Abstract
Human illness due to Camplyobacter jejuni infection is closely associated with consumption of poultry products. We previously demonstrated a 50 % shift in allele frequency (phase variation) in contingency gene Cj1139 (wlaN) during passage of C. jejuni NCTC11168 populations through Ross 308 broiler chickens. We hypothesized that phase variation in contingency genes during chicken passage could promote subsequent colonization and disease in humans. To test this hypothesis, we passaged C. jejuni strains NCTC11168, 33292, 81-176, KanR4 and CamR2 through broiler chickens and analysed the ability of passaged and non-passaged populations to colonize C57BL6 IL-10-deficient mice, our model for human colonization and disease. We utilized fragment analysis and nucleotide sequence analysis to measure phase variation in contingency genes. Passage through the chicken reservoir promoted phase variation in five specific contingency genes, and these ‘successful’ populations colonized mice. When phase variation did not occur in these same five contingency genes during chicken passage, these ‘unsuccessful’ populations failed to colonize mice. Phase variation during chicken passage generated small insertions or deletions (indels) in the homopolymeric tract (HT) in contingency genes. Single-colony isolates of C. jejuni strain KanR4 carrying an allele of contingency gene Cj0170 with a10G HT colonized mice at high frequency and caused disease symptoms, whereas single-colony isolates carrying the 9G allele failed to colonize mice. Supporting results were observed for the successful 9G allele of Cj0045 in strain 33292. These data suggest that phase variation in Cj0170 and Cj0045 is strongly associated with mouse colonization and disease, and that the chicken reservoir can play an active role in natural selection, phase variation and disease.
Introduction
Campylobacter jejuni and Salmonella are currently ranked the most important bacterial foodborne pathogens in the USA (Scallan et al., 2011). C. jejuni inhabits the gastrointestinal (GI) tract of poultry, cattle, sheep and swine (Blaser, 1997), and is most frequently transmitted to humans through carcass contamination that occurs during slaughter of meat animals (Jonsson et al., 2010). Poultry is the most important reservoir for human disease (Zhao et al., 2010; reviewed by Wassenaar, 2011). C. jejuni infection reportedly results in 850 000 cases of gastroenteritis and 76 deaths per year, which cost approximately $1.7 billion in the USA (Batz et al., 2011; Scallan et al., 2011; Snelling et al., 2005; Young et al., 2007). C. jejuni most frequently causes self-limiting gastroenteritis that does not require treatment. However, it can cause severe systemic infection in immunocompromised individuals and can trigger the onset of rare (1 per 1000 cases) autoimmune sequelae, such as Guillain–Barré syndrome (GBS) and Miller Fisher syndrome (MFS) (Humphrey et al., 2007). Colonization of the gut epithelium is the first step in triggering C. jejuni enteritis (Zhu et al., 2006). Therefore, it is important to reduce or prevent C. jejuni contamination of meat and poultry products and to detect and identify strains that have the greatest potential to cause severe disease. To pursue these goals, we studied the ability of C. jejuni to colonize and cause disease in a mouse model of human colonization and disease.
In previous studies, we have demonstrated that C. jejuni strains 33292 and 81-176 as well as 11168-Tn5CmR2 [CamR2] and 11168-23SKanR4 [KanR4], derived from NCTC11168, colonized commercial broilers but did not colonize C57BL/6 IL-10-deficient (IL-10−/−) mice at high frequency or cause disease (Wilson et al., 2010). In contrast, C. jejuni strain NCTC11168, sequenced by Parkhill et al. (2000), colonized both animals at high frequency and caused disease symptoms in the IL-10-deficient mouse model similar to those observed in human patients (Mansfield et al., 2007). This model has been utilized effectively in several subsequent studies (Mansfield et al., 2008; Bell et al., 2009; Wilson et al., 2010; Jerome et al., 2011).
C. jejuni carries several genes that carry homopolymeric tracts (HTs), or simple sequence repeats, embedded in the ORF or promoter (Guerry et al., 2002; Karlyshev et al., 2005; Linton et al., 2000; Parkhill et al., 2000; Wassenaar et al., 2002). The HT is subject to high-frequency frameshift via slip-strand mutation, which can result in a shift in gene function (phase variation). Because these genes only function under certain growth conditions, they are called contingency genes. In C. jejuni NCTC11168, more than 20 contingency genes have been observed to carry G/C hypervariable HTs with eight or more nucleotides (Parkhill et al., 2000). Contingency genes are also found in other bacteria, such as Yersinia pestis (Rosqvist et al., 1988), Bordetella pertussis (Willems et al., 1990) and Haemophilus influenzae (van Ham et al., 1993). Many bacterial contingency genes, including most of those in C. jejuni, encode enzymes involved in the synthesis or modification of surface structures such as lipooligosaccharide (LOS), pilus, flagella and capsule, suggesting that they play important roles in host interaction and virulence (Moxon et al., 2006; Parkhill et al., 2000).
The C. jejuni contingency gene wlaN (Cj1139, LOS synthesis) encodes a β-1,3-galactosyltransferase (Linton et al., 2000). Wilson et al. (2010) demonstrated that a population of C. jejuni strain NCTC11168 cultured in trypticase soy broth carried two wlaN alleles; the predominant 8G allele (carried by 70 % of the population) encodes a full-length ORF, and the 9G allele (carried by 30 % of the population) carries a 1 bp insertion in the HT (+1 reading frame) that presumably encodes in a non-functional protein (Wilson et al., 2010). C. jejuni NCTC11168 populations passaged through chickens carried a more complex mixture of alleles, including 13 % 8G (ORF), 80 % 9G (+1), 5 % 10G (+2) and 2 % 7G (−1). Two important shifts in genotype occurred during chicken passage. First, the number of wlaN alleles in the C. jejuni population increased from two to four. Second, 50 % of the population experienced phase variation from the predominant 8G allele to the alternative 9G allele in C. jejuni NCTC11168 isolated from three out of four birds. We hypothesized that phase variation and natural selection for alternative alleles in C. jejuni contingency genes during passage through chickens could promote subsequent colonization and disease in mice and possibly humans.
To test this hypothesis, we utilized fragment analysis (Wassenaar et al., 2002) to study mutation frequency in the HTs in 19 contingency genes in C. jejuni human disease isolates NCTC11168, 33292 and 81-176, and two derivatives of NCTC11168 (KanR4 and CamR2), before chicken passage, after chicken passage, and after passage through chickens and then mice. We also tested the ability of single-colony isolates of C. jejuni KanR4 and 33292 carrying specific alleles of Cj0170 and Cj0045 as the predominant allele (carried by >50 % of the population) to colonize and cause disease in mice. The data demonstrated that phase variation in contingency genes Cj0045 and Cj0170 strongly associates with colonization and disease in the C57BL/6 IL-10-deficient mouse model and that the chicken reservoir plays an active role in phase variation, natural selection, the genotype of C. jejuni contingency genes, and disease.
Methods
C. jejuni strains, media and growth conditions.
C. jejuni strains used in this study included human disease isolates NCTC11168 (ATCC 700819) (Parkhill et al., 2000), 81-176 (Bacon et al., 2000), 33292 [American Type Culture Collection (ATCC), Manassas, VA] (Mansfield et al., 2003) and two genetically marked mutants derived from 11168 designated KanR4 (kanamycin-resistant) and CamR2 (chloramphenicol-resistant) (Wilson et al., 2003). Construction of strains KanR4 and CamR2 has been described previously (Wilson et al., 2003, 2010).
C. jejuni strains were cultured on trypticase soy agar supplemented with 5 % sheep blood (TSBA) at 37 °C under standard conditions (10 % CO2, 10 % H2, 80 % N2). For selective growth of C. jejuni from caecal or cloacal swab/faecal samples, the medium was supplemented with cefoperazone (20 µg ml−1), vancomycin (10 µg ml−1) and amphotericin B (2 µg ml−1) (TSBA-CVA). For selection of KanR4 or CamR2 isolates from bacterial populations, the medium was supplemented with either kanamycin (30 µg ml−1) or chloramphenicol (20 µg ml−1).
Preparation of C. jejuni populations in standard laboratory growth media.
C. jejuni strains from frozen stock cultures (−80 °C) were grown on TSBA under standard conditions for 2 days, and the cells were suspended in trypticase soy broth (TSB) at OD600 0.1–0.2. Cell suspensions (100 µl) were spread on TSBA plates, which were incubated under standard conditions for 12–14 h. Bacteria on agar plates were resuspended in TSB at OD600 0.1–0.2 to generate populations of approximately 109 c.f.u. ml−1 (Wilson et al., 2010). These non-passaged populations were utilized to inoculate Ross 308 broilers or mice.
Preparation of chicken-passaged populations of C. jejuni.
