Abstract
Vibrio parahaemolyticus pandemic serotype O3 : K6 causes acute gastroenteritis, wound infections and septicaemia in humans. This organism encodes two type III secretion systems (T3SS1 and T3SS2); host-cell cytotoxicity has been attributed to T3SS1. Synthesis and secretion of T3SS1 proteins is positively regulated by ExsA, which is presumptively regulated by the ExsCDE pathway, similar to Pseudomonas aeruginosa. Herein we deleted the putative exsE from V. parahaemolyticus and found constitutive expression of the T3SS1 in broth culture as expected. More importantly, however, in a cell culture model, the ΔexsE strain was unable to induce cytotoxicity, as measured by release of lactate dehydrogenase (LDH), or autophagy, as measured by LC3 conversion. This is markedly different from P. aeruginosa, where deletion of exsE has no effect on host-cell cytolysis. Swarming and cytoadhesion were reduced for the deletion mutant and could be recovered along with T3SS1-induced HeLa cell cytotoxicity by in cis expression of exsE in the ΔexsE strain. Loss of adhesion and swarming motility was associated with the loss of flagella biogenesis in the exsE-deficient strain. Mouse mortality was unaffected by the deletion of exsE compared with a wild-type control, suggesting that additional adhesins are important for intoxication in vivo. Based on these data, we conclude that ExsE contributes to the negative regulation of T3SS1 and, in addition, contributes to regulation of an adherence phenotype that is requisite for translocation of effector proteins into HeLa cells.
Introduction
Vibrio parahaemolyticus, a Gram-negative, halophilic bacterial pathogen, can cause acute gastroenteritis following consumption of raw or undercooked seafood (Daniels et al., 2000; Hlady & Klontz, 1996; Morris & Black, 1985; Tison & Kelly, 1984). Wound infections and septicaemia can also result from V. parahaemolyticus exposure, with immune-compromised individuals being at highest risk (Daniels et al., 2000; Hlady & Klontz, 1996; Mertens et al., 1979; Qadri et al., 2003; Ryan, 1976). Pathogenicity of the pandemic V. parahaemolyticus serotype O3 : K6 has been attributed to several factors including thermostable direct haemolysin (TDH) (Nishibuchi et al., 1992), TDH-related haemolysin (Xu et al., 1994) and two distinct type III secretion systems (T3SSs) (Park et al., 2004; Piñeyro et al., 2010). Contact with host cells will induce synthesis of T3SS1 proteins, as has been demonstrated in vitro and in vivo by multiple independent investigations (Burdette et al., 2009; Hiyoshi et al., 2010; Ono et al., 2006; Park et al., 2004; Zhou et al., 2009). Importantly, it is unclear how host cell contact signals upregulate T3SS1 expression.
T3SSs were first discovered in Yersinia (Michiels et al., 1990; Salmond & Reeves, 1993), and later found in many Gram-negative pathogens including Escherichia coli, and Pseudomonas, Shigella, Salmonella and Vibrio strains (Coburn et al., 2007). T3SSs are structurally conserved and are composed of three parts: (i) a basal body that spans the periplasmic space; (ii) a needle-like complex that extends from the bacterial surface; and (iii) the translocation apparatus that forms a channel through the target cell membrane. This specialized molecular machine translocates effector proteins into the eukaryotic target cell where they are able to sabotage host cellular processes and modulate the target cell environment.
V. parahaemolyticus T3SS1 is phylogenetically related to the Ysc family of T3SS injectisomes and shares many common characteristics with both Yersinia and Pseudomonas aeruginosa T3SSs (Makino et al., 2003; Troisfontaines & Cornelis, 2005). Induction of the T3SS in P. aeruginosa is, in most cases, primarily dependent on contact with a eukaryotic host cell, although there are potential secondary inducing signals such as temperature, metabolic stress and environmental stimuli (Frank, 1997; Hornef et al., 2000; Urbanowski et al., 2007; Vallis et al., 1999). P. aeruginosa T3SS gene transcription is controlled by an AraC-like transcriptional activator, ExsA, which acts as a positive regulator and is coupled to secretion competency by the regulatory cascade ExsCDE (Yahr & Wolfgang, 2006). ExsD is an ‘anti-activator’ that binds ExsA and prevents transcriptional regulation (McCaw et al., 2002) while ExsC is an ‘anti-anti-activator’ that binds ExsD to permit promoter binding activity of ExsA (Dasgupta et al., 2004; Hovey & Frank, 1995). ExsC also acts as a chaperone for the protein ExsE (Rietsch et al., 2005; Urbanowski et al., 2007) and under non-inducing conditions the ExsC–ExsE protein interaction prevents ExsC from binding to ExsD, thereby preventing high-level expression of the T3SS. Upon host cell contact, ExsE is exported via a T3SS apparatus allowing ExsC to bind ExsD, thereby freeing the transcriptional regulator ExsA to upregulate T3SS expression. This model is based on the presence of low-level, constitutive expression of the T3SS.
Regulation of T3SS1 in V. parahaemolyticus is also controlled in a manner similar to P. aeruginosa (Kodama et al., 2010; Zhou et al., 2010a; Zhou et al., 2008). Beyond the interaction of the proximal regulators ExsA, ExsD and ExsC, however, little is known about the induction of T3SS1 other than studies showing that gene expression can be induced with host cell contact and when V. parahaemolyticus is cultured under specific conditions (Gode-Potratz et al., 2010; Zhou et al., 2008). Herein we further examine the applicability of the P. aeruginosa regulatory model in the control of T3SS1 expression in V. parahaemolyticus using a host cell contact model.
Kodama et al. (2010) recently demonstrated that deletion of the putative V. parahaemolyticus exsE (vp1702) results in constitutive high-level T3SS1 protein synthesis under non-inducing conditions, as would be predicted from the P. aeruginosa model (Rietsch et al., 2005). In the present study, we confirmed that deletion of exsE leads to constitutive protein synthesis under non-inducing conditions, and we also showed that this deletion does not negatively affect the T3SS1 regulon or its secretory function in the presence of HeLa cells. Despite successful secretory function, however, we found that deletion of exsE results in a loss of both cytolysis and induction of autophagy in HeLa cell culture; these phenotypes could be recovered by in cis complementation of exsE. Cytoadherence was also reduced in our mutant indicating that V. parahaemolyticus ExsE is involved with regulation of adhesion that may be requisite for successful translocation of effector proteins. Mouse mortality was unaffected compared with the wild-type strain, suggesting that either multiple adhesins are present to allow sufficient cell–cell contact during in vivo infection or adhesion required in vitro is simply unnecessary to affect in vivo host cell intoxication by T3SS1.
Methods
Strains and growth conditions.
V. parahaemolyticus strain NY-4 (serotype O3 : K6) was used as the wild-type strain for these experiments (Table 1) (Zhou et al., 2009). Bacteria were cultured at 37 °C in Luria–Bertani (LB) medium (Difco) supplemented with antibiotics where appropriate and 2.5 % (w/v) NaCl (LBS) for V. parahaemolyticus strains. HeLa cells were maintained at 37 °C, 5 % CO2 in DMEM (Thermo Scientific) supplemented with 10 % (v/v) fetal bovine serum (FBS) (Thermo Scientific).
Table 1. Strains and plasmids used in this study.
| Strain or plasmid | Genotype or description | Source or reference |
| E. coli strains | ||
| S17 λpir | thi pro hsdR hsdM+ recA RP-4-2-TC : : MU-Km : : Tn7 λpir | Milton et al. (1992) |
| S17pKN8-P1668-exsE | S17 containing P1668-exsE fusion gene located on plasmid pKN8 | This study |
| S17pKN8-P1668 | S17 containing P1668 located on plasmid pKN8 | This study |
| V. parahaemolyticus strains | ||
| NY-4 | Clinical isolate O3 : K6 | |
| NY-4 : pexsA | Wild-type strain containing exsA located in plasmid pMMB207 | Zhou et al. (2008) |
| ΔexsE | exsE deletion mutant | This study |
| ΔexsE : : pKN8-P1668-exsE | ΔexsE complemented with chromosomal insertion of pKN8-P1668-exsE | This study |
| ΔexsE : : pKN8-P1668 | ΔexsE complemented with chromosomal insertion of pKN8-P1668 | This study |
| NY-4 : pP1668-exsE-his | Wild-type strain containing P1668-exsE-His gene fusion located in plasmid pMMB207 | This study |
| ΔexsE:pP1668-exsE-his | ΔexsE containing P1668-exsE-His gene fusion located in plasmid pMMB207 | This study |
| ΔvcrD | T3SS-deficient mutant | Zhou et al. (2009) |
| ΔexsA | exsA deletion mutant | |
| ΔexsD | exsD deletion mutant | Zhou et al. (2008) |
| ΔlfgE | Lateral flagella-deficient mutant | This study |
| Plasmids | ||
| pDM4 | Suicide vector with ori R6K sacB Cmr | Milton et al. (1996) |
| pKN8 | Suicide vector with ori R6K Cmr mob+ (RP4) lacZYA | Falker et al. (2005) |
| pKN8-P1668-exsE | P1668-exsE gene fusion located on plasmid pKN8 | This study |
| pKN8-P1668 | Promoter P1668 located on plasmid pKN8 | This study |
| pMMB207 | RSF1010 derivative with IncQ Cmr Ptac oriT | Morales et al. (1991) |
| pP1668-exsE-His | P1668-exsE gene fusion located on plasmid pMMB207 | This study |
Construction of deletion mutants.
