Abstract
Proteolytic control can govern the levels of specific regulatory factors, such as Spx, a transcriptional regulator of the oxidative stress response in Gram-positive bacteria. Under oxidative stress, Spx concentration is elevated and upregulates transcription of genes that function in the stress response. When stress is alleviated, proteolysis of Spx catalysed by ClpXP reduces Spx concentration. Proteolysis is enhanced by the substrate recognition factor YjbH, which possesses a His–Cys-rich region at its N terminus. However, mutations that generate H12A, C13A, H14A, H16A and C31/34A residue substitutions in the N terminus of Bacillus subtilis YjbH (BsYjbH) do not affect functionality in Spx proteolytic control in vivo and in vitro. Because of difficulties in obtaining soluble BsYjbH, the Geobacillus thermodenitrificans yjbH gene was cloned, which yielded soluble GtYjbH protein. Despite its lack of a His–Cys-rich region, GtYjbH complements a B. subtilis yjbH null mutant, and shows high activity in vitro when combined with ClpXP and Spx in an approximately 30 : 1 (ClpXP/Spx : GtYjbH) molar ratio. In vitro interaction experiments showed that Spx and the protease-resistant SpxDD (in which the last two residues of Spx are replaced with two Asp residues) bind to GtYjbH, but deletion of 12 residues from the Spx C terminus (SpxΔC) significantly diminished interaction and proteolytic degradation, indicating that the C terminus of Spx is important for YjbH recognition. These experiments also showed that Spx, but not GtYjbH, interacts with ClpX. Kinetic measurements for Spx proteolysis by ClpXP in the presence and absence of GtYjbH suggest that YjbH overcomes non-productive Spx–ClpX interaction, resulting in rapid degradation.
Introduction
The global regulator Spx, which activates gene transcription in response to disulfide stress, is a substrate of the ATP-dependent protease ClpXP (Nakano et al., 2003). Proteolytic regulation is mediated by YjbH, a putative substrate recognition factor for ClpXP-catalysed Spx proteolysis, to maintain low or pre-stress levels of Spx (Larsson et al., 2007). As with Spx, YjbH is highly conserved in Gram-positive bacteria. Studies of YjbH have revealed its potential biological importance, for example, in control of haemolytic activity in Listeria monocytogenes (Zemansky et al., 2009) and glycopeptide sensitivity and desiccation tolerance in Staphylococcus aureus (Charbonnier et al., 2005; Chaibenjawong & Foster, 2011). Additionally, inactivation of YjbH leads to moderate resistance to oxacillin and other β-lactam antibiotics in S. aureus (Göhring et al., 2011). All of the studies suggested that YjbH plays important roles in pathogenicity, probably due to its contribution to the regulation of the oxidative stress response.
YjbH is functionally related to the adaptor proteins that confer substrate specificity to regulated proteolysis catalysed by ATP-dependent Clp proteases, such as ClpAP and ClpXP (Dougan et al. 2002; Levchenko et al., 2000). The proteases are composed of a hexameric, ATP-hydrolysing ‘unfoldase’ (ClpA or ClpX) and two heptameric rings of the ClpP subunit (Porankiewicz et al., 1999). The ClpP multimer forms the proteolytic chamber that receives the unfolded substrate protein from the hexameric ATPase component of the protease. ClpS and SspB are adaptor proteins that tether substrates to the protease ATPase component (ClpA or ClpX, respectively). This is accomplished through direct interaction of the adaptor with both substrate and protease.
YjbH protein of Bacillus subtilis has been obtained from an insoluble state by denaturation and renaturation during affinity column purification (Garg et al., 2009). Zinc was required for renaturation and solubility, and studies of intact and N-terminal-truncated protein have suggested that the N-terminal His–Cys rich region was necessary for Zn atom coordination. A proposed model of YjbH control predicted that oxidation would result in Zn release and inactivation of YjbH with subsequent release of Spx from proteolytic control (Garg et al., 2009). However, the putative Zn-binding N-terminal region is not conserved among all YjbH orthologues. Recent studies of S. aureus YjbH have provided evidence that the Cys residues associated with the N-terminal region of YjbH are not necessary for activity (Engman et al., 2012). Alternative explanations for regulation of YjbH-mediated proteolysis of Spx may have to be considered. For example, a small protein, YirB, has been shown to inhibit the Spx-proteolysis-enhancing activity of YjbH activity in vivo and in vitro through direct YjbH interaction (Kommineni et al., 2011), although YirB’s physiological role is uncertain at this time.
In this study, the N-terminal His–Cys-rich region of the YjbH protein was subjected to amino acid substitutions to create mutant YjbH derivatives that were tested in vivo and in vitro for proteolysis-enhancing activity. The results indicate that the residues do not function in proteolytic control of Spx in B. subtilis. Because of the uncertainties associated with assaying activity of renatured protein preparations, a YjbH orthologue (GtYjbH) was identified that is produced by Geobacillus thermodenitrificans. GtYjbH was produced from an Escherichia coli expression system in soluble form. The gene encoding GtYjbH can complement a yjbH null mutant of B. subtilis and the complemented strain is responsive to oxidative stress by induction of Spx activity. This was observed despite the absence in GtYjbH of most of the His–Cys rich region that coordinates Zn in the YjbH orthologue of B. subtilis. Unlike renatured B. subtilis YjbH (BsYjbH), GtYjbH is highly active, mediating rapid substrate degradation in reactions containing approximately 30-fold less GtYjbH than ClpXP or Spx in terms of molar equivalents. Unlike other well-studied adaptors (Dougan et al., 2002; Levchenko et al., 2000; Persuh et al., 2002; Schlothauer et al., 2003), no evidence of YjbH–ClpX interaction was detected. However, like the adaptor SspB (Levchenko et al., 2005), YjbH requires recognition of the C-terminal residues of Spx to enhance ClpXP-catalysed proteolysis.
Methods
Bacterial strains, plasmids and chemicals.
B. subtilis strains and plasmids are listed in Table S1 (available with the online version of this paper) and are all JH642 derivatives. G. thermodenitrificans was obtained from the Bacillus Genetic Stock Center (Columbus, Ohio). E. coli strain DH5α was used for general cloning procedures. Plasmid pET-23a and E. coli strain BL21(DE3)pLysS were from Novagen, and were used in heterologous protein production. Medium components were from Difco. All restriction/modifying enzymes were from New England Biolabs. Oligonucleotide primers (Table S2) were from Invitrogen. The Ni-nitrilotriacetic acid (NTA) resin was from 5 PRIME (PerfectPro Ni-NTA agarose) and PCR/plasmid purification kits were from Qiagen. High-Q anion exchange and heparin prepack columns were from Bio-Rad. All analytical grade chemicals were from Sigma–Aldrich unless otherwise stated.
Construction of yjbH mutants.
