Abstract
Purpose
The need for surgical excision in patients with ultrasound-guided core needle biopsy (CNB)-diagnosed atypical ductal hyperplasia (ADH) remains an issue of debate. The present study sought to validate a scoring system (the U score, for underestimation) that we have previously developed for predicting malignancy in CNB-diagnosed ADH.
Methods
The study prospectively enrolled 85 female patients with CNB-diagnosed ADH who underwent subsequent surgical excision. Underestimation was defined as a surgical specimen having malignant foci.
Results
The overall underestimation rate was 37% (31/85). Multivariate analysis showed that a clinically palpable mass, microcalcification on imaging, size >15 mm and a patient age of ≥50 years were independently associated with underestimation. When applied to the scoring system, the validation score was significant (p<0.001; area under the curve, 0.852). No patient with a U score <3.5 had an underestimated lesion.
Conclusion
The present study successfully validated the efficacy of our scoring system for predicting malignancy in CNB-diagnosed ADH. A U score of ≤3.5 indicates that surgical excision may not be necessary.
Keywords: Breast hyperplasia, Breast neoplasms, Diagnostic errors, Needle biopsy
INTRODUCTION
Ultrasound-guided core needle biopsy (CNB) has become an alternative to surgical biopsy as a primary diagnostic modality because of its accuracy and cost-effectiveness [1]. Its other advantages include that there is no requirement for ionizing radiation, non-dedicated equipment can be used, and real-time visualization of the biopsy needle is possible [2]. However, CNB has inherent limitations that can result in malignancy being missed.
Atypical ductal hyperplasia (ADH) belongs to the pathological category of 'proliferative disease with atypia.' The reported rate of underestimation of carcinoma in CNB-diagnosed ADH is as high as 48% [3-8]. Therefore, complete surgical excision is generally recommended for ADH lesions diagnosed using CNB. However, it may be possible to avoid surgical excision if clinicians are able to identify lesions with a low probability of harboring malignant foci. Therefore, we previously developed a scoring system for predicting malignancy in CNB-diagnosed ADH [1,5]. The score takes into account patient age, palpability, microcalcification, lesion size, and multiplicity. We have termed this score the U score, with U standing for underestimation.
The present study was designed to validate our scoring system. The study involved 85 women with CNB-diagnosed ADH who underwent subsequent surgical excision. We identified factors associated with underestimation, and determined whether the U score was a predictor of malignancy, mainly focusing on whether a 100% negative predictive value (NPV) (of cut-off 3.5) shows consistency as in the previous datasets.
METHODS
The study prospectively enrolled 85 women who consecutively underwent CNB with subsequent surgical excision between 2007 and 2011 in the Seoul National University Hospital. Patients who underwent CNB in other institutions were excluded due to the absence of information such as initial image findings and the gauge of the needle used. We have previously reported on our scoring system for predicting malignancy in CNB-diagnosed ADH [1], which is based on values given to five clinicopathological factors of age, palpability, microcalcification, size of lesion, and multiplicity [U score= 3.5×age (age ≤50=0, age >50 =1)+2.0×palpability (non-palpable=0, palpable=1)+2.0×microcalcification (no=0, yes=1)+3.5×sonographic size (≤1.5 cm=0, >1.5 cm=1)+ 3.5×multiplicity (focal=0, multiple=1)]. The constant of each variable was derived from the logistic regression model as previously described [1]. Histological underestimation was defined as upstaging of the CNB-based diagnosis to invasive carcinoma or carcinoma in situ after surgical excision. This study was approved by the Institutional Review Board (No. H-1106-069-366) of the Seoul National University Hospital.
All patients underwent CNB using the 14-gauge automated gun method (Bard Peripheral Technologies, Covington, USA) or the 8-, 11-gauge vacuum-assisted device method (Mammotome®; Ethicon-Endosurgery, Cincinnati, USA). The choice of method was made by the surgeon and the radiologist based on the pre-procedural condition of the lesion. For both methods, high resolution ultrasound guidance was carried out with 10 or 12 MHz linear transducers (Voluson 730, Kretz-Medison, Zipf, Austria; HDI 5000, Advanced Technology Laboratories, Bothell, USA).
