Abstract
Culture supernatants of leptospiral pathogens have long been known to haemolyse erythrocytes. This property is due, at least in part, to sphingomyelinase activity. Indeed, genome sequencing reveals that pathogenic Leptospira species are richly endowed with sphingomyelinase homologues: five genes have been annotated to encode sphingomyelinases in Leptospira interrogans. Such redundancy suggests that this class of genes is likely to benefit leptospiral pathogens in their interactions with the mammalian host. Surprisingly, sequence comparison with bacterial sphingomyelinases for which the crystal structures are known reveals that only one of the leptospiral homologues has the active site amino acid residues required for enzymic activity. Based on studies of other bacterial toxins, we propose that leptospiral sphingomyelinase homologues, irrespective of their catalytic activity, may possess additional molecular functions that benefit the spirochaete. Potential secretion pathways and roles in pathogenesis are discussed, including nutrient acquisition, dissemination, haemorrhage and immune evasion. Although leptospiral sphingomyelinase-like proteins are best known for their cytolytic properties, we believe that a better understanding of their biological role requires the examination of their sublytic properties as well.
Introduction
Sphingomyelinases are of great interest because of their potential to mediate key aspects of leptospiral pathogenesis. Leptospirosis is most prevalent in tropical countries where moist conditions favour environmental survival of pathogenic Leptospira species excreted by animal carriers of the spirochaete. Transmission occurs when contaminated soil or water comes into contact with cutaneous lacerations or mucous membranes of the mouth, eyes and nose (WHO, 2003). Leptospirosis is an invasive infection manifested by a broad spectrum of symptoms that are often mistaken for other infections. The disease is usually self-limiting but can progress to a severe form characterized by renal failure, haemorrhagic diathesis and jaundice. Pulmonary haemorrhage is a feared complication caused by damage to the endothelial lining of blood vessels (Dolhnikoff et al., 2007), possibly caused by a toxin as leptospires are often not detected at the site of the lesion (Miller et al., 1974). Another occasional complication is haemolytic anaemia (Feigin et al., 1975). Through their action on host cell membranes, leptospiral sphingomyelinases are potentially involved in aspects of pathogenesis, including tissue invasion, endothelial damage, immune evasion and nutrient acquisition.
Sphingomyelinases are enzymes that catalyse the hydrolysis of sphingomyelin into ceramide and phosphorylcholine. Biochemically, sphingomyelinases are classified as either acidic, neutral or alkaline, depending on their pH optimum for activation. Most of the neutral sphingomyelinases of bacteria and mammals form a family defined by a set of conserved catalytic core residues and overall sequence relatedness (Clarke et al., 2011). Mammalian members of the neutral sphingomyelinase family are membrane-associated, whereas the bacterial members are secreted. Mammalian sphingomyelinases act on the sphingomyelin present on the membranes and release ceramide, which controls cellular functions by acting as a signalling molecule and by altering the biophysical properties of the membrane (Hannun & Obeid, 2008). Ceramide is also the central hub of the sphingolipid signalling network, which includes other bioactive sphingolipids such as sphingosine and sphingosine-1-phosphate. The levels of ceramide and other sphingolipids are therefore tightly controlled (Breslow & Weissman, 2010), and their dysregulation contributes to the patho-biology of numerous infectious and non-infectious disease processes (Zeidan & Hannun, 2007). For example, cellular infection by diverse pathogens, including Neisseria gonorrhoea, rhinovirus and Cryptosporidium parvum, involves activation of the host acid sphingomyelinase by translocation of the enzyme from the endolysosomal to the plasma membrane (Grassmé et al., 2005; Zeidan & Hannun, 2007). Hydrolysis of sphingomyelin in the plasma membrane by acid sphingomyelinase leads to assembly of ceramide-enriched membrane platforms, which may be necessary to concentrate receptors to facilitate intracellular signal transduction and microbial internalization (Lafont & van der Goot, 2005).
Sphingomyelinases produced by Bacillus cereus, Staphylococcus aureus and Listeria (List.) ivanovii are the best characterized among the bacterial sphingomyelinases. As most bacteria do not synthesize sphingomyelin, bacterial sphingomyelinases probably target the sphingomyelin in the external leaflet of the host cell’s plasma membrane. Their inactivation in S. aureus and List. ivanovii diminished their infectivity in animal models (Bramley et al., 1989; González-Zorn et al., 1999). List. ivanovii sphingomyelinase enables the intracellular pathogen to escape from phagocytic vacuoles in epithelial cells by rupturing the membrane of the vacuole (González-Zorn et al., 1999). The sphingomyelinase activity of S. aureus β-toxin promotes excessive inflammation and vascular leakage in the lungs by inducing shedding of the ectodomain of the proteoglycan syndecan-1 in a mouse model of pneumonia (Hayashida et al., 2009). The response does not occur when the catalytic residues of β-toxin are altered, highlighting the importance of the enzymic activity of the toxin in triggering uncontrolled inflammation. In this review, we examine the evidence that sphingomyelinase-like proteins are involved in mechanisms of leptospiral pathogenesis.
Discovery of many leptospiral genes encoding sphingomyelinase-like proteins
Sphingomyelinase activity was first detected in Leptospira cultures in the 1960s (Kăsarov & Addamiano, 1969), yet cloning of a sphingomyelinase gene was not reported until 1989 (del Real et al., 1989), when a genomic expression library of Leptospira (Lept.) borgpetersenii serovar Hardjo was screened for haemolytic activity. Haemolytic and sphingomyelinase activities were expressed from a single gene that was later designated sphA (del Real et al., 1989; Segers et al., 1992). The sphingomyelinase encoded by sphA shared significant similarity to those found in S. aureus and Bacillus subtilis (Segers et al., 1990). Multiple sphingomyelinase sequences were detected in pathogenic members of Leptospira by low stringency Southern hybridization using Lept. borgpetersenii sphA as a probe (Segers et al., 1992).
SphH, one of the sphingomyelinase homologues in the genome of serovar Lai, was identified from a genomic library using sphA as the probe (Lee et al., 2000). The protein showed 75 % similarity to SphA. However, the clone failed to express sphingomyelinase (or phospholipase) activity, although the partially purified recombinant protein lysed sheep erythrocytes (Lee et al., 2000, 2002). The haemolytic activity of SphH was neutralized with rabbit antiserum raised against SphH, eliminating the possibility that haemolysis was due to the cryptic haemolysin of E. coli. Transmission electron microscopy of sheep erythrocytes incubated with the SphH preparation revealed pores in the membrane, suggesting that the haemolytic activity of SphH was due to pore-forming ability (Lee et al., 2002). However, another group was unable to confirm the haemolytic activity of a purified preparation of rSphH (Carvalho et al., 2010), possibly due to improper refolding of the insoluble recombinant protein.