Deletion mutants were constructed using a method of allelic exchange described previously (Milton et al., 1996). For ΔexsE, primers vp1702-1F, vp1702-1Rre, vp1702-2Fre and vp1702-2R were used to amplify regions flanking the exsE gene (Table 2). The resulting amplicons were digested with XbaI and ligated together. Primers vp1702-1F and vp1702-2R were then used to amplify the full-length fragment minus the deleted sequence. This amplicon was digested with XhoI and SphI followed by ligation into the suicide vector pDM4 (digested with the same) and electroporated into E. coli S17 λpir. For ΔlfgE, primers lfgE-1F, lfgE-1R, lfgE-2F and lfgE-2R were used to amplify regions flanking the lfgE gene (Table 2). The resulting amplicons were used as template in a splicing by overlap extension PCR to amplify the full-length amplicon. This amplicon was digested with XhoI and SacI followed by ligation into the suicide vector pDM4 (digested with the same) and electroporated into E. coli S17 λpir. Constructs were introduced to NY-4 by conjugation and positive insertions were selected on LBS agar supplemented with 34 µg chloramphenicol ml−1. To complete allelic exchange, cells were cultured in the presence of 5 % (w/v) sucrose. Chloramphenicol-sensitive, sucrose-resistant colonies were then screened for the gene deletion by PCR and confirmed by sequencing using primers delta-exsEseq-up and delta-exsEseq-down or lfgE-F and lfgE-R, respectively (Table 2).
Table 2. Primers used in this study.
| Primer | Sequence (5′–3′) |
| vp1702-1F | AGGTTACTCGAGTCCCCACGGACTGAGCGCAT |
| vp1702-1Rre | AGGATATCTAGAAAGACACCTAAACTC |
| vp1702-1Fre | AGGATATCTAGAACACAAGTTATCACGCAC |
| vp1702-2R | GAGTTTAGGTGTCTTACACAAGTTATCACGCAC |
| delta-exsEseq up | TGGCCGAAGAGACGCCTGCT |
| delta-exsEseq down | GGGGATCGGGCGTGAAACGC |
| lfgE-1F | AGGATAAACTCGAGTTCGCTACCGTGTATCAAAAAAC |
| lfgE-1R | CTATCCATGCAGAACTCCTAAAAAGACCTCTGATTTGCTT |
| lfgE-2F | AAGCAAATCAGAGGTCTTTTTAGGAGTTCTGCATGGATAG |
| lfgE-2R | AGTTAGTCTAGACGTGCCGACAACGCGCACAC |
| lfgE-F | CTTGGCTTAAAGCCGGGCAA |
| lfgE-R | CGCGTTGATGCAGAGTTTGTGAC |
| P1668F-XbaI | AGGATATCTAGAAATACTCATTCACTTGCACTC |
| P1668R-BamHI | AGTTAGGGATCCAATGTAAAAAATATGCGCAATG |
| P1668R-BglII | AGTTAGAGATCTAATGTAAAAAATATGCGCAATG |
| vp1702F-BamHI | AGGATAGGATCCATGTCTAATGACATCCAATC |
| vp1702R-BglII | AGTTAGAGATCTTTAATGGTGATGGTGATGGTGCCTTTCGCTTCGAGCAA |
| 1656-up | AGGATAGAATTCAGGAGATATACCATGTTGGATAAAATTGGTGGAAC |
| 1656-down | AGTTAGTCTAGATTAATGGTGATGGTGATGGTGCACTGTCGGGATAGATGCGC |
| 1680–600-up | TCTACGTTCCATAAAGGCCAC |
| 1680–600-down | CAACATCGCGTGACCAATGG |
| 1686–600-up | ATTCTAAATGAAGGCAAACTCAGC |
| 1686–600-down | GTTTAAATCCGTACTTGCGAGC |
| SecYF | TGGTGCTCTTGAGCGTGCATC |
| SecYR | CCTTGTTGACGCTTCGCGTAG |
| qExsA-F | TCCGTCAGCTTCCACTCTTT |
| qExsA-R | CTCGGGCTTGTTTTCTTTTG |
| qExsE-F | AACGTTTCAAGGTCGCAAAG |
| qExsE-R | TACCTTTCGCTTCGAGCAAT |
| qLafA-F | CGCAGCTATCACTGACGGTA |
| qLafA-R | TCCATGATACGGCCTTTAGC |
| qSecY-F | ACTGGCTCAGTGGTTTGGTC |
| qSecY-R | GGGTACGAATGCACCAGACT |
Complementation.
To complement the exsE deletion, we generated a gene fusion consisting of the ExsA-dependent promoter sequence upstream of vp1668 with the full-length exsE cloned into the suicide vector, pKN8 (Table 1). A control strain was also constructed consisting of the promoter sequence only. The gene fusion was constructed by amplifying approximately 220 bp DNA fragment upstream of vp1668 using primers P1668F-XbaI and P1668R-BamHI and the full-length exsE using primers vp1702F-BamHI and vp1702R-BglII (Table 2). Amplicons were digested with BamHI and ligated, and the resulting product was amplified by PCR using primers P1668F-XbaI and vp1702R-BglII. To generate the promoter-only control, primers P1668F-XbaI and P1668R-BglII (Table 2) were used to amplify the promoter sequence alone. The gene fusion and promoter sequence amplicons were digested with XbaI and BglII, and cloned into pKN8 digested with the same enzymes, resulting in the plasmids pKN8-P1668-exsE and pKN8-P1668 (Table 1). The plasmids were transformed into E. coli S17 λpir by electroporation, resulting in strains S17pKN8-P1668-exsE and S17pKN8-P1668, respectively (Table 1). pKN8-P1668-exsE and pKN8-P1668 were introduced into V. parahaemolyticus NY-4ΔexsE by conjugation, resulting in the single cross-over strains ΔexsE : : pKN8-P1668-exsE (in cis complemented strain) and ΔexsE : : pKN8-P1668 (in cis negative control), respectively, where each strain harbours a chromosomal insertion of the respective suicide vector upstream of vp1668 in the P1668 promoter region (Table 1). PCR was used to confirm these insertions (data not shown). In trans expression of exsE using the expression vector pMMB207 (Morales et al., 1991) was also used for this study but resulted in an overexpression of ExsE and subsequent repression of T3SS1 resulting in failure to complement the cytolysis phenotype (Fig. S1, available with the online version of this paper).
Western blot analysis.
Cells were harvested by centrifugation and the pellet was resuspended into SDS loading buffer and boiled for 5 min. Secreted proteins were collected from filtered supernatant by trichloroacetic acid precipitation (Zhou et al., 2008). Protein was loaded for each sample and separated using SDS-PAGE on pre-cast 4–20 % gels (Bio-Rad) and then transferred to PVDF membrane using a mini-PROTEAN tetra system following the manufacturer’s recommendations (Bio-Rad). Membranes were incubated with primary antibody (see below) for 2 h at room temperature or overnight at 4 °C, washed five times for 2 min each with PBS containing 0.05 % (v/v) Tween 20 (PBS-T) and incubated with either anti-mouse-DyLight 800 or anti-rabbit-DyLight 800 conjugate (1 : 10 000, Thermo Scientific) for 1 h at room temperature. Blots were scanned and images were obtained using the Odyssey Infrared Imaging system (LI-COR). Primary antibodies used at a 1 : 5000 dilution were anti-Vp1656 (Zhou et al., 2008), anti-DnaK (Zhou et al., 2010a) and anti-LC3 (Novus Biologicals).