Statistical analysis was performed using a statistical software package (SPSS version 17.0; SPSS Inc., Chicago, USA) and a two-sided p-value ≤0.05 was considered as indicating significance. The differences in the distribution of categorical variables were analyzed using the Pearson chi-square test and Student's t-test was used for continuous variables. The stepwise logistic regression model was used for multivariate analysis.
RESULTS
Predictors of underestimation
The mean patient age was 48.6 years (range, 24-70 years) and the mean lesion size was 15.5 mm (range, 3-67 mm). The overall underestimation rate was 37% (31/85). The excision specimens from the 85 patients showed that four (5%) had invasive carcinomas and 27 (32%) had in situ carcinomas (Table 1).
Table 1.
Patient demographics (n=85)
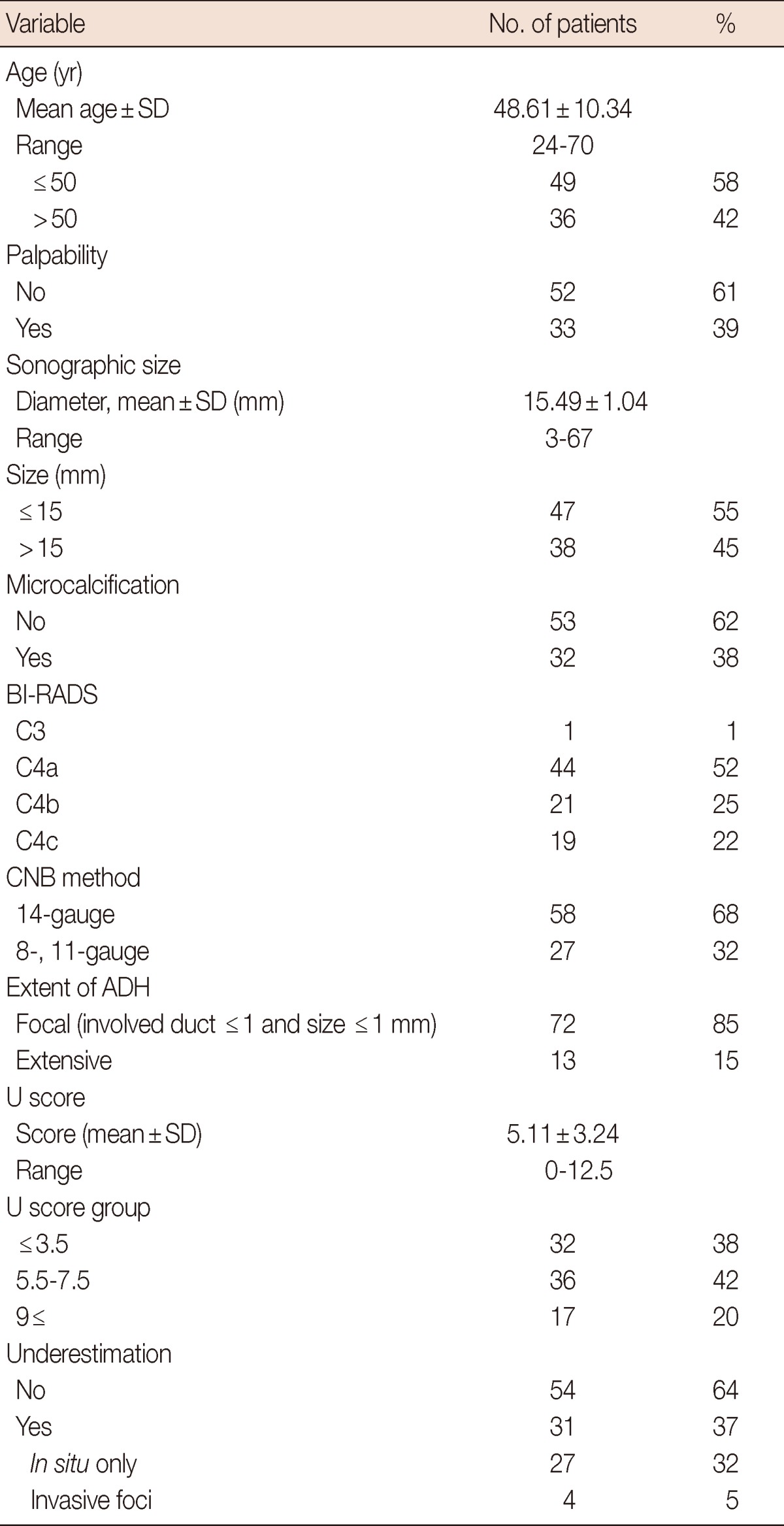
The demographic characteristics of the study patients are shown. The overall underestimation rate was 37% (32% with in situ carcinoma and 5% with invasive foci).
