Abstract
In this study, we tested the hypothesis that a surface functionalization delivery platform incorporating heparin onto strontium alginate microbeads surfaces would convert this “naive carriers” into “mini-reservoirs” for localized in vivo delivery of recombinant human bone morphogenetic protein-2 (rhBMP-2) that will induce functional bone regeneration. In vitro evaluation confirmed that (1) heparin incorporation could immobilize and prolong rhBMP-2 release for approximately 3 weeks; (2) a significant decrease (p<0.01) in rhBMP-2 burst release is attainable depending on initial protein load; and (3) rhBMP-2 released from surface functionalized microbeads retained bioactivity and stimulated higher alkaline phosphatase activity in cultured C2C12 cells when compared with daily administration of fresh bolus rhBMP-2. Subsequently, surface functionalized microbeads were used for in vivo delivery of rhBMP-2 at local sites of posterolateral spinal fusion surgery in rats. The microbeads were loaded into the pores of medical-grade polyepsilone caprolactone-tricalcium phosphate scaffolds before implantation. Results revealed robust bone formation and a biomechanically solid fusion after 6 weeks. When compared with a control group consisting of an equivalent amount of rhBMP-2 that was directly adsorbed onto bare-surfaced microbeads with no heparin, a 5.3-fold increase in bone volume fraction and a 2.6-fold increase in bending stiffness (flexion/extension) were observed. When compared with collagen sponge carriers of rhBMP-2, a 1.5-fold and a 1.3-fold increase in bone volume fraction and bending stiffness were observed, respectively. More importantly, 3D micro-computed tomography images enabled the visualization of a well-contained newly formed bone at ipsilateral implant sites with surface functionalized rhBMP-2 delivery. This was absent with collagen sponge carriers where newly formed bone tissue was poorly contained and crossed over the posterior midline to contralateral implants. These findings are important because of complications with current rhBMP-2 delivery method, including excessive, uncontrolled bone formation.
Introduction
Bone morphogenetic protein 2 (BMP-2) is a highly potent osteoinductive agent.1 Although it received regulatory approval about a decade ago for single-level interbody spinal fusion when delivered on collagen sponges, wider clinical application in multilevel spinal fusion and other similarly challenging bone healing applications continue to be “off-label” and associated with considerable risks.2 Many of these risks have been attributed to the use of “supra-physiological doses” necessitated, at least in part, by rapid growth factor washout from implant sites as well as short in vivo half life due to the rapid proteolytic degradation of exogenous BMP-2.
The use of heparin-like proteoglycans to control the release as well as protect the bioactivity of different growth factors has increasingly gained attention over the years.3–7 This growing interest has emerged as a result of significant advancements in our understanding of the storage and metabolism of endogenous cytokines. This, in turn, has led to the introduction of several attempts to optimize the in vivo delivery of bioactive exogenous BMPs and similar growth factors using heparin.3,8 Yang et al.9 evaluated the chemical conjugation of heparin with fibrinogen for rhBMP-2 delivery and showed ectopic bone formation in rats. Similarly, Lee et al.3 observed posterolateral spinal fusion in rabbits when heparin was conjugated with poly(lactic-co-glycolic acid) (PLGA) to deliver recombinant human BMP-2 (rhBMP-2). These in vivo results substantiate in vitro studies showing that heparin could indeed potentiate the osteoinductive activity as well as control the release of exogenous BMP-2.10,11 Although these and several other studies have demonstrated the bioactivity of heparin-based BMP-2 delivered using several different biomaterials, there is, unfortunately, still an unmet need for strategies that can improve the overall efficacy of growth factor therapy in the light of recent clinical reports.2
A possible method for incorporating heparin into biomaterials for growth factor delivery could be by ionic binding to biocompatible polycations using the principles of polyelectrolyte complexation (PEC).12,13 Besides eliminating the need for chemical reagents that could be detrimental to growth factor bioactivity, the incorporation of heparin in this oppositely charged self-assembly approach will more closely mimic many natural in vivo conditions (e.g., DNA strand assembly). In addition, this strategy would localize the heparin within the PEC membrane formed on the surfaces of biomaterials, and this will, in turn, immobilize and protect the uploaded biomolecules at implant surfaces, thus facilitating the spatiotemporal presentation as well as enhancing the minimization of administered cytokine.
The use of alginate hydrogel as matrices for the in vivo delivery of cells and biomolecules continues to attract research interest because of its biocompatibility and tailorable physical properties.8,14 Studies have shown that a coating layer could be formed on the surfaces of ionically cross-linked alginate beads using polycations such as poly-l-lysine (PLL).15,16 Furthermore, a previous study showed that heparin could irreversibly bind onto PLL-coated alginate beads.17 This presents a distinct opportunity to immobilize bioactive molecules within the nano-scale PEC membranes formed on the surfaces of these beads. We reasoned that while attention is being paid to encapsulation strategies with alginate bead matrices, surface functionalization could offer a superior spatiotemporal presentation of immobilized biomolecules.
In the present study, we hypothesize that heparin bonded to the surface of alginate microbeads will retain its affinity for macromolecular growth factors and further enhance the in vivo presentation of loaded rhBMP-2 to facilitate bone regeneration. The primary aim of this study was to compare in vivo bioactivity between heparin surface-modified alginate microbeads and bare unmodified alginate microbeads as rhBMP-2 carriers in posterolateral spinal fusion application. Subsequently, we compared this delivery method with the microencapsulation of rhBMP-2 and collagen sponges so as to evaluate the potential of this mode of delivery in the light of a contemporary clinical carrier of rhBMP-2.
Materials and Methods
Materials
Clinical grade rhBMP-2 (Medtronic sofarmor Danek) was reconstituted in water at a concentration of 0.5 mg/mL. Bioresorbable medical grade polyepsilone caprolactone tricalcium phosphate (mPCL–TCP) scaffolds spacers with a 0/90 degrees lay-down pattern, 75% total porosity, and 7×6×2 mm3 scaffold dimension were fabricated by fused deposition modeling from medical grade poly (epsilon-caprolactone), incorporating 20wt% beta-tricalcium phosphate (www.osteopore.com.sg).18 Ultra pure, medium viscosity, high G (MVG) alginate (Pronova MVG, FMC Biopolymer) and an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) were employed in this study. All other chemicals, including strontium chloride and PLL, were purchased from Sigma Aldrich, unless otherwise stated.
Microbeads preparation
Sodium alginate was dissolved overnight in deionized water at 4°C to a final concentration of 4% (w/v) and filter sterilized. Microdroplets generated from 5 mL alginate solution using an electrostatic bead generator (Nisco Engineering AG Zurich) were allowed to gel in 0.2 M strontium chloride (SrCl2) for 15 min before repeated washing in normal saline.
