Abstract
Activation of the phosphoinositide 3-kinase pathway is commonly observed in human prostate cancer. Loss of function of phosphatase and tensin homolog (PTEN) is associated with the activation of AKT and mammalian target of rapamycin (mTOR) in many cancer cell lines as well as in other model systems. However, activation of mTOR is also dependent of kinases other than AKT. Here, we show that activation of mTOR is not dependent on AKT in a prostate-specific PTEN-deficient mouse model of prostate cancer. Pathway bifurcation of AKT and mTOR was noted in both mouse and human prostate tumors. We demonstrated for the first time that cotargeting mTOR and AKT with ridaforolimus/MK-8669 and M1K-2206, respectively, delivers additive antitumor effects in vivo when compared to single agents. Our preclinical data suggest that the combination of AKT and mTOR inhibitors might be more effective in treating prostate cancer patients than current treatment regimens or either treatment alone.
Introduction
Prostate cancer, the third most common cause of cancer-related deaths in men, is particularly high in developed countries and the prevalence is now also increasing in developing nations [1]. Prostate cancer is the second most frequently diagnosed cancer in men in the United States with more than two million currently suffering from this disease. Although the death rate from prostate cancer is decreasing with the advent of early diagnosis, surgery, radiotherapy, and hormonal and chemotherapies, it still remains one of the leading causes of cancer-related death in men.
The androgen receptor (AR) signaling pathway plays a central role in the normal development and growth of the prostate gland, as well as the abnormal growth of prostate cancer (reviewed in [2]). The primary clinical strategy for prostate cancer treatment is to suppress AR signaling by androgen deprivation through castration, estrogen, antiandrogens, or their combination (reviewed in [3]). Androgen ablation by either surgical castration or luteinizing hormone-releasing hormone (LHRH) analogue treatments strongly inhibits the growth of localized advanced cancer by eliminating circulating testosterone [4,5]. Unfortunately, within a few years after androgen ablation therapy, most patients experience resurgence of aggressive disease that is refractory to anti-androgen therapy (reviewed in [6]). The mechanism of acquired resistance to antiandrogens remains unclear, yet emerging evidence suggests a central role for the phosphoinositide 3-kinase (PI3K) pathway [7–9]. We recently demonstrated that activation of the PI3K pathway is independent of AR signaling and combining a mammalian target of rapamycin (mTOR) inhibitor and AR antagonist displays significantly greater antitumor effect in an androgen-dependent model of prostate cancer [10].
The PI3K pathway plays an important role in regulating several cellular functions including cell growth, proliferation, differentiation, and survival. Loss or functional alteration of the tumor suppressor gene PTEN activates downstream PI3K signaling, resulting in increased cell proliferation and survival [11–15]. More than 20% of primary human prostate cancer is associated with the deletion or functional loss of PTEN, whereas more than 50% of human metastatic prostate cancer specimens harbor biallelic loss of this gene, suggesting a central role for phosphatase and tensin homolog (PTEN) loss in the lethal form of the disease [16–20]. Conventional deletion of both alleles of Pten leads to embryonic lethality in mice [21,22], whereas heterozygous Pten-null mice develop prostate intraepithelial neoplasia. These mice have been crossed with a number of other genetically engineered mice for further enhancement of the tumor phenotype (reviewed in Majumder and Sellers [23]). Loss-of-function mutations or lowered expression of PTEN increases the activity of important downstream targets of PI3K, such as AKT, in various cell lines and engineered mouse models (reviewed in [24,25]). Activation of AKT1 in the prostate alone induces the development of PIN but not invasive prostate cancer, whereas activation of AKT1 in a p27Kip1-null setting as well as prostate-specific deletion of Pten induces invasive cancer in mice [26–28]. To dissect the role of different components of the PI3K pathway signaling network, we have used a genetically engineered mouse model where PtenloxP/loxP mice (loxP—locus of X-over P1, [29]) were crossed with ARR2-Pb-Cre+ (rat probasin promoter with two androgen-responsive regions driving the Crerecombinase) transgenic mice [30]. These mice spontaneously develop prostate tumors that recapitulate most human prostate cancer characteristics [28]. AKT and mTOR, two important kinases of the PI3K pathway, are activated as measured by phosphorylation in this model. It has been reported that inhibition of mTOR by rapamycin analogues showed antitumor effects in preclinical models [10,13,14,27,31]. However, the negative feedback from mTOR inhibition on AKT might be an important factor to consider for treating PTEN-null tumors with rapalogs [30–32]. Here, we demonstrate that MK-2206, a small molecule inhibitor of AKT, is as efficacious as an mTOR inhibitor (ridaforolimus/MK-8669) for treatment of PTEN-null prostate tumors and that activation of mTOR is not dependent on AKT activity. Using both a genetically engineered mouse model and a human prostate cancer cell-based model, we demonstrate a combination benefit of these two inhibitors. Our findings support the combination of AKT and mTOR inhibitors for treatment of PTEN-null prostate cancer in patients.
