Abstract
A one-step concept for bone regeneration has been postulated in which human adipose stem cells (hASCs) are harvested, triggered to differentiate, seeded on carriers, and implanted in the same operative procedure. Toward this goal it was investigated whether short (minutes) incubation with bone morphogenetic protein-2 (BMP-2) suffices to trigger osteogenic differentiation of hASCs seeded on calcium phosphate carriers. hASCs were treated with or without BMP-2 (10 ng/mL) for 15 min, and seeded on β-tricalcium phosphate granules (β-TCP; sized <0.7 mm or >0.7 mm) or biphasic calcium phosphate (BCP; 60%/40% or 20%/80% hydroxyapatite/β-TCP). Attachment was determined after 10–30 min. Proliferation (DNA content) and osteogenic differentiation (alkaline phosphatase activity, gene expression) were analyzed up to 3 weeks of culture. hASC attachment to the different scaffolds was similar, and unaffected by BMP-2. It stimulated gene expression of the osteogenic markers core binding factor alpha 1, collagen-1, osteonectin, and osteocalcin in hASCs seeded on BCP and β-TCP. Downregulation of osteopontin expression by BMP-2 was seen in BCP-seeded cells only. BMP-2 treatment inhibited expression of the adipogenic marker peroxisome proliferator-activated receptor gamma. In conclusion, 15 min BMP-2 preincubation of hASCs seeded on BCP/β-TCP scaffolds had a long-lasting stimulating effect on osteogenic differentiation in vitro. These results strongly support a one-step clinical concept for bone regeneration.
Introduction
The use of an autologous bone graft is still the golden standard for bone reconstruction. It is the only type of bone graft supplying living bone cells, and has osteogenic, osteoinductive, as well as osteoconductive properties. This autograft does not provoke an immunological response since the tissue is retrieved from the same individual.1 There are many advantages using autologous bone, but there are also disadvantages associated with harvesting of the bone transplant, such as limited availability of bone volume, donor-site morbidity, and risk of infection. The use of allografts, xenografts, or biosynthetic substitutes eliminates these disadvantages associated with autologous bone harvesting.2
Biosynthetic substitutes, such as β-tricalcium phosphate (β-TCP), hydroxyapatite (HA), and mixtures of HA/β-TCP (biphasic calcium phosphates; BCP) have been successfully used as a graft material, because of their good biocompatibility and chemical composition, which resembles the composition of the natural bone matrix.3–6 An important issue to consider regarding graft material degradation and bone ingrowth is the pore size; large pore sizes (≥500 μm) promote neovascularization and favor mineralized bone ingrowth,7 whereas smaller pore sizes (90–120 μm) primarily induce endochondral bone formation.8 Therefore, in the present study, β-TCP and BCP biomaterials were used with a high porosity (60%–90%), and a pore size between 500 and 1400 μm for the β-TCP scaffolds and between 500 and 1000 μm for the BCP scaffolds. Nevertheless, despite these structural optimizations, the lack of osteogenic properties still results in a slower rate of new bone formation.9,10
Autologous adult mesenchymal stem cells (MSCs) provide new and innovative tools in tissue engineering, and may be combined with synthetic scaffolds to introduce osteogenic bioactivity. These bioactive scaffolds may then be used to restore or replace tissues or organs. Bone marrow is a common source for MSCs (BM-MSCs), but this is only available in limited amounts.11 Adipose tissue has been described as an alternative source for MSCs.12 The human adipose stem cells (ASCs) have similar surface marker profiles as the BM-MSCs, and also show multidifferentiation potential toward the adipogenic, chondrogenic, myogenic, neurogenic, and osteogenic lineage.13 In the field of bone tissue engineering, ASCs have been successfully used to repair critical-sized calvarial defects in animals14–17 as well as in a 7-year-old girl.18
It is as yet unclear whether stimulation of ASCs with bone morphogenetic protein-2 (BMP-2), a member of the transforming growth factor-β superfamily, should be performed for optimal osteogenesis, or that the local (orthotopic) microenvironment is sufficient. Earlier reports have shown beneficial effects of BMP-2 on osteogenic differentiation of stem cells/progenitors in vitro and in vivo,19–22 and on bone formation and bone repair in vivo.23,24 BMP-2 is available as an Food and Drug Administration-approved recombinant human protein24,25 and has been used extensively in clinical practice. Although initially, only studies were published showing beneficial effects of BMP-2 treatment, recently also, adverse effects, such as bone overgrowth and swelling, were reported.26,27 This clearly questions the high (mg-range) dosages of BMP-2 used in clinical studies.
Previously, a novel clinical concept has been postulated, in which ASCs are harvested from adipose tissue, seeded on scaffolds, and reimplanted during the same surgical procedure.28 Also, a short ex vivo preincubation of the ASC preparations with osteogenic factors was envisioned. Therefore, the aim of this study was to test whether a short (minutes) incubation with BMP-2 induces osteogenic differentiation of hASCs seeded on calcium phosphate carriers in vitro.
Materials and Methods
Calcium phosphate scaffolds
Four different calcium phosphate scaffolds were used: (1) Straumann® BoneCeramic (Straumann, Basel, Switzerland), a porous BCP with 60% HA and 40% β-TCP (BCP 60/40), (2) Straumann BoneCeramic, a porous BCP with 20% HA and 80% β-TCP (BCP 20/80), (3) Ceros® TCP (Mathys, Bettlach, Switzerland), a porous β-TCP with particle size 0.5–0.7 mm (β-TCP, <0.7 mm), and (4) Ceros TCP, a porous β-TCP with particle size 0.7–1.4 mm (β-TCP, >0.7 mm) (Table 1).
Table 1.
Characteristics of the Different Biphasic Calcium Phosphate and β-Tricalcium Phosphate Granules Scaffolds Used
| Scaffold | Composition | Particle size (μm) | Porosity (%) | Pore width (μm) |
|---|---|---|---|---|
| Straumann® BoneCeramic BCP 60/40 | 60% HA/40% β-TCP | 500–1000 | 90 | 500–1000 |
| Straumann BoneCeramic BCP 20/80 | 20% HA/80% β-TCP | 500–1000 | 90 | 500–1000 |
| Ceros® TCP β-TCP, <0.7 mm | 100% β-TCP | 500–700 | 60 | 100–500 |
| Ceros TCP β-TCP, >0.7 mm | 100% β-TCP | 700–1400 | 60 | 100–500 |
Composition, particle size, porosity, and pore width of Straumann BoneCeramic (60/40), Straumann BoneCeramic (20/80), Ceros TCP (<0.7 mm), and Ceros TCP (>0.7 mm).
HA, hydroxyapatite; β-TCP, β-tricalcium phosphate; BCP, biphasic calcium phosphate.
Donors
The subcutaneous adipose tissue was harvested from the abdominal wall of 10 healthy women (age 23–61) undergoing elective abdominal wall correction at the Tergooiziekenhuizen Hilversum, The Netherlands. The Ethics Review Board of the VU University Medical Center, Amsterdam, The Netherlands, approved the study protocol. Informed consent was obtained from all patients.
