procyon lotor | 3 | 1.3ef | ||
| Domestic sheep, Ovis aries | 3 | 1.3ef | ||
| Domestic cat, Felis catus | 2 | 0.9ef | ||
| Human, Homo sapiens | 2 | 1.9e | ||
| Total 30 Species | 107 | 231 | ||
Walk-in red box.
Percentages with different lowercase letters indicate that they are significantly different by chi-square test (P < 0.05).
Bird Population Estimates
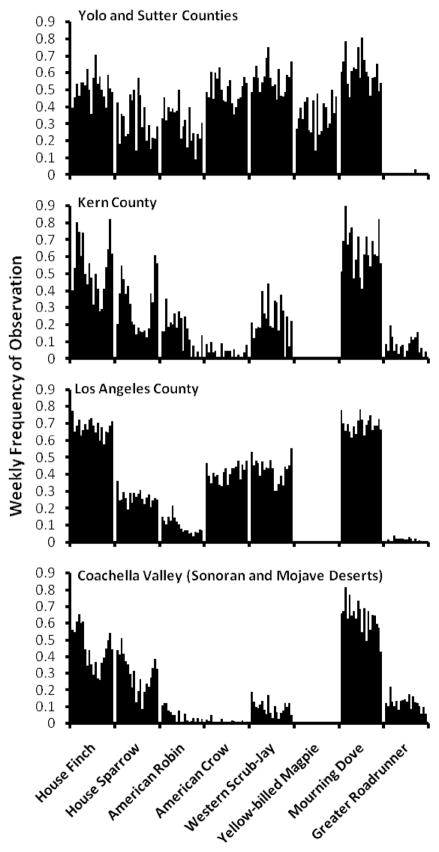
The frequency of occurrence of some common avian hosts in each study area is shown in Fig. 3. Weekly frequencies from May to September were averaged over the 3 yr of the study (2007–2009) (eBird 2011). Some species, such as house finch, house sparrow, and mourning dove, were found in all study areas. Yellow-billed magpie and greater roadrunner had limited ranges, whereas American robin occurrence increased in frequency in northern areas. These data provide a general and qualitative look at the occurrence of avian species across the study areas, but they do not provide avian abundance at specific mosquito sampling sites. These data helped explain why within mosquito species host use patterns varied spatially (Fig. 4).
Fig. 3.
Weekly frequency of some common avian host species, May–September 2007–2009, in California study areas. Frequency represents the proportion of citizen-submitted checklists reporting each species (eBird 2011).
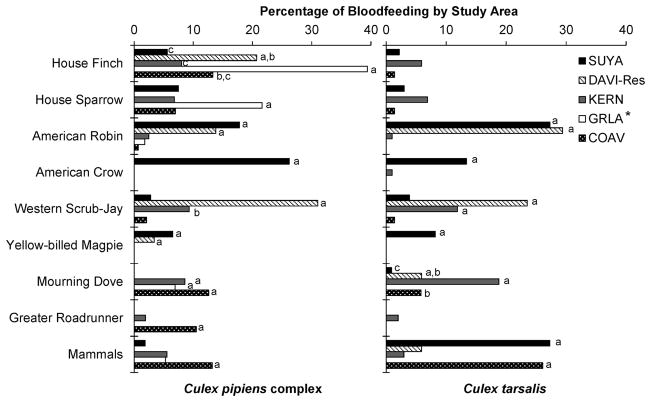
Fig. 4.
Percentage of bloodmeals from some common vertebrate hosts by the Cx. pipiens complex (left) and Cx. tarsalis (right) compared between five study areas in California: Sutter County (SUYA), Residential Yolo County in Davis (DAVI-Res), Kern County (KERN), Los Angeles (GRLA), and the Coachella Valley (COAV). Asterisk (*) indicates no Cx. tarsalis bloodmeals were identified from Los Angeles. Percentages with different letters within host species groups for each mosquito species denote significance by chi-square analysis (P < 0.05).
