Abstract
Creating a functional vascularized bone tissue remains one of the main goals of bone tissue engineering. Recently, a growing interest in the crosstalk between endothelial cells (EC) and osteoblasts (OB), the two main players in a new bone formation, has been observed. However, only a few reports have addressed a mutual influence of OB and EC on cell proliferation. Our study focuses on this issue by investigating cocultures of human bone-derived cells (HBDC) and human umbilical vein endothelial cells (HUVEC). Three various proportions of cells have been used that is, HBDC:HUVEC 1:1, 1:4, and 4:1 and the cocultures were investigated on day 1, 4, and 7, while HUVEC and HBDC monocultures served as reference. We have detected enhanced alkaline phosphatase (ALP) activity in a direct HBDC–HUVEC coculture. This effect was not observed when cells were separated by an insert, which is consistent with other reports on various OB–EC lineages. The appearance of gap-junctions in coculture was confirmed by a positive staining for connexin 43. The number of cells of both phenotypes has been determined by flow cytometry: CD-31-positive cells have been considered EC, while CD-31-negative have been counted as OB. We have observed an over 14-fold increase in OB number after a week in the 1:4 HBDC:HUVEC coculture as compared with less than fourfold in monoculture. The increase in HBDC number in 1:1 coculture has been less pronounced and has reached the value of about sevenfold. These results correspond well with the cell proliferation rate, which has been measured by 5-bromo-2′-deoxyuridine incorporation. Moreover, at day 7 EC have been still present in the coculture, which is inconsistent with some other reports. Real-time polymerase chain reaction analysis has revealed the upregulation of ALP and collagen type I genes, but not osteocalcin gene, in all the cocultures grown without pro-osteogenic additives. Our study indicates that HUVEC significantly promote HBDC expansion and upregulate collagen I gene expression in these cells. We believe that these findings have application potency in bone tissue engineering.
Introduction
In recent years, increasing attention has been given to cell coculture. The use of coculture systems mimicking the complex structures and regulation processes within the living tissue provides a superior tool for analysis of cellular interactions. Applying the coculture systems in tissue-engineered constructs might also result in a therapeutic advantage in the field of regenerative medicine and tissue engineering.1 For example, a better understanding of cellular interaction between endothelial cells (EC) and osteoblasts (OB) would significantly accelerate the development of the new bone tissue engineering applications. Despite an emerging body of research showing that the complex interactions between EC and OB is involved in the regulation of bone formation and angiogenesis, neovascularization still remains the limiting factor in successful implantation of voluminous bone grafts. Insufficient vascularity of the engineered construct results in its hypoxic cell death.2 Several studies have indicated that there are reciprocal advantages in functional relationship between OB and EC or their corresponding precursors.3–7 Rouwkema et al. have shown that osteoprogenitor cells were able to support the formation of EC network in a bone tissue engineering construct.8 It was demonstrated that the cocultures of EC with other cell types, such as bone marrow stem/stromal cells (BMSC), have a beneficial effect on the formation and stabilization of newly formed vascular structures after implantation.8–12 It seems, that at least in part, the beneficial effect of OB on EC is due to the release of diverse angiogenic growth factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF).13 At the same time, recent studies highlighted the stimulating influence of EC on alkaline phosphatase (ALP) activity in OB.2,6,7,14,15 The effect of EC on the induction of osteoblastic differentiation markers in osteoprogenitor cells, such as runt-related transcription factor 2 (Runx2), ALP, and osteocalcin represents another intensively investigated processes.6,13,16
Our knowledge about EC influence on OB differentiation remains far from complete. Nevertheless, a positive OB influence on EC organization in coculture seems to be a good reason to consider OB–EC coculture as a useful system in bone tissue engineering.2,3,17,18 To add an extra value to such a system we put particular attention to the possible EC influence on OB proliferation in vitro. Expansion of normal cells originating from primary cultures, which are candidates for transplantation in tissue engineered systems, is not as effective as of immortalized cell lines derived from tumors, often used for basic research experiments. Therefore, any advancement in enhancing cellular proliferation efficiency is of a high interest.
To the best of our knowledge, the first result that suggested EC influence on OB proliferation was obtained from the culture of OB derived from human bone exposed to human umbilical vein endothelial cells (HUVEC)-conditioned culture medium.19 OB proliferation after 72 h was enhanced as compared with the culture in a nonconditioned medium. This effect was also confirmed in a culture of marrow-derived MSC in HUVEC-conditioned medium, although in this case the effect occurred only after 12 days.20 These findings have prompted us to focus on the HUVEC influence on human bone-derived cells (HBDC) proliferation in a direct coculture with the intent to exploit this phenomenon in bone tissue engineering. To the best of our knowledge until now, no one has shown such an effect. Our preliminary observations revealed a significantly high increase in HBDC number after 7 days in coculture with HUVEC as compared with a monoculture.21 At the same time encouraging data have been published by Bidarra et al. showing stimulatory effect of HUVEC on the proliferation of marrow-derived MSC.22 Although in the case of MSC, unlike the HBDC, cell number was lower in the EC-coculture than in a monoculture after a week, stimulatory effect of EC on MSC number appeared in a prolonged culture, that is, after 14- and 21 days. Thus, similar to the results obtained in the conditioned media,19,20 mesenchymal cells responded to the EC-originating stimuli later in the culture than the cells isolated from bone chips. Obviously, from the perspective of practical use, the faster intensification of OB expansion the more profitable input in tissue engineered product preparation. The aim of this study was to prove the stimulatory effect of HUVEC on HBDC proliferation in a 1 week culture. Such finding might support OB–EC coculture as a valuable system toward obtaining bone tissue engineering constructs, indicating EC as a player in the OB expansion stage.
Materials and Methods
Isolation and culture of primary human OB
HBDC were isolated from pieces of bone explanted post-surgery. All the procedures were approved by the Local Ethics Committee of the Medical University of Warsaw (Decision No. KB/74/2005) and the donors provided informed consent. The isolation was based on the protocols described by Gallagher et al.23 with modifications.24 Briefly, after removing the soft tissue (including marrow), both mechanically and enzymatically (collagenase XI S 600 U/mL; Sigma), small (about 2 mm in length) bone chips were rinsed in Ca-free phosphate-buffered saline and placed in a 150 mL cell culture flask (Costar-Nunc) containing Dulbecco's modified Eagle medium (DMEM; Gibco), supplemented with fetal bovine serum (10%; Gibco), l-glutamine (1%; Gibco), antibiotic-antimycotic mixture (1%; Gibco), and l-ascorbic acid 2-phosphate (100 mM; Sigma) at 37°C in 5% CO2. The medium was changed every 7 days. Within 2–4 weeks, cell migration from the bone chips occurred. After reaching confluence, the HBDC were used in experiments. Only first passage cells were used in the experiments to avoid any changes in phenotype. According to the established practice used in our laboratory, every fifth sample of cell population obtained in accordance with the validated protocol is examined toward osteoblastic phenotype. Expression of the selected osteogenic genes and the enhanced ALP activity are verified in response to the osteogenic additives to a culture medium. Based on the repetitively positive results of these tests, HBDC obtained in our laboratory by the described method are considered OB.
