Abstract
Marfan syndrome (MFS) is an autosomal dominant disorder of the connective tissue characterized by early development of thoracic aortic aneurysms/dissections together with symptoms of the ocular and skeletal systems. While most patients/families with a classic phenotypic expression of MFS harbour mutations in the gene encoding fibrillin-1 (FBN1), genetic studies of the recent years revealed that the clinical features, as well as the mutated genes, show a high degree of overlap between MFS and other connective tissue diseases (e.g. Loeys-Dietz syndrome, Ehlers-Danlos syndrome, familial thoracic aneurysms and dissections and others). We summarize herein the current knowledge about the wide spectrum of differential diagnoses and their genetic background as well as novel therapeutic approaches in order to provide appropriate counselling and clinical follow-up for the patients.
Key Words: Connective tissue disorders, Ehlers-Danlos syndrome, Loeys-Dietz syndrome, Marfan syndrome
Marfan syndrome (MFS), first described by Antoine Marfan in 1896, is an autosomal dominant disorder of the connective tissue with a prevalence of approximately 1:5,000. Characteristic manifestations of MFS include involvement of the cardiovascular, ocular and skeletal systems, while lung, skin and dura are less frequently affected [Cañadas et al., 2010]. A key cardiovascular symptom of MFS is the development of an aneurysm or dissection of the thoracic aorta, especially in younger patients (<50 years of age). Additional to this life-threatening cardiovascular feature, ectopia lentis and a so-called marfanoid habitus with long and thin extremities, arachnodactyly, thorax deformities, scoliosis, joint hypermobility, and flat feet are considered to be cardinal symptoms of MFS [Cañadas et al., 2010]. Therapeutic options for MFS generally include regular (e.g. annual) echocardiographic evaluations, surgical repair of the aorta when the maximal measurement approaches certain thresholds as well as regular orthopaedic and ophthalmologic surveillance. So far, the only established pharmacological therapy is the application of β-receptor antagonists which has proven to reduce aneurysm progression by lowering the blood pressure and thus, reducing physical stress to the aortic wall [Hartog et al., 2012]. However, new therapeutic strategies based on recent pathogenetic findings are currently under development (see below).
In 1991, mutations in the gene encoding fibrillin 1 (FBN1) on chromosome 15q were identified as causative for MFS [Dietz et al., 1991]. While it still holds true that most patients/families with a complete classical phenotypic expression of MFS harbour FBN1 mutations, genetic studies of the recent years clearly emphasized that the clinical features, as well as the mutated genes, show a high degree of overlap between MFS and other connective tissue diseases (e.g. Loeys-Dietz syndrome (LDS), Ehlers-Danlos syndrome (EDS), familial thoracic aneurysms and dissections (fTAAD), and others) [von Kodolitsch et al., 2010]. Thus, correctly establishing the diagnosis of MFS or a related connective tissue disease may be challenging in clinical practice and should incorporate knowledge about the wide spectrum of differential diagnoses and their genetic background in order to provide appropriate counselling and clinical follow-up for the patients.
Marfan Syndrome
Diagnostic Criteria
Additional to the cardinal cardiovascular, ocular and skeletal symptoms of MFS presented above, Marfan patients often show high myopia, retinal detachment, mitral valve prolapse, distinct facial features (including dolichocephaly, high-arched palate, down-slanting palpebral fissures, and micrognathia), striae in the skin that do not result from extensive weight loss or pregnancy, and spontaneous pneumothorax [Cañadas et al., 2010]. Typical radiologic signs further include dural ectasia and protrusio acetabuli. Yet, the phenotypic expression can be very variable between patients, even within the same family. To facilitate the clinical diagnosis of MFS, the Ghent diagnostic criteria were established in 1991, distinguishing between major and minor symptoms in the involved organ systems [De Paepe et al., 1996]. These criteria were recently revised in order to better incorporate the variable clinical expression and the extended differential diagnosis [Loeys et al., 2010a]. In the revised Ghent nosology, summarized in table 1, more weight is laid on the cardinal clinical features aortic root dilation/dissection and ectopia lentis. Further, it highlights the impact of genetic analysis since a pathogenic FBN1 mutation constitutes an important criterion for the diagnosis of MFS: for example, the combination of an aortic aneurysm or dissection and a pathogenic FBN1 mutation is now sufficient to establish the diagnosis of MFS. On the other hand, all other cardiovascular and ocular manifestations, as well as symptoms in other organ systems (skeleton, dura, skin, and lung), were included in a systemic score (table 1). A systemic score ≥7 in combination with an aortic aneurysm/dissection or a positive family history also constitutes the diagnosis of MFS. Some of the less specific symptoms for MFS were either removed (e.g. joint hypermobility) or are less emphasized in the diagnostic evaluation (e.g. dural ectasia). Finally, the new nosology also allows the discrimination of several phenotypes closely related to MFS [Loeys et al., 2010a]. Ectopia lentis syndrome is proposed, irrespective of the systemic score, for patients with ectopia lentis but no aortic aneurysm/dissection that do not harbour an FBN1 mutation or carry an FBN1 mutation not known to be associated with aortic involvement. MASS syndrome (myopia, mitral valve prolapse, borderline and nonprogressive aortic root dilatation, skeletal findings, and striae) is defined by a mild aortic dilation (Z score <2) and a systemic score ≥5 without ectopia lentis, and mitral valve prolapse syndrome includes patients with mitral valve prolapse, mild aortic dilation and a systemic score >5 without ectopia lentis. As with the former Ghent criteria, establishing the correct diagnosis in children and adolescents may be difficult since some symptoms, especially aortic dilation, may evolve over time. For children with a systemic score <7 and borderline aortic root measurements, the term ‘nonspecific connective tissue disorder’ is proposed, whereas for children with a known FBN1 mutation but only with borderline aortic root dilation, ‘potential MFS’ is suggested [Loeys et al., 2010a]. Taken together, the authors conclude that the revised nosology may delay a definitive diagnosis of MFS in some patients but overall reduce the risk for premature diagnosis or misdiagnosis [Loeys et al., 2010a].
Table 1.
Revised Ghent criteria adapted from Loeys et al. [2010a]
| The diagnosis of MFS can be established if the following criteria applya: |
In the absence of a family history
|
In the presence of a family history
|
| Systematic score | |
| Feature | Points |
| Wrist and thumb sign | 3 |
| Wrist or thumb sign | 1 |
| Pectus carinatum | 2 |
| Pectus excavatum | 1 |
| Hindfoot deformity | 2 |
| Pes planus | 1 |
| Pneumothorax | 2 |
| Dural ectasia | 2 |
| Protrusio acetabuli | 2 |
| Reduced upper segment/lower segment ration and increased arm/height ratio without severe scoliosis | 1 |
| Scoliosis or thoracolumbar kyphosis | 1 |
| Reduced elbow extension | 1 |
| Facial features (≥3 of dolichocephaly, enophthalmos, downslanting palpebral fissures, malar hypoplasia, and retrognathia) | 1 |
| Skin striae | 1 |
| Myopia (>3 dpt) | 1 |
| Mitral valve proplaps | 1 |
Without discriminating features of Shprintzen-Goldberg syndrome, LDS or EDS.