Discussion
The blood-feeding patterns of the WNV vectors Cx. tarsalis and the Cx. pipiens complex were surveyed in five geographic regions of California spanning 850 km from the deserts of the southern Coachella Valley to the rice-growing regions of the northern Sacramento Valley. Both vector species fed on a wide variety of vertebrate hosts and exhibited significant spatial and interspecific differences in blood-feeding patterns. Nearly 100 different host species (avian, mammalian, and reptilian) were fed upon by these Culex mosquitoes across the state.
Both Cx. tarsalis and the Cx. pipiens complex were collected in nearly all study areas. As expected, few Cx. tarsalis were collected in highly urban Los Angeles where they remain a relatively rare species (Kwan et al. 2010), and no Cx. pipiens were collected in the Yolo Bypass Wildlife Area where most mosquitoes emerge from managed wetlands and rice fields. In general, when both species were collected from the same site, Cx. tarsalis fed on a greater diversity of hosts than did the Cx. pipiens complex (Fig. 2). Much of this diversity resulted from an increased feeding on mammals (χ2 = 49.04, df = 1, P < 0.0001), with Cx. tarsalis feeding on 17 mammalian species in total, whereas members of the Cx. pipiens complex fed on only four mammalian species. Even when the diversity of bloodmeals was similar between these vector species, such as near Yuba City, Cx. tarsalis fed more frequently on mammalian hosts than did the Cx. pipiens complex (27.3 vs 1.9%) (χ2 = 28.77, df = 1, P < 0.001). These findings were congruent with the idea that Cx. pipiens is primarily an avian feeder, whereas Cx. tarsalis is more of a generalist feeder (Reisen and Reeves 1990). There was no significant difference in the occurrence of human bloodmeals between these species (χ2 = 0.048, df = 1, P = 0.83), and only five (0.3% of total) human bloodmeals were identified throughout the study. Both Cx. tarsalis and the Cx. pipiens complex consistently have exhibited the highest WNV infection rates and presumably are responsible for transmitting virus to the human cases repeatedly reported from these areas. However, infrequent bloodmeals from humans, even in highly urbanized Los Angeles where several outbreaks have occurred, would indicate that these species are relatively inefficient bridge vectors.
Despite the long distances between study areas and the differences in climate and landscape, several vertebrate hosts were used repeatedly throughout the state. As in other areas around the United States (Molaei et al. 2007, Hamer et al. 2009, Kent et al. 2009, Molaei et al. 2010), house finch and house sparrow were ubiquitous (see avian frequency estimates in Fig. 3) and were fed upon by both Culex species (Fig. 4). These data agreed with previous studies at these same study areas that indicated both of these peridomestic passerine species were frequently infected with WNV (Table 7) and other arboviruses (Reeves et al. 1990, Reisen et al. 2000). House finches and house sparrows may be crucial for WNV transmission, especially in highly urban areas such as Los Angeles where these hosts account for >50% of the Culex bloodmeals (Table 2; Fig. 4; also see Molaei et al. 2010) and also in areas such as the Coachella Valley, where highly competent corvids were rare (Fig. 3) (Reisen et al. 2006a). Both house finch and house sparrow are competent experimental hosts (Komar et al. 2003, Langevin et al. 2005, Reisen et al. 2005) and have previously been implicated in virus maintenance transmission (Kwan et al. 2010). Western scrub-jay, a highly competent amplifying host in the laboratory (Reisen et al. 2005), was not previously identified as a frequent bloodmeal host, although this species frequently was WNV positive in dead bird (Wheeler et al. 2009) and seroprevalence surveys (Table 7). Our study revealed that Western scrub-jay may be an important contributor to WNV transmission in areas where it was fed upon frequently, especially Bakersfield (10.2%) and other parts of the Central Valley. Some less competent species also were repeatedly fed upon and may have functioned to divert Culex from competent hosts, thereby diluting or decreasing transmission. For example, mourning doves frequently were used as bloodmeal hosts (Fig. 4), as described previously in Texas (Molaei et al. 2007), Colorado (Kent et al. 2009), and California (Molaei et al. 2010, Montgomery et al. 2011). Although frequently antibody positive (Table 7), adults generally were poorly competent hosts for WNV (Komar et al. 2003, Reisen et al. 2005) and other arboviruses (Reisen et al. 2003). Nestling mourning doves, however, develop elevated viremias to St. Louis encephalitis virus (Mahmood et al. 2004) and could play an important role in transmission. When available, chickens also were frequently fed upon. This was important to document, because they are used as sentinel birds in arbovirus surveillance programs. Chickens and other galliforms, such as quail and turkey, have low competence for flaviviruses (Langevin et al. 2001, Reisen et al. 2006b), so frequent feeding on this group would dampen transmission (Keesing et al. 2006, Swaddle and Calos 2008).