Isolation of hASCs
The human adipose tissue was obtained by resection. hASCs were isolated from the resection material as described earlier with minor modifications.29 The adipose tissue was cut into small pieces, and enzymatically digested with 0.1% collagenase A (Roche Diagnostics GmbH, Mannheim, Germany) for 45 min at 37°C in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) under intermittent shaking. A single-cell suspension was obtained by filtration through a 100-μm mesh filter. After thorough washing with PBS containing 1% BSA, a Ficoll density centrifugation step (1.077 g/mL ficoll, 280±15 mOsm; Lymphoprep, Axis-Shield, Oslo, Norway) was performed to remove remaining erythrocytes from the hASC-containing cell suspension called the stromal vascular fraction (SVF). After centrifugation at 600 g for 10 min, the resulting SVF pellet containing the hASCs was resuspended in a medium composed of the Dulbecco's modified Eagle's medium (DMEM; LifeTechnologies™ Europe BV, Bleiswijk, The Netherlands) containing 10% fetal bovine serum (FBS; Hyclone Fetalclone I, Thermo Scientific, Logan, UT), 500 μg/mL streptomycin sulfate (Sigma-Aldrich), 500 μg/mL penicillin (Sigma-Aldrich), and 2.5 μg/mL amphotericin B (Gibco). Cell viability was assessed using the trypan blue exclusion assay. Cells were counted using a counting chamber (Burker-Turk, Marienfeld, Germany) and a light microscope at 10× magnification. Then, cells were immediately seeded and cultured on the different scaffolds, or resuspended in a Cryoprotective medium (Recovery™ Cell Culture Freezing medium; LifeTechnologies Europe BV), frozen under controlled rate conditions, and stored in liquid nitrogen until further use for attachment, proliferation, and differentiation studies. The latter cells are referred to as “fresh-frozen” cells below. Samples from different donors were studied individually in all experiments. Heterogeneity studies, including cell characterization and multipotent differentiation potential of these cells have been reported previously by our group.29–31 Recently, we determined that ∼90% of the ASCs within the freshly isolated SVF rapidly adhere to various scaffold types.32
BMP-2 treatment and hASC attachment to BCP and TCP scaffolds
Freshly isolated and “fresh-frozen” hASC-containing cell suspensions were either or not incubated for 15 min with 10 ng/mL BMP-2 (Peprotech®, London, United Kingdom) at room temperature or at 37°C, as previously described.20 Then, the cells were washed with PBS, centrifuged, and resuspended in DMEM without supplements. Cell suspensions were seeded at 1×105 cells per 25–35 mg of scaffold in 2-mL tubes (Eppendorf Biopur®, Hamburg, Germany). Cells were allowed to attach for 30 min. Then, hASC-seeded scaffolds were washed with PBS, the lysis buffer was added, and the DNA content (as a measure for cell number) was determined using the Cyquant Cell Proliferation Assay Kit (Molecular Probes/Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Absorption was read at 480 nm excitation and 520 nm emission in a microplate reader (BioRad Laboratories GmbH).
Culture of hASCs
Cryopreserved hASCs were thawed and seeded at 2.5×105 cells per 25–35 mg of BCP scaffold (Straumann BoneCeramic, 60/40, and Straumann BoneCeramic, 20/80) and β-TCP scaffold (Ceros TCP, <0.5 mm, and Ceros TCP, >0.5 mm). After osteogenic induction with BMP-2 as described in “BMP-2 treatment and hASC attachment to BCP and TCP scaffolds”, the hASC-seeded scaffolds were cultured up to 21 days in 12-well plates with Costar® Transwell® containers (Corning Life Sciences, Lowell, MA) containing the expansion medium (DMEM) supplemented with 10% FBS, antibiotics, and 50 μM ascorbic acid (Merck, Darmstadt, Germany). hASCs seeded on tissue culture plastic (control) were cultured in the expansion medium in the presence of 10 mM β-glycerol phosphate (Sigma) to provide a phosphate donor. The hASC-seeded scaffolds were incubated at 37°C under 5% CO2 in a humidified atmosphere, and the medium was changed three times per week.
hASC proliferation on BCP and β-TCP scaffolds
hASC proliferation was assessed by determination of the DNA content of hASC cultures. Cells were seeded on BCP and β-TCP scaffolds, allowed to attach, and cultured as described above in the paragraphs “BMP-2 treatment and hASC attachment to BCP and TCP scaffolds” and “Culture of hASCs.” After 4, 14, and 21 days of culture, the hASC-seeded scaffolds were washed with PBS, and transferred to Eppendorf tubes. The Cyquant lysis buffer was added and the DNA content per tube was determined using the Cyquant Cell Proliferation Assay Kit as described above in paragraph“BMP-2 treatment and hASC attachment to BCP and TCP scaffolds”.
Osteogenic differentiation of hASCs on BCP and β-TCP scaffolds
Alkaline phosphatase (ALP) activity was measured to assess the osteoblastic phenotype of hASC cultures. Cells were seeded on BCP and β-TCP scaffolds, allowed to attach, and cultured as described above in the paragraphs “BMP-2 treatment and hASC attachment to BCP and TCP scaffolds” and “Culture of hASCs.” After 4, 14, and 21 days of culture, the cells were lysed with the CyQuant® lysis buffer as described above in “hASC proliferation on BCP and β-TCP scaffolds” to determine the ALP activity and protein content. P-nitrophenyl-phosphate (Merck) at pH 10.3 was used as a substrate for ALP as described earlier.19 The absorbance was read at 410 nm. ALP activity was expressed as μmol/μg cellular protein. The amount of protein was determined by using a BCA Protein Assay reagent Kit (Pierce, Rockford, IL), and the absorbance was read at 540 nm with a microplate reader (BioRad Laboratories). Donors were also used in the study by Jurgens et al.32 In this study, expression of mature osteogenic differentiation markers was demonstrated both at the gene expression level as well as at the protein level. In our study, the ALP enzyme activity was quantitatively determined in addition to the mRNA expression profiles to verify the osteogenic differentiation at the protein level.
Colony-forming unit fibroblasts assay and colony-forming unit fibroblasts depletion assay
Colony-forming unit fibroblasts (CFU-f) assays were performed to assess if the CFU capacity of hASCs within the isolated SVF was affected by BMP-2 treatment, as described elsewhere.33 A total of 1×103 or 1×104 cells were seeded in six-well plates (Greiner Bio-One, Alphen a/d Rijn, The Netherlands). After 14 days of culture, the cells were fixed in 4% formaldehyde and stained with a 0.2% toluidine blue in the borax buffer (pH 12) for 1 min. A colony was defined as a group of cells consisting of ≥10 clustered cells. The number of colonies was counted using a light microscope at 100× magnification. The percentage of CFU-f per total number of hASCs seeded was calculated.
The CFU-f depletion assay was used as an indirect measurement to determine hASC attachment to the scaffolds.32 The scaffold washing steps were collected to obtain the nonadhered cells, which were pelleted and used for CFU-f frequency determination as described above. After 14 days of culture, the percentage of retrieved colonies was divided by the percentage of colonies obtained from the plastic-seeded hASCs, as an indirect measurement of hASC attachment and viability.
Analysis of gene expression
Total RNA was extracted from hASCs of eight donors cultured on tissue culture plastic and BCP (60/40 and 20/80) and β-TCP (<0.7 and >0.7 mm) scaffolds for 4, 14, and 21 days, using the TRIzol® reagent (LifeTechnologies) according to the manufacturer's instructions, and stored at −80°C before assay. The cDNA synthesis was performed in a thermocycler GeneAmp® PCR System 9700 PE (Applied Biosystems, Foster City, CA), using the SuperScript® VILO™ cDNA Synthesis Kit (LifeTechnologies) with 0.1 μg total RNA in a 20 μL reaction mix containing the VILO Reaction Mix and the SuperScript Enzyme Mix. cDNA was stored at −20°C before real-time polymerase chain reaction (PCR) analysis.
Real-time PCR reactions were performed using 2.5 μL cDNA and SYBR® Green Supermix (Roche Laboratories, Indianapolis, IN) according to the manufacturer's instructions in a LightCycler® (Roche Diagnostics). The target and reference genes were amplified in separate wells. All reactions were performed in triplicate. In each run, the reaction mixture without cDNA was used as a negative control. All primers used for real-time PCR were from LifeTechnologies. For quantitative real-time PCR, the values of relative target gene expression were normalized for relative YWHAZ housekeeping gene expression. Real-time PCR was used to assess expression of the following genes: core binding factor alpha 1/runx2 (CBFA1), collagen 1 (COL1), ALP, osteonectin (ON), osteopontin (OPN), osteocalcin (OC), and peroxisome proliferator-activated receptor gamma (PPARγ). In each assay for osteogenic markers, mRNA preparations of osteoblasts were used as a reference and internal control for the primer sets to pick up the specific mRNA of interest. Human primary osteoblasts were used as a positive control. Gene expression was compared between cells seeded on BCP and β-TCP scaffolds with or without BMP-2 treatment.
