Abstract
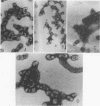
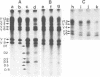
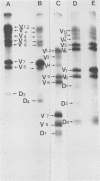
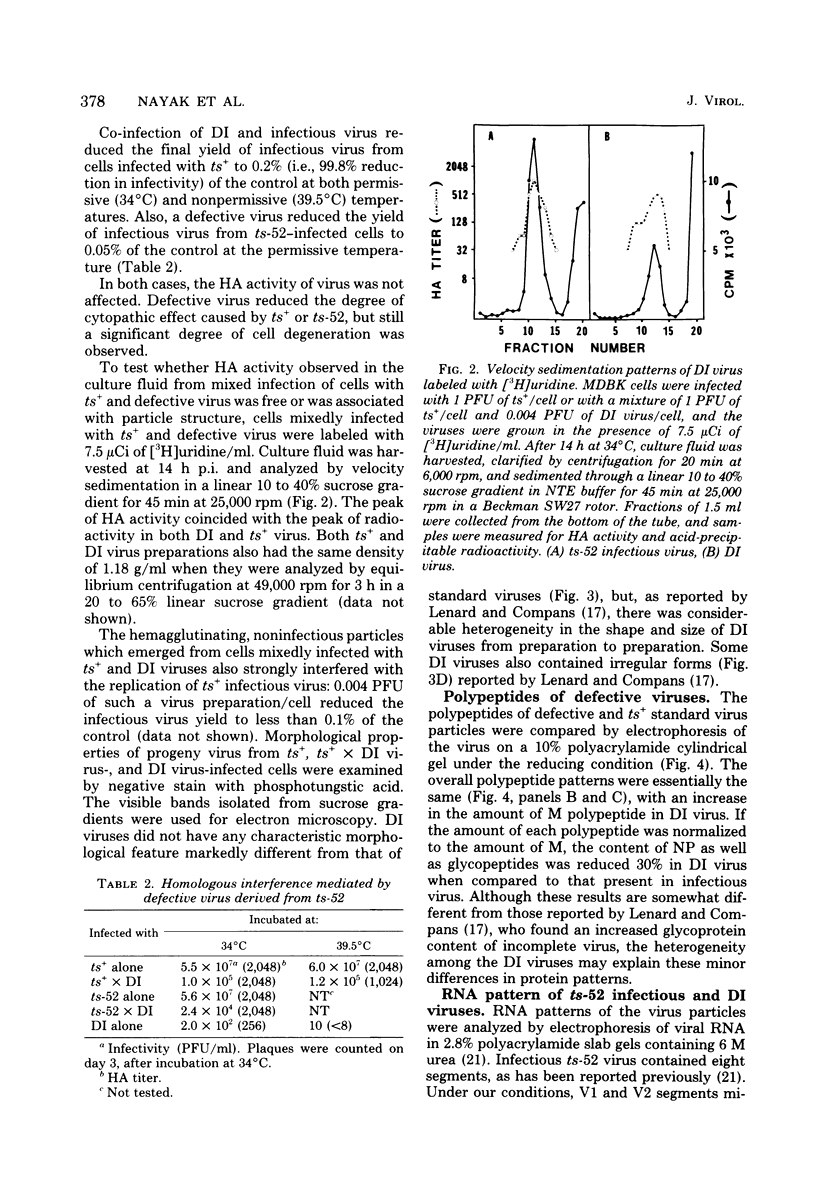
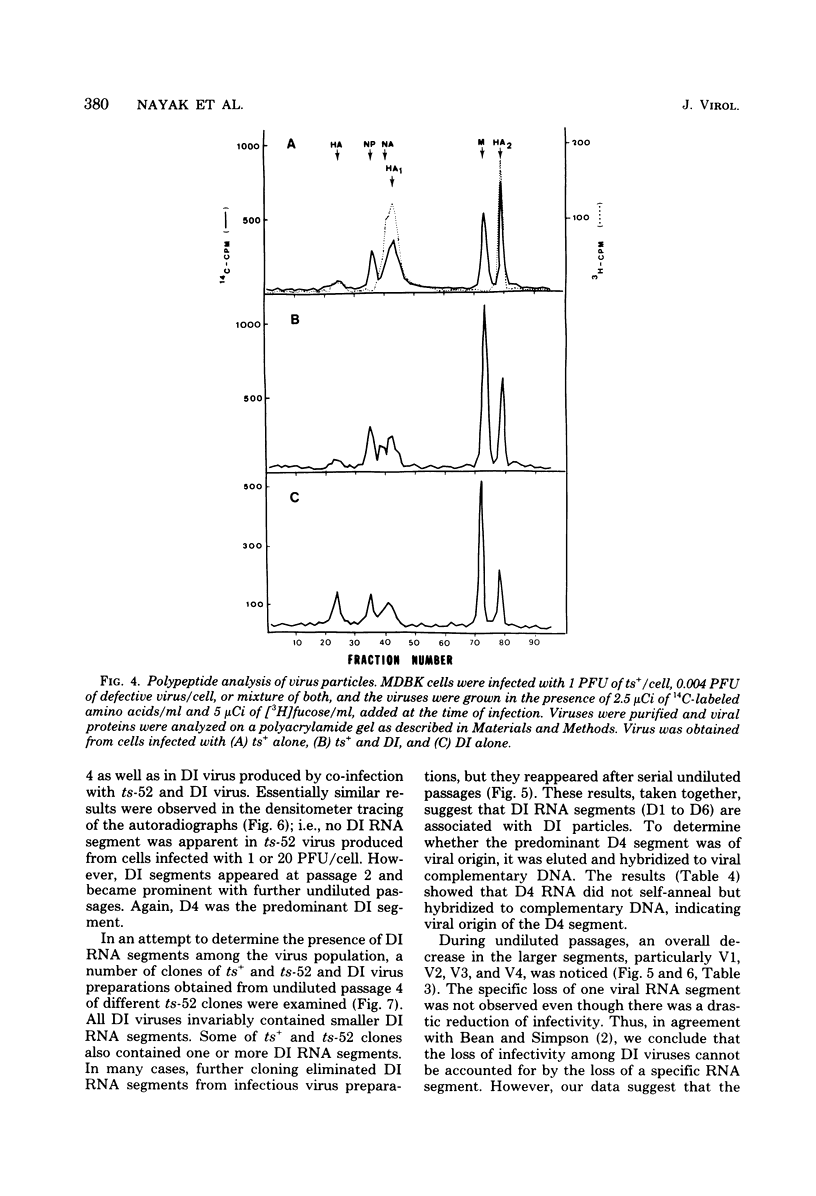
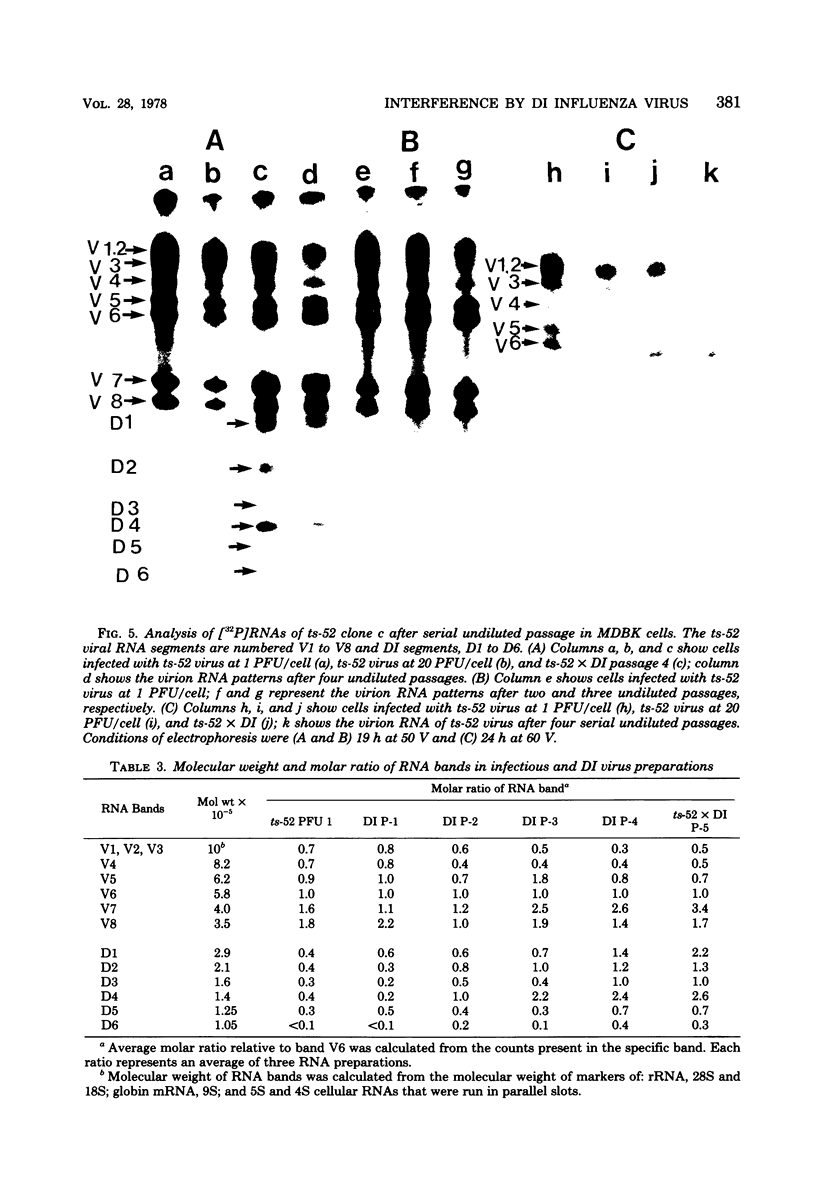
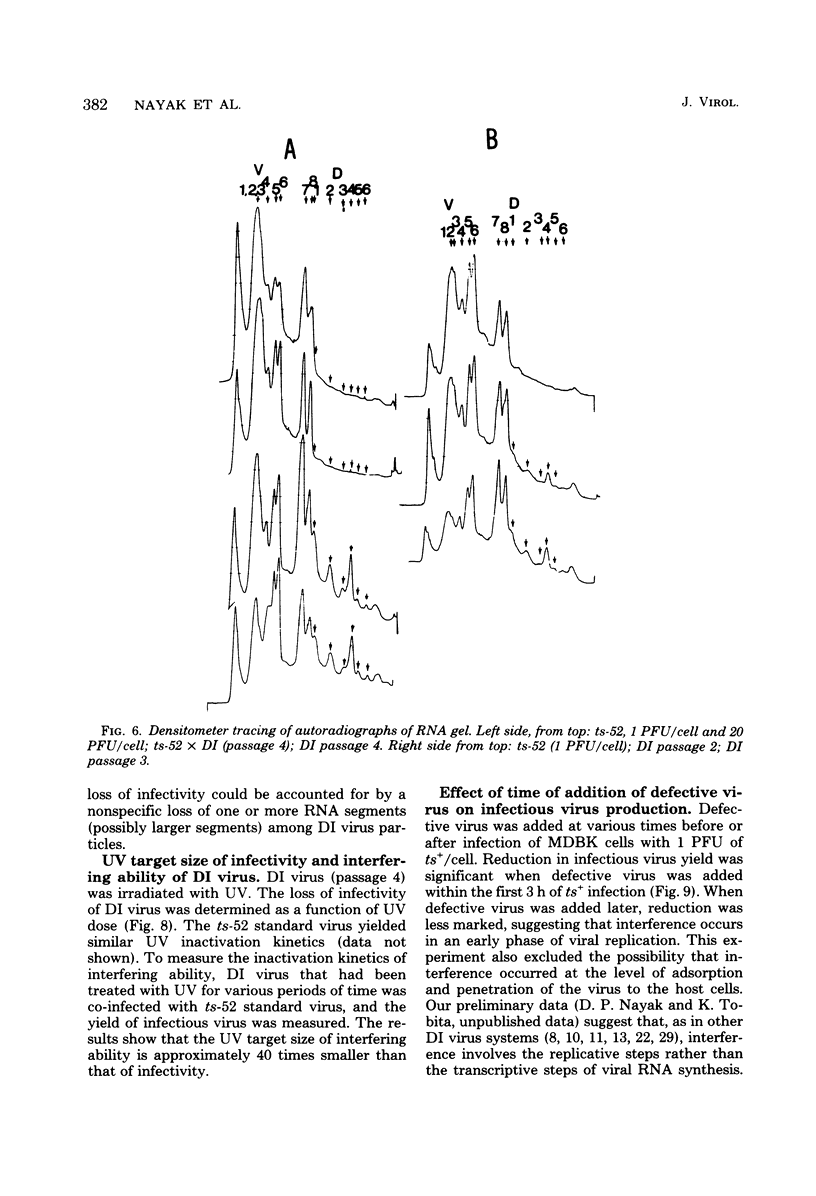
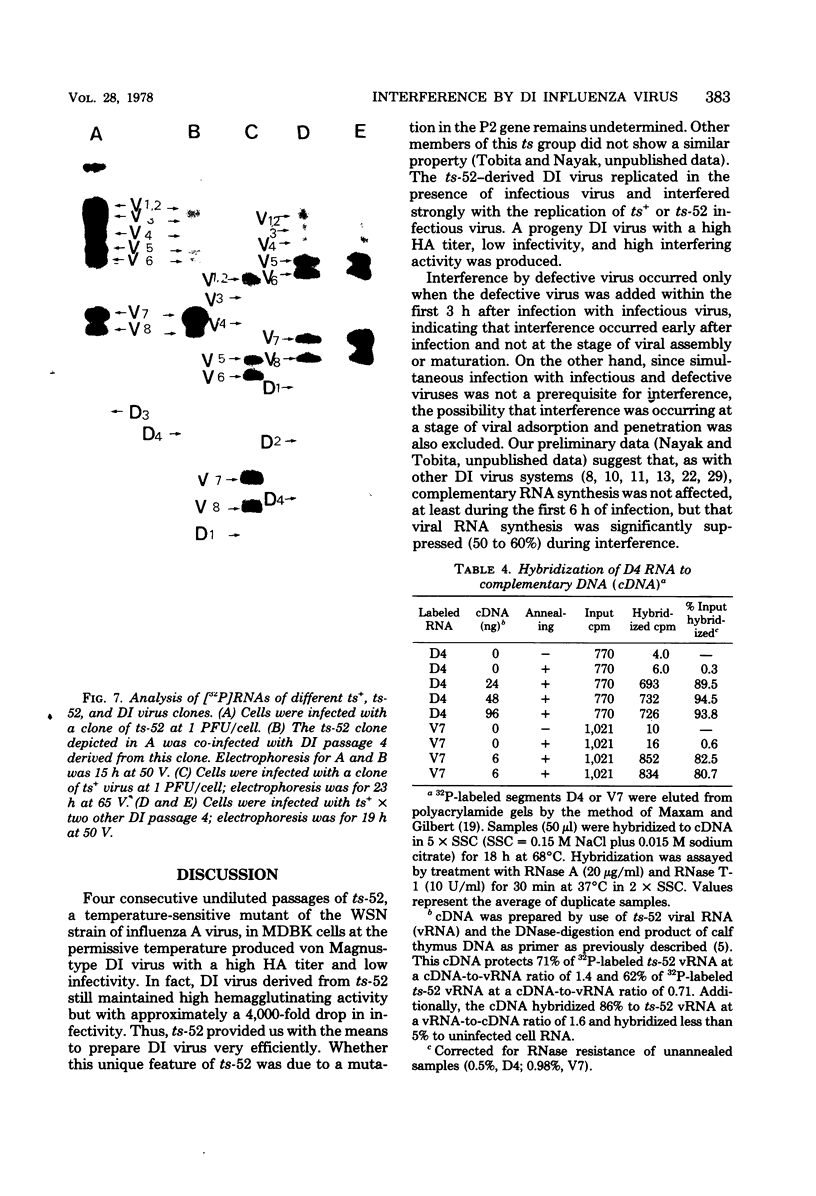
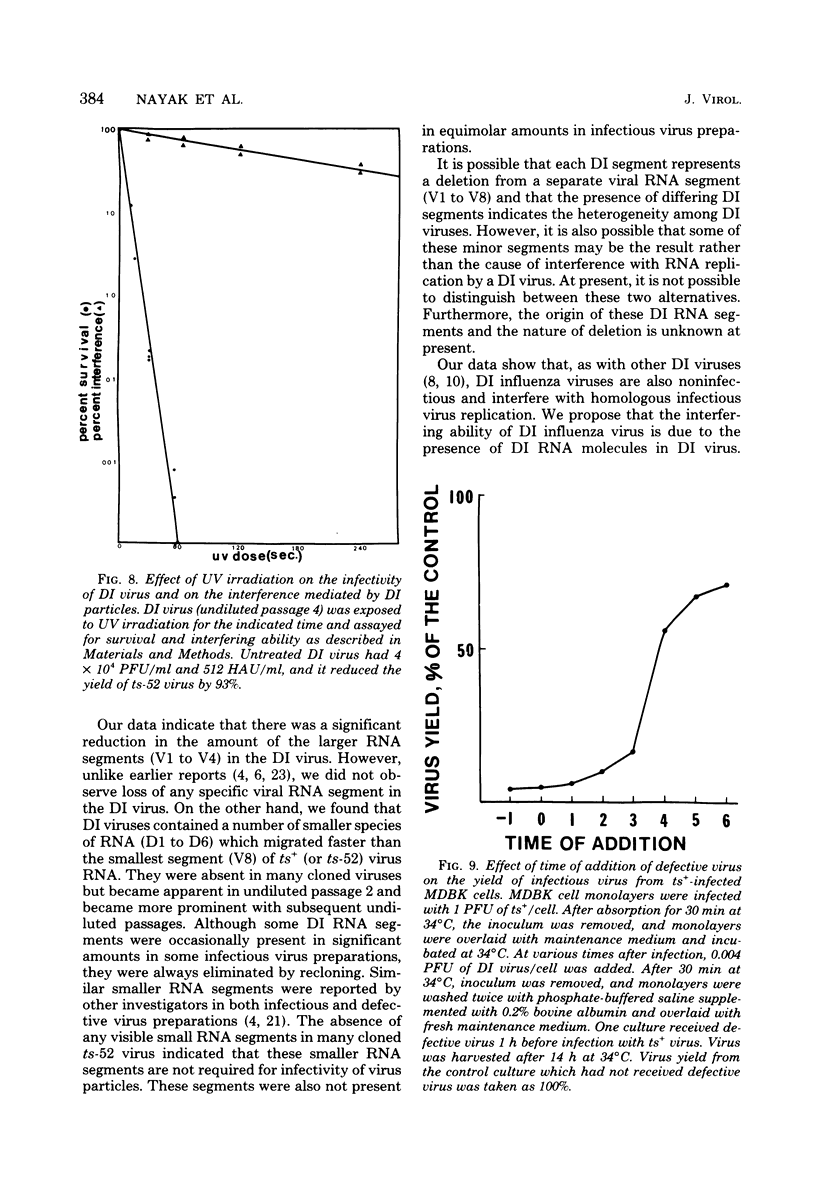
A temperature-sensitive group II mutant of influenza virus, ts-52, with a presumed defect in viral RNA synthesis, readily produced von Magnus-type defective interfering virus (DI virus) when passed serially (four times) at high multiplicity in MDBK cells. The defective virus (ts-52 DI virus) had a high hemagglutinin and a low infectivity titer, and strongly interfered with the replication of standard infectious viruses (both ts-52 and wild-type ts+) in co-infected cells. Progeny virus particles produced by co-infection of DI virus and infectious virus were also defective and also had low infectivity, high hemagglutinating activity, and a strong interfering property. Infectious viruses ts+ and ts-52 were indistinguishable from ts-52 DI viruses by sucrose velocity or density gradient analysis. Additionally, these viruses