BI-RADS=Breast Imaging Reporting and Data System; CNB=core needle biopsy; ADH=atypical ductal hyperplasia.
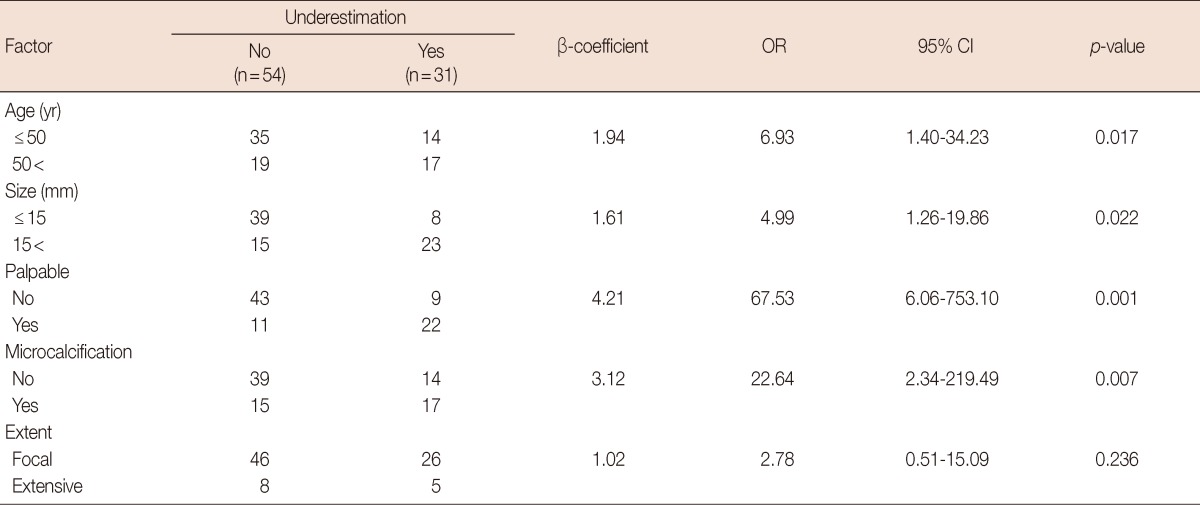
Univariate analysis showed that palpability, microcalcification, and the sonographic size of the lesion were all associated with underestimation (p<0.001, p=0.013, and p=0.020, respectively) (Table 2). The CNB method and the pathological extent of ADH in the CNB specimen were not found to be factors. Multivariate analysis showed that palpability, microcalcification, a sonographic size >15 mm and an age >50 years were independent factors associated with underestimation (p=0.001, p=0.007, p=0.022, and p=0.017, respectively) (Table 3).
Table 2.