Statistical analysis
Data were obtained from hASCs of 10 donors in total. All data were expressed as mean±standard error of the mean. The effect of BMP-2 treatment compared to nontreated cells was tested with the Student's t-test for single group mean, or with the Wilcoxon Signed Rank test for single group median. Differences in the DNA content, ALP activity, and gene expression between groups were tested with the Student's paired two-tailed t-test. Two-way analysis of variance was used to compare attachment data between BCP and β-TCP scaffolds, and to compare the DNA content and ALP activity between the different time points (day 4, 14, and 21). To determine a time-dependent increase of osteogenic gene expression levels, the linear regression coefficiency was determined. Differences were considered significant if p<0.05. Statistical analysis was performed using SPSS 17.0 (SPSS, Inc., Chicago, IL), Kyplot v2.0 beta 15 (Japan), and GraphPad Prism 5.01 (GraphPad Software, Inc., La Jolla, CA).
Results
Does BMP-2 prestimulation affect hASCs similar to goat ASCs on tissue culture plastic?
BMP-2 increased the CFU frequency of hASCs seeded on tissue culture plastic
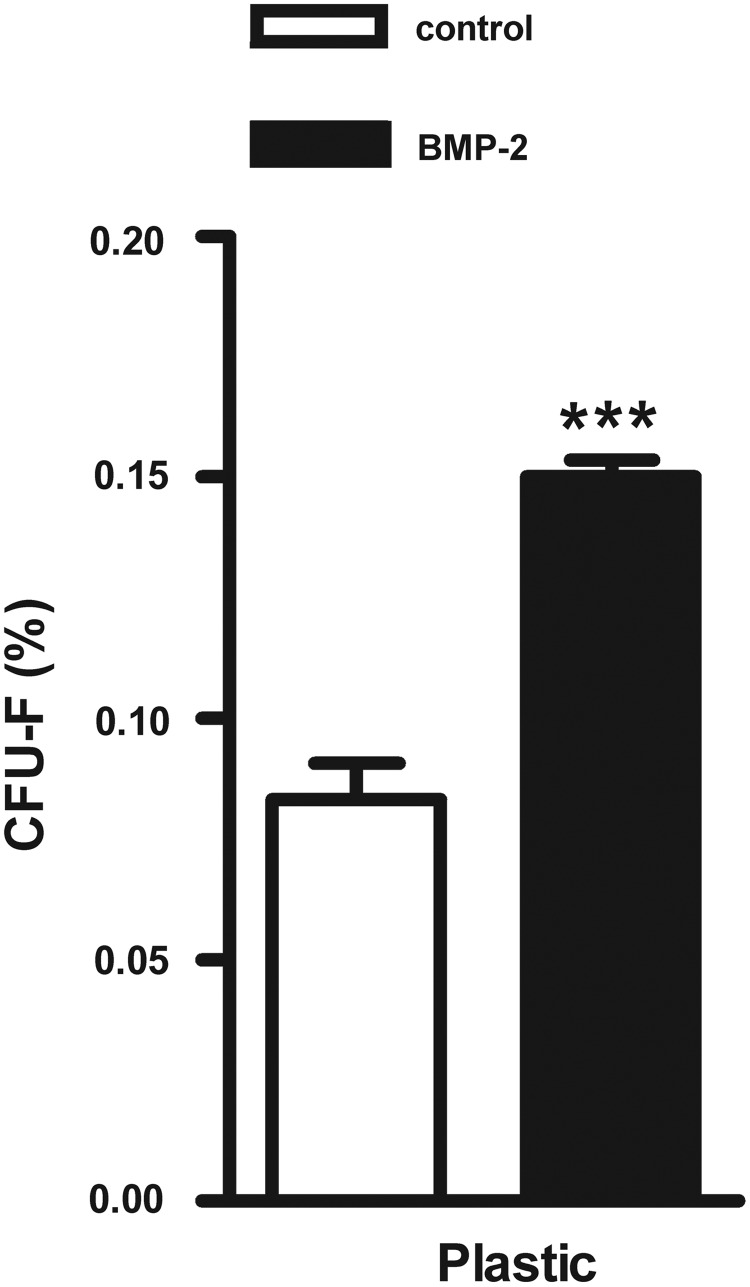
The number of CFU-f was counted 14 days after seeding of freshly isolated hASC preparations, which were prestimulated with or without BMP-2 for 15 min. Cells were cultured on plastic during this 14-day culture period (n=6). BMP-2 treatment significantly increased the percentage of CFU-f by 1.8-fold (p<0.0001; Fig. 1).
FIG. 1.
Effect of short stimulation with BMP-2 on colony-forming unit formation from hASCs cultured on tissue culture plastic. A 15-min BMP-2 pretreatment increased the colony-forming potential of hASCs cultured on tissue culture plastic for 2 weeks (p=5.5×10−7), reflecting the number of viable hASCs in adipose tissue. Values are mean±SEM (n=6). ***Significant effect of BMP-2, p<0.001. CFU-f, colony-forming unit fibroblasts; BMP-2, bone morphogenetic protein-2; hASCs, human adipose stem cells; plastic, tissue culture plastic; SEM, standard error of the mean.
hASCs, prestimulated for 15 min with BMP-2, did not show upregulation of osteogenic gene expression, nor downregulation of PPAR-γ expression, when seeded on tissue culture plastic
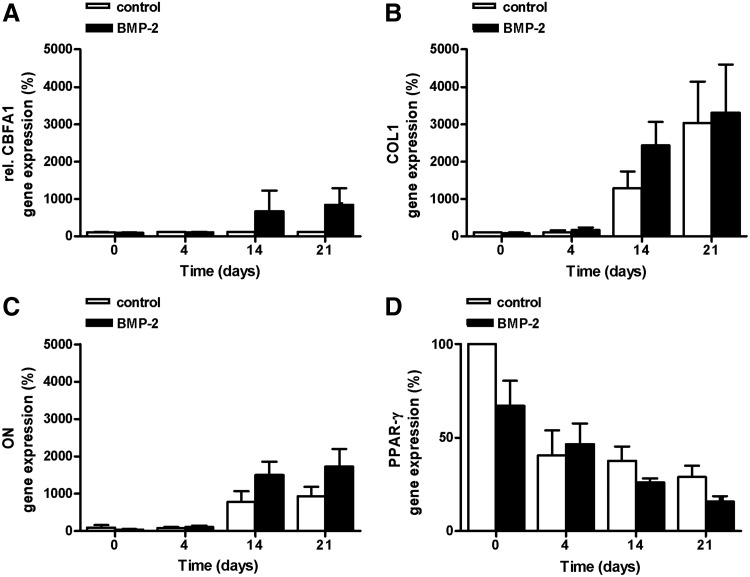
BMP-2 did not significantly upregulate the expression levels of CBFA1, COL1, and ON in hASC cultured on tissue culture plastic compared to control cultures (Fig. 2A–C). BMP-2 also did not significantly inhibit PPAR-γ expression (Fig. 2D).
FIG. 2.
Effect of short stimulation with BMP-2 on osteogenic gene expression and PPAR-γ gene expression in hASCs cultured on tissue culture plastic. A 15-min BMP-2 pretreatment did not increase the expression levels of (A) CBFA1, (B) COL1, or (C) ON after 14 or 21 days of culture. (D) BMP-2 also did not inhibit PPAR-γ expression levels after 21 days (p=0.08). Values are mean±SEM (n=2–6), and relative to the control value at time point 0. CBFA1, core binding factor A1 (or Runx-2); COL1, collagen type1; ON, osteonectin; PPAR-γ, peroxisome proliferator-activated receptor gamma.
Interestingly, osteogenic gene expression, especially COL1 and ON gene expression, increased in hASCs during 3 weeks of culture. To confirm a correlation between the osteogenic gene expression levels and the culture time, the correlation coefficient was calculated. These calculations indicated that culture time positively affected the increase in gene expression of CBFA1, COL1, and ON in BMP-2-treated cells (Table 2). The correlation coefficient between culture time and the decreasing PPAR-γ gene expression levels was also calculated, and a positive correlation in BMP-2-treated cells was found.
Table 2.
Correlation Between Osteogenic and Peroxisome Proliferator-Activated Receptor Gamma Gene Expression and Culture Time of Human Adipose Stem Cells Cultured on Tissue Culture Plastic
| |
Control |
BMP-2 |
||
|---|---|---|---|---|
| R2 | p | R2 | P | |
| CBFA1 | 0.44 | 0.33 | 0.96 | 0.02a |
| COL1 | 0.92 | 0.04a | 0.97 | 0.01a |
| ON | 0.94 | 0.03a | 0.94 | 0.03a |
| PPAR-γ | 0.60 | 0.20 | 0.95 | 0.03a |
Regression coefficient (R2) and their p-values (p) calculated from the gene expression levels of CBFA1, COL1, ON, and PPAR-γ of hASCs cultured on tissue culture plastic.