Several studies recently aimed at comparing the old and revised Ghent criteria in patient groups with suspected MFS and a known FBN1 mutation. Overall, the level of agreement between both nosologies was high: 89% versus 83% of patients met the old and new criteria, respectively, in 1,009 probands from the Marfan database (see below) [Faivre et al., 2012] and 81% versus 79% in 106 Korean patients [Yang et al., 2011]. However, up to 15% of cases received a different diagnosis such as Ectopia lentis syndrome or MASS according to the revised criteria [Faivre et al., 2012]. It has to be considered, though, that these patients may develop classic MFS over time. Further, one group criticized that the definition of the cardinal criterion aortic dilatation is based exclusively on Z-scores which may, in their opinion, underestimate aortic involvement [Radonic et al., 2011]. Another analysis revealed that for the revised criteria, genotype information is essential for diagnosis or exclusion of MFS [Sheikhzadeh et al., 2011], emphasizing the importance of molecular genetic analysis in patients with suspected MFS.
Mutational Analysis
The FBN1 gene on chromosome 15q21 comprises 65 coding and 3 alternatively spliced exons and encodes fibrillin-1, a cysteine-rich glycoprotein with a molecular weight of 350 kD. Fibrillin-1 and the related fibrillin-2 constitute major components of the extracellular microfibrils that interact with other extracellular matrix proteins and form sheaths around elastic fibers, thus, playing an important role for stability and elasticity of connective tissues [Bonetti, 2009]. Fibrillin-1 is composed of 3 characteristic modules: an epidermal growth factor (EGF)-like motif, a latent transforming growth factor beta (TGFβ)-binding protein motif and a fusion module. Of the 47 EGF-like modules, 43 include a highly conserved calcium-binding sequence (cbEGF) [Turner et al., 2009]. More than 600 mutations in the FBN1 gene have been described so far (Universal Marfan database UMD-FBN1, http://www.umd.be), two-thirds of which are missense mutations that mainly affect the cbEGF modules [Turner et al., 2009]. Other mutations include frameshift, splice site and nonsense mutations. With the implementation of MLPA analysis, partial and whole-gene deletions have been described in a few patients [Furtado et al., 2011; Hilhorst-Hofstee et al., 2011]. So far, analyses of genotype-phenotype correlations revealed only few consolidated findings. It was shown that patients presenting with a severe phenotype in early childhood, also referred to as ‘neonatal Marfan syndrome’, mostly harbour mutations in exons 24–32 of the FBN1 gene; however, not all patients with a severe phenotype carry mutations in this region, and some patients with mutations in exons 24–32 show a mild or classic disease [Faivre et al., 2009]. While deletions of one or few exons within the FBN1 gene mostly showed a more severe phenotype, whole-gene deletions were associated with mild to classic disease expression [Hilhorst-Hofstee et al., 2011]. Overall, however, the phenotypic expression is highly variable, and thus, knowledge about the specific FBN1 mutation has little prognostic value for an individual patient and cannot reliably guide individual management. In order to facilitate the interpretation in genetic analyses, the revised Ghent nosology includes criteria under which an FBN1 mutation should be considered as causative for MFS [Loeys et al., 2010a]. Disease-causing mutations comprise mutations that either segregate in an MFS family or occur de novo and fall into the groups of nonsense mutations, deletions, splice site mutations, and missense mutations affecting cysteine residues or residues of the conserved EGF consensus sequence; other missense mutations must be further evaluated by segregation analyses or investigation in at least 400 controls [Loeys et al., 2010a].
In 2004, it was first described that some patients/families with a Marfanoid phenotype in which no FBN1 mutation or no linkage to FBN1 was recognized, harbour mutations in the gene encoding transforming growth factor beta receptor 2 (TGFBR2) on chromosome 3p24 [Mizuguchi et al., 2004]. Shortly thereafter, Loeys and Dietz presented a new aortic aneurysm syndrome characterized by hypertelorism, bifid uvula or cleft palate and generalized arterial tortuosity, subsequently called Loeys-Dietz syndrome (LDS), that was caused by mutations in either TGFBR1 or TGFBR2 [Loeys et al., 2005]. Thus, first evidence emerged that there may be substantial overlap between phenotypes including aortic aneurism.
Loeys-Dietz Syndrome
LDS patients share several features with MFS, especially the tendency towards early-onset dilatation of the aortic root, leading to increased risk for aortic dissection at younger age. Arachnodactyly, joint hypermobility, pectus deformity, and scoliosis are also frequently seen in both disorders. However, there are some differences in clinical appearance. First of all, LDS patients tend to show generalized arterial tortuosity with aneurysm formation throughout the arterial tree [Kalra et al., 2011]. Thus, echocardiographic screening is not sufficient for LDS patients who should instead have regular magnetic resonance angiography and/or CT scans [Van Hemelrijk et al., 2010]. Further, dissection appears to occur at smaller aortic diameters than in MFS, requiring earlier and more aggressive surgical intervention [Williams et al., 2007]. Pregnancy-related complications such as uterine rupture or arterial dissection during pregnancy have also been reported at higher frequency in LDS, and overall life expectancy appears to be lower in LDS compared to MFS [Kalra et al., 2011]. Approximately three-quarters of LDS patients show facial dysmorphologic features that are not typical for MFS, including hypertelorism, cleft palate/bifid uvula, craniosynostosis, blue sclerae or strabismus [Van Hemelrijk et al., 2010] (sometimes called LDS type 1). Furthermore, contractures of feet and fingers are common features of LDS, but not MFS. On the other hand, ectopia lentis, as one of the cardinal symptoms of MFS, has not been reported in LDS patients [Van Hemelrijk et al., 2010]. Some LDS patients also show features suggestive of EDS (see below), such as translucent skin with visible veins, a tendency to bruising and disturbed wound healing.
LDS is caused by heterozygous mutations in the TGFBR1 or TGFBR2 genes, located on chromosomes 9q33–34 and 3p22, respectively, and inherited in an autosomal dominant manner. Approximately two-thirds of LDS patients harbour a mutation in TGFBR2 and one-third in TGFBR1 [Stheneur et al., 2008]. Most mutations are missense mutations affecting the highly conserved serine-threonine kinase domains of each receptor [Stheneur et al., 2008]. So far, no phenotypic differences have been reported between patients with mutations in TGFBR1 or TGFBR2. Recently, a 20-month-old female with microcephaly and developmental delay, but no typical features of LDS, was found to carry a microdeletion including TGFBR2 [Campbell et al., 2011]. The authors concluded that TGFBR2 haploinsufficiency may cause a phenotype that is different from LDS. Duplication of TGFBR1 on the other hand was found in a patient with symptoms suggestive of LDS [Campbell et al., 2011]. These findings are in line with the hypothesis that an increase in TGFβ signalling, rather than haploinsufficiency, is the main pathogenetic mechanism underlying LDS, and in part also MFS. Additionally, mutations in the SMAD3 gene on chromosome 15q22, encoding a member of the TGFβ signalling pathway (see below), were recently identified in families with thoracic aortic aneurysms plus craniofacial, skeletal and cutaneous features reminiscent of LDS as well as early-onset osteoarthritis [van de Laar et al., 2011]. On the other hand, a recent study did not find evidence that TGFBR3 variation constitutes a common cause of Marfan- or LDS-like syndromes [Singh et al., 2012].