Univariate analysis
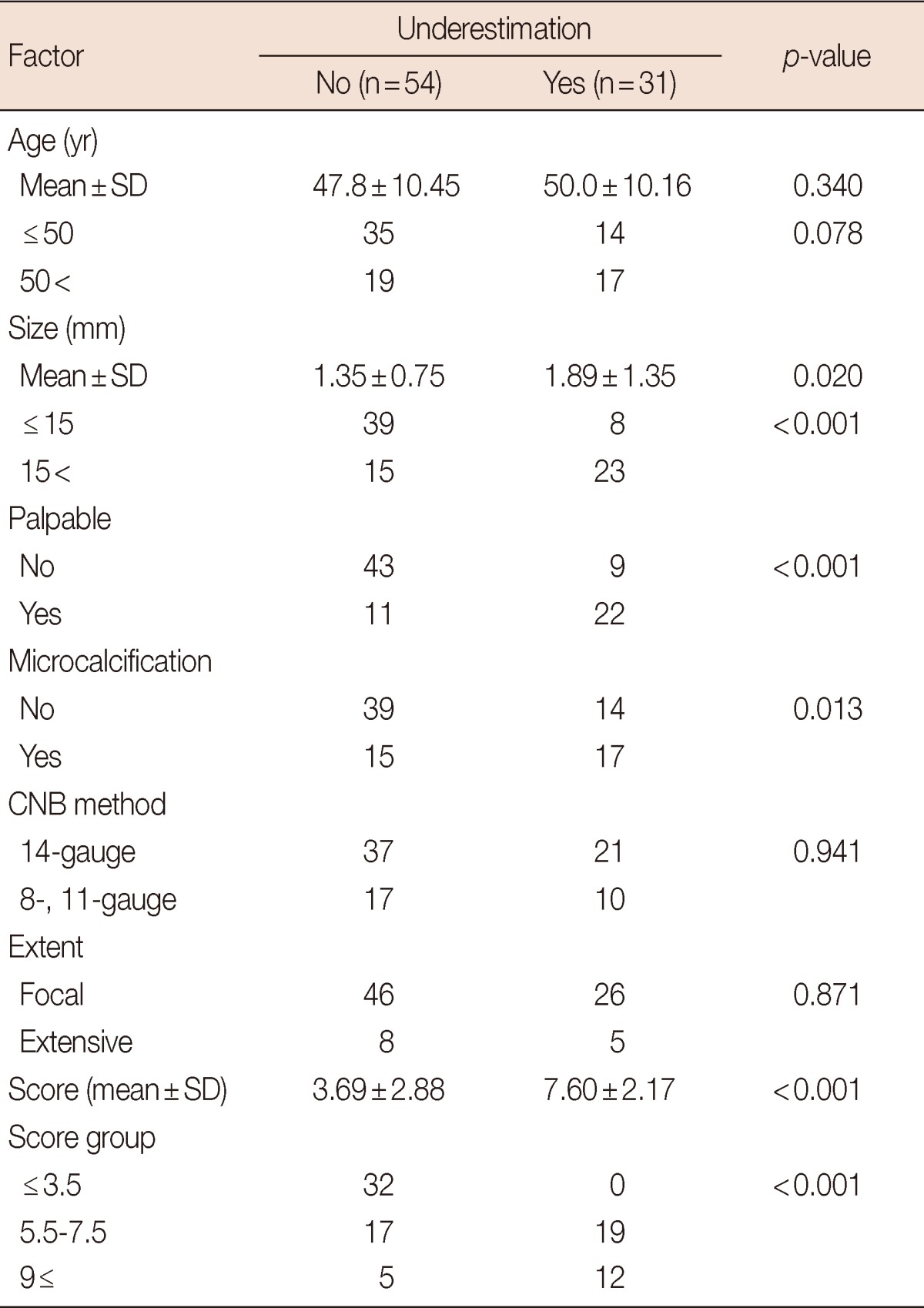
Univariate analysis was performed to identify clinicopathological factors associated with underestimation.
CNB=core needle biopsy.
Table 3.
Multivariate analysis
Multivariate analysis was performed. Palpability, microcalcification, sonographic size >1.5 cm and age >50 years were found to be independent factors (p-values of 0.001, 0.007, 0.022, and 0.017, respectively).
OR=odds ratio; CI=confidence interval.
