Abstract
Puerto Rican adults have a greater prevalence of type 2 diabetes (T2D) and lower HDL-cholesterol (HDL-C) than the general U.S. population. Carbohydrate nutrition may play a role in this disparity. Cross-sectional analyses included data from 1219 Puerto Ricans aged 45–75 y enrolled in the Boston Puerto Rican Health Study. Using the Pearson chi-square test and ANCOVA, lifestyle characteristics and dietary intake, as assessed by semiquantitative FFQ, were compared by T2D status based on fasting plasma glucose concentration and medication use. Food source rankings for carbohydrate, dietary glycemic load (GL), and fiber were obtained using the SAS procedure PROC RANK. Geometric mean plasma HDL-C and TG concentrations were compared across quintiles of dietary carbohydrate, glycemic index (GI), GL, and fiber by using ANCOVA and tests for linear trend. In multivariable analyses, individuals with T2D (39.5%) had lower dietary carbohydrate, GL, and total sugar intake from lower intake of sugar, fruit drinks, and soda compared with those without T2D. In individuals without T2D, dietary carbohydrate and GL were inversely associated with HDL-C (P < 0.0001). Associations between dietary fiber and HDL-C were confounded by carbohydrate intake, apparently from concurrent consumption of legumes with white rice, a refined carbohydrate food. No associations were observed between carbohydrate, dietary GI, GL, or fiber and TG. In conclusion, individuals with T2D showed evidence of dietary modification. Among those without diabetes, a high intake of refined carbohydrates was associated with decreased HDL-C. Longitudinal research on carbohydrate nutrition in relation to diabetes risk factors and blood lipids in Puerto Ricans is warranted.
Introduction
Type 2 diabetes (T2D)6 is a critical public health concern and is particularly prevalent among Hispanics, the fastest growing ethnic group in the US (1). Puerto Ricans, the second largest U.S. Hispanic subgroup, are reported to have the second highest prevalence of T2D of all ethnic groups (2), and this is often accompanied by obesity (3, 4), hypertension (5), and dyslipidemia (4, 6). Together, this constellation of cardiometabolic risk factors in individuals with T2D contributes to a high risk of cardiovascular disease (CVD).
The American Diabetes Association (ADA) provides dietary recommendations to reduce disease progression among those with T2D (7). These include monitoring carbohydrate intake, both quantity and quality, to improve glycemic control. In addition to restricting total carbohydrate intake and choosing high-fiber foods such as fruits, vegetables, whole grains, and legumes, the ADA has stated that the use of glycemic index (GI) and glycemic load (GL) as measures of carbohydrate quality may provide a modest additional benefit over carbohydrate counting alone (7).
In addition to affecting glycemia, carbohydrate nutrition has effects on plasma lipids such that a diet of poor carbohydrate quality can cause or contribute to dyslipidemia. Low HDL-cholesterol (HDL-C) and high TG have been reported with high carbohydrate intake (8, 9) as well as with high dietary GI or GL (8, 10, 11); however, dietary fiber has been associated with improvements in HDL-C, even among individuals at high risk of CVD (12). In primarily Asian populations, where rice is the main carbohydrate source, dietary GI and/or GL have been linked to low HDL-C and high TG (13–15).
Puerto Rican adults suffer disproportionately from T2D and dyslipidemia, yet few data exist on their lifestyle and dietary characteristics by T2D status or on their dietary associations with blood lipids. In the Boston area, Puerto Rican adults consuming a traditional rice, beans, and oils dietary pattern were found to have higher total and central obesity (16) and a higher prevalence of low HDL-C and metabolic syndrome (17), a predisposition to T2D, compared with those consuming other dietary patterns. The traditional Puerto Rican dietary pattern is interesting in terms of its potential metabolic effects, given that the 2 main carbohydrate sources, white rice and legumes, reflect different sources of carbohydrate quality. However, there are few investigations on the effects of carbohydrate nutrition, i.e., total carbohydrate intake, dietary fiber, GI, and GL, on blood lipids in Puerto Rican or Hispanic populations with or without T2D. The aims of this analysis were to characterize carbohydrate nutrition in Puerto Rican adults with and without T2D and investigate associations between these carbohydrate nutrition measures and plasma HDL-C and TG.
Participants and Methods
Study population.
The Boston Puerto Rican Health Study is an ongoing cohort study of 1500 Puerto Rican adults aged 45–75 y in the greater Boston area, which began in May 2004. Details of the study methodology have been published (18). Briefly, eligible participants were identified through enumeration of high-Hispanic density blocks, based on year 2000 U.S. Census data, as well as through additional methods in collaboration with a local community organization. Individuals were considered eligible if they were of Puerto Rican origin based on self-report, aged 45–75 y, not planning to move out of Massachusetts within the 2 y following the baseline interview, and capable of answering questions in Spanish or English (based on a Mini-Mental State Examination score >10 points). The present cross-sectional analyses included 1219 individuals [i.e., 345 (28%) men and 874 (72%) women] with complete data on sociodemographics, lifestyle/behavioral variables, medication use, fasting blood, and FFQ. The Institutional Review Board of Tufts Medical Center approved the study protocol and procedures and all participants provided written informed consent.
Data collection.
Bilingual interviewers conducted 3- to 4-h interviews in participants’ homes. Questions included education level, smoking, and alcohol use, and the names of all current prescription and over-the-counter medications were recorded. Physical activity was estimated from open-ended questions modeled after the Paffenbarger Harvard Alumni Questionnaire (19) and a summary score was derived based on time spent in different activities and the metabolic equivalents. Poverty was defined using the U.S. Department of Health and Human Services guidelines (20) based on information on total household income, family size, and year of the study interview. Height, weight, and waist circumference were measured, in duplicate, using standard techniques and the means of each were used. Dietary intake was estimated using an interviewer-administered, semiquantitative FFQ that was designed for this population. The FFQ format was initially derived from that of the National Cancer Institute/Block FFQ but was modified using data from Hispanic Health and Nutrition Examination Survey dietary recalls in Puerto Rican adults to more accurately capture foods and portion sizes in the Puerto Rican diet (21). Intake patterns during the previous 12 mo were captured with detailed questions on frequency and portion size of individual foods and mixed meals, beverages, and dietary supplements.