Materials and Methods
Animals
A conditional Pten-deficient model of prostate cancer (Ptenloxp/loxp/Pb-Cre4) in which Pten is homozygously deleted in the prostate gland because of prostate-specific expression of Cre recombinase [28] was used for these studies. This model was licensed from Dr Hong Wu's laboratory at the University of California (Los Angeles, CA). Both founder lines, the ARR2Probasin-Cre (C+) transgenic mice ([33]; on C57BL/6xDBA2 background) and the PtenLoxp/Loxp mice (on a 129/Balb/c background), were maintained using our contract facility. The heterozygous PtenLoxp/+;C/+ males were bred to PtenLoxp/Loxp females to obtain the PtenLoxp/loxp;C/+ genotype. Male PtenLoxp/loxp;C/+ mice between 36 and 46 weeks of age were used for experiments. Mice were using an isolated barrier unit system on woodchip bedding and fed standard rodent chow ad libitum.
Treatment
Animals were prescreened by three-dimensional (3D) ultrasound imaging to obtain a baseline tumor volume for enrollment and were randomized into groups based on tumor volumes. Ten animals were treated with an orally active AKT inhibitor (MK-2206) [34] at a dose of either 120 mg/kg or 240 mg/kg thrice a week (Monday, Wednesday, and Friday), and other 10 animals were kept as controls (vehicle treated). Mice were imaged weekly. Tumor-bearing mice were also treated intraperitoneally with an mTOR inhibitor (ridaforolimus) [35] at 1.0 mg/kg, once a day (Monday to Friday), or with the combination of MK-2206 and ridaforolimus at their above indicated doses, or by 0.5% methylcellulose + normal saline (placebo) for 9 weeks.
Prostate Tumor Measurement
Prostate tumors were imaged using a 3D ultrasound imaging system Vevo770 (VisualSonics, Toronto, Canada). 3D images were reconstructed from 2D section images using the Vevo770 system software following acquisition. Tumor volumes were determined using the 3D Quantify Version 3.5 software (Robarts Research Institute, London, Canada) as described [10]. For each study, animals were randomized into groups based on their baseline tumor volumes measured by ultrasound imaging and animals were imaged weekly for 9 to 10 weeks. All animal procedures were reviewed and approved by the Merck Research Laboratories-Boston Institutional Animal Care and Use Committee.
Endpoint Tumor Assessment
Mice were killed using CO2, and the entire tumors were collected and weighed. Animals were killed, and tumor samples were collected when tumor volumes reached 10% of body weight before study termination. Tumor samples were collected for histologic and biochemical analyses.
Histologic and Immunohistochemical Analyses
Formalin-fixed paraffin-embedded tissues were sectioned at 5-µm thickness. Tissue sections were stained with hematoxylin and eosin (Fisher Scientific, Waltham, MA) and analyzed for cell proliferation [bromodeoxyuridine (BrdU), 1:500 (BD Biosciences, San Jose, CA); Ki-67, 1:500, Clone SP6 (Lab Vision, Kalamazoo, MI)]. The status of phospho-S6 ribosomal protein (1:75; Cell Signaling Technology, Inc, Danvers, MA) and p-AKT (S473) (1:50; Cell Signaling Technology, Inc) were also measured. Automated staining was performed using the ChromoMap Kit on the Discovery XT (Ventana Medical System, Inc, Tucson, AZ) under standard conditions as described [36].
Cell Line
Human prostate cancer cell line (LNCaP) was cultured in RPMI with 10% fetal bovine serum, 1% glutamine, penicillin/streptomycin, and 50 µM β-mercaptoethanol at 37°C under 5% CO2. To inhibit AKT signaling, we plated cells at 1 x 106 in a 10-cm dish in the presence of either MK-2206 at 0.1, 0.5, or 1 µM, or ridaforolimus at 0.1, 0.5, or 1.0 µM, or a combination of MK-2206 and ridaforolimus. Mock-treated cells were cultured with DMSO at a final concentration of 0.01%. Cell growth was measured by ATPlite assay as per manufactured kits (PerkinElmer Life Sciences, Waltham, MA). Statistical analysis to determine highest single-agent response (HSA) and confidence interval (CI) values was performed.