Significant correlation, p<0.05.
BMP-2, bone morphogenetic protein-2; CBFA1, core binding factor alpha 1; COL1, collagen type 1; ON, osteonectin; PPAR-γ, peroxisome proliferator-activated receptor-gamma; hASCs, human adipose stem cells.
How do hASCs behave on calcium phosphate scaffolds?
BMP-2 did not affect cell attachment on different scaffolds and increased hASC number
Before starting the analyses of proliferation and differentiation, the optimal conditions for the hASCs to be handled in vitro were investigated, thereby keeping in mind, the conditions preferred for a one-step surgical procedure. Freshly isolated hASCs as well as “fresh-frozen” hASCs were seeded on BCP and β-TCP scaffolds (without any pretreatment for 10, 20, and 30 min, and at room temperature or 37°C). After removal of the nonattached cells, the DNA content was determined as a measure of cell number. The results indicated that the maximum number of cells did attach to the carriers within 10 min (data not shown; data are in line with previously reported data.32 No marked difference was observed between attachment at room temperature and at 37°C.
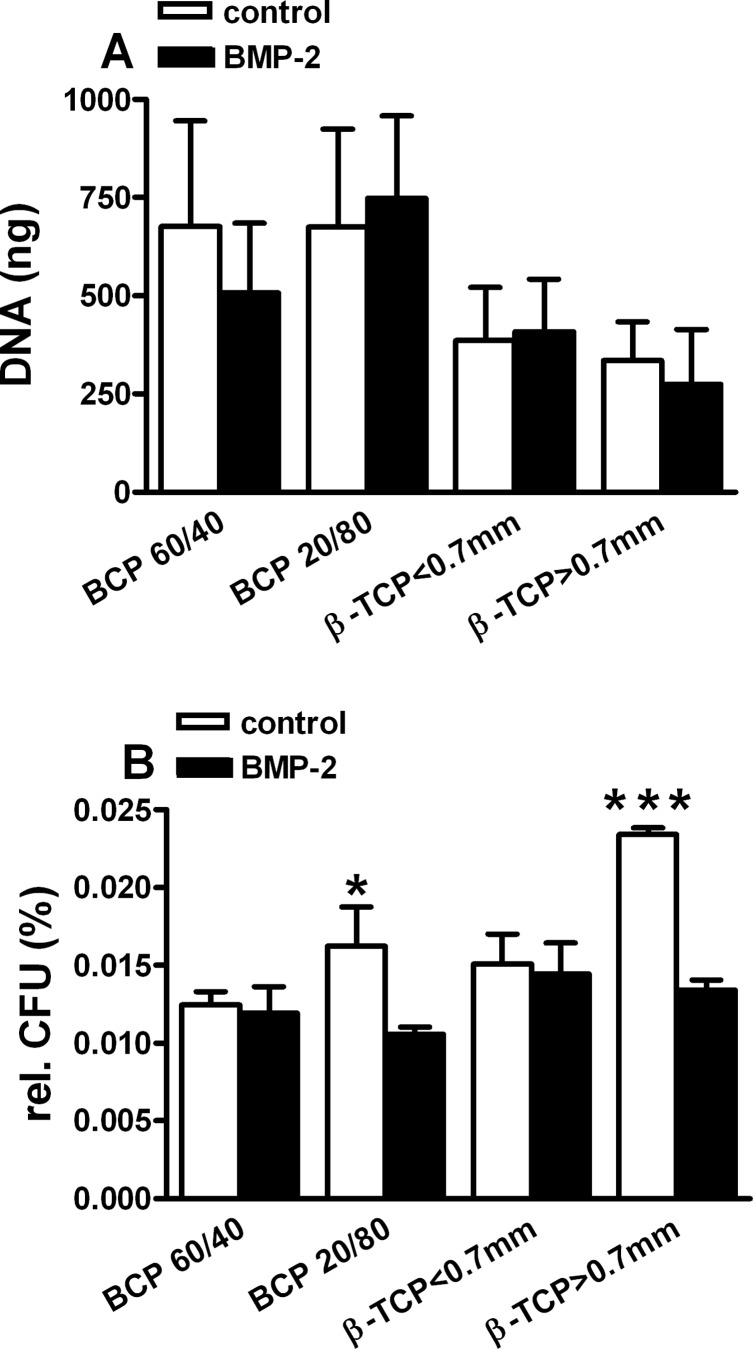
BMP-2 did not affect cell attachment to the BCP and/or β-TCP scaffolds (Fig. 3A). Remarkably, it seemed that a higher number of cells was attached to both types of BCP scaffolds used, whether hASCs had been BMP-2 treated or not, compared to TCP scaffolds.
FIG. 3.
Short stimulation with BMP-2 did not affect the attachment of human SVF cells to calcium phosphate scaffolds. (A) A 15-min BMP-2 pretreatment did not change the cell number (expressed as DNA, ng) of hASCs on different BCP and β-TCP scaffolds after allowing attachment for 30 min. More hASCs seemed to attach to the BCP scaffolds compared to β-TCP scaffolds. (B) A 15-min BMP-2 pretreatment affected the percentage of CFU-f from nonattached cells dependent on the scaffold. More BMP-2-treated cells were attached to BCP 20/80 (p=0.05) and β-TCP>0.7 mm (p=2.2×10−7). Values are calculated relative to the CFU-f proliferation assay data (Fig. 1). Values are mean±SEM (n=6). Significant difference between scaffolds, *p<0.05, ***p<0.001. SVF, stromal vascular fraction; BCP, biphasic calcium phosphate; β-TCP, β-tricalcium phosphate.
The number of CFU-f cultured from the BCP and β-TCP washings was less than 0.03% of the total number of CFU-f (Fig. 3B). This indicated that at least the hASCs within the heterologous primary hASC isolates did adhere to the scaffolds, as previously described for other types of scaffolds.33 Intriguingly, the number of CFU-f of the hASCs collected from BCP 20/80 and β-TCP>0.7 mm was significantly lower (p=0.05 and p<0.0001, respectively), compared to the number of CFU-f collected from the two other scaffolds (BCP 60/40 and β-TCP<0.7 mm). These findings indicate that hASCs may have a higher affinity for these specific BCP and β-TCP scaffolds.
BMP-2 affected hASC proliferation
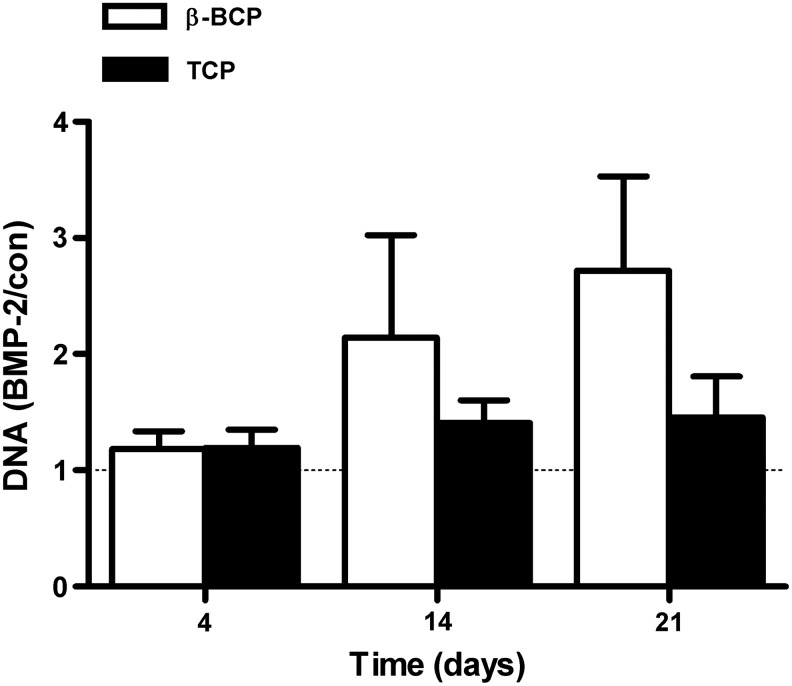
BMP-2 pretreatment increased the DNA content of hASCs seeded on the scaffolds compared to nontreated controls. On β-TCP, a marginally significant increase was noted at day 14 by 2.1±0.9-fold (p=0.06), and on BCP at day 21 by 2.7±0.8-fold (p=0.06, Fig. 4).