GI and GL.
GI is calculated as the ratio of the glycemic response to a test food over the response to a reference, such as glucose or white bread. For the present study, we used the 2008 International Tables of Glycemic Index and Glycemic Load Values (22) and additional publications (23–25) to create a database of GI and GL values for foods and beverages consumed by our study population. Foods with ≥5 g total carbohydrate/medium portion size were assigned a GI value (26) and the remainder of foods were assigned a GI value of zero. Detailed food preparation data from the FFQ were used to choose appropriate GI values for foods such as plantains, based on whether they were consumed green compared with ripe or boiled compared with fried (27). We used GI values with glucose as the referent and if multiple values were available, we used the mean. For foods without an identical published GI value, we imputed the value from the most similar food based on macronutrient and fiber content as well as preparation method. To calculate each food’s GL and each individual’s dietary GL and dietary GI, the following formulas were applied: GL per food = GI × AvCHO/100, where AvCHO = grams of available carbohydrate in one serving, dietary GL (summed over all foods) = Σ [GL × (number of servings/day)], and dietary GI = dietary GL/total AvCHO × 100. For mixed dishes such as rice and beans or pigeon peas, we utilized the following formula: GImixed meal = Σ GIa × AvCHOa/AvCHOtot, where GIa = GI of the ath food, AvCHOa = grams of available carbohydrate in ath food, and AvCHOtot = grams of available carbohydrate in a mixed dish. We calculated the GL of foods, and therefore dietary GI and GL, using available carbohydrate, as done elsewhere (11, 28), because this has been reported to be more biologically meaningful than using total carbohydrate.
Blood lipids and diabetes classification.
Participants provided a 12-h fasting blood sample within a few days after their study interview. Serum glucose was measured using an enzymatic kinetic reaction on the Olympus AU400e using Olympus Glucose Reagents (OSCR6121) (Olympus America) with an intra-assay CV of 2%. Blood lipids were obtained from EDTA plasma used for the lipoprotein profile with an enzymatic endpoint reaction on the Olympus AU400e with Olympus HDL-C (OSR6156) and TG (OSR6033) Reagents (Olympus America); the intra-assay CV for TG was 2.8%. For the present study, we classified participants as having T2D if their fasting plasma glucose concentration was ≥126 mg/dL (7.0 mmol/L) and/or they were taking oral diabetes medication or insulin (29).
Statistical analyses.
Nutrient intakes were computed using Nutrition Data System for Research software, version 2007 (Nutrition Coordinating Center, University of Minnesota) and statistical analyses were conducted in SAS for WINDOWS, version 9.1 (SAS Institute). All P values were 2-sided. Individuals with an invalid FFQ, based on implausible total energy intakes of <600 or >4800 kcal and/or missing food items (≥7), were excluded from the present analyses (n = 66). Lifestyle variables by T2D status were compared using Student’s t test for age and Pearson’s chi-square test for categorical variables. To obtain the adjusted mean intake of selected dietary characteristics, including nutrients, dietary GI and GL, and food groups, we first winsorized the data (30) by identifying data points that were >2 SD from the remainder of the distribution and substituting them with the next closest data point to avoid undue influence by outliers. This was necessary only for intake outliers at the high end of the distribution. Dietary intake means were then computed with ANCOVA using untransformed data adjusting for age, sex, and total energy intake; however, significant differences by T2D were assessed using log-transformed data, for all variables except dietary GI, to fulfill the homoscedasticity assumption of multivariate linear regression. Food rankings for dietary carbohydrate, GL, and fiber were obtained using the PROC RANK procedure.
Finally, quintile categories of dietary carbohydrate, GI, GL, and fiber were separately created in individuals with or without T2D to evaluate associations with blood lipids. All of these except dietary GI were energy adjusted using the residual method (31) prior to creating quintiles; dietary GI was not energy adjusted because it is a measure of carbohydrate quality, which is not directly correlated with energy intake. As plasma HDL-C and TG concentrations were log-transformed to improve normality, geometric mean concentrations across quintiles of dietary variables were computed using ANCOVA in 2 models. Model 1 was adjusted for age (y), sex, education level (≤ or >8th grade), smoking (never, past, or current), alcohol use (never, past, or current), physical activity level (sedentary/light or moderate/heavy), waist circumference (cm), and lipid-lowering medication use (yes/no). For dietary carbohydrate, GI, and GL analyses, model 2 was additionally adjusted for dietary fat and fiber intake. For dietary fiber analyses, model 2 was additionally adjusted for dietary fat and carbohydrate intake. Tests for linear trend of blood lipids across quintiles of dietary carbohydrate, GI, GL, and fiber were conducted by including the median value per quintile as a continuous variable in multivariate linear regression models. Analyses were tested for effect modification by sex, lipid-lowering medication use, and waist circumference, but no significant interaction was detected.
Results
The prevalence of T2D in this sample of Puerto Rican adults aged 45–75 y was 39.5%. Individuals with T2D tended to be older (P < 0.0001) and have less education (P < 0.005) and were less likely to be current smokers (P = 0.02) or alcohol consumers (P = 0.0002) than those without T2D (Table 1). Individuals with T2D were more likely to be obese (BMI ≥30 kg/m2; 68.4 vs. 49.9%, respectively) and display central obesity (79.6 vs. 65.9%, respectively) and they were approximately twice as likely to be taking antihypertensive (76.9 vs. 40.4%, respectively) or lipid-lowering medications (62.1 vs. 26.3%, respectively) compared with those without T2D (P < 0.0001 for each characteristic). Glycemic control among those with T2D was relatively poor, as measured by glycosylated hemoglobin; mean (SD) was 8.4 (2.0)%, and 74% had glycosylated hemoglobin ≥7% (data not shown). Further, individuals with T2D had lower levels of acculturation than those without T2D according to proxy measures including language acculturation.