Validation
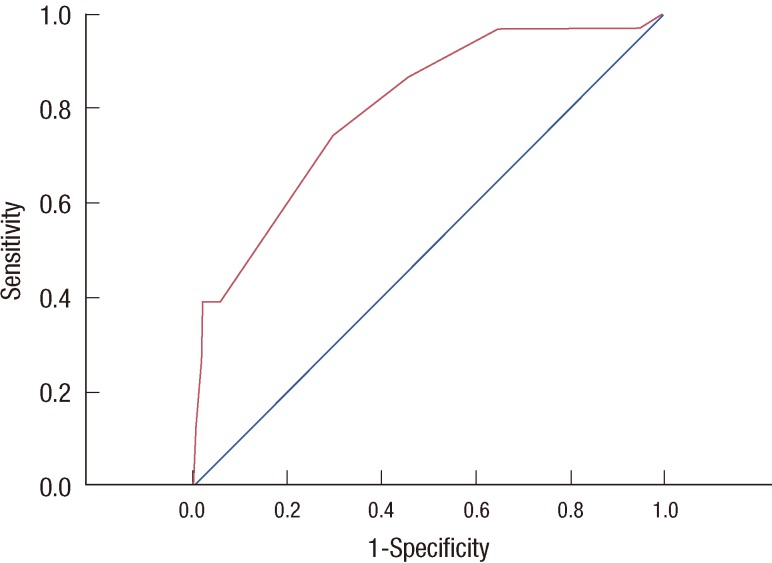
The mean U score was 5.11 (range, 0-12.5). For analysis, U scores were divided into three groups: ≤3.5, 5.5 to 7.5, and >9 as in the previous study [1]. The U score was found to be associated with underestimation (p<0.001). The area under the curve (AUC) of the receiver operating characteristic (ROC) curve of the U score was analyzed to evaluate the performance of the scoring system. The curve was articulated using SPSS by calculating the probability of being underestimated (no underestimation=0, underestimation=1) and the likelihood of being predicted with the scoring system. The AUC was 0.852 (p<0.001; 95% CI, 0.73-0.91) (Figure 1). No patient with a U score ≤3.5 was found to have a lesion that was underdiagnosed, which indicates a NPV of 100%.
Figure 1.
The receiver operating characteristic curve for the U scoring system. U score=3.5×age (age ≤50=0, age >50=1)+2.0×palpability (non-palpable=0, palpable=1)+2.0×microcalcification (no=0, yes=1) +3.5×sonographic size (≤1.5 cm=0, >1.5 cm=1)+3.5×multiplicity (focal=0, multiple=1). Area under the curve=0.852 (p<0.001; 95% confidence interval, 0.729-0.907).
DISCUSSION
The overall underestimation rate was 37%, which was lower than that in the previous study (46%) from an analysis of 74 patients between 2000 and 2007. Palpability, microcalcification, sonographic size and age >50 years were independent factors associated with underestimation. The extent didn't reach statistical significance compared to the previous study. The U score was validated with an AUROC of 0.852. Chae et al. [9] have reported a 22.2% (10/45) underestimation rate with age >50 as an independent risk factor, which is consistent with our data. Nonetheless, 'size >1 cm' and 'microcalcification combined with mass finding' lost their statistical power in multivariate analysis, which is thought to be due to the small number of analyzed data.
ADH is a borderline proliferative intraductal breast lesion that involves a four to five times greater risk of developing a subsequent breast carcinoma. There are two histological types: one with the cytological features of low-grade ductal carcinoma in situ (DCIS) with insufficient architectural atypia, and the other called 'mini-DCIS' with similar cytology and architectural atypia but of a smaller size (<2 mm) [3]. It is difficult to histologically differentiate between ADH and DCIS [4]. Moreover, it is much more difficult to differentiate ADH from DCIS in a CNB specimen, which explains the high upgrade rate of ADH to DCIS after complete excision following CNB.
Some studies have shown a lower underestimation rate when using the 11-gauge vacuum-assisted device compared to the 14-gauge automated gun device, suggesting that a greater amount of biopsy tissue reduces the risk of underestimation [1,5,6]. However, the present study found that the rate of underestimation was similar for both the 11- and 14-gauge methods. We compared the demographics of 11- and 14-gauge methods to identify any potential selection bias. In univariate comparison, non-palpable lesion and lesions with microcalcification were selected for the 11-gauge method (32/58 vs. 21/27, p=0.045, and 13/58 vs. 18/27, p<0.001, respectively). When stepwise logistic regression analysis was performed adjusting for the confounding factors, microcalcification was the only independent different factor between two groups (odds ratio [OR], 6.54; 95% confidence interval [CI], 2.37-18.07; p<0.001). From these analyses, it is quite acceptable that no significant difference of underestimation was observed between two groups, considering that microcalcification is a risk factor of underestimation. The use of the 11-gauge vacuum-assisted device or excision has also become more popular for benign breast lesions especially in younger women [7,8,10]. But since there is not enough data to confirm the significant benefit of vacuum-assisted device excision in terms of underestimation, patient selection should be done with caution considering major issues such as whether adequate margin evaluation is possible, and that later surgery may be difficult due to the associated tissue destruction [11].