The culture of HUVEC
HUVEC were purchased from Invitrogen Life Technologies. Cells were grown in EBM-2 basal medium supplemented with the EGM-2 SingleQuot kit (Lonza). For all experiments HUVEC up to passage five were used.
Direct coculture
The investigated cell populations were mixed and seeded on 6-well plates at density 1.5×105 cells/well, on 24-well plates at a density 3×104 cells/well, and on 96-well plates at density 5×103 cells/well in EBM-2 basal medium supplemented with the EGM-2 SingleQuot kit. The seeding ratio of OB:EC was 1:1, 4:1, and 1:4. The monocultures of HUVEC and HBDC grown in EBM-2 basal medium supplemented with the EGM-2 SingleQuot kit were used as controls. The cells were cultured for 7 days.
Indirect coculture
HUVEC were seeded on the microporous membrane inserts (Nunc) at a density of 3×104 cells/well. After adherence of cells (2–3 h), inserts with HUVEC were carefully placed in 24-well plates containing the same number of HBDC seeded at the bottom. HUVEC and HBDC seeded alone at the bottom of the plates and grown in EBM-2 basal medium supplemented with the EGM-2 SingleQuot kit were used as controls. The cells were cultured for 7 days.
Cell proliferation assay
Cell proliferation was assessed with a commercial cell proliferation ELISA kit (Roche), based on the detection of 5-bromo-2′-deoxyuridine (BrdU) incorporated during DNA synthesis in replicating cells. Assays were performed according to the manufacturer's protocol. Cell proliferation was measured at day 4 and 7 of culture.
ALP assay
ALP activity was determined by detecting the formation of p-nitrophenol, a product of p-nitrophenyl phosphate catalyzed by ALP, following the colorimetric procedure of alkaline phosphatase activity kit (Sigma). An absorbance was measured at 405 nm. The activity of ALP was measured in cell lysates at day 4 and 7 of culture.
Determination of cell number of individual cell types in coculture
For flow cytometry analysis cells were grown in six-well dishes. At day 4 and 7, cells were treated with collagenase type I for 10 min at 37°C and then for 3 min at 37°C with a cell detachment solution Accutase (Thermo Electron). Cells were counted and labeled with phycoerythrin-conjugated monoclonal anti-human CD31 antibodies (Becton Dickinson), as described by the manufacturer. The CD31-positive cells were considered EC and the CD31-negative cells were counted as OB. Total number of cells and their relative proportions allowed the calculation of the number of each type cells in coculture.
All quantitative assays (cell number, cell proliferation assay, ALP activity assay, and flow cytometry). Were performed in the five independent experiments. Each experiment evaluated cells harvested from one donor and the donor was different for each experiment (five donors altogether).
Relative quantification of gene expression by real-time polymerase chain reaction
Total RNA was isolated from monocultures and cocultures using the RNeasy Micro Kit (Qiagen). Total RNA (50 ng) was reverse transcribed into cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer's protocol. Gene amplification was performed on Applied Biosystems Step One 7500 Real-Time PCR System with the following TaqMan gene expression assays: glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Hs99999905_m1), ALP (Hs01029144_m1), collagen type I (Coll I; Hs00164004_m1), osteocalcin (OC; Hs00609452_g1) and VEGF (Hs 00900054_m1). The number of cycles and annealing temperature were selected according to the manufacturer's instructions. The expression of each gene was normalized to the expression of the reference GAPDH. The expression of investigated genes in HBDC seeded for the experiment, that is, on day 1, was used as a reference. Each sample was processed in triplicate. Changes in the expression of the target gene were calculated using 2−ΔΔCt method.
VEGF measurement by enzyme-linked immunosorbent assay
VEGF released into the culture medium was quantified using enzyme-linked immunosorbent assay (ELISA; Invitrogen). Supernatants collected from cultures of HBDC, HUVEC, and cocultures were assayed according to manufacturer's manuals. Results are expressed in picogram of VEGF per milliliter of sample.
CD31 ELISA
Supplementary to the flow cytometry measurements, the presence of EC in the investigated cocultures was verified by the detection of CD31 protein, which is one of EC markers, in all the investigated time points. Measurements were performed in lysates with a commercial ELISA kit (Abcam, Inc.) in the three independent experiments, from at least two wells per time point and per cell number proportion (n=6).
Gap junctions immunostaining
Mouse monoclonal anti-connexin 43 (Cx43) antibodies (Becton Dickinson) in combination with biotin-conjugated (Santa Cruz Biotechnology) secondary antibodies (dilution 1:100 in blocking buffer) with extravidin-TRITC (dilution 1:100 in blocking buffer) were used for detection of gap junctions in cocultures grown on coverslips. Cx43 staining was followed by von Willebrand factor labeling with mouse monoclonal antibodies (Becton Dickinson) and goat anti-mouse fluorescein isothiocyanate (FITC)-conjugated antibody (Becton Dickinson). Specimens were mounted in Ultra Cruz Mounting Medium containing fluorescent stain DAPI (Santa Cruz Biotechnology) and observed in fluorescence (Leica TCS confocal microscope).
Cell morphology
Morphological phenotypes were observed under an inverted phase contrast microscope on selected days of each type of cell culture (Nikon Eclipse TE-2000). Additionally, in the HBDC:HUVEC coculture of the initial 1:4 cell proportion, EC were visualized by CD31 labeling with mouse monoclonal antibodies (Sigma) in combination with goat anti-mouse FITC-conjugated antibody (Becton Dickinson). For the fluorescence Nikon Eclipse Ti was used.
Statistical analysis
The data are presented as mean±95% confidence interval. Statistical analysis was performed with Statistica Software using analysis of variance combined with a post-hoc Tukey Multiple Comparisons Test. In the case of gene expression—data from real time polymerase chain reaction (PCR)—Kruskall–Wallis test was applied. Results were considered significant at p<0.05. Details are given in the captions to figures.