RNA isolation and reverse transcriptase (RT)-PCR.
After 4 h infection, total RNA was isolated by using a RiboPure-Bacteria kit (Ambion). Reverse transcription was performed using 500 ng RNA, 200 ng random hexamers and Superscript III (Invitrogen). PCR was performed according to the manufacturer’s instructions (1 U Master Taq polymerase, 200 µM each of the four dNTPs and 1 µM each primer). Primer pairs for amplification of cDNA used for analysis of gene expression are listed in Table 2. Cycling parameters were identical for all primer sets: one cycle of 94 °C for 4 min; 30 cycles of 95 °C for 1 min, 52 °C for 1 min and 72 °C for 1 min; and a final incubation at 72 °C for 5 min. Amplicons were separated using 1 % (w/v) agarose gel electrophoresis and visualized using MultiImage light cabinet and AlphaEase FC software (Alpha Innotech).
Lactate dehydrogenase (LDH)-release assay.
Growth media was removed from 70–80 % confluent HeLa cell monolayers and replaced with DMEM supplemented with 1 % (v/v) FBS prior to infection at an m.o.i. of 100. After infection, cell cultures were centrifuged briefly and a 50 µl aliquot of the supernatant was removed for LDH-release assay using CytoTox 96 non-radioactive cytotoxicity assay performed as per the manufacturer’s instructions (Promega).
Swarming assay.
Semi-solid swarming motility agar was prepared based on the method described by Niu et al. (2005) using antibiotic-free LBS broth with the addition of 0.5 % (w/v) agar, 0.04 % (v/v) sterile Tween 80 and supplemented with 1 mM IPTG. A single isolated colony was selected for each strain listed and inoculated to a freshly prepared swarm agar plate and incubated at 37 °C for 8 h. Images were obtained using MultiImage light cabinet and AlphaEase FC software. The assay was repeated three times with similar results and representative photographs are shown here.
Adhesion assay.
Strains cultured overnight in LBS broth were used to infect HeLa cell monolayers cultured on four-well glass slides (Nunc) in DMEM supplemented with 10 % (v/v) FBS at an m.o.i. ~100. After a 30 min incubation, slides were washed five times with sterile PBS and stained using Diff-Quik (Siemens). Slides were fixed with coverslips and images were obtained using an Axio Imager system with a 63× oil objective (Zeiss). For each biological replicate, a minimum of 20 HeLa cells were observed for each strain per well and the number of attached bacteria were enumerated by direct count.
quantitative RT-PCR (qPCR).
HeLa cell monolayers were infected at an m.o.i. of 100. Over the course of infection, cells were harvested at the indicated time points and total RNA was isolated using a RiboPure-Bacteria kit (Ambion). cDNA was prepared by reverse-transcription using 2 µg RNA, 200 ng random hexamers and Superscript III (Invitrogen). qPCR was performed using SsoFast EvaGreen reagent according to the manufacturer’s instructions (Bio-Rad). Primer pairs used for analysis of gene expression are listed in the last eight rows of Table 2. Cycling parameters were identical for all primer sets: one cycle of 95 °C for 30 s; 38 cycles of 95 °C for 1 s, 52 °C for 5 s and 72 °C for 15 s. Reactions were performed using the CFX 96 real-time PCR system (Bio-Rad) and relative expression levels were calculated using the ΔΔCt method with secY as the control transcript.
Mouse intrapulmonary infections.
Mice were housed in microisolator cages and allowed ad libitum access to food and water. In vivo infections were performed as described by Piñeyro et al. (2010) with minor modifications. Briefly, 4–5-month-old C57-B6 mice (n = 8 per strain) weighing 20–35 g were sedated with 150–200 mg Avertin kg−1 administered intraperitoneally and were restrained ventrally. An inoculum of ~105 c.f.u. bacteria was delivered via gavage tube just proximal to the larynx to induce aspiration. Mice were monitored every hour for 12 h and every 3 h thereafter for mortality; moribund animals were euthanized immediately with time of death documented. Lungs were collected following death or euthanasia, homogenized in sterile PBS and an aliquot was plated to thiocitrate bile salts (TCBS) agar. Recovered bacteria were strain confirmed by PCR. Animal challenge experiments conformed to a protocol approved by the Washington State University Institutional Animal Care and Use Committee.
Statistical analysis.
Tests for significance were performed using NCSS (v.7.1.19; www.ncss.com/ncss.html) and the resulting data were plotted using Sigma Plot (v.11.0; www.sigmaplot.com/). One-way ANOVA using the Dunnett’s two-sided multiple-comparison test versus control group was employed for LDH release, LC3 conversion, cytoadherence assays and qPCR assays. For in vivo mortality, Kaplan–Meier curves were plotted and statistical significance between strains was determined using the log-rank test. For all tests, P = 0.05 was selected for determining significance.
Results
ExsE is not required for T3SS1 synthesis or secretion
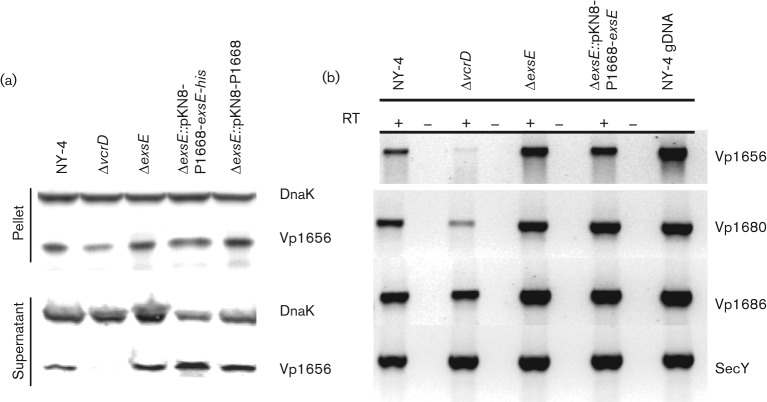
Vp1656 (a putative PopD orthologue) is synthesized in an ExsA-dependent manner and is secreted in a T3SS1-dependent manner (Ono et al., 2006; Zhou et al., 2008). Thus, Vp1656 serves as a simple marker for T3SS1-dependent secretion. We infected HeLa cell monolayers with wild-type NY-4, ΔvcrD, ΔexsE, ΔexsE : : pKN8-P1668-exsE and ΔexsE : : pKN8-P1668 strains for 4 h before collecting whole-cell lysates and cell-free culture supernatants. All strains synthesized Vp1656 (Fig. 1a, pellet) and only the T3SS1-deficient ΔvcrD strain failed to secrete Vp1656 (Fig. 1a, supernatant). We also performed RT-PCR for three T3SS1 genes (vp1656, vp1680 and vp1686) and a housekeeping gene (secY) following HeLa cell infection and found that transcripts were produced in all strains (Fig. 1b) although with a potential reduction in transcript levels for the ΔvcrD strain. Consequently, deletion of exsE has no apparent impact on the synthesis of T3SS1 proteins or the T3SS1-dependent secretion of Vp1656 when V. parahaemolyticus is co-cultured with HeLa cells.
Fig. 1.
Deletion of exsE does not negatively affect synthesis or secretion of Vp1656. (a) The indicated strains were used to infect HeLa cells cultured in DMEM supplemented with 10 % (v/v) FBS at an m.o.i. of 100 for 4 h followed by centrifugation to separate cell pellet and supernatant fractions. Cell-associated and secreted proteins obtained from whole-cell lysate and trichloroacetic acid-precipitated cell-free supernatant, respectively, were separated using 4–20 % SDS-PAGE, transferred to PVDF and probed using anti-Vp1656 and anti-DnaK antibodies. DnaK was used as a loading control. This experiment was replicated three times and a representative blot is shown here. (b) RT-PCR was used to detect mRNA transcripts for a subset of T3SS1 genes after infection. RT+ reactions included reverse transcriptase; RT− reactions contained no reverse transcriptase. The latter indicated no evidence for contaminating DNA in the reaction.