Genome sequencing uncovered the multiple sphingomyelinase-like proteins encoded in several pathogenic Leptospira. The Lai, Copenhageni, Manilae and Pomona strains each carried genes annotated as sph1, sph2, sph3, sph4 and sphH (Bulach et al., 2006b; Nascimento et al., 2004; Ren et al., 2003) (B. Adler, personal communication). In contrast, the genomes of two Lept. borgpetersenii strains harboured only sphA, sphB and sph4 (Bulach et al., 2006b). The non-pathogen Leptospira biflexa lacks sph coding sequences (Picardeau et al., 2008).
Domains of leptospiral sphingomyelinase-like proteins
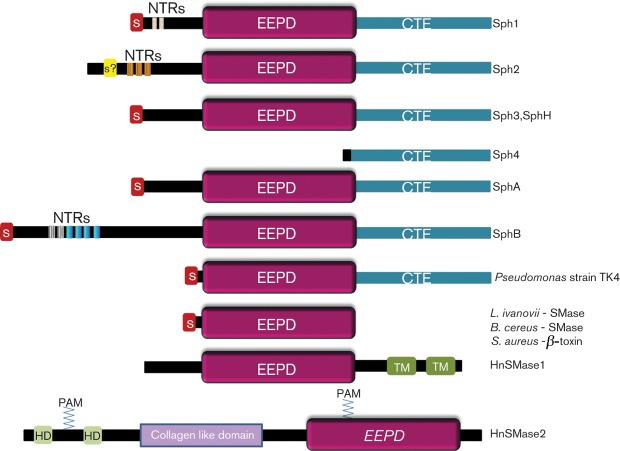
Multi-sequence alignment of all available leptospiral sphingomyelinase-like sequences reveals the modular nature of the proteins (Fig. 1). In addition to signal sequences, there are N-terminal and C-terminal extensions flanking the central enzymic domain. The region of sequence similarity among the proteins comprises the enzymic domain and C-terminal extensions
Fig. 1.
Schematic representation of leptospiral sphingomyelinase-like proteins. NTRs, N-terminal repetitive sequences; S, signal peptide; EEPD, exo-endo phosphatase domain; CTE, C-terminal extension; TM, transmembrane domain; HD, hydrophobic domain; PAM, palmitate.
Enzymic domain
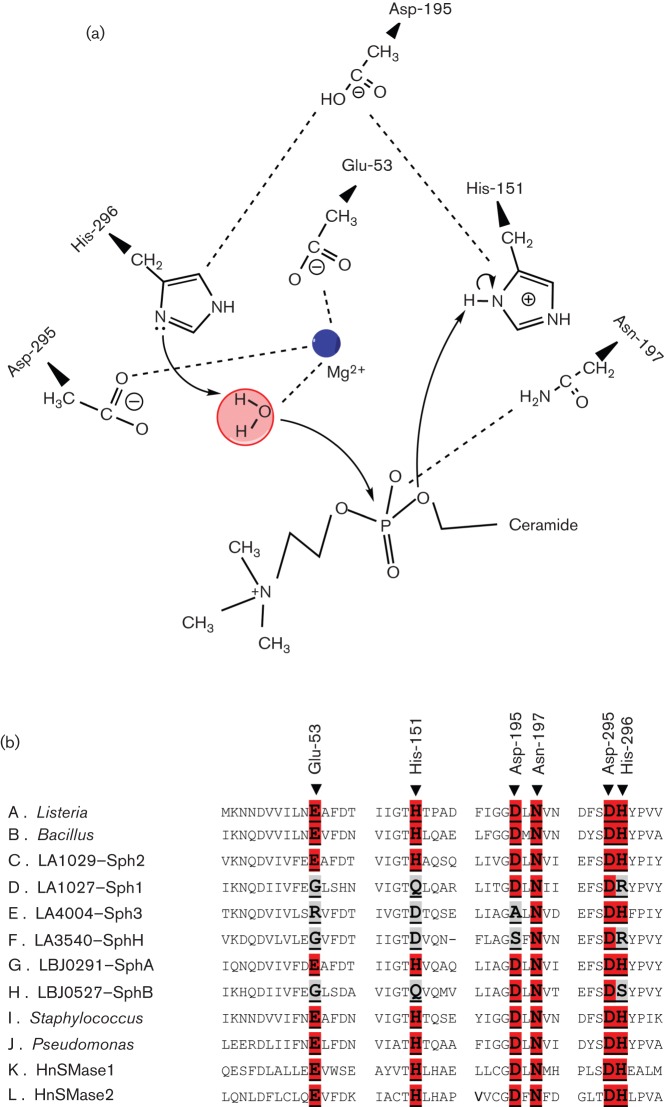
The crystal structures of the sphingomyelinases of List. ivanovii (Openshaw et al., 2005), B. cereus (Ago et al., 2006) and S. aureus (Huseby et al., 2007) have been determined. These structures revealed the active site configuration of the conserved residues shown to be crucial for sphingomyelinase activity in mutagenesis studies (Huseby et al., 2007; Obama et al., 2003a, b). The active site of B. cereus sphingomyelinase contained the divalent metal cation necessary for catalytic activity (Ago et al., 2006). Using the numbering for B. cereus sphingomyelinase, essential residues include Glu-53, His-151, Asp-195 and His-296, the metal-binding and catalytic functions of which are shown in Fig. 2(a). Surprisingly, the multi-sequence alignment shows that only Lept. borgpetersenii SphA and Leptospira interrogans Sph2 possess these four amino acid residues (Fig. 2b). In contrast, Sph1 and Sph3 of Lept. interrogans and SphB of Lept. borgpetersenii have non-conservative amino acid substitutions for three or all four of these critical residues. This raises the possibility that these latter Sph proteins are not true sphingomyelinases, despite their overall sequence similarity with other bacterial sphingomyelinases. This observation is consistent with the finding that SphH lacks sphingomyelinase activity (Lee et al., 2002). Although one study reported sphingomyelinase activity for recombinant Sph1, Sph3 and Sph4 expressed in E. coli (Zhang et al., 2005), their conclusions are in doubt for several reasons. First of all, Sph4 lacks the entire enzymic domain (Fig. 1) and therefore should not have exhibited any sphingomyelinase activity. Secondly, their results are difficult to interpret because data from the negative control experiment were not presented. Thirdly, the observed reduction of the sphingomyelinase peak as measured by HPLC could have resulted from the activity of E. coli lipases in the extract. This is possible because of the high protein concentrations of the crude extracts (100 mg ml−1) in their assays (Zhang et al., 2005). In conclusion, we propose that pathogenic Leptospira species have only one true sphingomyelinase (Sph2 or SphA) and that all of the sphingomyelinase-like proteins may possess additional molecular functions.