Table 7.
Percentage of sera collected from frequently selected avian bloodmeal hosts that tested positive for WNV antibodies by enzyme immunoassay
| Avian species | COAV (2003–2005)a
|
GRLA (2003–2008)b
|
KERN (2003–2007)c
|
YOLO (2006–2009)d
|
||||
|---|---|---|---|---|---|---|---|---|
| % | N | % | N | % | N | % | N | |
| House sparrow | 2.3 | 656 | 2.3 | 5,476 | 5.1 | 972 | 1.9 | 270 |
| House finch | 4.7 | 615 | 11.3 | 8,249 | 16.8 | 1,381 | 5.9 | 187 |
| Western scrub-jay | 39.7 | 552 | 13.7 | 153 | ||||
| Mourning dove | 4 | 1,761 | 24.1 | 137 | 30.8 | 1020 | 28.6 | 7 |
Coachella Valley MVCD (Reisen et al. 2008c).
Greater Los Angeles County VCD (Kwan et al. 2010).
Kern MVCD (Reisen et al. 2009).
Yolo County published in part (Wheeler et al. 2009).
Variation in blood-feeding patterns across the state (Tables 1–6; Fig. 4) was due, in part, to the limited distributions of some host species (Fig. 3). For example, the greater roadrunner was a common host (10.5%) in the Coachella Valley but was not identified elsewhere where it is less abundant or absent. Likewise, yellow-billed magpie was only identified as a host within its restricted range in the Central Valley. Similarly blood-feeding on American robin, a frequent and preferred host in previous studies (Kilpatrick et al. 2006a, Savage et al. 2007, Kent et al. 2009), was more frequent in the northern part of California (also see Montgomery et al. 2011) where this species is abundant during summer (Fig. 3).
There has been much discussion about the role of American crows in WNV transmission. American crows originally were thought to be a key amplifying host in WNV transmission due to extremely elevated viremias and 100% mortality after experimental infection (Komar et al. 2003), high WNV infection prevalence in dead bird surveys (Reisen et al. 2006a, Wheeler et al. 2009), and significant population declines associated with outbreaks (Caffrey et al. 2003, LaDeau et al. 2007, Wheeler et al. 2009). In contrast, the near absence of crows in bloodmeal identification studies has led to speculation about their involvement in mosquito infection, and therefore bird–mosquito–bird transmission (Kramer et al. 2008, Hamer et al. 2009, Molaei et al. 2010). In our studies, American crow was identified as a host in two areas, a farmstead north of Davis (Thiemann et al. 2011) and the area in and around Yuba City, where the American crow was one of the most frequently fed upon hosts by the Cx. pipiens complex (26.2%) and accounted for 13.4% of the Cx. tarsalis bloodmeals (Table 6). These blood-meals were collected during spring and early summer, not near a large late season communal crow roost, and probably resulted from only a few nesting family groups. Interestingly, these crow bloodmeals were all detected in fully engorged Culex collected resting in red boxes. It may be that American crows are tolerant of mosquito feeding, so bloodmeals are less likely to be identified from CO2 collections, which mostly collect females with partial interrupted meals. Given the complex staging and roosting behavior of crows, sampling in a manner to find crow bloodmeals may be difficult and require collections adjacent to nocturnal roosts, but from our study it seems that when available American crows are fed upon by Culex mosquitoes, even when house finches, American robins, and other avian hosts are present.