Codon substitutions introduced into the N-terminal coding end of the yjbH gene were generated by a two-step PCR procedure described previously (Nakano et al., 2010). Complementary oligonucleotides (Table S2) specifying each nucleotide substitution that would change YjbH codons to Ala codons were designed: for H12A, oligonucleotides SG-08-13 and SG-08-14; C13A, oligonucleotides SG-08-15 and SG-08-16; H14A, oligonucleotides SG-08-17 and SG-08-18; H16A, oligonucleotides SG-08-19 and SG-08-20; C31A/34A, oligonucleotides SG-08-23 and SG-08-24. The reverse primer of the mutagenic complementary pair was combined with SG-08-4 for PCR of the N-terminal coding end of the YjbH coding sequence, and the forward primer of the mutagenic complementary pair was combined with oligonucleotide SG-07-4 to amplify the C-terminal ~900 bp of the YjbH coding sequence. The two products of the separate PCRs were then combined together with oligonucleotides SG-08-4 and SG-07-4 (Table S2) in a PCR to generate the intact mutant yjbH coding sequence. For production of mutant YjbH protein, the fragments were cleaved with NheI and XhoI and ligated into NheI/XhoI-cleaved pET23a. The ligations were used to transform strain DH5α, and then in strain BL21(DE3)pLysS for overexpression. The plasmids generated are listed in Table 1. To construct mutant versions of pDR111 plasmids bearing yjbH, PCR products were obtained with primers SG-08-8 and SG-08-9 (Table S2) for PCR amplification using pET23a plasmids carrying yjbH mutant alleles as templates. The PCR fragment generated contained the complete mutant yjbH coding sequence with a His6 tag. The fragment was cleaved with HindIII and NheI, followed by ligation with HindIII/NheI-cleaved pDR111 (Britton et al., 2002). The ligations were used to transform strain DH5α. The resulting mutant plasmids (Table S1) were transformed into B. subtilis strains for complementation using the same method as for pEC-22 (GtyjbH) described below.
Table 1. Kinetic measurements of Spx proteolysis by ClpXP in the presence and absence of GtYjbH.
| Substrate | His-tag adaptor | Vmax (µM min−1) | Km (µM) | kcat (s−1) | kcat/Km (µM s−1) |
| BsSpx | − GtYjbH | 0.24±0.06 | 0.42±0.17 | 0.0081±0.0020 | 21324±7956 |
| BsSpx | + GtYjbH | 1.98±0.48 | 1.79±0.66 | 0.0659±0.0161 | 38320±8729 |
Assay of β-galactosidase activity.
Cells were grown in Difco sporulation medium (DSM) (Nicholson & Setlow, 1990) to OD600 0.3–0.4 at 37 °C, followed by induction with 1 mM IPTG, and samples were withdrawn at 30 min intervals during growth. β-Galactosidase activity in each sample was determined as described previously (Nakano et al., 1988) and is presented as Miller units (Miller, 1972).
Diamide treatment.
Mid-exponential-phase cells in minimal TSS medium (Fouet et al., 1990) were treated with 0.5 mM IPTG to induce mutant yjbH expression at 37 °C. After 30 min, cells were treated with 1 mM diamide (1 M stock). Culture samples were collected at 0, 10 and 25 min after diamide treatment. Whole-cell lysates were prepared by the protoplast isolation method (Harwood & Cutting, 1990). Protein samples (20 µg) were resolved by 15 % SDS-PAGE. The protein was transferred onto a nitrocellulose membrane and immunoblotting was performed to detect Spx, BsYjbH and GtYjbH with anti-Spx (Nakano et al., 2001), anti-BsYjbH (Kommineni et al., 2011) and anti-His antibodies, respectively.
In vitro proteolysis reactions.
In vitro proteolysis was carried out in 60 µl reaction mixtures containing 50 mM HEPES-KOH (pH 7.6), 50 mM KCl, 10 mM magnesium acetate, 5 mM DTT, 5 mM ATP, 5 mM creatine phosphate, 0.05 U creatine kinase ml−1 (Sigma), and ClpX, ClpP, Spx and YjbH (concentrations are indicated in figure legends) at 37 °C. At the indicated time intervals (see figure legends), a 12 µl sample from each reaction mixture was collected, treated with 3 µl 5× SDS loading dye (with 0.1 M DTT), and heated at 95 °C for 5 min. The protein samples were resolved on a 15 % SDS-PAGE gel, visualized by Coomassie blue R-250 staining. Levels of Spx after proteolysis were determined as ratios of Spx to ClpP band intensities, since ClpP concentrations in each set of reactions were equal. The Spx : ClpP value in a reaction mixture at 0 min time point was defined as 100 % (Zhang & Zuber, 2007).
Protein purification.
For G. thermodenitrificans YjbH–His6, the yjbH coding sequence was amplified by PCR from G. thermodenitrificans genomic DNA, using the forward primer EC-26 and the reverse primer EC-28 (Table S2). PCR products were inserted into pET23a to construct pEC-21. The pEC-21-transformed E. coli BL21(DE3)pLysS cells were grown at 37 °C in LB broth containing 50 µg ampicillin ml−1 and 5 µg chloramphenicol ml−1 to OD600 0.4–0.5, and expression was induced with 0.5 mM IPTG for 3 h at 30 °C. Cells from a 1 l culture were suspended in 20 ml buffer A (20 mM Tris/HCl, pH 7.6, 200 mM NaCl). One tablet of EDTA-free protease inhibitor cocktail (Roche Applied Science) was added per 20 ml suspended cells. The cells were subjected to freeze–thaw cycles and disrupted by French press. The lysate was cleared by centrifugation at 15 000 r.p.m. (Sorvall, rotor SL-50T) for 30 min. The soluble fraction from the whole-cell lysate was applied to a 5 ml Ni2+-NTA affinity agarose (5 PRIME) column pre-equilibrated with buffer A. The column was incubated and gently rotated for 1 h at 4 °C. The column was washed with 20 column volumes (CVs) buffer W (20 mM Tris/HCl, pH 7.6, 200 mM NaCl, 30 mM imidazole). The bound protein was eluted in buffer E (20 mM Tris/HCl, pH 7.6, 200 mM NaCl, 250 mM imidazole). Eluted fractions that contain GtYjbH–His6 were pooled and diluted with buffer QA [20 mM Tris/HCl (pH 8.5), 2 % glycerol (v/v), 1 mM DTT] to lower the salt concentration to 40 mM. The diluted sample was applied to a High Q or heparin column equilibrated with buffer QA. The bound proteins were eluted with buffer QB (buffer QA containing 1 M NaCl) in a 0–100 % gradient. Fractions containing GtYjbH–His6 were resolved on a 15 % SDS-PAGE gel, visualized by Coomassie blue staining, concentrated and stored in buffer (20 mM Tris/HCl, pH 7.6, 100 mM NaCl, 10 % glycerol) at −80 °C. Protein concentration was measured using Bio-Rad protein assay, and BSA was used to generate the standard curve.
ClpX and ClpP were purified as described in previous studies using the intein-mediated purification with an affinity chitin-binding tag (IMPACT) system (Liu et al., 1999; Nakano et al., 2002). His6-tagged wild-type Spx and SpxDD proteins were purified using a previously published procedure (Nakano et al., 2003). The histidine tag on Spx was removed using γTEV (tobacco etch virus) protease (provided by Kelly Chacon, Ohio State University) (Nakano et al., 2005). SpxHA and SpxΔCHA were purified using the IMPACT system and RNA polymerase was purified using a Ni affinity column as described in previous studies (Nakano et al., 2005).