TGFβ Signalling in Aneurysm Formation: Proposed Pathogenetic Background for MFS and LDS and New Therapeutic Options
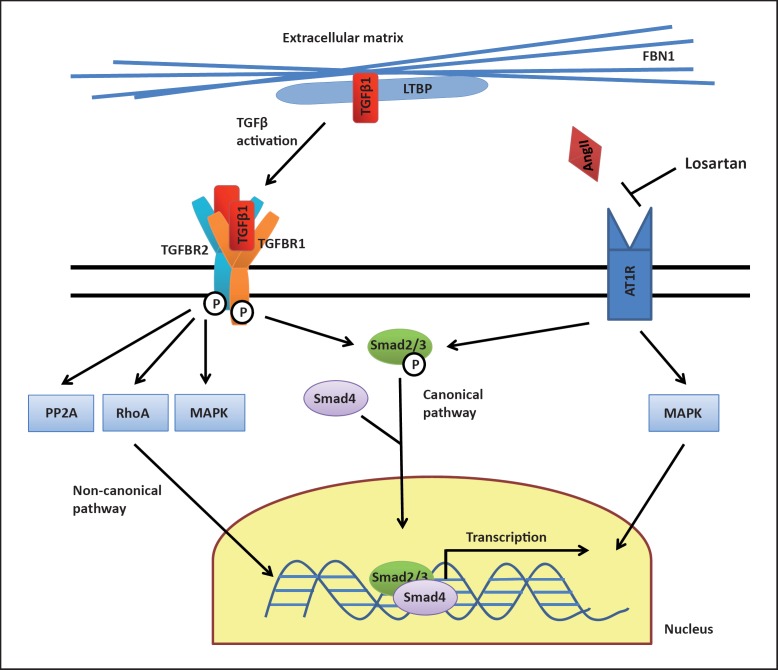
The TGFβ signalling pathway plays an important role in numerous cellular processes, and dysregulation of this pathway has been implicated in several human disorders including cancer, autoimmune and cardiovascular diseases [Lin and Yang, 2010]. Binding of TGFβ1 to the TGFBR2 leads to phosphorylation and activation of TGFBR1, resulting in activation of Smad2/3 transcription factors. After association with Smad4, these transcription factors enter the nucleus and increase the transcription of several target genes (canonical pathway, see fig. 1). Additionally, recent work has shown that TGFβ also induces other (noncanonical) pathways, including for example the mitogen-activated protein kinase or RhoA cascades [Lin and Yang, 2010].
Fig. 1.
TGFβ signalling pathway. Fibrillin-1 (FBN1), as a major component of the extracellular matrix, interacts with large latent TGFβ-binding protein (LTBP) which sequesters TGFβ1 and thus prevents excessive TGFβ1 signalling. Binding of TGFβ1 to TGFBR2 leads to phosphorylation and activation of TGFBR1, resulting in activation of Smad2/3 transcription factors. After association with Smad4, these transcription factors enter the nucleus and increase the transcription of several target genes (canonical pathway). Additionally, TGFβ1 induces other (noncanonical) pathways, including for example the mitogen-activated protein kinase (MAPK), PP2A or RhoA cascades. Both defective FBN1 as well as heterozygous mutations in TGFBR1/TGFBR2 were shown to result in increased TGFβ1 signalling, promoting aneurysm formation. Angiotensin II (AngII) regulates TGFβ activation and signalling through the angiotensin II type 1 receptor (AT1R). The AT1R blocker Losartan was found to down-regulate TGFβ signalling and is currently evaluated as a therapeutic option for MFS.
Although the exact pathogenetic mechanism remains unclear, heterozygous mutations in the TGFBR1 and TGFBR2 genes were shown to be associated with increased TGFβ signalling, as indicated by enhanced nuclear accumulation of phosphorylated Smad2/3 and increased expression of TGFβ-responsive genes [Loeys et al., 2005]. Additionally, there is growing evidence for a narrow relationship between fibrillin-1 and TGFβ. Mice deficient for fibrillin-1 were found to have a marked increase in TGFβ signalling [Neptune et al., 2003]. It was shown that fibrillin-1, as a major component of the extracellular matrix, can regulate the bioavailability of TGFβ by interacting with large latent TGFβ-binding protein [Massam-Wu et al., 2010]. While intact fibrillin-1 appears to hold a reservoir of inactive TGFβ1 preventing excessive signalling, this mechanism seems to be perturbed in MFS, leading to an increase in TGFβ-mediated aneurysm formation [Goumans et al., 2009]. Interestingly, dysregulated TGFβ/Smad2 signalling was found in both syndromic (e.g. MFS) and nonsyndromic (e.g. degenerative) forms of aortic aneurysm, suggesting, at least in part, a shared pathogenetic background [Gomez et al., 2009, 2011].
Growing insight into the important role of TGFβ signalling for MFS and LDS has led to promising new therapeutic approaches in the last years. Mouse models suggested that administration of the angiotensin II type 1 receptor (AT1) blocker losartan could downregulate TGFβ signalling, prevent aneurysm formation and even partially reverse manifestations of MFS in fibrillin-deficient mice [Habashi et al., 2006]. Several clinical trials to evaluate the efficacy of losartan therapy in MFS patients are currently under way [Gambarin et al., 2009; Detaint et al., 2010; Radonic et al., 2010; Möberg et al., 2011], and there is legitimate hope that this therapy may reduce the risk for aneurysm formation in MFS- and perhaps LDS-patients in the future. Furthermore, noncanonical (Smad-independent) TGFβ signalling has also been shown to promote aortic disease in MFS mice, opening additional potential therapeutic strategies [Holm et al., 2011]. Additional novel therapeutic approaches for MFS apart from the TGFβ pathway include the application of doxycycline and statins as inhibitors of matrix metalloproteinases [Hartog et al., 2012].