For polyelectrolyte surface modification of microbeads, a polycation coating layer was applied by suspension in 0.1% PLL (w/v) for 10 min followed by repeated washings to remove unbound PLL. Subsequently, a polyanion layer was applied by suspension in 0.05% heparin solution (w/v). Microbeads were finally washed thrice with normal saline and suspended in a solution containing reconstituted rhBMP-2 of predetermined concentration for 1 h. To enable confocal laser scanning microscopy, fluorescein isothiocyanate (FITC)-labeled heparin (Invitrogen, Life Technologies) was used in a batch to examine heparin uptake and distribution.
In vitro bioactivity of rhBMP-2
The retention of in vitro osteoinductive activity of rhBMP-2 released from surface functionalized microbeads was evaluated using alkaline phosphatase immunohistochemical assay on C2C12 cells as previously reported.19 The culture media consist of Dulbecco's modified Eagle's medium (DMEM) (Gibco, Invitrogen) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The incubator was maintained at 37°C temperature and 5% CO2 atmosphere. For all experiments, cells seeded in six-well plates at 104 cells per well were allowed to grow for 3 days. Subsequently, surface functionalized microbeads were loaded into the pores of mPCL-TCP scaffolds and allowed to suspend and completely immerse in the culture media using a 100 μm pore-sized cell strainer. Another group of cells (positive control) received a daily bolus administration of free rhBMP-2 (without heparin) at doses predetermined from previous in vitro release study, while cells that received no rhBMP-2 served as a negative control.
To measure ALP activity, cells were lysed with 100 μL of 0.5% Triton-X100. Subsequently, ALP activity was measured following the manufacturer's instructions (p-nitrophenylphosphate substrate, Sigma-Aldrich). The ALP activity was expressed as micromoles of p-nitrophenyl produced per min per mg of protein. The total protein content of each sample was determined using the bicinchoninic acid protein assay kit. Visualization of alkaline phosphatase activity in cultured cells was conducted after 9 days of culture.19 Briefly, cells were fixed in citrate buffer (pH 5.4) containing 60% acetone and 10% methanol at room temperature followed by incubation with a mixture of 0.1 mg/mL of TR/Naphthol AS-MX Phosphate phosphate (SIGMA FAST Fast Red).
Preparation of implants
Microbeads prepared from 0.2 mL of alginate were loaded with rhBMP-2 (10 μg total). To directly adsorb rhBMP-2 onto microbeads, the heparin coating step was omitted, while microencapsulated rhBMP-2 were prepared by premixing alginate solution with rhBMP-2 before gelation in SrCl2. All groups of microbeads (approximately 550 μm diameter) were manually loaded into the pores of rapid prototyped mPCL-TCP scaffolds (350–500 μm diameter pore diameter) using press-fit approach. Loaded scaffolds were stored at −20°C until use (commonly within 2 h). For collagen sponge carriers, mPCL-TCP scaffold (4×2×2 mm) was bathed in 0.2 mL of 3% type I collagen (Symatese) before freezing (−20°C) and lyophilization as commonly practiced in our lab.20 Each scaffold was loaded with 10 μg of rhBMP-2 and stored at −20°C, which was similar to other spacer constructs.
Surgical protocol
All animal-related procedures were preapproved by the institutional ethics committee. Thirty-two mature male Sprague Dawley rats (16 weeks old) underwent posterolateral (inter-transverse) spinal fusion with implantation of mPCL-TCP spacers, incorporating four different components (n=8), namely (Gp A) surface functionlized alginate microbeads + BMP-2; (Gp B) collagen sponges + BMP-2; (Gp C) BMP-2 directly adsorbed onto alginate microbeads with no heparin; and (Gp D) BMP-2 encapsulated in alginate microbeads. Each implant was aseptically placed onto decorticated L4/5 and L5/6 transverse processes for posterolateral spinal fusion following procedures previously described.21 Animals were sacrificed after 6 weeks by injection of lethal dose of sodium pentobarbital. Operated lumbar segments were harvested after 6 weeks of the implantation period and were stored at −80°C until use.
In vivo osteogenic activity evaluation
Micro-computed tomography (micro-CT) imaging (Shimadzu SMX-100 CT Kyoto Japan) was performed on all harvested samples (n=8). Bone and total tissue ingrowth volume into the scaffold spacer were evaluated using the volume graphics software v1.2 (VGStudio MAX, GmBH). Histology was subsequently performed on two randomly selected specimens from each group following protocols previously reported.20 Briefly, each specimen was fixed in 10% neutral buffered formalin, dehydrated in graded ethanol, decalcified in formic acid, and embedded in paraffin wax. Thin tissue sections (5 μm) were cut in sagittal plane of the fusion mass. Tissue sections were stained with eosin and haematoxylin to visualize new bone formation, connective tissue infiltration, alginate degradation, and cellular aggregations.
Biomechanical testing
The biomechanical stiffness testing protocol for rat spinal segments employed in this study was adapted from previously reported protocols with slight modifications.22 Briefly, the inferior endplate of harvested specimens (n=6) was potted in dental cement. Stiffness was determined by measuring dorsal-ventral loading force using a material-testing system (Instron 5543) with a±50 N load cell at a rate of 0.005 mm/s. The first cycle of each test was always discarded, and the second was used for evaluation. The mean slope of these cycles was used to determine the average stiffness expressed in N mm/degree.
Statistical analysis
Data were analyzed by Student's t-test to assess the statistical significance between experimental groups. Results were expressed as means±standard error.
Results
Morphological evaluation
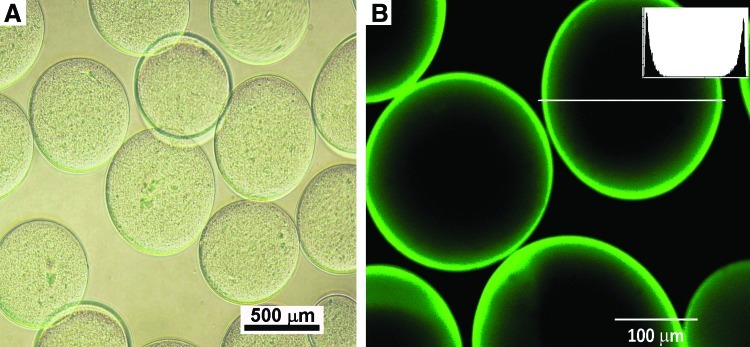
Strontium alginate microbeads of uniform morphology were consistently generated with the electrostatic beads generator (Fig. 1A). The microbeads remained spherical even after surface functionalization and repeated washings (Fig. 1B). Confocal laser scanning microscopy with FITC-labeled heparin confirmed uniform uptake of heparin (Fig. 1B). It also shows that loaded heparin was mainly distributed around the circumference of the microbeads.
FIG. 1.