Complementation of yjbH with His-tagged GtyjbH.
To obtain a GtyjbH–His6 gene, including the ribosome-binding site, for complementation analysis, DNA was amplified by PCR from pEC-21 using the forward primer EC-4 and the reverse primer EC-29 (Table S2). PCR products were digested with HindIII and NheI and cloned at identical sites in plasmid pDR111 to generate pEC-22. pEC-22 was used to transform strain ORB4541 (JH642, trxB : : trxB-lacZ), and transformants were selected on DSM agar containing 75 µg spectinomycin ml−1 and 5 µg chloramphenicol ml−1. To confirm the integration of GtyjbH–His6 at the amyE locus, clones were streaked on an LB-starch plate and grown for 16 h at 37 °C. The resulting amylase-negative strain (ORB8166, amyE : : GtyjbH–His6, trxB : : trxB–lacZ) was transformed with chromosomal DNA of ORB6952 (JH642, yjbH : : tetf) with selection on DSM agar containing 75 µg spectinomycin ml−1, 12.5 µg tetracycline ml−1 and 5 µg chloramphenicol ml−1. The resulting strain (ORB8169) was used to determine if GtyjbH–His6 complements the yjbH-null mutation by testing Spx protein level in the absence and presence of diamide and by assaying for trxB-directed β-galactosidase activity as described above.
In vitro affinity interaction assays using Ni-chelate chromatography.
Different combinations of proteins (indicated in figure legends) Spx or His6–Spx (2.5 µM) or SpxDD (1.8 µM), GtYjbH (2.5 µM), ClpX (4 µM), ClpP (4 µM), ATP or ATP-γ-S (80 µM) were incubated in buffer A (100 mM Tris/HCl, pH 8.5, 50 mM NaCl) for 10 min at room temperature. The protein mixtures were then applied to a 50 µl Ni2+-NTA affinity agarose column (5 PRIME) that was pre-equilibrated with buffer A. The column was washed with 20 CVs buffer W. The bound protein was eluted three times with 1 CV each time in 20 mM Tris/HCl, pH 7.6, 200 mM NaCl, 250 mM imidazole. The input, flow-through, wash and eluent samples were resolved on a 15 % SDS-PAGE gel, followed by ‘Blue silver’ colloidal Coomassie G-250 protein staining: gels were washed with water, then gently shaken in washing/fixing solution (40 % methanol, 10 % phosphoric acid) twice for 30 min per wash and then in staining solution (0.12 % Coomassie G-250, 10 % phosphoric acid, 10 % ammonium sulfate, 20 % methanol) for a minimum of overnight to a maximum of 24 h.
Results
Mutagenesis studies on the His–Cys-rich domain of B. subtilis YjbH
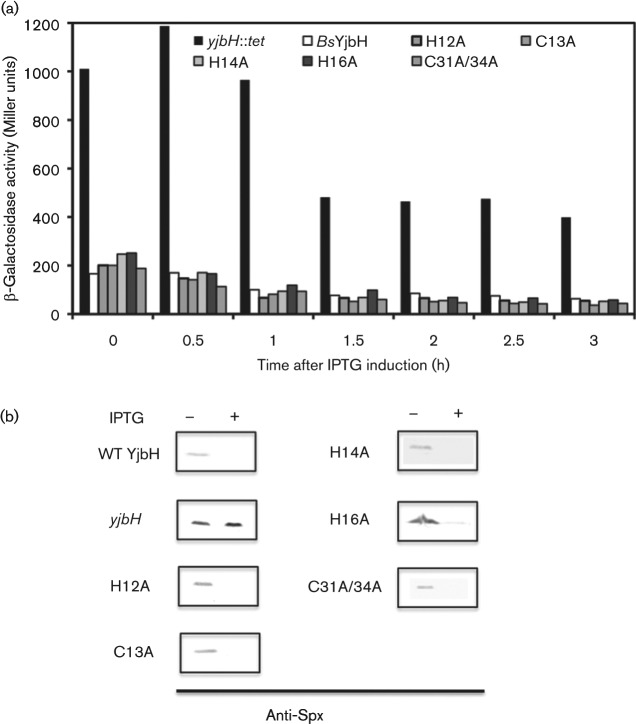
Sequence alignments show that B. subtilis and G. thermodenitrificans share a conserved YjbH [identities = 150/273 (55 %), similarities = 199/273 (73 %)] with a putative redox CxxC motif, but Geobacillus lacks a His–Cys-rich region at the N terminus (Fig. S1). Previous studies suggested that the His–Cys-rich region is important for Zn2+ binding, for interaction between Spx and YjbH, and also for the enhancement of ClpXP-dependent Spx proteolysis (Garg et al., 2009). To study the roles of the N-terminal His and Cys residues in the BsYjbH, mutants bearing amino acid substitutions (H12A, C13A, H14A, H16A and C31A/34A) were constructed and tested by complementation in a yjbH-null strain. Mutated yjbH encoding a product with six histidines appended to the C terminus was introduced into the amyE locus as a single-copy, IPTG-inducible construct in the B. subtilis. A yjbH-null strain with only the IPTG-inducible promoter (Physpank) in the amyE locus was used as negative control. The Spx level was determined by monitoring the β-galactosidase activity of a promoterless lacZ gene, which is fused with the promoter region of trxB (thioredoxin reductase gene) – a gene that requires Spx for optimal expression. The β-galactosidase activities of all strains producing the BsYjbH mutants upon IPTG induction were similar to those of strains producing wild-type (WT) BsYjbH (Fig. 1a). Also, immunoblot analysis of samples collected 1 h after IPTG induction showed decreased levels of Spx in WT and in strains producing mutant BsYjbH (Fig. 1b), suggesting that the mutant YjbH proteins are functional in ClpXP-dependent proteolytic control of Spx following recovery from oxidative stress. These results indicate that all the mutants showed little if any defect in YjbH-dependent enhancement of Spx proteolysis. An oxidant treatment experiment in which the thiol-specific oxidant, diamide (1 mM) was added to the cultures showed that BsYjbH mutants responded to oxidant in the same fashion as WT BsYjbH as is evident by the increase in the Spx level (data not shown). In vitro proteolysis assays showed that WT and mutant BsYjbH enhanced ClpXP-catalysed Spx proteolysis, and addition of the YjbH inhibitor YirB reduced proteolysis (Fig. S2). Taken together, the data support the conclusion that the His and Cys residues in the N terminus are not essential for function of BsYjbH in the enhancement of ClpXP-catalysed Spx proteolysis.
Fig. 1.
BsYjbH mutants show similar activity to wild-type BsYjbH. (a) Effect of the wild-type and mutant on Spx-dependent expression of trxB–lacZ. The graph shows the IPTG induction time-course of β-galactosidase activity encoded by the trxB–lacZ fusion in yjbH mutant cells bearing the Physpank–BsyjbH (white) and mutants [coloured (see key)] or the Physpank promoter (black) in a yjbH-null mutant background. β-Galactosidase activity is expressed as Miller units. (b) Immunoblot analysis of Spx level using anti-Spx antiserum (Nakano et al., 2002). Cell samples were collected before IPTG induction and at 1 h after IPTG induction from the strains in (a).