To summarize, Cx. tarsalis and the Cx. pipiens complex were generalist blood feeders and fed on a wide variety of avian and mammalian hosts throughout California. Both species fed predominantly on avian hosts, with Cx. tarsalis feeding more frequently on nonhuman mammals, including horses, than did the Cx. pipiens complex. Both vectors fed frequently on abundant and competent avian hosts, such as house finch and house sparrow, allowing for the maintenance and amplification of WNV, even in the absence of highly competent amplifying hosts. Corvids, such as American crow, western scrub-jay and yellow-billed magpie, were fed upon by both Culex species when these hosts were available and probably contributed to WNV transmission particularly in the Central Valley. Similar to previous bloodmeal identification studies (Reisen and Reeves 1990, Montgomery et al. 2011, Molaei et al. 2010), few human bloodmeals were identified throughout the state in both Cx. tarsalis and the Cx. pipiens complex, even in highly urban Los Angeles. Similarly infrequent human bloodmeals were reported in studies designed specifically to detect human blood feeding at a park near Bakersfield (Tempelis et al. 1965) and at residential habitats in the Los Angeles basin (Reisen et al. 1990). This low frequency of human bloodmeals may explain the continued low incidence of human infection with encephalitis viruses detected historically (Reisen et al. 1996, Reisen and Chiles 1997) and during the current WNV epidemic (http://westnile.ca.gov/reports.php).
Acknowledgments
We thank H. Lu for help with DNA extractions and C. M. Barker for help with statistical analysis. We also thank Drs. A. C. Brault and H. B. Ernest who provided laboratory space and equipment for sample analysis. We thank the employees of Sutter-Yuba MVCD, Kern MVCD, Greater Los Angeles County VCD, and Coachella Valley MVCD for assistance with sample collection and identification. Funding for this work was provided by the Mosquito Research Foundation of California, with contributions from Coachella Valley MVCD, Sacramento-Yolo MVCD, and Turlock MAD; a Hazeltine student research fellowship from the University of California–Davis Department of Entomology; and research grant AI55607 from the National Institutes of Health National Institute of Allergy and Infectious Diseases. W.K.R. acknowledges support from the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Science & Technology Directorate, Department of Homeland Security and Fogarty International Center, National Institutes of Health.
References Cited
- Aplin KP, Suzuki H, Chinen AA, Chesser RT, ten Have J, Donnellan SC, Austin J, Frost A, Gonzalez JP, Herbreteau V, et al. Multiple geographic origins of commensalism and complex dispersal history of black rats. PloS ONE. 2011;6:e26357. doi: 10.1371/journal.pone.0026357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AR. Occurrence and distribution of the Culex pipiens complex. Bull WHO. 1967;37:293–296. [PMC free article] [PubMed] [Google Scholar]
- Caffrey CL, Weston TJ, Smith SCR. High mortality among marked crows subsequent to the arrival of West Nile virus. Wildl Soc B. 2003;31:870–872. [Google Scholar]
- Cooper JK, Sykes G, King S, Cottrill K, Ivanova NV, Hanner R, Ikonomi P. Species identification in cell culture: a two-pronged molecular approach. In Vitro Cell Dev Biol Anim. 2007;43:344–351. doi: 10.1007/s11626-007-9060-2. [DOI] [PubMed] [Google Scholar]
- Cornel AJ, McAbee R, Rasgon J, Stanich MA, Scott TW, Coetzee M. Differences in extent of genetic introgression between sympatric Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in California and South Africa. J Med Entomol. 2003;40:36–51. doi: 10.1603/0022-2585-40.1.36. [DOI] [PubMed] [Google Scholar]
- Cummings RF. Design and use of a modified Reiter gravid mosquito trap for mosquito-borne encephalitis surveillance in Los Angeles County, California. Proc Mosq Vector Control Assoc Calif. 1992;60:170–176. [Google Scholar]