FIG. 4.
Short stimulation with BMP-2 affected hASC proliferation. hASCs were pretreated for 15 min with BMP-2 and cultured on different BCP and β-TCP scaffolds for 4, 14, and 21 days. A 15-min stimulation with BMP-2 resulted in increased proliferation of hASCs cultured on β-TCP at day 14 (p=0.06), and on BCP at day 21 (p=0.06). DNA content is expressed as BMP-2-treated–over-untreated control ratio. Values are mean±SEM (n=6). con, control.
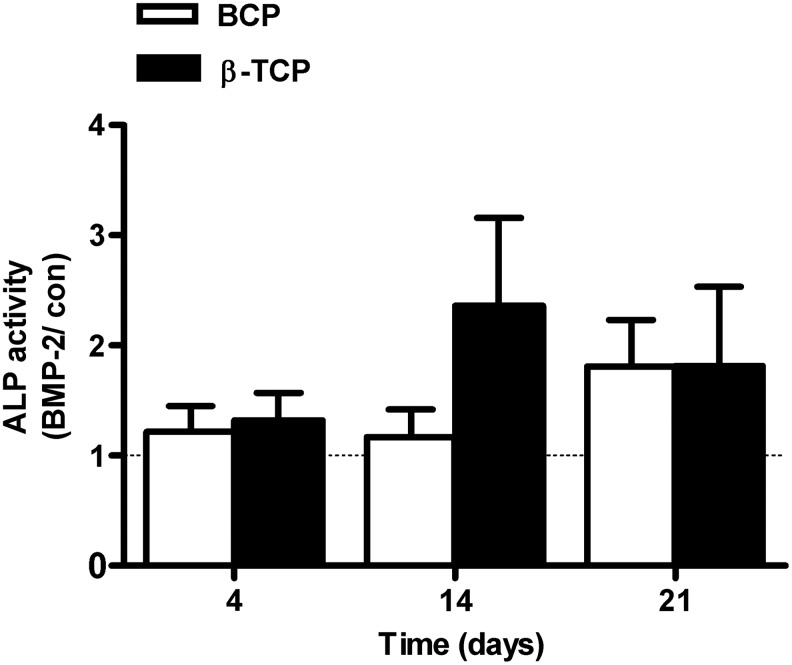
BMP-2 did not stimulate ALP activity in hASCs seeded on BCP or β-TCP scaffolds
ALP activity was measured after 4, 14, and 21 days of culture under control or BMP-2 prestimulated conditions. The ALP activity was expressed as fold-increase of BMP-2-treated cells versus nontreated cells. Although ALP activity increased in both BMP-2-treated and untreated hASCs during 21 days of culture, it did not reach significance at any time point measured (Fig. 5).
FIG. 5.
Short stimulation with BMP-2 did not increase ALP activity in hASCs cultured on BCP. hASCs were pretreated for 15 min with BMP-2 and cultured on different BCP and TCP scaffolds for 4, 14, and 21 days. BMP-2 did not increase ALP activity in hASCs seeded on BCP and β-TCP. Values are mean±SEM (n=6–7). ALP activity is expressed as BMP-2-treated–over-untreated control ratio. ALP, alkaline phosphatase activity.
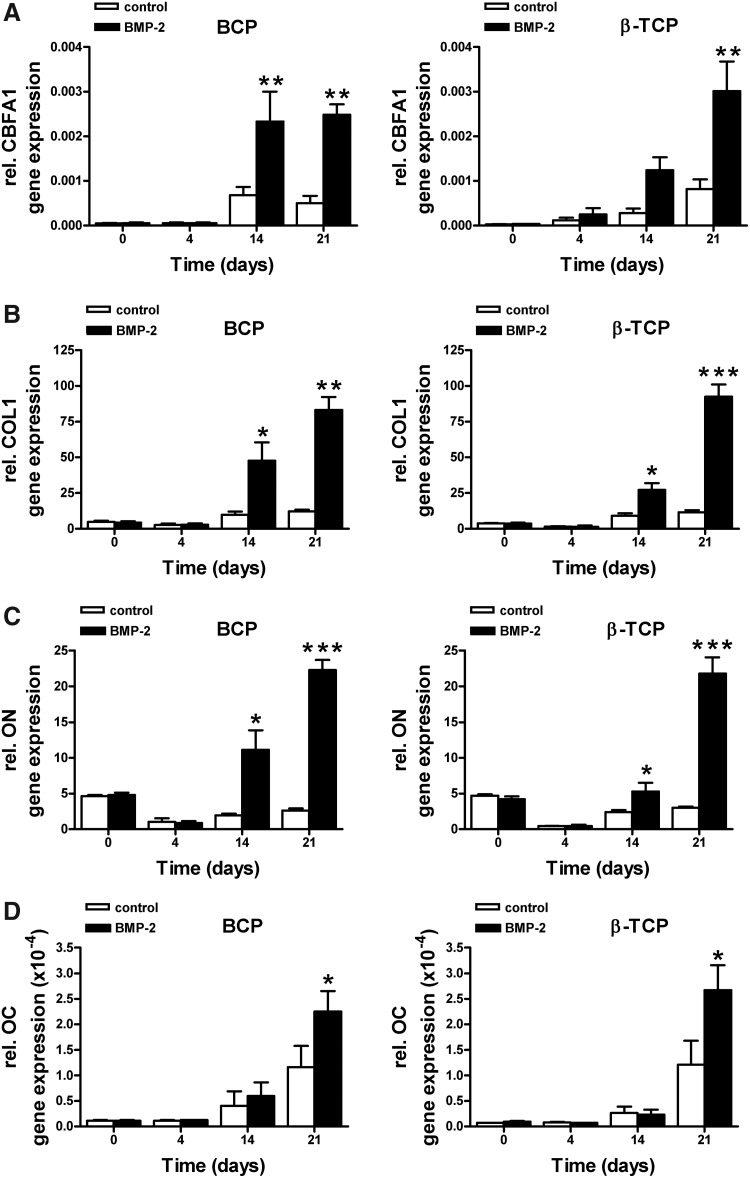
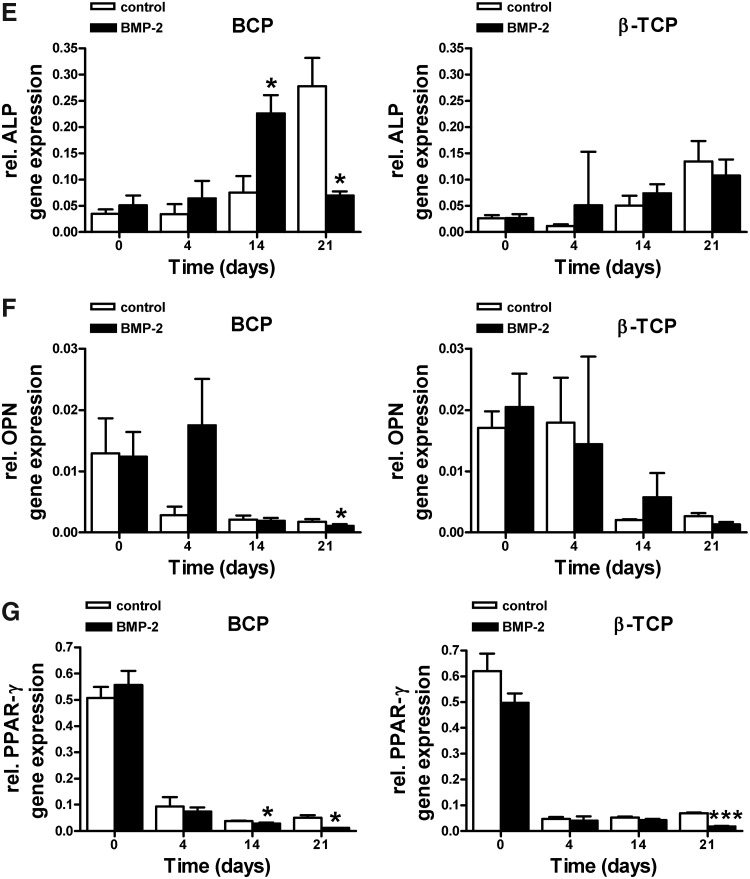
BMP-2 increased osteogenic gene expression, and decreased PPAR-γ expression in hASCs seeded on calcium phosphate scaffolds
BMP-2 pretreatment significantly increased mRNA gene expression levels of CBFA1, COL1, ON, and OC in hASCs cultured on BCP and β-TCP (Fig. 6A–D) compared to unstimulated controls. BMP-2 also upregulated ALP gene expression by hASCs cultured on BCP. On these BCP scaffolds, ALP gene expression was significantly upregulated at day 14, and decreased back to day-0-levels at day 21 (Fig. 6E). Since unstimulated controls showed a similar, but delayed pattern of ALP gene expression (significant upregulation at day 21), the osteogenic induction may be primarily governed by the scaffold properties, and only facilitated by BMP-2 pretreatment of hASCs seeded on these scaffolds. On β-TCP, a gradual, but lower increase in ALP gene expression was observed, which never reached significance at any time point tested. OPN gene expression showed a decrease in time for both hASCs seeded on BCP and β-TCP. Only at day 21, and only on BCP scaffolds, the inhibition of OPN gene expression by BMP-2 was significant (Fig. 6F). BMP-2 strongly inhibited PPAR-γ gene expression by cells cultured on BCP as well as β-TCP (Fig. 6G).