Campylobacter-free Ross 308 broiler chickens were obtained, inoculated, housed and fed as described previously (Wilson et al., 2010). C. jejuni populations (108–109 c.f.u. in 200 µl) were gavaged orally into 1–2 days old, Campylobacter-free Ross 308 broilers. TSB was gavaged into the negative control group. Chickens were housed individually and fed ad libitum with gamma-irradiated (1 mRad) starter feed (Miller Poultry-Broiler Starter #120/#220 with Clostat) and sterile water. After 14 days, chickens were humanely killed with CO2, and caecal tissue (60–200 mg) was removed during necropsy. C. jejuni colonization was analysed, and chicken-passaged (CP) populations were prepared from the caecum as described previously (Wilson et al., 2010).
Preparation of C. jejuni populations from single-colony isolates.
KanR4 and 33292 CP populations were spread on TSBA-CVA after serial dilution of frozen stock cultures. After 2–3 days of incubation under standard conditions, single colonies were isolated and streaked onto fresh TSBA-CVA plates. After 1–2 days of incubation under standard conditions, the cultures were harvested using cotton swabs and suspended in TSB at OD600 0.1–0.3. Frozen stock cultures (−80 °C) of individual single-colony isolates were prepared as described previously (Wilson et al., 2010). Cultures for inoculation of mice were prepared from frozen stock cultures of single-colony isolates as described above.
Colonization of chickens and mice.
Mice or chickens were gavaged orally with C. jejuni populations as described previously (Wilson et al., 2010). To confirm the initial absence of C. jejuni in experimental animals, cloacal swab samples (BD ProbeTec, Specimen Collection and Dry Transport kit) or faecal pellets were collected from chickens and mice, respectively (one animal per treatment group) prior to oral gavage. The samples were spread on TSBA-CVA and incubated under standard growth conditions. After oral gavage, cloacal swab samples (chickens) and faecal pellets (mice) were collected on days 1, 4, 7 and 10 post-gavage to monitor colonization in chicken–mouse (CM) experiments 1 and 2 (see description in Results). In CM3, as well as genotype experiments 1 and 2 (G1 and G2), faecal pellets were collected from mice on days 1, 8 and 15 post-gavage (see description in Results). These samples were suspended in sterile TSB, serially diluted in TSB, and 100 and 10−1 dilutions (cloacal swabs) or 100, 10−2 and 10−4 dilutions (faecal pellets) were spread on TSBA-CVA. Agar plates were incubated under standard conditions and colonies were counted after 2–3 days of incubation as described previously (Wilson et al., 2010).
Gross pathology score.
Gross pathology was evaluated and scored during mouse necropsy to estimate the severity of intestinal symptoms (Mansfield et al., 2007). Six intestinal sites were studied, including jejunum, caecum, colon, ileocecocolic junction lymph node, mesenteric lymph node and spleen. The extent of thickening or enlargement at each site was recorded based on a scale ranging from 0 (no symptoms) to 3 (severe symptoms). Scores were assigned for all six sites based on ‘blind’ analysis by two examiners. Scores for each of the six sites were combined to generate a composite gross pathology score for each mouse.
Motility assay.
Bacterial cell suspensions (OD600 0.1) were prepared as described above. Pipette tips were dipped into the suspension and then stabbed into the surface of Mueller–Hinton soft agar (0.4 %). Plates were incubated right-side-up under standard growth conditions. Motility was measured by the diameter (cm) of the growth halo surrounding the inoculation site at 48 h.
DNA extraction and PCR.
Bacterial cells were harvested in 5–10 ml TSB from agar plates by scraping with a Falcon cell scraper (BD). Cells in 1 ml of cell suspension (OD600 0.1–0.3) were pelleted by centrifugation (8000 r.p.m., 10 min, 25 °C) and resuspended in 1× PBS (137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, pH 7.4). Chromosomal DNA was extracted using an Easy-DNA kit (Invitrogen) and analysed as described previously (Wilson et al., 2010). Working stocks of DNA samples (10 ng µl−1−1) were prepared, and samples from the same treatment group were pooled. Groups included non-passaged populations, CP populations, or populations passaged through chickens and then mice. PCR was conducted essentially as described previously (Wilson et al., 2010), using primers and annealing conditions presented in Supplementary Table S1 available with the online version of this paper.
Fragment analysis.
Either the forward or the reverse primer in each PCR primer pair (Table S1) was labelled with 6-carboxyfluorescein (6-FAM) fluorescent dye (Integrated DNA Technologies). The target DNAs were amplified by PCR and resolved on a 3 % agarose gel to confirm identity. PCR products were purified, diluted to 0.1–1.0 ng µl−1 and analysed using a 3130xl Genetic Analyzer (Applied Biosystems) at the Research Technology Support Facility (RTSF) at Michigan State University. Fragment analysis data were analysed with Peak Scanner Software v1.0 (Applied Biosystems).
High-throughput DNA sequence analysis.
DNA sequence analysis was conducted using primers and annealing conditions described in Table S1 to confirm fragment analysis data, essentially as described previously (Wilson et al., 2010).
Direct PCR on chicken caecal tissue.
PCR was performed on DNA samples directly purified from chicken caecal tissue using the DNeasy Blood & Tissue kit (Qiagen) following the manufacturer’s instructions.
Statistical analysis.
Fisher’s exact test was used to compare the number of colonized mice between groups for statistical significance (P<0.05) using R free statistical software (http://cran.mtu.edu). A t test was used to compare the level of mouse colonization between groups for statistical significance (P<0.05).
Results
Passage through the chicken GI tract promotes mouse colonization by C. jejuni
C. jejuni human disease isolates NCTC11168, 33292 and 81-176, and isolates KanR4 and CamR2 derived from NCTC11168, colonized caecal tissue of Ross 308 broilers at high frequency in previous studies (Wilson et al., 2010) and in two independent experiments [chicken-mouse (CM)1 and 2] conducted in the current study. C. jejuni populations recovered from caecal tissue were pooled from groups of four colonized chickens; these CP populations were compared with non-passaged populations grown under standard conditions on TSB medium with respect to their ability to colonize C57BL/6 IL-10-deficient mice in CM1 and CM2. CP and non-passaged populations of strain NCTC11168 (positive control) each colonized eight out of eight mice at equally high levels [103–106 c.f.u. (mg dry caecal tissue)−1]. No mice inoculated with TSB (negative control) were colonized with C. jejuni. Non-passaged populations of strains KanR4, CamR2, 33292 and 81-176 either did not colonize mice or colonized at low frequency; only a non-passaged population of KanR4 colonized one mouse at high levels [104–105 c.f.u. (mg dry caecal tissue)−1] in CM2. Striking differences were observed in the ability of independently prepared CP populations of the same strain to colonize mice in CM1 and CM2. For example, independently prepared CP populations of KanR4 colonized none out of four mice in CM1 and four out of five mice in CM2; independently prepared CP populations of CamR2 colonized four out of four mice in CM1 and none out of five mice in CM2; and independently prepared CP populations of 33292 colonized four out of four mice in CM1 and none out of five mice in CM2. A single CP population of 81-176 colonized none out of four mice in CM2.
We defined ‘successful’ populations as those CP populations able to colonize mice at high frequency (e.g. CP populations of CamR2 and 33292 in CM1, and the CP population of KanR4 in CM2). We defined ‘unsuccessful’ populations as those CP populations unable to colonize mice (e.g. CP populations of CamR2 and 33292 in CM2, and a CP population of KanR4 in CM1). Consistent differences in mouse colonization by non-passaged and CP populations were observed in CM1 and CM2 but they were not statistically significant. However, statistically significant differences in mouse colonization were observed between successful and unsuccessful CP populations of the same strain in CM1 and CM2 (P<0.05).
We conducted CM3 with eight mice per group to confirm data obtained in CM1 and CM2 and to test their statistical significance. Successful CP populations of CamR2, 33292 and KanR4 generated from frozen caecal tissue obtained in CM1 and CM2 colonized six out of eight mice, eight out of eight mice, and five out of eight mice, respectively (Fig. 1). In contrast, one out of eight mice inoculated with the CamR2 non-passaged population was colonized by C. jejuni [1.5×104 c.f.u. (mg caecal tissue)−1] and none out of eight mice inoculated with either the 33292 or KanR4 non-passaged population were colonized. We observed a statistically significant difference in mouse colonization by successful CP populations and non-passaged populations of the same strain in CM3 (P<0.05). Of critical importance, fragment analysis (below) demonstrated that the genotype of contingency genes did not differ significantly between CP populations prepared from frozen caecal samples in CM3 or prepared from fresh caecal samples in CM1 and CM2 (Table 2). The data demonstrated enhanced colonization of mice after chicken passage in both CM1 and CM2, and this observation was confirmed in CM3. Of particular significance, successful CP populations arose in three different C. jejuni strains in CM1 and CM2.
Fig. 1.