TABLE 1.
Lifestyle characteristics of Puerto Rican adults with and without T2D1
| No diabetes (n = 738, 60.5%) | Diabetes (n = 481, 39.5%) | P value | |
| Age, y | 56.3 ± 0.3 | 58.9 ± 0.3 | <0.0001 |
| Female, % | 72.0 | 71.3 | 0.81 |
| Education ≤8th grade, % | 44.9 | 53.2 | 0.005 |
| Poverty, % | 57.2 | 59.4 | 0.47 |
| Born in Puerto Rico, % | 95.6 | 97.5 | 0.09 |
| Length of stay in the US, y | 33.5 ± 0.5 | 35.1 ± 0.6 | 0.03 |
| Language acculturation,2 % | 25.8 ± 0.8 | 21.9 ± 1.0 | 0.003 |
| Physical activity, % | 0.13 | ||
| Sedentary/light | 94.9 | 96.7 | |
| Moderate/heavy | 5.2 | 3.3 | |
| Current smoking, % | 26.9 | 20.9 | 0.02 |
| Current alcohol use, % | 43.5 | 32.8 | 0.0002 |
| BMI, % | <0.0001 | ||
| Normal (<25) | 15.9 | 8.3 | |
| Overweight (25–29) | 34.2 | 23.3 | |
| Obese class I (30–34) | 29.1 | 31.6 | |
| Obese class II (35–39) | 12.6 | 20.6 | |
| Obese class III (≥40) | 8.2 | 16.2 | |
| Central obesity,3 % | 65.9 | 79.6 | <0.0001 |
| Antihypertensive medication use, % | 40.4 | 76.9 | <0.0001 |
| Lipid-lowering medication use, % | 26.3 | 62.1 | <0.0001 |
| Diabetes medication use, % | |||
| None | – | 17.7 | – |
| Oral only | – | 56.3 | – |
| Oral and insulin | – | 26.0 | – |
Values are mean ± SEM or percentages. T2D, type 2 diabetes.
Language acculturation was assessed using a modified Bidimensional Acculturation Scale for Hispanics (54), which asks about language(s) typically used for 7 daily activities. Responses were recorded on a 5-point Likert scale, with 1 being “only Spanish” and 5 being “only English,” and a summary score was constructed with a range of 0 to 100, with 0 representing 0% acculturation (Spanish only) and 100, 100% acculturation (English only).
Central obesity was defined as waist circumference >102 cm for men and >88 cm for women.
Compared with those without T2D, individuals with T2D consumed less carbohydrate and had a lower dietary GL (P < 0.0001 for both), which appeared to be mainly from lower intake of total sugars (99.4 vs. 110 g/d; P < 0.0001) (Table 2). Total dietary fiber was similar between groups, with the main source being legume fiber, comprising ~33% of total fiber (data not shown). With respect to food sources of dietary fiber, individuals with T2D consumed more hot breakfast cereal (74.2 vs. 60.5 g/d; P = 0.0008) than did those without T2D. For other main carbohydrate food sources, individuals with T2D reported lower intakes of fruit juice (P = 0.02) and sugar-sweetened beverages such as fruit drinks and soda (P = 0.02 and P < 0.0001, respectively) as well as sugar added to coffee and/or tea (P < 0.0001). Dietary GL food sources were similar to carbohydrate food sources but included more medium- and high- (vs. low-) GI foods (Table 3). Regardless of T2D status, legumes (beans and peas) were the top contributor to dietary fiber intake, followed by grains, both whole (oatmeal and whole wheat bread) and refined (rice with beans/peas, white bread, and pasta), and starchy vegetables (plantains and sweet potatoes).
TABLE 2.
Dietary characteristics of Puerto Rican adults with and without T2D1
| No diabetes (n = 738, 60.5%) | Diabetes (n = 481, 39.5%) | P value2 | |
| Carbohydrate, g/d | 272 ± 2 | 265 ± 2 | <0.0001 |
| Dietary GI | 57.4 ± 0.2 | 57.2 ± 0.2 | 0.25 |
| Dietary GL | 145 ± 1 | 140 ± 1 | <0.0001 |
| Total dietary fiber, g/d | 18.9 ± 0.2 | 19.4 ± 0.3 | 0.30 |
| Total energy, kJ/d | 9530 ± 142 | 9350 ± 172 | 0.24 |
| Total sugars, g/d | 110 ± 2 | 99 ± 2 | <0.0001 |
| Protein, g/d | 89.7 ± 0.7 | 94.7 ± 0.8 | 0.0002 |
| Total fat, g/d | 77.5 ± 0.6 | 79.9 ± 0.7 | 0.02 |
| Food groups,3 g/d | |||
| Beans | 70.2 ± 2.4 | 69.9 ± 2.9 | 0.81 |
| Cold breakfast cereal | 10.1 ± 0.5 | 9.9 ± 0.6 | 0.35 |
| Hispanic root crops | 43.7 ± 1.6 | 43.4 ± 2.0 | 0.70 |
| Hot breakfast cereal4 | 60.5 ± 2.8 | 74.2 ± 3.3 | 0.0008 |
| 100% fruit juice | 177 ± 9 | 169 ± 11 | 0.02 |
| Fruit drinks (not 100% juice) | 97.2 ± 6.8 | 93.9 ± 8.1 | 0.02 |
| Milk, plain or in cereal | 182 ± 7 | 189 ± 9 | 0.09 |
| Pasta | 35.4 ± 1.6 | 39.6 ± 2.0 | 0.48 |
| Soft drinks, regular | 80.2 ± 6.7 | 61.1 ± 8.0 | <0.0001 |
| Sugar in coffee or tea | 11.9 ± 0.5 | 5.9 ± 0.6 | <0.0001 |
| White bread and bagels | 32.9 ± 1.2 | 32.7 ± 1.4 | 0.60 |
| White rice including mixed dishes | 196 ± 5 | 189 ± 6 | 0.14 |
Values are mean ± SEM. Means of dietary variables by diabetes status were obtained using ANCOVA, adjusting for age, sex, and total energy. GI, glycemic index; GL, glycemic load; T2D, type 2 diabetes.