Complementation of GtYjbH in a B. subtilis yjbH strain
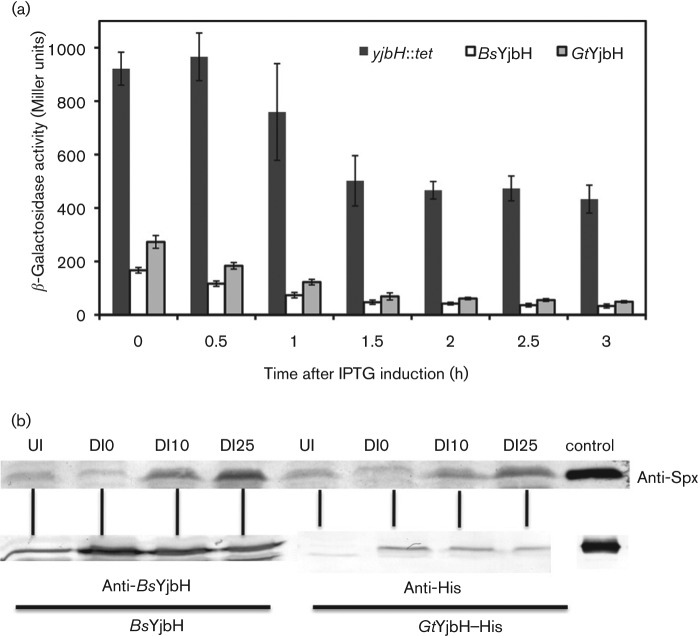
As stated earlier, GtYjbH lacks the putative Zn-binding motif at the N terminus, but still shares significant sequence similarities with BsYjbH, including the putative redox-sensing CxxC motif. Experiments were conducted to test if GtYjbH was able to complement the B. subtilis yjbH-null strain, and to determine if GtYjbH activity was responsive to oxidant treatment. A strain was constructed that bears an IPTG-inducible GtyjbH–His6 construct at the amyE locus. A complementation assay was conducted in the same way as described in the previous section. GtYjbH proteins behaved in the same fashion as BsYjbH as shown in two in vivo assays: (1) both showed greatly decreased trxB-directed β-galactosidase activity compared with yjbH-null mutation (Fig. 2a); (2) both were sensitive to oxidant, with increased Spx concentration observed after treatment with 1 mM diamide (Fig. 2b). The data show that although GtYjbH lacks the His–Cys-rich motif, it exhibits similar functionality to BsYjbH with respect to control of Spx proteolysis in vivo.
Fig. 2.
GtYjbH mutants show similar activities to BsYjbH. (a) BsYjbH and GtYjbH affect Spx-dependent expression of trxB–lacZ. The graph shows the time-course of β-galactosidase activity encoded by the trxB–lacZ fusion in cells bearing the Physpank–BsyjbH (white) and GtyjbH (grey) or the Physpank promoter (black) in a yjbH-null mutant background. (b) Immunoblot analysis of Spx level before and after 1 mM diamide treatment at 0 (DI0), 10 (DI10), 25 (DI25) min time points. UI denotes uninduced. Cells were induced by IPTG for 30 min before diamide treatment. Anti-Spx antiserum, anti-BsYjbH antiserum and anti-His antibodies were used to detect Spx, BsYjbH and GtYjbH–His6 levels. Control is purified Spx, BsYjbH–Strep and GtYjbH–His6 protein.
GtYjbH enhances ClpXP-catalysed proteolysis of Spx
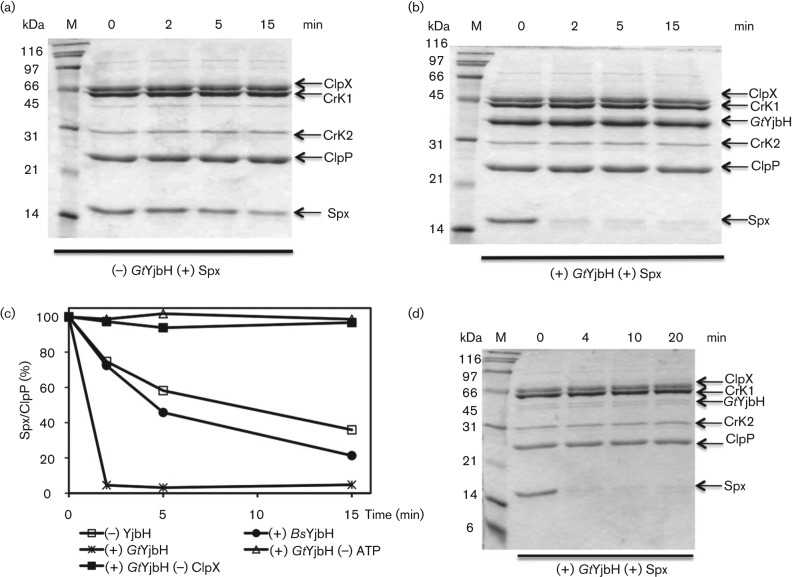
In vivo studies showed that GtYjbH was functional in B. subtilis. Expression of GtyjbH complemented a B. subtilis yjbH-null mutant and was responsive to diamide treatment. To test the ability of GtYjbH to facilitate Spx proteolysis in vitro, a proteolysis assay reaction composed of Spx, ClpXP, ATP and a creatine kinase/creatine-phosphate ATP-regenerating system was performed in the presence and absence of GtYjbH (Fig. 3a, b). Notably, in contrast with BsYjbH, GtYjbH was partially soluble, and could be purified using a Ni-column and HiQ or heparin column chromatography to reach >95 % purity (Fig. S3). Compared with renatured BsYjbH, the same concentration of GtYjbH (4 µM) showed a much higher activity in terms of Spx (8 µM) degradation. In 2 min, 90 % of Spx was degraded by ClpXP (Fig. 3c). Interestingly, a low concentration (0.16 µM) of GtYjbH added to the proteolysis reaction resulted in 90 % degradation of Spx (5 µM) in 4 min (Fig. 3d). This result suggested that the adaptor GtYjbH may act catalytically to enhance Spx proteolysis by ClpXP. As expected, this activity is also ATP- and ClpX-dependent (Fig. 3c).
Fig. 3.
GtYjbH enhanced Spx proteolysis mediated by ClpXP, which is ATP- and ClpX-dependent. (a, b) SDS-PAGE shows the effect of GtYjbH on ClpXP-catalysed proteolysis of Spx in vitro in a time-course experiment. Spx (8 µM), ClpX (3 µM) and ClpP (8 µM) with an ATP-generating system (creatine kinase) were incubated at 37 °C for the times (min) indicated in the absence (a) or presence (b) of GtYjbH (4 µM). (c) Plot of Spx band intensities against the reaction time in the experiments shown in the graph. The intensities of ClpP protein in each lane were used as internal controls (Zhang & Zuber, 2007). The Spx : ClpP ratio at 0 min was defined as 100 %. (d) Proteolysis assay under the same conditions as in (a, b) but in the presence of 0.16 µM GtYjbH.