FIG. 6.
Effect of short stimulation with BMP-2 on osteogenic gene expression levels and PPAR-γ expression levels in hASCs. hASCs were pretreated for 15 min with BMP-2 and cultured on different BCP and β-TCP scaffolds for 4, 14, and 21 days. (A) BMP-2 increased CBFA1 gene expression in hASCs seeded on BCP at day 21 (14;p=0.009), and in hASCs seeded on β-TCP at day 14 and 21 (14;p=0.004 and p=0.003, respectively). (B) BMP-2 increased COL1 expression at day 14 (14;p=0.05) and day 21 (14;p=0.05) in hASCs seeded on BCP. BMP-2 also increased COL1 expression at day 14 (14;p=0.016) and day 21 (14;p=0.00005) in hASCs seeded on β-TCP. (C) BMP-2 increased ON gene expression at day 14 (14;p=0.04) and day 21 (14;p=0.0005) in BCP seeded cells. BMP-2 also increased ON expression at day 14 (14;p=0.05) and day 21 (14;p=7.0×10−5) in β-TCP seeded cells. (D) BMP-2 increased OC expression levels at day 21 in both BCP (14;p=0.04) and β-TCP (14;p=0.02). (E) BMP-2 increased ALP gene expression in cells seeded on BCP at day 14 (14;p=0.04), but inhibited ALP expression at day 21 (14;p=0.04). A similar trend (not significant) was observed in cells seeded on β-TCP. (F) BMP-2 inhibited OPN gene expression at day 21 in cells seeded on BCP (14;p=0.04), but did not affect OPN gene expression in ACSs seeded on β-TCP. (G) BMP-2 decreased PPAR-γ expression levels at day 14 (14;p=0.03) and day 21 (14;p=0.03) in cells seeded on BCP. BMP-2 decreased PPAR-γ expression levels at day 21 (14;p=6.5×10−7) in cells seeded on β-TCP. Values are mean±SEM (n=4–8). Significant effect of BMP-2, *p<0.05, **p<0.01, ***p<0.0001. Expression levels are normalized to the housekeeping gene YWHAZ. OC, osteocalcin.
Furthermore, a significant correlation between the expression levels of CBFA1, ALP, and OPN expression and culture time in BMP-2-treated cells cultured on β-TCP was observed, similar as in cells cultured on plastic (Table 3).
Table 3.
Correlation Between Osteogenic and Peroxisome Proliferator-Activated Receptor Gamma Gene Expression and Culture Time of Human Adipose Stem Cells Cultured on BCP and β-TCP
| |
BCP |
β-TCP |
||||||
|---|---|---|---|---|---|---|---|---|
| |
Control |
BMP-2 |
Control |
BMP-2 |
||||
| R2 | p | R2 | p | R2 | p | R2 | P | |
| CBFA1 | 0.70 | 0.15 | 0.90 | 0.50 | 0.87 | 0.07 | 0.93 | 0.04a |
| ALP | 0.76 | 0.13 | 0.13 | 0.63 | 0.80 | 0.11 | 0.97 | 0.015a |
| COL1 | 0.86 | 0.07 | 0.96 | 0.02a | 0.87 | 0.07 | 0.84 | 0.09 |
| ON | 0.10 | 0.69 | 0.85 | 0.08 | 0.009 | 0.90 | 0.68 | 0.17 |
| OPN | 0.55 | 0.26 | 0.76 | 0.13 | 0.85 | 0.08 | 0.98 | 0.01a |
| OCN | 0.85 | 0.08 | 0.81 | 0.10 | 0.76 | 0.12 | 0.67 | 0.18 |
| PPAR-γ | 0.55 | 0.26 | 0.56 | 0.25 | 0.43 | 0.34 | 0.50 | 0.29 |
Regression coefficient (R2) and their p-values (p) calculated from the gene expression levels of CBFA1, COL1, ALP, COL1, ON, OPN, OCN, and PPAR-γ of ASCs cultured on tissue culture plastic.
Significant correlation, p<0.05.
CBFA1, core binding factor alpha 1; ALP, alkaline phosphatase; COL1, collagen type 1; ON, osteonectin; OPN, osteopontin; OCN, osteocalcin, PPAR-γ, peroxisome proliferator-activated receptor-gamma.
Discussion
The aim of this study was to investigate whether a short preincubation of hASCs with BMP-2 would result in a long-lasting osteogenesis-promoting effect in vitro as previously reported for goat ASCs. Furthermore, we investigated whether this osteogenic induction could be enhanced by seeding of BMP-2 prestimulated cells on BCP and β-TCP calcium phosphate scaffolds. The ultimate goal was to validate the BMP-2 prestimulation and hASC seeding steps for their feasibility to fit within the time frame of a one-step surgical procedure as described earlier by Helder et al.28
We found that (1) hASCs showed differential responses when compared to goat ASCs on tissue culture plastic after prestimulation of hASCs for 15 min with a 10 ng/mL dose of BMP-2; (2) BMP-2 prestimulation significantly increased the frequency of CFUs of hASC preparations; (3) hASCs adhered rapidly (within 10 min) to the BCP and β-TCP scaffolds, independent of BMP-2 pretreatment; (4) proliferation and osteogenic differentiation after seeding on the BCP/β-TCP scaffolds were enhanced by BMP-2 pretreatment, with concomitant downregulation of adipogenic gene expression; and (5) BCP effects appeared more pronounced and/or differentiation accelerating compared to their β-TCP counterparts.
Our osteogenic differentiation data obtained from BMP-2 prestimulated hASCs cultured on plastic did not reveal a stimulation of osteogenic differentiation within 4 days, as reported by Knippenberg et al. using goat ASCs.20 A plausible explanation for this discrepancy could be the origin of the cells used, since species-determined differences may exist. For example, rat and human MSCs differ in their properties regarding metabolism and proliferation.34 An alternative explanation of this discrepancy may be that hASCs respond stronger to the cofactors ascorbic acid and β-glycerol phosphate present in the culture medium; the cells already showed upregulation of differentiation markers without BMP-2 pretreatment, which was observed to a much lower degree for goat ASCs.
The increase in CFU frequency upon BMP-2 prestimulation is surprising. Increased proliferation rates (see below) may be part of the explanation, since this will enhance the chance of colonies growing fast enough to reach the threshold size (i.e., ≥10 clustered hASCs). Alternatively, BMP-2 pretreatment may enhance hASC attachment rates on tissue culture plastic and/or efficiency of the hASCs within the fresh isolates, which increases the number of colonies. We intend to address this issue in more detail in follow-up studies.