Bright-field microscopy revealed microbeads of uniform morphology (A); Microbeads retained their morphological uniformity even after surface functionalization with heparin and heparin-binding proteins as observed on confocal laser scanning microscopy (B); this also confirms the uptake of fluorescein isothiocyanate-labeled heparin. Inset is histograph demonstrating the circumferential localization of green fluorescent dye intensity. Color images available online at www.liebertpub.com/tea
Growth factors uptake and release profile
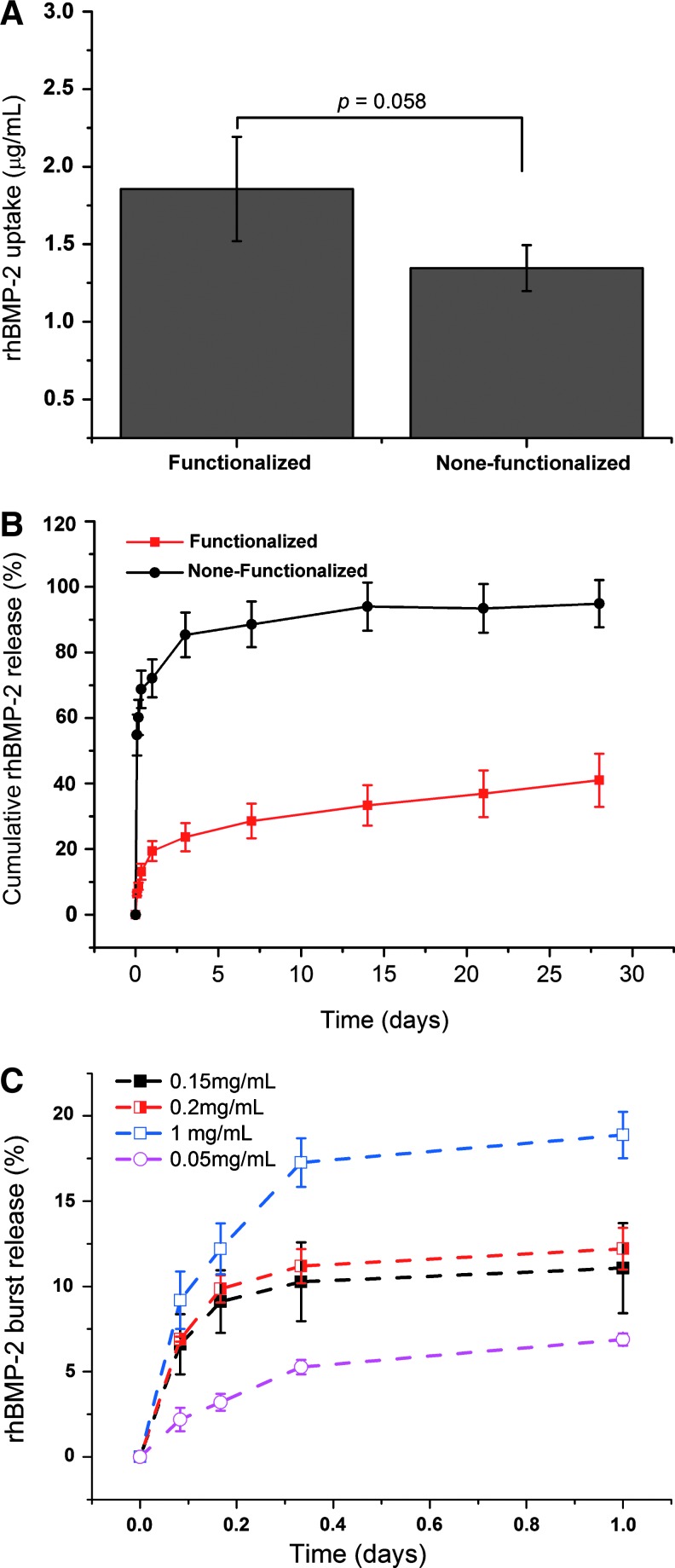
The capacity of alginate microbeads to bind and retain rhBMP-2 from the solution was significantly improved when heparin was incorporated onto the PLL-coated microbeads (Fig. 2A). A release study further shows that over 70% of rhBMP-2 adsorbed directly onto alginate microbeads is released in a burst within approximately 2 h; whereas only about 10% of rhBMP-2 bound to heparin functionalized alginate microbeads are released within this time (Fig. 2B). Furthermore, about 90% of rhBMP-2 adsorbed directly to alginate microbeads are released within 5 days; whereas less than 40% of rhBMP-2 loaded onto heparin functionalized microbeads are released over 28 days. This indicates excellent immobilization of the growth factor within the polyelectrolyte membrane formed with heparin and PLL on the alginate microbead surfaces. A further evaluation of the influence of protein concentration in the loading buffer shows that initial burst could be reduced by lowering the concentration of the loading buffer (Fig. 2C).
FIG. 2.
Monitoring the uptake (A) and release patterns (B and C) of recombinant human bone morphogenetic protein-2 (rhBMP-2) embedded within the surfaces of strontium crosslinked alginate microbeads using enzyme-linked immunosorbent assay methods. Color images available online at www.liebertpub.com/tea
In vitro bioactivity
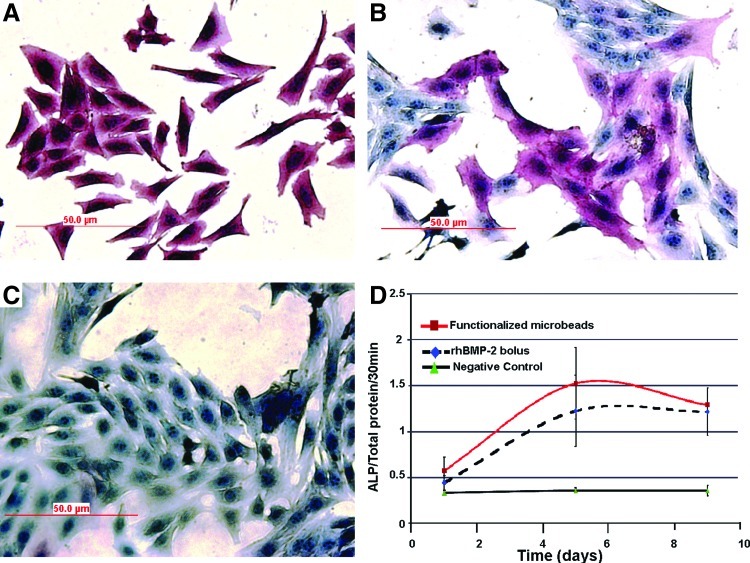
Figure 3 are data obtained from bioactivity evaluation studies. Cells exposed to rhBMP-2 from surface functionalized microbeads stained uniformly (Fig. 3A), whereas cells exposed to a daily bolus administration of equivalent rhBMP-2 doses in free form (without heparin) stained rather unevenly on ALP immunohistochemistry (Fig. 3B). A quantitative evaluation of ALP enzyme activity showed that both experimental groups resulted in significantly enhanced ALP activity when compared with negative controls (Fig. 3C) over the 9-day culture period (Fig. 3D). Significantly, the ALP activity measured among cells exposed to rhBMP-2 from surface functionalized microbeads was moderately higher than in positive controls, but this difference was not statistically significant. This demonstrates that the rhBMP-2 released from surface functionalized beads remained bioactive over the 9-day culture period in this study.