Results
Determination of cell number of individual cell types in coculture
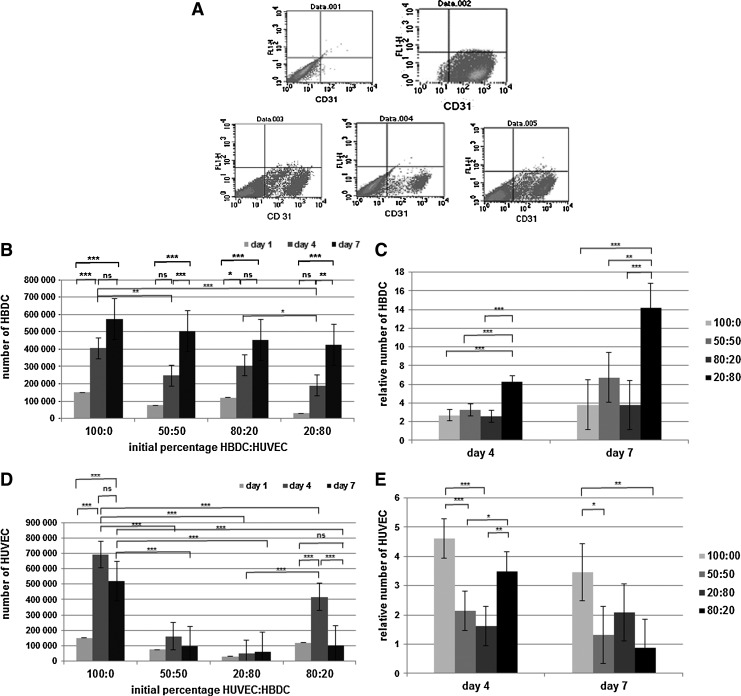
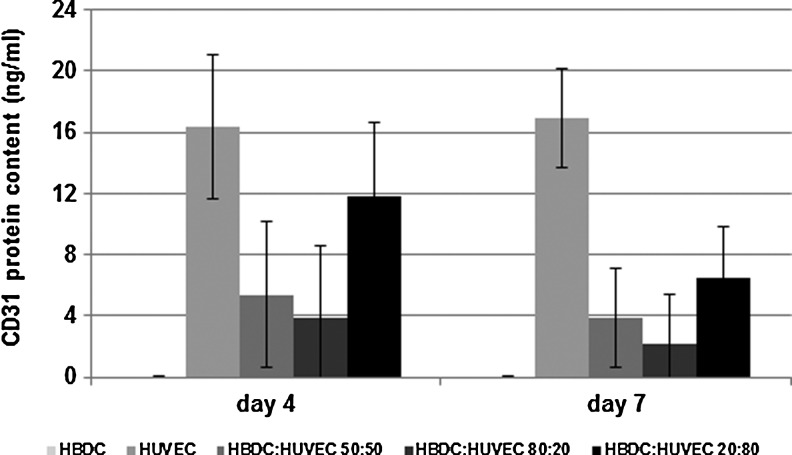
We observed that the expansion of HBDC population in each of the three types of coculture was significantly higher than in monoculture. The highest OB proliferation was detected when the coculture EC:OB ratio was 1:4. On day 7 we detected over 14-fold increase of OB number as compared with less than fourfold in monoculture (Fig. 1C). The results were confirmed in independently performed experiments with cells obtained from five different donors. Although HUVEC grew poorer in coculture than in monoculture, they were still present in coculture on day 7, which was confirmed by CD31 ELISA (Fig. 2).
FIG. 1.
Determination of cell number in monocultures and cocultures using flow cytometry. The CD-31-positive (HUVEC) and the CD31-negative population (HBDC) were quantified using CellQuest software. (A) An exemplary dot plot for HBDC monoculture (Data. 001), HUVEC monoculture (Data. 002), 1:1 (Data. 003), 4:1 (Data. 004), and 1:4 coculture (Data. 005) at day 7 of culture. (B, D) Number of HBDC (B) and HUVEC (D) in monoculture and in the three types of coculture (different in the HBDC:HUVEC number initial ratio-initial percentage HUVEC:HBDC is shown at the x-axis) at day 1 (light grey), day 4 (dark grey bars), and day 7 (black bars) of culture. (C, E) Relative number of HBDC (D) and HUVEC (E) in monoculture and in cocultures—data from monocultures and particular cocultures (see legend) are grouped in correspondence to the observation periods, that is, day 4 and 7. Relative cell number is calculated as 100×CNi)/CNn, where CNi means the number of cells on the given (i) day, and CNn—the number of cells on day 1, that is, the number of cells seeded for the experiment. Initial percentage HUVEC:HBDC is shown in the legend. At the (B–D) diagrams results are shown as the mean values from the five independent experiments. Error bars represent the 95% confidence interval (CI) of a mean. One-way ANOVA and post-hoc Tukey test have been performed. Independent variable is proportion of HBDC to HUVEC in culture. Dependent variable is number (B, C) or relative number (D, E) of HBDC. *p<0.05, **p<0.01, ***p<0.001 and ns, not statistically significant. HUVEC, human umbilical vein endothelial cells; HBDC, human bone-derived cells; ANOVA, analysis of variance.
FIG. 2.
CD31 protein concentration measured by enzyme-linked immunosorbent assay in monocultures and cocultures on day 4 (grey bars) and day 7 (black bars). The mean values from three independent experiments are shown. Initial percentage HUVEC:HBDC is shown in the legend. Error bars represents the 95% CI of a mean. One-way ANOVA and post-hoc Tukey test have been performed. Independent variable is proportion of HBDC to HUVEC in culture. Dependent variable is CD31 protein concentration.
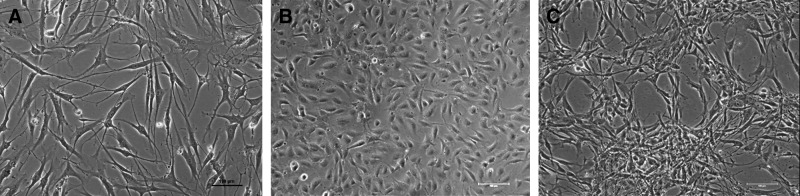
Cell morphology in monoculture and coculture of HBDC and HUVEC
Inverted light microscopic observation of all types of cocultures, HBDC monoculture and HUVEC monoculture, revealed normal cell morphology and an increase of cell number during the time of culture. OB demonstrated an elongated, spindle shape. HUVEC had characteristic polygonal shape during the first days of all types of culture. After 3–4 days in coculture HUVEC became elongated in shape, similar to HBDC. Only in HUVEC cocultured with HBDC at a ratio of 1:4 we observed lumen-like structures (Fig. 3C). They were formed by CD-31-positive cells considered EC (Supplementary Fig. S1; Supplementary data are available online at www.liebertpub.com/tea).