P values based on significance tests with logged data for all variables except dietary GI.
Food groups listed were the top contributors to dietary carbohydrate in the study sample.
Hot breakfast cereal included oatmeal, grits, cream of wheat, and cream of rice cereal.
TABLE 3.
Top contributors to carbohydrate, dietary GL, and dietary fiber in Puerto Rican adults with and without T2D1
| Food group | No diabetes (n = 738, 60.5%) |
Diabetes (n = 481, 39.5%) |
GI | GI class2 | ||
| % | Rank | % | Rank | |||
| Carbohydrate | ||||||
| White rice | 6.2 | 1 | 6.2 | 1 | 79 | High |
| Rice with beans or pigeon peas | 5.7 | 2 | 5.6 | 2 | 74 | High |
| Beans | 4.4 | 3 | 4.7 | 3 | 31 | Low |
| Sugar in coffee and/or tea | 4.4 | 4 | 2.2 | 9 | 65 | Medium |
| Orange juice (100% juice) | 3.3 | 5 | 3.3 | 4 | 50 | Low |
| White bread | 3.3 | 6 | 3.2 | 5 | 71 | High |
| Cold cereal | 3.1 | 7 | 3.2 | 6 | 71 | High |
| Soft drinks, regular | 3.0 | 8 | 2.5 | 7 | 57 | Medium |
| Plantains, green bananas | 2.4 | 9 | 2.4 | 8 | 50 | Low |
| Pasta | 1.8 | 10 | 2.0 | 11 | 44 | Low |
| Oatmeal | 1.5 | 14 | 2.0 | 10 | 65 | Medium |
| Dietary GL | ||||||
| White rice | 9.0 | 1 | 9.2 | 1 | 79 | High |
| Rice with beans or pigeon peas | 6.4 | 2 | 6.4 | 2 | 74 | High |
| Sugar in coffee and/or tea | 5.3 | 3 | 2.5 | 7 | 65 | Medium |
| White bread | 4.3 | 4 | 4.2 | 3 | 71 | High |
| Cold cereal | 3.8 | 5 | 4.1 | 4 | 71 | High |
| Soft drinks, regular | 3.2 | 6 | 2.7 | 6 | 57 | Medium |
| Orange juice (100% juice) | 3.0 | 7 | 3.1 | 5 | 50 | Low |
| Plantains, green bananas | 2.3 | 8 | 2.3 | 8 | 50 | Low |
| Rice with chicken | 2.2 | 9 | 2.2 | 9 | 74 | High |
| Cranberry juice | 2.0 | 10 | 2.2 | 11 | 59 | Medium |
| Oatmeal | 1.5 | 13 | 2.2 | 10 | 65 | Medium |
| Dietary fiber | ||||||
| Beans | 21.4 | 1 | 21.4 | 1 | 31 | Low |
| Rice with beans or pigeon peas | 7.4 | 2 | 6.8 | 2 | 74 | High |
| Oatmeal | 3.3 | 3 | 4.3 | 3 | 65 | Medium |
| Peas (pigeon peas or cowpeas) | 3.0 | 4 | 2.9 | 4 | 45 | Low |
| Cold cereal | 3.0 | 5 | 2.9 | 5 | 71 | High |
| Plantains, green bananas | 2.6 | 6 | 2.5 | 6 | 50 | Low |
| White bread | 1.9 | 7 | 2.1 | 7 | 71 | High |
| Salad greens | 1.9 | 8 | 1.9 | 8 | 0 | n/a |
| Pasta | 1.8 | 9 | 1.9 | 10 | 44 | Low |
| Sweet potatoes, yams | 1.8 | 10 | 1.9 | 11 | 70 | High |
| 100% whole wheat bread | 1.6 | 12 | 1.9 | 9 | 69 | Medium |
Data are percentage of the nutrient contributed by each food. GI, glycemic index; GL, glycemic load; T2D, type 2 diabetes.
GI classifications are: low GI: ≤55; medium GI: 56–69; and high GI: ≥70 (55).