- eBird. eBird: an online database of bird distribution and abundance. Version 2. Cornell Laboratory of Ornithology; Ithaca, NY: 2011. ( http://www.ebird.org) [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. Host selection by Culexpipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg. 2009;80:268–278. [PubMed] [Google Scholar]
- Iltis WG. PhD dissertation. University of California–Davis; Davis: 1966. Biosystematics of the Culex pipiens complex in northern California. [Google Scholar]
- Ivanova NV, Dewaard JR, Hebert PDN. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol Ecol Notes. 2006;6:998–1002. [Google Scholar]
- Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Kent RJ. Molecular methods for arthropod blood-meal identification and applications to ecological and vector-borne disease studies. Mol Ecol Resour. 2009;9:4–18. doi: 10.1111/j.1755-0998.2008.02469.x. [DOI] [PubMed] [Google Scholar]
- Kent RJ, Juliusson L, Weissmann M, Evans S, Komar N. Seasonal blood-feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J Med Entomol. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, LaDeau SL, Marra PP. Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk. 2007;124:1121–1136. [Google Scholar]
- Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proc R Soc B Biol Sci. 2006a;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006b;4:0606–0610. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- Kwan JL, Kluh S, Madon MB, Reisen WK. West Nile Virus Emergence and Persistence in Los Angeles, California, 2003–2008. Am J Trop Med Hyg. 2010;83:400–412. doi: 10.4269/ajtmh.2010.10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDeau SL, Kilpatrick AM, Marra PP. West Nile virus emergence and large-scale declines of North American bird populations. Nature. 2007;447:710–U713. doi: 10.1038/nature05829. [DOI] [PubMed] [Google Scholar]
- Langevin SA, Bunning M, Davis B, Komar N. Experimental infection of chickens as candidate sentinels for west nile virus. Emerg Infect Dis. 2001;7:726–729. doi: 10.3201/eid0704.010422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin SA, Brault AC, Panella NA, Bowen RA, Komar N. Variation in virulence of West Nile virus strains for house sparrows (Passer domesticus) Am J Trop Med Hyg. 2005;72:99–102. [PubMed] [Google Scholar]
- Mahmood F, Chiles RE, Fang Y, Barker CM, Reisen WK. Role of nestling mourning doves and house finches as amplifying hosts of St. Louis encephalitis virus. J Med Entomol. 2004;41:965–972. doi: 10.1603/0022-2585-41.5.965. [DOI] [PubMed] [Google Scholar]
- Meyer RP. The “walk-in” type red box for sampling resting adult mosquitoes. Proc NJ Mosq Control Assoc. 1987;72:104. [Google Scholar]
- Molaei G, Andreadis TA, Armstrong PM, Anderson JF, Vossbrinck CR. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Bueno R, Dennett JA, Real SV, Sargent C, Bala A, Randle Y, Guzman H, et al. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County, Texas. Am J Trop Med Hyg. 2007;77:73–81. [PubMed] [Google Scholar]
- Molaei G, Cummings RF, Su T, Armstrong PM, Williams GA, Cheng ML, Webb JP, Andreadis TG. Vector-host interactions governing epidemiology of West Nile virus in Southern California. Am J Trop Med Hyg. 2010;83:1269–1282. doi: 10.4269/ajtmh.2010.10-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery MJ, Thiemann TC, Macedo P, Brown DA, Scott TW. Blood-Feeding Patterns of the Culex pipiens complex in Sacramento and Yolo counties, California. J Med Entomol. 2011;48:398–404. doi: 10.1603/me10067. [DOI] [PubMed] [Google Scholar]
- Newhouse VF, Chamberlain RW, Johnston JG, Jr, Sudia WD. Use of dry ice to increase mosquito catches of the CDC miniature light trap. Mosq News. 1966;26:30–35. [Google Scholar]