ExsE is required for in vitro cytotoxicity
Epithelial cells infected with wild-type NY-4 undergo rapid cytolysis in vitro through a T3SS1-dependent mechanism that includes rounding and lysis (Burdette et al., 2008; Zhou et al., 2009). Based on the secretion and RT-PCR results (see above), we hypothesized that HeLa cells infected with ΔexsE would undergo cytolysis equivalent to the wild-type strain (rounding followed by lysis). Unexpectedly, the HeLa cell monolayers infected with the ΔexsE strain remained visibly unaffected (no rounding) up to 8 h post-infection (data not shown). LDH release after 4 h of infection was measured using NY-4 and ΔvcrD as positive and negative controls, respectively. LDH release from ΔexsE infected cells was reduced significantly compared to NY-4 and the phenotype was recovered by in cis complementation while the negative insertion control (ΔexsE : : pKN8-P1668) did not induce cytolysis (Fig. 2). Consequently, ExsE appears to be requisite for T3SS1-dependent cell lysis.
Fig. 2.
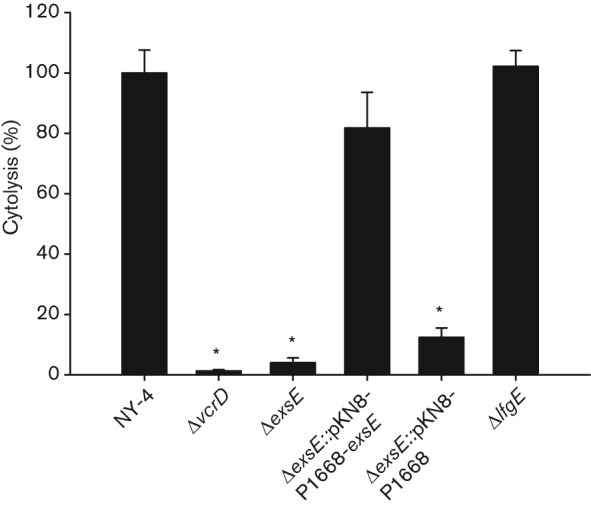
Deletion of exsE results in loss of HeLa cell cytotoxicity. The indicated strains were used to infect HeLa cells cultured in DMEM supplemented with 1 % FBS (v/v) at an m.o.i. of 100 for 4 h and assayed for T3SS1-dependent cytotoxicity. Percentage cytotoxicity was calculated based on the release of LDH relative to the uninfected control (0 %) and wild-type NY-4 strain (100 %). The reported values represent the mean±sem for three independent replicates. Asterisks, statistically significant difference in mean value compared with wild-type NY-4 by one-way ANOVA (P<0.05).
ExsE is required for induction of host cell autophagy
Our results show that deletion of exsE results in unimpaired T3SS1 secretion (Fig. 1), but loss of T3SS1-dependent host cell cytolysis (Fig. 2). Consequently, we hypothesized that deletion of exsE results in a loss of effector protein translocation. To test this, we measured the induction of autophagy for infected HeLa cells. Conversion of cytosolic LC3-I to vesicle-bound LC3-II can be used to measure induction of autophagy (Kabeya et al., 2000) and this assay has been used to assess induction of autophagy by V. parahaemolyticus (Burdette et al., 2008; Zhou et al., 2010b). Furthermore, VopQ (Vp1680) is necessary and sufficient to induce autophagy in HeLa cells infected with V. parahaemolyticus and VopQ is translocated in a T3SS1-dependent manner (Burdette et al., 2009). Thus, loss of autophagy would indicate probable failure to translocate VopQ. For this experiment, NY-4 served as a positive control while ΔvcrD and uninfected cells served as negative controls. Deletion of exsE abrogated LC3-I to LC3-II conversion to a level equivalent to a T3SS1 knockout (ΔvcrD, Fig. 3). Complementation of exsE recovered the autophagy phenotype, indicating that ExsE is necessary for successful translocation of VopQ.
Fig. 3.
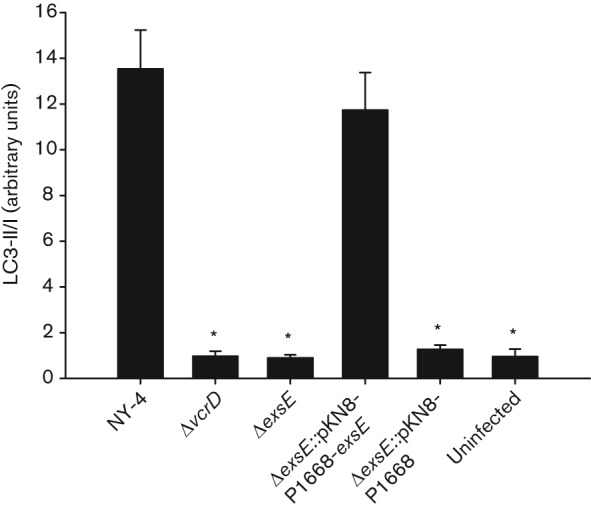
Deletion of exsE results in loss of LC3 conversion. The indicated strains were used to infect HeLa cell monolayers at an m.o.i. of 100. Whole-cell lysates were separated using 4–20 % SDS-PAGE, transferred to a membrane and probed using anti-LC3 and anti-actin antibody. Densitometry was used to determine relative LC3-II accumulation and to calculate the LC3-II/I ratio. The reported values represent the mean±sem for three independent experiments. Asterisks, statistically significant difference in mean value compared with wild-type NY-4 by one-way ANOVA (P<0.05).
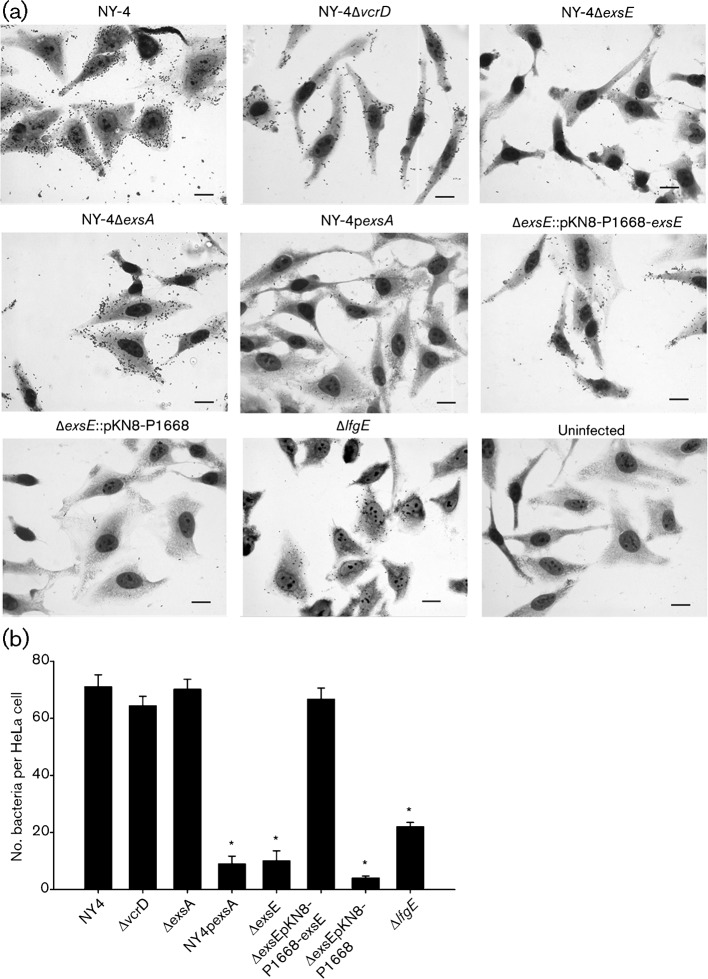
ExsE is required for adhesion to HeLa cells and swarming motility
Loss of translocation could result from a defective translocon apparatus or from some other change in requisite cell–cell interaction. We tested for changes in host cell adhesion (Fig. 4a) and found that ΔexsE exhibits a significant reduction in cytoadherence compared with NY-4 (Fig. 4b). This phenotype is recovered by in cis complementation indicating that ExsE is required for V. parahaemolyticus adherence to HeLa cells.
Fig. 4.
Deletion of exsE results in loss of adhesion. The indicated strains were used to infect HeLa cell monolayers for 30 min. Slides were washed to remove non-adherent bacteria and stained using Diff-Quik. (a) Representative fields for each strain are shown here (63×). Bar, 20 µm. (b) Attached bacteria were enumerated by direct count and the reported values represent the mean±sem for three biological replicates. Asterisks, statistically significant difference in mean value compared with wild-type NY-4 by one-way ANOVA (P<0.05).