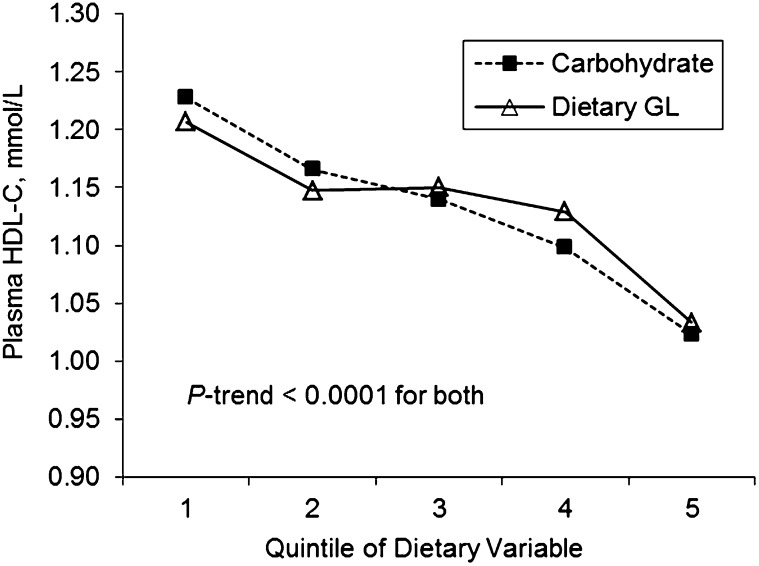
In the total sample, simple correlations among measures of carbohydrate nutrition showed that total carbohydrate was highly correlated with dietary GL and fiber (r = 0.98 and 0.79, respectively; P < 0.0001) but not with dietary GI (r = 0.0014; P = 0.96), although dietary GI was correlated with dietary GL (r = 0.17; P < 0.0001) and inversely with dietary fiber (r = −0.10; P = 0.0003) (data not shown). In individuals with T2D, no significant associations were observed between measures of carbohydrate nutrition and HDL-C or TG. Among individuals without T2D, multivariate analyses with adjustment for dietary fiber intake showed strong inverse trends between HDL-C and both carbohydrate and dietary GL (P-trend < 0.0001) (Fig. 1). However, no association was observed between dietary GI and HDL-C (data not shown). An inverse association was initially found between dietary fiber and HDL-C; however, this association was attenuated and no longer significant after adjusting for total carbohydrate intake (data not shown). No significant associations were observed between TG and carbohydrate, dietary GI, GL, or fiber intake in individuals without T2D.
FIGURE 1.
Adjusted mean plasma HDL-C concentrations across quintiles of carbohydrate and dietary GL in Puerto Rican adults without T2D, n = 738. Carbohydrate and dietary GL were energy adjusted using the residual method before creating quintiles. Values are geometric means obtained by back-transforming logged HDL-C concentrations and using ANCOVA, adjusted for age, sex, education level, smoking, alcohol use, physical activity, waist circumference, lipid-lowering medication use, total energy, total fat, and dietary fiber intake. P-trend < 0.0001 for both. GL, glycemic load; HDL-C, HDL-cholesterol; T2D, type 2 diabetes.
Discussion
To our knowledge, this is the first cross-sectional study to investigate measures of carbohydrate nutrition in Puerto Rican adults in relation to T2D status and to report significant inverse associations between HDL-C and total carbohydrate and dietary GL in Puerto Rican adults without T2D. Dietary intake differed between groups, suggesting that individuals with T2D may have modified their diet as a consequence of the disease, specifically, toward lower intake of carbohydrate, dietary GL, and total sugars. Among individuals without T2D, significant inverse associations were observed between total carbohydrate and dietary GL in relation to HDL-C, but no association was observed between any carbohydrate nutrition measure and TG. An initial association between dietary fiber and HDL-C was confounded by carbohydrate intake, likely due to frequent consumption of legumes, the primary dietary fiber source, with white rice, a refined-carbohydrate food.
In the present study, Puerto Rican adults with T2D appeared to have made some dietary modifications toward improved carbohydrate quality, including reduced consumption of sugar-sweetened beverages such as fruit drinks and soda, less sugar added to coffee or tea, and greater intake of hot breakfast cereal. Consequently, those with T2D consumed lower quantities of carbohydrate and total sugars, which translated into lower dietary GL. Although national trends in carbohydrate intake have shown an increase from 1988 to 2004 among adults with self-reported T2D (32), evidence suggests that individuals are making some dietary modifications (33), such as switching to diet soda (34). Nonetheless, in the present study, only 22–25% of individuals met the dietary recommendations for fiber of 25–38 g/d for women and men aged ≤50 y and 21–30 g/d for those aged >50 y (35) despite a high intake of legume fiber (6.4 ± 0.2 g/d), their main fiber source, compared with the general U.S. population (36).
Among individuals with T2D, no associations were observed between measures of carbohydrate nutrition and HDL-C or TG. This may be due to the high prevalence of lipid-lowering medication use (62% of individuals) and the possibility that individuals may have changed their diets in response to their diagnosis. Further, our previous investigation of CVD risk factors in this study population (4) documented low HDL-C concentrations among 62 and 73% of men and women with T2D, respectively. Across categories of glucose dysregulation, prevalent obesity, hyperglycemia, and dyslipidemia were observed, but prevalence was particularly high in individuals with impaired fasting glucose (i.e., prediabetes) or T2D. Among those with T2D, achievement of the ADA treatment goals was low, with only 4% meeting all 3 goals.
In individuals without T2D, consistent with the literature, we found strong inverse associations between HDL-C and carbohydrate intake (8, 11, 13) as well as dietary GL (8, 10, 11, 13), independent of sociodemographic characteristics, lifestyle and behavioral factors, anthropometrics, and medication use. Previous studies have documented the high prevalence of low HDL-C in Puerto Rican adults (4, 6), which contributes to dyslipidemia in those with T2D, and our results suggest a possible link between carbohydrate quantity and quality and low HDL-C in this group. Unlike many reports, we did not detect an association between TG and carbohydrate (8, 9), dietary GI (8, 9), or dietary GL (8, 10, 11, 13–15), which could be due to the relatively lower prevalence of hypertriglyceridemia in Hispanic than in non-Hispanic white adults (37).