Western Blot Analysis and Reverse Phase Protein Array
Tumor samples or cells were collected, flash frozen in liquid nitrogen, and stored at -80°C until processed. Frozen samples were thawed in cold RIPA lysis buffer [0.5 M Tris-HCl (pH 7.4), 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, and 10 mM EDTA; Upstate USA, Inc, Billerica, MA] containing complete protease inhibitor cocktail (Roche, Indianapolis, IN). Protein concentrations in cell and tissue lysates were determined by the Bicinchoninic Acid Protein Assay Kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions. Twenty nanoliters of denatured lysates from each mice was immobilized onto nitrocellulose-backed glass slides and probed with validated corresponding phosphoepitope-specific antibodies for the said analyte. Arrays were developed with a 3,3′-diaminobenzidine tetrahydrochloride chromogen after which intensity values were measured by ImageQuant for each sample. Phospho-AKT (S473 and T308), phospho-S6RP (S235/236), total AKT, and total S6RP were measured. All proteins were detected by resolving proteins on criterion 4 to 15% Tris-HCl sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad Laboratories, Hercules, CA) and blotted onto nitrocellulose membrane. Residual binding sites were blocked by 5% nonfat dry milk and blotted with antibodies against phospho-AKT (S473 and T308; 1:1000), phospho-S6RP (1:1000; Cell Signaling Technology, Inc), and tubulin (1:1000; Abcam Inc, Cambridge, MA). Detection of protein bands was performed using SuperSignal West Pico and Femto Chemiluminescent Substrate (Pierce Biotechnology) after incubation with the HRP-conjugated secondary antibody (1:5000; Jackson Immuno-Research Laboratories, Inc, West Grove, PA).
mRNA Microarray Expression Analysis
Tumors were dissected from Pten wild-type and Pten-null mice at the age of 6, 10, 14, 21, 30, and 36 weeks. Tumor or normal prostate samples for RNA analysis were snap frozen in 1 ml of Triazol (Invitrogen, Grand Island, NY) per 100 mg of tissue. RNA was extracted using a RNeasy midi kit (Qiagen, Valencia, CA) according to the manufacturer's instructions but with additional treatment with RNase-free DNAse Set (Qiagen) before elution. cDNA was synthesized using the high-capacity archive kit (Applied Biosystems, Carlsbad, CA). mRNA and microarray studies were conducted as previously described [37].
Statistical Analysis
A repeated measures analysis of variance followed by Dunnett's multiple comparison tests were performed for longitudinal studies and Student's t test was conducted to evaluate statistically significant differences between groups for the endpoint analyses.
Results
Prostate Tumors in PtenLoxp/Loxp;C+ Mice Are Sensitive to AKT Inhibitor
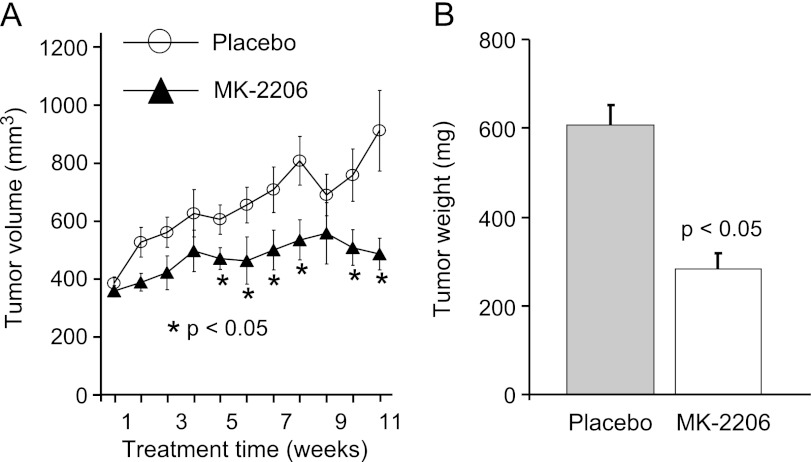
Activation of AKT either through loss-of-function tumor suppressor gene PTEN or by the activation of PI3K has been identified in human prostate cancer. Phosphorylation of AKT occurs at both S473 and T308 sites in PTEN-deleted mouse models of prostate cancer. Activation of AKT, along with other downstream targets of PTEN, is observed in this model [10,28]. To determine whether this model is dependent on AKT, we have treated tumor-bearing mice with an allosteric inhibitor of AKT (MK-2206). Twenty mice with tumor volumes ranging from 300 to 400 mm3 (ages ranged from 36 to 46 weeks old) were randomly assigned to two groups. One group was treated by 0.5% methylcellulose (placebo), and the remaining mice were treated with MK-2206 at the dose of 240 mg/kg thrice a week. Mice were imaged weekly by ultrasound following enrollment into the study to monitor tumor volume. Within the first 3 weeks following treatment, we observed no difference in tumor growth between placebo and AKT inhibitor (Figure 1A). By week four, tumor volumes in the MK-2206-treated mice were significantly smaller compared to the placebo controls (Figure 1A). Our results show a drop of tumor volume in control group due to death of two large tumor-bearing mice. Four hours after the last dose at 11 weeks, mice were killed and tumor weight was measured. Data showed a tumor net weight reduction along with a significant reduction in ultrasound-measured volume following MK-2206 treatment (Figure 1B). These data demonstrate that AKT plays a significant role in tumor growth progression in this model of prostate cancer. However, AKT inhibition alone was not sufficient to completely suppress, suggesting other PI3K pathway nodes and/or other oncogenic signaling networks might also play a critical role in tumor maintenance and growth.