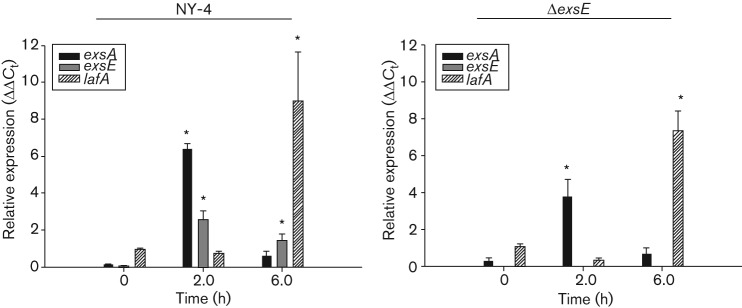
Lateral flagella plays a role in adherence to HeLa cells and these structures are required for a swarming phenotype (Park et al., 2005; Shinoda & Okamoto, 1977). Consequently, we hypothesized that deletion of exsE results in a loss of lateral flagella synthesis and subsequent swarming and adhesion phenotypes due to unrestricted ExsA activity (Gode-Potratz et al., 2010). To test this hypothesis we first measured swarming on semi solid agar media and found that deletion of exsE results in the loss of a swarming phenotype (Fig. 5) consistent with a loss of functional lateral flagella in the ΔexsE strain. Next we tested the hypothesis that the loss of functional lateral flagella could be responsible for lost adhesion by the ΔexsE strain (Fig. 4) using qPCR to measure levels of exsA, exsE and lafA transcripts relative to the control transcript secY. Time points of 0, 2 and 6 h post-infection were chosen to reflect pre-infection, peak infection (>90 % cell rounding and/or cytolysis) and post-infection (100 % cytolysis) periods. Our results show that stationary phase bacteria cultured in LBS broth have relatively low level exsA, exsE and lafA gene transcripts in both the wild-type and ΔexsE prior to infection (t = 0). Both strains show a statistically significant increase in exsA and exsE transcript at 2 h followed by a reduction at 6 h. As expected, wild-type lafA transcript was relatively low at 0 h and then increased significantly at 6 h, consistent with expression of lafA after contact with a solid surface. Surprisingly, this pattern was also observed in the ΔexsE mutant that lacked a swarming phenotype after 8 h (Fig. 5). Visualization of individual ΔexsE cells using transmission electron microscopy (TEM) confirmed the loss of lateral flagella synthesis (Fig. S2). In addition, a lateral flagella-deficient mutant (ΔlfgE) was negative for swarming (data not shown) and deficient in lateral flagella (Fig. S2). This mutant also had reduced cytoadhesion compared with NY-4 (Fig. 4), although not as reduced as the ΔexsE strain (P = 0.005). Despite the reduced level of cytoadhesion, the ΔlfgE strain was still cytotoxic (Fig. 2). These data are consistent with (1) lateral flagella playing a role in cytoadhesion, (2) a potential role for ExsE in lateral flagella biogenesis and (3) only partial cytoadherence being necessary for host-cell intoxication.
Fig. 5.
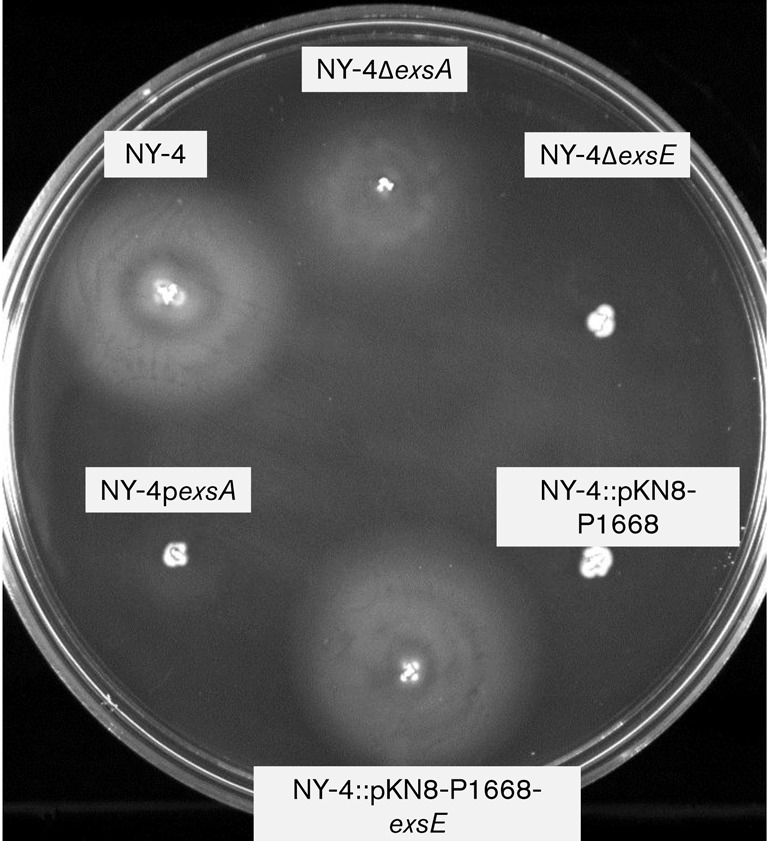
Deletion of exsE results in a non-swarming phenotype. Swarm agar was inoculated with the indicated strains and incubated at 37 °C for 8 h. In cis complementation of exsE recovered the swarming phenotype. The assay was repeated three times with similar results and a representative photograph is shown here.
Deletion of exsE does not negatively affect pathogenicity in vivo
Based on our in vitro cytolysis (Fig. 2) and cytoadherence results (Fig. 4), we hypothesized that our deletion mutant would be attenuated for an intrapulmonary infection model (Piñeyro et al., 2010). Our results show no reduction in mortality compared with the wild-type strain, indicating that loss of in vitro cytoadhesion does not prevent T3SS1-dependent mortality in a mouse-challenge model (Fig. 6).
Fig. 6.
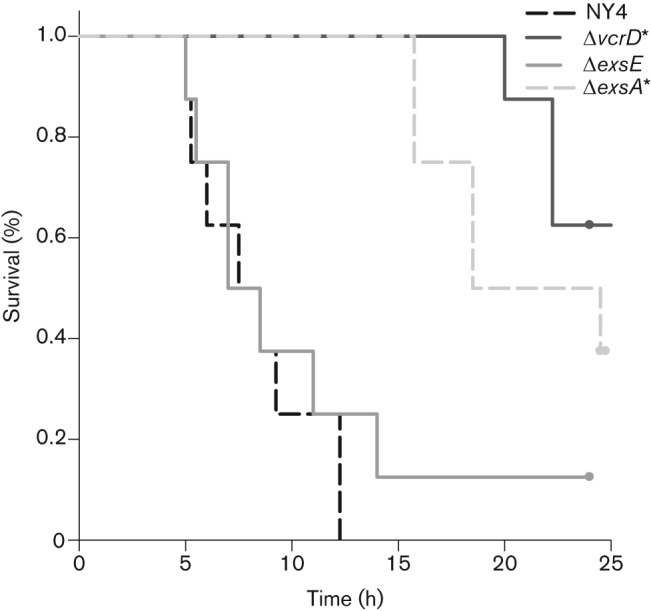
Deletion of exsE does not affect in vivo lethality. Indicated strains were used to infect C57-B6 mice as described by Piñeyro et al. (2010). Mice were observed over the course of 24 h and mortality was recorded. Kaplan–Meier survival plots for all strains are shown here; asterisks represent values with a statistically significant difference in mortality compared with wild-type NY-4 by log-rank analysis.
Discussion
Many T3SSs use a regulatory cascade involving a T3SS protein at the top of the cascade leading to the activation of an AraC-like transcriptional activator at the bottom. Examples of this coupled secretion model include members of the Ysc family of T3SSs found in P. aeruginosa, Yersinia and Aeromonas. In P. aeruginosa, ExsE indirectly regulates expression of T3SS proteins by binding ExsC, allowing ExsD to bind ExsA and prevent transcription of T3SS genes. In silico evaluation of the V. parahaemolyticus O3 : K6 genome revealed probable orthologues for exsA, exsC and exsD based on sequence identity (40, 34 and 30 %, respectively). Zhou et al. (2010a, 2008) verified that exsA, exsC and exsD are functional orthologues of their P. aeruginosa counterparts. No exsE homologue has been identified by sequence similarity.