Familial Thoracic Aortic Aneurysms and Dissections
In addition to syndromic forms of TAAD (e.g. as part of MFS or LDS), many families have been described that show increased incidence of TAAD – mostly with an autosomal dominant form of transmission – without clear syndromic features (fTAAD). Occasionally, mutations in FBN1, TGFBR1 or TGFBR2 are found in these families [Milewicz et al., 2008] further underlining the wide phenotypic spectrum of the syndromic forms of disease. Apart from this, several other loci have been identified in linkage studies, and recently 3 new genes for fTAAD have been reported. In 2007, mutations in the gene encoding myosin heavy chain protein 11 (MYH11) on chromosome 16p13 were identified in 2 large families with autosomal dominant inheritance of TAAD and patent ductus arteriosus [Zhu et al., 2006]. Subsequently, 2 additional families with MYH11 mutations were described that showed substantial smooth muscle cell (SMC) disarray and focal hyperplasia of SMCs in the aortic media [Pannu et al., 2007]. Shortly thereafter, the gene encoding SMC α-actin (ACTA2) on chromosome 10q22–24 was identified in TAAD families that also showed a variety of additional symptoms such as livedo reticularis, patent ductus arteriosus and iris flocculi [Guo et al., 2007]. Interestingly, ACTA2 mutations seem to increase risk not only for aneurysms/dissections, but also for occlusive arterial disease including premature stroke, premature coronary artery disease and Moya-Moya disease [Guo et al., 2009]. While MYH11 mutations appear to be rare (only 5 mutations reported so far) and have been exclusively described in association with patent ductus arteriosus, ACTA2 mutations were found to be responsible for approximately 14–21% of fTAAD and are believed to interfere with the normal assembly of actin filaments [Hoffjan et al., 2011]. Recently, mutations in the gene encoding the kinase that controls SMC contractile function (myosin light chain kinase (MYLK) on chromosome 3q21) were also shown to cause familial aortic dissections [Wang et al., 2010].
Taken together, these findings strongly indicate an important role of the SMC contractile apparatus for stability and integrity of the aortic wall, although the exact pathogenetic mechanisms remain unclear to date [Milewicz et al., 2008]. Interestingly, upregulated TGFβ signalling in the aortic wall – similar to the findings in MFS and LDS –was recently demonstrated also in patients with MYH11 and ACTA2 mutations [Renard et al., 2011], pointing again towards the TGFβ pathway as key regulator for aneurysm formation in different disorders.
Ehlers-Danlos Syndrome
EDS comprises a heterogeneous group of connective tissue disorders caused by defective synthesis of collagen. While the ‘classical’ EDS (type 1 and 2) is mainly characterized by joint hypermobility, hyperelasticity of the skin, easy bruising, abnormal wound healing, and scar formation (without vascular complications), EDS type IV goes along with increased risk of arterial aneurysms/dissections and thus, constitutes another important differential diagnosis for MFS [Beridze and Frishman, 2012]. Like MFS and LDS, EDS type IV (also called the vascular type of EDS) is inherited in an autosomal dominant manner. It is caused by mutations in the gene encoding the alpha1-chain of collagen III (COL3A1) on chromosome 2q31 [Beridze and Frishman, 2012]. Cardinal clinical criteria for EDS IV comprise easy bruising, thin skin with visible veins, characteristic facial features (including thin lips and philtrum, small chin, thin nose, and large eyes), and rupture of arteries, uterus or intestines [Beighton et al., 1998]. Joint hypermobility and hyperextensibility of the skin are rather unusual in the vascular type. In contrast to MFS, only about half of arterial complications in EDS type IV affect the thoracic or abdominal aorta, while the rest occurs in arteries in the head, neck and limbs [Pepin and Byers, 1993]. Further, bowel rupture is a common feature of EDS type IV, and pregnancy for affected women was found to have a 12% risk for death from peripartum arterial rupture or uterine rupture [Pepin and Byers, 1993]. In order to sustain the clinical suspicion for EDS type IV, biochemical testing in cultured dermal fibroblasts is available. Further, electron microscopy from a small skin biopsy can be used to confirm or reject the diagnosis of EDS [Morais et al., 2011].
More than 100 mutations in the COL3A1 gene have been reported to date, two-thirds of which are missense mutations affecting glycine residues in the triple helical domain [Pepin and Byers, 1993]. Null mutations leading to haploinsufficiency were found to be associated with a milder form of disease: compared to missense and splice site mutations, the occurrence of the first complication was delayed by almost 15 years, and almost exclusively vascular complications were observed [Leistritz et al., 2011]. Apart from this finding, no other genotype-phenotype correlations were demonstrated for COL3A1 mutations [Pepin and Byers, 1993]. Rarely, vascular complications can also be observed in the kyphoscoliotic type of EDS (EDS VIA), characterized by a deficiency of collagen lysyl hydroxylase 1 and caused by mutations in the PLOD1 gene [Rohrbach et al., 2011]. Unlike the vascular type, this form of EDS is inherited automosal recessively and characterized by progressive scoliosis, muscular hypotonia, tissue fragility with easy bruising, and sometimes cognitive delay [Rohrbach et al., 2011].
Surveillance and therapy of EDS type IV (including regular cardiovascular screening, thorough monitoring of pregnancies and operative procedures) have proven challenging, and no preventive therapy has been established yet. Recently, first evidence emerged form a prospective trial that application of celiprolol, a long-acting β2 receptor antagonist, might reduce the incidence of arterial rupture or dissection in EDS IV patients [Ong et al., 2010]. Whether increased TGFβ signalling plays a pathogenetic role also for this connective tissue disorder has not been evaluated yet, but will certainly be addressed in the near future.
Arterial Tortuosity Syndrome
Arterial tortuosity syndrome (ATS) is another rare connective tissue disorder that shows substantial clinical overlap with LDS but is inherited autosomal recessively [Coucke et al., 2006]. It is characterized by tortuosity of the large and medium-sized arteries as well as aneuryms of large arteries and pulmonary stenosis. Further, the patients often show joint hypermobility, arachnodactyly and distinct facial features including microretrognathia, downslanting palpebral fissures, hypertelorism and cleft palate, and/or bifid uvula. In 2007, mutations in the SLC2A10 gene on chromosome 20q13, encoding the glucose transporter GLUT10, were identified as causative for ATS in large families [Coucke et al., 2006]. Analysis of additional patients also revealed increased risk for ischemic events [Callewaert et al., 2008]. While previous clinical reports indicated a severe clinical course with a 40% mortality rate before the age of 5 years, recent investigations revealed a far more variable phenotype [Callewaert et al., 2008]. Since the SLC2A10 gene is located in a region linked to type 2 diabetes and presumably plays a role in glucose metabolism, it was considered a potential candidate gene for diabetes [Dawson et al., 2001]; however, an association with type 2 diabetes could not be demonstrated [Bento et al., 2005; Mohlke et al., 2005]. In ATS families, neither heterozygous nor homozygous mutation carriers showed increased risk for diabetes [Callewaert et al., 2008], arguing against a major role of this gene in diabetes pathogenesis. Interestingly, an increase in TGFβ signalling, which has proven to be a cardinal pathogenetic mechanism in both MFS and LDS (see above), has also been found in ATS. It was hypothesized that GLUT10 acts as a glucose transporter into the nucleus where it modulates the expression of glucose-responsive genes [Coucke et al., 2006]. Deficiency of GLUT10 in ATS would then down-regulate inhibitors of the TGFβ cascade, leading to increased TGFβ signalling that stimulates vessel wall cell formation. As an alternative hypothesis, however, GLUT10 has also been implicated in ascorbic acid metabolism [Segade, 2010].