Figure 1.
PTEN-deficient tumors are sensitive to AKT inhibition. (A) Tumor response to AKT inhibitor MK-2206. Prostate tumors in PTEN-deficient mice were imaged, and the mice were randomized into two groups (n = 10/group) based on tumor volume (∼400 mm3). Mice in group 1 underwent placebo treatment and those in group 2 were treated with MK-2206 at a dose of 240 mg/kg thrice a week. Tumors were imaged and volume was measured weekly for 11 weeks and represented as mean ± SEM. (B) Mice were killed; tumors were dissected and weight was measured. Significant (P ≤ 0.05) tumor growth progression was observed when mice were treated with MK-2206 compared to the corresponding placebo-treated mice.
Activation of the mTOR Pathway Is Independent of AKT in a PTEN-Deficient Model of Prostate Cancer
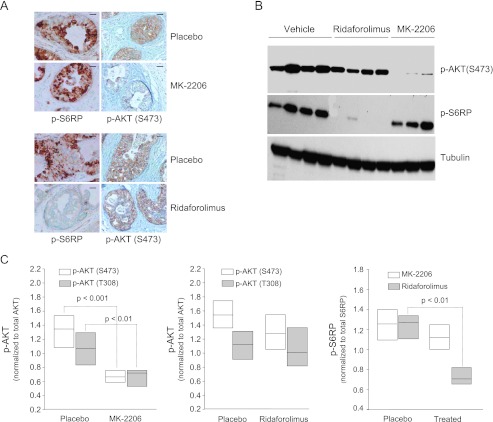
Loss of function of PTEN is associated with activation of both AKT and mTOR kinases evidenced by increased phosphorylation of both AKT and S6RP, a downstream target of mTOR kinase in these tumors ([10,28], and data not shown). However, it is not clear whether the activation of mTOR downstream signaling is due to activation of AKT. Inhibition of AKT phosphorylation at S473 and T308 was noted in tumors treated with MK-2206 (Figure 2A, upper panel and data not shown). Surprisingly, tumor sections stained with p-S6RP indicate no inhibition of the mTOR signaling network when tumors were treated with MK-2206 (Figure 2A, lower panel). Similarly, immunoblots showed that MK-2206 had no effect on the phosphorylation status of S6RP while completely blocking p-AKT, whereas mTOR inhibitor completely inhibited p-S6RP with no effect on p-AKT (Figure 2B). To determine the quantitative inhibition of phosphorylation with both AKT and mTOR inhibitors, we performed reverse phase phosphoproteomic analysis. These data further demonstrated that AKT inhibition by MK-2206 significantly decreased both serine (S473) and threonine (T308) phosphorylation sites of AKT (Figure 2C, left panel), whereas no effect was observed on p-AKT by mTOR inhibitor (Figure 2C, middle panel). The phosphorylation sites of S6RP were significantly decreased by mTOR inhibitor but remained unaltered by AKT inhibitor (Figure 2C, right panel). Together, these data suggest that AKT is not the rate-limiting step for the activation of mTOR signaling in this model of prostate cancer.
Figure 2.
