Abstract
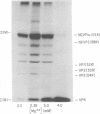
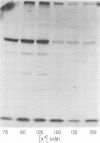
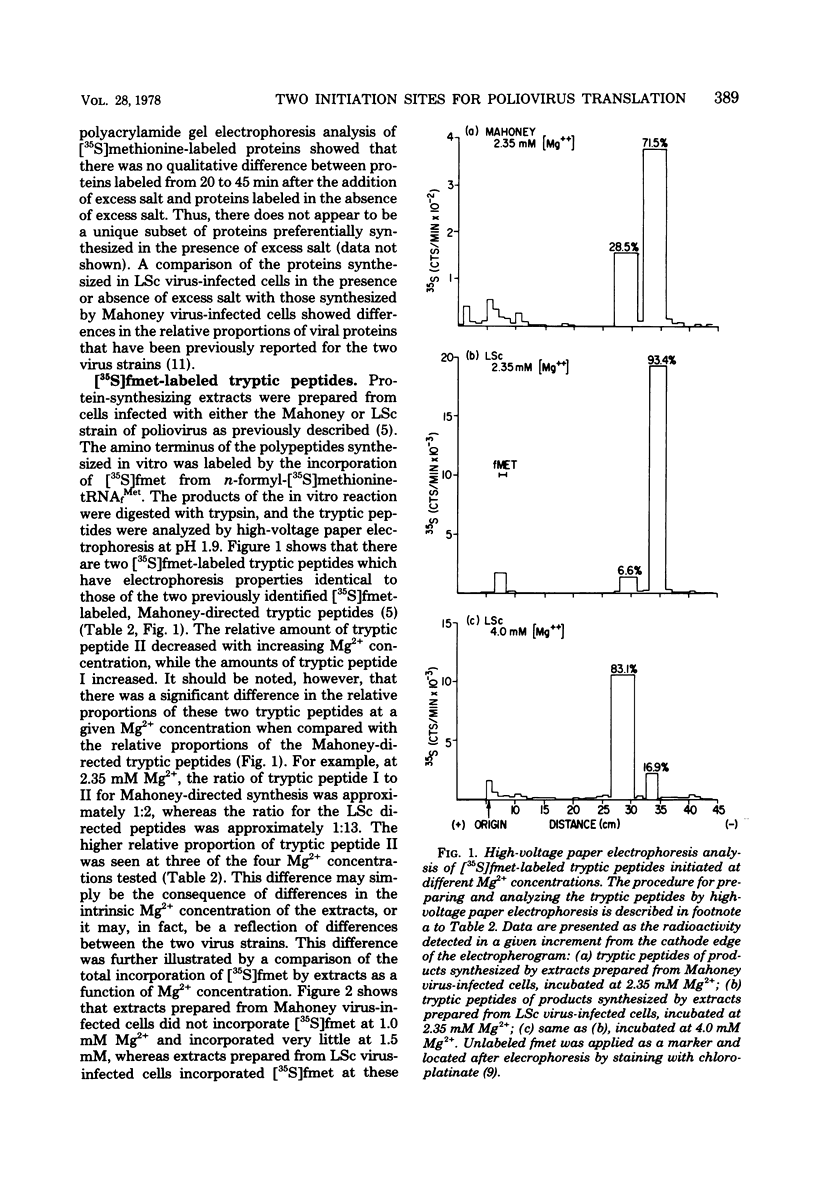
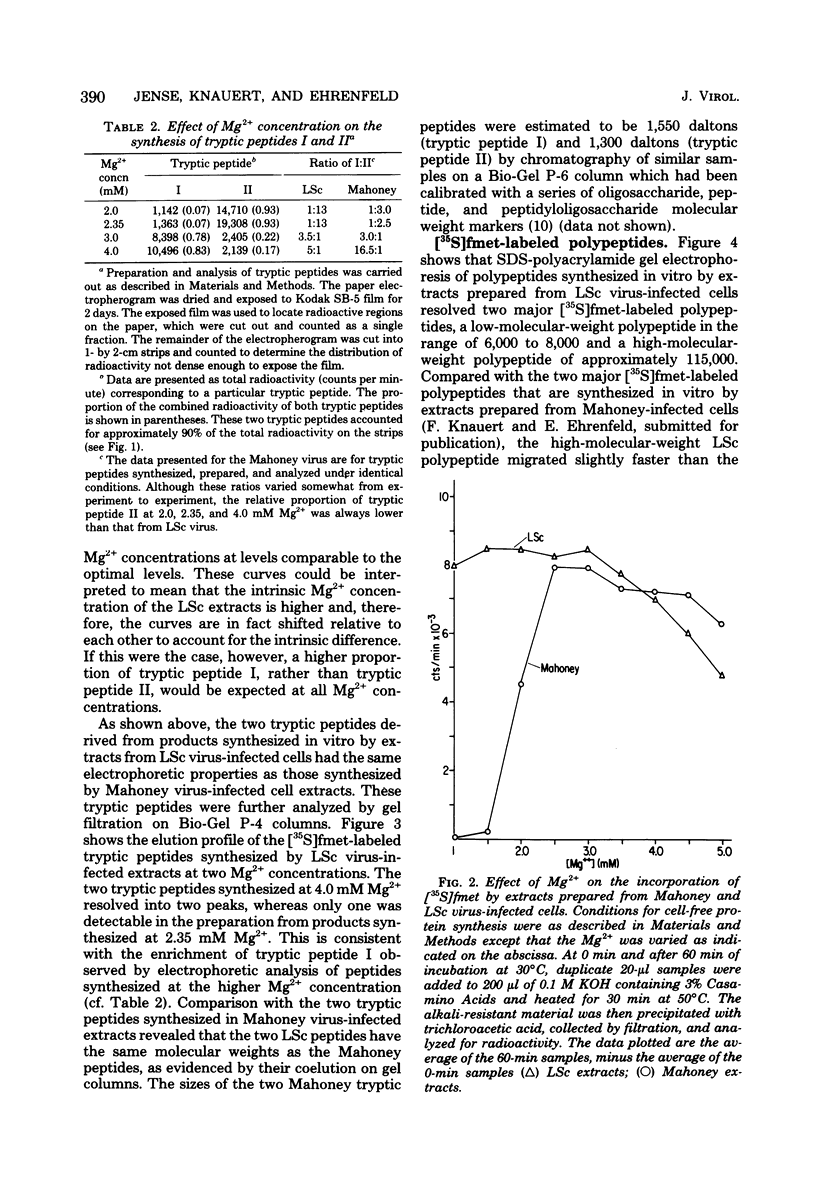
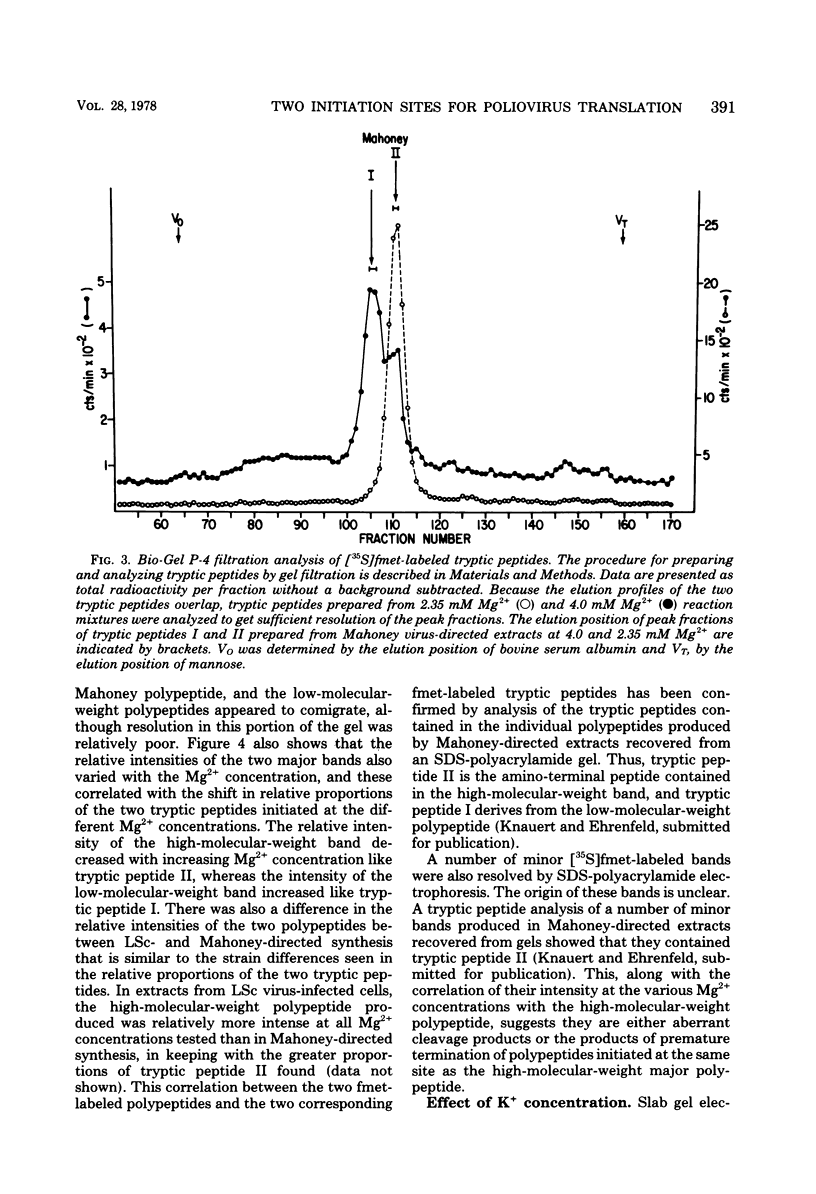
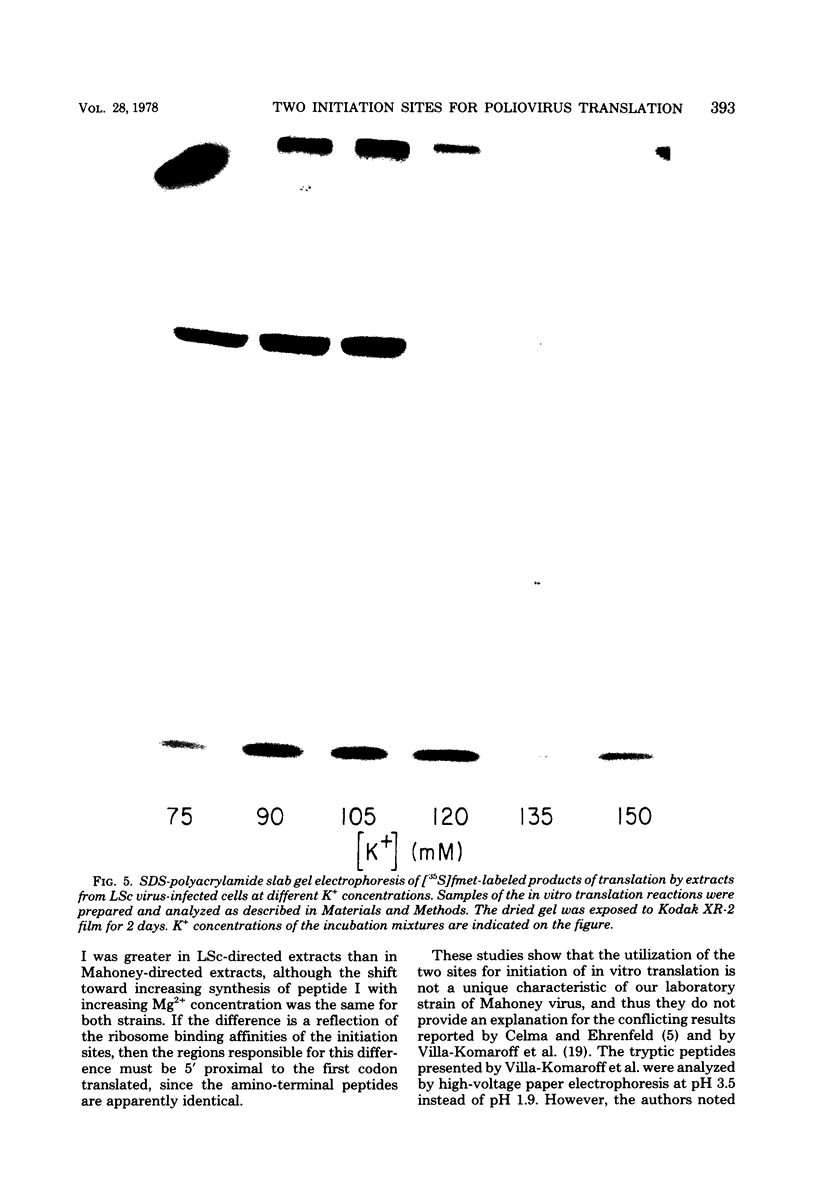
Previous studies in our laboratory provided evidence that the initiation of translation by the Mahoney strain of poliovirus type 1 RNA in vitro occurs at two unique sites. This study shows that the LSc strain of poliovirus type 1, a multistep, temperature-sensitive mutant of the Mahoney strain, also utilizes two sites for the initiation of translation in vitro. Incorporation of formyl-[35S]methionine into the amino terminus of newly synthesized polypeptides revealed the production of two labeled tryptic peptides which are identical in size and electrophoretic mobility with those produced by Mahoney virus. The polypeptides containing amino-terminal label showed similar patterns on sodium dodecyl sulfate-acrylamide gels, although one of the LSc polypeptides had a slightly faster mobility. The relative proportion