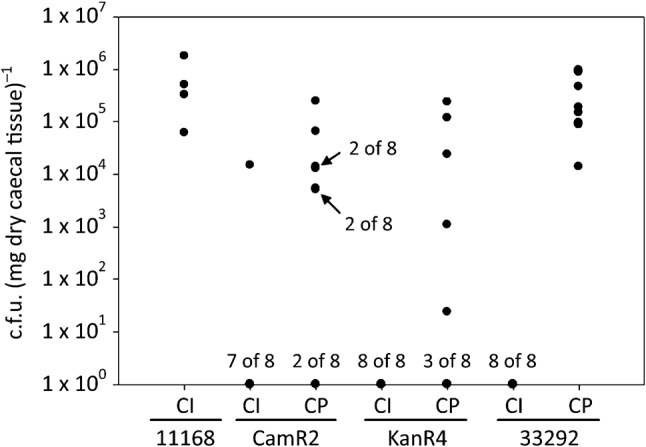
Colonization of caecal tissue of C57BL/6 IL-10-deficient mice by C. jejuni recovered from frozen chicken caeca obtained in CM1 and CM2. Frozen chicken caecal samples were macerated and spread on selective growth media. CamR2 (CM1), KanR4 (CM2) and 33292 (CM1) CP populations were recovered on selective medium. Then, eight mice per group were gavaged with CP or non-passaged (CI) C. jejuni populations. At 21 days post-gavage, caecal tissue was homogenized, serially diluted and plated onto selective growth media (see Methods). For all three strains tested, CP populations colonized mice at significantly higher levels than CI populations (P<0.05). Single dots represent colonization in individual mice. Numbers adjacent to dots indicate data for multiple mice.
Table 2. Fragment/sequence analysis data for successful CP populations recovered from frozen or fresh caeca.
Fresh caecal samples were analysed immediately after necropsy; frozen caecal samples were stored at −20 °C prior to fragment or sequence analysis.
| Strain | Gene | Analysis | Caecal samples | Allele frequency | ||||
| CamR2 | Cj0685 | Fragment | 8C (−1) | 9C (ORF) | 10C (+1) | |||
| Frozen (CM3) | 43 % | 57 % | ||||||
| Fresh (CM1) | 48 % | 51 % | 1 % | |||||
| Cj1139 | Fragment | 7G (−1) | 8G (ORF) | |||||
| Frozen (CM3) | 55 % | 45 % | ||||||
| Fresh (CM1) | 55 % | 45 % | ||||||
| Cj1421 | Sequence | 8G (−1) | 9G (ORF) | 10G (+1) | 11G (+2) | |||
| Frozen (CM3) | 4 % | 35 % | 61 % | |||||
| Fresh (CM1) | 4 % | 50 % | 38 % | 8 % | ||||
| Cj1426 | Fragment | 9G (−1) | 10G (ORF) | 11G (+1) | ||||
| Frozen (CM3) | 57 % | 43 % | ||||||
| Fresh (CM1) | 55 % | 43 % | 2 % | |||||
| KanR4 | Cj0170 | Fragment | 8G (ORF) | 9G (+1) | 10G (+2) | |||
| Frozen (CM3) | 5 % | 72 % | 23 % | |||||
| Fresh (CM2) | 4 % | 76 % | 20 % | |||||
| Cj1422 | Sequence | 8G (−1) | 9G (ORF) | 10G (+1) | ||||
| Frozen (CM3) | 4 % | 87 % | 9 % | |||||
| Fresh (CM2) | 100 % | |||||||
| Cj1426 | Fragment | 9G (−1) | 10G (ORF) | 11G (+1) | ||||
| Frozen (CM3) | 12 % | 75 % | 13 % | |||||
| Fresh (CM2) | 8 % | 84 % | 8 % | |||||
| 33292 | Cj0045 | Fragment | <8G | 9G | 10G | 11G | 12G | |
| Frozen (CM3) | 4 % | 25 % | 66 % | 5 % | ||||
| Fresh (CM1) | 4 % | 26 % | 48 % | 21 % | 1 % | |||
| Cj1420 | Fragment | 8G (−1) | 9G (ORF) | |||||
| Frozen (CM3) | 85 % | 15 % | ||||||
| Fresh (CM1) | 84 % | 16 % | ||||||
Fragment analysis identifies insertions/deletions (indels) in hypervariable HTs
We hypothesized that the enhancement in colonization of mice by successful CP populations resulted from changes in the frequency of alleles carrying indel mutations within the HTs of contingency genes. To test this hypothesis, we conducted fragment analysis to track mutation frequency in 19 of the 23 hypervariable contingency genes identified in the genome of C. jejuni NCTC11168 (Parkhill et al., 2000). These 19 genes were placed into 15 individual or small groups of closely related genes (Table 1). Fragment analysis resolved PCR products generated from regions carrying HTs based on a single base pair difference. We estimated the number of alleles for each contingency gene by counting the total number of peaks, and allele frequency by calculating the area under each peak. To confirm fragment analysis data, sequence analysis was conducted on up to 24 clones of individual PCR products carrying the HT. The number and frequency of each allele in fragment analysis agreed with sequence analysis (Table S2). The genotype of 19 contingency genes in a non-passaged population of strain NCTC11168 as determined by fragment analysis was confirmed by Illumina sequence analysis of strain NCTC11168 grown under similar culture conditions (Jerome et al., 2011).
Table 1. Polymorphic contingency genes with HTs and their functions.
| Region | Gene, locus | HT that gives complete ORF | Function |
| Unspecified | Cj0031–32* | 9G | Putative type IIS restriction–modification enzyme |
| Cj0045c | 9G | Putative iron-binding protein | |
| Cj0170–71* | 8G | Hypothetical protein | |
| Cj0685c, cipA | 9C | Invasion protein CipA | |
| Protein glycosylation and LOS biosynthesis | Cj1139c, wlaN | 8G | β-1,3-Galactosyltransferase |
| Cj1144–45c* | 19 (G+A) | Hypothetical protein | |
| Flagellin modification | Cj1305c/06c/10c† | 9G | Hypothetical proteins |
| Cj1318, maf1/35–36*, maf4† | 11G | Motility accessory factor | |
| Cj1321 | 10G‡ | Acetyltransferase | |
| Cj1325–26* | 9G | Putative methyltransferase | |
| Cj1342c, maf7 | 9G | Motility accessory factor | |
| Capsule biosynthesis | Cj1420c | 9G | Putative methyltransferase |
| Cj1421c/22c† | 9G | O-Methyl phosphoramidate transferases | |
| Cj1426c | 10G | Putative methyltransferase family protein | |
| Cj1429c | 10G | Hypothetical protein |
Genes separated by (–) were merged as a result of the C. jejuni NCTC11168 reannotation.
Genes separated by (/) are separate genes but share high sequence similarity.
HT located in promoter region rather than ORF.
Phase variation in specific contingency genes in C. jejuni CP populations is strongly associated with enhanced mouse colonization
We observed large shifts in allele frequency (a large shift occurs in >70 % of the bacterial population) from the predominant allele to an alternative allele in six C. jejuni contingency genes (Cj0045, Cj0685, Cj1139, Cj1421, Cj1422 and Cj1426) after passage through chickens and then mice (Fig. 2). Moderate shifts in allele frequency (a shift that occurs in 40–69 % of the bacterial population) were observed in five of these contingency genes (Cj0045, Cj0685, Cj1139, Cj1421 and Cj1426) in successful CP populations prior to mouse passage, and these shifts in allele frequency were not observed in the corresponding unsuccessful CP populations. To illustrate, the successful CP CamR2 population from CM1 experienced a moderate shift in allele frequency for the predominant allele for contingency genes Cj0685, Cj1139, Cj1421 and Cj1426 (Fig. 2); allele shifts in these four genes resulted in phase variation from a complete ORF to a truncated and presumably non-functional protein. However, these moderate shifts in allele frequency were not observed in the unsuccessful CP CamR2 population from CM2. Similarly, in human disease isolate 33292, a moderate shift in allele frequency from a predominant 11G allele to a 9G allele (the 9G allele encodes a complete ORF) occurred in contingency gene Cj0045 in a successful CP population from CM1 (Fig. 2), but this shift was not observed in an unsuccessful CP population from CM2 (Fig. 2). For contingency genes Cj0045, Cj0685, Cj1139, Cj1421 and Cj1426, the portion of the successful CP population carrying the predominant allele shifted almost completely to the alternative allele after mouse passage (Fig. 2a).
Fig. 2.