all possessed similar morphology. However, when the RNA of DI viruses was analyzed by use of polyacrylamide gels containing 6 M urea, there was a reduction in the amount of large RNA species (V1 to V4), and a number of new smaller RNA species (D1 to D6) with molecular weights ranging from 2.9 X 10(5) to 1.05 X 10(5) appeared. Since these smaller RNA species (D1 to D6) were absent in some clones of infectious viruses, but were consistently associated with DI viruses and increased during undiluted passages and during co-infection of ts-52 with DI virus, they appeared to be a characteristic of DI viruses. Additionally, the UV target size of interfering activity and infectivity of DI virus indicated that interfering activity was 40 times more resistant to UV irradiation than was infectivity, further implicating small RNA molecules in interference. Our data suggest that the loss of infectivity observed among DI viruses may be due to nonspecific loss of a viral RNA segment(s), and the interfering property of DI viruses may be due to interfering RNA segments (DIRNA, D1 to D6). ts-52 DI virus interfered with the replication of standard virus (ts+) at both permissive (34 degrees C) and nonpermissive temperatures. The infectivity of the progeny virus was reduced to 0.2% for ts+ and 0.05% for ts-52 virus without a reduction in hemagglutinin titer. Interference was dependent on the concentration of DI virus. A particle ratio of 1 between DI virus (0.001 PFU/cell) and infectious virus (1.0 PFU/cell) produced a maximal amount of interference. Infectious virus yield was reduced 99.9% without any reduction of the yield of DI viruses Interference was also dependent on the time of addition of DI virus. Interference was most effective within the first 3 h of infection by infectious virus, indicating interference with an early function during viral replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean W. J., Jr, Simpson R. W. Transcriptase activity and genome composition of defective influenza virus. J Virol. 1976 Apr;18(1):365–369. doi: 10.1128/jvi.18.1.365-369.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W., Pons M. W. The RNAs of infective and incomplete influenza virions grown in MDBK and HeLa cells. Virology. 1970 Nov;42(3):603–610. doi: 10.1016/0042-6822(70)90306-5. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Nayak D. P. Expression of endogenous retroviral genes in leukemic guinea pig cells. J Virol. 