Abstract
In this work, we have developed chitosan hydrogel/nanofibrin composite bandages (CFBs) and characterized using Fourier transform-infrared spectroscopy and scanning electron microscopy. The homogeneous distribution of nanofibrin in the prepared chitosan hydrogel matrix was confirmed by phosphotungstic acid–hematoxylin staining. The mechanical strength, swelling, biodegradation, porosity, whole-blood clotting, and platelet activation studies were carried out. In addition, the cell viability, cell attachment, and infiltration of the prepared CFBs were evaluated using human umbilical vein endothelial cells (HUVECs) and human dermal fibroblast (HDF) cells. It was found that the CFBs were microporous, flexible, biodegradable, and showed enhanced blood clotting and platelet activity compared to the one without nanofibrin. The prepared CFBs were capable of absorbing fluid and this was confirmed when immersed in phosphate buffered saline. Cell viability studies on HUVECs and HDF cells proved the nontoxic nature of the CFBs. Cell attachment and infiltration studies showed that the cells were found attached and proliferated on the CFBs. In vivo experiments were carried out in Sprague-Dawley rats and found that the wound healing occurred within 2 weeks when treated with CFBs than compared to the bare wound and wound treated with Kaltostat. The deposition of collagen was found to be more on CFB-treated wounds compared to the control. The above results proved the use of these CFBs as an ideal candidate for skin tissue regeneration and wound healing.
Introduction
Skin, the largest organ of the integumentary system in the body, which primarily serves as a protective barrier and guards the underlying muscles, bones, ligaments, and internal organs from the external environment. Any type of injury in which the skin is torn, cut, or punctured can be called as a wound. Different types of wounds can be generally classified into (1) Wounds with tissue loss and (2) Wounds without tissue loss.1 Wounds with tissue loss may be chronic and extensive and these wounds are usually associated with a lack of adequate blood supply and sufficient oxygen for the normal wound-healing process. Hence, it takes several weeks or months to heal and proper care has to be given for the wound management. Wound healing is a complex biological process, which involves the restoration of cellular structures and tissue layers by the coordination of a variety of cellular activities, such as inflammation, migration of cells to the wound area, proliferation, angiogenesis, and remodeling of the extracellular matrix (ECM).2 As skin plays an important role in maintaining homeostasis and acts as a first line defense mechanism of the body, it needs to be covered with a dressing material immediately after the damage.3 With the introduction of advanced biomaterials that are biocompatible and biodegradable, many new varieties of wound dressing materials in the form of membranes, bandages, fibers, and sponges are made available currently in the market. However, all have their own advantages as well as disadvantages. An ideal dressing should be nontoxic, nonallergic, and nonadherent. It should maintain a moist environment at the wound interface, remove excess exudates from the wound surface, and allow proper gaseous exchange.3
Chitosan has been widely used as a wound dressing material due to its properties.4 The notable properties of chitosan include its nontoxicity, hemostatic action, anti-inflammatory effect, biodegradability, biocompatibility, antimicrobial activity, retention of fibroblast growth factors, release of glucosamine, N-acetyl glucosamine monomers, oligomers, and stimulation of human dermal fibroblast (HDF) cellular activities.1,3,5–7 It stimulates cell adhesion and proliferation and helps in the organization of the extracellular matrix.1,6
It has been reported that the chitosan mesh membrane possesses potential wound-healing effects by enhancing the re-epithelialization and granular layer.8 Another study reported the use of composite hydrogel sheets made from chitosan, honey, and gelatin for burn wound healing.9 A study on chitosan film enriched with an antioxidant agent reported that chitosan can stimulate fibroblasts and promote collagen deposition.10
Fibrin is an insoluble protein involved in blood clotting. When an injury occurs, fibrin is deposited around the wound in the form of a mesh, which dries and hardens, so that bleeding stops. This is an enzyme-mediated process in which the soluble fibrinogen is converted into insoluble fibrin in the presence of thrombin.11–13 Thrombin mediates the cleavage of fibrinopeptide A from the Aα chains and fibrinopeptide B from the Bβ chains14 of fibrinogen and exposes the polymerization sites.11 The subsequent conformational changes result in the formation of fibrin monomers, which can self associate to form insoluble fibrin.11 Furthermore, the blood coagulation factor, XIIIa, also plays an important role in the fibrin clot formation.15,16 Nanoparticles have drawn increasing interest in the biomedical and tissue engineering field. When fibrin is used in its nanoform, because of its high surface to volume ratio, more cells get attached on to the bandages and thereby enhance the cell migration and proliferation, which can promote the wound-healing process. Nanofibrin can increase the clotting efficiency and accelerate the healing process. There are many studies that support the use of fibrin as a good delivery vehicle for cells, such as fibroblast, keratinocytes, drugs, growth factors, and genes.17–19 Fibrin can act as a reservoir for growth factors, cells, and enzymes and also provide an excellent substrate for the cells to attach and proliferate during wound healing.19 Also, it can behave as a scaffold for the synthesis of ECM and thereby tissue regeneration. It has been also reported that fibrin can decrease the length of the lag phase of keratinocyte activation and increase the consistency of the healing response.20 So far, no work has been reported regarding the use of nanofibrin in the wound-dressing applications.
Usually when the skin is injured, blood loss occurs from the damaged blood vessels and immediately after the injury the blood vessels in that area constrict and prevent the excessive blood loss. Platelets are activated by thrombin and release a number of clotting factors into the blood plasma, which in turn, begin the coagulation cascade. The next step is the actual formation of a clot, which is composed of fibrin, platelets, and red blood cells.21 However, individuals who suffer from bleeding disorders usually diagnosed with hemophilia, experience excessive bleeding when their skin is cut or injured. In such patients, chitosan hydrogel/nanofibrin composite bandages (CFBs) can be effectively used as a supplement of fibrin, which can promote the clot formation. Though there are many works based on chitosan as a wound-dressing material, nanofibrin-incorporated chitosan bandage is a novel dressing material, which can be used as a good candidate for wound-dressing applications. In this study, the feasibility of using fibrin nanoparticles incorporated chitosan hydrogel as a bioactive wound-dressing material for healing of wounds and regeneration of skin was investigated in detail.
Materials and Methods
Materials
Chitosan (MW; 150 kDa, degree of deacetylation—85%) was purchased from Koyo Chemical Ltd. Acetic acid and sodium hydroxide were purchased from Qualigens. Hen lysozyme was purchased from Fluka. 4′,6-Diamidino-2-phenylindole (DAPI), Alamar Blue, trypsin-ethylenediaminetetraacetic acid, fetal bovine serum (FBS), and tetramethyl rhodamine iso-thiocyanate (TRITC) were obtained from Gibco, Invitrogen Corporation. A minimum essential medium and thrombin were purchased from Sigma-Aldrich Company. Fibrinogen was purchased from Himedia. The chemicals were used without further purification.
Methods
Synthesis of fibrin nanoparticles
The fibrin nanoparticles were synthesized by a surfactant-free water-in-oil emulsification–diffusion method as reported from our laboratory.22 In brief, the aqueous suspensions of both fibrinogen-FXIIIa cryoprecipitate and thrombin were simultaneously administrated into a preheated vegetable oil system. This was kept under constant magnetic stirring at 400 rpm and maintained at a temperature of 70–80°C for the aqueous suspensions to crosslink and emulsify in the oil phase. The stirring was continued for 6–8 h, for the crosslinking of the fibrin clot and its uniform dispersion in oil to occur. The nanoparticles thus formed in the emulsion were then centrifuged at 10,000 rpm for 15 min. This resulted in the formation of fibrin nanoconstructs at the oil–water interface, which was collected from the emulsion. Later on, the fibrin nanoparticles were purified by lyophilization and the obtained powder form was used for further studies.
Fabrication of CFBs
The chitosan hydrogel was prepared according to the reported method by our group.23 The chitosan solution was prepared by dissolving 2 g of chitosan powder in 1% of acetic acid followed by vigorous stirring until it dissolves at room temperature. The solution was neutralized to 7 by adding 1% of NaOH solution dropwise under vigorous stirring. The obtained chitosan hydrogel was then centrifuged at 8000 rpm for 10 min and the supernatant was discarded. The prepared nanofibrin was suspended in distilled water and probe sonication was carried out for 20 min. Nanofibrin suspensions were mixed with chitosan hydrogel under vigorous stirring for 1 h to get 1% and 2% of nanofibrin-incorporated CFBs. Stirring enables the homogeneous distribution of nanofibrin on chitosan hydrogel. The homogenized chitosan hydrogel/nanofibrin mixture was poured on to a Teflon mould and kept at −20°C overnight. The frozen samples were then lyophilized for 24 h (Martin Christ) to obtain porous CFBs.