We have not specifically tested to what extent BMP-2 is washed off from the cell surface. It may well be that at least part of the BMP-2 is still sequestered by the BMP-2 receptors on the cell surface. However, even if some aspecific sticking of BMP-2 occurs, this will never exceed the administered concentration of 10 ng/mL, as also used in the study of Knippenberg et al.20
The rapid attachment of the hASCs to the scaffolds was in line with previous studies of our group for other classes of scaffolds, consisting of polymeric and collagenous biomaterials.32 Interestingly, our data set indicates a slightly higher attachment rate of hASCs (as determined by measuring DNA content) on BCP scaffolds versus their β-TCP counterparts. We speculate, but did not study in detail, that this may be due to different scaffold surface characteristics caused by surface topography and/or material compositional differences (either or not HA-containing), which were shown previously to have major differences on osteoconductivity, osteoinductivity, and cell behavior.35,36
The enhancement of proliferation and osteogenic differentiation of the hASCs on calcium phosphate scaffolds by BMP-2 pretreatment resembles the outcome of a study, where rat ASCs were cultured on a β-TCP scaffold.37 However, an obvious difference between that study and our study is that the BMP-2 in the study with rat ASCs was continuously present in the culture medium at 100 ng/mL concentration, whereas our study employed only a 15-min exposure of 10 ng/mL BMP-2 followed by culture in a plain expansion medium for the full culture period. Nevertheless, the study using rat ASCs confirms the clear difference in differentiation efficiencies on tissue culture plastic versus calcium phosphate scaffolds. The efficacy of a 15-min stimulation has been shown earlier to be similar for both BMP-2 and BMP-7 (OP-1).20 Interestingly, the responses to both growth factors were divergent; BMP-2 induced an osteogenic response, while BMP-7 resulted in a chondrogenic phenotype of ASCs.
In conjunction with the observed osteogenic differentiation, the differentiation toward adipogenesis was strongly downregulated in hASCs. These findings are in line with data presented in the literature and support the view that stimulation into the osteogenic direction simultaneously inhibits differentiation along the adipogenic pathway. The level of PPAR-γ gene expression is an important parameter for adipogenesis; previous research showed that activation of the PPAR-γ receptor induces adipogenesis in BM stromal cells,38 whereas PPAR-γ haploinsufficiency stimulates osteoblastogenesis in mouse stem cells.39 More recently, it has been shown that two signaling cascades promote osteoblastic differentiation from MSCs through two distinct modes of PPAR-γ transrepression40 and that BMPs might interfere with the adipogenic differentiation of MSCs dependent on the type of BMP-receptor involved.41 Thus, the positive effect of BMP-2 on hASC differentiation is also confirmed by the concomitant downregulation of PPAR-γ expression.
The observation that osteogenic differentiation may be enhanced and/or accelerated in hASCs seeded on BCP versus β-TCP scaffolds may be explained by surface topography and/or material compositional differences as described above. However, another consideration may be that the HA component in the BCP may provide additional stiffness to the scaffold, which favors bone differentiation as highlighted in recent reports.42–45 This study clearly shows that the interaction with the calcium phosphate scaffolds markedly enhanced the osteogenic phenotype of the cells compared with culture plates. We hypothesize that this rapid attachment to the stiff bone-like surface may contribute positively to the osteogenic differentiation process, and may possibly at least, in part, overrule possible diverting signals, such as microenvironmental cytokines, growth factors, and BMP antagonists, which may affect in vivo outcome.44,46–48.
Conclusions
In conclusion, this study revealed that a 15-min incubation with a low dose of BMP-2 is sufficient to stimulate hASCs to gain an osteogenic phenotype in vitro after culturing on either BCP or β-TCP. Our findings indicate that this short pretreatment is a very promising tool for its use in a clinical one-step surgical procedure.
These results will be extrapolated and applied in further development of the one-step surgical concept28 in a clinical maxillary sinus floor elevation model. Whether the differences in osteogenic gene expression by hASCs seeded on the different scaffold types will influence bone formation in this clinical setting needs yet to be established.
Acknowledgments
The work of J.R. Overman was supported by the Research School of the Academic Centre for Dentistry Amsterdam (ACTA), The Netherlands. The work of E. Farré Guasch was partly supported by a travel grant from AGAUR, Spain, grant number 2009 BE2 00114 and a fellowship from the International University of Catalonia. The authors wish to extend their thanks to the surgical staff of Tergooiziekenhuizen Hilversum Hospital, The Netherlands, for kindly providing the human adipose tissue for this study. They also thank J. Hogervorst for excellent technical assistance in the isolation of ASCs, and V. Everts and A.D. Bakker for critical scientific feedback on the manuscript.
Disclosure Statement
No competing financial interests exist.
References
- 1.Van den Bergh J.P. ten Bruggenkate C.M. Krekeler G. Tuinzing D.B. Sinus floor elevation and grafting with autogenous iliac crest bone. Clin Oral Implants Res. 1998;9:429. doi: 10.1034/j.1600-0501.1996.090608.x. [DOI] [PubMed] [Google Scholar]
- 2.Meijer G.J. de Bruijn J.D. Koole R. van Blitterswijk C.A. Cell-based bone tissue engineering. PloS Med. 2007;4:e9. doi: 10.1371/journal.pmed.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frenken J.W. Bouwman W.F. Bravenboer N. Zijderveld S.A. Schulten E.A. ten Bruggenkate C.M. The use of Straumann® BoneCeramic in a maxillary sinus floor elevation procedure: a clinical, radiological, histological and histomorphometric evaluation with a 6-month healing period. Clin Oral Implants Res. 2010;21:201. doi: 10.1111/j.1600-0501.2009.01821.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee J.H. Jung U.W. Kim C.S. Choi S.H. Cho K.S. Histologic and clinical evaluation for maxillary sinus augmentation using macroporous biphasic calcium phosphate in human. Clin Oral Implants Res. 2008;19:767. doi: 10.1111/j.1600-0501.2008.01520.x. [DOI] [PubMed] [Google Scholar]
- 5.Zerbo I.R. Bronckers A.L.J.J. de Lange G. Burger E.H. Localisation of osteogenic and osteoclastic cells in porous beta-tricalcium phosphate particles used for human maxillary sinus floor elevation. Biomaterials. 2005;26:1445. doi: 10.1016/j.biomaterials.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Zijderveld S.A. Zerbo I.R. van den Bergh J.P. Schulten E.A.J.M. ten Bruggenkate C.M. Maxillary sinus floor augmentation using a beta-tricalcium phosphate (Cerasorb) alone compared to autogenous bone grafts. Int J Oral Maxillofac Implants. 2005;20:432. [PubMed] [Google Scholar]
- 7.Kühne J.H. Bartl R. Frisch B. Hammer C. Jansson V. Zimmer M. Bone formation in coralline hydroxyapatite. Effects of pore size studied in rabbits. Acta Orthop Scand. 1994;65:46. doi: 10.3109/17453679408995448. [DOI] [PubMed] [Google Scholar]
- 8.Kuboki Y. Jin Q. Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg Am. 2001;83:S105. [PubMed] [Google Scholar]
- 9.Petite H. Viateau V. Bensaïd W. Meunier A. de Pollak C. Bourguignon M. Oudina K. Sedel L. Guillemin G. Tissue-engineered bone regeneration. Nat Biotechnol. 2009;18:959. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 10.Zerbo I.R. Zijderveld S.A. de Boer A. Bronckers A.L.J.J. de Lange G. ten Bruggenkate C.M. Burger E.H. Histomorphometry of human sinus floor augmentation using a porous beta-tricalcium phosphate: a prospective study. Clin Oral Implants Res. 2004;15:724. doi: 10.1111/j.1600-0501.2004.01055.x. [DOI] [PubMed] [Google Scholar]