Cutis Laxa Syndrome
Cutis laxa is a rare connective tissue disorder characterized by redundant, loose and hypoelastic skin due to abnormal elastic fibers [Berk et al., 2012]. The disease may be acquired or inherited with various modes of transmission (autosomal dominant, autosomal recessive or X-linked). Recently, mutations in the fibulin-4 gene (FBLN4) on chromosome 11q13 were identified as causative for a rare autosomal recessive form of Cutis laxa that also led to altered TGFβ signalling [Renard et al., 2010]. In contrast to other types of Cutis laxa, the patients carrying homozygous FBLN4 mutations showed major cardiovascular events, including aortic aneurysms and arterial tortuosity [Renard et al., 2010], with only minor skin involvement, suggesting that this rare disorder of elastic tissue constitutes another differential diagnosis for the cardiovascular features of MFS.
Bicuspid Aortic Valve
The bicuspid aortic valve (BAV) is a relatively common congenital heart disease (prevalence approximately 1–2%) that is characterized by the presence of only 2 instead of 3 semilunar valves, often followed by calcification [Siu and Silversides, 2010]. Affected individuals have an increased risk for aortic stenosis and/or regurgitation and also for aortic dissection. Both isolated valve defects and familial clustering have been documented. In families with BAV and severe valve calcification, causative mutations in the NOTCH1 gene were identified [Garg et al., 2005], and overrepresentation of NOTCH1 missense mutations was subsequently confirmed in additional nonrelated patients with BAV and thoracic aortic aneurysms [Mohamed et al., 2006; McKellar et al., 2007]. NOTCH1 is a signalling and transcriptional regulator that is involved in cell differentiation and determination during organogenesis. It was suggested that NOTCH1 mutations may cause an early developmental defect in the aortic valve and later affect calcium deposition. However, mutations in this gene explain only a small fraction of isolated as well as familial BAV, and locus heterogeneity [Ellison et al., 2007] as well as multifactorial pathogenesis have been suggested.
Differential Diagnosis of the Ophthalmologic and Skeletal Features of Marfan Syndrome
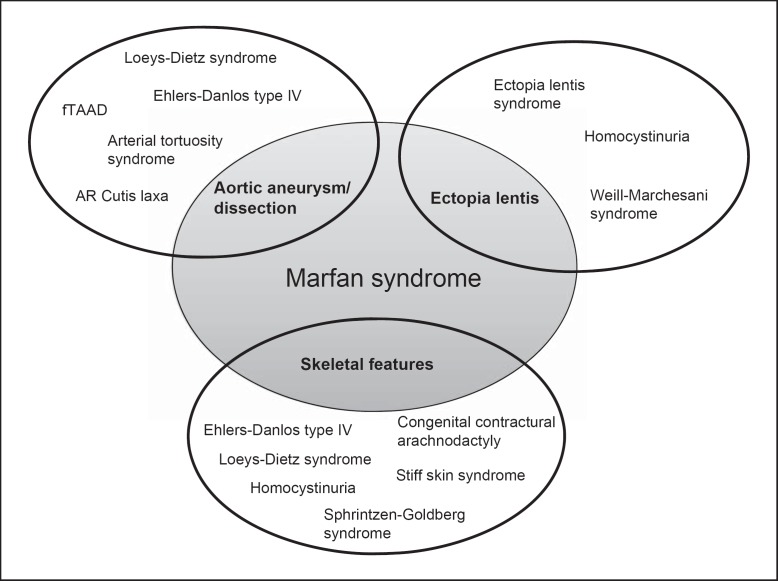
Additional to the many differential diagnoses predominantly characterized by aortic aneurysm/dissection that have been described above, there are also some rare disorders that share ophthalmologic and skeletal features with MFS (see fig. 2). Besides the closely-related Ectopia lentis syndrome (patients with ectopia lentis but no aortic aneurysm/dissection that do not harbour FBN1 mutations, see above), ectopia lentis is also a typical feature of Weill-Marchesani syndrome (WMS) and homocystinuria. WMS is a rare connective tissue disorder characterized by short (not tall!) stature, joint stiffness, brachydactyly, and characteristic eye abnormalities including ectopia lentis, microspherophakia, severe myopia, and glaucoma. Both autosomal dominant and autosomal recessive forms of inheritance have been described for this condition. For the recessive form, mutations have been identified in the ADAMTS10 [Dagoneau et al., 2004] and ADAMTS17 [Morales et al., 2009] genes, suggesting that ADAMTS proteinases may constitute potential therapeutic targets for this group of connective tissue disorders [Jones, 2006]. In one family with autosomal dominant WMS, a 24-nt in-frame deletion in the FBN1 gene was found [Faivre et al., 2003], highlighting again the complexity of clinical and genetic findings. Homocystinuria is an autosomal recessive metabolic disorder caused by cystathionine β-synthase deficiency due to mutations in the CBS gene [Yap, 2003]. Like MFS patients, individuals with homocystinuria are often tall and slender, show ectopia lentis, severe myopia, and skeletal abnormalities; in contrast to MFS, developmental delay/intellectual disability and thromboembolism are usually present. Although homocystinuria is very rare, it is important to consider this differential diagnosis since an effective dietary therapy is available [Yap, 2003].
Fig. 2.
Differential diagnosis for Marfan syndrome and overlapping phenotypes. The cardinal symptoms of MFS, including thoracic aneurysm/dissection, ectopia lentis and skeletal features (sometimes called Marfanoid build) show a high degree of overlap with various other diseases that have to be considered for differential diagnosis. The discriminating features of the respective disorders are given in the text and summarized in table 2.
Skeletal features reminiscent of MFS (e.g. arachnodactyly, camptodactyly, pes planus, pectus excavatum or carinatum, scoliosis, and joint hypermobility or contractures) are also evident for Sphrintzen-Goldberg syndrome (SGS); further, SGS is characterized by craniosynostosis which is a common feature of LDS, but not MFS [Robinson et al., 2005]. Additional symptoms that discriminate SGS from the other connective tissues disorders include mild-to-moderate intellectual disability and brain anomalies. While a few SGS patients were found to carry a FBN1 mutation, the genetic background of most cases is unknown to date [Robinson et al., 2005]. Interestingly, mutations in a specific domain of the FBN1 gene were recently also found to cause stiff skin syndrome, a rare autosomal dominant congenital form of scleroderma characterized by hard, thick skin and joint contractures but without the typical ocular and cardiovascular findings of MFS [Loeys et al., 2010b]. Additionally, mutations in the TGFβ-binding protein-like domain 5 of the FBN1 gene were held responsible for geleophysic and acromimic dysplasia, characterized by short stature, short extremities and stiff joints [Le Goff et al., 2011]. Another rare differential diagnosis, especially for neonatal MFS, is autosomal dominantly inherited congenital contractural arachnodactyly (CCA, also called Beals syndrome), caused by mutations in the gene encoding fibrillin-2 (FBN2) [Callewaert et al., 2009]. Arachnodactyly, severe kyphoscoliosis and muscular hypotonia are found in both MFS and CCA; features suggestive of CCA that are not commonly seen in MFS include multiple joint contractures and crumpled ears [Tuncbilek and Alanay, 2006]. However, while the first studies did not find evidence for aortic root dilatation in CCA, newer reports indicated that aortic dilatation may also occur in some CCA patients [Callewaert et al., 2009].