Dietary patterns in Puerto Rican adults characterized by frequent consumption of rice and beans have been associated with metabolic syndrome and low HDL-C (17) as well as higher BMI and waist circumference (16). Of the few studies conducted on dietary GI and GL in U.S. Hispanics, 2 studies in Mexican adults reported dyslipidemia (low HDL-C and high TG) with higher dietary GI or GL (15) as well as higher total cholesterol and LDL-C with a high-GI, low-fiber diet (38). However, another study in Cuban Americans found no adverse effects of dietary GI or GL on HDL-C (33). A high intake of white rice has been linked to harmful effects on cardiometabolic risk factors, including low HDL-C and other components of metabolic syndrome (39, 40) as well as T2D (41–43), whereas brown rice intake has been associated with elevated HDL-C (44) and reduced risk of T2D (42). Further, legume intake has shown beneficial cardiometabolic effects, including improved lipid profiles (45, 46) and reduced risk of CVD (47). In the general U.S. population, the diets of rice consumers (48) and bean consumers (36) tend to be more nutrient rich and include more vegetables and dietary fiber than those of nonconsumers, offering greater protection against CVD risk factors (49). However, in our sample, an inverse trend in HDL-C was detected across dietary fiber quintiles, which was confounded by total carbohydrate intake. This could be explained by a unique dietary pattern characterizing dietary fiber intake in this population, in which the main contributors to dietary fiber were both high-fiber, low- to medium-GI foods such as beans and oatmeal as well as low-fiber, high-GI refined grains such as white rice and white bread. Similarly, in a Japanese population, an observed protective effect of steamed white rice intake on CVD outcomes was likely confounded by dietary fiber, as rice was their second major fiber source (50, 51). In populations that consume white rice as a staple, not only is white rice often an important source of fiber, but its concurrent consumption with legumes may muddle metabolic effects. Within a Costa Rican staple dietary pattern, in which consumption of white rice and beans was directly correlated, high-GI foods such as white rice seemed to overcome the potential beneficial effects of beans on low HDL-C and myocardial infarction risk (39). Similarly, for Puerto Rican adults, the relative composition of meals consisting of mainly refined carbohydrate, high-GI white rice consumed with lesser quantities of high-fiber, low-GI legumes may override the favorable effects of legumes and dietary fiber on blood lipids such as HDL-C. The present findings suggest that, whereas legume consumption may be an indicator of a healthy diet in the general U.S. population (36), it was associated with a high-GI dietary pattern in this Puerto Rican population, a novel research finding that deserves future study.
The implications of the study findings for Puerto Rican adults with prediabetes or T2D include the importance of considering carbohydrate source, with a focus on increasing consumption of high-fiber foods and decreasing consumption of refined grain foods as well as an emphasis on behavioral modifications to decrease obesity and other CVD risk factors. We previously found low physical activity levels in this sample of Puerto Rican adults, with ~90% reporting sedentary/light activity (4). Low socioeconomic status, as represented by low levels of education and poverty status, may have been correlated with low physical activity, and low socioeconomic status was also associated with a higher likelihood of T2D and poorer diabetes control. In contrast, being above the poverty level was associated with better carbohydrate quality (lower dietary GI and starch intake and greater fruit and non-starchy vegetable intake) with increased language acculturation in this study sample (52). Taken together, these findings underscore the importance of increased attention on CVD risk profiles as well as culturally sensitive lifestyle intervention and education programs in Puerto Rican adults.
This study has several strengths. First, study participants completed a specially designed FFQ, which allowed the estimation of aspects of carbohydrate nutrition, including dietary GI and GL and categories of dietary fiber, in Puerto Rican adults. Detailed information on traditional Hispanic dishes, including plantains and white rice-based mixed dishes, was used to assign appropriate GI values to foods and to create an extensive GI database for future studies in this population. Collection of fasting blood samples and information on prescribed medications allowed objective classification of individuals with T2D, minimizing error of self-reports. Finally, this was a large sample of community-dwelling Puerto Rican adults with extensive questionnaire, anthropometric, and biological data. Data from biological samples, medication use, and dietary intake provided the opportunity to investigate associations between blood lipids and carbohydrate nutrition measures in this understudied population.
The limitations of this research include the cross-sectional design and lack of data on duration of T2D; however, the extensive diabetes, antihypertensive, and lipid-lowering medication use suggests that, on average, individuals may have been experiencing an advanced stage of the disease. A single, fasting blood measure may have misclassified some individuals with T2D, but this is a valid method of disease classification recommended by the ADA (29). Finally, for some foods, an exact GI value was not available and thus GI assignments were based on imputed values from similar foods or estimated using mixed-meal calculations. Nonetheless, we think that foods and mixed meals were appropriately ranked, producing a valid estimate of dietary GI and GL.
Puerto Rican adults with T2D had evidence of dietary improvement toward better carbohydrate quality, which included lower intakes of carbohydrate, GL, and sugars compared with those without T2D. In individuals without T2D, inverse associations with HDL-C reflected the harmful effects of a high-carbohydrate, high-dietary GL diet and the confounding effect of refined carbohydrate intake on dietary fiber in this population. Dietary recommendations for Puerto Rican adults should include consuming a greater proportion of beans to white rice, as has been associated with more favorable cardiometabolic profiles in Costa Rican adults (53), and replacement of white rice with brown rice (42, 44). In addition, continued research on the effects of white rice, legumes, and other possible factors influencing glucose metabolism, dyslipidemia, T2D, and CVD risk in this ethnic group is warranted.
Acknowledgments
M.I.V.R. designed the research, analyzed the data, and wrote the manuscript; and N.M.M., C.C.-S., J.M.O., and K.L.T. provided feedback on the analyses and critical comments on the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ADA, American Diabetes Association; CVD, cardiovascular disease; GI, glycemic index; GL, glycemic load; HDL-C, HDL-cholesterol; T2D, type 2 diabetes.