FIG. 3.
Alkaline phosphatase enzyme activity of cultured C2C12 cells exposed to rhBMP-2 from surface functionalized microbeads (A) compared with bolus daily addition of equivalent rhBMP-2 doses (B) and negative control (beads with neither heparin nor rhBMP-2) (C). Measurements of alkaline phosphatase activity showed a similar trend between surface functionalized microbeads and the positive control group with a moderate increase in the surface functionalized group (D). Color images available online at www.liebertpub.com/tea
Micro-CT
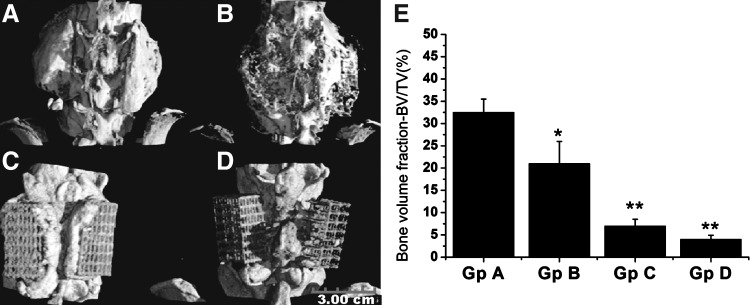
Figure 4 are data derived from micro-CT scanning of harvested spinal segments 6 weeks after implantation. Three-dimensional (3D) reconstructed images showed consistent and abundant bone formation in all rats implanted with surface functionalized microbeads as well as collagen sponges (Fig. 4A, B). However, minimal mineralized tissue formation along mPCL-TCP scaffold periphery was observed among rats implanted with rhBMP-2 adsorbed directly on microbeads with no heparin incorporation, while a complete failure of bone formation was observed when rhBMP-2 was encapsulated (Fig. 4C, D). Comparing surface functionalized microbeads with collagen sponge carriers of rhBMP-2, 3D micro-CT reconstruction enables the visualization of a clear difference in the morphology of deposited new bone tissue (Fig. 4A, B). A distinctly localized, uniformly cortical, bone mass could be seen on either sides of the spinal column (at implant sites) in the surface functionalized group, whereas new bone lay down was poorly localized, visibly trabeculated, in the collagen group with filament-like or trabecular extensions and bone mass crossing over the midline to contralacteral implants. Similarly, the percentage bone volume (in relation to total tissue volume) of the fusion mass measured from micro-CT slices revealed a significant increase in bone volume fraction induced by surface functionalized microbeads when compared with all control groups, including collagen sponges (Fig. 4E).
FIG. 4.
Reconstructed micro-CT images (A–D) and bone volumetric data (E) of harvested spinal segments after 6 weeks of implantation. New bone formation was observed in animals implanted with surface functionalized microbeads (A) and collagen sponge rhBMP-2 carriers (B). Peri-implant tissue mineralization and failure of bone formation were observed, respectively, in animals implanted with rhBMP-2 adsorbed directly onto alginate microbeads with no heparin (C) and rhBMP-2 microencapsulated in alginate core (D). Quantitative estimation of bone volume fraction (bone volume/total volume) showed significantly increased bone volume among surface functionalized alginate microbeads treatment group compared with the three control groups (E). *p<0.05 and **p<0.01.
Histological evaluation
A histological examination confirmed micro-CT observations of extensive new bone formation at implant sites in the functionalized microbeads and the collagen sponge implant groups (Fig. 5A, B). Broad plates of woven bone tissue displaying osteocytes in lacunae were observed in both experimental groups. Bone formation was by direct rather than endochondrial ossification with no evidence of cartilage tissue formation. Among animals receiving rhBMP-2 adsorbed microbeads without heparin as well as microencapsulated rhBMP-2, there was however, no histological evidence of bone ingrowth in the scaffold pore spaces (Fig. 5C, D). The pore spaces of mPCL-TCP scaffold spacers were mainly filled with soft tissues in these two control groups. In addition, although collagen sponges were all but degraded after 6 weeks, remnants of alginate at varying stages of degradation or contracture could be seen in all scaffold spacers containing alginate microbeads (Fig. 5A, C, D). There was no evidence of chronic inflammation or inflammatory cell aggregation in any implant group at 6 weeks.
FIG. 5.
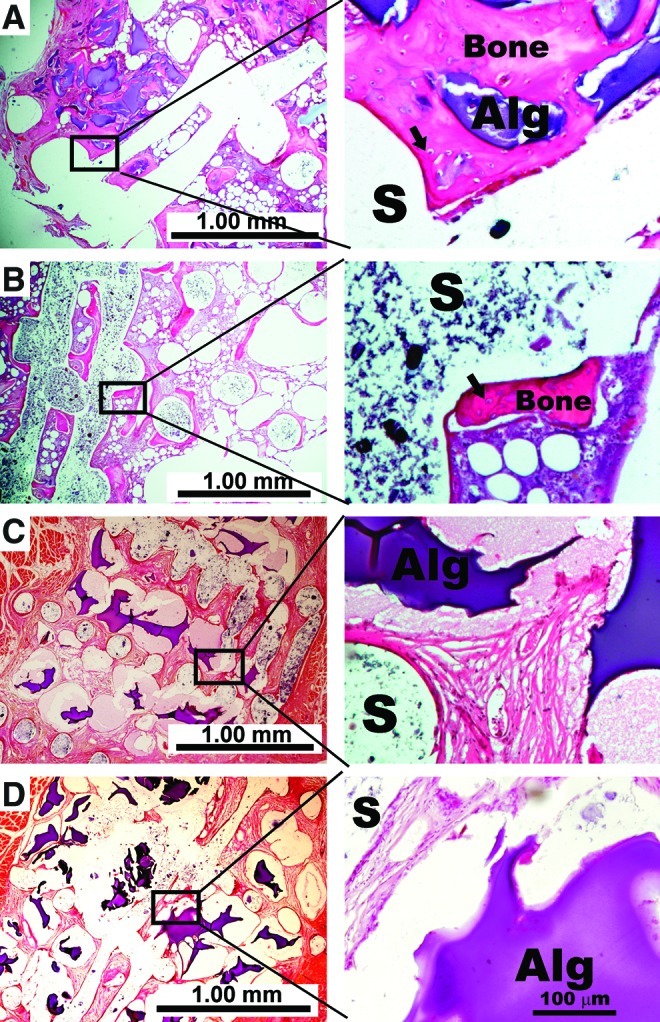
Representative photomicrographs of histology sections stained with eosin and hematoxylin. New bone formation within medical grade polyepsilone caprolactone-tricalcium phosphate scaffold (S) pore spaces was confirmed among surface functionalized microbeads (A) and collagen sponge implants groups (B) at 6 weeks. Among animals implanted with rhBMP-2 adsorbed directly to microbeads without heparin (C) and animals implanted with microencapsulated rhBMP-2 (D), there was no evidence of new bone formation within the scaffold pore spaces. Bone formation was by direct ossification in the two groups with new bone formation. High magnification images show osteocytes in their lacunae (arrows) as well as fragmented remnants of alginate (Alg) matrix with no evidence of fibrous tissue encapsulation or massive cell infiltration. Color images available online at www.liebertpub.com/tea
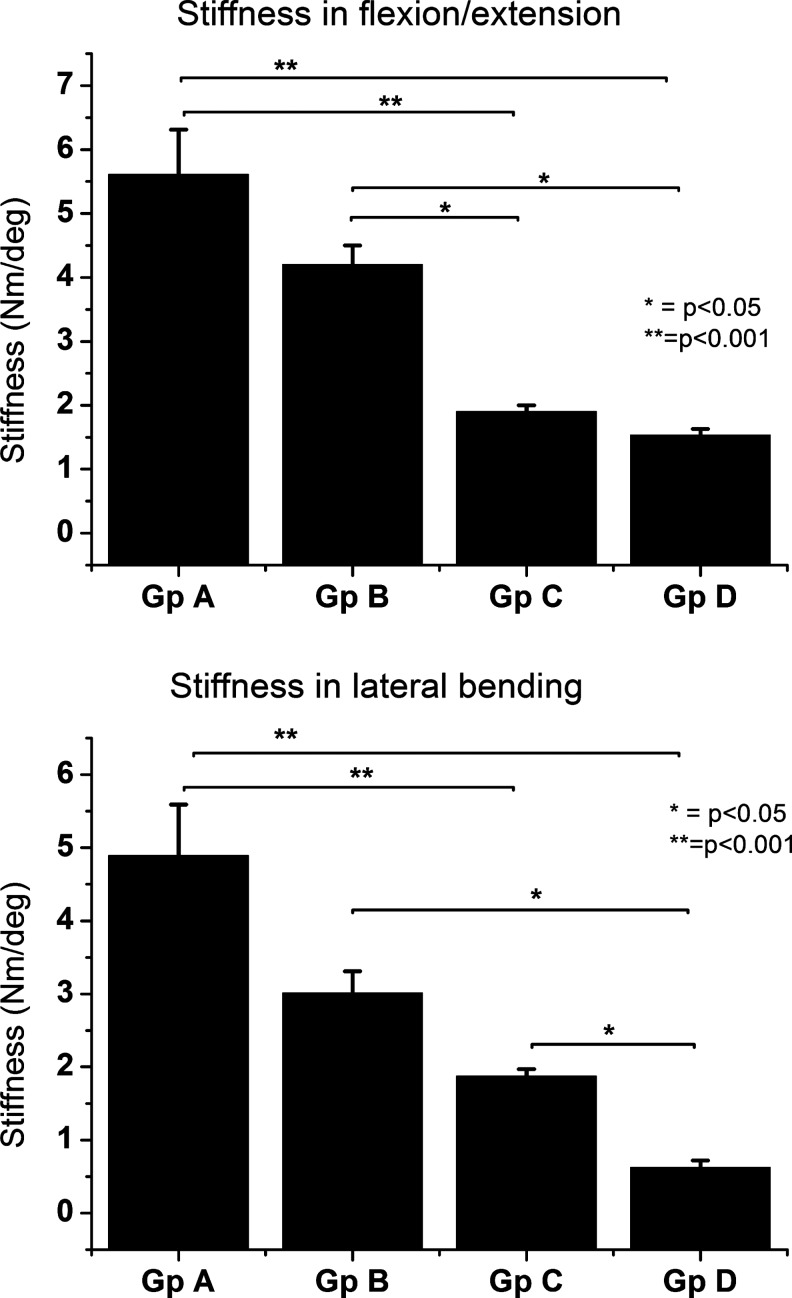
Biomechanical evaluation