- 11.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 12.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 13.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H. Alfonso Z.C. Fraser J.K. Benhaim P. Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan C.M. Shi Y.Y. Aalami O.O. Chou Y.F. Mari C. Thomas R. Quarto N. Contag C.H. Wu B. Longaker M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 15.Levi B. James A.W. Nelson E.R. Vistnes D. Wu B. Lee M. Gupta A. Longaker M.T. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLos One. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui L. Liu B. Liu G. Zhang W. Cen L. Sun J. Yin S. Liu W. Cao Y. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials. 2007;28:5477. doi: 10.1016/j.biomaterials.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 17.Yoon E. Dhar S. Chun D.E. Gharibjanian N.A. Evans G.R. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13:619. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]
- 18.Lendeckel S. Jödicke A. Christophis P. Heidinger K. Wolff J. Fraser J.K. Hedrick M.H. Berthold L. Howaldt H.P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Kroeze R.J. Knippenberg M. Helder M.N. Osteogenic differentiation strategies for adipose-derived stem cells. In: Gimble J.M., editor; Bunnell B.A., editor. Adipose-Derived Stem Cells: Methods and Protocols, Methods in Molecular Biology. Clifton, NJ: Springer Science+Business Media; 2011. pp. 233–248. [DOI] [PubMed] [Google Scholar]
- 20.Knippenberg M. Helder M.N. Zandieh Doulabi B. Wuisman P.I. Klein-Nulend J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun. 2006;342:902. doi: 10.1016/j.bbrc.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 21.Dragoo J.L. Choi J.Y. Lieberman J.R. Huang J. Zuk P.A. Zhang J. Hedrick M.H. Benhaim P. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 22.Yamagiwa H. Endo N. Tokunaga K. Hayami T. Hatano H. Takahashi H.E. In vivo bone-forming capacity of human bone marrow-derived stromal cells is stimulated by recombinant human bone morphogenetic protein-2. J Bone Miner Metab. 2001;19:20. doi: 10.1007/s007740170056. [DOI] [PubMed] [Google Scholar]
- 23.Peterson B. Zhang J. Iglesias R. Kabo M. Hedrick M. Benhaim P. Lieberman J.R. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 24.Smith D.M. Afifi A.M. Cooper G.M. Mooney M.P. Marra K.G. Losee J.E. BMP-2-based repair of large-scale calvarial defects in an experimental model: regenerative surgery in cranioplasty. ANZ J Surg. 2007;77:626. doi: 10.1097/SCS.0b013e3181843369. [DOI] [PubMed] [Google Scholar]
- 25.Gautschi O.P. BMP-2-based repair of large-scale calvarial defects in an experimental model: regenerative surgery in cranioplasty. J Craniofac Surg. 2008;19:1315. doi: 10.1097/SCS.0b013e3181843369. [DOI] [PubMed] [Google Scholar]
- 26.Chao M. Donovan T. Sotelo C. Carstens M.H. In situ osteogenesis of hemimandible with rhBMP-2 in a 9-year-old boy: osteoinduction via stem cell concentration. J Craniofac Surg. 2006;17:405. doi: 10.1097/00001665-200605000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Carragee E.J. Hurwitz E.L. Weiner B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;6:471. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Helder M.N. Knippenberg M. Klein-Nulend J. Wuisman P.I. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng. 2007;13:1799. doi: 10.1089/ten.2006.0165. [DOI] [PubMed] [Google Scholar]
- 29.Oedayrajsingh-Varma M.J. Breuls R.G. Schouten T.E. Jürgens W.J. Bontkes H.J. Schuurhuis G.J. van Ham S.M. van Milligen F.J. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16:91. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 30.Varma M.J. Breuls R.G. Schouten T.E. Jurgens W.J. Bontkes H.J. Schuurhuis G.J. van Ham S.M. van Milligen F.J. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16:91. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 31.Jurgens W.J.F.M. van Dijk A. Zandieh Doulabi B. Niessen F.B. Ritt M.J.P.F. van Milligen F.J. Helder M.N. Freshly isolated stromal cells from the infrapatellar fat pad are suitable for a one-step surgical procedure to regenerate cartilage tissue. Cytotherapy. 2009;11:1. doi: 10.3109/14653240903219122. [DOI] [PubMed] [Google Scholar]
- 32.Jurgens W.J. Kroeze R.J. Bank R.A. Ritt M.J. Helder M.N. Rapid attachment of adipose stromal cells on resorbable polymeric scaffolds facilitates the one-step surgical procedure for cartilage and bone tissue engineering purposes. J Orthop Res. 2011;29:853. doi: 10.1002/jor.21314. [DOI] [PubMed] [Google Scholar]
- 33.Jurgens W.J. Oedayrajsingh-Varma M.J. Helder M.N. Zandieh Doulabi B. Schouten T.E. Kuik D.J. Ritt M.J.P.F. van Milligen F.J. Effect of tissue harvesting-site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332:415. doi: 10.1007/s00441-007-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schop D. Janssen F.W. van Rijn L.D.S. Fernandes H. Bloem R.M. de Bruijn J.D. van Dijkhuizen-Radersma R. Growth, metabolism and growth inhibitors of mesenchymal stem cells. Tissue Eng Part A. 2009;15:1877. doi: 10.1089/ten.tea.2008.0345. [DOI] [PubMed] [Google Scholar]
- 35.Wilson C.E. Kruyt M.C. de Buijn J.D. van Blitterswijk C.A. Oner F.C. Verbout A.J. Dhert W.J.A. A new in vivo screening model for posterior spinal bone formation: comparison of ten calcium phosphate ceramic material treatments. Biomaterials. 2006;27:302. doi: 10.1016/j.biomaterials.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 36.Guha A.K. Singh S. Kumaresan R. Nayar S. Sinha A. Mesenchymal cell response to nanosized biphasic calcium phosphate composites. Colloids Surf B Biointerfaces. 2009;73:146. doi: 10.1016/j.colsurfb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 37.E L.L. Xu L.L. Wu X. Wang D.S. Lv Y. Wang J.Z. Lui H.C. The interactions between rat-adipose-derived stromal cells, recombinant human bone morphogenetic protein-2, and beta-tricalcium phosphate play an important role in bone tissue engineering. Tissue Eng Part A. 2010;16:2927. doi: 10.1089/ten.TEA.2010.0018. [DOI] [PubMed] [Google Scholar]
- 38.Gimble J.M. Robinson C.E. Wu X. Kelly K.A. Rodriguez B.R. Kliewer S.A. Lehmann J.M. Morris D.C. Peroxisome proliferator-activated receptor-γ activation by thialzolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087. [PubMed] [Google Scholar]
- 39.Akune T. Ohba S. Kamekura S. Yamaguchi M. Chung U.I. Kubota N. Terauchi Y. Harada Y. Azuma Y. Kadowaki T. Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takada I. Kouzmenko A.P. Kato S. Molecular switching of osteoblastogenesis versus adipogenesis: implications for targeted therapies. Expert Opin Ther Targets. 2009;13:593. doi: 10.1517/14728220902915310. [DOI] [PubMed] [Google Scholar]
- 41.Muruganandan S. Roman A.A. Sinal C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66:236. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Santis G. Lennon A.B. Boschetti F. Verhegghe B. Verdonck P. Prendergast P.J. How can cells sense the elasticity of a substrate? An analysis using a cell tensegrity model. Eur Cells Mater. 2011;22:202. doi: 10.22203/ecm.v022a16. [DOI] [PubMed] [Google Scholar]
- 43.Breuls R.G. Jiya T.U. Smit T.H. Scaffold stiffness influences cell behavior: opportunities for skeletal tissue engineering. Open Orthop J. 2008;2:103. doi: 10.2174/1874325000802010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 45.Disscher D.E. Janmey P. Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 46.McBeath R. Pirone D.M. Nelson C.M. Bhadriraju K. Chen C.S. Cell shape, cytoeskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 47.Knippenberg M. Helder M.N. Zandieh-Doulabi B. Semeins C.M. Wuisman P.I.J.M. Klein-Nulend J. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005;11:1780. doi: 10.1089/ten.2005.11.1780. [DOI] [PubMed] [Google Scholar]
- 48.Matsushima A. Kotobuki N. Tadokoro M. Kawate K. Yajima H. Takakura Y. Ohgushi H. In vivo osteogenic capability of human mesenchymal cells cultured on hydroxyapatite and on beta-tricalcium phosphate. Artif Organs. 2009;33:474. doi: 10.1111/j.1525-1594.2009.00749.x. [DOI] [PubMed] [Google Scholar]