Characterization of fibrin nanoparticles
The structural morphology of the prepared fibrin nanoparticles were analyzed by scanning electron microscopy (SEM; JEOL-JSM-6490LA). The freeze-dried fibrin nanoparticles were properly diluted in Milli Q water and were dropped on an aluminum stub and sputter coated with gold using an automatic fine gold coater (JEOL JFC-1600) at 10 mA for 120 s before observing under SEM.
Mallory's phosphotungstic acid hematoxylin (PTAH) staining technique was adopted to specifically stain the core fibrin nanoparticles as reported recently from our laboratory.22 In brief, the freeze-dried fibrin nanoparticles were dehydrated in 80% ethanol and prestained with eosin for 5 min. After that, the particles were washed with double distilled water and stained with PTAH. After 30 min of incubation at 60°C, the particles were viewed under the bright field mode of a fluorescent microscope (Olympus-BX-51). The other physicochemical characterization of the fibrin nanoparticle was also studied recently from our laboratory and has been published.22
Characterization of CFBs
The structural morphology of the developed CFBs was examined using SEM. The lyophilized samples were cut into thin pieces, fixed on aluminium stubs, and sputter coated with gold for the observation.
The characteristic absorption peaks of chitosan, nanofibrin, and CFBs were found out using Fourier transform-infrared spectroscopy (FT-IR; Perkin-Elmer Co.; Model SPECTRUM RXI, FT-IR). For FT-IR analysis, 2 mg of the freeze-dried chitosan, nanofibrin, and CFBs were pelletized with 175 mg of KBr. The spectrum was recorded in the frequency range of 650–2100 cm−1.
PTAH staining was adopted to stain specifically the fibrin nanoparticles, which were incorporated in the CFBs.22 In brief, the lyophilized CFBs were dehydrated in 80% ethanol and prestained with eosin for 5 min. After washing with distilled water, PTAH staining was done and incubated at 60°C for 30 min. The materials were dehydrated in 80% and 100% ethanol, respectively. The bandages were then blotted dry with filter paper and viewed under the bright field mode of a fluorescent microscope (Olympus-BX-51).
Porosity evaluation
For determining the porosity of the CFBs, cylindrical-shaped bandages were prepared. Chitosan bandages were used as the control and all the samples were triplicated in the experiment. The height and diameter of the cylindrical bandages were found out using a vernier calliper to calculate the volume. Then, the bandages were immersed in absolute ethanol until it is saturated. The weights of the bandages before and after immersion in alcohol were noted. The porosity was calculated using the following formula,23
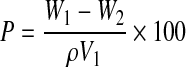 |
In the equation, W1 and W2 indicate the weight of CFBs before and after immersion in alcohol, respectively. V1 is the volume before immersion in alcohol; ρ is a constant (density of alcohol).
Swelling studies
The swelling study was carried out in phosphate buffered saline (PBS) containing 3.97 g of NaCl, 0.096 g of KCl, 0.88 g of Na2HPO4, and 0.119 g of KH2PO4 in 500 mL of deionized water. The pH of the PBS solution was maintained at 7.4. Bare chitosan and commercially available wound dressing material “Kaltostat™” were used as the control along with CFBs. The lyophilized samples were cut into small pieces and for each samples triplicates were taken. The samples were cut so as to fit into a 50-mL falcon tube containing 5 mL of the PBS solution. The samples were immersed into the PBS solution in the falcon tube. After that, all the falcon tubes were placed at 37°C for incubation. At definite time intervals, the samples were taken out of the falcon tube, gently blotted with filter paper, and the wet weight was noticed. The swelling studies were done till the day 12. The buffer uptake was evaluated by the following formula,24
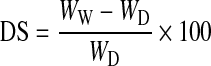 |
where DS is the degree of swelling, WW and WD represents the wet weight and dry weight of the bandages, respectively.
Biodegradation studies
A small piece of each lyophilized samples having a dimension of 0.5×0.5 cm were taken and the dry weight was found out. For each sample, triplicates were taken and the enzyme used for the in vitro biodegradation study was lysozyme (hen-egg lyophilized powder from fluka) of concentration 84,612 U/mg. The required concentration of lysozyme was 104 U/mg and it was mixed with the PBS. Bare chitosan and Kaltostat were used as the control along with CFBs. Each falcon tube was filled with 5 mL of the PBS-lysozyme solution and the samples were put into the tubes correspondingly. The tubes were placed at 37°C for incubation. At definite time intervals, the samples were taken out and washed with PBS. The study was carried up to 2 weeks. Again these samples were freeze-dried and the dry weight was noted. The degradation was calculated by the formula,25
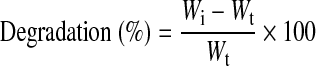 |
where Wi is the initial weight and Wt is the dry weight of the bandage after lyophilization.
Mechanical properties
Mechanical properties were evaluated by measuring the tensile strength and elongation at break of the CFBs. Kaltostat was used as the positive control along with chitosan. The bandage specimens with dimensions of 10×2×0.4 cm were prepared.23 The tensile strength and percentage of elongation at break of the bandages were measured by a universal testing machine (Instron; Model 3365) with a load cell of 5 kN and the crosshead speed was 25-mm min−1 at room temperature. Measurements were run six times for each bandages and the average value was taken.
Whole-blood clotting
A blood clotting study was done as follows:23 blood was drawn from the human ulnar vein and anticoagulated with acid citrate dextrose (ACD) (20 mM citric acid, 110 mM sodium citrate, 5 mM dextrose) at a v/v ratio of 8:2. All the samples were cut into small pieces weighing 10 mg and the citrated whole blood was dispensed on to the dressings, so as the blood covers the entire area of the dressings. Bare chitosan and Kaltostat were used as the control. All samples were triplicated. A 10 μL of 0.2 M CaCl2 solution was added to initiate the clotting process, followed by incubation at 37°C for 15 min; the red blood cells that were not trapped on the bandages were hemolyzed with 2 mL of water. The blood clotting ability was analyzed by measuring the absorbance of the supernatant at 540 nm using a plate reader (BioTek PowerWave XS).
Platelet activation studies
A platelet activation study was conducted by isolating platelet-rich plasma (PRP) from the blood.26 Centrifugation of blood at 2500 rpm for 5 min resulted in the formation of PRP. One hundred microliters of PRP was added on to the bandage pieces weighing 10 mg and incubated at 37°C for 20 min. Here also bare chitosan and “Kaltostat” were used as the control along with CFBs. The bandages were then washed with PBS solution and fixed using 0.1% glutaraldehyde solution. The bandages were dried, fixed on aluminium stubs, and sputter coated for the SEM images to be taken.
Isolation and maintenance of human umbilical vein endothelial cells
As per the established protocol, human umbilical vein endothelial cells (HUVECs) were isolated from the umbilical cord.27 From the ladies who underwent normal delivery at the Gynaecology Department of Amrita Institute of Medical Sciences and Research Centre (AIMS), the umbilical cord was collected with their prior written consent and also with the ethics clearance of the institutional body of AIMS. The detached cells were washed in the serum-free Iscove's modified Dulbecco's medium (IMDM) and resuspended in the complete IMDM (containing 20% fetal calf serum [Gibco, Invitrogen], 100 U mL−1 pen–strep antibiotic solutions [Gibco, Invitrogen], and 150 μg mL−1 endothelial cell growth factor [ECGF]). The HUVECs were grown on 2% gelatin (Sigma Aldrich)-coated tissue culture plates in the complete IMDM in a humidified atmosphere of 5% CO2 at 37°C.27
Cell viability using Alamar blue assay
The cell viability of the prepared CFBs on HUVECs and HDF cells were evaluated by Alamar Blue assay.22,23 The bandages were cut into small pieces and were sterilized by ethylene oxide gas. HDF cells were cultured in a fibroblast growth medium provided by Promocell. The sterilized bandage pieces were placed in 24-well plates and 5×104 cells of each HDF and HUVEC were seeded separately on the bandages. Alamar Blue Assay was performed by adding Alamar blue into the plates containing the bandage materials along with the cells. This was followed by incubation up to 48 h. The optical density (OD) measurement at 570 nm, with 620 nm set as the reference wavelength, using a microplate spectrophotometer (Biotek PowerWave XS,) was taken.
Cell adhesion and proliferation studies
SEM was used to observe the cell morphologies within the bandages.23 Both HUVECs and HDF cells were seeded separately on the CFBs in a 24-well plate at a concentration of 1×105 cells/well. After 24 and 48 h of incubation, the bandages were rinsed by PBS and fixed with 2.5% gluteraldehyde for 1 h. The samples were thoroughly washed with PBS and dehydrated through a series of graded-ethanol solutions and air-dried. After gold sputtering in vacuum, the samples were examined by SEM.