The C terminus of Spx is required for YjbH binding
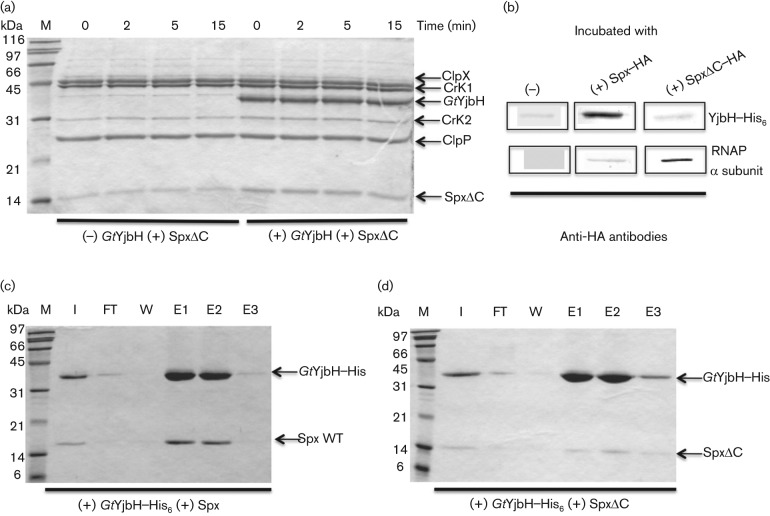
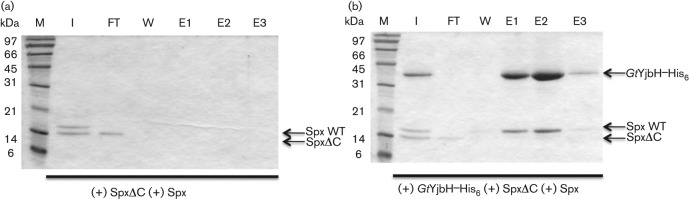
A series of experiments were carried out to uncover the determinants mediating interaction between Spx and GtYjbH. Previous studies showed that a mutant Spx having a 12 amino acid deletion of its C-terminal end (SpxΔC) was present at high concentration in vivo (Lin & Zuber, 2012), suggesting that removal of the C-terminal residues renders Spx resistant to proteolysis. An in vitro proteolysis assay showed that SpxΔC resisted proteolysis by ClpXP in the presence or absence of GtYjbH (Fig. 4a). Moreover, in the presence of both SpxΔC and Spx WT, the rate of Spx WT proteolysis was not affected (Fig. S4). These results suggested that SpxΔC is unable to interact with GtYjbH or ClpX, and thus did not compete with wild-type Spx for contact with YjbH or protease in the proteolysis reaction. We hypothesized that the C terminus of Spx is required for effective GtYjbH interaction. In vitro affinity interaction experiments were performed using GtYjbH–His6 and Ni-chelate chromatography to detect the interaction between GtYjbH and Spx. As expected, most of the WT Spx was bound to immobilized GtYjbH–His6 and co-eluted with GtYjbH after application of imidazole to the Ni column (Fig. 4c). On the other hand, little SpxΔC was bound to and co-eluted with GtYjbH–His6 (Fig. 4d). A negative control with only WT Spx and SpxΔC showed no non-specific Spx binding to the Ni column (Fig. S5a, b and Fig. 5a). WT Spx is observed in the wash fractions and was not detected in elution or as immobilized protein on the column beads (data not shown). In vitro competition affinity interaction experiments with reactions containing WT Spx and SpxΔC showed that only WT Spx co-eluted with GtYjbH–His6, indicating interaction between GtYjbH and WT Spx and loss of recognition by GtYjbH when the 12 C-terminal residues of Spx are deleted (Fig. 5b).
Fig. 4.
GtYjbH recognizes the C terminus of Spx. Deletion of 12 amino acids at the Spx C terminus (SpxΔC) greatly diminished GtYjbH binding. (a) SDS-PAGE shows the effect of GtYjbH on ClpXP-catalysed proteolysis of SpxΔC in vitro in a time-course experiment. SpxΔC (4 µM), ClpX (3 µM) and ClpP (8 µM) with an ATP-generating system (creatine kinase) were incubated at 37 °C for the times (min) indicated in the absence (a) or presence (b) of GtYjbH (4 µM). (b) Far-Western blotting to detect Spx interaction with GtYjbH. Proteins that interact with SpxHA or SpxΔCHA were detected by anti-HA antibodies. RNA polymerase α subunit was used as a positive control for Spx interaction. (c, d) In vitro Ni-affinity interaction experiments to detect contact between GtYjbH–His6 and Spx (c) or SpxΔC (d). M, marker; I, input; FT, flowthrough; W, last wash; E1–3, elution fractions 1–3.
Fig. 5.
Competition in vitro Ni-affinity interaction experiments show Spx outcompetes SpxΔC for GtYjbH binding. (a) Spx WT and SpxΔC proteins applied to a Ni affinity column. Column fractions separated by SDS-PAGE and stained with ‘Blue silver’ colloidal Coomassie G-250 protein. (b) Spx WT, SpxΔC and GtYjbH–His6 reaction applied to Ni affinity column. Fractions separated by SDS-PAGE with staining as in (a). M, marker; I, input; FT, flowthrough; W, last wash; E1–3, elution fractions 1–3.
A far-Western blot was performed to confirm that the C terminus of Spx is required for interaction between Spx and YjbH. For these experiments, an influenza haemagglutinin (HA) epitope-tagged version of Spx was utilized. An allele of spx was constructed that encodes a product with a C-terminal HA tag. SpxΔCHA, with the HA tag appended to the Spx deletion derivative, resists proteolysis. SpxHA, full-length Spx with the C-terminal HA tag, behaves similarly to Spx WT with respect to YjbH-mediated proteolysis in vivo (Fig. S6). Both SpxHA and SpxΔCHA were used as far-Western substrates. Each was incubated with filters onto which gel-resolved GtYjbH, ClpX and RNA polymerase were electro-blotted. RNA polymerase was used as a positive control, since both epitope-tagged Spx derivatives were expected to show interaction with the RNA polymerase α subunit. The filters that were incubated with the Spx derivatives were then treated with anti-HA monoclonal antiserum, and Western analysis was conducted. The far-Western results indicated that only SpxHA showed strong binding to GtYjbH–His6, as evident by reaction of the GtYjbH band with anti-HA antibodies after SpxHA treatment (Fig. 4b). The other two membranes incubated with and without SpxΔCHA showed minimal interaction and little non-specific binding of anti-HA antibodies, respectively. Overall, the data presented herein indicate that the deletion of 12 amino acids from the Spx C terminus mostly abolished the binding to GtYjbH, and this region increases the specificity of the GtYjbH–Spx interaction.