Literature Cited
- 1.U.S. Census Bureau U.S. Hispanic population surpasses 45 million. Now 15 percent of total [news release]; 2008. [cited 2009 Nov 17]. Available from: http://www.census.gov/Press-Release/www/releases/archives/population/011910.html
- 2.National Institute of Diabetes and Digestive and Kidney Diseases National diabetes statistics; 2011 [cited 2012 Oct 17]. Available from: http://diabetes.niddk.nih.gov/dm/pubs/statistics/DM_Statistics_508.pdf
- 3.Fitzgerald N, Himmelgreen D, Damio G, Segura-Perez S, Peng YK, Perez-Escamilla R. Acculturation, socioeconomic status, obesity and lifestyle factors among low-income Puerto Rican women in Connecticut, U.S., 1998–1999. Rev Panam Salud Publica. 2006;19:306–13 [DOI] [PubMed] [Google Scholar]
- 4.Van Rompay MI, Castaneda-Sceppa C, McKeown NM, Ordovas JM, Tucker KL. Prevalence of cardiovascular disease risk factors among older Puerto Rican adults living in Massachusetts. J Immigr Minor Health. 2011;13:825–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin H, Bermudez OI, Falcon LM, Tucker KL. Hypertension among Hispanic elders of a Caribbean origin in Massachusetts. Ethn Dis. 2002;12:499–507 [PubMed] [Google Scholar]
- 6.Bermudez OI, Velez-Carrasco W, Schaefer EJ, Tucker KL. Dietary and plasma lipid, lipoprotein, and apolipoprotein profiles among elderly Hispanics and non-Hispanics and their association with diabetes. Am J Clin Nutr. 2002;76:1214–21 [DOI] [PubMed] [Google Scholar]
- 7.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31 Suppl 1:S61–78 [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Li Y, Chiriboga DE, Olendzki BC, Hebert JR, Li W, Leung K, Hafner AR, Ockene IS. Association between carbohydrate intake and serum lipids. J Am Coll Nutr. 2006;25:155–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKeown NM, Meigs JB, Liu S, Rogers G, Yoshida M, Saltzman E, Jacques PF. Dietary carbohydrates and cardiovascular disease risk factors in the Framingham offspring cohort. J Am Coll Nutr. 2009;28:150–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Manson JE, Stampfer MJ, Holmes MD, Hu FB, Hankinson SE, Willett WC. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr. 2001;73:560–6 [DOI] [PubMed] [Google Scholar]
- 11.Liese AD, Gilliard T, Schulz M, D'Agostino RB, Jr, Wolever TM. Carbohydrate nutrition, glycaemic load, and plasma lipids: the Insulin Resistance Atherosclerosis Study. Eur Heart J. 2007;28:80–7 [DOI] [PubMed] [Google Scholar]
- 12.Estruch R, Martinez-Gonzalez MA, Corella D, Basora-Gallisa J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Escoda R, et al. Effects of dietary fibre intake on risk factors for cardiovascular disease in subjects at high risk. J Epidemiol Community Health. 2009;63:582–8 [DOI] [PubMed] [Google Scholar]
- 13.Radhika G, Ganesan A, Sathya RM, Sudha V, Mohan V. Dietary carbohydrates, glycemic load and serum high-density lipoprotein cholesterol concentrations among South Indian adults. Eur J Clin Nutr. 2009;63:413–20 [DOI] [PubMed] [Google Scholar]
- 14.Nakashima M, Sakurai M, Nakamura K, Miura K, Yoshita K, Morikawa Y, Ishizaki M, Murakami K, Kido T, Naruse Y, et al. Dietary glycemic index, glycemic load and blood lipid levels in middle-aged Japanese men and women. J Atheroscler Thromb. 2010;17:1082–95 [DOI] [PubMed] [Google Scholar]
- 15.Denova-Gutierrez E, Huitron-Bravo G, Talavera JO, Castanon S, Gallegos-Carrillo K, Flores Y, Salmeron J. Dietary glycemic index, dietary glycemic load, blood lipids, and coronary heart disease. J Nutr Metab. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin H, Bermudez OI, Tucker KL. Dietary patterns of Hispanic elders are associated with acculturation and obesity. J Nutr. 2003;133:3651–7 [DOI] [PubMed] [Google Scholar]
- 17.Noel SE, Newby PK, Ordovas JM, Tucker KL. A traditional rice and beans pattern is associated with metabolic syndrome in Puerto Rican older adults. J Nutr. 2009;139:1360–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paffenbarger RS, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–45 [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department of Health & Human Services The 2009. HHS Poverty Guidelines [cited 2009 Jul 9]. Available from: http://aspe.hhs.gov/poverty/09poverty.shtml
- 21.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–18 [DOI] [PubMed] [Google Scholar]
- 22.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31:2281–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noriega E, Rivera L, Peralta E. Glycaemic and insulinaemic indices of Mexican foods high in complex carbohydrates. Diabetes Nutr Metab. 2000;13:13–9 [PubMed] [Google Scholar]
- 24.Noriega E, Peralta E, Rivera L, Saucedo S. Glycaemic and insulinaemic indices of Mexican foods high in complex carbohydrates in Type 2 diabetic subjects. Diabetes Nutr Metab. 2001;14:43–50 [PubMed] [Google Scholar]
- 25.Frati-Munari AC, Roca-Vides RA, Lopez-Perez RJ, de Vivero I, Ruiz-Velazco M. [The glycemic index of some foods common in Mexico] Gac Med Mex. 1991;127:163–70, discussion 170–1 [PubMed] [Google Scholar]
- 26.Neuhouser ML, Tinker LF, Thomson C, Caan B, Horn LV, Snetselaar L, Parker LM, Patterson RE, Robinson-O'Brien R, Beresford SA, et al. Development of a glycemic index database for food frequency questionnaires used in epidemiologic studies. J Nutr. 2006;136:1604–9 [DOI] [PubMed] [Google Scholar]
- 27.Bahado-Singh PS, Wheatley AO, Ahmad MH, Morrison EY, Asemota HN. Food processing methods influence the glycaemic indices of some commonly eaten West Indian carbohydrate-rich foods. Br J Nutr. 2006;96:476–81 [PubMed] [Google Scholar]