FIG. 3.
Cell morphology of HBDC monoculture (A), HUVEC monoculture (B), coculture of HBDC:HUVEC 1:4. Lumen-like structures visible in the coculture were confirmed to be formed by CD-31-positive (endothelial cells) cells—shown in the Supplementary data (Supplementary Fig. S1) Scale bars=100 μm.
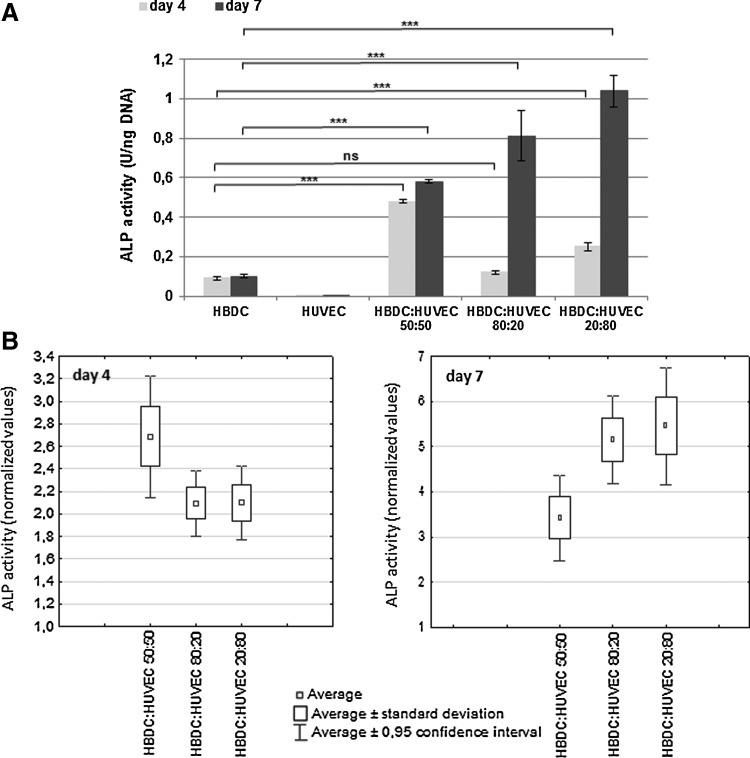
Effect of direct contact of HBDC and HUVEC in coculture on ALP activity
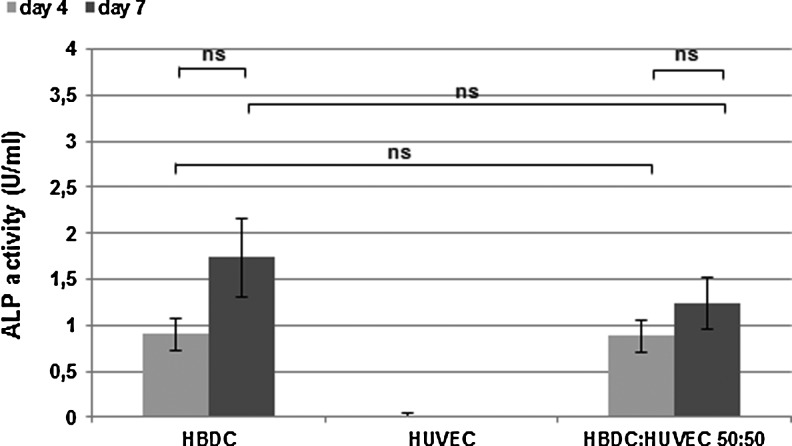
ALP activity was significantly higher in direct coculture than in OB monoculture in all three tested ratios, both on the fourth and seventh day of culture. The ALP activity of HBDC was almost three-times higher when cells were seeded at OB:EC ratio 1:4, after 4 days and over five-times higher after 7 days, in comparison to OB monoculture (Fig. 4). Since there were big differences in the initial value of ALP activity in HBDC depending on a donor, instead of showing the average values from all experiments, data from the one repetition are given in Figure 4A. To analyze data from all experiments, ALP activity in the cocultures were normalized to the value in HBDC at given time point (Fig. 4B). The analysis revealed significantly higher ALP activity in all cocultures compared with HBDC monoculture after both observation periods. EC expressed negligible ALP activity. To confirm that the increase in ALP activity is mediated by direct cell–cell contact, HBDC and HUVEC were cultured in the same well but separated with an insert. In that case, the ALP activity of OB grown on the bottom of culture plates and of OB in monoculture was comparable (Fig. 5).
FIG. 4.
Effect of coculture of HBDC with HUVEC grown in direct contact on ALP activity. (A) Data obtained in one from the five experiments are shown as the mean values (n=6). Error bars represents the 95% CI of a mean.***p<0,001, ns, not statistically significant. (B) Values obtained in all cocultures are normalized to the ALP activity in HBDC monoculture. Mean values from five independent experiments are shown; n=30 (five experiments×six repetitions). For each repetition cells from different donors were used. CIs and standard errors for relative ALP activity have been shown. The CIs do not include 1, so ALP activity is significantly higher in cocultures than in HBDC culture. Initial percentage HUVEC:HBDC is shown at the x-axis. ALP, alkaline phosphatase.
FIG. 5.
Effect of coculture of HBDC with HUVEC grown in nondirect contact on ALP activity. Data are presented as the mean (n=18). Error bars represent the 95% CI of a mean. Results show data from three independent experiments, ns—not statistically significant. One-way ANOVA and post-hoc Tukey test have been performed.
Osteogenic activity assessed by gene expression for ALP, Coll I, and osteocalcin
Cells were analyzed for their expression of ALP and Coll I, which are characteristic for osteogenic activity. Results of the real-time PCR analysis are presented according to Willems et al.25 and Livak and Schmittgen.26 Data are shown in relation to the given gene expression in HBDC population on day 1 (Fig. 6). The mean values from three independent experiments are presented. The values for the both investigated genes expression were significantly different in the investigated cell populations, which is probably due to their origin from different donors. Even though it resulted in considerable deviation of the results, several relations were confirmed to be statistically significant.