- Nielsen CF, Armijos MV, Wheeler S, Carpenter TE, Boyce WM, Kelley K, Brown D, Scott TW, Reisen WK. Risk factors associated with human infection during the 2006 West Nile virus outbreak in Davis, a residential community in northern California. Am J Trop Med Hyg. 2008;78:53–62. [PMC free article] [PubMed] [Google Scholar]
- Ratnasingham S, Hebert PDN. BOLD: the bar-code of life data system. Mol Ecol Notes. 2007;7:355–364. doi: 10.1111/j.1471-8286.2007.01678.x. ( www.barcodinglife.org) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [RDC] R Development Core. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. [Google Scholar]
- Reeves WC, Asman SM, Hardy JL, Milby MM, Reisen WK. Epidemiology and control of mosquito-borne arboviruses in California, 1943–1987. California Mosquito Vector Control Association; Sacramento, CA: 1990. [Google Scholar]
- Reisen WK, Chiles RE. Prevalence of antibodies to western equine encephalomyelitis and St. Louis encephalitis viruses in residents of California exposed to sporadic and consistent enzootic transmission. Am J Trop Med Hyg. 1997;57:526–529. doi: 10.4269/ajtmh.1997.57.526. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Reeves WC. Epidemiology and control of mosquito-borne arboviruses in California, 1943–1987. Vol. 1. California Mosquito and Vector Control Association; Sacramento, CA: 1990. Bionomics and ecology of Culex tarsalis and other potential mosquito vector species; pp. 254–329. [Google Scholar]
- Reisen WK, Meyer RP, Tempelis CH, Spoehel JJ. Mosquito abundance and bionomics in residential communities in Orange and Los Angeles Counties, California. J Med Entomol. 1990;27:356–367. doi: 10.1093/jmedent/27.3.356. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Chiles RE, Lothrop HD, Presser SB, Hardy JL. Prevalence of antibodies to mosquito-borne encephalitis viruses in residents of the Coachella Valley. Am J Trop Med Hyg. 1996;55:667–671. doi: 10.4269/ajtmh.1996.55.667. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lundstrom JO, Scott TW, Eldridge BF, Chiles RE, Cusack R, Martinez VM, Lothrop HD, Gutierrez D, Wright S, et al. Patterns of avian seroprevalence to western equine encephalomyelitis and St. Louis encephalitis viruses in California, USA. J Med Entomol. 2000;37:507–527. doi: 10.1603/0022-2585-37.4.507. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Chiles RE, Martinez VM, Fang Y, Green EN. Experimental infection of California birds with western equine encephalomyelitis and St. Louis encephalitis viruses. J Med Entomol. 2003;40:968–982. doi: 10.1603/0022-2585-40.6.968. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J Med Entomol. 2005;42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Barker CM, Carney R, Lothrop HD, Wheeler SS, Wilson JL, Madon MB, Takahashi R, Carroll B, Garcia S, et al. Role of corvids in epidemiology of West Nile virus in southern California. J Med Entomol. 2006a;43:356–367. doi: 10.1603/0022-2585(2006)043[0356:rocieo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, V, Martinez M, Fang Y, Garcia S, Ashtari S, Wheeler SS, Carroll BD. Role of California (Callipepla californica) and Gambel’s (Callipepla gambelii) quail in the ecology of mosquito-borne encephalitis viruses in California, USA. Vector Borne Zoonotic Dis. 2006b;6:248–260. doi: 10.1089/vbz.2006.6.248. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Barker CM, Fang Y, Martinez VM. Does variation in Culex (Diptera: Culicidae) vector competence enable outbreaks of West Nile virus in California? J Med Entomol. 2008a;45:1126–1138. doi: 10.1603/0022-2585(2008)45[1126:dvicdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, Wheeler SS, Kennsington M, Gutierrez A, Fang Y, Garcia S, Lothrop B. Persistent West Nile virus transmission and the apparent displacement St. Louis encephalitis virus in southeastern California, 2003–2006. J Med Entomol. 