Fig. 2.
Catalytic site functions and multi-sequence alignment of the active-site amino acid residues required for sphingomyelinase activity. (a) The proposed function of amino acids at the catalytic site of B. cereus sphingomyelinase (adapted from Obama et al., 2003a). Asn-197 interacts with the phosphate group of sphingomyelin, and Glu-53 and Asp-295 coordinate a divalent cation. His-296 and His-151 function as the acid-base catalytic residues; His-296 and the metal ion activate the water molecule that attacks the phosphorus of sphingomyelin, resulting in its hydrolysis to phosphocholine and ceramide. Asp-195 maintains the appropriate spatial arrangement of the catalytic histidine residues. (b) Multi-sequence alignment showing six of the amino acids (highlighted in red) conserved in all members of the extended neutral sphingomyelinase family, including the two human neutral sphingomyelinases. Note that Glu-53, His-151, Asp-195 and/or His-296 are not conserved (highlighted in grey) in Sph1, Sph3, SphH and Sph4.
What, then, could be the additional functions of the ‘enzymic’ domain of the leptospiral sphingomyelinase-like proteins? Their non-catalytic function may target host sphingomyelin on membrane surfaces for attachment of the protein. For example, the Helicobacter pylori toxin VacA uses sphingomyelin as a receptor to enter the target cell (Gupta et al., 2008). The domain may also possess surfaces that bind other host receptors. This is reminiscent of the leptospiral haemolysin-like protein TlyC, which lacks haemolytic activity yet binds to extracellular matrix proteins fibronectin, collagen IV and laminin (Carvalho et al., 2009). A novel role for sphingomyelinase has been described for the S. aureus β-toxin. In the process of biofilm formation, β-toxin covalently interacts with extracellular DNA, forming insoluble nucleoprotein complexes. Biofilm assembly occurred even when the two histidine residues responsible for catalytic activity were altered by mutation, indicating that the residues involved in biofilm formation are distinct from the ones involved in catalysis (Huseby et al., 2010).
The crystal structures of the sphingomyelinases of B. cereus, List. ivanovii and S. aureus revealed a protruding hydrophobic β-hairpin and a second external hydrophobic loop adjacent to the active site. The surface hydrophobic loops may be important in properly positioning the catalytic site in relation to the sphingomyelin substrate in the target membrane. Replacement of the hydrophobic residues in the β-hairpin with alanine in B. cereus sphingomyelinase impaired its binding to sphingomyelin liposomes and disrupted its sphingomyelin hydrolytic activity (Ago et al., 2006; Narayanavari et al., 2012). The leptospiral sphingomyelinases lack the hydrophobic β-hairpin (Openshaw et al., 2005). Hence the initial interaction of the leptospiral sphingomyelinase-like proteins with the target membrane may involve sequences located outside of the enzymic domain.
C-terminal extension
The leptospiral sphingomyelinase-like proteins and Pseudomonas strain TK4 sphingomyelinase have a carboxy-terminal extension of approximately 186 aa that is missing in the other bacterial sphingomyelinases (Narayanavari et al., 2012; Sueyoshi et al., 2002). The role of the C-terminal extension in the Pseudomonas sphingomyelinase has been examined. Deletion of 186 aa from the C-terminal end of Pseudomonas sphingomyelinase completely abolished the haemolytic activity without affecting the sphingomyelinase activity, indicating that the C-terminal extension is indispensable for haemolytic activity (Sueyoshi et al., 2002). This observation suggests that the function of the C-terminal extension is to interact with the target host membrane to position the enzymic domain near the sphingomyelin substrate (Sueyoshi et al., 2002).
Export and secretion signals
Sphingomyelinase activity has been detected in the culture fluids of several strains of pathogenic Leptospira (Bernheimer & Bey, 1986). The secreted sphingomyelinase is most likely to be SphA or Sph2 because only these enzymes possess the essential catalytic residues. Sph2 has been detected in the culture supernatant with specific antiserum (Carvalho et al., 2010; Matsunaga et al., 2007). However, the mechanism by which Sph2 is secreted is unknown because the protein appears to lack an amino-terminal signal peptide (Fig. 1). In contrast, Sph1, Sph3, SphB and SphH are predicted to have a cleavable amino-terminal signal peptide, suggesting that they are exported out of the cytoplasm to an unknown destination. Lept. interrogans also releases sphingomyelinase in membrane vesicles under some culture conditions (Velineni et al., 2009).
Transport of Sph2 and SphA out of the leptospiral cell could involve either the type I or type II secretion pathway (Bulach et al., 2006a). Recently a 63 kDa TolC homologue (LA0957) was immunoprecipitated from an outer membrane preparation of Lept. interrogans with antiserum raised against the enzymic domain of Sph3 (Velineni et al., 2009). Although further experimentation is necessary to confirm the association of the proteins, this observation suggests that at least one of the sphingomyelinase-like proteins is secreted via the TolC-based type I secretory pathway (Jenewein et al., 2009). Another TolC homologue (LA3927/LIC13135) was also noted as potentially functioning in sphingomyelinase secretion (Louvel et al., 2006).
N-terminal repeats
Analysis of the sequences attached to the N-termini of the enzymic domain using RADAR (Heger & Holm, 2000) revealed between two and seven short N-terminal imperfect repeats (NTRs) in Sph1, Sph2 and SphB (Table 1). The repeats are enriched in disorder-promoting amino acids (Tompa, 2005). Based on the known functions of intrinsically disordered sequences, the NTRs may harbour proteolytic sites, function as a flexible linker between the signal peptide and the enzymic domain, or bind macromolecules or small ligands (Tompa, 2005).