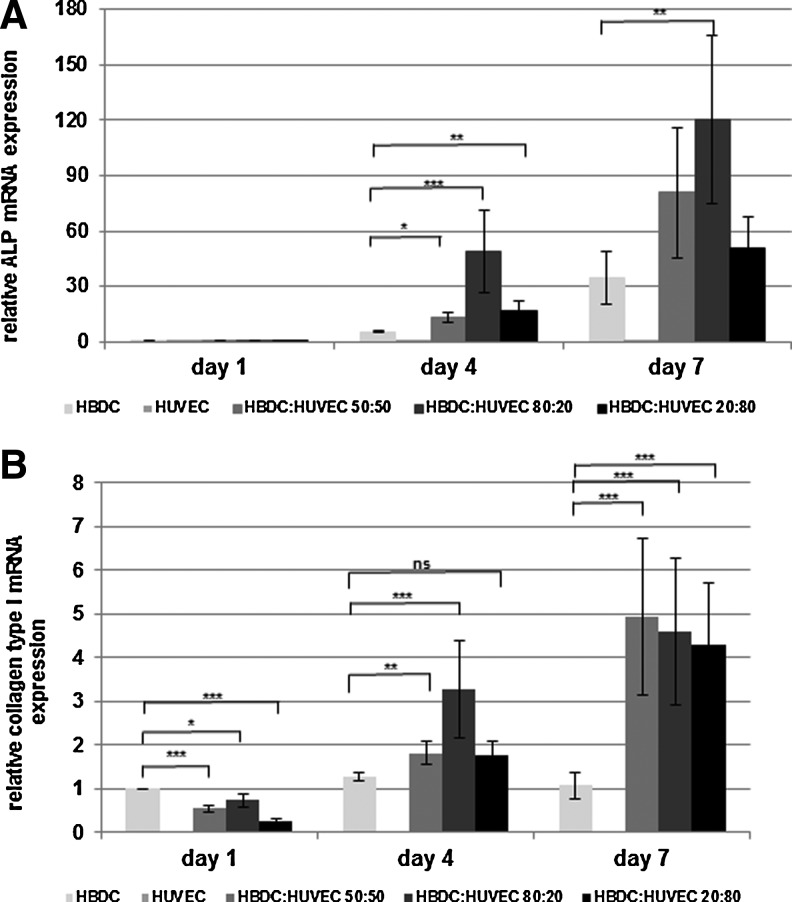
FIG. 6.
Effect of HUVEC on osteogenic activity. Gene expression of ALP (A) and Coll I (B) was normalized to GAPDH. HBDC monoculture on day 1 was used as a relative control. Data from three independent experiments are shown. Bars represent mean (n=18).Error bars represent the 95% CI of a mean. Initial percentage HUVEC:HBDC is shown at the x-axis. (*p<0.05, **p<0.01, and ***p<0.001). Coll I, collagen type I; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
In HBDC–HUVEC coculture, the expression of ALP and Coll I was higher than in OB monoculture after 4- and 7 days of culture. This effect was the strongest for HBDC:HUVEC initial proportion of 4:1, when the difference was statistically significant for both genes. The highest expression of the genes was observed on day 7. In the case of Coll I, it was significantly higher in all cocultures in comparison with the HBDC monoculture, while this effect for ALP mRNA expression, although still remarkable, was significant only for the proportion of 4:1. The expression of OC gene was very low in HBDC culture while in coculture it was about zero after both 4- and 7 days (Supplementary Fig. S2).
Determination of VEGF gene and protein expression
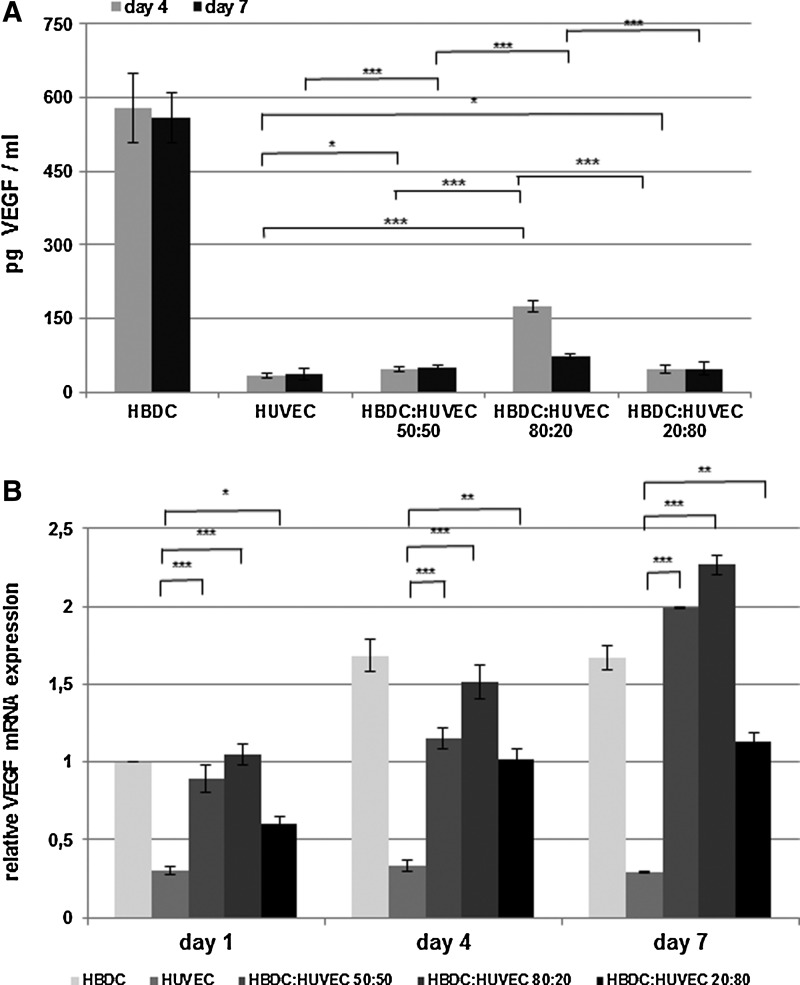
The highest VEGF concentration was detected in culture medium collected from HBDC monoculture. In the cocultures it was at the same low level as in the HUVEC monoculture with the exception of the coculture with the initial HBDC content of 80% (Fig. 7A). The analysis of VEGF gene expression revealed an increase in VEGF mRNA expression in time in all cocultures. In HBDC monocultures the maximal values were reached on day 4 (Fig. 7B).
FIG. 7.
VEGF release (A) and VEGF mRNA expression (B) in the investigated cultures. (A) Data are shown as the mean from three independent experiments. Error bars represents the 95% CI of a mean (n=9). The amount of VEGF released in HBDC monoculture compared with other type of culture was significantly higher (p<0,001, while there were no statistically significant differences between HUVEC monoculture and 1:1 or 1:4 coculture. Significance of the other differences are marked: *p<0.05 and ***p<0.001). Two-way ANOVA and post-hoc Tukey test has been performed. Initial percentage HUVEC:HBDC is shown at the x-axis. (B) VEGF mRNA expression was normalized to GAPDH. HBDC monoculture on day 1 was used as a relative control. Data are shown as the mean (n=18).Error bars represent the 95% CI of a mean. The results show data from three independent experiments (n=18). The Kruskal–Wallis test was used for statistical analysis of data. Initial percentage HUVEC:HBDC is indicated in the legend. VEGF, vascular endothelial growth factor.