ExsE is a small (81 aa) and highly charged protein that is secreted in a T3SS-dependent manner (Rietsch et al., 2005; Urbanowski et al., 2007). For P. aeruginosa, the protein is translocated into the host cell but has no defined function once inside the cell, suggesting that its major function involves regulation of T3SS expression through interaction with ExsC. Deletion of this gene leads to constitutive expression of the T3SS operon in P. aeruginosa and subsequent T3SS-dependent cytopathic effect when co-cultured with A549 cells (Rietsch et al., 2005). Expression of an exsE allele resulting in an ExsE secretion and translocation-deficient mutant showed significant reduction in T3SS-dependent cytotoxicity and loss of T3SS gene expression when exposed to host cells (Urbanowski et al., 2007). Based on synteny of T3SS genes found in P. aeruginosa and V. parahaemolyticus T3SSs, we and others (Kodama et al., 2010) hypothesized that vp1702 encodes the functional orthologue of ExsE. vp1702 is similar to P. aeruginosa exsE in that it encodes a small (103 aa, 11.6 kDa), highly charged protein and the gene sequence is located immediately downstream of vp1701 (exsC), suggesting conserved synteny with P. aeruginosa.
We created the vp1702-deletion mutant by gene knockout and found constitutive T3SS1 protein synthesis and secretion in broth culture (Fig. S3) similar to findings by Kodama et al. (2010), and this is analogous to the function of ExsE in P. aeruginosa. Overexpression of ExsE in wild-type NY-4 using a plasmid-based expression vector repressed T3SS1 upregulation, which is also consistent with the role of ExsE as a negative regulator of T3SS1 (Fig. S1). It was therefore unexpected to find that the V. parahaemolyticus ΔexsE strain was not cytotoxic compared with the highly cytotoxic wild-type strain (Fig. 2).
Two important V. parahaemolyticus effector proteins have been described, VopQ and VopS, that are responsible for inducing autophagy, activating MAPK signalling pathways, and actin destabilization in epithelial cells (Broberg et al., 2010; Burdette et al., 2009; Casselli et al., 2008; Matlawska-Wasowska et al., 2010; Yarbrough et al., 2009). Autophagy and cytoskeleton destabilization phenotypes are readily observed through Western blot analysis that shows conversion of LC3-I to LC3-II, or by visualization of cell rounding, respectively. Both effectors require T3SS1-dependent translocation to induce these phenotypes and therefore they are ideal markers for measuring T3SS1-dependent translocation. Deletion of exsE does not affect T3SS1-dependent secretion of our marker protein Vp1656 (Fig. 1a), and transcription of vopQ and vopS appears to be unaffected (Fig. 1b). Nevertheless, deletion of exsE eliminates cell lysis (Fig. 2), cell rounding (data not shown) and LC3 conversion (Fig. 3), indicating that ExsE is required for translocation of V. parahaemolyticus effector proteins.
There are several possibilities for why we see normal T3SS1 induction during HeLa cell infection (Fig. 1) but apparent loss of effector translocation. Firstly, ExsE may be a structural component of the T3SS1 needle or translocon. Presumably, only a minimally formed needle structure is sufficient to secrete proteins into the extracellular milieu. V-tip proteins are found in the Ysc family of T3SSs (reviewed by Sato & Frank, 2011) but there is no apparent sequence homology with Vp1702. Another possibility is that ExsE may act as a chaperone required for effective stabilization of an unidentified protein needed for translocon functionality, but we have no evidence for this role. VopQ is necessary and sufficient to induce autophagy (Broberg et al., 2010; Burdette et al., 2009) and thus it is unlikely that ExsE is functionally partnered with this protein. In any case, the basal body and at least a portion of the needle complex are apparently assembled, as shown by the secretion of Vp1656 into cell culture supernatant. This leaves the possibility that ExsE impacts other factors that are required for successful HeLa-cell cytolysis.
Synthesis of lateral flagella, encoded by the laf regulon in V. parahaemolyticus, is associated with swarming motility, adherence and cytotoxicity (Belas & Colwell, 1982a; Belas & Colwell, 1982b; Gode-Potratz et al., 2011; McCarter, 2004; Park et al., 2005). Cross-talk between bacterial T3SSs and flagellar systems is a common phenomenon (Bleves et al., 2002; Diaz et al., 2011; Eichelberg & Galán, 2000; Iyoda et al., 2006; Soscia et al., 2007). In trans expression of ExsA in V. parahaemolyticus reduces laf gene expression and swarming motility through an undefined mechanism (Gode-Potratz et al., 2010) but does not negatively affect cytolysis (Zhou et al., 2008). Thus the reduction in swarming (Fig. 5) and adhesion observed with ΔexsE in this study (Fig. 4) may simply be a result of unmitigated basal level ExsA activity leading to suppression of the laf regulon. We tested this hypothesis using the NY-4 : pexsA strain and found reduced swarming motility (Fig. 5) and cytoadhesion (Fig. 4) as expected. Deletion of exsA, however, had no effect on either cytoadhesion or swarming phenotypes (Figs 4 and 5, respectively). It is interesting that our NY-4 : pexsA strain also retains wild-type level cytolysis despite a significant decrease in cytoadhesion. We speculate that this is due to the artificially high level of ExsA in NY-4 : pexsA prior to infection, which probably results in an abnormal abundance of assembled and functional T3SS1 components (including both structural and effector proteins) such that only minimal cell–cell contact is necessary for successful translocation of effector proteins. This is in contrast with the wild-type and ΔexsE strains that require intimate host cell contact to both upregulate exsA expression (Fig. 7) and subsequently translocate effectors to the host cell.
Fig. 7.
Deletion of exsE does not affect relative transcription levels of exsA or lafA. HeLa cell monolayers were infected with either NY-4 or ΔexsE at an m.o.i. of 100. Total RNA was extracted and cDNA was synthesized for qPCR analysis as described in the main text. Reported values represent the mean±sem for relative expression level of four biological replicates at each time point. Asterisks, statistically significant difference in mean value compared with t = 0 h by one-way ANOVA.
Next, we tested a lateral flagella-deficient mutant and found that cytoadhesion was reduced compared with NY-4, yet was significantly higher than ΔexsE (Fig. 4, P = 0.005). Cytolysis was unaffected with ΔlfgE compared with NY-4, suggesting that the number of adherent bacteria, while reduced compared with the wild-type strain, was sufficient to intoxicate HeLa cells (Fig. 2). Nevertheless, while transcription of lafA was unaffected by deletion of exsE (Fig. 7), neither lateral nor polar flagella were observed by TEM (Fig. S2). We did find, however, that lafA was highly upregulated at 6 h for both wild-type and ΔexsE (Fig. 7), which is after the point when most, if not all, HeLa cells have been lysed by V. parahaemolyticus (Fig. 2). Thus, lafA appears to be upregulated post-infection. This is consistent with data from Gode-Potratz et al. (2010) who hypothesized that increased ExsA expression during host cell intoxication is coordinated with reduced swarming motility. After infection, swarming motility would be induced, presumably, to assist dispersal of V. parahaemolyticus. Deletion of exsE still prevents swarming with the agar plate assay and there were no observable lateral flagella, although this could be related to overexpression of ExsA in this assay [we did not measure mRNA levels for bacteria grown on agar, which enhances T3SS1 gene expression (Gode-Potratz et al., 2011)]. The actual mechanism of how lateral flagella biogenesis is affected by ExsA remains unclear, though our results suggest it may occur post-translationally or at the protein level. Importantly, deletion of exsE dramatically impacts T3SS1-dependent cytolysis and this is consistent with a loss of adhesion (Fig. 4b), likely due in part to the loss of lateral flagella and possibly from loss of the polar flagella (Fig. S2). Additional adhesins might be affected by ExsE [e.g. MAM7 (Krachler & Orth, 2011)] or it is possible that ExsE itself must be translocated into the host cell to affect secure adhesion and subsequent translocation of effector proteins.