AKT inhibition does not alter the activation of mTOR. Tumor-bearing mice were surgically treated with inhibitors of AKT at 240 mg/kg or mTOR at 1 mg/kg for 9 weeks and harvested 4 hours after the last dose. (A) Tumor sections from 0.5% methylcellulose (placebo), inhibitor of AKT (MK-2206) (upper panel), and mTOR inhibitor treatment (lower panel) were stained with antibody against p-S6RP and p-AKT. These are representative pictures of four mice per group. Scale bar is 50 µm. (B) Fresh tissues were lysed and total protein was isolated. Protein was separated by gel electrophoresis, blotted onto membrane, and hybridized with antibody against p-AKT (S473) and p-S6RP(S235/236). Tubulin was used as a loading control. (C) Reverse phase protein array analysis of tumor lysates for p-AKT and total AKT from MK-2206-treated samples (left panel), from mTOR inhibitor-treated samples (middle panel), and p-S6RP and total S6RP were performed from ridaforolimus or MK-2206-treated tumors (right panel). Results are represented in a standard box blot format in which the boundaries of the box are the smallest and largest intensity observations, and the notch denotes the median.
Simultaneous Inhibition of Both mTOR and AKT Demonstrates Additive Antitumor Efficacy
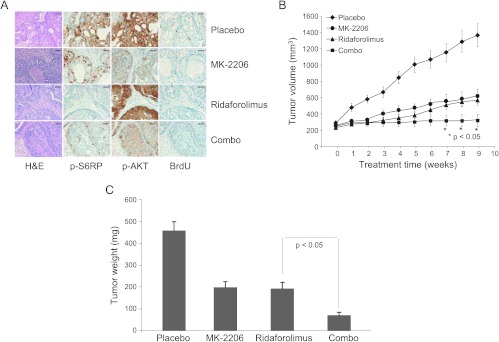
Both AKT and mTOR are phosphorylated in a subset of human prostate cancer. Our data show that AKT and mTOR signaling pathways are independently active in this model of prostate cancer (Figure 2), suggesting that simultaneous inhibition of both pathways may result in a better therapeutic response. Tumor-bearing mice were treated with placebo, ridaforolimus, MK-2206, or a combination of ridaforolimus and MK-2206 for a duration of 9 weeks. Immunohistochemical analysis was performed to evaluate the inhibition of p-AKT or p-S6RP by these treatments and their effects on tumor cell proliferation. MK-2206 alone reduced p-AKT-positive cells, while mTOR activity remains unaffected (Figures 2 and 3A). As expected, combination treatment with MK-2206 and ridaforolimus completely inhibited both AKT and phospho-S6RP levels, whereas each agent alone only inhibited their respective targets (Figures 2 and 3A). BrdU staining showed that inhibition of tumor cell proliferation by either MK-2206 or mTOR inhibitor was further enhanced by the combination treatment (Figure 3A). Inhibition of tumor cell proliferation by simultaneously blocking both AKT and mTOR kinases in these tumors resulted in an increased antitumor effect (Figure 3B). After 9 weeks of treatment, the tumor volumes were 1366 ± 141, 618.8 ± 87, 568 ± 62, and 320 ± 75 mm3 for placebo control, MK-2206, ridaforolimus, and the combination, respectively. These data demonstrate a significant additive antitumor effect when mice were treated with the combination of ridaforolimus and MK-2206 (Figure 3B) without affecting body weight (data not shown). Postmortem tumor weight data further confirmed ultrasound-based tumor volume data (Figure 3C). Together, these data demonstrate that the combination treatment of PTEN-deficient prostate tumors with MK-2206 and ridaforolimus attenuates both AKT pathway and AKT-independent mTOR pathway, leading to increased inhibition of tumor cell proliferation and the additive antitumor efficacy.
Figure 3.
AKT and mTOR inhibitor combinations induce an additive antitumor effect. Tumor-bearing mice were enrolled when tumor size was 250 to 350 mm3 and randomized into four groups (n = 12 mice/group). Mice were treated with either vehicle (placebo), MK-2206 at 120 mg/kg, ridaforolimus at 1 mg/kg, or in combination for 9 weeks. (A) Mice were killed and tumors were harvested after 4 hours of the last dose. Tumors were fixed, paraffin embedded, and sectioned. Five-micrometer-thick tumor sections were stained with hematoxylin and eosin. Adjacent tumors sections were also stained with antibodies against p-S6RP, p-AKT (S473), or proliferation marker BrdU. Data represented six tumors in each treatment type. Scale bar is 50 µm. (B) Tumor volume was measured by ultrasound imaging. Data were plotted as mean ± SEM over treatment time. (C) Mice were killed and tumor weights were measured, with the mean ± SEM plotted against treatment.