DAPI is a fluorescent stain, which can particularly stain the nucleus of cells.23 For DAPI staining, the HUVECs were seeded on both the concentrations of CFBs and the bandages were fixed with 4% paraformaldehyde for 20 min. Later, the permeabilization step was carried out by adding 0.5% Triton X-100 (in PBS) for 5 min.24 The bandages were then treated with 1% FBS and washed with PBS. The cell-seeded bandages were stained with 50 μL of DAPI (1:30 dilution with PBS). The bandages were then incubated in darkness for 5 min and viewed under a fluorescent microscope (Olympus-BX-51).
Cell infiltration studies
For cell infiltration evaluation, the bandages were incubated with HUVECs for 24 h. After decanting the medium, permeabilization and blocking steps were carried out as mentioned above. Then, the bandages were stained with TRITC-conjugated Phalloidin dye and the images were taken using a laser confocal microscope (Leica; Model SP 5 II).
Creation of skin wounds and treatment with CFBs
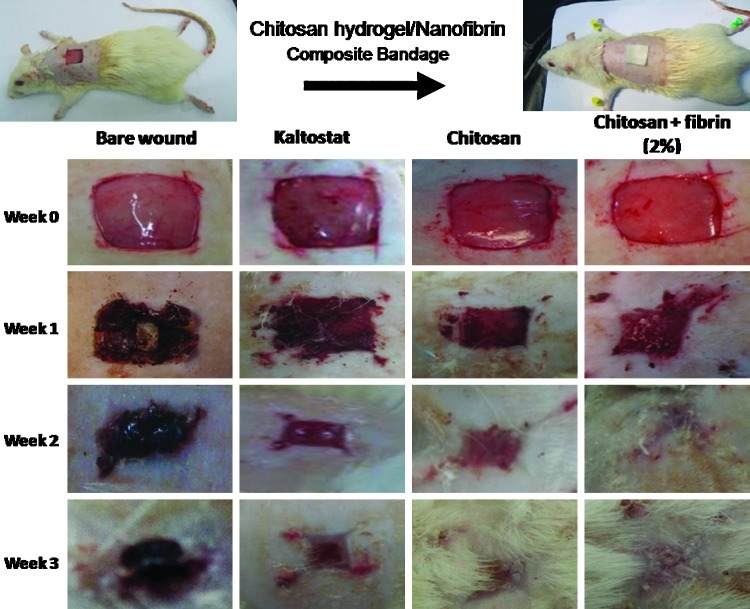
An in vivo animal study was approved by the Institutional Animal Ethics Committee, at AIMS. Briefly, male Sprague-Dawley (SD) rats, weighing 200–250 g and 4–6 weeks of age, were given a single intramuscular injection of 35.0 mg/kg ketamine and 5.0 mg/kg xylazine and were anesthetized. Totally, four groups of rats were taken and each group contained six rats (n=6). The groups were chitosan control, Kaltostat, CFB-treated wounds, and bare wounds. The dorsal area of the rats was totally depilated and the skin area was wiped with alcohol. A partial thickness skin wound (5 mm) was created by excising the dorsum of the rat using a pair of sharp surgical scissors and forceps. Chitosan, Kaltostat, and CFBs were sterilized by ethylene oxide gas before the treatment. The prepared wounds were then covered with the CFB, chitosan bandage, and Kaltostat. Rats with bare wound were kept as negative control. After applying the dressing materials, the rats were housed individually in cages.
Wound closure evaluation
The dressing materials were changed at weeks 1, 2, and 3 and the photographs were taken, while changing the dressings. The wound closure evaluation was done by measuring the wound area.23 A transparent polyethylene sheet was placed on top of the wound and the wound area was drawn using a marker pen. The marked area was then transferred to a graph sheet and areas were calculated. The wound closure area was calculated by the formula
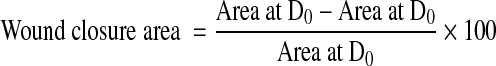 |
where D0 is the day 0 and Dt is the day 7/14/21.
Hematoxylin–eosin staining and quantification of re-epithelialization
After first week and third week, the healed portions of the skin tissue were excised and were fixed in 10% formalin. The tissue processing was carried out by placing the tissues in 70% alcohol (overnight). This was followed by two changes of xylene (10 and 15 min, respectively), two changes of paraffin wax (60 min each), and three changes of acetone (20 min each). After that, the processed tissues were embedded in paraffin wax and tissue sections were taken with a thickness of nearly 5 μm. After that, they were fixed on glass slides and stained with the hematoxylin–eosin (H&E) reagent for histological observations. The re-epithelialization was quantified using Image-Pro software. The total length of the wound and the re-epithelialized wound length was measured and the percentage of re-epithelialization was calculated by the formula,28
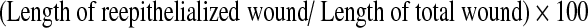 |
Picrosirius red staining for collagen quantification
Picrosirius red staining was carried out to determine the collagen deposition and the collagen content was quantified by histomorphometry. The excised skin tissue was fixed with 10% formalin and processed in alcohol and sections were taken. Thereafter, sections were stained with Picrosirius red for collagen detection. The collagen deposition was quantified by histomorphometry using the formula
 |
Statistical analysis
Each experiment was done in triplicate. The statistical significance was determined by the Student's two-tailed t-test and a p-value of <0.05 was considered to be statistically significant. Data are expressed as the mean±standard deviation.
Results
Characterization of fibrin nanoparticles
Morphological analysis was performed through SEM and is shown in Figure 1A. The SEM images showed that the obtained nanofibrin particles were spherical and monodispersed in nature having the size range of 240±5 nm.22
FIG. 1.
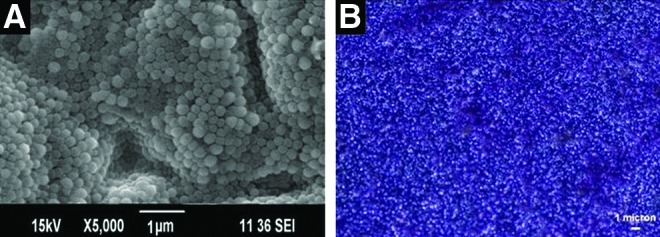
Characterization of nanofibrin: (A) SEM image nanofibrin. (B) PTAH staining of fibrin nanoparticles. SEM, scanning electron microscopy; PTAH, phosphotungstic acid hematoxylin. Color images available online at www.liebertpub.com/tea
For visualizing the physiologically unaltered fibrin component, histochemical staining using PTAH was employed22 and the results are shown in Figure 1B. PTAH can specifically stain the fibrin particles as blue in color is evident from Figure 1B.
Synthesis and characterization of CFBs
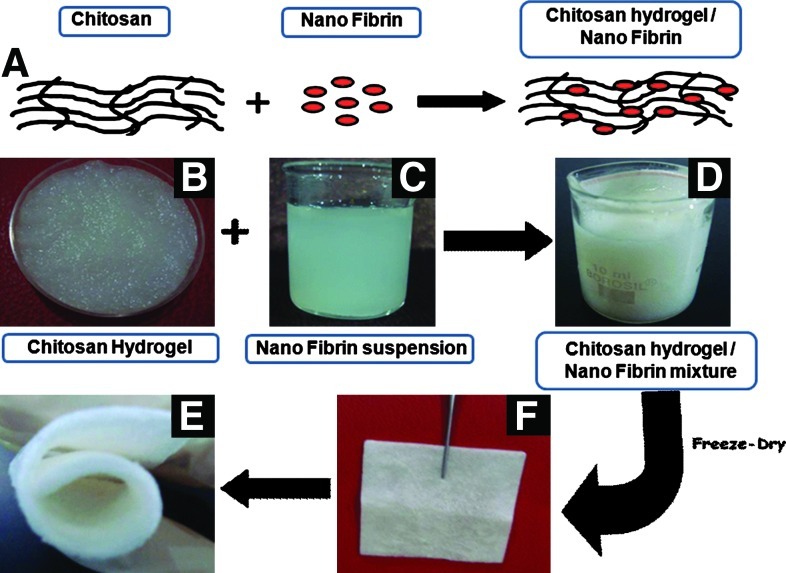
The schematic representation of the synthesis of CFBs is shown in Figure 2A. The photographs of the chitosan hydrogel, nanofibrin suspension, and chitosan hydrogel/nanofibrin mixture are shown in Figure 2B–D. Photographs of CFBs are represented in Figure 2E and F. From Figure 2F, the flexibility of the CFB is clearly evident.