of initiation at each site varied with the magnesium concentration for both viruses, but the LSc strain favored initiation at one site more so than did the Mahoney strain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Cooper P. D. Poliovirus polypeptides examined in more detail. J Gen Virol. 1975 Nov;29(2):199–213. doi: 10.1099/0022-1317-29-2-199. [DOI] [PubMed] [Google Scholar]
- Abraham G., Cooper P. D. Relations between poliovirus polypeptides as shown by tryptic peptide analysis. J Gen Virol. 1975 Nov;29(2):215–221. doi: 10.1099/0022-1317-29-2-215. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Celma M. L., Ehrenfeld E. Effect of poliovirus double-stranded RNA on viral and host-cell protein synthesis. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2440–2444. doi: 10.1073/pnas.71.6.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma M. L., Ehrenfeld E. Translation of poliovirus RNA in vitro: detection of two different initiation sites. J Mol Biol. 1975 Nov 15;98(4):761–780. doi: 10.1016/s0022-2836(75)80009-x. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Smoler D., Wimmer E., Baltimore D. Defective interfering particles of poliovirus. I. Isolation and physical properties. J Virol. 1971 Apr;7(4):478–485. doi: 10.1128/jvi.7.4.478-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley C. W. Combinations of specific color reactions useful in the peptide mapping technique. Biochim Biophys Acta. 1965 Sep 13;107(2):386–388. doi: 10.1016/0304-4165(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Etchison J. R., Robertson J. S., Summers D. F. Partial structural analysis of the oligosaccharide moieties of the vesicular stomatitis virus glycoprotein by sequential chemical and enzymatic degradation. Virology. 1977 May 15;78(2):375–392. doi: 10.1016/0042-6822(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Fiszman M., Reynier M., Bucchini D., Girard M. Thermosensitive block of the Sabin strain of poliovirus type I. J Virol. 1972 Dec;10(6):1143–1151. doi: 10.1128/jvi.10.6.1143-1151.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Goldstein E., Penman S. Poliovirus-induced inhibition of polypeptide initiation in vitro on native polyribosomes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1834–1838. doi: 10.1073/pnas.73.6.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg B. F., Shatkin A. J. Initiation of picornavirus protein synthesis in ascites cell extracts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3589–3593. doi: 10.1073/pnas.69.12.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E. The initiation of protein synthesis directed by the RNA from encephalomyocarditis virus. Eur J Biochem. 1973 Mar 1;33(2):301–313. doi: 10.1111/j.1432-1033.1973.tb02684.x. [DOI] [PubMed] [Google Scholar]
- Tershak D. R. Effect of hypertonic medium on protein synthesis of Mahoney and LSc poliovirus. J Virol. 1976 Dec;20(3):597–603. doi: 10.1128/jvi.20.3.597-603.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Guttman N., Baltimore D., Lodishi H. F. Complete translation of poliovirus RNA in a eukaryotic cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4157–4161. doi: 10.1073/pnas.72.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]