Conclusion and Future Prospects
In conclusion, due to substantial clinical and genetic overlap, correctly establishing the diagnosis of MFS or a related connective tissue disease is often challenging in clinical practice. Yet, the genetic findings of recent years have considerably contributed to unraveling the genetic background of connective tissue disorders, and molecular genetic testing is more and more integrated into the diagnostic evaluations, since different disease entities go along with different therapeutic and surveillance requirements [von Kodolitsch et al., 2010]. Furthermore, increased insight into the pathogenetic background has already opened the way for novel therapeutic approaches [Hartog et al., 2012]. However, analysis of the many genes involved in the varying tissue disorders is time-consuming and expensive (especially for the FBN1 gene comprising >60 exons), and at the time being, testing for a whole panel of genes involved in connective tissue disorders in patients with overlapping phenotypes is not yet available on a routine basis. Therefore, at the moment, the decision which genes to evaluate in a patient/family is still based on the clinical observation of discriminating phenotypes (table 2). For example, in a patient with thoracic aneurysm/dissection and additional symptoms suggestive of MFS, analysis of the FBN1 and TGFR2 genes appears sensible, while the existence of peripheral arterial aneurysms, especially in combination with generalized arterial tortuosity, should rather point to LDS (autosomal dominant, TGFBR1 and TGFBR2 genes) or ATS (autosomal recessive, SLC2A10 gene). If a patient shows features reminiscent of EDS such as translucent skin and bruising, an electron microscopy can be performed before COL3A1 analysis in order to confirm or exclude EDS. Comprehensive analysis of TAAD genes in affected families has not been incorporated into routine diagnostic procedures yet.
Table 2.
Genes involved in Marfan syndrome and related connective tissue disorders
| Disorder | Genes | Mode of inheritance | Discriminating clinical features |
|---|---|---|---|
| Marfan syndrome | FBN1 TGFBR2 | AD |
|
| Loeys-Dietz syndrome |
|
AD |
|
| Ehlers-Danlos type IV | COL3A1 | AD |
|
| Ehlers-Danlos type VIA | PLOD1 | AR |
|
| fTAAD |
|
AD |
|
| Ectopia lentis syndrome |
|
AD |
|
| Cutis laxa, AR type 1 | FBLN4 | AR |
|
| Bicuspid aortic valve with calcification | NOTCH1 | AD |
|
| Sphrintzen-Goldberg syndrome | ? | ? |
|
| Weill-Marchesani syndrome |
|
|
|
| Stiff skin syndrome | FBN1 | AD |
|
| Geleophysic and acrophysic dysplasia | FBN1 | AD |
|
| Congenital contractural arachnodactyly | FBN2 | AD |
|
| Homocystinuria | CBS | AR |
|
| AD = Autosomal dominant; AR = autosomal recessive; fTAAD = familial thoracic aortic aneurysms/dissections. |
However, new technologies are already in development that will lead to more time- and cost-effective strategies in the near future, allowing analysis of several genes simultaneously. For example, a method using multiplex PCR followed by next-generation sequencing (massive parallel sequencing) was shown to reliably detect mutations in the FBN1, TGFBR1 and TGFBR2 genes in MFS patients fulfilling the Ghent criteria [Baetens et al., 2011]. Further, a custom-based duplicate resequencing assay (Affymetrix) and a long-range PCR protocol were recently presented to cover 8 genes previously associated with MFS and related disorders (FBN1, TGFBR1, TGFBR2, COL3A1, MYH11, ACTA2, SLC2A10, and NOTCH1) [Kathiravel et al., 2012]. Finally, exome (and whole-genome) sequencing strategies become more and more employed for both targeted diagnostic testing and genome-wide searches for the mutations that underlie unexplained disorders [Thorogood et al., 2012]. With rapidly decreasing costs, these strategies may also be applied to the complex field of inheritable connective tissue disorders in the near future and potentially identify additional genetic variation underlying these complex phenotypes. Yet, there is still considerable dispute about how to address the many ethical issues that go along with whole-genome sequencing strategies [Thorogood et al., 2012]. Therefore, it is not yet clear when these approaches will be used on a routine basis for MFS and related disorders.
References
- Baetens M, Van Laer L, De Leeneer K, Hellemans J, De Schrijver J, et al. Applying massive parallel sequencing to molecular diagnosis of Marfan and Loeys-Dietz syndromes. Hum Mutat. 2011;32:1–10. doi: 10.1002/humu.21525. [DOI] [PubMed] [Google Scholar]
- Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) Am J Med Genet. 1998;77:31–37. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bento JL, Bowden DW, Mychaleckyj JC, Hirakawa S, Rich SS, et al. Genetic analysis of the GLUT10 glucose transporter (SLC2A10) polymorphisms in Caucasian American type 2 diabetes. BMC Med Genet. 2005;6:42. doi: 10.1186/1471-2350-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beridze N, Frishman WH. Vascular Ehlers-Danlos syndrome: pathophysiology, diagnosis, and prevention and treatment of its complications. Cardiol Rev. 2012;20:4–7. doi: 10.1097/CRD.0b013e3182342316. [DOI] [PubMed] [Google Scholar]
- Berk DR, Bentley DD, Bayliss SJ, Lind A, Urban Z. Cutis laxa: a review. J Am Acad Dermatol. 2012;66:842.e1–e17. doi: 10.1016/j.jaad.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Bonetti MI. Microfibrils: a cornerstone of extracellular matrix and a key to understand Marfan syndrome. Ital J Anat Embryol. 2009;114:201–224. [PubMed] [Google Scholar]
- Callewaert BL, Willaert A, Kerstjens-Frederikse WS, De Backer J, Devriendt K, et al. Arterial tortuosity syndrome: clinical and molecular findings in 12 newly identified families. Hum Mutat. 2008;29:150–158. doi: 10.1002/humu.20623. [DOI] [PubMed] [Google Scholar]
- Callewaert BL, Loeys BL, Ficcadenti A, Vermeer S, Landgren M, et al. Comprehensive clinical and molecular assessment of 32 probands with congenital contractural arachnodactyly: report of 14 novel mutations and review of the literature. Hum Mutat. 2009;30:334–341. doi: 10.1002/humu.20854. [DOI] [PubMed] [Google Scholar]
- Campbell IM, Kolodziejska KE, Quach MM, Wolf VL, Cheung SW, et al. TGFBR2 deletion in a 20-month-old female with developmental delay and microcephaly. Am J Med Genet A. 2011;155A:1442–1447. doi: 10.1002/ajmg.a.34015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañadas V, Vilacosta I, Bruna I, Fuster V. Marfan syndrome. Part 1: pathophysiology and diagnosis. Nat Rev Cardiol. 2010;7:256–265. doi: 10.1038/nrcardio.2010.30. [DOI] [PubMed] [Google Scholar]
- Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, et al. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat Genet. 2006;38:452–457. doi: 10.1038/ng1764. [DOI] [PubMed] [Google Scholar]
- Dagoneau N, Benoist-Lasselin C, Huber C, Faivre L, Mégarbané A, et al. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am J Hum Genet. 2004;75:801–806. doi: 10.1086/425231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Mychaleckyj JC, Fossey SC, Mihic SJ, Craddock AL, Bowden DW. Sequence and functional analysis of GLUT10: a glucose transporter in the Type 2 diabetes-linked region of chromosome 20q12–13.1. Mol Genet Metab. 2001;74:186–199. doi: 10.1006/mgme.2001.3212. [DOI] [PubMed] [Google Scholar]