To further evaluate the functionality of regenerated bone tissue, we conducted biomechanical testing of segmental stiffness in flexion/extension and lateral bending (Fig. 6A, B). Results revealed stiffer spinal segments among the surface functionalized microbeads implant groups when compared with all control implant groups, including collagen sponge. Although the difference with collagen sponge was only moderate and not statistically significant; rhBMP-2 adsorbed directly onto microbeads and rhBMP-2 encapsulated in the alginate microbead core showed a significantly lower segmental stability, indicating failure of fusion.
FIG. 6.
Stiffness analysis (mean±standard deviation) of rat spinal segments evaluated in flexion/extension and lateral bending after 6 weeks of posterolateral fusion surgery. Gp A=surface functionalized alginate microbeads with heparin and rhBMP-2, Gp B=rhBMP-2 loaded unto collagen sponges, Gp C=rhBMP-2 adsorbed directly to microbeads without heparin, and Gp D=microencapsulated rhBMP-2.
Discussion
The present study set out to address two specific questions: (1) whether commercially available heparin, bonded with PLL by electrostatic complexation, retains its affinity to entrap and localize macromolecular growth factors for tissue-engineering applications and (2) whether such newly formed tissue is structurally and functionally comparable to established carriers. The results presented here clearly demonstrate that rhBMP-2 immobilized in this manner preserved their osteoinductive ability; inducing a larger volume of new bone and facilitating a biomechanically superior fusion when compared with experimental controls in this model of spinal fusion (Figs. 4–6). More importantly, we show for the first time that besides promoting a higher volume of tissue ingrowth, this strategy could exert superior control over the morphological pattern of bone tissue deposition when assembled with structural scaffolds as implantable, tissue-engineered constructs.
In a previous study, Yang et al.9 employed a covalent linkage to conjugate heparin with fibrinogen for BMP-2 delivery. The authors showed ectopic bone formation in a rat muscle pouch. Similarly, Lee et al.3 conjugated heparin with PLGA nanospheres as a long-term delivery system for BMP-2. They reported a higher rate of bone formation and increase in Young's modulus with long-term release when compared with short-term delivery. In the present study, we reasoned that due to its high negative charge density, heparin could be employed as a “triple duty” molecule; first, for physical substrates formation by PEC; second, for entrapment/localization and controlled release BMP-2; and third, for the enhancement of BMP-2 bioactivity. This approach will eliminate the need for covalent reactions that could be detrimental, at least theoretically, to the conformation and presentation of the bioactive growth factor.23 We observed a 5.3-fold increase in the fractional bone volume with surface functionalized alginate microbeads when compared with bare-surfaced microbeads lacking heparin. This resulted in a statistically significant increase in biomechanical stability of spinal segments receiving surface functionalized microbeads. Similarly, a 1.5-fold increase in bone volume fraction and a 1.3-fold increase in biomechanical stability of the fused spinal segments was observed in the functionalized alginate experimental group compared with collagen sponge carriers (Figs. 4 and 6). Comparing surface functionalization with microencapsulation as a delivery platform for rhBMP-2, we observed a complete failure of bone ingrowth into scaffold pores with the microencapsulation of rhBMP-2. This is consistent with a previous report.24 Empirically interpreted, the findings documented here suggest that the immobilization of rhBMP-2 on PLL-heparin modified alginate hydrogel surfaces could have facilitated the entrapment and controlled the release and presentation of rhBMP-2. This, in turn, augmented the volume and ordered the morphological deposition of bone formed at implant sites. Other contributory mechanisms underlying these findings would include (1) prolongation of the biological half life of rhBMP-2 by heparin; (2) inhibition of noggin (a molecule known to inhibit endogenous BMP-2); and (3) alterations of cellular responsiveness to growth factors by heparin.10,25–27
Several different interactive effects of heparin-like molecules with growth factors in bone metabolism have been reported. Recently, Bramono et al.6 showed that the long-term supplementation of BMP-2 with heparan sulfate could enhance BMP-2-induced bone formation in vitro and in vivo. Hausser and Brenner28 showed that low concentrations of heparin promoted matrix deposition and its subsequent mineralization. Zhao et al.10 demonstrated that heparin potentiates the in vivo ectopic bone formation by protecting BMPs from degradation and inhibition by BMP antagonists, while Kim et al.,29 showed that by grafting heparin and immobilizing BMP-2 on Ti surfaces, inflammation can be inhibited and osteoblast function can be promoted.