FIG. 2.
(A) Schematic representation of the CFBs preparation. (B–D) Photographs of the chitosan hydrogel, nanofibrin suspension and chitosan hydrogel/nanofibrin mixture, respectively. (E, F) Photographs of CFBs. CFBs, chitosan, hydrogel/nano fibrin composite bandages. Color images available online at www.liebertpub.com/tea
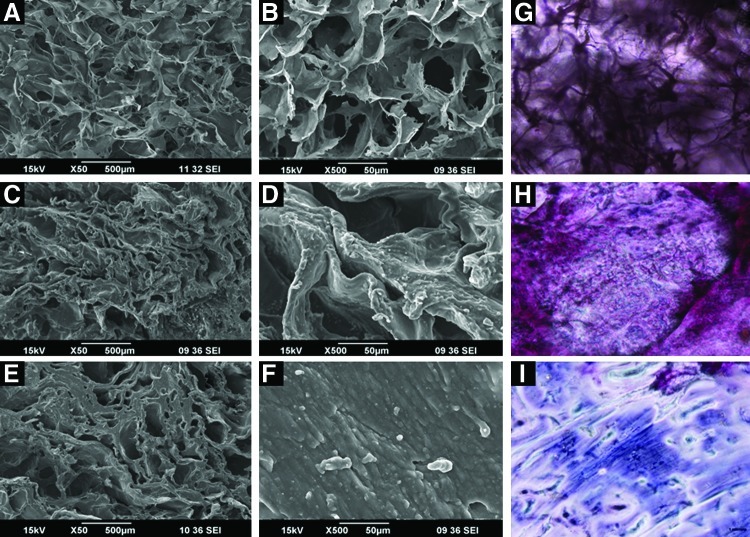
SEM images of the chitosan control and CFBs with 1% and 2% concentrations of nanofibrin are shown in Figure 3A–F. In SEM images, all these bandages showed a well-interconnected porous structure and the pore size was in the range of 200–300 μm. It was found that the structural morphology of the bandages remain unchanged even after the incorporation of nanofibrin.
FIG. 3.
SEM images of CFBs: (A, C, E) are the lower magnification images of chitosan control, chitosan+1% fibrin, and chitosan+2% fibrin. (B, D, F) are the higher magnification images of chitosan control, chitosan+1% fibrin, and chitosan+2% fibrin. (G) Shows the PTAH stained image of chitosan control. (H) Shows the image of chitosan nanofibrin-stained bandages. (I) Shows the magnified image of chitosan nanofibrin-stained bandages. Color images available online at www.liebertpub.com/tea
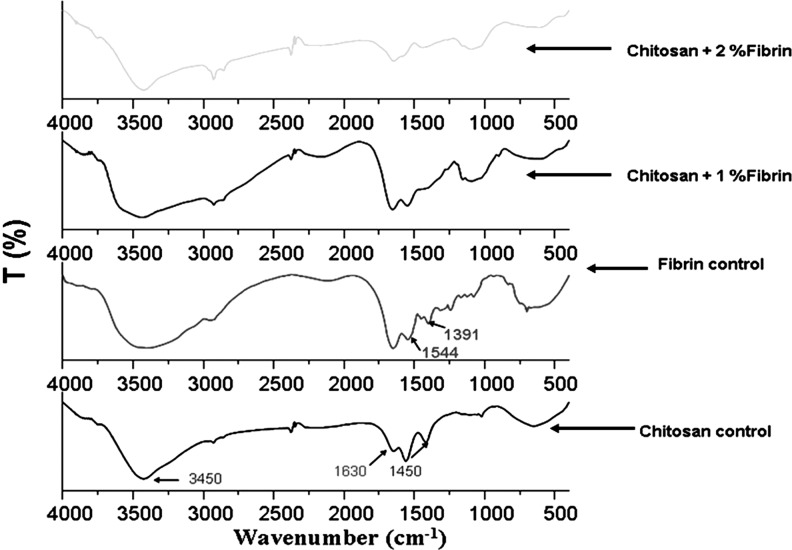
From the FT-IR analysis, the characteristic peaks of chitosan were obtained at 3450, 1450, and 1630 cm−1. The peak at 3450 cm−1 represents the OH group and the peaks at 1450 and 1630 cm−1 represents the amine groups.23 The characteristic peaks of fibrin were obtained at 1391 and 1544 cm−1. The peak at 1391 cm−1 shows the CH2 deformation vibration of the amino acid and the peak at 1544 cm−1 denotes the amide II group.22 In the FT-IR spectrum of CFBs, the characteristic peaks of both chitosan and nanofibrin were present and are shown in Figure 4.
FIG. 4.
Fourier transform-infrared spectroscopy spectrum analysis of chitosan, nanofibrin, and fibrin incorporated chitosan bandages.
The PTAH staining of the CFBs showed the homogeneous distribution of nanofibrin over the bandages. PTAH can specifically stain the fibrin particles in the CFBs as blue in color22 and is evident from Figure 3H.
Porosity studies
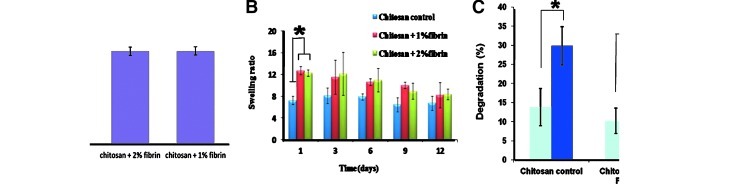
Porosity of CFBs were evaluated by the alcohol displacement method.23 All bandages showed porosity in the range of 40–50% and is shown in Figure 5A; it was found that after the incorporation of nanofibrin, there was no significant change in the porosity compared to the control.
FIG. 5.
(A) Porosity studies of CFBs. (B) Swelling ratio analysis of CFBs. (C) In vitro biodegradation of CFBs (*p<0.05). Color images available online at www.liebertpub.com/tea
Swelling studies
The swelling study was carried up to the day 12. Swelling ratio analysis revealed that at day 1, 1% and 2% fibrin incorporated CFBs showed swelling ratios in the range of 10–15% (Fig. 5B). At the same time, the swelling ratios exhibited by the chitosan bandages were nearly 8%. When compared to the chitosan control, the 1% and 2% fibrin-incorporated CFBs showed a significant difference in the swelling ratio at day 1. Thereafter, the swelling ratio was retained and there was no significant difference in the swelling ability shown by the fibrin-contained bandages and chitosan bandages. At day 9 and 12, there was some difference in the swelling ratio exhibited by all the bandages, but the difference was not significant.
Degradation studies
In the in vitro biodegradation studies, after 1 week, all the bandages, including the control, showed 10–20% degradation. After 2 weeks, the degradation was about 25–35%. While comparing the degradation percentage of week 1 and 2, all the bandages showed a significant difference in the in vitro biodegradation study. This revealed the controlled biodegradation behavior of CFBs (Fig. 5C).
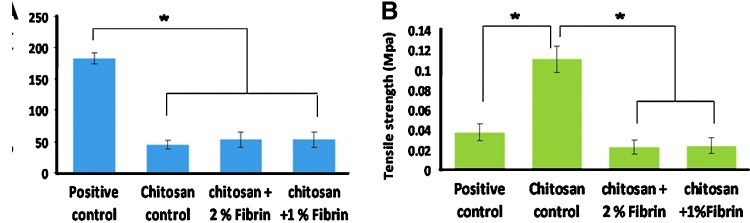
Mechanical properties
Adequate mechanical strength is required for the bandages in wound-dressing applications. The elongation at break and the tensile strength of the prepared bandages were shown in the Figure 6A and B. The elongation at break values indicates the flexibility of the material. Control and CFBs showed elongation in the range of 40–60% at break points (Fig. 6A), less compared with Kaltostat, which showed nearly 200% of elongation at break. This may be because of the nonwoven nature of the material. However, the obtained data were adequate to show the flexible nature of the CFBs.
FIG. 6.
Mechanical properties evaluation: (A) Elongation at break and (B) tensile strength of CFBs (*p<0.05). Color images available online at www.liebertpub.com/tea
The tensile strength of the chitosan control and CFBs were measured, and the chitosan control showed a tensile strength of 0.1 MPa, which was adequate for a wound-dressing material (Fig. 6B). The incorporation of nanofibrin decreased the tensile strength to 0.02–0.04 MPa, than the chitosan control. Both Kaltostat and CFBs exhibited a significant difference in the strength than compared to the chitosan. This may be due to the interaction between nanofibrin and chitosan. The obtained results showed that the CFBs are flexible and had sufficient strength to withstand the force applied on them.