1977 Aug;23(2):263–271. doi: 10.1128/jvi.23.2.263-271.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. The RNA of influenza virus. Proc Natl Acad Sci U S A. 1968 Mar;59(3):930–937. doi: 10.1073/pnas.59.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. K., Pons M. W. Mechanism of influenza recombination. II. Virus aggregation and its effect on plaque formation by so-called noninfective virus. Virology. 1973 Dec;56(2):620–631. doi: 10.1016/0042-6822(73)90063-9. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Ueda M., Palese P. Temperature-sensitive mutants of influenza WSN virus defective in virus-specific RNA synthesis. J Virol. 1975 Oct;16(4):790–796. doi: 10.1128/jvi.16.4.790-796.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamnson R. N., Reichmann M. E. The RNA of defective vesicular stomatitis virus particles in relation to viral cistrons. J Mol Biol. 1974 Jan 5;85(4):551–568. doi: 10.1016/0022-2836(74)90315-5. [DOI] [PubMed] [Google Scholar]
- Lenard J., Compans R. W. Polypeptide composition of incomplete influenza virus grown in MDBK cells. Virology. 1975 Jun;65(2):418–426. doi: 10.1016/0042-6822(75)90047-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Sugiura A. Isolation of an influenza virus temperature-sensitive mutant of a new recombination-complementation group. Virology. 1977 May 15;78(2):365–374. doi: 10.1016/0042-6822(77)90114-3. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Differences in RNA patterns of influenza A viruses. J Virol. 1976 Mar;17(3):876–884. doi: 10.1128/jvi.17.3.876-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E. L., Perlman S. M., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. VI. Correlation of defective particle RNA synthesis with standard RNA replication. J Mol Biol. 1974 May 5;85(1):127–136. doi: 10.1016/0022-2836(74)90133-8. [DOI] [PubMed] [Google Scholar]
- Pons M., Hirst G. K. The single- and double-stranded RNA's and the proteins of incomplete influenza virus. Virology. 1969 May;38(1):68–72. doi: 10.1016/0042-6822(69)90128-7. [DOI] [PubMed] [Google Scholar]
- ROTT R., SCHOLTISSEK C. INVESTIGATIONS ABOUT THE FORMATION OF INCOMPLETE FORMS OF FOWL PLAGUE VIRUS. J Gen Microbiol. 1963 Nov;33:303–312. doi: 10.1099/00221287-33-2-303. [DOI] [PubMed] [Google Scholar]