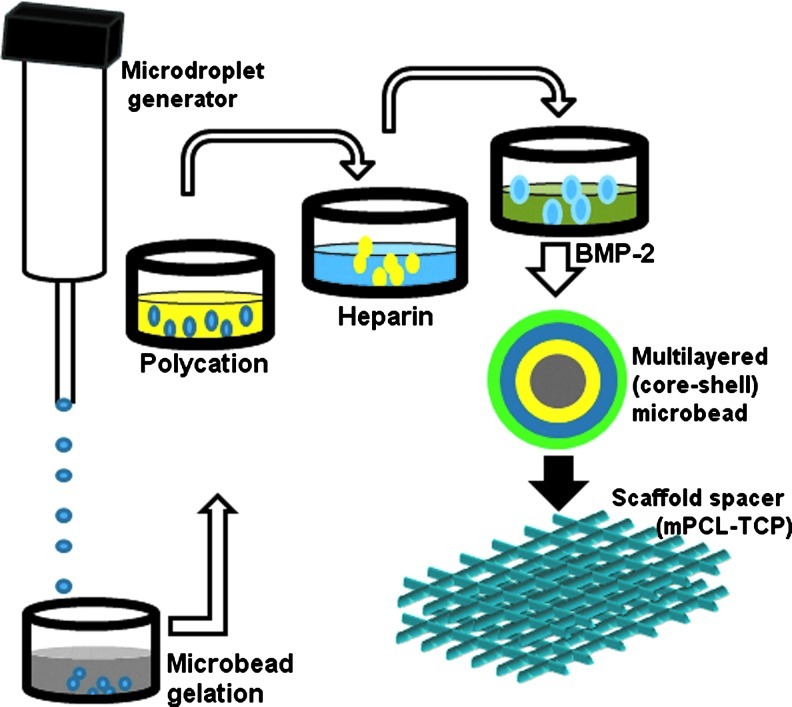
Given the importance of heparin and heparan sulfate in osteogenesis, increasing number of studies are exploring the use of heparin to control the release of BMP-2 by covalently conjugating these molecules unto different implantable substrates such as: fibrin,9 collagen,30 demineralized bone matrix,31 alginate,32 and hyaluronic acid-based hydrogel Xu et al.11 In the present study, we show that the initial protein burst could be significantly reduced when employing polyelectrolyte complexes that are devoid of covalent linkage as the immobilizing substrate. We further observed that the deposition of newly formed bone tissue could be regulated in an orderly manner using this delivery platform in vivo (Fig. 4A, B). Although robust new bone formation and solid spinal fusion was observed with both carriers, bone deposition was poorly contained or “uncontrolled” with collagen carriers. New bone tissue was observed to form across the posterior midline and obliterated the morphology of the spacer implants (mPCL-TCP) on both sides of the vertebral column in this group. In contrast, surface functionalized microbeads stimulated a highly localized bone deposition at ipsilateral implant sites with clearly designable spacer morphology that was relative to the spinal column. Taken together, the success of this strategy in controlling the pattern of bone deposition could be attributed to different design features of the implanted tissue-engineered construct working together in concert, including stepwise polyelectrolyte deposition, rhBMP-2 spatio-temporal location and loading of beads into a structural scaffold (Fig. 7).
FIG. 7.
Schematic of the surface functionalization procedure depicting layer-by-layer polyelectrolyte deposition for condensing heparin onto the surface of alginate microbeads and further entrapping rhBMP-2. Color images available online at www.liebertpub.com/tea
When rhBMP-2 is delivered on collagen sponge carriers, excessive, uncontrolled, heterotopic bone formation has continued to attract attention.2 Although this may not always provoke clinically relevant symptoms, especially in single-level interbody lumbar fusion, the compression of vital tissues such as nerve roots, blood vessels, and airway tubes, in addition to the risk of vertebral stenosis and radiculopathy, has been reported.2,33 Acute and chronic inflammation (including seroma formation and radiculities), retrograde ejaculation, and other potentially serious complications have been associated with the use of BMP-2 delivered on collagen sponges in contemporary clinical applications.34–36 Therefore, uncontrolled bone formation currently represents a significant limiting factor to the wider clinical application of rhBMP-2(2). A delivery platform that would ensure robust in vivo bone formation and further control the pattern of bone tissue deposition could widen the horizon for rhBMP-2 and other similar growth factor applications. One possible reason for the enhancement in bone localization observed in the present study could arise from possible differences in the ability of the two delivery platforms to immobilize and release loaded bioactive proteins. In one of our previous studies, we observed a higher burst release from collagen sponges when compared with data obtained in the present study from in vitro protein release analyses.18 This is in agreement with other studies which show that covalently crosslinked heparin could indeed suppress the initial burst release of bounded proteins.37
The failure of bone induction and spinal fusion observed with alginate microencapsulation and direct rhBMP-2 adsorption without heparin in this study is not surprising (Figs. 4–6). Several previous studies have suggested that alginate matrix with no modifications could be a rather “naive” or poor substrate for rhBMP-2 delivery. In one report, Fu et al.24 documented that rhBMP-2 mixed with unmodified alginate resulted in the complete failure of bone formation and posterolateral spinal fusion in rabbits. In contrast, Simmons et al.38 employed RGD-modified alginate to concurrently deliver two growth factors and bone marrow stem cells for ectopic bone formation. More recently, Kolambkar et al.39 employed a hybrid mixture containing gamma-irradiated, RGD-modified alginate; lyophilized rhBMP-2; and calcium sulphate slurry loaded onto nanofiber mesh tubes to successfully repair critically sized segmental defects in rats. These previous reports coupled with findings from the present study indicate that nonspecific protein bindings to unmodified alginate matrix might be either insufficient or too weak to immobilize and deliver rhBMP-2 for bone regeneration applications.