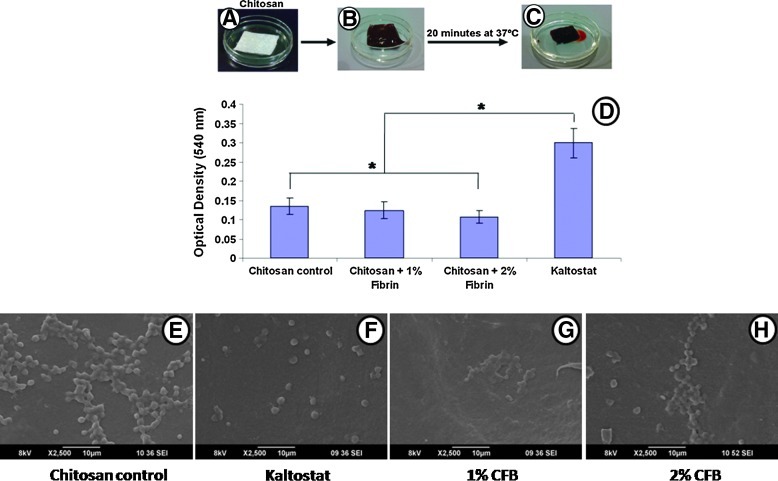
Whole-blood clotting
The hemostatic potential of CFBs was assessed and the Figure 7A–C show representative photographs of the blood clotting caused by the CFBs. CFBs showed the enhanced blood clotting ability in comparison with Kaltostat and Chitosan control (Fig. 7D). When compared to chitosan, the 1% fibrin-incorporated CFB did not show any significant difference in blood clotting, while the 2% fibrin-incorporated CFBs showed significant difference. When compared to Kaltostat, all the bandages possessed a significant difference in blood clotting. The lower OD value indicates the high blood clotting.
FIG. 7.
Blood clotting analysis: (A–C) Photographs of the chitosan bandage, blood on the CFB, and clotted blood on the CFB, respectively. (D) Whole-blood clotting evaluation of bandages. (E–H) SEM images of platelet activation of bandages. (*p<0.05). Color images available online at www.liebertpub.com/tea
Platelet activation
The platelet activation analysis was done by SEM to confirm the blood clotting. Kaltostat, which is the negative control, did not activate the platelets (Fig. 7F), while the chitosan control and CFBs promoted platelet activation. The SEM images show that the activated platelets have some deformations in the shapes and spread all over the bandage surface (Fig. 7E, G, H).
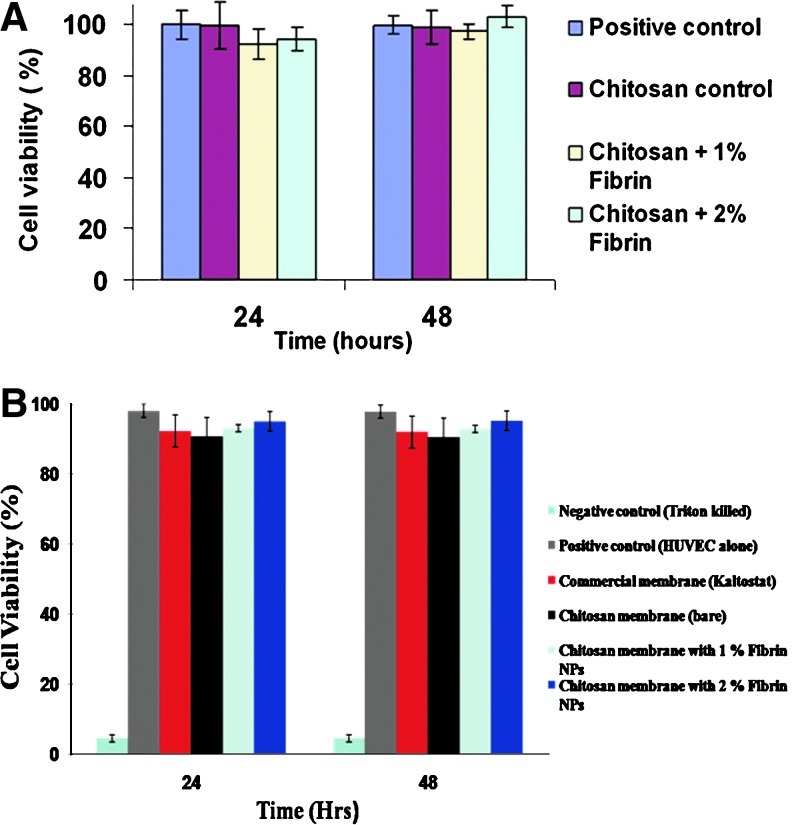
Cell viability using Alamar blue assay
Cell viability data (Fig. 8) showed that both positive control and chitosan control did not show any toxicity at 24 and 48 h in contact with the HDF cells and HUVECs. CFBs having 1% and 2% nanofibrin showed 80–90% viability after 24 h of incubation. Cell viability of CFBs was increased up to 100% after 48 h of incubation. After 24 h, the remaining viable cells began to multiply and hence, the viability increased.
FIG. 8.
Cell viability studies: (A) Cell viability study using HDF cells. (B) Cell viability study using HUVECs. HDF, human dermal fibroblast; HUVECS, human umbilical vein endothelial cells. Color images available online at www.liebertpub.com/tea
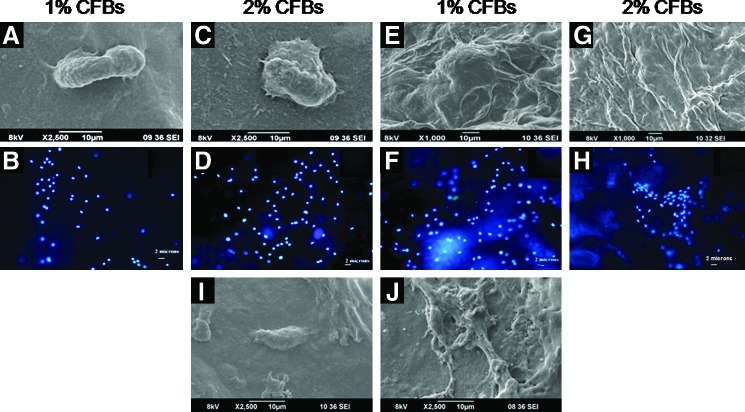
Cell adhesion and proliferation studies
The cytocompatible nature of the CFBs was determined by performing cell attachment studies via SEM. The SEM images revealed that HDF cells and HUVECs were attached onto the CFBs as well as chitosan control bandages and began to spread on the bandages after 24 h of incubation. After 48 h of incubation, the attached cells started proliferating and further confirmation was done by the DAPI staining. The images showed that more cells were attached on CFBs containing 2% of nanofibrin (Fig. 9).
FIG. 9.
Cell attachment and proliferation: (A, C) SEM images of HUVECs attachment on bandages after 24 h. (E, G) SEM images of HUVECs attachment on bandages after 48 h. (B, D) DAPI staining of HUVECs attached on bandages after 24 h. (F, H) DAPI staining of HUVECs attached on bandages after 48 h. (I, J) SEM images of HDF attachment on 2% CF bandages after 24 and 48 h, respectively. DAPI, 4′,6-diamidino-2-phenylindole. Color images available online at www.liebertpub.com/tea
Cell infiltration studies
The cell infiltration images revealed that the HUVECs penetrated into the interior of the CFBs up to 200 μm after 24 h of incubation (Fig. 10B). The red color was due to the actin filaments stained with the Phalloidin dye. The cell penetration was because of the porous nature of the control and CFBs. The cells that filtered into the interior of the bandages would be helpful for the vascularization, which is very essential in the wound-healing process.
FIG. 10.