Table 1. N-terminal repeats.
| Protein | Locus tag | Species/serovar | Signal peptide* | No. of repeats | Repetitive sequences† |
| Sph1 | LA1027 | List. interrogans serovar Lai | Yes (39–40) | 2 | 60–70 (NVNEKIEDSTN) |
| 76–86 (NVNEEDENSIN) | |||||
| LIC12632 | List. interrogans serovar Copenhageni | Yes (38–39) | 2 | 59–69 (NVNEKIEDSTN) | |
| 75–85 (NVNEEDENSIN) | |||||
| LIP0979 | List. interrogans serovar Pomona | Yes (38–39) | 2 | 59–69 (NVNEKIEDSTN) | |
| 75–85 (NVNEEDENSTN) | |||||
| LiL49501006 | List. interrogans serovar Manilae | No | 2 | 59–69 (NVNEENENVTN) | |
| 75–85 (NVNEKDENATN) | |||||
| Sph2 | LA1029 | List. interrogans serovar Lai | No | 3 | 49–67 (NQVNSVSINNDPANPNPVN) |
| 74–92 (NQVNAVPENDDPANLNPVN) | |||||
| 99–117 (NQVNAAPENGSPADPNPAN) | |||||
| LIC12631 | List. interrogans serovar Copenhageni | No | 3 | 49–67 (NQVNSVSINNDPANPNPVN) | |
| 74–92 (NQVNAVPENDNPANLNPVN) | |||||
| 99–117 (NQVNAAPENGSSADPNPAN) | |||||
| LIP0980 | List. interrogans serovar Pomona | No | 4 | 55–77 (SINNDPANPNPVNPASANNNQVN) | |
| 80–102 (PENDNPANLNPVNPASANSNQVN) | |||||
| 105–127 (PENDNPANLNPVNPASANSNQVN) | |||||
| 130–152 (PENGSPTDPNPANLASANNNQVN) | |||||
| LiL49501008 | List. interrogans serovar Manilae | No | 3 | 27–48 (DPTNPNPVNPASATSNQVNAVP) | |
| 52–73 (DPANPNPVNPASANNNQVNAVP) | |||||
| 77–98 (NPADPNPANSASANNNQVNAVP) | |||||
| Sph3 | LA4004 | List. interrogans serovar Lai | Yes (38–39) | – | – |
| LIC13198 | List. interrogans serovar Copenhageni | Yes (38–39) | – | – | |
| LIP0774 | List. interrogans serovar Pomona | No | – | – | |
| LiL49503485 | List. interrogans serovar Manilae | No | – | – | |
| SphH | LA3540 | List. interrogans serovar Lai | No | – | – |
| LIC10657 | List. interrogans serovar Copenhageni | No | – | – | |
| LIP2950 | List. interrogans serovar Pomona | Yes (44–45) | – | – | |
| LiL49503095 | List. interrogans serovar Manilae | No | – | – | |
| SphB | LBJ 0527 | Lept. borpetersenii serovar Hardjobovis strain JB197 | Yes (38–39) | 6 | 71–90 (GYDPISSGPASPTSpAGPGP) |
| 92–110 (DLDPSNPDTANSSS–TNSGS) | |||||
| 112–130 (NSSSTSSGSANSSS–TSSGS) | |||||
| 142–160 (NSSSTSSGSANSSS–TSSGS) | |||||
| 162–180 (NSSSTSSGSANSSS–TSSGS) | |||||
| 182–199 (NSSSTSSGSANSSS––KAPP) | |||||
| LBL 2552 | Lept. borpetersenii serovar Hardjobovis strain L550 | Yes (38–39) | 7 | 79–109 (PASPTSPAgpgpGDLDPSNPDTANSSSTSSG) | |
| 110–139 (SANPDTAN–sssTSSGSANPDTANSSSTSSG) | |||||
| 140–169 (SANPDTAN–sssTSSGSANPDTANSSSTNSG) | |||||
| 170–184 (SANPDTAN––––––––––––––––SSSTSSG) | |||||
| 185–214 (-ANPDTAN–sssTNSGSANPDTANSSSTSSG) | |||||
| 215–244 (SANPDTA–-sssTNSGSANPDTANSSSTSSG) | |||||
| 245–274 (SANPDTA––sssTNSGSANPDTANSSSTSSG) | |||||
| SphA | LBJ 0291 | Lept. borpetersenii serovar Hardjobovis strain JB197 | Yes (26–27) | – | – |
| LBL 2785 | Lept. borpetersenii serovar Hardjobovis strain L550 | Yes (26–27) | – | – |
The number in parentheses represents the amino acids flanking the putative signal peptidase cleavage site.
The number represents the amino acid position in the protein sequence. Lower case characters are used for amino acid residues that are not aligned. Gaps are represented by –.
Phylogenetic analysis of leptospiral sphingomyelinase-like proteins
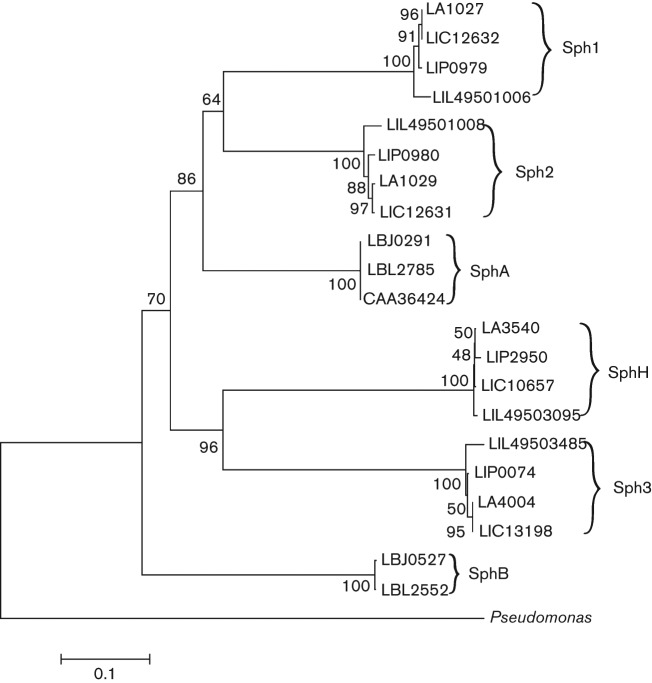
A phylogenetic tree was constructed from a multi-sequence alignment of the amino acid sequences of the leptospiral sphingomyelinase-like proteins from four strains of Lept. interrogans and two strains of Lept. borgpetersenii (Fig. 3). Sph4 was excluded from the analysis because it lacks the enzymic domain. The dendrogram shows that the leptospiral sphingomyelinase-like proteins can be grouped into six clusters. The Lept. interrogans and Lept. borgpetersenii proteins form separate clusters. The genes encoding Sph1 and Sph2 in Lept. interrogans appear to have arisen from a relatively recent duplication event, consistent with sph1 and sph2 being located next to each other on the Lept. interrogans chromosome. In contrast, only one copy of sphA is present in the same genomic position in Lept. borgpetersenii.