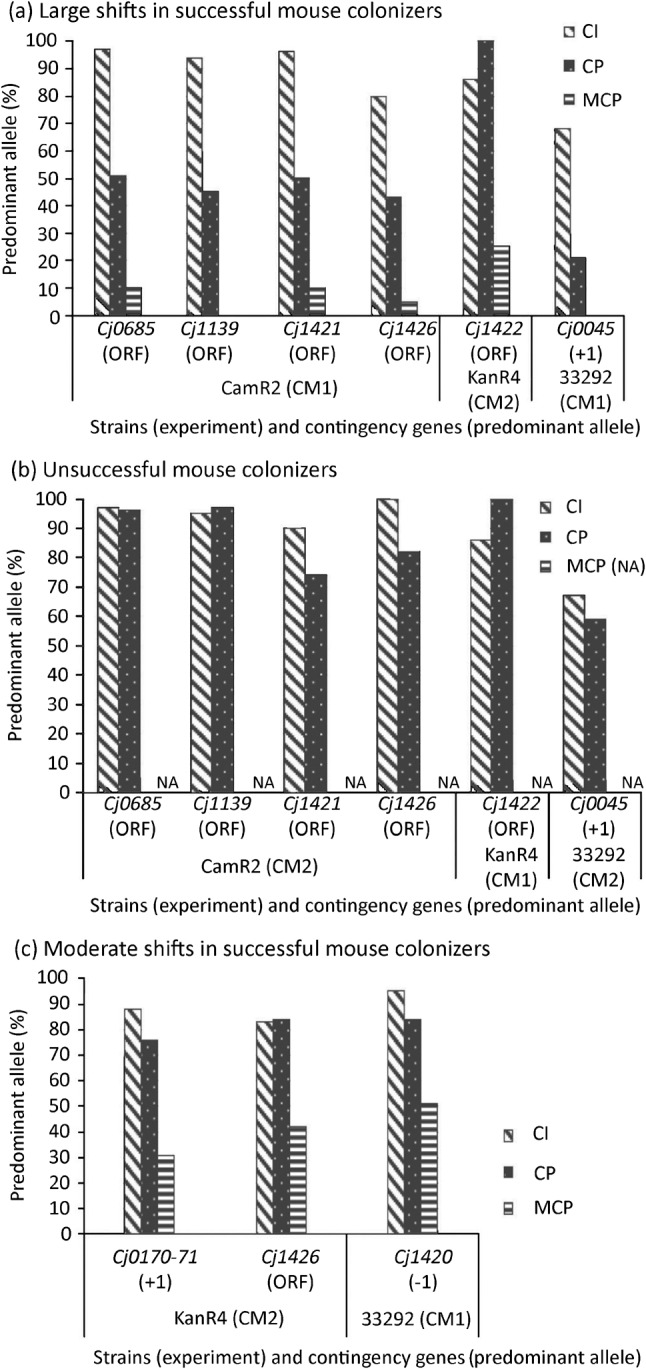
Frequency (percentage) of the predominant allele in C. jejuni populations. The initial predominant allele is defined as the highest-frequency allele in a non-passaged C. jejuni (CI) population grown in culture. Contingency genes were amplified by PCR, and fragment and/or sequence analyses were used to determine allele frequencies in a non-passaged population (CI), after chicken passage (CP), or after passage through chickens and then mice (MCP). (a) ‘Successful mouse colonizers’ colonized mice at high frequency after passage through chickens. Predominant allele frequencies in CI and CP populations were significantly different (P<0.05), except for Cj1422 in KanR4 populations in CM2 (P = 0.23). Predominant allele frequencies in CP and MCP populations also were significantly different (P<0.05). (b) ‘Unsuccessful mouse colonizers’ did not colonize mice at high frequency after chicken passage. We did not observe significant differences in predominant allele frequencies between CI and CP populations (P>0.05), except for Cj1426 in the CamR2 population (CM2) (P = 3.3×10−6). MCP populations were not available (na) because mice were not colonized. With the exception of locus Cj1422, allele frequencies for all contingency loci in successful CP populations shown in (a) were significantly different from unsuccessful CP populations shown in (b) (P<0.05). (c) MCP populations experienced moderate allele shifts after passage through chickens and then mice. Allele frequencies for contingency loci in CI and MCP populations were significantly different (P<0.05). Allele frequencies for contingency loci in CI and CP populations were significantly different (P<0.05), except for Cj1426 in KanR4 populations (CM2). For alleles predicted to encode a complete ORF, see Table 1.
A moderate shift in allele frequency in contingency gene Cj0170 in KanR4 in CM2 altered the relative frequency of two alleles, each of which encodes a truncated protein. Of the non-passaged population, 88 % carried the predominant 9G allele (+1 reading frame) and 4 % of the population carried the alternative 10G allele (+2 reading frame). After passage through chickens and then mice, the frequency of the predominant allele (9G) decreased to 31 % and the frequency of the alternative allele (10G) increased to 68 % (Fig. 2).
Large shifts in allele frequency in contingency genes did not occur in strain 11168 during passage through chickens and/or mice; this strain colonizes mice with or without chicken passage. However, we did observe moderate shifts in allele frequency in contingency genes Cj0170, Cj1139, Cj1305 and Cj1421 during passage through chickens and mice (data not shown). Nevertheless, moderate shifts in allele frequency in strain 11168 were not associated with either the number of mice colonized or the level of colonization, suggesting that another factor plays a role in the ability of strain 11168 to colonize mice at high frequency.
Phase variation in contingency genes depends on animal passage and does not occur during growth in culture
Several independent observations demonstrated that phase variation in contingency genes depends on passage through chickens and/or mice. (1) Transfer of NCTC11168 in TSB growth medium every 2 days for up to 2 weeks did not result in significant changes in genotype in contingency genes (data not shown). (2) Growth of C. jejuni in laboratory media under standard conditions did not result in significant changes in genotype in contingency genes (Table S2). (3) Preparation of CP populations from either fresh caeca (CM1 and CM2) or frozen caeca (CM3) did not result in significant changes in the genotype of contingency genes (Table 2). (4) The genotype for four contingency genes in C. jejuni populations isolated from fresh caecal tissue by growth on selective culture media was not significantly different from the genotype for the same four contingency genes in C. jejuni populations analysed directly in caecal tissue (data not shown; direct PCR analysis of caecal tissue, see Methods).
Contingency gene allele frequency remains stable in single-colony isolates during inoculum preparation
We identified pairs of single-colony isolates that carry successful or unsuccessful alleles of three contingency genes (Cj0045, Cj0170 or Cj1420) and analysed the ability of these isolates to colonize and cause disease in the mouse model. First, it was necessary to demonstrate that single-colony isolates remained genetically stable during preparation of C. jejuni populations for oral gavage into mice. Single colonies were isolated from a successful KanR4 CP population from CM2 and a successful 33292 CP population from CM1. Then, fragment analysis was used to compare the genotypes of populations of these single-colony isolates in frozen glycerol stocks with the genotypes of populations after culture (Table 3). Two observations confirmed that the genotype of the predominant allele in the glycerol stock remained stable during preparation of the mouse inoculum. (1) The predominant allele for Cj0170, Cj0045 and Cj1420 in the glycerol stock remained the predominant allele in all eight single-colony isolates during culture for mouse inoculum preparation. (2) The observed decrease in frequency of the predominant allele after culture ranged from 0 (minimum) to 18 % (maximum) as compared with the glycerol stock. Nucleotide sequence analysis of Cj0170 and Cj0045 in single-colony isolates of KanR4 and 33292 confirmed the fragment analysis data.
Table 3. Comparison of allele frequencies for contingency genes Cj0170, Cj0045 and Cj1420 in single-colony isolates prepared from glycerol stocks or prepared for mouse inocula.
| Isolate | Cj0170 | Cj0045 | Cj1420 | |||
| Glycerol* | Inoculum† | Glycerol | Inoculum | Glycerol | Inoculum | |
| SCK1‡ (S) | 81 (10G) | 78 | ||||
| SCK7 (S) | 84 (10G) | 80 | ||||
| SCK18 (U) | 90 (9G) | 89 | ||||
| SCK21 (U) | 90 (9G) | 90 | ||||
| SC4‡ (S) | 86 (9G) | 85 | 97 (9G) | 100 | ||
| SC13 (S) | 96 (9G) | 84 | 100 (9G) | 95 | ||
| SC5 (U) | 72 (11G) | 58 | 96 (8G) | 92 | ||
| SC9 (U) | 71 (11G) | 53 | 97 (8G) | 95 | ||
Percentage frequency of the predominant allele in population derived from glycerol stock (see Methods).
Percentage frequency of the predominant allele in population after culture in growth medium during preparation of inocula for oral gavage into mice.
Single-colony isolates SCK1 and SCK7 were derived from a successful population of C. jejuni KanR4 and carry the successful allele (S) of Cj0170 (10G HT). Single-colony isolates SCK18 and 21 were derived from the same successful KanR4 population and carry the unsuccessful allele (U) of Cj0170 (9G HT). Single-colony isolates SC4 and SC13 were derived from a successful population of C. jejuni 33292 and carry successful alleles (S) of Cj0045 (9G HT) and Cj1420 (9G HT). Single-colony isolates SC5 and SC9 were derived from the same successful population of C. jejuni 33292 and carry unsuccessful alleles (U) of Cj0045 (11G HT) and Cj1420 (8G HT).