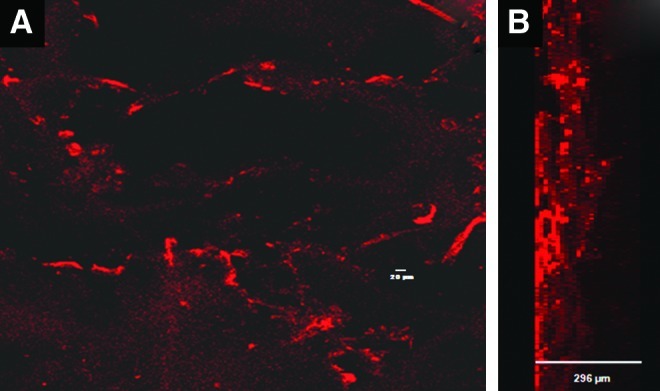
Laser confocal images of CFBs with HUVECs after 24 h of incubation (A) surface view of CFBs and (B) lateral view of CFBs. Color images available online at www.liebertpub.com/tea
In vivo evaluation
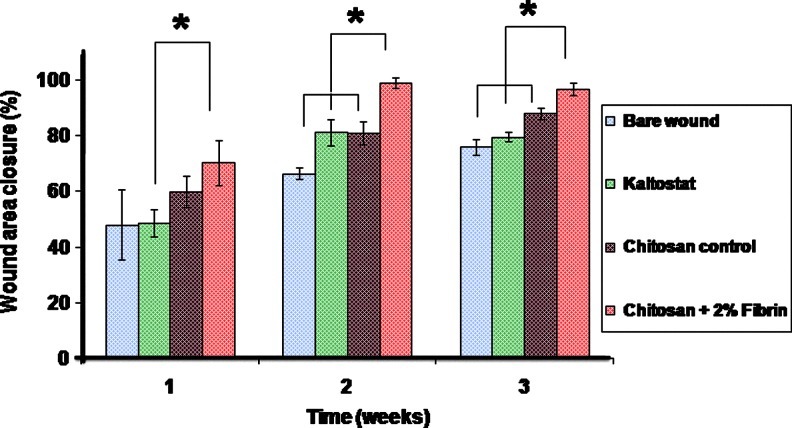
The in vivo study conducted in SD rats proved the enhanced wound-healing ability of the prepared CFBs. Figure 11 shows photographs of the in vivo wound-healing study. The extent of wound closure was evaluated macroscopically. After week 1 itself, both chitosan control and CFBs showed a significant difference in the wound closure when compared to Kaltostat. After 2 weeks, the wounds treated with CFBs achieved a significant closure to nearly 98% than compared to the chitosan control, Kaltostat-treated wound, and bare wounds, which showed nearly 70% of wound closure (Fig. 12). After 3 weeks, chitosan control showed 90% and CFB-treated wounds showed 100% closure than compared to control and Kaltostat.
FIG. 11.
Photographs of the in vivo wound-healing study in Sprague-Dawley rats using CFBs. Color images available online at www.liebertpub.com/tea
FIG. 12.
Wound closure area evaluation (*p<0.05). Color images available online at www.liebertpub.com/tea
H&E staining and quantification of re-epithelialization
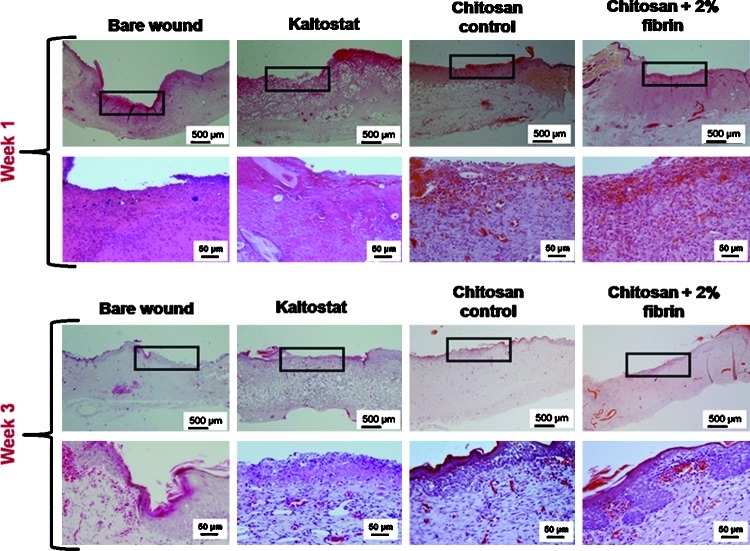
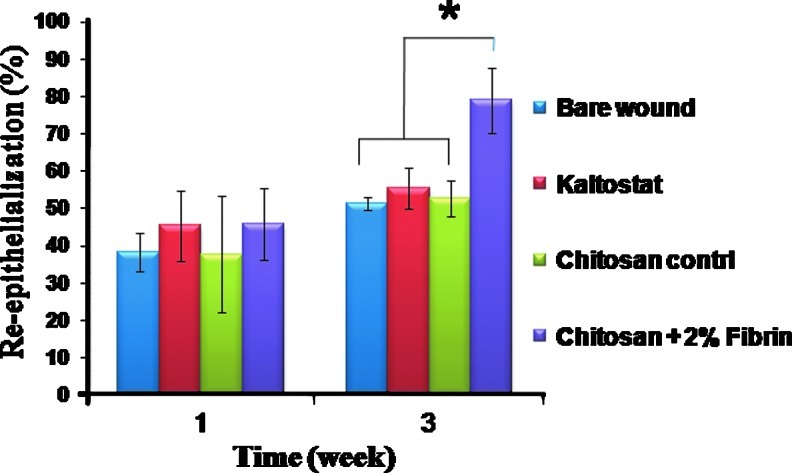
H&E staining of the tissue sections showed enhanced granulation tissue formation and densely packed keratinocytes in the epidermis when treated with chitosan control and CFBs, compared to the Kaltostat and bare wound (Fig. 13). Hematoxylin will stain nucleus in purple color and eosin will stain cytoplasm in pink color. The blood vessels were clearly visible and the epidermis was intact in the wounds treated with CFBs after week 3. In the quantification of re-epithelialization, after week 1, there was no significant difference among the bandages. However, after week 3, the CFB-treated wounds showed 90% re-epithelialization, which was significantly higher than the other groups. (Fig. 14)
FIG. 13.
Photomicrographs of hematoxylin–eosin stained histology sections of re-epithelialized tissue. The section panels representing the histological sections of re-epithelialized tissue of week-1 and week-3, respectively. The area indicated in the box is the region of interest of the histological sections. The magnified images of those regions are provided in the corresponding lower panel. Color images available online at www.liebertpub.com/tea
FIG. 14.
Quantification of re-epithelialization (*p<0.05). Color images available online at www.liebertpub.com/tea
Picrosirius red staining for collagen quantification
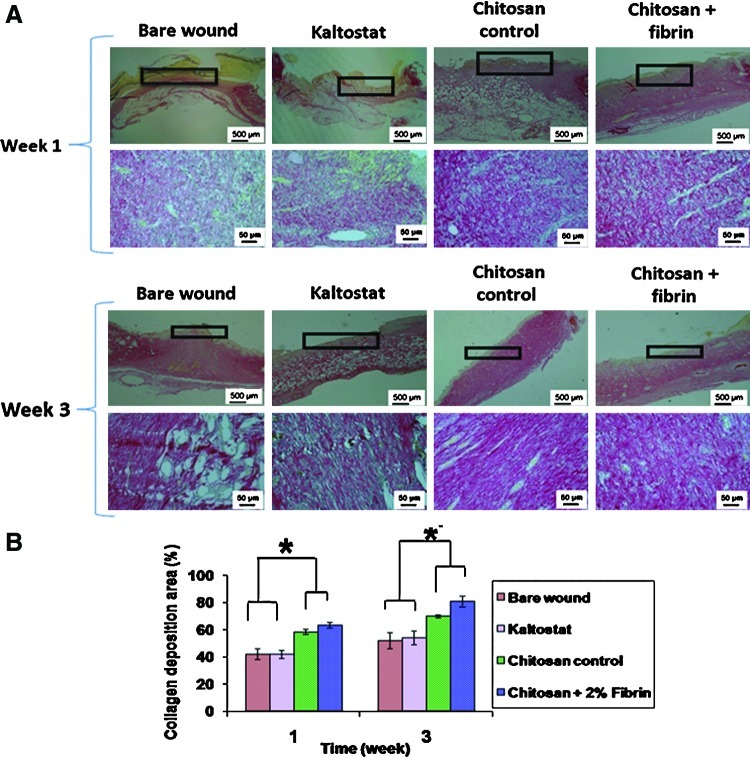
Picrosirius red staining was done to quantify the collagen deposition on the wound site. After week 1, the collagen deposition was found less in the bare wound and in the wound treated with Kaltostat. After 3 weeks of healing, there was enhanced collagen deposition in the wound healing assisted by the chitosan controls and the CFBs, compared to the Kaltostat and the bare wounds (Fig. 15A). Picrosirius red can stain collagen in pink color. The white colored empty spaces in the images showed the lack of collagen in the bare wound and Kaltostat-treated wounds. After week 1, the collagen deposition area of chitosan- and CFB-treated wounds were considerably higher compared with the bare wound and Kaltostat-treated wounds and the collagen deposition exhibited by the chitosan- and CFB-treated wounds exhibited a significant difference. After week 3, the CFB-treated wounds showed a significant difference in the collagen deposition area than the bare wound, the Kaltostat- and the chitosan-treated wound by 90% (Fig. 15B).