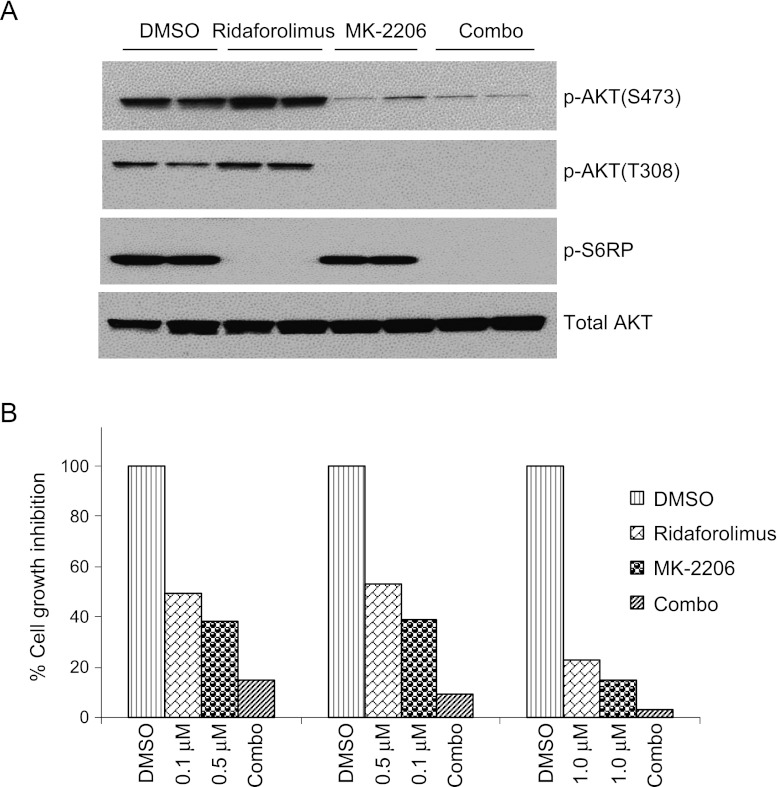
Inhibition of AKT did not alter the activation of S6RP in the tumors of PTEN-null prostate. To understand whether this effect is due to the tumor microenvironment, we tested MK-2206 and ridaforolimus in an androgen-dependent human prostate cancer cell line (LNCaP) with loss of function of PTEN, where a negative feedback mechanism of mTOR inhibition was observed [35–37]. Immunoblot analysis data show the inhibition of AKT and S6RP phosphorylation by MK-2206 and ridaforolimus, respectively, and negative feedback activation of AKT was observed by ridaforolimus (Figure 4A). Consistent with our in vivo data, AKT inhibition had no effect on the phosphorylation of S6RP (Figure 4A). To further investigate whether inhibition of both AKT and mTOR results in an additive antitumor efficacy, we treated LNCaP cells with DMSO, MK-2206, ridaforolimus, or the combination of MK-2206 and ridaforolimus. Cell growth inhibition was measured with three different combinations of AKT and mTOR inhibitors. Cell growth inhibition data demonstrated a significant combinational benefit in LNCaP cells (Figure 4B). Results indicate that the combination of AKT and mTOR inhibitors exhibits an additive to synergistic effect. These data further confirm that AKT-independent activation of mTOR is not limited to the tumor microenvironment. Together, these data suggest that the activation of mTOR is independent of AKT, and simultaneous inhibition of AKT and mTOR induces a significant tumor growth inhibition.
Figure 4.
Inhibition of AKT and mTOR exhibits combinational benefit in human prostate cancer cells. (A) LNCaP cells were treated with DMSO, MK-2206, or ridaforolimus for 6 and 24 hours. Cells were harvested and total protein lysates were made and blotted against indicated antibodies. (B) LNCaP cells were treated with either DMSO, MK-2206, ridaforolimus, or in combinations as indicated for 3 days. Cells were harvested; the number of cells was counted, and percent against DMSO was calculated and plotted.
Discussion
The AKT/mTOR signaling network is deregulated in more than 50% of solid tumors and plays a key role in cancer cell growth, survival, and tumor angiogenesis. PTEN is a tumor suppressor that negatively regulates this pathway. Loss of function of PTEN activates downstream substrates of PI3K signaling network in many model systems [23]. Both AKT and mTOR are activated in human primary prostate cancer specimens [27]. However, the dependency of AKT and mTOR activation on tumor growth has not been dissected. Here, we show that PTEN-deficient models of prostate cancer with evidence of AKT and mTOR pathway activation responded to MK-2206 and ridaforolimus combination therapy. Inhibition of either kinase alone had no effect on the status of the other kinase (Figures 1 and 2). These findings strongly indicate that the mTOR signaling network in the PTEN-null tumor is independent of AKT activity and that both AKT and mTOR downstream signaling pathways play a part in PTEN-deficient prostate tumors. These results support the rationale for using AKT and mTOR inhibitors in combination.