The 10G allele of Cj0170 in C. jejuni strain KanR4 is strongly associated with mouse colonization
To help confirm the observed association between Cj0170 genotype and ability to colonize mice, we compared pairs of single-colony isolates derived from a successful CP KanR4 population that differed in genotype at the Cj0170 locus. Single-colony isolates carrying either the successful 10G allele (SCK1 and SCK7) or unsuccessful 9G allele (SCK18 and SCK21) as the predominant allele were gavaged orally into C57BL/6 IL-10-deficient mice in two independent experiments, designated genotype 1 (G1) and genotype 2 (G2). After 21 days, colonization was estimated by enumerating C. jejuni recovered from the mouse caecum at necropsy. In G1, four mice each were inoculated with SCK1 (10G allele in Cj0170) or SCK21 (9G allele in Cj0170). SCK1 (successful allele) colonized two out of four mice, and SCK21 (unsuccessful allele) failed to colonize any mice (none out of four). This initial association, although promising, was not statistically significant (P>0.05). In experiment G2, eight mice were inoculated with each of SCK1, SCK7 (with the successful 10G allele), SCK18 or SCK21 (with the unsuccessful 9G allele). SCK1 and SCK7 colonized 11 out of 16 mice (Fig. 3). Of the colonized mice, eight carried between 103 and 105 c.f.u. (mg caecum)−1; the remaining three mice carried between 1 and 10 c.f.u. (mg caecum)−1. In contrast, SCK18 and SCK21 colonized none out of 16 mice. The number of mice colonized by SCK1 and SCK7 (10G allele) was significantly higher than the number of mice colonized by SCK18 and SCK21 (9G allele; P<0.05). These data were consistent with the dramatic increase in the frequency of the 10G allele observed after mouse passage of the KanR4 CP population illustrated above (Fig. 2), suggesting that the 10G allele imparts a selective advantage during mouse colonization. Alternatively, the 9G allele lacks a selective advantage or is selected against. These data strongly suggest that the 10G allele of Cj0170 in C. jejuni KanR4 is directly involved in mouse colonization and in virulence.
Fig. 3.
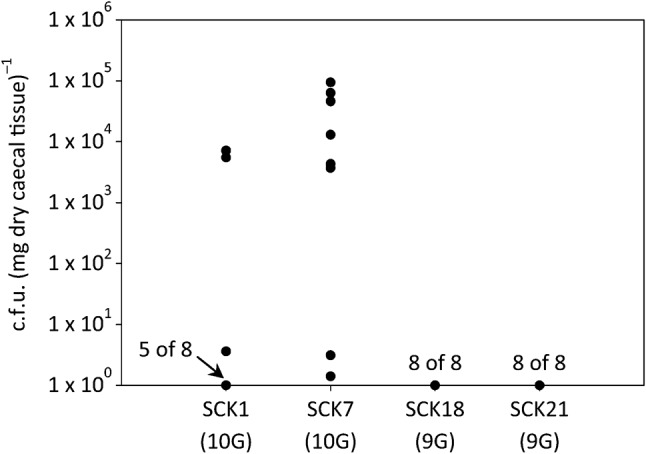
Colonization of mouse caeca by KanR4 single-colony isolates carrying two different Cj0170 alleles (HT carrying either 9G or 10G). Eight mice were gavaged orally with each KanR4 single-colony isolate. Mice were sacrificed at 21 days post-gavage and caecal tissue was homogenized, serially diluted and plated onto selective growth media (see Methods). Single dots represent colonization in individual mice; in specific cases, numbers adjacent to dots indicate data for multiple mice (e.g. the single dots for SCK18 and SCK21 each represent eight mice). The number of mice colonized by SCK1 and SCK7 carrying the 10G allele of Cj0170 was significantly higher than the number of mice colonized by SCK18 and SCK21 carrying the 9G allele of Cj0170 (P<0.05).
The 9G allele of Cj0045 in strain 33292 is associated with mouse colonization
Single-colony isolates SC5 and SC9 carrying unsuccessful alleles of Cj0045 (11G) and Cj1420 (8G) were compared with single-colony isolates SC4 and SC13 carrying successful alleles (9G in Cj0045, 9G in Cj1420) with respect to their ability to colonize C57BL/6 IL-10-deficient mice. In experiment G1, isolate SC5 (unsuccessful alleles) colonized three out of four mice and SC13 (successful alleles) colonized four out of four mice. The observed difference in the number of mice colonized between these isolates was not statistically significant. However, the level of colonization by SC13 was significantly higher than that by SC5 (P<0.05), suggesting that the 9G allele of Cj0045 and Cj1420 imparted a selective advantage in the GI tract of mice as compared with the 11G allele of Cj0045 and the 8G allele of Cj1420.
To confirm these initial observations, we conducted experiment G2 using eight mice per group. Single-colony isolates SC4 and SC13 carried the successful 9G allele in Cj0045 and Cj1420, and single-colony isolates SC5 and SC9 carried the unsuccessful 11G allele in Cj0045 and the unsuccessful 8G allele in Cj1420 (Table 3). Isolates SC4 and SC13 colonized 13 out of 16 mice, and isolates SC5 and SC9 colonized 12 out of 16 mice. We did not observe a statistically significant difference in the number of mice colonized or in the level of colonization between the two sets of duplicate isolates in experiment G2 (P>0.05).
C. jejuni populations recovered from caeca of colonized mice were subjected to fragment analysis to track allele frequency in Cj0045. In mice colonized by SC4 and SC13, the successful 9G allele remained the predominant allele in 12 out of 13 mice (Table 4). In one mouse, the 9G allele experienced a shift to a predominant successful 10G allele. In contrast, in mice colonized by isolates SC5 and SC9, the unsuccessful 11G allele shifted to the successful 9G, 10G or 12G alleles. Overall, 21 of 25 mice colonized by C. jejuni populations carried the predominant successful 9G allele of Cj0045, strongly suggesting either that the 9G allele of Cj0045 imparts a selective advantage in the mouse GI tract and is important for colonization by C. jejuni 33292, or that the 11G allele experiences strong negative selection.
Table 4. Shifts in allele frequency in contingency gene Cj0045 in single-colony isolates of 33292 during mouse passage.
| Experiment | Single-colony isolates | Non-passaged or mouse passaged | Number of mice | Allele frequency (%) in Cj0045 HT* | |||||
| <8G | 9G | 10G | 11G | 12G | >13G | ||||
| G1 | 33292-SC5 | Non-passaged | na† | 3 | 28 | 66‡ | 3 | ||
| Mouse passaged | 2 | 10 | 4 | 24 | 61 | 2 | |||
| Mouse passaged | 1 | 7 | 6 | 85 | 2 | ||||
| 33292-SC13 | Non-passaged | na | 15 | 85 | |||||
| Mouse passaged | 3 | 12 | 88 | ||||||
| G2 | 33292-SC5 | Non-passaged | na | 2 | 3 | 34 | 58 | 3 | |
| Mouse passaged | 5 | 7 | 83 | 8 | 1 | 1 | |||
| Mouse passaged | 1 | 9 | 41 | 49 | 1 | ||||
| Mouse passaged | 1 | 4 | 30 | 63 | 3 | ||||
| 33292-SC9 | Non-passaged | na | 2 | 5 | 37 | 53 | 3 | ||
| Mouse passaged | 4 | 10 | 79 | 9 | 1 | 2 | |||
| Mouse passaged | 1 | 1 | 5 | 26 | 65 | 3 | |||
| 33292-SC4 | Non-passaged | na | 8 | 85 | 7 | ||||
| Mouse passaged | 5 | 9 | 87 | 4 | |||||
| Mouse passaged | 1 | 5 | 15 | 77 | 3 | ||||
| 33292-SC13 | Non-passaged | na | 13 | 84 | 3 | ||||
| Mouse passaged | 7 | 9 | 88 | 2 | |||||
Fragment analysis was performed on C. jejuni in the mouse inoculum (non-passaged) or recovered from individual mice (mouse-passaged). Numbers represent the mean allele frequencies.
na, Not applicable.
Predominant alleles in a population are in bold type. SC5 and SC9 mouse inocula carry the 11G unsuccessful allele as the predominant allele. SC4 and SC13 mouse inocula carry the 9G successful allele as the predominant allele.
Analysis of Cj1420 in colonized mice was more complicated. A shift in allele frequency from the predominant successful 9G allele was not observed in any mice colonized by SC4 or SC13 (successful alleles). However, three out of 12 mice colonized by SC5 experienced a shift in allele frequency from the predominant unsuccessful 8G allele to a predominant successful 9G allele; no major shift in allele frequency from the 8G allele to the 9G allele was observed in the remaining nine mice. The data do not support a strong association between Cj1420 allele frequency and mouse colonization, suggesting that Cj1420 does not play a major role in this process.