Fig. 3.
Phylogenetic analysis of leptospiral sphingomyelinase-like proteins. Multi-sequence alignment was performed using Geneious software utilizing the blosum62 score matrix. The phylogenetic tree was constructed using mega tool version 5 utilizing the neighbour-joining method. The robustness of the tree was determined using bootstrapping with 500 replicates. The tree was rooted with the Pseudomonas species TK4 sphingomyelinase (GenBank accession no. BAB69072.1).
Expression of leptospiral sphingomyelinase-like proteins during infection
Clear evidence for expression of a sphingomyelinase-like protein during a natural leptospiral infection came from a study of equine leptospirosis. Sera from mares infected with Lept. interrogans serovar Pomona strongly recognized recombinant Sph2 protein (Artiushin et al., 2004). A more recent study showed that IgG antibodies present in the sera of leptospirosis patients recognized recombinant Sph2 but not Sph1, Sph4 or SphH (Carvalho et al., 2010). Moreover anti-Sph2 and anti-SphH antisera reacted with renal tubular epithelium of laboratory hamsters infected with Lept. interrogans. These results indicate that Sph2 and possibly SphH are expressed during infection.
The expression of sph2 can be regulated by simulating host-like conditions. Except in several strains of serovar Pomona, Sph2 was not detected by Western blot analysis in Lept. interrogans strains cultivated in the standard leptospiral culture medium EMJH (Artiushin et al., 2004; Carvalho et al., 2010; Matsunaga et al., 2007). When sodium chloride or sucrose was added to raise the osmolarity of the culture medium to equal that found in the mammalian host, Sph2 was detected in the Lept. interrogans strain Fiocruz L1-130 cell lysates and in a processed form in the culture supernatant fluid, suggesting that the increase in osmolarity experienced by leptospires entering the host triggers sph2 expression (Matsunaga et al., 2007).
Possible roles of leptospiral sphingomyelinase-like proteins in leptospirosis
A role in nutrient acquisition has been proposed for the leptospiral sphingomyelinases (Bulach et al., 2006a). Leptospira depend on β-oxidation of fatty acid to meet their carbon and energy needs in vitro (Henneberry & Cox, 1970). Inside the host, cell membranes could provide a rich source of fatty acids as nutrients. However, sphingomyelinase would seem to be an inefficient means for obtaining fatty acid. Since the genomes of pathogenic Leptospira do not encode a ceramidase homologue, a host ceramidase would be necessary to release fatty acid molecules from ceramide for utilization by Leptospira. Leptospira also express phospholipases that yield fatty acid from abundant glycerophospholipids directly, seemingly rendering sphingomyelinases unnecessary for acquisition of fatty acid (Kasărov, 1970).
Cell lysis by sphingomyelinase or the pore-forming activity of SphH may also be important in iron acquisition. Haem released from damaged erythrocytes is a potential source of iron for Leptospira during infection. Expression of the haemin-binding protein HbpA, identified in Lept. interrogans (Sritharan et al., 2005) is induced upon iron limitation and acquires iron from haemin (Asuthkar et al., 2007). Although the expression and release of a 42 kDa sphingomyelinase-like protein in outer membrane vesicles in the presence of the chelator EDDA may support a role for an Sph protein in iron acquisition by Lept. interrogans serovar Lai (Velineni et al., 2009), microarray analysis with a strain of serovar Manilae failed to show changes in sph transcript levels when iron was depleted with 2,2′-dipyridyl (Lo et al., 2010). The different strains or chelators selected for the studies may account for the discrepancies in the results.
Another case where membrane damage may be critical to leptospiral survival is immune evasion. Although Leptospira is primarily an extracellular pathogen, it is able to escape from the phagosome of cultured mouse macrophages (Toma et al., 2011). As observed for several Listeria species, escape from the phagocytic vacuole may require the cooperation of lipases and pore-forming activities (González-Zorn et al., 1999; Schnupf & Portnoy, 2007), which may be provided by the sphingomyelinase activity of Sph2 and the pore-former SphH.
Sphingomyelinases may also have a role in cytotoxicity as part of the pathogenesis of leptospirosis. Recombinant Sph2 was cytotoxic towards mouse lymphocytes and macrophages (Zhang et al., 2008). Some evidence suggests that the immune cells undergo a proinflammatory form of apoptosis when exposed to Sph2 in vitro (Zhang et al., 2008). Additionally, damage to the vascular endothelium may be responsible for the haemorrhage observed during severe disease (Carvalho & Bethlem, 2002). Recombinant Sph2 (Lk73.5) from a Pomona strain of Lept. interrogans was cytotoxic to equine pulmonary endothelial cells (Artiushin et al., 2004). However, disruption of endothelial cell layer integrity by Lept. interrogans crossing the monolayer did not affect the viability of the cells (Martinez-Lopez et al., 2010). Thus, the evidence accumulated to date does not support a cytotoxic role for sphingomyelinases in leptospiral dissemination or haemorrhage.
The true relevance of sphingomyelinase in leptospiral pathogenesis may lie in sublytic effects that do not damage the host cell membrane. For example, alteration of vascular permeability is caused in part by generation of ceramide by acid sphingomyelinase (Göggel et al., 2004), which may explain the ability of sphingomyelinase-producing Leptospira to cross the endothelial layer without cytolytic effects. Excessive ceramide production induced by leptospiral sphingomyelinase could also explain the pulmonary oedema observed in some cases of severe leptospirosis. Alterations of sphingolipid homeostasis and lipid rafts have also been linked to altered renal function (Zager, 2000). The activity of the renal Na+/H+ NH3 transporter, whose levels are diminished in the proximal tubule of severe leptospirosis patients, depends on formation of lipid rafts (Araujo et al., 2010; Murtazina et al., 2006). Finally, the novel non-catalytic role of S. aureus sphingomyelinase in biofilm formation described earlier may also be an important function of leptospiral sphingomyelinase-like proteins during infection (Huseby et al., 2010). β-Toxin also promoted biofilm formation in vivo in a rabbit model of S. aureus endocarditis (Huseby et al., 2010). Pathogenic leptospires have been shown to form biofilms in vitro (Ristow et al., 2008), and biofilm formation may be essential for long-term leptospiral survival in the renal tubules of the reservoir host.