In vitro affinity interaction shows that Spx binds to ClpX in the absence of GtYjbH
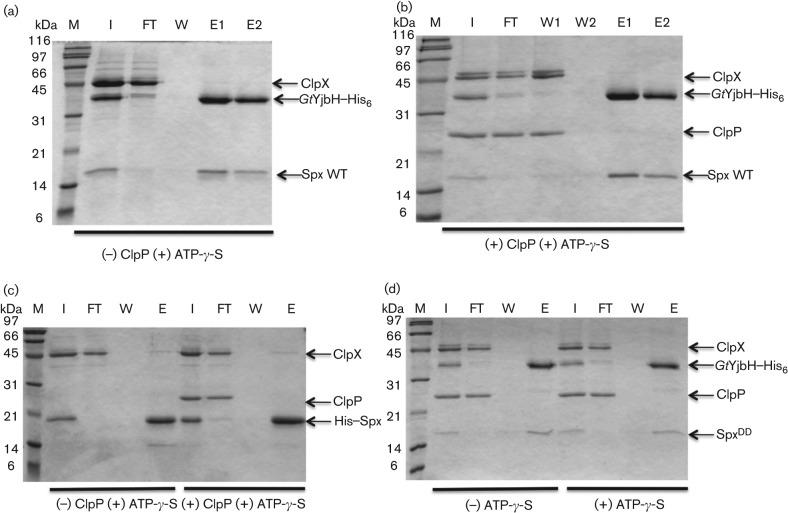
Further in vitro affinity interaction assays were performed to test interactions between Spx, GtYjbH, ClpX and ClpP. Protein mixtures composed of GtYjbH–His6, Spx, and ClpX with or without ClpP were applied to a Ni-NTA column in the presence of non-hydrolysable ATP-γ-S, but only GtYjbH–His6 and Spx co-eluted from the column, which suggested again strong interaction between GtYjbH and its substrate (Fig. 6a, b). In a reaction containing His6–Spx, ATP-γ-S and ClpX with and without ClpP, the result showed that a small amount of ClpX co-eluted with His6–Spx (Fig. 6c). Similar affinity interaction experiments performed with or without ATP produced the same results (Fig. S7). From these in vitro interaction studies, we concluded that Spx establishes a weak interaction with ClpX and that GtYjbH does not contact ClpX. spxDD is an allele encoding a product in which the last two amino acid residues of Spx (Ala and Asn) are replaced with Asp (Nakano et al., 2003). This change renders the protein impervious to ClpXP-catalysed degradation. An affinity interaction assay showed that the change does not affect Spx interaction with GtYjbH (Fig. 6d).
Fig. 6.
In vitro Ni-affinity interaction experiments to detect interaction between GtYjbH–His6, Spx and ClpX, in the absence (a) or presence (b) of ClpP; between His6–Spx and ClpX, in the absence or presence (c) of ClpP, all reactions contain ATP-γ-S; between GtYjbH–His6, SpxDD, ClpX and ClpP, in the presence or absence (d) of ATP-γ-S. Column fractions were applied to SDS-PAGE gels that were stained with ‘Blue silver’ colloidal Coomassie G-250 protein. M, marker; I, input; FT, flowthrough; W, last wash; E, elution.
Kinetics of ClpXP-catalysed Spx proteolysis with and without GtYjbH
Kinetic measurements were conducted to provide insight into the mechanism of YjbH-enhanced Spx proteolysis. Increasing concentrations of Spx were incubated with GtYjbH, ClpXP and creatine kinase, an ATP-generating system, and the remaining Spx was quantified from SDS-PAGE in order to obtain initial rates (Fig. S8). Experiments were performed in triplicate to obtain a standard deviation. The results are listed in Table 1 as two sets of data, in the absence (Km = 0.42±0.17 µM, Vmax = 0.24±0.06 µM min−1) or presence (Km = 1.79±0.66 µM, Vmax = 1.98±0.48 µM min−1) of 0.5 µM GtYjbH. In the presence of GtYjbH, the turnover rate is approximately eightfold higher with an increased Km value. The increased Vmax value but increased Km value may be explained as an uncompetitive ‘inhibitor’ effect of GtYjbH, suggesting that YjbH overcomes low Km, non-productive Spx binding to ClpX to enhance the rate of proteolysis.
Discussion
Previous studies (Garg et al., 2009; Kommineni et al., 2011; Larsson et al., 2007) showed that YjbH acts as a substrate recognition factor to enhance Spx proteolysis by ClpXP. YjbH bears a His–Cys-rich region on the N terminus which is important for Zn metal binding and for enhancement of proteolysis of Spx by ClpXP (Garg et al., 2009). To investigate the roles of the histidines and cysteines in the YjbH N terminus, YjbH mutants bearing amino acid substitutions (H12A, C13A, H14A, H16A and C31A/C34A) were tested for in vivo activity and responsiveness to diamide treatment. Results showed that all the mutants behaved like the wild-type YjbH, indicating that mutation on the N terminus did not affect YjbH functionality with respect to Spx regulation. The loss of function when YjbH lacks the 23 aa N terminus may be caused by gross changes in YjbH protein structure. At present, we do not know if YjbH activity is regulated in response to stress. The small protein YirB can exert a negative effect on YjbH activity when overproduced, and has been shown to interact with YjbH in vivo and in vitro (Kommineni et al., 2011). However, no Spx-related phenotype has been uncovered in the yirB deletion mutant. Response to thiol stress that proteolytically controls Spx concentration might be accomplished through oxidation of the ClpX protein itself, which bears a Cys-coordinated Zn atom in its N-terminal domain that can undergo oxidation (Zhang & Zuber, 2007). Alternatively, YjbH might be titrated by increasing concentrations of Spx upon disulfide stress, leading to further accumulation of Spx protein. Such a mechanism has been proposed for the SspB–ClpXP substrate of E. coli, RecN, which overwhelms the proteolytic complex responsible for its degradation by titration when cells undergo DNA damage (Neher et al., 2006). The conserved CxxC motif of YjbH, while not essential for Spx proteolytic control, may play a role in some other function fulfilled by YjbH that is, perhaps, unrelated to Spx turnover.
Expression of B. subtilis yjbH produced insoluble product in an E. coli expression system. In previous studies, renatured BsYjbH was used for biochemical studies including in vitro proteolysis assay, Zn measurement (Garg et al., 2009). In order to study YjbH in a native form, we cloned and expressed yjbH from G. thermodenitrificans, which yielded soluble protein when produced in E. coli. GtYjbH possesses a similar protein function to BsYjbH both in vivo (lacZ assay, responses to diamide) and in vitro (proteolysis assay). No His–Cys-rich region is found at the N terminus of GtYjbH except the putative redox CxxC motif, but both YjbH orthologues share high sequence similarity (Fig. S1).