Cell proliferation in monocultures and cocultures
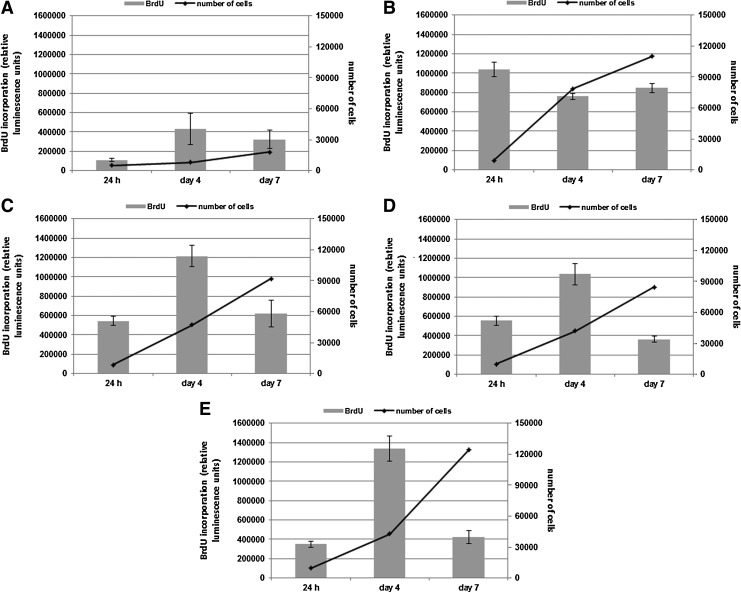
Cell proliferation in monocultures and cocultures was determined by incorporation of BrdU into cellular DNA during replication. For all three types of cocultures and HBDC monoculture, the highest proliferation rate was on day 4 (Fig. 8A, C–E); then the BrdU incorporation significantly declined. It was still at the same level on day 7 only in the HUVEC monoculture. To confirm the population growth, cells were counted in Burker chamber. The increase in cell number in all types of culture was observed up to 7 days (Fig. 8A–E). On day 7, cell populations reached confluence and stopped proliferating.
FIG. 8.
5-Bromo-2′-deoxyuridine incorporation into DNA during S phase (grey bars) and number of cells (black lines) in monoculture of HBDC (A), monoculture of HUVEC (B) and HBDC–HUVEC coculture [1:1 (C); 4:1 (D); 1:4 (E)]. Values are shown as mean from the experiments (n=6 in each of them). Error bars represent the 95% CI of a mean. Experiments were performed thrice.
Gap junction communication between HBDC and HUVEC
Cx43 is the predominant protein of gap junctions in OB and EC. Fluorescence staining of Cx43 performed in HBDC and HUVEC and in cocultures demonstrated that both cell types express this protein. Cx43 was localized in cell contact area. Additional staining of von Willebrand factor allowed us the identification of EC. A distinguishable labeling of Cx43 between EC (von Willebrand positive) and OB (von Willebrand negative) was observed (Supplementary Fig. S3). It confirms the existence of gap junctions between HBDC and HUVEC.
Discussion
Creating a functional vascularized bone tissue remains one of the main goals of bone tissue engineering. However, a better understanding of the mechanisms that regulate the communication processes between bone forming cells—OB and vessel forming cells—EC, is necessary to achieve this goal.
In our study HBDC:HUVEC coculture was investigated at different time points, and with different proportions of cells of both phenotypes. Our main finding is the tremendous impact of EC on the OB expansion in a direct coculture leading to over 14-fold increase of OB number compared with less than fourfold in monoculture (Fig. 1C). Since this tendency was confirmed in five independent experiments using cells derived from different donors, we report this finding as a systematic phenomenon. To the best of our knowledge, such apparent influence of HUVEC on HBDC expansion in coculture as shown in our study has not been yet documented. Only few reports have briefly noted a mutual influence of OB and EC on cell proliferation. Wang et al. noticed that conditioned medium of HBDC stimulated the proliferation of HUVEC, and conditioned medium of HUVEC slightly promoted HBDC proliferation.19 Similar effects were shown for BMSC and HUVEC.14,20 Only recently, a promoting influence of HUVEC on BMSC was confirmed in a direct coculture.22 This observation is consistent with our findings. However, with BMSC the effect appeared only in the prolonged (14- and 21 days) culture, while there was no effect on day 7. Significant EC-dependent increase in BMSC number was observed even later, that is, only on day 28 by Ma et al.7 The most probable reason for these discrepancies is the osteogenic cell population put into observation. BMSC are quiescent cells in vivo,27 but may reversibly change between proliferation and quiescence in vitro,28 while OB push other cells to proliferation. Therefore, the more rapid HBDC response to EC compared with BMSC may reflect the in vivo interplay between cells present in bone and EC. Contrary to Bidarra et al.,22 we have also found that for the observed phenomenon cell proportion is important. The medium used for cell culture could be another possible factor influencing the results. The proliferation rate of EC grown in M199 medium, which has been used by Bidarra et al.,22 has shown to be ∼2 times slower than in EBM-2 medium, which we used. The significance of the medium applied for OB–EC coculture was clearly shown also by Ma et al.7 In the preliminary observations we confirmed that the HBDC cultured in EC medium (EBM-2 basal medium supplemented with the EGM-2 SingleQuot kit) grew even better than in the DMEM-based medium routinely used in our lab for cells of osteogenic lineages. Therefore, we used EBM-2 basal medium supplemented with the EGM-2 SingleQuot kit for all investigated cultures that could additionally promote EC-induced effects.
Morphological observation revealed that the 1:4 coculture, which was the most profitable for OB expansion, was the only one in which we have noticed a nonuniform cell organization. It resembled the structures that were described by Grellier et al. as tube-like ones in a two-dimensional coculture of human osteoprogenitors and EC.29 This is another reason for which this OB:EC proportion seems the most suitable for tissue engineering applications. Even though EC expansion was limited after the day 4, EC presence was systematically confirmed at the end of the observation, that is, until the day 7 in all the experiments.