The level of caecum colonization affects gross pathology in mice colonized by C. jejuni KanR4 or 33292
Gross pathology in all mice was scored upon necropsy. All mice in the negative control group gavaged with TSB scored 0, while the positive control group gavaged with C. jejuni NCTC11168 scored 2.5 (Table 5), confirming that this strain causes disease symptoms in C57BL/6 IL-10-deficient mice (Mansfield et al., 2007). Mice gavaged with C. jejuni KanR4 or 33292 that were either not colonized[(0 c.f.u. (mg dry caeca)−1] or colonized at a low level [<103 c.f.u. (mg dry caeca)−1] exhibited a relatively low gross pathology score (1.4 and 1.0, respectively), and there was no significant difference between these groups (P>0.05) (Table 5). However, mice colonized at higher levels by either KanR4 or 33292 [>103 c.f.u. (mg dry caecum)−1] exhibited severe disease symptoms (average score 2.7) and this group scored significantly more highly than non-colonized mice (P<0.05). These data strongly suggest that contingency gene allele and colonization level greatly affect the severity of disease symptoms during C. jejuni infection.
Table 5. Association between mouse colonization level and gross pathology score in mice infected with single-colony isolates of KanR4 and 33292.
| Inoculum* | c.f.u. (mg dry caecum)−1† | Number of mice with level | Number of mice with gross pathology score (percentage) | Mean score‡ | ||||
| ≤1 | 2 | 3 | 4 | ≥5 | ||||
| TSB | 0 | 4 | 4 (100 %) | 0.0 | ||||
| 11168 | >103 | 8 | 4 (50 %) | 1 (13 %) | 1 (13 %) | 2 (25 %) | 2.5 | |
| KanR4 (S) | 0 | 5 | 3 (60 %) | 1 (20 %) | 1 (20 %) | 1.8 | ||
| KanR4 (S) | 1–103 | 3 | 3 (100 %) | 1.0 | ||||
| KanR4 (S) | >103 | 8 | 3 (37 %) | 1 (13 %) | 4 (50 %) | 2.1 | ||
| KanR4 (U) | 0 | 16 | 10 (62 %) | 4 (25 %) | 2 (13 %) | 1.2 | ||
| 33292 | 0 | 7 | 4 (57 %) | 1 (14 %) | 2 (29 %) | 1.6 | ||
| 33292 | 1–103 | 2 | 1 (50 %) | 1 (50 %) | 1.0 | |||
| 33292 | >103 | 23 | 6 (26 %) | 8 (35 %) | 4 (17 %) | 1 (4 %) | 4 (17 %) | 2.9 |
(S), Successful genotypes SCK1 and SCK7; (U), unsuccessful genotypes SCK18 and SCK21.
Colonization level in the caecum at the time of necropsy.
Mean gross pathology score.
The predominant allele of Cj0170 in C. jejuni KanR4 is strongly associated with motility and colony morphology
A C. jejuni KanR4 CP population was spread on an appropriate agar medium and two colony morphologies were observed. Seventeen large and seven small colonies were picked randomly and screened for allele frequency in Cj0170 using fragment analysis. All 17 large colonies carried the successful 10G (+2) allele and all seven small colonies carried the unsuccessful 9G (+1) allele as the predominant allele. We suspected that colony size could be a function of motility, so we tested motility on Mueller–Hinton soft agar (0.4 %). All large colonies with the successful 10G allele were significantly more motile (motility halo between 3.0 and 3.7 cm) than all small colonies carrying the unsuccessful 9G allele (motility halo between 1.1 and 1.3 cm) (P<0.01), suggesting that allele frequency in Cj0170 affects colony size and motility in C. jejuni KanR4. SCK1 carrying the 10G allele and SCK21 carrying the 9G allele were grown in Mueller–Hinton broth under standard conditions. Isolate SCK21 with lower motility and smaller colony size grew 1.4-fold faster than SCK1, suggesting that the observed increase in size of the motility halo of SCK1 (10G allele) as compared with SCK21 (9G allele) was due to increased motility. The 100 % association between colony size, Cj0170 allele frequency and motility in KanR4 single-colony isolates strongly suggested that increased mouse colonization by single-colony isolates carrying the successful 10G allele of Cj0170 was due to increased motility (Morooka et al., 1985).
Discussion
The current study advances our understanding of the role of contingency genes in C. jejuni colonization and disease. The data demonstrate that passage through the chicken reservoir can promote phase variation in specific contingency genes in the limited number of C. jejuni strains under analysis, and this modified genotype contributes to enhanced colonization by and disease due to C. jejuni in our mouse model. It would be instructive to broaden the scope of the current study to include a variety of genetically divergent C. jejuni strains. We also demonstrate that specific alleles of contingency genes Cj0170 and Cj0045 are enriched during passage in chickens and/or mice, and that the emergence of subpopulations carrying these successful alleles is strongly associated with mouse colonization and disease for the stains under analysis. Although chicken passage has been observed to enhance virulence of C. jejuni strains during infection of 9 day-old mice, the underlying mechanisms were not identified (Sang et al., 1989). The current study provides new detail about the underlying mechanisms associated with increased virulence due to phase variation in contingency genes. Below we discuss several potential mechanisms by which phase variation in contingency genes could affect virulence.
Phase variation alters the antigenicity of cell surface structures
Phase variation in contingency genes generates antigenic variation in surface structures in many bacterial pathogens (van der Woude & Bäumler, 2004) and these structures elicit a host immune response to C. jejuni (Guerry et al., 2002; Yuki, 2010). C. jejuni contingency genes Cj1139, Cj1421, Cj1422 and Cj1426 are involved in capsule biosynthesis/modification or LOS biosynthesis (Linton et al., 2000; McNally et al., 2007), and they experienced phase variation to a non-functional protein or protein with modified function. So, phase variation could enhance colonization and disease by altering the ability of mice to mount an effective innate or adaptive immune response (Ang et al., 2010).
Phase variation alters motility and gene expression
C. jejuni motility is strongly associated with colonization and virulence (Dasti et al., 2010; Guerry, 2007). Our data demonstrate that phase variation in Cj0170 affects both motility and virulence (Table 5) in mice. Analysis of the C. jejuni 11168 genome sequence by Parkhill et al. (2000) and Gundogdu et al. (2007) did not identify the function of the Cj0170 gene. However, we searched the NCBI database using the Cj0170 peptide sequence as a query and detected strong identity between Cj0170 and methyltransferases from a variety of organisms [e.g. 68 % identity, 82 % similarity to a DNA adenine (Dam) methyltransferase in Campylobacter lari; 31 % identity, 49 % similarity to a type 11 methyltransferase of Aminomonas paucivorans]. Electron microscopy of C. jejuni KanR4 single-colony isolates carrying a successful 10G allele or unsuccessful 9G allele of Cj0170 revealed no detectable differences in gross flagellar structure (data not shown). These observations prompted us to propose two potential mechanisms by which Cj0170 could affect motility. (1) Alteration of gene expression. Phase-variable Dam methyltransferases regulate expression of genes involved in motility and chemotaxis in Escherichia coli, as well as virulence genes in Salmonella and Yersinia enterocolitica (Balbontín et al., 2006; Fälker et al., 2005; Oshima et al., 2002; Srikhanta et al., 2010); genes regulated in this fashion belong to a ‘phase varion’. Since Cj0170 does not appear to affect flagellar structure or assembly, genes involved in chemotaxis (e.g. cheY), post-translational modification of flagella and/or flagellar rotation top our list of candidate target genes for activation by the Cj0170 Dam methyltransferase (Karlyshev et al., 2002; Yao et al., 1997). (2) Post-translational modification of flagella. Post-translational modification of flagellin subunits by pseudaminic acid (PseAc) promotes proper flagellar assembly and motility in C. jejuni (Guerry, 2007; Guerry & Szymanski, 2008). Genes encoding methyltransferase (Cj1325–1326) and acetyltransferase (Cj1321) activities localize in the flagella modification cluster (Guerry, 2007; Guerry & Szymanski, 2008). An acetylated version of PseAc (PseOAc) was identified in flagella, supporting a role for the Cj1321 acetyltransferase in flagellar modification. Cj0170 exhibits 57 % identity and 68 % similarity to the Cj1325–1326 methyltransferase, supporting a possible role in flagellar modification.
Cj0045 has been tentatively identified as haemerythrin, a non-haem oxygen binding protein (French et al., 2008), in the reannotation of the C jejuni 11168 genome (Gundogdu et al., 2007). In support of this assignment, we observed 99 % identity to a putative iron binding protein in another sequenced isolate of C. jejuni. Iron uptake is necessary for C. jejuni survival in the host, and C. jejuni utilizes various mechanisms to acquire iron (Miller et al., 2009). So the Cj0045 iron-binding protein might enhance iron uptake, providing a selective advantage in the GI tract.