Our recent publication demonstrated that this model is androgen dependent and that androgen blockade attenuates tumor growth progression [10]. In the clinic, antiandrogens or complete androgen blockade and conventional chemotherapeutic approaches are effective in androgen-dependent prostate cancer [38]. However, these agents do not result in complete tumor regression and are commonly associated with the reemergence of an aggressive and androgen therapy-resistant form of the disease. Therefore, more effective therapeutic strategies are needed in addition to antiandrogen therapies to prevent or delay the emergence of castrate-resistant prostate cancer.
Recent preclinical and clinical findings suggest that AR signaling remains critical throughout the course of the disease (reviewed in [2]). This hypothesis has been validated by the clinical successes of a new generation of potent AR antagonists such as MDV3100 or antiandrogen agents such as abiraterone [2]. Recently, it has also been demonstrated that the AR and PI3K signaling pathways are feedback regulated in a reciprocal manner [39]. That is, androgen independence, commonly associated with PTEN loss, is associated with the activation PI3K signaling; conversely, PI3K inhibitors can reactivate androgen signaling and sensitivity to antiandrogen therapy.
Here, we report MK-2206 and ridaforolimus combination efficacy in the castrate-sensitive setting. Although robust, the antitumor response generally resulted in tumor stasis rather than significant regression. This finding suggests that other pathways may need to be cotargeted, such as extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) [40,41] or androgen signaling pathway. In this regard, it would be interesting to test the MK-2206 and ridaforolimus combination in both castrate-sensitive and castrate-resistant settings. It has been shown that mTOR inhibitors reverse PI3K-mediated neoplasia and reduce tumor volumes in genetically engineered mice models of prostate cancer [10,13,14,43]. We and others have noted combination benefit of ridaforolimus with anti-AR or antiandrogen inhibitors in the partially castrate-resistant and PTEN-deficient C4-2 model (unpublished data and [44]). Unfortunately, there is lack of preclinical models available to rigorously test AKT, mTOR, and antiandrogen cotargeting strategies in the castrate-resistant setting. Tolerability issues associated with multidrug therapies are also a potential concern and will require optimization of dose schedules. Nevertheless, this report provides a conceptual framework for cotargeting AKT and mTOR in PTEN-deficient prostate cancer. MK-2206 in combination with ridaforolimus is currently in early phase clinical development (clinicaltrials.gov NCT01295632).
Acknowledgments
We thank Nirah Shomer, Merck Research Laboratories-Boston, for providing animal resource support. We also thank Shubing Wang, Merck Research Laboratories-Rahway, for statistical analysis and members of AKT and mTOR project teams for their critical comments in this manuscript.
Footnotes
All authors are currently employed by Merck & Co, Inc or were employed by Merck & Co, Inc when the experimental work was completed.
References
- 1.Baade PD, Coory MD, Aitken JF. International trends in prostate-cancer mortality: the decrease is continuing and spreading. Cancer Causes Control. 2004;15:237–241. doi: 10.1023/B:CACO.0000024212.66334.26. [DOI] [PubMed] [Google Scholar]
- 2.Yap TA, Zivi A, Omlin A, de Bono JS. The changing therapeutic landscape of castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011;8:597–610. doi: 10.1038/nrclinonc.2011.117. [DOI] [PubMed] [Google Scholar]
- 3.Michaelson MD, Cotter SE, Gargollo PC, Zietman AL, Dahl DM, Smith MR. Management of complications of prostate cancer treatment. CA Cancer J Clin. 2008;58:196–213. doi: 10.3322/CA.2008.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Gil T, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 5.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, Yao SL. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pomerantz M, Kantoff P. Advances in the treatment of prostate cancer. Annu Rev Med. 2007;58:205–220. doi: 10.1146/annurev.med.58.101505.115650. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–7792. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 8.Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ. Activation of phosphatidylinositol 3-kinase/AKT pathway by androgen through interaction of p85α, androgen receptor, and Src. J Biol Chem. 2003;278:42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- 9.Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004;279:14579–14586. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Zhu J, Efferson CL, Ware C, Tammam J, Angagaw M, Laskey J, Bettano KA, Kasibhatla S, Reilly JF, et al. Inhibition of tumor growth progression by antiandrogens and mTOR inhibitor in a Pten-deficient mouse model of prostate cancer. Cancer Res. 2009;69:7466–7472. doi: 10.1158/0008-5472.CAN-08-4385. [DOI] [PubMed] [Google Scholar]