The EC survival was confirmed by flow cytometry and by the presence of EC-specific protein—CD31in all experimental conditions (Fig. 2). It is inconsistent with the data reported by Unger et al.4 In their experiments, both for the primary OB and MG-63, human dermal microvascular EC survived in cocultures with OB only when they were more numerous, that is, OB:EC ratios were equal to 1:5 and 1:10. EC survival in the 4:1 coculture in our study may be supported by VEGF produced by HBDC (see Fig. 7A). Such effect has been already reported for EC exposed to the BMSC-conditioned medium.30
The EC influence on OB differentiation comprises another important aspect of OB–EC coculture. Until now it has not been well understood, although some data indicate that EC may regulate OB differentiation. Meury et al. reported a reversible inhibitory effect of HUVEC on dexamethasone-induced differentiation of BMSC, which was indicated by inhibited expression of Osterix—a transcription factor for OB differentiation.31 Downregulation of runx2 and osteocalcin in human osteoprogenitors after 48 h of coculture with HUVEC was shown by Guillotin et al.6 However, it was accompanied by an upregulation of ALP. An enhanced ALP activity, as compared with monoculture, was found also in longer observation of similar cocultures.22,32 The evidence that ALP activity is promoted by the presence of EC in coculture is very strong.2,7–9,13,15,19,29,33 In our study, ALP activity was significantly higher in all investigated OB:EC proportions compared with the HBDC monoculture on day 7 (see Fig. 4).
It is commonly accepted that this phenomenon requires the direct contact of OB and EC,32–34 which is also the case in our study. It is postulated that the mutual influence of OB and EC is gap-junction mediated.13,32 The detection of Cx43, which is characteristic for gap junctions, between HBDC and HUVEC shown in this study strengthens this hypothesis.
The enhanced ALP activity in the cocultures investigated in our study corresponds well with the changes in ALP gene expression measured by real time PCR (see Fig. 6A). Moreover, the promoting effect of HUVEC on Coll I expression in HBDC has been shown, too (Fig. 6B). Until now, only a moderate upregulation of Coll I in BMSC cocultured with HUVEC has been reported.22,29,33 In our study, this effect was remarkable, as we have detected up to fivefold increase in Coll I expression. However, we did not find stimulating effect of EC on OC expression (Supplementary Fig. S2). Downregulation of OC within the first 48 h of BMSC and HUVEC coculture was found by Guillotin et al.6 Similar results were shown up to 72 h in human OB–HUVEC coculture by Hager et al.2 On the contrary, in rat cells Sun et al. reported the enhanced OC synthesis on day 5 in coculture of BMSC and kidney vascular EC, compared with monoculture.35 Interestingly, as shown by Villars et al., in a coculture of OC-positive BMSC with HUVEC the level of OC synthesis was lower than in the BMSC monoculture on day 3, 6, and 9.20 This result is in a good coincidence with our finding. However, Kaigler et al. have documented significantly higher OC level in a culture medium collected after both 7- and 14 days of BMSC-EC coculture, compared with the OB monoculture.9 Despite all these ambiguities, it is interesting that the relation between OB monoculture and OB–EC coculture with respect to all the investigated osteogenic genes expression that we have obtained is similar to the results reported for MG63 osteosarcoma cell line.15 Clearly, this aspect needs further investigation.
In conclusion, our study indicates that HUVEC significantly promote HBDC expansion. Our observations revealed the occurrence of this effect much earlier in coculture than previously reported for BMSC. We have shown that this effect is dependent on HBDC:HUVEC ratio and is the strongest when EC comprise the majority of cells in coculture. At the same time HUVEC survive well and may form tubular structures. We have also confirmed that EC are involved in OB differentiation, that is, they not only enhance ALP gene expression and ALP activity in a direct coculture, but also upregulate Coll I gene in OB. Our findings, and particularly the beneficial EC influence on OB expansion, could be used in bone tissue engineering.
Supplementary Material
Acknowledgments
The authors wish to thank Prof. Andrzej Górecki and Dr. Marcin Kowalski from the Department of Orthopedics and Traumatology, Medical University of Warsaw for their collaboration. This work was supported by the European Regional Development Fund within the Innovative Economy Operational Program in the frame of project BIO-IMPLANT (Grant No. POIG.01.01.02-00-022/09).
Disclosure Statement
No competing financial interests exist.
References
- 1.Fuchs S. Jiang X. Schmidt H. Dohle E. Ghanaati S. Orth C. Hofmann A. Motta A. Migliaresi C. Kirkpatrick C.J. Dynamic processes involved in the pre-vascularization of silk fibroin constructs for bone regeneration using outgrowth endothelial cells. Biomaterials. 2009;30:1329. doi: 10.1016/j.biomaterials.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Hager S. Lampert F.M. Orimo H. Stark G.B. Finkenzeller G. Up-regulation of alkaline phosphatase expression in human primary osteoblasts by cocultivation with primary endothelial cells is mediated by p38 mitogen-activated protein kinase-dependent mRNA stabilization. Tissue Eng Part A. 2009;15:3437. doi: 10.1089/ten.TEA.2009.0133. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs S. Hofmann A. Kirkpatrick C.J. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007;13:2577. doi: 10.1089/ten.2007.0022. [DOI] [PubMed] [Google Scholar]
- 4.Unger R.E. Sartoris A. Peters K. Motta A. Migliaresi C. Kunkel M. Bulnheim U. Rychly J. Kirkpatrick C.J. Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. Biomaterials. 2007;28:3965. doi: 10.1016/j.biomaterials.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 5.Scherberich A. Galli R. Jaquiery C. Farhadi J. Martin I. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells. 2007;25:1823. doi: 10.1634/stemcells.2007-0124. [DOI] [PubMed] [Google Scholar]
- 6.Guillotin B. Bareille R. Bourget C. Bordenave L. Amedee J. Interaction between human umbilical vein endothelial cells and human osteoprogenitors triggers pleiotropic effect that may support osteoblastic function. Bone. 2008;42:1080. doi: 10.1016/j.bone.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Ma J. van den Beucken J.J. Yang F. Both S.K. Cui F.Z. Pan J. Jansen J.A. Coculture of osteoblasts and endothelial cells: optimization of culture medium and cell ratio. Tissue Eng Part C Methods. 2011;17:349. doi: 10.1089/ten.TEC.2010.0215. [DOI] [PubMed] [Google Scholar]