Seeking to understand the substrate recognition mechanism of YjbH, in vitro affinity interaction assays and far-Western blotting experiments were conducted and showed that the 12 aa C terminus of Spx is important for GtYjbH binding and for effective proteolysis by ClpXP (Fig. 6). While GtYjbH–His6 binds to Spx in the presence of ClpX or ClpXP, there is negligible binding of ClpX with GtYjbH. In other binding experiments utilizing His6–Spx and ClpX or ClpXP, a small amount of ClpX was found co-eluted with His6–Spx, which suggested interaction between His6–Spx and ClpX (Fig. 6). To date, there are five classes of degradation tags proposed that are recognized by ClpXP: two at the C terminus and three at the N terminus (Flynn et al., 2003). In vivo and in vitro data showed that SpxDD and SpxΔC resist proteolysis, which indicates C terminus recognition by ClpXP. The data we provide here suggest that the C terminus of Spx is not only important for ClpX recognition, but also important for YjbH binding. Interestingly, the SpxDD product was found to interact with GtYjbH, but was not susceptible to proteolysis. By analogy with the ClpXP system of E. coli, the extreme C-terminal residues may be required to engage the ClpX unfoldase, while the adjacent region contacts YjbH. This is similar to the way in which SspB–ClpXP interacts with peptides bearing the C-terminal SsrA tag, which is appended during clearance from ribosomes of products resulting from interrupted translation (Chien et al., 2007; Levchenko et al., 2000). SspB and ClpX contact adjacent amino acid sequence motifs on the SsrA tag, with ClpX recognizing the terminal 3 aa. Unlike SspB, which interacts with the N-terminal Zn-binding domain of ClpX (Bolon et al., 2004), no evidence for ClpX–YjbH interaction could be uncovered in the current study.
We present (Table 1) the kinetic measurements of YjbH-enhanced Spx proteolysis. In the presence of GtYjbH, a weaker Km and increased Vmax value (Km = 1.79±0.66 µM, Vmax = 1.98±0.48 µM min−1) were observed, while in the absence of GtYjbH, proteolysis was characterized by a lower Km and lower Vmax (Km = 0.42±0.17 µM, Vmax = 0.24±0.06 µM min−1). However, the turnover rate was approximately eightfold enhanced when GtYjbH was present. The increased Vmax value, but increased Km may be explained as an uncompetitive ‘inhibitor’ effect of GtYjbH. Our in vitro interaction experiments showed that GtYjbH binds Spx tightly, but ClpX only shows weak interaction with Spx, and both GtYjbH and ClpX bind to the C terminus of Spx. This resulted in a weaker Km when GtYjbH is present. Our data support the hypothesis that GtYjbH binds the C terminus of Spx, altering Spx structure to expose a structural element for productive recognition by ClpX, and reducing non-productive binding of Spx with ClpX. Hence the weaker Km would result in faster degradation, since the unfolding of native protein is a rate-limiting step for protein degradation by ClpX (Kenniston et al., 2003). The strong interaction of GtYjbH and Spx could increase the effective concentration of Spx to the pore of ClpX, and then the equilibrium would shift towards the generation of degraded products (Davis et al., 2009). Finally, a model mechanism is proposed of how GtYjbH is an adaptor mediating Spx proteolysis by ClpXP. In the absence of YjbH, ClpXP recognizes Spx, but degrades the substrate slowly due to the lower Km value, non-productive interaction. However, YjbH binds to the C-terminal region of Spx, elevating the Km value, possibly reflecting the elimination of inhibitory Spx–ClpX interactions, and resulting in accelerated Spx proteolysis (Fig. 7).
Fig. 7.
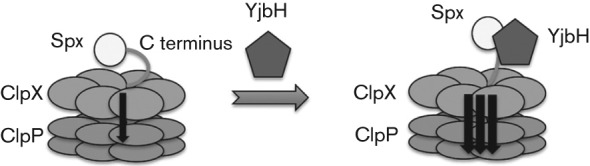
A model for the mechanism of YjbH-enhanced proteolysis is proposed. In the absence of YjbH, the Spx C terminus contacts ClpX, but ClpXP proteolysis is slow. YjbH binds to the Spx C-terminal region, which may alter Spx folding, expose a structural element of Spx or eliminate Spx–ClpX contacts that might be inhibitory, thus accelerating Spx proteolysis.
In vitro studies of adaptor-mediated proteolysis catalysed by Clp proteases involve reactions in which the adaptor, such as SspB and ClpS, is present at equimolar or near equimolar amounts with respect to protease and substrate (Levchenko et al., 2000). In one case, SspB was present in 10-fold molar excess over ClpXP (Chowdhury et al., 2010), while in another study, ClpS was present in a ClpAP-catalysed proteolytic reaction in 0.5 molar equivalent to protease and substrate (Dougan et al., 2002). Thus, the adaptor–protease complex acts catalytically in the reaction that causes substrate degradation, but the adaptor acts stoichiometrically with respect to interaction with the protease. Importantly, YjbH behaves quite differently, in that concentrations approximately 30-fold less than those of substrate or protease subunit result in rapid, efficient elimination of Spx substrate. This suggests a novel mechanism of proteolytic enhancement carried out by YjbH, which is unlike that of other Clp protease adaptors. The mechanism of YjbH-mediated proteolytic enhancement warrants further investigation.
Acknowledgements
The authors wish to thank Kelly Chacon and Ninian Blackburn for providing TEV protease and Michiko M. Nakano and members of the Zuber lab for helpful discussions. The research reported herein was supported by the National Institutes of Health grant GM045898 to P. Z.
Abbreviations:
- CV
column volumes
- NTA
nitrilotriacetic acid
Footnotes
Two supplementary tables and eight supplementary figures are available with the online version of this paper.