FIG. 15.
Photomicrographs of Picrosirius red-stained histology sections showing increased collagen deposition on re-epithelialized tissue. (A) The upper and lower section panels representing the histological sections of stained tissues of week-1 and week-3, respectively. The area indicated in the box is the region of interest of the histological sections. The magnified images of those regions are provided in the corresponding lower panel. (B) Collagen deposition area evaluation (*p<0.05). Color images available online at www.liebertpub.com/tea
Discussion
The characterization of fibrin nanoparticles confirmed that the obtained nanofibrin particles were in the size range of 243±5 nm. Morphological analysis was also performed by SEM and a tubular protein array was observed. The unaltered state of fibrin is evident from the PTAH stained image. The evenly spaced phenolic-OH groups of the hematin ligand chelate with the unaltered amino groups of fibrin, and thereby, specifically impart a blue color to the unaltered amino peptides of fibrin.29
The SEM images of CFBs showed that the prepared bandages have an adequate microporous structure and the pores were interconnected. The addition of nanofibrin did not cause any change in the morphology as well as porosity of CFBs. Peptide moieties of fibrin are comprised of amine and hydroxyl groups.22 These functional groups will possibly get reacted with the verso groups of chitosan (hydroxyl and amine groups) by means of intermolecular hydrogen bonding providing a firm attachment of nanofibrin within the chitosan hydrogel matrix.
The bandages were porous enough to absorb the excess exudates from the wound surface. The FT-IR data of CFBs revealed the incorporation of nanofibrin in the chitosan bandages by showing the characteristic peaks of chitosan (3450, 1450, and 1630 cm−1) as well as nanofibrin (1391 and 1544 cm−1) in the spectra of nanofibrin-incorporated CFBs.22,23,30 The broadening of peaks in the CFB spectra at 3450 cm−1 attributed to the intermolecular hydrogen bonding between the nanofibrin and chitosan. The PTAH staining showed the homogeneous distribution of nanofibrin in the bandages. The binding of PTAH by fibrin is due to hydrogen bonding occurring between two phenolic-OH groups of each hematein ligand chelated within the suitably spaced sites of amino peptides.22 The evenly spaced phenolic-OH groups of the chelated ligand specifically impart a blue color to the unaltered amino peptides of fibrin, thereby proving the unaltered state of fibrin.29 Furthermore, the porous nature of bandages enhances the distribution of nutrients and provides gaseous exchange and helps in the wound-healing process.
Swelling ratio data revealed that the bandages were saturated with the liquid medium in which it was immersed after 24 h. The swelling ratio was found decreased after 24 h and it was due to the degradation of the bandages. The differences in the swelling ratio between the groups were not significant even after the incorporation of nanofibrin. The swelling ability of the bandages would be helpful to absorb wound exudates from the wound surface.
The reported mechanism of degradation was that the lysozyme can cleave the glycosidic linkage between the monomers.27,29 The obtained results revealed that the incorporation of nanofibrin can alter the degradation nature of bandages. It has been reported that the degradation of products of chitosan were D-glucosamine and glycosaminoglycan. They are nontoxic to the human beings since they are already present in our body.31,32 The degradation products would be helpful to initiate the migration of fibroblast and keratinocyte cells toward the wound site. This migration of cells enhances the collagen deposition and re-epithelialization.32
It is suggested that bandages suitable for wound dressing should preferably be strong, but flexible.33 If the elongation at break of a material is high, then it is more soft and flexible.33 Here the results showed that the Kaltostat is more flexible and soft because of the fibrous nature of the material, but the tensile strength was low. Chitosan possessed high strength, but low flexibility. When fibrin is incorporated, it reduced the strength, but as far as bandages are concerned the tensile strength of 0.02–0.04 was sufficient and had considerable flexibility. The incorporation of fibrin might have disrupted the compact structure of the chitosan bandages, and thereby decreasing the strength.
In healthy biological systems, the blood clots occurs when fibrin, an insoluble coagulation protein forms a plug at the wound site through chemical crosslinking of fibrinogen by enzymatic cleavage of thrombin. The fibrin strands thus formed at the wound site will then trap the platelets that later results in platelet aggregation and contraction of damaged blood vessel. The advantage of using fibrin in nanoformulation relies here, as the bandages designed in this study itself provide an ideal biomimetic matrix comprising of active fibrin moieties for the ready activation of platelets, and thus inducing a rapid wound-healing process. The nanofibrin in the CFBs enhanced the clotting to a great extend, and thereby proved the hemostatic potential of the CFBs. That was due to the interaction between cationic chitosan and negatively charged blood cells.34,35
The cell studies on HDF and HUVECs revealed the cytocompatible nature of the CFBs. So, the cells can adhere well on to the bandages and result in the further growth and proliferation that can enhance the ECM formation, and thereby, tissue regeneration. The infiltration of HUVECs into the bandages proved that the bandages can promote the vascularization36 and provide an adequate blood supply that is essential for the skin tissue regeneration.
In the in vivo study, granulation tissue formation was more on the wounds treated with CFBs after 1 and 3 weeks. The healing was significantly faster in the CFB-treated wounds compared to the other groups. The prepared CFB improved the healing compared to the control bandage. Fibrin, a potent chemotactic agent, enhances the migration of more fibroblast and keratinocyte cells toward the wound site. The migration of keratinocyte helped to form an intact epidermal layer after 3 weeks of the application of the bandages. Also, the presence of fibrin enhances the vascularization and hence, the supply of nutrients and other necessary factors to the wound site. The presence of fibrin in the composite bandage helped to form a fibrin plug over the wound surface faster compared to the control bandage and bare wound. The wounds treated with the CFBs and chitosan control showed enhanced collagen deposition compared to the bare wound and Kaltostat-treated wounds. The migration of fibroblast cells and their proliferation on the wound site caused the enhanced deposition of collagen on the wound site. The deposition of collagen could have further accelerated the healing process and remodeling of the damaged tissue.
The re-epithelialization was complete and intact on wounds treated with CFBs compared to the bare wound, the Kaltostat-treated wound, and the chitosan-treated wound. Keratinocytes of the dermal tissue is well known for their integrative role in the re-epithelialization process.23 Fibrin nanoparticles and chitosan matrix caused the migration of more number of keratinocyte cells to the wound area and the migrated cells caused the faster re-epithelialization of the wounds treated with CFB compared to the controls. The presence of fibrin improved the vascularization22 and hence, the cells got enhanced nutrients to grow and proliferate on the wound site. This may infer to be the possible reason for improved re-epithelialization on wounds treated with CFBs.
It is important to analyze the deposition of collagen on the wound surface because skin is composed of collagen and cells. The enhanced collagen deposition after week 3 on the CFB-treated wound and the chitosan-treated wound was due to the presence of more fibroblast cells.18 When the wound occurs, the collagen degrades and the strength of skin will be lost. So it is important to make sure that collagen deposition is happening while wound healing, to form the remodeled and intact skin tissue.18 The study revealed the enhanced deposition and ordered arrangement of collagen fibers on the wound site. This matrix deposition would help the wound to heal faster without any undesired contraction of skin at the wound area.
Conclusions
Chitosan is one of the major players in wound management materials and is known to contribute substantially in terms of a physiologic support for wound healing. Modern clinical approaches are aimed at further improving the functionality of chitosan, which would ultimately aid the proper regeneration of healed tissue. In such an attempt, we fabricated composite bandages of chitosan and fibrin, which were found to be superior to bare chitosan as well as commercially used Kaltostat bandages. The prepared CFBs were microporous enough to absorb the excess exudates from the wound interface, was found to be biocompatible and biodegradable, and have adequate flexibility as well as tensile strength. The enhanced blood clotting and excellent platelet activation ability of the CFBs proved that they can be used as an effective treatment for the patients having blood clotting disabilities. The cell viability and infiltration showed by the bandages revealed the cyto-friendly nature of the CFBs, which could play an important role in promoting vascularization. In vivo evaluation of CFBs in SD rats revealed that the wounds treated with the CFBs healed much faster compared to the control and Kaltostat. The deposition of collagen was more on the wounds treated with CFBs compared to the control. Moreover, the wounds treated with the CFBs showed an intact epithelial layer with fully matured epidermis which was not there on the control groups. Apparently, the CFBs helped to attain complete re-epithelialization compared to the control. Altogether, these findings suggest that the composite bandage we prepared is much better in terms of supporting the healing process and it helped in the regeneration of the skin tissue at the wound site. Future studies are aimed at using this composite bandage for treating more complicated scenarios, such as burn, chronic ulcers, and diabetic wound infections.