- 11.Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK. The Akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H, Sawyers CL. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Podsypanina K, Lee RT, Politis C, Hennessy I, Crane A, Puc J, Neshat M, Wang H, Yang L, Gibbons J, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/- mice. Proc Natl Acad Sci USA. 2001;98:10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stiles B, Gilman V, Khanzenzon N, Lesche R, Li A, Qiao R, Liu X, Wu H. Essential role of AKT-1/protein kinase Bα in PTEN-controlled tumorigenesis. Mol Cell Biol. 2002;22:3842–3851. doi: 10.1128/MCB.22.11.3842-3851.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray IC, Phillips SM, Lee SJ, Neoptolemos JP, Weissenbach J, Spurr NK. Loss of the chromosomal region 10q23-25 in prostate cancer. Cancer Res. 1995;55:4800–4803. [PubMed] [Google Scholar]
- 17.Komiya A, Suzuki H, Ueda T, Yatani R, Emi M, Ito H, Shimazaki J. Allelic losses at loci on chromosome 10 are associated with metastasis and progression of human prostate cancer. Genes Chromosomes Cancer. 1996;17:245–253. doi: 10.1002/(SICI)1098-2264(199612)17:4<245::AID-GCC6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 19.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, Isaacs WB, Bova GS. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58:204–209. [PubMed] [Google Scholar]
- 21.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 22.Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 24.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 25.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 26.Majumder PK, Yeh JJ, George DJ, Febbo PG, Kum J, Xue Q, Bikoff R, Ma H, Kantoff PW, Golub TR, et al. Prostate intraepithelial neoplasia induced by prostate restricted AKT activation: the MPAKT model. Proc Natl Acad Sci USA. 2003;100:7841–7846. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majumder PK, Grisanzio C, O'Connell F, Barry M, Brito JM, Xu Q, Guney I, Berger R, Herman P, Bikoff R, et al. A prostatic intraepithelial neoplasiadependent p27Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression. Cancer Cell. 2008;14:146–155. doi: 10.1016/j.ccr.2008.06.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 29.Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 30.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 32.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 34.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, et al. MK-2206, an allosteric Akt inhibitor, enhances anti-tumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9(7):1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 35.Rivera VM, Squillace RM, Miller D, Berk L, Wardwell SD, Ning Y, Pollock R, Narasimhan NI, Iuliucci JD, Wang F, et al. Ridaforolimus (AP23573; MK-8669), a potent mTOR inhibitor, has broad antitumor activity and can be optimally administered using intermittent dosing regimens. Mol Cancer Ther. 2011;10:1059–1071. doi: 10.1158/1535-7163.MCT-10-0792. [DOI] [PubMed] [Google Scholar]
- 36.Efferson CL, Winkelmann CT, Ware C, Sullivan T, Giampaoli S, Tammam J, Patel S, Mesiti G, Reilly JF, Gibson RE, et al. Downregulation of Notch pathway by a γ-secretase inhibitor attenuates AKT/mTOR signaling and glucose uptake in an ERBB-2 transgenic breast cancer model. Cancer Res. 2010;70:2476–2484. doi: 10.1158/0008-5472.CAN-09-3114. [DOI] [PubMed] [Google Scholar]
- 37.Tammam J, Ware C, Efferson C, O'Neil J, Rao S, Qu X, Gorenstein J, Angagaw M, Kim H, Kenific C, et al. Down-regulation of the Notch pathway mediated by a γ-secretase inhibitor induces anti-tumour effects in mouse models of T-cell leukaemia. Br J Pharmacol. 2009;158:1183–1195. doi: 10.1111/j.1476-5381.2009.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pienta KJ, Smith DC. Advances in prostate cancer chemotherapy: a new era begins. CA Cancer J Clin. 2005;55:300–318. doi: 10.3322/canjclin.55.5.300. quiz 23-5. [DOI] [PubMed] [Google Scholar]
- 39.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, et al. Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, et al. mTOR inhibition reverses AKT-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10(6):594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 44.Squillace RM, Miller D, Wardwell SD, Wang F, Clackson T, Rivera VM. Synergistic activity of the mTOR inhibitor ridaforolimus and the anti-androgen bicalutamide in prostate cancer models. Int J Oncol. 2012;41:425–432. doi: 10.3892/ijo.2012.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]