However, the Cj0045 HT lies near the carboxyl terminus of the ORF, so frameshifts induced by phase variation do not generate truncated Cj0045 proteins; they add one or six amino acids to the carboxyl terminus. So, an alternative mechanism by which Cj0045 affects virulence is by modulating expression of Cj0044. We propose that the 9G allele of Cj0045 is a successful allele because the translation termination codon of Cj0045 does not overlap the translation initiation codon of Cj0044, allowing normal expression of the Cj0044 protein. Furthermore, we propose that the 11G allele of Cj0045 is unsuccessful because the translation stop codon of Cj0045 overlaps the translation initiation codon in Cj0044. This could reduce levels of reinitiation at Cj0044 and downregulate synthesis and activity of this protein. Analysis of the C. jejuni NCTC11168 sequence (Parkhill et al., 2000; Gundogdu et al., 2007) failed to assign a protein function to Cj0044, and little work has been done on either Cj0045 or Cj0044. However, the three genes immediately upstream from Cj0044 were assigned putative roles in flagella assembly, including Cj0041 (motility and invasion), Cj0042 (flgD basal body) and Cj0043 (flgE, flagellar hook protein) (Gundogdu et al., 2007; Parkhill et al., 2000), suggesting a role for Cj0044 in motility.
Emergence of successful populations of C. jejuni
We propose the following model to explain the emergence of successful C. jejuni populations through natural selection. Our data suggest that, prior to chicken passage, populations of C. jejuni strains 33292, CamR2 and KanR4 carry a small number of cells with the appropriate mix of successful contingency gene alleles (successful genotypes), and these cells are not present in sufficient numbers to assure high-frequency colonization of mice. However, selective pressure in the chicken GI tract enriches these low-abundance populations or enables the emergence of new successful subpopulations. These successful subpopulations carry large numbers of cells with successful genotypes; this alters surface structure or regulates the expression of virulence factors such as motility or iron uptake, resulting in enhanced colonization and disease in mice.
Single-colony isolates provide support for our model. (1) Single colonies of C. jejuni 11168, 33292, 81-176, KanR4 and CamR2 isolated from laboratory culture media prior to chicken passage do not colonize mice or cause disease (Wilson et al., 2010). (2) Single-colony isolates of C. jejuni KanR4 or 33292 from the same successful CP population can carry either successful or unsuccessful alleles of Cj0170 and Cj0045, and colonization and disease in the mouse model depend on which allele is present in the single-colony isolate.
The work by Jerome et al. (2011) supports the proposed model. These researchers demonstrated that passage of C. jejuni NCTC11168 through C57Bl6 IL10-deficient mice increased colonization and disease symptoms, and this process was accompanied by phase variation in specific contingency genes. Illumina sequence analysis of these bacterial populations demonstrated that the genetic changes observed during animal passage occurred almost exclusively in contingency genes, strongly suggesting that phase variation in contingency genes during passage in chickens and/or mice in our study is directly responsible for enhanced colonization and disease. Jerome et al. (2011) did not, however, directly link changes in any one contingency gene to increased colonization and disease.
Human disease isolates 81-176 and 33292 exhibit a high level of genetic divergence from 11168 within gene clusters for synthesis/modification of flagella, LOS and capsule (Wilson et al., 2010), and many contingency genes lie within these three divergent gene clusters. In our study, only five contingency genes (Cj0045, Cj0170, Cj0685, Cj1305/06/10 and Cj1420) were sufficiently conserved to enable fragment analysis in all three human disease isolates. We hypothesize that these five conserved contingency loci play an important role in successful colonization of humans. In support of this hypothesis, four of these five conserved contingency genes exhibited large (Cj0045 and Cj0685) or moderate (Cj0170 and Cj1305) allele shifts after passage through chickens and/or mice. We propose that fragment analysis of predominant alleles for these specific contingency genes may eventually be useful in predicting the potential virulence of C. jejuni strains isolated from the chicken reservoir or from human patients. These data may allow more informed treatment of patients and may help in developing novel and effective vaccines targeted at stable cell-surface antigens.
Acknowledgements
This work was funded by NIH grant R21 AI081714-01, the National Food Safety and Toxicology Center (Michigan State University) and the Michigan Agricultural Experiment Station. We greatly appreciate assistance with animal care by Linda Mansfield, Julia Bell, Jamie Kopper and John Jerome. We also thank Wenzhao Yang for statistical analysis. The technical support of the staff at RTSF is also greatly appreciated. The views expressed in this article do not necessarily represent the views of the FDA or the United States Government.
Abbreviations:
- CP
chicken-passaged
- GI
gastrointestinal
- HT
homopolymeric tract
- LOS
lipooligosaccharide
Footnotes
Two supplementary tables, listing PCR primer sequences for fragment and sequence analysis used in this study, and results of sequence and fragment analysis to confirm large shifts in allele frequency, with a supplementary reference, are available with the online version of this paper.
References
- Ang C. W., Dijkstra J. R., de Klerk M. A., Endtz H. P., van Doorn P. A., Jacobs B. C., Jeurissen S. H. M., Wagenaar J. A. (2010). Host factors determine anti-GM1 response following oral challenge of chickens with Guillain-Barré syndrome derived Campylobacter jejuni strain GB11. PLoS ONE 5, e9820. 10.1371/journal.pone.0009820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon D. J., Alm R. A., Burr D. H., Hu L., Kopecko D. J., Ewing C. P., Trust T. J., Guerry P. (2000). Involvement of a plasmid in virulence of Campylobacter jejuni 81-176. Infect Immun 68, 4384–4390. 10.1128/IAI.68.8.4384-4390.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbontín R., Rowley G., Pucciarelli M. G., López-Garrido J., Wormstone Y., Lucchini S., García-Del Portillo F., Hinton J. C., Casadesús J. (2006). DNA adenine methylation regulates virulence gene expression in Salmonella enterica serovar Typhimurium. J Bacteriol 188, 8160–8168. 10.1128/JB.00847-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batz M. B., Hoffmann D., Morris J. G. (2011). Report. Ranking the Risks: the 10 Pathogen-Food Combinations with the Greatest Burden on Public Health. Gainesville FL: University of Florida, Emerging Pathogens Institute. [Google Scholar]
- Bell J. A., St Charles J. L., Murphy A. J., Rathinam V. A., Plovanich-Jones A. E., Stanley E. L., Wolf J. E., Gettings J. R., Whittam T. S., Mansfield L. S. (2009). Multiple factors interact to produce responses resembling spectrum of human disease in Campylobacter jejuni infected C57BL/6 IL-10−/− mice. BMC Microbiol 9, 57. 10.1186/1471-2180-9-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J. (1997). Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis 176 (Suppl. 2), S103–S105. 10.1086/513780 [DOI] [PubMed] [Google Scholar]
- Dasti J. I., Tareen A. M., Lugert R., Zautner A. E., Groß U. (2010). Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol 300, 205–211. 10.1016/j.ijmm.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Fälker S., Schmidt M. A., Heusipp G. (2005). DNA methylation in Yersinia enterocolitica: role of the DNA adenine methyltransferase in mismatch repair and regulation of virulence factors. Microbiology 151, 2291–2299. 10.1099/mic.0.27946-0 [DOI] [PubMed] [Google Scholar]
- French C. E., Bell J. M. L., Ward F. B. (2008). Diversity and distribution of hemerythrin-like proteins in prokaryotes. FEMS Microbiol Lett 279, 131–145. 10.1111/j.1574-6968.2007.01011.x [DOI] [PubMed] [Google Scholar]
- Guerry P. (2007). Campylobacter flagella: not just for motility. Trends Microbiol 15, 456–461. 10.1016/j.tim.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Guerry P., Szymanski C. M. (2008). Campylobacter sugars sticking out. Trends Microbiol 16, 428–435. 10.1016/j.tim.2008.07.002 [DOI] [PubMed] [Google Scholar]