2008b;45:494–508. doi: 10.1603/0022-2585(2008)45[494:pwnvta]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Takahashi RM, Carroll BD, Quiring R. Delinquent mortgages, neglected swimming pools, and West Nile Virus, California. Emerg Infect Dis. 2008c;14:1747–1749. doi: 10.3201/eid1411.080719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Carroll BD, Takahashi R, Fang Y, Garcia S, Martinez VM, Quiring R. Repeated West Nile virus epidemic transmission in Kern County, California, 2004–2007. J Med Entomol. 2009;46:139–157. doi: 10.1603/033.046.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, Anderson M, Charnetzky D, McMillen L, Unnasch EA, et al. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector Borne Zoonotic Dis. 2007;7:365–386. doi: 10.1089/vbz.2006.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sella M. Relazione della campagna antianofelica di Fiumicino (1919) con speciale riguardo alla biologia degli Anofeli ed agli Anofeli infetti. Ann Igiene. 1920;30(Suppl 85) [Google Scholar]
- Sudia WD, Chamberlain RW. Battery-operated light trap, an improved model. Mosq News. 1962;22:126–129. [PubMed] [Google Scholar]
- Sullivan BL, Wood CL, Iliff MJ, Bonney RE, Fink D, Kelling S. eBird: a citizen-based bird observation network in the biological sciences. Biol Conserv. 2009;142:2282–2292. [Google Scholar]
- Swaddle JP, Calos SE. Increased avian diversity is associated with lower incidence of human West Nile infection: observation of the dilution effect. PloS ONE. 2008;3:e2488. doi: 10.1371/journal.pone.0002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick WJ, Powell JR. Genetic analysis of Culex pipiens populations in the central valley of California. Ann Entomol Soc Am. 1983;76:715–720. [Google Scholar]
- Tempelis CH, Reeves WC. Feeding habits of one anopheline and three culicine mosquitoes by the precipitin test. J Med Entomol. 1964;1:148–151. [Google Scholar]
- Tempelis CH, Washino RK. Host-feeding patterns of Culex tarsalis in the Sacramento Valley, California, with notes on other species. J Med Entomol. 1967;4:315–318. doi: 10.1093/jmedent/4.3.315. [DOI] [PubMed] [Google Scholar]
- Tempelis CH, Reeves WC, Bellamy RE, Lofy MF. A three-year study of the feeding habits of Culex tarsalis in Kern County, California. Am J Trop Med Hyg. 1965;14:170–177. doi: 10.4269/ajtmh.1965.14.170. [DOI] [PubMed] [Google Scholar]
- Tempelis CH, Reeves WC, Nelson RL. Species identification of blood meals from Culex tarsalis that had fed on passeriform birds. Am J Trop Med Hyg. 1976;25:744–746. doi: 10.4269/ajtmh.1976.25.744. [DOI] [PubMed] [Google Scholar]
- Thiemann TC, Reisen WK. Evaluating sampling method bias in Culex tarsalis and Culex quinquefasciatus(Diptera: Culicidae) bloodmeal identification studies. J Med Entomol. 2012;49:143–149. doi: 10.1603/me11134. [DOI] [PubMed] [Google Scholar]
- Thiemann TC, Wheeler SS, Barker CM, Reisen WK. Mosquito host selection varies seasonally with host availability and mosquito density. PLoS Negl Trop Dis. 2011;5:e1452. doi: 10.1371/journal.pntd.0001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemann TC, Brault AC, Ernest HB, Reisen WK. Development of a high-throughput microsphere-based molecular assay to identify 15 common bloodmeal hosts of Culex mosquitoes. Mol Ecol Resour. 2012;12:238–246. doi: 10.1111/j.1755-0998.2011.03093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanelli S, Silvestrini F, Reisen WK, deVito E, Bullini L. California hybrid zone between Culex pipiens pipiens and Cx. p. quinquefasciatus revisited (Diptera: Culicidae) J Med Entomol. 1997;34:116–127. doi: 10.1093/jmedent/34.2.116. [DOI] [PubMed] [Google Scholar]
- Wheeler SS, Barker CM, Fang Y, Armijos MV, Carroll BD, Husted S, Johnson WO, Reisen WK. Differential impact of West Nile virus on California birds. Condor. 2009;111:1–20. doi: 10.1525/cond.2009.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [WHO] World Health Organization. Methods and techniques. World Health Organization; Geneva, Switzerland: 1975. Practical methods in malaria. Part II. [Google Scholar]