Kodama et al. (2010) reported that vp1702 may encode a functional orthologue of P. aeruginosa ExsE and they showed that Vp1702 acts in a similar regulatory capacity for T3SS1 in V. parahaemolyticus as it does in P. aeruginosa. Kodama et al. (2010) measured synthesis and secretion using broth culture consisting of LBS with and without the addition of 5 mM CaCl2, but they did not examine the effect of host cell co-culture and contact. In contrast, we found that while growth under high-calcium conditions (5 mM) results in an apparent decrease in T3SS1 synthesis, it does not block host cell cytolysis (Fig. S4). Zhou et al. (2008) showed previously that growth in LBS alone does not induce T3SS1 gene expression and we found the same results for this study (Figs 7 and S3). The difference between the current study and that of Kodama et al. may be due to use of different strains of V. parahaemolyticus, or due to reliance on LBS to test the induction of T3SS1; Luria Broth is a complex and undefined media that probably introduces a variety of confounding variables that vary between batches and manufacturers (Pavankumar et al., 2012). Observations from the current study support findings with P. aeruginosa where treatment of A549 epithelial cells with calcimycin was used to increase intracellular calcium concentration, but this had no effect on expression of exoS, suggesting that cytoplasmic calcium concentrations may not play a significant role in effector induction (Cisz et al., 2008). Alternatively, physical contact is necessary and sufficient to enhance expression of T3SS genes subsequent to cytotoxicity for both V. parahaemolyticus and P. aeruginosa (Gode-Potratz et al., 2011; Vallis et al., 1999). Unlike the LB broth model, host cell co-culture and contact appears to be an unambiguous induction model for T3SS synthesis and function.
The data presented herein provide further characterization of the putative ExsE of V. parahaemolyticus. Importantly, besides acting as a functional orthologue to ExsE in P. aeruginosa, we demonstrate that the putative ExsE from V. parahaemolyticus is a T3SS1-associated protein necessary for regulating cytoadhesion that is apparently required for the translocation of effector proteins and subsequent T3SS1-dependent cytolysis in vitro. To our knowledge, this is the first report of an association between the T3SS1-regulatory protein ExsE and a bacterial cytoadhesion phenotype. In addition, we report here for the first time expression kinetics for genes encoding the T3SS1 regulatory proteins ExsA and ExsE that support the coupled-secretory induction model. These data also suggest the presence of a host cell contact-dependent signal for upregulation of exsA expression that is independent of the ExsACDE pathway, and this signal has yet to be identified.
Acknowledgements
We gratefully acknowledge technical assistance and discussions with Lisa Orfe, Jim Deringer and Christine Davitt from Washington State University. This project was supported in part by the National Institutes of Health, Department of Health and Human Services under the contract number NO1-AI-30055, and by the Agricultural Animal Health Program, College of Veterinary Medicine, Washington State University, and by the Agricultural Research Center, Washington State University.
Abbreviations:
- LDH
lactate dehydrogenase
- qPCR
quantitative RT-PCR
- TEM
transmission electron microscopy
- T3SS
type III secretion system
Footnotes
Four supplementary figures are available with the online version of this paper.
References
- Belas M. R., Colwell R. R. (1982a). Adsorption kinetics of laterally and polarly flagellated Vibrio. J Bacteriol 151, 1568–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas M. R., Colwell R. R. (1982b). Scanning electron microscope observation of the swarming phenomenon of Vibrio parahaemolyticus. J Bacteriol 150, 956–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleves S., Marenne M. N., Detry G., Cornelis G. R. (2002). Up-regulation of the Yersinia enterocolitica yop regulon by deletion of the flagellum master operon flhDC. J Bacteriol 184, 3214–3223. 10.1128/JB.184.12.3214-3223.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg C. A., Zhang L., Gonzalez H., Laskowski-Arce M. A., Orth K. (2010). A Vibrio effector protein is an inositol phosphatase and disrupts host cell membrane integrity. Science 329, 1660–1662. 10.1126/science.1192850 [DOI] [PubMed] [Google Scholar]
- Burdette D. L., Yarbrough M. L., Orvedahl A., Gilpin C. J., Orth K. (2008). Vibrio parahaemolyticus orchestrates a multifaceted host cell infection by induction of autophagy, cell rounding, and then cell lysis. Proc Natl Acad Sci U S A 105, 12497–12502. 10.1073/pnas.0802773105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette D. L., Seemann J., Orth K. (2009). Vibrio VopQ induces PI3-kinase-independent autophagy and antagonizes phagocytosis. Mol Microbiol 73, 639–649. 10.1111/j.1365-2958.2009.06798.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casselli T., Lynch T., Southward C. M., Jones B. W., DeVinney R. (2008). Vibrio parahaemolyticus inhibition of Rho family GTPase activation requires a functional chromosome I type III secretion system. Infect Immun 76, 2202–2211. 10.1128/IAI.01704-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisz M., Lee P. C., Rietsch A. (2008). ExoS controls the cell contact-mediated switch to effector secretion in Pseudomonas aeruginosa. J Bacteriol 190, 2726–2738. 10.1128/JB.01553-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn B., Sekirov I., Finlay B. B. (2007). Type III secretion systems and disease. Clin Microbiol Rev 20, 535–549. 10.1128/CMR.00013-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels N. A., MacKinnon L., Bishop R., Altekruse S., Ray B., Hammond R. M., Thompson S., Wilson S., Bean N. H. & other authors (2000). Vibrio parahaemolyticus infections in the United States, 1973–1998. J Infect Dis 181, 1661–1666. 10.1086/315459 [DOI] [PubMed] [Google Scholar]
- Dasgupta N., Lykken G. L., Wolfgang M. C., Yahr T. L. (2004). A novel anti-anti-activator mechanism regulates expression of the Pseudomonas aeruginosa type III secretion system. Mol Microbiol 53, 297–308. 10.1111/j.1365-2958.2004.04128.x [DOI] [PubMed] [Google Scholar]
- Diaz M. R., King J. M., Yahr T. L. (2011). Intrinsic and extrinsic regulation of type III secretion gene expression in Pseudomonas aeruginosa. Front Microbiol 2, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelberg K., Galán J. E. (2000). The flagellar sigma factor FliA (sigma28) regulates the expression of Salmonella genes associated with the centisome 63 type III secretion system. Infect Immun 68, 2735–2743. 10.1128/IAI.68.5.2735-2743.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fälker S., Schmidt M. A., Heusipp G. (2005). DNA methylation in Yersinia enterocolitica: role of the DNA adenine methyltransferase in mismatch repair and regulation of virulence factors. Microbiology 151, 2291–2299. 10.1099/mic.0.27946-0 [DOI] [PubMed] [Google Scholar]
- Frank D. W. (1997). The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol 26, 621–629. 10.1046/j.1365-2958.1997.6251991.x [DOI] [PubMed] [Google Scholar]
- Gode-Potratz C. J., Chodur D. M., McCarter L. L. (2010). Calcium and iron regulate swarming and type III secretion in Vibrio parahaemolyticus. J Bacteriol 192, 6025–6038. 10.1128/JB.00654-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gode-Potratz C. J., Kustusch R. J., Breheny P. J., Weiss D. S., McCarter L. L. (2011). Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol Microbiol 79, 240–263. 10.1111/j.1365-2958.2010.07445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyoshi H., Kodama T., Iida T., Honda T. (2010). Contribution of Vibrio parahaemolyticus virulence factors to cytotoxicity, enterotoxicity, and lethality in mice. Infect Immun 78, 1772–1780. 10.1128/IAI.01051-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlady W. G., Klontz K. C. (1996). The epidemiology of Vibrio infections in Florida, 1981–1993. J Infect Dis 173, 1176–1183. 10.1093/infdis/173.5.1176 [DOI] [PubMed] [Google Scholar]
- Hornef M. W., Roggenkamp A., Geiger A. M., Hogardt M., Jacobi C. A., Heesemann J. (2000). Triggering the ExoS regulon of Pseudomonas aeruginosa: A GFP-reporter analysis of exoenzyme (Exo)S, ExoT and ExoU synthesis. Microb Pathog 29, 329–343. 10.1006/mpat.2000.0398 [DOI] [PubMed] [Google Scholar]
- Hovey A. K., Frank D. W. (1995). Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol 177, 4427–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyoda S., Koizumi N., Satou H., Lu Y., Saitoh T., Ohnishi M., Watanabe H. (2006). The GrlR–GrlA regulatory system coordinately controls the expression of flagellar and LEE-encoded type III protein secretion systems in enterohemorrhagic Escherichia coli. J Bacteriol 188, 5682–5692. 10.1128/JB.00352-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. (2000). LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19, 5720–5728. 10.1093/emboj/19.21.5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Yamazaki C., Park K. S., Akeda Y., Iida T., Honda T. (2010). Transcription of Vibrio parahaemolyticus T3SS1 genes is regulated by a dual regulation system consisting of the ExsACDE regulatory cascade and H-NS. FEMS Microbiol Lett 311, 10–17. 10.1111/j.1574-6968.2010.02066.x [DOI] [PubMed] [Google Scholar]