Wagoner et al. [12] have reported an underestimation rate of 17.9% after excision of core needle biopsy diagnosed ADH. Ninety-four point three percent cases were diagnosed using a stereotactic vacuum-assisted device with 11-gauge needle. In this study, the extent of the ADH lesion and histologic type were associated with underestimation. Lesions with 1 to 2 ADH foci (n=82), three foci (n=23), and four or more foci (n=18) were upstaged to DCIS in 7%, 13%, and 72% of cases, respectively. They also demonstrated that lesions with micro-papillary feature had a higher number of ADH foci compared to the cribriform type, resulting in a significantly higher rate of underestimation. In the same context, Koo et al. [13] found that the presence of stromal alterations around the ADH lesion is an independent risk factor of underestimation, after analyzing 50 cases of ADH with subsequent surgical excision. These findings indicate the necessity of more discrete and categorized pathologic review followed by detailed description of CNB diagnosed ADH lesions, in terms of predicting the underestimation risk so as to enable surgeons to determine the treatment strategy.
Sixty-eight cases of mammographic findings in ADH were reviewed to identify the difference which discriminates DCIS from pure ADH [14]. It was found that 14 out of 29 granular calcification were underestimated and 6 out of 8 cases showing segmental/linear branching distribution of microcalcification were upstaged to DCIS. No underestimation was found in lesions with fine, rounded microcalcification. More detailed assessment of the mammographic finding of microcalcification would provide more accurate predictive information about underestimation when added to the U score.
The main issue when predicting underestimation is deciding whether to surgically excise the lesion or not when core needle biopsy diagnoses atypical ductal hyperplasia. McGhan et al. [15] have reviewed 114 cases of CNB-diagnosed ADH with subsequent excisional biopsy. Young age (<50 years) with focal atypia and no residual calcifications after post-biopsy may represent a low risk of being underestimated, and thus the potential avoidance of surgical excision. There are also similar data showing that when post-biopsy mammography reveals no residual lesion, surgical excision may be unnecessary in small sized ADH with less than three foci [12,16]. In this study, after initial CNB-diagnosis, no post-biopsy imaging follow-up was performed as this is not a routine practice in our center. However, these data support the potential role of post-biopsy images in decision-making about whether to perform surgical excision or close observation. Linda et al. [17] analyzed the role of post-biopsy MRI in CNB diagnosed ADH and reported a low negative predictive value, i.e., that magnetic resonance imaging is not helpful in predicting residual malignancies. Mammography would be beneficial as post-biopsy imaging. When added to the U score, post-biopsy findings may have more predictive value.
The present study successfully validated the efficacy of our scoring system for predicting malignancy in CNB-diagnosed ADH. The study found that a score of ≤3.5 indicates that surgical excision may not be necessary. This scoring system can be applied in clinical settings.
Footnotes
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korean government (20110031417 and 20110032148).
The authors declare that they have no competing interests.
References
- 1.Ko E, Han W, Lee JW, Cho J, Kim EK, Jung SY, et al. Scoring system for predicting malignancy in patients diagnosed with atypical ductal hyperplasia at ultrasound-guided core needle biopsy. Breast Cancer Res Treat. 2008;112:189–195. doi: 10.1007/s10549-007-9824-0. [DOI] [PubMed] [Google Scholar]