References
- Bolon D. N., Wah D. A., Hersch G. L., Baker T. A., Sauer R. T. (2004). Bivalent tethering of SspB to ClpXP is required for efficient substrate delivery: a protein-design study. Mol Cell 13, 443–449. 10.1016/S1097-2765(04)00027-9 [DOI] [PubMed] [Google Scholar]
- Britton R. A., Eichenberger P., Gonzalez-Pastor J. E., Fawcett P., Monson R., Losick R., Grossman A. D. (2002). Genome-wide analysis of the stationary-phase sigma factor (sigma-H) regulon of Bacillus subtilis. J Bacteriol 184, 4881–4890. 10.1128/JB.184.17.4881-4890.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaibenjawong P., Foster S. J. (2011). Desiccation tolerance in Staphylococcus aureus. Arch Microbiol 193, 125–135. 10.1007/s00203-010-0653-x [DOI] [PubMed] [Google Scholar]
- Charbonnier Y., Gettler B., François P., Bento M., Renzoni A., Vaudaux P., Schlegel W., Schrenzel J. (2005). A generic approach for the design of whole-genome oligoarrays, validated for genomotyping, deletion mapping and gene expression analysis on Staphylococcus aureus. BMC Genomics 6, 95. 10.1186/1471-2164-6-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien P., Perchuk B. S., Laub M. T., Sauer R. T., Baker T. A. (2007). Direct and adaptor-mediated substrate recognition by an essential AAA+ protease. Proc Natl Acad Sci U S A 104, 6590–6595. 10.1073/pnas.0701776104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury T., Chien P., Ebrahim S., Sauer R. T., Baker T. A. (2010). Versatile modes of peptide recognition by the ClpX N domain mediate alternative adaptor-binding specificities in different bacterial species. Protein Sci 19, 242–254. 10.1002/pro.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. H., Baker T. A., Sauer R. T. (2009). Engineering synthetic adaptors and substrates for controlled ClpXP degradation. J Biol Chem 284, 21848–21855. 10.1074/jbc.M109.017624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan D. A., Reid B. G., Horwich A. L., Bukau B. (2002). ClpS, a substrate modulator of the ClpAP machine. Mol Cell 9, 673–683. 10.1016/S1097-2765(02)00485-9 [DOI] [PubMed] [Google Scholar]
- Engman J., Rogstam A., Frees D., Ingmer H., von Wachenfeldt C. (2012). The YjbH adaptor protein enhances proteolysis of the transcriptional regulator Spx in Staphylococcus aureus. J Bacteriol 194, 1186–1194. 10.1128/JB.06414-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. M., Neher S. B., Kim Y. I., Sauer R. T., Baker T. A. (2003). Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol Cell 11, 671–683. 10.1016/S1097-2765(03)00060-1 [DOI] [PubMed] [Google Scholar]
- Fouet A., Jin S. F., Raffel G., Sonenshein A. L. (1990). Multiple regulatory sites in the Bacillus subtilis citB promoter region. J Bacteriol 172, 5408–5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S. K., Kommineni S., Henslee L., Zhang Y., Zuber P. (2009). The YjbH protein of Bacillus subtilis enhances ClpXP-catalyzed proteolysis of Spx. J Bacteriol 191, 1268–1277. 10.1128/JB.01289-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göhring N., Fedtke I., Xia G., Jorge A. M., Pinho M. G., Bertsche U., Peschel A. (2011). New role of the disulfide stress effector YjbH in β-lactam susceptibility of Staphylococcus aureus. Antimicrob Agents Chemother 55, 5452–5458. 10.1128/AAC.00286-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. R., Cutting S. M. (1990). Molecular Biological Methods for Bacillus. Chichester, UK: Wiley. [Google Scholar]
- Kenniston J. A., Baker T. A., Fernandez J. M., Sauer R. T. (2003). Linkage between ATP consumption and mechanical unfolding during the protein processing reactions of an AAA+ degradation machine. Cell 114, 511–520. 10.1016/S0092-8674(03)00612-3 [DOI] [PubMed] [Google Scholar]
- Kommineni S., Garg S. K., Chan C. M., Zuber P. (2011). YjbH-enhanced proteolysis of Spx by ClpXP in Bacillus subtilis is inhibited by the small protein YirB (YuzO). J Bacteriol 193, 2133–2140. 10.1128/JB.01350-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J. T., Rogstam A., von Wachenfeldt C. (2007). YjbH is a novel negative effector of the disulphide stress regulator, Spx, in Bacillus subtilis. Mol Microbiol 66, 669–684. 10.1111/j.1365-2958.2007.05949.x [DOI] [PubMed] [Google Scholar]
- Levchenko I., Seidel M., Sauer R. T., Baker T. A. (2000). A specificity-enhancing factor for the ClpXP degradation machine. Science 289, 2354–2356. 10.1126/science.289.5488.2354 [DOI] [PubMed] [Google Scholar]
- Levchenko I., Grant R. A., Flynn J. M., Sauer R. T., Baker T. A. (2005). Versatile modes of peptide recognition by the AAA+ adaptor protein SspB. Nat Struct Mol Biol 12, 520–525. 10.1038/nsmb934 [DOI] [PubMed] [Google Scholar]
- Lin A. A., Zuber P. (2012). Evidence that a single monomer of Spx can productively interact with RNA polymerase in Bacillus subtilis. J Bacteriol 194, 1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cosby W. M., Zuber P. (1999). Role of Lon and ClpX in the post-translational regulation of a sigma subunit of RNA polymerase required for cellular differentiation in Bacillus subtilis. Mol Microbiol 33, 415–428. 10.1046/j.1365-2958.1999.01489.x [DOI] [PubMed] [Google Scholar]
- Miller J. H. (1972). Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Nakano M. M., Marahiel M. A., Zuber P. (1988). Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol 170, 5662–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Hajarizadeh F., Zhu Y., Zuber P. (2001). Loss-of-function mutations in yjbD result in ClpX- and ClpP-independent competence development of Bacillus subtilis. Mol Microbiol 42, 383–394. 10.1046/j.1365-2958.2001.02639.x [DOI] [PubMed] [Google Scholar]
- Nakano S., Zheng G., Nakano M. M., Zuber P. (2002). Multiple pathways of Spx (YjbD) proteolysis in Bacillus subtilis. J Bacteriol 184, 3664–3670. 10.1128/JB.184.13.3664-3670.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S., Nakano M. M., Zhang Y., Leelakriangsak M., Zuber P. (2003). A regulatory protein that interferes with activator-stimulated transcription in bacteria. Proc Natl Acad Sci U S A 100, 4233–4238. 10.1073/pnas.0637648100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S., Erwin K. N., Ralle M., Zuber P. (2005). Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx. Mol Microbiol 55, 498–510. 10.1111/j.1365-2958.2004.04395.x [DOI] [PubMed] [Google Scholar]
- Nakano M. M., Lin A., Zuber C. S., Newberry K. J., Brennan R. G., Zuber P. (2010). Promoter recognition by a complex of Spx and the C-terminal domain of the RNA polymerase α subunit. PLoS ONE 5, e8664. 10.1371/journal.pone.0008664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher S. B., Villén J., Oakes E. C., Bakalarski C. E., Sauer R. T., Gygi S. P., Baker T. A. (2006). Proteomic profiling of ClpXP substrates after DNA damage reveals extensive instability within SOS regulon. Mol Cell 22, 193–204. 10.1016/j.molcel.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Nicholson W., Setlow P. (1990). Sporulation, germination and outgrowth. In Molecular Biological Methods for Bacillus, pp. 391–450. Edited by Harwood C. R., Cutting S. M. Chichester, UK: Wiley. [Google Scholar]
- Persuh M., Mandic-Mulec I., Dubnau D. (2002). A MecA paralog, YpbH, binds ClpC, affecting both competence and sporulation. J Bacteriol 184, 2310–2313. 10.1128/JB.184.8.2310-2313.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porankiewicz J., Wang J., Clarke A. K. (1999). New insights into the ATP-dependent Clp protease: Escherichia coli and beyond. Mol Microbiol 32, 449–458. 10.1046/j.1365-2958.1999.01357.x [DOI] [PubMed] [Google Scholar]
- Schlothauer T., Mogk A., Dougan D. A., Bukau B., Turgay K. (2003). MecA, an adaptor protein necessary for ClpC chaperone activity. Proc Natl Acad Sci U S A 100, 2306–2311. 10.1073/pnas.0535717100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemansky J., Kline B. C., Woodward J. J., Leber J. H., Marquis H., Portnoy D. A. (2009). Development of a mariner-based transposon and identification of Listeria monocytogenes determinants, including the peptidyl-prolyl isomerase PrsA2, that contribute to its hemolytic phenotype. J Bacteriol 191, 3950–3964. 10.1128/JB.00016-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zuber P. (2007). Requirement of the zinc-binding domain of ClpX for Spx proteolysis in Bacillus subtilis and effects of disulfide stress on ClpXP activity. J Bacteriol 189, 7669–7680. 10.1128/JB.00745-07 [DOI] [PMC free article] [PubMed] [Google Scholar]