- De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417–426. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Detaint D, Aegerter P, Tubach F, Hoffman I, Plauchu H, et al. Rationale and design of a randomized clinical trial (Marfan Sartan) of angiotensin II receptor blocker therapy versus placebo in individuals with Marfan syndrome. Arch Cardiovasc Dis. 2010;103:317–325. doi: 10.1016/j.acvd.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Ellison JW, Yagubyan M, Majumdar R, Sarkar G, Bolander ME, et al. Evidence of genetic locus heterogeneity for familial bicuspid aortic valve. J Surg Res. 2007;142:28–31. doi: 10.1016/j.jss.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Faivre L, Gorlin RJ, Wirtz MK, Godfrey M, Dagoneau N, et al. In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J Med Genet. 2003;40:34–36. doi: 10.1136/jmg.40.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre L, Collod-Beroud G, Callewaert B, Child A, Binquet C, et al. Clinical and mutation-type analysis from an international series of 198 probands with a pathogenic FBN1 exons 24–32 mutation. Eur J Hum Genet. 2009;17:491–501. doi: 10.1038/ejhg.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre L, Collod-Beroud G, Adès L, Arbustini E, Child A, et al. The new Ghent criteria for Marfan syndrome: what do they change? Clin Genet. 2012;81:433–442. doi: 10.1111/j.1399-0004.2011.01703.x. [DOI] [PubMed] [Google Scholar]
- Furtado LV, Wooderchak-Donahue W, Rope AF, Yetman AT, Lewis T, et al. Characterization of large genomic deletions in the FBN1 gene using multiplex ligation-dependent probe amplification. BMC Med Genet. 2011;12:119. doi: 10.1186/1471-2350-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambarin FI, Favalli V, Serio A, Regazzi M, Pasotti M, et al. Rationale and design of a trial evaluating the effects of losartan vs. nebivolol vs. the association of both on the progression of aortic root dilation in Marfan syndrome with FBN1 gene mutations. J Cardiovasc Med (Hagerstown) 2009;10:354–362. doi: 10.2459/JCM.0b013e3283232a45. [DOI] [PubMed] [Google Scholar]
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- Gomez D, Al Haj Zen A, Borges LF, Philippe M, Gutierrez PS, et al. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J Pathol. 2009;218:131–142. doi: 10.1002/path.2516. [DOI] [PubMed] [Google Scholar]
- Gomez D, Coyet A, Ollivier V, Jeunemaitre X, Jondeau G, et al. Epigenetic control of vascular smooth muscle cells in Marfan and non-Marfan thoracic aortic aneurysms. Cardiovasc Res. 2011;89:446–456. doi: 10.1093/cvr/cvq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans MJ, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, et al. Mutations in smooth muscle α-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84:617–627. doi: 10.1016/j.ajhg.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartog AW, Franken R, Zwinderman AH, Groenink M, Mulder BJ. Current and future pharmacological treatment strategies with regard to aortic disease in Marfan syndrome. Expert Opin Pharmacother. 2012;13:647–662. doi: 10.1517/14656566.2012.665446. [DOI] [PubMed] [Google Scholar]
- Hilhorst-Hofstee Y, Hamel BC, Verheij JB, Rijlaarsdam ME, Mancini GM, et al. The clinical spectrum of complete FBN1 allele deletions. Eur J Hum Genet. 2011;19:247–252. doi: 10.1038/ejhg.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffjan S, Waldmüller S, Blankenfeldt W, Kötting J, Gehle P, et al. Three novel mutations in the ACTA2 gene in German patients with thoracic aortic aneurysms and dissections. Eur J Hum Genet. 2011;19:520–524. doi: 10.1038/ejhg.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GC. ADAMTS proteinases: potential therapeutic targets? Curr Pharm Biotechnol. 2006;7:25–31. doi: 10.2174/138920106775789656. [DOI] [PubMed] [Google Scholar]
- Kalra VB, Gilbert JW, Malhotra A. Loeys-Dietz syndrome: cardiovascular, neuroradiological and musculoskeletal imaging findings. Pediatr Radiol. 2011;41:1495–1504. doi: 10.1007/s00247-011-2195-z. [DOI] [PubMed] [Google Scholar]
- Kathiravel U, Keyser B, Hoffjan S, Koetting J, Müller M, et al. : High-density oligonucleotide-based resequencing assay for mutations causing syndromic and non-syndromic forms of thoracic aortic aneurysms and dissections. (submitted 2012). [DOI] [PubMed]
- Le Goff C, Mahaut C, Wang LW, Allali S, Abhyankar A, et al. Mutations in the TGFbeta binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am J Hum Genet. 2011;89:7–14. doi: 10.1016/j.ajhg.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistritz DF, Pepin MG, Schwarze U, Byers PH. COL3A1 haploinsufficiency results in a variety of Ehlers-Danlos syndrome type IV with delayed onset of complications and longer life expectancy. Genet Med. 2011;13:717–722. doi: 10.1097/GIM.0b013e3182180c89. [DOI] [PubMed] [Google Scholar]
- Lin F, Yang X. TGF-beta signaling in aortic aneurysm: another round of controversy. J Genet Genomics. 2010;37:583–591. doi: 10.1016/S1673-8527(09)60078-3. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010a;47:476–485. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Gerber EE, Riegert-Johnson D, Iqbal S, Whiteman P, et al. Mutations in fibrillin-1 cause congenital scleroderma: stiff skin syndrome. Sci Transl Med. 2010b;2(23ra20) doi: 10.1126/scitranslmed.3000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massam-Wu T, Chiu M, Choudhury R, Chaudhry SS, Baldwin AK, et al. Assembly of fibrillin microfibrils governs extracellular deposition of latent TGF beta. J Cell Sci. 2010;123:3006–3018. doi: 10.1242/jcs.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar SH, Tester DJ, Yagubyan M, Majumdar R, Ackerman MJ, Sundt TM., 3rd Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007;134:290–296. doi: 10.1016/j.jtcvs.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, et al. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet. 2008;9:283–302. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet. 2004;36:855–860. doi: 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möberg K, De Nobele S, Devos D, Goetghebeur E, Segers P, et al. : The Ghent Marfan Trial – a randomized, double-blind placebo controlled trial with losartan in Marfan patients treated with β-blockers. Int J Cardiol 2011, E-pub ahead of print. [DOI] [PubMed]
- Mohamed SA, Aherrahrou Z, Liptau H, Erasmi AW, Hagemann C, et al. Novel missense mutations (p.T596M and p.P1797H) in NOTCH1 in patients with bicuspid aortic valve. Biochem Biophys Res Commun. 2006;345:1460–1465. doi: 10.1016/j.bbrc.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Mohlke KL, Skol AD, Scott LJ, Valle TT, Bergman RN, et al. Evaluation of SLC2A10 (GLUT10) as a candidate gene for type 2 diabetes and related traits in Finns. Mol Genet Metab. 2005;85:323–327. doi: 10.1016/j.ymgme.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Morais P, Mota A, Eloy C, Lopes JM, Torres F, et al. Vascular Ehlers-Danlos syndrome: a case with fatal outcome. Dermatol Online J. 2011;17:1. [PubMed] [Google Scholar]
- Morales J, Al-Sharif L, Khalil DS, Shinwari JM, Bavi P, et al. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am J Hum Genet. 2009;85:558–568. doi: 10.1016/j.ajhg.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Ong KT, Perdu J, De Backer J, Bozec E, Collignon P, et al. Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers-Danlos syndrome: a prospective randomised, open, blinded-endpoints trial. Lancet. 2010;376:1476–1484. doi: 10.1016/S0140-6736(10)60960-9. [DOI] [PubMed] [Google Scholar]
- Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, et al. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet. 2007;16:2453–2462. doi: 10.1093/hmg/ddm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin MG, Byers PH: Ehlers-Danlos Syndrome Type IV, in Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP (eds): Gene Review™ [Internet] (University of Washington, Seattle 1993).