- Guerry P., Szymanski C. M., Prendergast M. M., Hickey T. E., Ewing C. P., Pattarini D. L., Moran A. P. (2002). Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect Immun 70, 787–793. 10.1128/IAI.70.2.787-793.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogdu O., Bentley S. D., Holden M. T., Parkhill J., Dorrell N., Wren B. W. (2007). Re-annotation and re-analysis of the Campylobacter jejuni NCTC11168 genome sequence. BMC Genomics 8, 162. 10.1186/1471-2164-8-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey T., O’Brien S., Madsen M. (2007). Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol 117, 237–257. 10.1016/j.ijfoodmicro.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Jerome J. P., Bell J. A., Plovanich-Jones A. E., Barrick J. E., Brown C. T., Mansfield L. S. (2011). Standing genetic variation in contingency loci drives the rapid adaptation of Campylobacter jejuni to a novel host. PLoS ONE 6, e16399. 10.1371/journal.pone.0016399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M. E., Norström M., Sandberg M., Ersbøll A. K., Hofshagen M. (2010). Space–time patterns of Campylobacter spp. colonization in broiler flocks, 2002–2006. Epidemiol Infect 138, 1336–1345. 10.1017/S0950268810000051 [DOI] [PubMed] [Google Scholar]
- Karlyshev A. V., Linton D., Gregson N. A., Wren B. W. (2002). A novel paralogous gene family involved in phase-variable flagella-mediated motility in Campylobacter jejuni. Microbiology 148, 473–480. [DOI] [PubMed] [Google Scholar]
- Karlyshev A. V., Champion O. L., Churcher C., Brisson J. R., Jarrell H. C., Gilbert M., Brochu D., St Michael F., Li J. & other authors (2005). Analysis of Campylobacter jejuni capsular loci reveals multiple mechanisms for the generation of structural diversity and the ability to form complex heptoses. Mol Microbiol 55, 90–103. 10.1111/j.1365-2958.2004.04374.x [DOI] [PubMed] [Google Scholar]
- Linton D., Gilbert M., Hitchen P. G., Dell A., Morris H. R., Wakarchuk W. W., Gregson N. A., Wren B. W. (2000). Phase variation of a β-1,3 galactosyltransferase involved in generation of the ganglioside GM1-like lipo-oligosaccharide of Campylobacter jejuni. Mol Microbiol 37, 501–514. 10.1046/j.1365-2958.2000.02020.x [DOI] [PubMed] [Google Scholar]
- Mansfield L. S., Gauthier D. T., Abner S. R., Jones K. M., Wilder S. R., Urban J. F. (2003). Enhancement of disease and pathology by synergy of Trichuris suis and Campylobacter jejuni in the colon of immunologically naïve swine. Am J Trop Med Hyg 68, 70–80. [PubMed] [Google Scholar]
- Mansfield L. S., Bell J. A., Wilson D. L., Murphy A. J., Elsheikha H. M., Rathinam V. A. K., Fierro B. R., Linz J. E., Young V. B. (2007). C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect Immun 75, 1099–1115. 10.1128/IAI.00833-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield L. S., Patterson J. S., Fierro B. R., Murphy A. J., Rathinam V. A., Kopper J. J., Barbu N. I., Onifade T. J., Bell J. A. (2008). Genetic background of IL-10−/− mice alters host–pathogen interactions with Campylobacter jejuni and influences disease phenotype. Microb Pathog 45, 241–257. 10.1016/j.micpath.2008.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally D. J., Lamoureux M. P., Karlyshev A. V., Fiori L. M., Li J. J., Thacker G., Coleman R. A., Khieu N. H., Wren B. W. & other authors (2007). Commonality and biosynthesis of the O-methyl phosphoramidate capsule modification in Campylobacter jejuni. J Biol Chem 282, 28566–28576. 10.1074/jbc.M704413200 [DOI] [PubMed] [Google Scholar]
- Miller C. E., Williams P. H., Ketley J. M. (2009). Pumping iron: mechanisms for iron uptake by Campylobacter. Microbiology 155, 3157–3165. 10.1099/mic.0.032425-0 [DOI] [PubMed] [Google Scholar]
- Morooka T., Umeda A., Amako K. (1985). Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol 131, 1973–1980. [DOI] [PubMed] [Google Scholar]
- Moxon R., Bayliss C., Hood D. (2006). Bacterial contingency loci: the role of simple sequence DNA repeats in bacterial adaptation. Annu Rev Genet 40, 307–333. 10.1146/annurev.genet.40.110405.090442 [DOI] [PubMed] [Google Scholar]
- Oshima T., Wada C., Kawagoe Y., Ara T., Maeda M., Masuda Y., Hiraga S., Mori H. (2002). Genome-wide analysis of deoxyadenosine methyltransferase-mediated control of gene expression in Escherichia coli. Mol Microbiol 45, 673–695. 10.1046/j.1365-2958.2002.03037.x [DOI] [PubMed] [Google Scholar]
- Parkhill J., Wren B. W., Mungall K., Ketley J. M., Churcher C., Basham D., Chillingworth T., Davies R. M., Feltwell T. & other authors (2000). The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403, 665–668. 10.1038/35001088 [DOI] [PubMed] [Google Scholar]
- Rosqvist R., Skurnik M., Wolf-Watz H. (1988). Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature 334, 522–524. 10.1038/334522a0 [DOI] [PubMed] [Google Scholar]
- Sang F. C., Shane S. M., Yogasundram K., Hagstad H. V., Kearney M. T. (1989). Enhancement of Campylobacter jejuni virulence by serial passage in chicks. Avian Dis 33, 425–430. 10.2307/1591100 [DOI] [PubMed] [Google Scholar]
- Scallan E., Hoekstra R. M., Angulo F. J., Tauxe R. V., Widdowson M. A., Roy S. L., Jones J. L., Griffin P. M. (2011). Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis 17, 7–15. 10.3201/eid1701.09-1101p1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelling W. J., Matsuda M., Moore J. E., Dooley J. S. G. (2005). Campylobacter jejuni. Lett Appl Microbiol 41, 297–302. 10.1111/j.1472-765X.2005.01788.x [DOI] [PubMed] [Google Scholar]
- Srikhanta Y. N., Fox K. L., Jennings M. P. (2010). The phasevarion: phase variation of type III DNA methyltransferases controls coordinated switching in multiple genes. Nat Rev Microbiol 8, 196–206. 10.1038/nrmicro2283 [DOI] [PubMed] [Google Scholar]
- van der Woude M. W., Bäumler A. J. (2004). Phase and antigenic variation in bacteria. Clin Microbiol Rev 17, 581–611. 10.1128/CMR.17.3.581-611.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ham S. M., van Alphen L., Mooi F. R., van Putten J. P. (1993). Phase variation of H. influenzae fimbriae: transcriptional control of two divergent genes through a variable combined promoter region. Cell 73, 1187–1196. 10.1016/0092-8674(93)90647-9 [DOI] [PubMed] [Google Scholar]
- Wassenaar T. M. (2011). Following an imaginary Campylobacter population from farm to fork and beyond: a bacterial perspective. Lett Appl Microbiol 53, 253–263. 10.1111/j.1472-765X.2011.03121.x [DOI] [PubMed] [Google Scholar]
- Wassenaar T. M., Wagenaar J. A., Rigter A., Fearnley C., Newell D. G., Duim B. (2002). Homonucleotide stretches in chromosomal DNA of Campylobacter jejuni display high frequency polymorphism as detected by direct PCR analysis. FEMS Microbiol Lett 212, 77–85. 10.1111/j.1574-6968.2002.tb11248.x [DOI] [PubMed] [Google Scholar]
- Willems R., Paul A., van der Heide H. G. J., ter Avest A. R., Mooi F. R. (1990). Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. EMBO J 9, 2803–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. L., Bell J. A., Young V. B., Wilder S. R., Mansfield L. S., Linz J. E. (2003). Variation of the natural transformation frequency of Campylobacter jejuni in liquid shake culture. Microbiology 149, 3603–3615. 10.1099/mic.0.26531-0 [DOI] [PubMed] [Google Scholar]
- Wilson D. L., Rathinam V. A. K., Qi W., Wick L. M., Landgraf J., Bell J. A., Plovanich-Jones A., Parrish J., Finley R. L. & other authors (2010). Genetic diversity in Campylobacter jejuni is associated with differential colonization of broiler chickens and C57BL/6J IL10-deficient mice. Microbiology 156, 2046–2057. 10.1099/mic.0.035717-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R., Burr D. H., Guerry P. (1997). CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol 23, 1021–1031. 10.1046/j.1365-2958.1997.2861650.x [DOI] [PubMed] [Google Scholar]
- Young K. T., Davis L. M., Dirita V. J. (2007). Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol 5, 665–679. 10.1038/nrmicro1718 [DOI] [PubMed] [Google Scholar]
- Yuki N. (2010). Human gangliosides and bacterial lipo-oligosaccharides in the development of autoimmune neuropathies. Methods Mol Biol 600, 51–65. 10.1007/978-1-60761-454-8_4 [DOI] [PubMed] [Google Scholar]
- Zhao S., Young S. R., Tong E., Abbott J. W., Womack N., Friedman S. L., McDermott P. F. (2010). Antimicrobial resistance of Campylobacter isolates from retail meat in the United States between 2002 and 2007. Appl Environ Microbiol 76, 7949–7956. 10.1128/AEM.01297-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. G., Hua X. G., Wu Z. L., Yi M. M. (2006). Molecular mechanisms of pathogenesis of Campylobacter jejuni. Rev Med Microbiol 17, 39–43. 10.1097/01.revmedmi.0000244133.66040.7e [DOI] [Google Scholar]