The “gold standard” clinical procedure in posterolateral spinal fusion is the implantation of autologous bone tissue (autograft bone) in an instrumented, mechanically stabilized fusion bed.40,41 However, absorbable collagen sponges soaked in rhBMP-2 solution have been assembled with different structural devices such as titanium and polyetheretherketone implants and employed as substitutes or as adjuncts to autograft bone.42 Although the use of this bone graft substitute is considered “off-label” in posterolateral spinal fusion, high fusion rates are often reported.43 In small animal models, however, the harvesting of a sufficient amount of autograft bone to be used as an appropriate positive control is a technically difficult challenge because of the small size of the iliac bone in these animals.44 Similarly, scaling down the implant devices to fit into a rodent's posterolateral fusion gutter could represent a daunting task. Therefore, in this study, we opted to use a customizable mPCL-TCP scaffold, fabricated by fused deposition modeling. This scaffold was coated with absorbable collagen sponge and has been shown previously to deliver rhBMP-2 that induced excellent bone formation in various animal models.18,20,45,46
One limitation of the present study is that the dose effects of heparin and rhBMP-2 have not been evaluated. This was not the object of the present study. However, this question and other questions such as the influence of alginate degradation on this mode of delivery will form part of future studies.
In conclusion, surface functionalized alginate microbeads embedding heparin delivered bioactive rhBMP-2 that induced in vivo bone formation and posterolateral spinal fusion, which was superior to traditional microbead encapsulation. Enhanced containment of newly formed bone tissue was observed when compared with conventional collagen sponge delivery. This delivery platform may offer potential therapeutic versatility for multiple growth factor delivery in tissue-engineering applications.
Acknowledgments
The authors would like to appreciate the contributions of Ms Toh Soo Yein in the animal surgery and the entire team at SBIC-Nikon Imagine Center, Singapore, for their assistance with microscopic evaluations. Funding support was provided by a grant from the National Medical Research Council (NMRC) A*Star Singapore Grant #: NMRC/EDG/0022/2008.
Disclosure Statement
No competing financial interests exist.
Reference
- 1.Reddi A.H. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000;6:351. doi: 10.1089/107632700418074. [DOI] [PubMed] [Google Scholar]
- 2.Carragee E.J. Hurwitz E.L. Weiner B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Lee J.W. Lee S. Lee S.H. Yang H.S. Im G.I. Kim C.S., et al. Improved spinal fusion efficacy by long-term delivery of bone morphogenetic protein-2 in a rabbit model. Acta Orthop. 2011;82:756. doi: 10.3109/17453674.2011.636675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan S.A. Nelson M.S. Pan C. Gaffney P.M. Gupta P. Endogenous heparan sulfate and heparin modulate bone morphogenetic protein-4 signaling and activity. Am J Physiol Cell Physiol. 2008;294:1387C. doi: 10.1152/ajpcell.00346.2007. [DOI] [PubMed] [Google Scholar]
- 5.Dejima K. Kanai M.I. Akiyama T. Levings D.C. Nakato H. Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans. J Biol Chem. 2011;286:17103. doi: 10.1074/jbc.M110.208082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramono D.S. Murali S. Rai B. Ling L. Poh W.T. Lim Z.X., et al. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2) Bone. 2011;50:954. doi: 10.1016/j.bone.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon O. Krebs M. Alsberg E. Controlled and sustained gene delivery from injectable, porous PLGA scaffolds. J Biomed Mater Res A. 2011;98:72. doi: 10.1002/jbm.a.33098. [DOI] [PubMed] [Google Scholar]
- 8.Jeon O. Powell C. Ahmed S.M. Alsberg E. Biodegradable, photocrosslinked alginate hydrogels with independently tailorable physical properties and cell adhesivity. Tissue Eng Part A. 2010;16:2915. doi: 10.1089/ten.TEA.2010.0096. [DOI] [PubMed] [Google Scholar]
- 9.Yang H.S. La W.G. Bhang S.H. Jeon J.Y. Lee J.H. Kim B.S. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng Part A. 2010;16:1225. doi: 10.1089/ten.TEA.2009.0390. [DOI] [PubMed] [Google Scholar]
- 10.Zhao B. Katagiri T. Toyoda H. Takada T. Yanai T. Fukuda T., et al. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem. 2006;281:23246. doi: 10.1074/jbc.M511039200. [DOI] [PubMed] [Google Scholar]
- 11.Xu X. Jha A.K. Duncan R.L. Jia X. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomater. 2011;7:3050. doi: 10.1016/j.actbio.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu H. Johnson N.R. Mason N.S. Wang Y. A [polycation:heparin] complex releases growth factors with enhanced bioactivity. J Control Release. 2011;150:157. doi: 10.1016/j.jconrel.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Zern B.J. Chu H. Wang Y. Control growth factor release using a self-assembled [polycation:heparin] complex. PLoS One. 2010;5:e11017. doi: 10.1371/journal.pone.0011017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rokstad A.M. Brekke O.L. Steinkjer B. Ryan L. Kollarikova G. Strand B.L., et al. Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater. 2011;7:2566. doi: 10.1016/j.actbio.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 15.de Vos P. Faas M.M. Strand B. Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27:5603. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Strand B.L. Morch Y.A. Espevik T. Skjak-Braek G. Visualization of alginate-poly-L-lysine-alginate microcapsules by confocal laser scanning microscopy. Biotechnol Bioeng. 2003;82:386. doi: 10.1002/bit.10577. [DOI] [PubMed] [Google Scholar]
- 17.Varghese M.S. Hildebrandt D. Glasser D. Rubin D.M. Crowther N.J. The effect of poly-L-lysine/alginate bead membrane characteristics on the absorption of heparin. Artif Cells Blood Substit Immobil Biotechnol. 2009;37:13. doi: 10.1080/10731190802664171. [DOI] [PubMed] [Google Scholar]
- 18.Abbah S.A. Lam C.X. Hutmacher D.W. Goh J.C. Wong H.K. Biological performance of a polycaprolactone-based scaffold used as fusion cage device in a large animal model of spinal reconstructive surgery. Biomaterials. 2009;30:5086. doi: 10.1016/j.biomaterials.2009.05.067. [DOI] [PubMed] [Google Scholar]
- 19.Hoemann C.D. El-Gabalawy H. McKee M.D. In vitro osteogenesis assays: influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol Biol (Paris) 2009;57:318. doi: 10.1016/j.patbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Abbah S.A. Lam C.X. Ramruttun A.K. Goh J.C. Wong H.K. Fusion performance of low dose rhbmp-2 and bmscs in biodegradable scaffolds: a comparative study in a large animal model of anterior lumbar interbody fusion. Spine (Phila Pa 1976) 2011;36:1752. doi: 10.1097/BRS.0b013e31822576a4. [DOI] [PubMed] [Google Scholar]