Acknowledgments
The authors acknowledge Department of Biotechnology (DBT), India, for the financial support. We are also grateful to Nanomission, Department of Science and Technology, India, which supported this work, under a grant of the Nanoscience and Nanotechnology Initiative program. P.T. Sudheesh Kumar acknowledges the Council of Scientific and Industrial Research, India for the Senior Research Fellowship (Award No. 9/963 (0011) 2K11- EMR-1). G. Praveen acknowledges the CSIR, India for the Senior Research Fellowship (Award No. 9/963(0019)2K12-EMR-I). We are also grateful to Mr. Sajin P. Ravi for his help in SEM analysis. We acknowledge K.S. Sarath for his help in confocal imaging. The authors are grateful to Dr. Sabareeswaran for the help during histopathology analysis. We are grateful to Dr. P. Reshmi, Dr. A.K.K. Unni, Chandini, Sajith, Sunil, and Sunitha for their help during the in vivo study. We thank the Amrita Centre for Nanosciences and Molecular Medicine for the infrastructure support.
Disclosure Statement
No competing financial interests exist.
References
- 1.Paul W. Sharma C.P. Chitosan and alginate wound dressings: a short review. Trends Biomater Artif Organs. 2004;18:18. [Google Scholar]
- 2.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 3.Jayakumar R. Prabaharan M. Sudheesh K.P.T. Nair S.V. Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29:322. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Jayakumar R. Prabaharan M. Nair S.V. Tamura H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv. 2010;28:142. doi: 10.1016/j.biotechadv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Bruin P. Jonkman M.F. Meijer H.J. Pennings A.J. A new porous polyetherurethane wound covering. J Biomed Mater Res. 1990;24:217. doi: 10.1002/jbm.820240208. [DOI] [PubMed] [Google Scholar]
- 6.Muzzarelli R.A.A. Muzzarelli C. Chitosan chemistry: relevance to the biomedical sciences. Adv Polym Sci. 2005;186:151. [Google Scholar]
- 7.Jayakumar R. Menon D. Manzoor K. Nair S.V. Tamura H. Biomedical applications of chitin and chitosan based nanomaterials-a short review. Carbohydr Polym. 2010;82:227. [Google Scholar]
- 8.Abul K.A. Niwet S. Suwalee C. Willem F.S. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res Part B Appl Biomater. 2004;69:216. doi: 10.1002/jbm.b.30000. [DOI] [PubMed] [Google Scholar]
- 9.Tao W. Xiao K.Z. Xu T.X. Da Y.W. Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohydr Polym. 2012;88:75. [Google Scholar]
- 10.Nurdan O. Ozcan G. Haytac C.M. Alaaddinoglu E.E. Mustafa F.S. Sevda S. Chitosan film enriched with an antioxidant agent, taurine, in fenestration defects. J Biomed Mater Res. 2000;51:500. doi: 10.1002/1097-4636(20000905)51:3<500::aid-jbm26>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Tamer A.E. Ahmed E.V. Dare M.H. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B. 2008;14:2. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 12.Mosesson M.W. Fibrinogen and fibrin structure and functions (review) J Thromb Haemost. 2005;3:1894. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 13.Weisel J.W. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247. doi: 10.1016/S0065-3233(05)70008-5. [DOI] [PubMed] [Google Scholar]
- 14.Mosesson M.W. Siebenlist K.R. Meh D.A. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11. doi: 10.1111/j.1749-6632.2001.tb03491.x. [DOI] [PubMed] [Google Scholar]
- 15.Horan J.T. Francis C.W. Fibrin degradation products, fibrin monomer and soluble fibrin in disseminated intravascular coagulation. Semin Thromb Hemost. 2001;27:657. doi: 10.1055/s-2001-18870. [DOI] [PubMed] [Google Scholar]
- 16.Schense J.C. Hubbell J.A. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug Chem. 1999;10:75. doi: 10.1021/bc9800769. [DOI] [PubMed] [Google Scholar]
- 17.Cox S. Cole W. Tawil B. Behavior of human dermal fibroblasts in three-dimensional fibrin clots: dependence on fibrinogen and thrombin concentration. Tissue Eng Part A. 2004;10:942. doi: 10.1089/1076327041348392. [DOI] [PubMed] [Google Scholar]
- 18.Currie L.J. Sharpe J.R. Martin R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast Reconstr Surg. 2001;108:1713. doi: 10.1097/00006534-200111000-00045. [DOI] [PubMed] [Google Scholar]
- 19.Breen A. O'Brien T. Pandit A. Fibrin as a delivery system for therapeutic drugs and biomolecules. Tissue Eng Part B. 2009;15:201. doi: 10.1089/ten.TEB.2008.0527. [DOI] [PubMed] [Google Scholar]
- 20.David J.G. Daniel D.S. Stelios T.A. Fibrin promotes migration in a three dimensional in vitro model of wound regeneration. Tissue Eng Part A. 2002;8:787. doi: 10.1089/10763270260424141. [DOI] [PubMed] [Google Scholar]
- 21.Alexander M.H. Wei K. Charito S.B. Anne J.G. Tim R.M. Effects of fibrin pad haemostat on the wound healing process in vivo and in vitro. Biomaterials. 2011;32:9594. doi: 10.1016/j.biomaterials.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Praveen G. Sreerekha P.R. Menon D. Nair S.V. Chennazhi K.P. Fibrin nanoconstructs: a novel processing method and their use as controlled delivery agents. Nanotechnology. 2012;23:102. doi: 10.1088/0957-4484/23/9/095102. [DOI] [PubMed] [Google Scholar]
- 23.Sudheesh K.P.T. Lakshmanan V.K. Anilkumar T.V. Ramya C. Reshmi P. Unnikrishnan A.G. Nair S.V. Jayakumar R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation. ACS Appl Mater Interfaces. 2012;4:2618. doi: 10.1021/am300292v. [DOI] [PubMed] [Google Scholar]
- 24.Sudheesh K.P.T. Abhilash S. Manzoor K. Nair S.V. Tamura H. Jayakumar R. Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohydr Polym. 2010;80:761. [Google Scholar]
- 25.Madhumathi K. Sudheesh K.P.T. Abilash S. Sreeja V. Tamura H. Manzoor K. Development of novel chitin/nanosilver composite scaffolds for wound dressing applications. J Mater Sci Mater Med. 2010;21:807. doi: 10.1007/s10856-009-3877-z. [DOI] [PubMed] [Google Scholar]
- 26.Ong S.Y. Wu J. Moochhala S.M. Tan M.H. Lu J. Development of a chitosan- based wound dressing with improved haemostatic and antimicrobial properties. Biomaterials. 2008;29:4323. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 27.Asahara T. Murohara T. Sullivan A. Silver M. Van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 28.Anilkumar T.V. Jaseer M. Anumol J. Arun J. Mohanan P.V. Lissy K.K. Advantages of hyaluronic acid as a component of fibrin sheet for care of acute wound. Biologicals. 2011;39:81. doi: 10.1016/j.biologicals.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Mossesson M.W. Fibrinogen structure and fibrin clot assembly. Semin Thromb Hemost. 1998;24:169. doi: 10.1055/s-2007-995837. [DOI] [PubMed] [Google Scholar]
- 30.Aoyagi S. Onishi H. Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int J Pharm. 2007;330:138. doi: 10.1016/j.ijpharm.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Sowmya S. Jayakumar R. Chennazhi K.P. Levorson E.J. Mikos A.G. Nair S.V. Multiscale fibrous scaffolds in regenerative medicine. Adv Polym Sci. 2012;246:1. [Google Scholar]
- 32.Muzzarelli R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr Polym. 2009;76:167. [Google Scholar]
- 33.Tanveer A.K. Kok K.P. Mechanical, bioadhesive strength and biological evalutions of chitosan films for wound dressing. J Pharm Pharmaceut Sci. 2000;3:303. [PubMed] [Google Scholar]
- 34.Burkatovskaya M. Castano A.P. Demidova R.T.N. Tegos G.P. Hamblin M.R. Effect of chitosan acetate bandage on wound healing in infected and non infected wounds in mice. Wound Rep Reg. 2008;16:425. doi: 10.1111/j.1524-475X.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meloan S.N. Puchtler H. On the chemistry of phosphotungstic acid-hematein: development of a rapidly ripening PTAH solution. J Histotechnol. 1988;11:153. [Google Scholar]
- 36.Tseng H.J. Tsou T.L. Wang H.J. Hsu S.H.J. Characterization of chitosan-gelatin scaffolds for dermal tissue engineering. Tissue Eng Regen Med. 2011;4:76. doi: 10.1002/term.492. [DOI] [PubMed] [Google Scholar]