Abstract
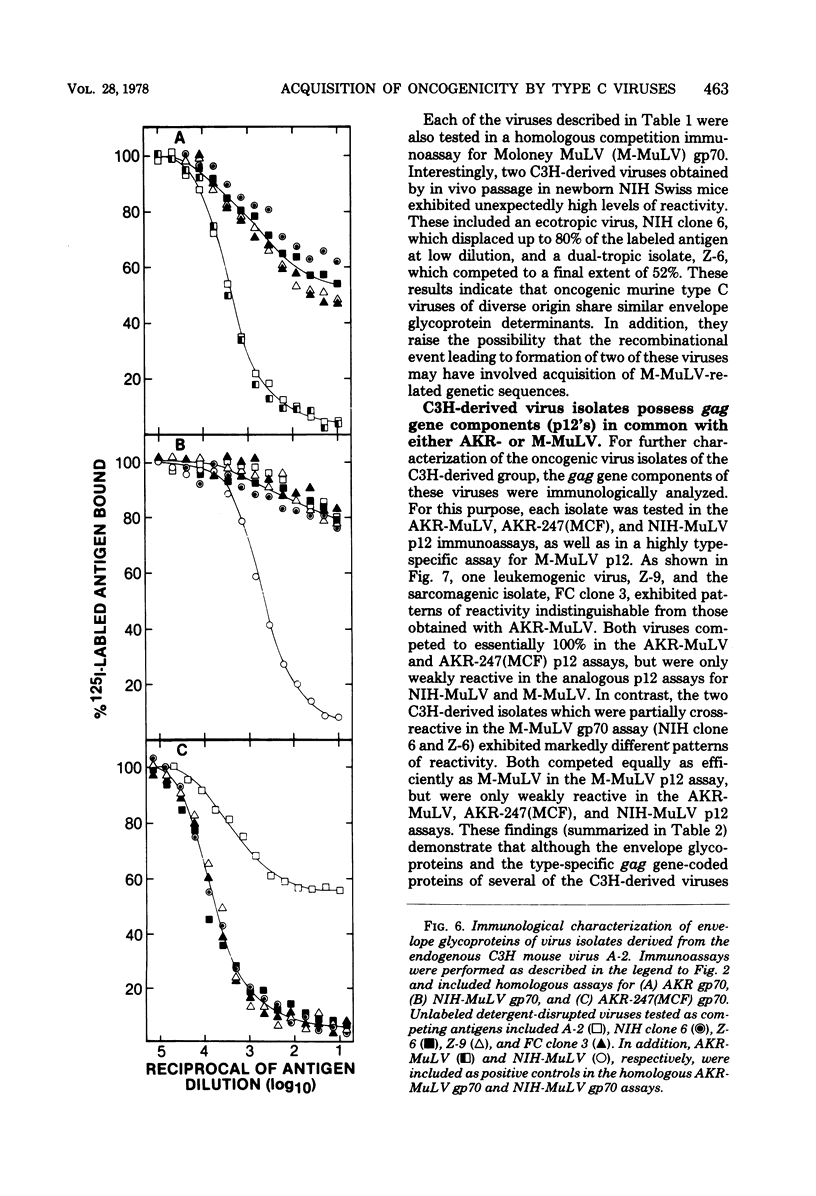
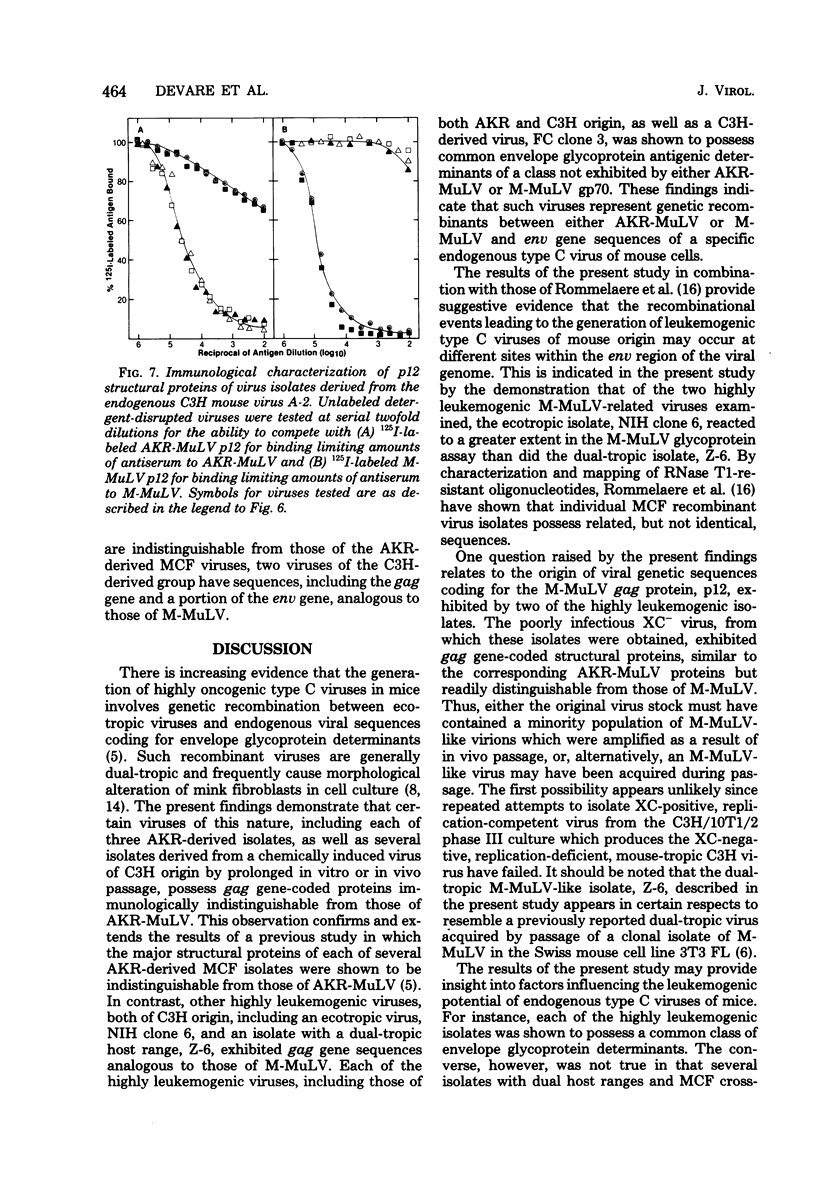
Several dual-tropic isolates derived from the thymuses of preleukemic or leukemic AKR mice and a more recrnt group of viruses generated by in vitro or in vivo passage of a poorly infectious endogenous virus of C3H mouse cells have been shown to be highly oncogenic. By analysis of the immunological properties of their gag gene-coded structural proteins, each of the AKR-derived isolates and two dual-tropic C3H-derived isolates were found to closely resemble AKR murine leukemia virus. In contrast, gag gene-coded proteins of two other leukemogenic isolates of C3H origin, including one ecotropic and one dual-tropic virus, were indistinguishable from those of Moloney murine leukemia virus. All of the oncogenic isolates, including those of AKR and C3H origin, were found to possess common envelope glycoprotein determinants of a unique class not shared by the nononcogenic ecotropic viruses from which they were derived. These findings support the possibility that oncogenic variants of endogenous ecotropic mouse type C viruses are derived by genetic recombination. This recombinational event appears to involve the acquisition, by different ecotropic viruses, of a common class of endogenous virus-coded envelope glycoprotein determinants which are presumably required, but not necessarily sufficient, for oncogenicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Endogenous type-C