- 21.Morisue H. Matsumoto M. Chiba K. Matsumoto H. Toyama Y. Aizawa M., et al. A novel hydroxyapatite fiber mesh as a carrier for recombinant human bone morphogenetic protein-2 enhances bone union in rat posterolateral fusion model. Spine (Phila Pa 1976) 2006;31:1194. doi: 10.1097/01.brs.0000217679.46489.1b. [DOI] [PubMed] [Google Scholar]
- 22.Ziran B.H. Pineda S. Pokharna H. Esteki A. Mansour J.M. Moskowitz R.W. Biomechanical, radiologic, and histopathologic correlations in the pathogenesis of experimental intervertebral disc disease. Spine (Phila Pa 1976) 1994;19:2159. doi: 10.1097/00007632-199410000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Arakawa T. Wen J. Philo J.S. Stoichiometry of heparin binding to basic fibroblast growth factor. Arch Biochem Biophys. 1994;308:267. doi: 10.1006/abbi.1994.1037. [DOI] [PubMed] [Google Scholar]
- 24.Fu T.S. Chen W.J. Chen L.H. Lin S.S. Liu S.J. Ueng S.W. Enhancement of posterolateral lumbar spine fusion using low-dose rhBMP-2 and cultured marrow stromal cells. J Orthop Res. 2009;27:380. doi: 10.1002/jor.20644. [DOI] [PubMed] [Google Scholar]
- 25.Rolny C. Spillmann D. Lindahl U. Claesson-Welsh L. Heparin amplifies platelet-derived growth factor (PDGF)- BB-induced PDGF alpha -receptor but not PDGF beta -receptor tyrosine phosphorylation in heparan sulfate-deficient cells. Effects on signal transduction and biological responses. J Biol Chem. 2002;277:19315. doi: 10.1074/jbc.M111805200. [DOI] [PubMed] [Google Scholar]
- 26.Damon D.H. Lobb R.R. D'Amore P.A. Wagner J.A. Heparin potentiates the action of acidic fibroblast growth factor by prolonging its biological half-life. J Cell Physiol. 1989;138:221. doi: 10.1002/jcp.1041380202. [DOI] [PubMed] [Google Scholar]
- 27.Zakrzewska M. Wiedlocha A. Szlachcic A. Krowarsch D. Otlewski J. Olsnes S. Increased protein stability of FGF1 can compensate for its reduced affinity for heparin. J Biol Chem. 2009;284:25388. doi: 10.1074/jbc.M109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausser H.J. Brenner R.E. Low doses and high doses of heparin have different effects on osteoblast-like Saos-2 cells in vitro. J Cell Biochem. 2004;91:1062. doi: 10.1002/jcb.20007. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.E. Song S.H. Yun Y.P. Choi B.J. Kwon I.K. Bae M.S., et al. The effect of immobilization of heparin and bone morphogenic protein-2 (BMP-2) to titanium surfaces on inflammation and osteoblast function. Biomaterials. 2011;32:366. doi: 10.1016/j.biomaterials.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Johnson M.R. Boerckel J.D. Dupont K.M. Guldberg R.E. Functional restoration of critically sized segmental defects with bone morphogenetic protein-2 and heparin treatment. Clin Orthop Relat Res. 2011;469:3111. doi: 10.1007/s11999-011-2012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin H. Zhao Y. Sun W. Chen B. Zhang J. Zhao W., et al. The effect of crosslinking heparin to demineralized bone matrix on mechanical strength and specific binding to human bone morphogenetic protein-2. Biomaterials. 2008;29:1189. doi: 10.1016/j.biomaterials.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Jeon O. Powell C. Solorio L.D. Krebs M.D. Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J Control Release. 2011;154:258. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klimo P., Jr. Peelle M.W. Use of polyetheretherketone spacer and recombinant human bone morphogenetic protein-2 in the cervical spine: a radiographic analysis. Spine J. 2009;9:959. doi: 10.1016/j.spinee.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Carragee E.J. Ghanayem A.J. Weiner B.K. Rothman D.J. Bono C.M. A challenge to integrity in spine publications: years of living dangerously with the promotion of bone growth factors. Spine J. 2011;11:463. doi: 10.1016/j.spinee.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Anderson C.L. Whitaker M.C. Heterotopic ossification associated with recombinant human bone morphogenetic protein-2 (infuse) in posterolateral lumbar spine fusion: a case report. Spine (Phila Pa 1976) 2012;37:E502. doi: 10.1097/BRS.0b013e318238870b. [DOI] [PubMed] [Google Scholar]
- 36.Salisbury E.A. Olmsted-Davis E.A. Davis A.R. Adverse events and bone morphogenetic protein-2. Spine J. 2011;11:802. doi: 10.1016/j.spinee.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Crouzier T. Szarpak A. Boudou T. Auzely-Velty R. Picart C. Polysaccharide-blend multilayers containing hyaluronan and heparin as a delivery system for rhBMP-2. Small. 2010;6:651. doi: 10.1002/smll.200901728. [DOI] [PubMed] [Google Scholar]
- 38.Simmons C.A. Alsberg E. Hsiong S. Kim W.J. Mooney D.J. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35:562. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 39.Kolambkar Y.M. Dupont K.M. Boerckel J.D. Huebsch N. Mooney D.J. Hutmacher D.W., et al. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32:65. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kho V.K. Chen W.C. Posterolateral fusion using laminectomy bone chips in the treatment of lumbar spondylolisthesis. Int Orthop. 2008;32:115. doi: 10.1007/s00264-006-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gazdag A.R. Lane J.M. Glaser D. Forster R.A. Alternatives to Autogenous Bone Graft: Efficacy and Indications. J Am Acad Orthop Surg. 1995;3:1. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Ponnappan R.K. Serhan H. Zarda B. Patel R. Albert T. Vaccaro A.R. Biomechanical evaluation and comparison of polyetheretherketone rod system to traditional titanium rod fixation. Spine J. 2009;9:263. doi: 10.1016/j.spinee.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Dawson E. Bae H.W. Burkus J.K. Stambough J.L. Glassman S.D. Recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge with an osteoconductive bulking agent in posterolateral arthrodesis with instrumentation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91:1604. doi: 10.2106/JBJS.G.01157. [DOI] [PubMed] [Google Scholar]
- 44.Rao R.D. Bagaria V.B. Cooley B.C. Posterolateral intertransverse lumbar fusion in a mouse model: surgical anatomy and operative technique. Spine J. 2007;7:61. doi: 10.1016/j.spinee.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Sawyer A.A. Song S.J. Susanto E. Chuan P. Lam C.X. Woodruff M.A., et al. The stimulation of healing within a rat calvarial defect by mPCL-TCP/collagen scaffolds loaded with rhBMP-2. Biomaterials. 2009;30:2479. doi: 10.1016/j.biomaterials.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 46.Abbah S.A. Lam C.X. Ramruttun K.A. Goh J.C. Wong H.K. Autogenous bone marrow stromal cell sheets-loaded mPCL/TCP scaffolds induced osteogenesis in a porcine model of spinal interbody fusion. Tissue Eng Part A. 2011;17:809. doi: 10.1089/ten.TEA.2010.0255. [DOI] [PubMed] [Google Scholar]