- Reichmann M. E., Pringle C. R., Follett E. A. Defective particles in BHK cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Aug;8(2):154–160. doi: 10.1128/jvi.8.2.154-160.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P. Identification of the defective genes in three mutant groups of influenza virus. J Virol. 1977 Mar;21(3):1196–1204. doi: 10.1128/jvi.21.3.1196-1204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Genome homology of vesicular stomatitis virus and defective T particles and evidence for the sequential transcription of the virion ribonucleic acid. J Virol. 1972 Jun;9(6):946–955. doi: 10.1128/jvi.9.6.946-955.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., Tobita K., Kilbourne E. D. Isolation and preliminary characterization of temperature-sensitive mutants of influenza virus. J Virol. 1972 Oct;10(4):639–647. doi: 10.1128/jvi.10.4.639-647.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., Ueda M., Tobita K., Enomoto C. Further isolation and characterization of temperature-sensitive mutants of influenza virus. Virology. 1975 Jun;65(2):363–373. doi: 10.1016/0042-6822(75)90042-2. [DOI] [PubMed] [Google Scholar]
- VON MAGNUS P. Incomplete forms of influenza virus. Adv Virus Res. 1954;2:59–79. doi: 10.1016/s0065-3527(08)60529-1. [DOI] [PubMed] [Google Scholar]