- 2.Cho N, Moon WK, Cha JH, Kim SM, Kim SJ, Lee SH, et al. Sonographically guided core biopsy of the breast: comparison of 14-gauge automated gun and 11-gauge directional vacuum-assisted biopsy methods. Korean J Radiol. 2005;6:102–109. doi: 10.3348/kjr.2005.6.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingegnoli A, d'Aloia C, Frattaruolo A, Pallavera L, Martella E, Crisi G, et al. Flat epithelial atypia and atypical ductal hyperplasia: carcinoma underestimation rate. Breast J. 2010;16:55–59. doi: 10.1111/j.1524-4741.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 4.Masood S, Rosa M. Borderline breast lesions: diagnostic challenges and clinical implications. Adv Anat Pathol. 2011;18:190–198. doi: 10.1097/PAP.0b013e31821698cc. [DOI] [PubMed] [Google Scholar]
- 5.Jang M, Cho N, Moon WK, Park JS, Seong MH, Park IA. Underestimation of atypical ductal hyperplasia at sonographically guided core biopsy of the breast. AJR Am J Roentgenol. 2008;191:1347–1351. doi: 10.2214/AJR.07.3643. [DOI] [PubMed] [Google Scholar]
- 6.Lee JW, Han W, Ko E, Cho J, Kim EK, Jung SY, et al. Sonographic lesion size of ductal carcinoma in situ as a preoperative predictor for the presence of an invasive focus. J Surg Oncol. 2008;98:15–20. doi: 10.1002/jso.21077. [DOI] [PubMed] [Google Scholar]
- 7.Londero V, Zuiani C, Linda A, Battigelli L, Brondani G, Bazzocchi M. Borderline breast lesions: comparison of malignancy underestimation rates with 14-gauge core needle biopsy versus 11-gauge vacuum-assisted device. Eur Radiol. 2011;21:1200–1206. doi: 10.1007/s00330-010-2053-7. [DOI] [PubMed] [Google Scholar]
- 8.Ancona A, Capodieci M, Galiano A, Mangieri F, Lorusso V, Gatta G. Vacuum-assisted biopsy diagnosis of atypical ductal hyperplasia and patient management. Radiol Med. 2011;116:276–291. doi: 10.1007/s11547-011-0626-9. [DOI] [PubMed] [Google Scholar]
- 9.Chae BJ, Lee A, Song BJ, Jung SS. Predictive factors for breast cancer in patients diagnosed atypical ductal hyperplasia at core needle biopsy. World J Surg Oncol. 2009;7:77. doi: 10.1186/1477-7819-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park HL, Kim LS. The current role of vacuum assisted breast biopsy system in breast disease. J Breast Cancer. 2011;14:1–7. doi: 10.4048/jbc.2011.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forgeard C, Benchaib M, Guerin N, Thiesse P, Mignotte H, Faure C, et al. Is surgical biopsy mandatory in case of atypical ductal hyperplasia on 11-gauge core needle biopsy? A retrospective study of 300 patients. Am J Surg. 2008;196:339–345. doi: 10.1016/j.amjsurg.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 12.Wagoner MJ, Laronga C, Acs G. Extent and histologic pattern of atypical ductal hyperplasia present on core needle biopsy specimens of the breast can predict ductal carcinoma in situ in subsequent excision. Am J Clin Pathol. 2009;131:112–121. doi: 10.1309/AJCPGHEJ2R8UYFGP. [DOI] [PubMed] [Google Scholar]
- 13.Koo JS, Kim MJ, Kim EK, Jung W. Factors in the breast core needle biopsies of atypical ductal hyperplasia that can predict carcinoma in the subsequent surgical excision specimens. J Breast Cancer. 2010;13:132–137. [Google Scholar]
- 14.Hoang JK, Hill P, Cawson JN. Can mammographic findings help discriminate between atypical ductal hyperplasia and ductal carcinoma in situ after needle core biopsy? Breast. 2008;17:282–288. doi: 10.1016/j.breast.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 15.McGhan LJ, Pockaj BA, Wasif N, Giurescu ME, McCullough AE, Gray RJ. Atypical ductal hyperplasia on core biopsy: an automatic trigger for excisional biopsy? Ann Surg Oncol. 2012;19:3264–3269. doi: 10.1245/s10434-012-2575-0. [DOI] [PubMed] [Google Scholar]
- 16.Sneige N, Lim SC, Whitman GJ, Krishnamurthy S, Sahin AA, Smith TL, et al. Atypical ductal hyperplasia diagnosis by directional vacuum-assisted stereotactic biopsy of breast microcalcifications. Considerations for surgical excision. Am J Clin Pathol. 2003;119:248–253. doi: 10.1309/0GYV-4F2L-LJAV-4GFN. [DOI] [PubMed] [Google Scholar]
- 17.Linda A, Zuiani C, Furlan A, Lorenzon M, Londero V, Girometti R, et al. Nonsurgical management of high-risk lesions diagnosed at core needle biopsy: can malignancy be ruled out safely with breast MRI? AJR Am J Roentgenol. 2012;198:272–280. doi: 10.2214/AJR.11.7040. [DOI] [PubMed] [Google Scholar]