- 28.Murakami K, Sasaki S, Okubo H, Takahashi Y, Hosoi Y, Itabashi M. Dietary fiber intake, dietary glycemic index and load, and body mass index: a cross-sectional study of 3931 Japanese women aged 18–20 years. Eur J Clin Nutr. 2007;61:986–95 [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association Standards of medical care in diabetes: 2009. Diabetes Care. 2009;32 Suppl 1:S13–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon W, Yuen K. Trimming and winsorization: a review. Statist Papers. 1974;15:157–70 [Google Scholar]
- 31.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65 Suppl 4:S1220–8; discussion S1229–31. [DOI] [PubMed] [Google Scholar]
- 32.Oza-Frank R, Cheng YJ, Narayan KM, Gregg EW. Trends in nutrient intake among adults with diabetes in the United States: 1988–2004. J Am Diet Assoc. 2009;109:1173–8 [DOI] [PubMed] [Google Scholar]
- 33.Huffman FG, Zarini GG, Cooper V. Dietary glycemic index and load in relation to cardiovascular disease risk factors in Cuban American population. Int J Food Sci Nutr. 2010;61:690–701 [DOI] [PubMed] [Google Scholar]
- 34.Mackenzie T, Brooks B, O'Connor G. Beverage intake, diabetes, and glucose control of adults in America. Ann Epidemiol. 2006;16:688–91 [DOI] [PubMed] [Google Scholar]
- 35.Food and Nutrition Board Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2005 [DOI] [PubMed] [Google Scholar]
- 36.Mitchell DC, Lawrence FR, Hartman TJ, Curran JM. Consumption of dry beans, peas, and lentils could improve diet quality in the US population. J Am Diet Assoc. 2009;109:909–13 [DOI] [PubMed] [Google Scholar]
- 37.Sharma MD, Pavlik VN. Dyslipidaemia in African Americans, Hispanics and whites with type 2 diabetes mellitus and hypertension. Diabetes Obes Metab. 2001;3:41–5 [DOI] [PubMed] [Google Scholar]
- 38.Jimenez-Cruz A, Turnbull WH, Bacardi-Gascon M, Rosales-Garay P. A high-fiber, moderate-glycemic index, Mexican style diet improves dyslipidemia in individuals with type 2 diabetes. Nutr Res. 2004;24:19–27 [Google Scholar]
- 39.Martínez-Ortiz JA, Fung TT, Baylin A, Hu FB, Campos H. Dietary patterns and risk of nonfatal acute myocardial infarction in Costa Rican adults. Eur J Clin Nutr. 2006;60:770–7 [DOI] [PubMed] [Google Scholar]
- 40.Radhika G, Van Dam RM, Sudha V, Ganesan A, Mohan V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57). Metabolism. 2009;58:675–81 [DOI] [PubMed] [Google Scholar]
- 41.Villegas R, Liu S, Gao YT, Yang G, Li H, Zheng W, Shu XO. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med. 2007;167:2310–6 [DOI] [PubMed] [Google Scholar]
- 42.Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik VS, Willett WC, Hu FB. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med. 2010;170:961–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ. 2012;344:e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JY, Kim JH. Lee da H, Kim SH, Lee SS. Meal replacement with mixed rice is more effective than white rice in weight control, while improving antioxidant enzyme activity in obese women. Nutr Res. 2008;28:66–71 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, Lanza E, Kris-Etherton PM, Colburn NH, Bagshaw D, Rovine MJ, Ulbrecht JS, Bobe G, Chapkin RS, Hartman TJ. A high legume low glycemic index diet improves serum lipid profiles in men. Lipids. 2010;45:765–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazzano LA, Thompson AM, Tees MT, Nguyen CH, Winham DM. Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21:94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagura J, Iso H, Watanabe Y, Maruyama K, Date C, Toyoshima H, Yamamoto A, Kikuchi S, Koizumi A, Kondo T, et al. Fruit, vegetable and bean intake and mortality from cardiovascular disease among Japanese men and women: the JACC Study. Br J Nutr. 2009;102:285–92 [DOI] [PubMed] [Google Scholar]
- 48.Batres-Marquez SP, Jensen HH, Upton J. Rice consumption in the United States: recent evidence from food consumption surveys. J Am Diet Assoc. 2009;109:1719–27 [DOI] [PubMed] [Google Scholar]
- 49.Papanikolaou Y, Fulgoni VL III. Bean consumption is associated with greater nutrient intake, reduced systolic blood pressure, lower body weight, and a smaller waist circumference in adults: results from the National Health and Nutrition Examination Survey 1999–2002. J Am Coll Nutr. 2008;27:569–76 [DOI] [PubMed] [Google Scholar]
- 50.Eshak ES, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Wakai K, Tamakoshi A. Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. J Nutr. 2010;140:1445–53 [DOI] [PubMed] [Google Scholar]
- 51.Eshak ES, Iso H, Date C, Yamagishi K, Kikuchi S, Watanabe Y, Wada Y, Tamakoshi A. Rice intake is associated with reduced risk of mortality from cardiovascular disease in Japanese men but not women. J Nutr. 2011;141:595–602 [DOI] [PubMed] [Google Scholar]
- 52.van Rompay MI, McKeown NM, Castaneda-Sceppa C, Falcon LM, Ordovas JM, Tucker KL. Acculturation and sociocultural influences on dietary intake and health status among Puerto Rican adults in Massachusetts. J Acad Nutr Diet. 2012;112:64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mattei J, Hu FB, Campos H. A higher ratio of beans to white rice is associated with lower cardiometabolic risk factors in Costa Rican adults. Am J Clin Nutr. 2011;94:869–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marin G, Gamba RJ. A new measurement of acculturation for Hispanics: The Bidimensional Acculturation Scale for Hispanics (BAS). Hisp J Behav Sci. 1996;18:297–316 [Google Scholar]
- 55.Monro JA. Glycaemic glucose equivalent: combining carbohydrate content, quantity and glycaemic index of foods for precision in glycaemia management. Asia Pac J Clin Nutr. 2002;11:217–25 [DOI] [PubMed] [Google Scholar]