- Krachler A. M., Orth K. (2011). Functional characterization of the interaction between bacterial adhesin multivalent adhesion molecule 7 (MAM7) protein and its host cell ligands. J Biol Chem 286, 38939–38947. 10.1074/jbc.M111.291377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino K., Oshima K., Kurokawa K., Yokoyama K., Uda T., Tagomori K., Iijima Y., Najima M., Nakano M. & other authors (2003). Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361, 743–749. 10.1016/S0140-6736(03)12659-1 [DOI] [PubMed] [Google Scholar]
- Matlawska-Wasowska K., Finn R., Mustel A., O’Byrne C. P., Baird A. W., Coffey E. T., Boyd A. (2010). The Vibrio parahaemolyticus Type III Secretion Systems manipulate host cell MAPK for critical steps in pathogenesis. BMC Microbiol 10, 329. 10.1186/1471-2180-10-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L. L. (2004). Dual flagellar systems enable motility under different circumstances. J Mol Microbiol Biotechnol 7, 18–29. 10.1159/000077866 [DOI] [PubMed] [Google Scholar]
- McCaw M. L., Lykken G. L., Singh P. K., Yahr T. L. (2002). ExsD is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol Microbiol 46, 1123–1133. 10.1046/j.1365-2958.2002.03228.x [DOI] [PubMed] [Google Scholar]
- Mertens A., Nagler J., Hansen W., Gepts-Friedenreich E. (1979). Halophilic, lactose-positive Vibrio in a case of fatal septicemia. J Clin Microbiol 9, 233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels T., Wattiau P., Brasseur R., Ruysschaert J. M., Cornelis G. (1990). Secretion of Yop proteins by Yersiniae. Infect Immun 58, 2840–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton D. L., Norqvist A., Wolf-Watz H. (1992). Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J Bacteriol 174, 7235–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton D. L., O’Toole R., Horstedt P., Wolf-Watz H. (1996). Flagellin A is essential for the virulence of Vibrio anguillarum. J Bacteriol 178, 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales V. M., Bäckman A., Bagdasarian M. (1991). A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97, 39–47. 10.1016/0378-1119(91)90007-X [DOI] [PubMed] [Google Scholar]
- Morris J. G., Jr, Black R. E. (1985). Cholera and other vibrioses in the United States. N Engl J Med 312, 343–350. 10.1056/NEJM198502073120604 [DOI] [PubMed] [Google Scholar]
- Nishibuchi M., Fasano A., Russell R. G., Kaper J. B. (1992). Enterotoxigenicity of Vibrio parahaemolyticus with and without genes encoding thermostable direct hemolysin. Infect Immun 60, 3539–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu C., Graves J. D., Mokuolu F. O., Gilbert S. E., Gilbert E. S. (2005). Enhanced swarming of bacteria on agar plates containing the surfactant Tween 80. J Microbiol Methods 62, 129–132. 10.1016/j.mimet.2005.01.013 [DOI] [PubMed] [Google Scholar]
- Ono T., Park K. S., Ueta M., Iida T., Honda T. (2006). Identification of proteins secreted via Vibrio parahaemolyticus type III secretion system 1. Infect Immun 74, 1032–1042. 10.1128/IAI.74.2.1032-1042.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. S., Ono T., Rokuda M., Jang M. H., Okada K., Iida T., Honda T. (2004). Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect Immun 72, 6659–6665. 10.1128/IAI.72.11.6659-6665.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. S., Arita M., Iida T., Honda T. (2005). vpaH, a gene encoding a novel histone-like nucleoid structure-like protein that was possibly horizontally acquired, regulates the biogenesis of lateral flagella in trh-positive Vibrio parahaemolyticus TH3996. Infect Immun 73, 5754–5761. 10.1128/IAI.73.9.5754-5761.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavankumar A. R., Ayyappasamy S. P., Sankaran K. (2012). Small RNA fragments in complex culture media cause alterations in protein profiles of three species of bacteria. Biotechniques 52, 167–172. [DOI] [PubMed] [Google Scholar]
- Piñeyro P., Zhou X., Orfe L. H., Friel P. J., Lahmers K., Call D. R. (2010). Development of two animal models to study the function of Vibrio parahaemolyticus type III secretion systems. Infect Immun 78, 4551–4559. 10.1128/IAI.00461-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri F., Alam M. S., Nishibuchi M., Rahman T., Alam N. H., Chisti J., Kondo S., Sugiyama J., Bhuiyan N. A. & other authors (2003). Adaptive and inflammatory immune responses in patients infected with strains of Vibrio parahaemolyticus. J Infect Dis 187, 1085–1096. 10.1086/368257 [DOI] [PubMed] [Google Scholar]
- Rietsch A., Vallet-Gely I., Dove S. L., Mekalanos J. J. (2005). ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 102, 8006–8011. 10.1073/pnas.0503005102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan W. J. (1976). Marine vibrios associated with superficial septic lesions. J Clin Pathol 29, 1014–1015. 10.1136/jcp.29.11.1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond G. P., Reeves P. J. (1993). Membrane traffic wardens and protein secretion in gram-negative bacteria. Trends Biochem Sci 18, 7–12. 10.1016/0968-0004(93)90080-7 [DOI] [PubMed] [Google Scholar]
- Sato H., Frank D. W. (2011). Multi-functional characteristics of the Pseudomonas aeruginosa type III needle-tip protein, PcrV; comparison to orthologs in other Gram-negative bacteria. Front Microbiol 2, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda S., Okamoto K. (1977). Formation and function of Vibrio parahaemolyticus lateral flagella. J Bacteriol 129, 1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soscia C., Hachani A., Bernadac A., Filloux A., Bleves S. (2007). Cross talk between type III secretion and flagellar assembly systems in Pseudomonas aeruginosa. J Bacteriol 189, 3124–3132. 10.1128/JB.01677-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tison D. L., Kelly M. T. (1984). Vibrio species of medical importance. Diagn Microbiol Infect Dis 2, 263–276. 10.1016/0732-8893(84)90057-9 [DOI] [PubMed] [Google Scholar]
- Troisfontaines P., Cornelis G. R. (2005). Type III secretion: more systems than you think. Physiology (Bethesda) 20, 326–339. 10.1152/physiol.00011.2005 [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Brutinel E. D., Yahr T. L. (2007). Translocation of ExsE into Chinese hamster ovary cells is required for transcriptional induction of the Pseudomonas aeruginosa type III secretion system. Infect Immun 75, 4432–4439. 10.1128/IAI.00664-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallis A. J., Yahr T. L., Barbieri J. T., Frank D. W. (1999). Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect Immun 67, 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Yamamoto K., Honda T., Xu M. (1994). Construction and characterization of an isogenic mutant of Vibrio parahaemolyticus having a deletion in the thermostable direct hemolysin-related hemolysin gene (trh). J Bacteriol 176, 4757–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr T. L., Wolfgang M. C. (2006). Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol Microbiol 62, 631–640. 10.1111/j.1365-2958.2006.05412.x [DOI] [PubMed] [Google Scholar]
- Yarbrough M. L., Li Y., Kinch L. N., Grishin N. V., Ball H. L., Orth K. (2009). AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science 323, 269–272. 10.1126/science.1166382 [DOI] [PubMed] [Google Scholar]
- Zhou X., Shah D. H., Konkel M. E., Call D. R. (2008). Type III secretion system 1 genes in Vibrio parahaemolyticus are positively regulated by ExsA and negatively regulated by ExsD. Mol Microbiol 69, 747–764. 10.1111/j.1365-2958.2008.06326.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Konkel M. E., Call D. R. (2009). Type III secretion system 1 of Vibrio parahaemolyticus induces oncosis in both epithelial and monocytic cell lines. Microbiology 155, 837–851. 10.1099/mic.0.024919-0 [DOI] [PubMed] [Google Scholar]
- Zhou X., Konkel M. E., Call D. R. (2010a). Regulation of type III secretion system 1 gene expression in Vibrio parahaemolyticus is dependent on interactions between ExsA, ExsC, and ExsD. Virulence 1, 260–272. 10.4161/viru.1.4.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Konkel M. E., Call D. R. (2010b). Vp1659 is a Vibrio parahaemolyticus type III secretion system 1 protein that contributes to translocation of effector proteins needed to induce cytolysis, autophagy, and disruption of actin structure in HeLa cells. J Bacteriol 192, 3491–3502. 10.1128/JB.01493-09 [DOI] [PMC free article] [PubMed] [Google Scholar]