The pore-forming activity of SphH may also have profound biological consequences. The pore-forming proteins α-toxin of S. aureus and pneumolysin of Streptococcus pneumoniae activate the metalloprotease ADAM10, which cleaves E-cadherin, an intercellular protein essential for epithelial barrier function (Inoshima et al., 2011). ADAM10 is required by α-toxin to disturb the alveolar barrier function in the mouse model of pneumonia (Inoshima et al., 2011). These results raise the possibility that SphH promotes the acute lung injury that is observed in many cases of severe leptospirosis.
Conclusion
In this review, we have examined a number of potential roles for sphingomyelinase and its non-enzymic homologues in leptospirosis. In Lept. interrogans, only Sph2 retains all of the active-site amino acid residues essential for catalysis. Because the other sphingomyelinase homologues lack at least three of the residues, experimental studies are still needed to settle the fundamental issue of whether Sph1, Sph3 and SphH have sphingomyelinase activity. Irrespective of their catalytic activity, the proteins may dock onto sphingomyelin or some other host molecule as a prelude to performing their effector function, which may include the type of pore-forming activity described for SphH. Even in the case of Sph2, sphingomyelin hydrolysis is likely to be relevant to pathogenesis in ways that go beyond mere host cell membrane damage. Previous studies that addressed the biological functions of leptospiral Sph2 have focused on its cytotoxic potential. However, disruption of sphingolipid homeostasis by leptospiral sphingomyelinase activity also has the potential to alter cellular functions in ways that do not necessarily kill the host cell. Future studies should therefore also seek non-cytotoxic effects of Sph2 on host cells. We hope that by broadening our view of the potential biological activities of the Sph proteins, we can acquire the evidence we need to truly understand the role of leptospiral sphingomyelinases and sphingomyelinase-like proteins in leptospiral pathogenesis.
Acknowledgements
S. A. N. would like to acknowledge the United States India Educational Foundation (USIEF) for financial support in the form of Fulbright Nehru Doctoral and Professional Research Fellowship. This study was supported by Public Health Service National Institute of Allergy and Infectious Diseases grant AI-034431 (to D. A. H.) and VA Medical Research Funds (to J. M. and D. A. H.). We thank Ben Adler (Monash University) for providing the sphingomyelinase sequences of Lept. interrogans serovars Manilae and Pomona.
References
- Ago H., Oda M., Takahashi M., Tsuge H., Ochi S., Katunuma N., Miyano M., Sakurai J. (2006). Structural basis of the sphingomyelin phosphodiesterase activity in neutral sphingomyelinase from Bacillus cereus. J Biol Chem 281, 16157–16167. 10.1074/jbc.M601089200 [DOI] [PubMed] [Google Scholar]
- Araujo E. R., Seguro A. C., Spichler A., Magaldi A. J., Volpini R. A., De Brito T. (2010). Acute kidney injury in human leptospirosis: an immunohistochemical study with pathophysiological correlation. Virchows Arch 456, 367–375. 10.1007/s00428-010-0894-8 [DOI] [PubMed] [Google Scholar]
- Artiushin S., Timoney J. F., Nally J., Verma A. (2004). Host-inducible immunogenic sphingomyelinase-like protein, Lk73.5, of Leptospira interrogans. Infect Immun 72, 742–749. 10.1128/IAI.72.2.742-749.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuthkar S., Velineni S., Stadlmann J., Altmann F., Sritharan M. (2007). Expression and characterization of an iron-regulated hemin-binding protein, HbpA, from Leptospira interrogans serovar Lai. Infect Immun 75, 4582–4591. 10.1128/IAI.00324-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Bey R. F. (1986). Copurification of Leptospira interrogans serovar pomona hemolysin and sphingomyelinase C. Infect Immun 54, 262–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley A. J., Patel A. H., O’Reilly M., Foster R., Foster T. J. (1989). Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun 57, 2489–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow D. K., Weissman J. S. (2010). Membranes in balance: mechanisms of sphingolipid homeostasis. Mol Cell 40, 267–279. 10.1016/j.molcel.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulach D., Seemann T., Zuerner R., Adler B. (2006a). The organization of Leptospira at a genomic level. In Bacterial Genomes and Infectious Diseases, pp. 109–123. Edited by Chan V. L., Sherman P. M., Bourke B. Totowa, USA: Humana press; 10.1007/978-1-59745-152-9_7 [DOI] [Google Scholar]
- Bulach D. M., Zuerner R. L., Wilson P., Seemann T., McGrath A., Cullen P. A., Davis J., Johnson M., Kuczek E. & other authors (2006b). Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc Natl Acad Sci U S A 103, 14560–14565. 10.1073/pnas.0603979103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C. R., Bethlem E. P. (2002). Pulmonary complications of leptospirosis. Clin Chest Med 23, 469–478. 10.1016/S0272-5231(01)00010-7 [DOI] [PubMed] [Google Scholar]
- Carvalho E., Barbosa A. S., Gómez R. M., Cianciarullo A. M., Hauk P., Abreu P. A., Fiorini L. C., Oliveira M. L., Romero E. C., Gonçales A. P. (2009). Leptospiral TlyC is an extracellular matrix-binding protein and does not present hemolysin activity. FEBS Lett 583, 1381–1385. 10.1016/j.febslet.2009.03.050 [DOI] [PubMed] [Google Scholar]
- Carvalho E., Barbosa A. S., Gómez R. M., Oliveira M. L., Romero E. C., Gonçales A. P., Morais Z. M., Vasconcellos S. A., Ho P. L. (2010). Evaluation of the expression and protective potential of leptospiral sphingomyelinases. Curr Microbiol 60, 134–142. 10.1007/s00284-009-9519-3 [DOI] [PubMed] [Google Scholar]