- Radonic T, de Witte P, Baars MJ, Zwinderman AH, Mulder BJ, et al. Losartan therapy in adults with Marfan syndrome: study protocol of the multi-center randomized controlled COMPARE trial. Trials. 2010;11:3. doi: 10.1186/1745-6215-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonic T, de Witte P, Groenink M, de Bruin-Bon R, Timmermans J, et al. : Critical appraisal of the revised Ghent criteria for diagnosis of Marfan syndrome. Clin Genet 2011, E-pub ahead of print. [DOI] [PubMed]
- Renard M, Holm T, Veith R, Callewaert BL, Adès LC, et al. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur J Hum Genet. 2010;18:895–901. doi: 10.1038/ejhg.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M, Callewaert B, Baetens M, Campens L, Macdermot K, et al. : Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGFbeta signaling in FTAAD. Int J Cardiol 2011, E-pub ahead of print. [DOI] [PMC free article] [PubMed]
- Robinson PN, Neumann LM, Demuth S, Enders H, Jung U, et al. Shprintzen-Goldberg syndrome: fourteen new patients and a clinical analysis. Am J Med Genet A. 2005;135:251–262. doi: 10.1002/ajmg.a.30431. [DOI] [PubMed] [Google Scholar]
- Rohrbach M, Vandersteen A, Yiş U, Serdaroglu G, Ataman E, et al. Phenotypic variability of the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VIA): clinical, molecular and biochemical delineation. Orphanet J Rare Dis. 2011;6:46. doi: 10.1186/1750-1172-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segade F. Glucose transporter 10 and arterial tortuosity syndrome: the vitamin C connection. FEBS Lett. 2010;584:2990–2994. doi: 10.1016/j.febslet.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Sheikhzadeh S, Kade C, Keyser B, Stuhrmann M, Arslan-Kirchner M, et al. : Analysis of phenotype and genotype information for the diagnosis of Marfan syndrome. Clin Genet 2011, E-pub ahead of print. [DOI] [PubMed]
- Singh KK, Schmidtke J, Keyser B, Arslan-Kirchner M. TGFBR3 variation is not a common cause of Marfan-like syndrome and Loeys-Dietz-like syndrome. J Negat Results Biomed. 2012;11:9. doi: 10.1186/1477-5751-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–2800. doi: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- Stheneur C, Collod-Béroud G, Faivre L, Gouya L, Sultan G, et al. Identification of 23 TGFBR2 and 6 TGFBR1 gene mutations and genotype-phenotype investigations in 457 patients with Marfan syndrome type I and II, Loeys-Dietz syndrome and related disorders. Hum Mutat. 2008;29:E284–295. doi: 10.1002/humu.20871. [DOI] [PubMed] [Google Scholar]
- Thorogood A, Knoppers BM, Dondorp WJ, de Wert GM. Whole-genome sequencing and the physician. Clin Genet. 2012;81:511–513. doi: 10.1111/j.1399-0004.2012.01868.x. [DOI] [PubMed] [Google Scholar]
- Tuncbilek E, Alanay Y. Congenital contractural arachnodactyly (Beals syndrome) Orphanet J Rare Dis. 2006;1:20. doi: 10.1186/1750-1172-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CL, Emery H, Collins AL, Howarth RJ, Yearwood CM, et al. Detection of 53 FBN1 mutations (41 novel and 12 recurrent) and genotype-phenotype correlations in 113 unrelated probands referred with Marfan syndrome, or a related fibrillinopathy. Am J Med Genet A. 2009;149A:161–170. doi: 10.1002/ajmg.a.32593. [DOI] [PubMed] [Google Scholar]
- van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–126. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- Van Hemelrijk C, Renard M, Loeys B. The Loeys-Dietz syndrome: an update for the clinician. Curr Opin Cardiol. 2010;25:546–551. doi: 10.1097/HCO.0b013e32833f0220. [DOI] [PubMed] [Google Scholar]
- von Kodolitsch Y, Rybczynski M, Bernhardt A, Mir TS, Treede H, et al. Marfan syndrome and the evolving spectrum of heritable thoracic aortic disease: do we need genetics for clinical decisions? Vasa. 2010;39:17–32. doi: 10.1024/0301-1526/a000002. [DOI] [PubMed] [Google Scholar]
- Wang L, Guo DC, Cao J, Gong L, Kamm KE, et al. Mutations in myosin light chain kinase cause familial aortic dissections. Am J Hum Genet. 2010;87:701–707. doi: 10.1016/j.ajhg.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Loeys BL, Nwakanma LU, Dietz HC, Spevak PJ, et al. Early surgical experience with Loeys-Dietz: a new syndrome of aggressive thoracic aortic aneurysm disease. Ann Thorac Surg. 2007;83:S757–763. doi: 10.1016/j.athoracsur.2006.10.091. [DOI] [PubMed] [Google Scholar]
- Yang JH, Han H, Jang SY, Moon JR, Sung K, et al. A comparison of the Ghent and revised Ghent nosologies for the diagnosis of Marfan syndrome in an adult Korean population. Am J Med Genet A. 2011;158A:989–995. doi: 10.1002/ajmg.a.34392. [DOI] [PubMed] [Google Scholar]
- Yap S. Classical homocystinuria: vascular risk and its prevention. J Inherit Metab Dis. 2003;26:259–265. doi: 10.1023/a:1024497419821. [DOI] [PubMed] [Google Scholar]
- Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38:343–349. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]