- 8.Rouwkema J. de Boer J. Van Blitterswijk C.A. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 9.Kaigler D. Krebsbach P.H. West E.R. Horger K. Huang Y.C. Mooney D.J. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19:665. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 10.Kaigler D. Krebsbach P.H. Wang Z. West E.R. Horger K. Mooney D.J. Transplanted endothelial cells enhance orthotopic bone regeneration. J Dent Res. 2006;85:633. doi: 10.1177/154405910608500710. [DOI] [PubMed] [Google Scholar]
- 11.Sanz L. Santos-Valle P. Alonso-Camino V. Salas C. Serrano A. Vicario J.L. Cuesta A.M. Compte M. Sanchez-Martin D. Alvarez-Vallina L. Long-term in vivo imaging of human angiogenesis: critical role of bone marrow-derived mesenchymal stem cells for the generation of durable blood vessels. Microvasc Res. 2008;75:308. doi: 10.1016/j.mvr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Au P. Tam J. Fukumura D. Jain R.K. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos M.I. Unger R.E. Sousa R.A. Reis R.L. Kirkpatrick C.J. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials. 2009;30:4407. doi: 10.1016/j.biomaterials.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Xue Y. Xing Z. Hellem S. Arvidson K. Mustafa K. Endothelial cells influence the osteogenic potential of bone marrow stromal cells. Biomed Eng Online. 2009;8:34. doi: 10.1186/1475-925X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y. Schedle A. Matejka M. Rausch-Fan X. Andrukhov O. The proliferation and differentiation of osteoblasts in co-culture with human umbilical vein endothelial cells: an improved analysis using fluorescence-activated cell sorting. Cell Mol Biol Lett. 2010;15:517. doi: 10.2478/s11658-010-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs S. Ghanaati S. Orth C. Barbeck M. Kolbe M. Hofmann A. Eblenkamp M. Gomes M. Reis R.L. Kirkpatrick C.J. Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials. 2009;30:526. doi: 10.1016/j.biomaterials.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 17.Rouwkema J. Westerweel P.E. de Boer J. Verhaar M.C. van Blitterswijk C.A. The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Eng Part A. 2009;15:2015. doi: 10.1089/ten.tea.2008.0318. [DOI] [PubMed] [Google Scholar]
- 18.Buschmann J. Welti M. Hemmi S. Neuenschwander P. Baltes C. Giovanoli P. Rudin M. Calcagni M. Three-dimensional co-cultures of osteoblasts and endothelial cells in DegraPol foam: histological and high-field magnetic resonance imaging analyses of pre-engineered capillary networks in bone grafts. Tissue Eng Part A. 2011;17:291. doi: 10.1089/ten.TEA.2010.0278. [DOI] [PubMed] [Google Scholar]
- 19.Wang D.S. Miura M. Demura H. Sato K. Anabolic effects of 1,25-dihydroxyvitamin D3 on osteoblasts are enhanced by vascular endothelial growth factor produced by osteoblasts and by growth factors produced by endothelial cells. Endocrinology. 1997;138:2953. doi: 10.1210/endo.138.7.5275. [DOI] [PubMed] [Google Scholar]
- 20.Villars F. Bordenave L. Bareille R. Amedee J. Effect of human endothelial cells on human bone marrow stromal cell phenotype: role of VEGF? J Cell Biochem. 2000;79:672. doi: 10.1002/1097-4644(20001215)79:4<672::aid-jcb150>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Leszczynska J. Koziak K. Lewandowska-Szumiel M. Coculture with endothelial cells promotes expansion of osteoblasts–to be used in bone tissue engineering. Tissue Eng Part A. 2011;17:A16. [Google Scholar]
- 22.Bidarra S.J. Barrias C.C. Barbosa M.A. Soares R. Amedee J. Granja P.L. Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells. Stem Cell Res. 2011;7:186. doi: 10.1016/j.scr.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher J.A. Gundle R. Beresford J.N. Isolation and culture of bone-forming cells (osteoblasts) from human bone. Methods Mol Med. 1996;2:233. doi: 10.1385/0-89603-335-X:233. [DOI] [PubMed] [Google Scholar]
- 24.Kudelska-Mazur D. Lewandowska-Szumiel M. Mazur M. Komender J. Osteogenic cell contact with biomaterials influences phenotype expression. Cell Tissue Bank. 2005;6:55. doi: 10.1007/s10561-005-1911-z. [DOI] [PubMed] [Google Scholar]
- 25.Willems E. Leyns L. Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal Biochem. 2008;379:127. doi: 10.1016/j.ab.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 26.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Bianco P. Riminucci M. Gronthos S. Robey P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 28.Glauche I. Moore K. Thielecke L. Horn K. Loeffler M. Roeder I. Stem cell proliferation and quiescence—two sides of the same coin. PLoS Comput Biol. 2009;5:e1000447. doi: 10.1371/journal.pcbi.1000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grellier M. Ferreira-Tojais N. Bourget C. Bareille R. Guillemot F. Amedee J. Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J Cell Biochem. 2009;106:390. doi: 10.1002/jcb.22018. [DOI] [PubMed] [Google Scholar]
- 30.Kaigler D. Krebsbach P.H. Polverini P.J. Mooney D.J. Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng. 2003;9:95. doi: 10.1089/107632703762687573. [DOI] [PubMed] [Google Scholar]
- 31.Meury T. Verrier S. Alini M. Human endothelial cells inhibit BMSC differentiation into mature osteoblasts in vitro by interfering with osterix expression. J Cell Biochem. 2006;98:992. doi: 10.1002/jcb.20818. [DOI] [PubMed] [Google Scholar]
- 32.Villars F. Guillotin B. Amedee T. Dutoya S. Bordenave L. Bareille R. Amedee J. Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am J Physiol Cell Physiol. 2002;282:C775. doi: 10.1152/ajpcell.00310.2001. [DOI] [PubMed] [Google Scholar]
- 33.Stahl A. Wenger A. Weber H. Stark G.B. Augustin H.G. Finkenzeller G. Bi-directional cell contact-dependent regulation of gene expression between endothelial cells and osteoblasts in a three-dimensional spheroidal coculture model. Biochem Biophys Res Commun. 2004;322:684. doi: 10.1016/j.bbrc.2004.07.175. [DOI] [PubMed] [Google Scholar]
- 34.Guillotin B. Bourget C. Remy-Zolgadri M. Bareille R. Fernandez P. Conrad V. Amedee-Vilamitjana J. Human primary endothelial cells stimulate human osteoprogenitor cell differentiation. Cell Physiol Biochem. 2004;14:325. doi: 10.1159/000080342. [DOI] [PubMed] [Google Scholar]
- 35.Sun H. Qu Z. Guo Y. Zang G. Yang B. In vitro and in vivo effects of rat kidney vascular endothelial cells on osteogenesis of rat bone marrow mesenchymal stem cells growing on polylactide-glycoli acid (PLGA) scaffolds. Biomed Eng Online. 2007;6:41. doi: 10.1186/1475-925X-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.