- Clarke C. J., Wu B. X., Hannun Y. A. (2011). The neutral sphingomyelinase family: identifying biochemical connections. Adv Enzyme Regul 51, 51–58. 10.1016/j.advenzreg.2010.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Real G., Segers R. P., van der Zeijst B. A., Gaastra W. (1989). Cloning of a hemolysin gene from Leptospira interrogans serovar hardjo. Infect Immun 57, 2588–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolhnikoff M., Mauad T., Bethlem E. P., Carvalho C. R. (2007). Pathology and pathophysiology of pulmonary manifestations in leptospirosis. Braz J Infect Dis 11, 142–148. 10.1590/S1413-86702007000100029 [DOI] [PubMed] [Google Scholar]
- Feigin R. D., Anderson D. C., Heath C. W. (1975). Human leptospirosis. CRC Crit Rev Clin Lab Sci 5, 413–467. 10.3109/10408367509107050 [DOI] [PubMed] [Google Scholar]
- Göggel R., Winoto-Morbach S., Vielhaber G., Imai Y., Lindner K., Brade L., Brade H., Ehlers S., Slutsky A. S. & other authors (2004). PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med 10, 155–160. 10.1038/nm977 [DOI] [PubMed] [Google Scholar]
- González-Zorn B., Domínguez-Bernal G., Suárez M., Ripio M. T., Vega Y., Novella S., Vázquez-Boland J. A. (1999). The smcL gene of Listeria ivanovii encodes a sphingomyelinase C that mediates bacterial escape from the phagocytic vacuole. Mol Microbiol 33, 510–523. 10.1046/j.1365-2958.1999.01486.x [DOI] [PubMed] [Google Scholar]
- Grassmé H., Riehle A., Wilker B., Gulbins E. (2005). Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem 280, 26256–26262. 10.1074/jbc.M500835200 [DOI] [PubMed] [Google Scholar]
- Gupta V. R., Patel H. K., Kostolansky S. S., Ballivian R. A., Eichberg J., Blanke S. R. (2008). Sphingomyelin functions as a novel receptor for Helicobacter pylori VacA. PLoS Pathog 4, e1000073. 10.1371/journal.ppat.1000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Obeid L. M. (2008). Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9, 139–150. 10.1038/nrm2329 [DOI] [PubMed] [Google Scholar]
- Hayashida A., Bartlett A. H., Foster T. J., Park P. W. (2009). Staphylococcus aureus beta-toxin induces lung injury through syndecan-1. Am J Pathol 174, 509–518. 10.2353/ajpath.2009.080394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heger A., Holm L. (2000). Rapid automatic detection and alignment of repeats in protein sequences. Proteins 41, 224–237. [DOI] [PubMed] [Google Scholar]
- Henneberry R. C., Cox C. D. (1970). Beta-oxidation of fatty acids by Leptospira. Can J Microbiol 16, 41–45. 10.1139/m70-007 [DOI] [PubMed] [Google Scholar]
- Huseby M., Shi K., Brown C. K., Digre J., Mengistu F., Seo K. S., Bohach G. A., Schlievert P. M., Ohlendorf D. H., Earhart C. A. (2007). Structure and biological activities of beta toxin from Staphylococcus aureus. J Bacteriol 189, 8719–8726. 10.1128/JB.00741-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseby M. J., Kruse A. C., Digre J., Kohler P. L., Vocke J. A., Mann E. E., Bayles K. W., Bohach G. A., Schlievert P. M. & other authors (2010). Beta toxin catalyzes formation of nucleoprotein matrix in staphylococcal biofilms. Proc Natl Acad Sci U S A 107, 14407–14412. 10.1073/pnas.0911032107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshima I., Inoshima N., Wilke G. A., Powers M. E., Frank K. M., Wang Y., Bubeck Wardenburg J. (2011). A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med 17, 1310–1314. 10.1038/nm.2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenewein S., Barry Holland I., Schmitt L. (2009). Type I bacterial secretion systems. In Bacterial Secreted Proteins: Secretory Mechanisms and Role in Pathogenesis, pp. 45–65. Edited by Wooldridge K. Hethersett, Norwich, UK: Caister Academic Press. [Google Scholar]
- Kasărov L. B. (1970). Degradiation of the erythrocyte phospholipids and haemolysis of the erythrocytes of different animal species by leptospirae. J Med Microbiol 3, 29–37. 10.1099/00222615-3-1-29 [DOI] [PubMed] [Google Scholar]
- Kăsarov L. B., Addamiano L. (1969). Degradation of the phospholipids of the serum lipoproteins by leptospirae. J Med Microbiol 2, 243–248. 10.1099/00222615-2-3-243 [DOI] [PubMed] [Google Scholar]
- Lafont F., van der Goot F. G. (2005). Bacterial invasion via lipid rafts. Cell Microbiol 7, 613–620. 10.1111/j.1462-5822.2005.00515.x [DOI] [PubMed] [Google Scholar]
- Lee S. H., Kim K. A., Park Y. G., Seong I. W., Kim M. J., Lee Y. J. (2000). Identification and partial characterization of a novel hemolysin from Leptospira interrogans serovar lai. Gene 254, 19–28. 10.1016/S0378-1119(00)00293-6 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Kim S., Park S. C., Kim M. J. (2002). Cytotoxic activities of Leptospira interrogans hemolysin SphH as a pore-forming protein on mammalian cells. Infect Immun 70, 315–322. 10.1128/IAI.70.1.315-322.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M., Murray G. L., Khoo C. A., Haake D. A., Zuerner R. L., Adler B. (2010). Transcriptional response of Leptospira interrogans to iron limitation and characterization of a PerR homolog. Infect Immun 78, 4850–4859. 10.1128/IAI.00435-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvel H., Bommezzadri S., Zidane N., Boursaux-Eude C., Creno S., Magnier A., Rouy Z., Médigue C., Saint Girons I. & other authors (2006). Comparative and functional genomic analyses of iron transport and regulation in Leptospira spp. J Bacteriol 188, 7893–7904. 10.1128/JB.00711-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lopez D. G., Fahey M., Coburn J. (2010). Responses of human endothelial cells to pathogenic and non-pathogenic Leptospira species. PLoS Negl Trop Dis 4, e918. 10.1371/journal.pntd.0000918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga J., Medeiros M. A., Sanchez Y., Werneid K. F., Ko A. I. (2007). Osmotic regulation of expression of two extracellular matrix-binding proteins and a haemolysin of Leptospira interrogans: differential effects on LigA and Sph2 extracellular release. Microbiology 153, 3390–3398. 10.1099/mic.0.2007/007948-0 [DOI] [PubMed] [Google Scholar]
- Miller N. G., Allen J. E., Wilson R. B. (1974). The pathogenesis of hemorrhage in the lung of the hamster during acute leptospirosis. Med Microbiol Immunol (Berl) 160, 269–278. 10.1007/BF02121442 [DOI] [PubMed] [Google Scholar]
- Murtazina R., Kovbasnjuk O., Donowitz M., Li X. (2006). Na+/H+ exchanger NHE3 activity and trafficking are lipid Raft-dependent. J Biol Chem 281, 17845–17855. 10.1074/jbc.M601740200 [DOI] [PubMed] [Google Scholar]
- Narayanavari S. A., Nanda Kishore M., Sritharan M. (2012). Structural analysis of the leptospiral sphingomyelinases: in silico and experimental evaluation of Sph2 as an Mg++-dependent sphingomyelinase. J Mol Microbiol Biotechnol 22, 24–34. [DOI] [PubMed] [Google Scholar]
- Nascimento A. L., Ko A. I., Martins E. A., Monteiro-Vitorello C. B., Ho P. L., Haake D. A., Verjovski-Almeida S., Hartskeerl R. A., Marques M. V. & other authors (2004). Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J Bacteriol 186, 2164–2172. 10.1128/JB.186.7.2164-2172.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obama T., Fujii S., Ikezawa H., Ikeda K., Imagawa M., Tsukamoto K. (2003a). His151 and His296 are the acid-base catalytic residues of Bacillus cereus sphingomyelinase in sphingomyelin hydrolysis. Biol Pharm Bull 26, 920–926. 10.1248/bpb.26.920 [DOI] [PubMed] [Google Scholar]
- Obama T., Kan Y., Ikezawa H., Imagawa M., Tsukamoto K. (2003b). Glu-53 of Bacillus cereus sphingomyelinase acts as an indispensable ligand of Mg2+ essential for catalytic activity. J Biochem 133, 279–286. 10.1093/jb/mvg038 [DOI] [PubMed] [Google Scholar]
- Openshaw A. E., Race P. R., Monzó H. J., Vázquez-Boland J. A., Banfield M. J. (2005). Crystal structure of SmcL, a bacterial neutral sphingomyelinase C from Listeria. J Biol Chem 280, 35011–35017. 10.1074/jbc.M506800200 [DOI] [PubMed] [Google Scholar]
- Picardeau M., Bulach D. M., Bouchier C., Zuerner R. L., Zidane N., Wilson P. J., Creno S., Kuczek E. S., Bommezzadri S. & other authors (2008). Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS ONE 3, e1607. 10.1371/journal.pone.0001607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S. X., Fu G., Jiang X. G., Zeng R., Miao Y. G., Xu H., Zhang Y. X., Xiong H., Lu G. & other authors (2003). Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422, 888–893. 10.1038/nature01597 [DOI] [PubMed] [Google Scholar]
- Ristow P., Bourhy P., Kerneis S., Schmitt C., Prevost M. C., Lilenbaum W., Picardeau M. (2008). Biofilm formation by saprophytic and pathogenic leptospires. Microbiology 154, 1309–1317. 10.1099/mic.0.2007/014746-0 [DOI] [PubMed] [Google Scholar]
- Schnupf P., Portnoy D. A. (2007). Listeriolysin O: a phagosome-specific lysin. Microbes Infect 9, 1176–1187. 10.1016/j.micinf.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Segers R. P., van der Drift A., de Nijs A., Corcione P., van der Zeijst B. A., Gaastra W. (1990). Molecular analysis of a sphingomyelinase C gene from Leptospira interrogans serovar hardjo. Infect Immun 58, 2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers R. P., van Gestel J. A., van Eys G. J., van der Zeijst B. A., Gaastra W. (1992). Presence of putative sphingomyelinase genes among members of the family Leptospiraceae. Infect Immun 60, 1707–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sritharan M., Ramadevi S., Pasupala N., Tajne S., Asuthkar S. (2005). In silico identification and modelling of a putative iron-regulated TonB dependent outer membrane receptor protein from the genome of Leptospira interrogans serovar Lai. Online Journal of Bioinformatics 6, 74–90. [Google Scholar]
- Sueyoshi N., Kita K., Okino N., Sakaguchi K., Nakamura T., Ito M. (2002). Molecular cloning and expression of Mn2+-dependent sphingomyelinase/hemolysin of an aquatic bacterium, Pseudomonas sp. strain TK4. J Bacteriol 184, 540–546. 10.1128/JB.184.2.540-546.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma C., Okura N., Takayama C., Suzuki T. (2011). Characteristic features of intracellular pathogenic Leptospira in infected murine macrophages. Cell Microbiol 13, 1783–1792. 10.1111/j.1462-5822.2011.01660.x [DOI] [PubMed] [Google Scholar]
- Tompa P. (2005). The interplay between structure and function in intrinsically unstructured proteins. FEBS Lett 579, 3346–3354. 10.1016/j.febslet.2005.03.072 [DOI] [PubMed] [Google Scholar]
- Velineni S., Ramadevi S., Asuthkar S., Sritharan M. (2009). Effect of iron deprivation on expression of sphingomyelinase in pathogenic serovar Lai. Online J Bioinform 10, 241–258. [Google Scholar]
- WHO (2003). Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control. World Health Organization; http://whqlibdoc.who.int/hq/2003/WHO_CDS_CSR_EPH_2002.23.pdf [Google Scholar]
- Zager R. A. (2000). Plasma membrane cholesterol: a critical determinant of cellular energetics and tubular resistance to attack. Kidney Int 58, 193–205. 10.1046/j.1523-1755.2000.00154.x [DOI] [PubMed] [Google Scholar]
- Zeidan Y. H., Hannun Y. A. (2007). Translational aspects of sphingolipid metabolism. Trends Mol Med 13, 327–336. 10.1016/j.molmed.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Zhang Y. X., Geng Y., Bi B., He J. Y., Wu C. F., Guo X. K., Zhao G. P. (2005). Identification and classification of all potential hemolysin encoding genes and their products from Leptospira interrogans serogroup Icterohaemorrhagiae serovar Lai. Acta Pharmacol Sin 26, 453–461. 10.1111/j.1745-7254.2005.00075.x [DOI] [PubMed] [Google Scholar]
- Zhang Y. X., Geng Y., Yang J. W., Guo X. K., Zhao G. P. (2008). Cytotoxic activity and probable apoptotic effect of Sph2, a sphigomyelinase hemolysin from Leptospira interrogans strain Lai. BMB Rep 41, 119–125. 10.5483/BMBRep.2008.41.2.119 [DOI] [PubMed] [Google Scholar]