RNA viruses of mammalian cells. Biochim Biophys Acta. 1976 Dec 23;458(4):323–354. doi: 10.1016/0304-419x(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Stephenson J. R., Aaronson S. A. gag Gene of mammalian type-C RNA tumour viruses. Nature. 1976 Aug 12;262(5569):554–559. doi: 10.1038/262554a0. [DOI] [PubMed] [Google Scholar]
- Declève A., Lieberman M., Ihle J. N., Kaplan H. S. Biological and serological characterization of radiation leukemia virus. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4675–4679. doi: 10.1073/pnas.73.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Hanson R. E., Jr, Stephenson J. R. Primate retroviruses: envelope glycoproteins of endogenous type C and type D viruses possess common interspecies antigenic determinants. J Virol. 1978 May;26(2):316–324. doi: 10.1128/jvi.26.2.316-324.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Bolognesi D. P. A novel murine oncornavirus with dual eco- and xenotropic properties. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J Virol. 1971 Nov;8(5):778–785. doi: 10.1128/jvi.8.5.778-785.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Collins J. J., Lee J. C., Fischinger P. J., Moennig V., Schäfer W., Hanna M. G., Jr, Bolognesi D. P. Characterization of the immune response to the major glycoprotein (gp 71) of Friend leukemia virus. I. Response in BALB/c mice. Virology. 1976 Nov;75(1):74–87. doi: 10.1016/0042-6822(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Kawashima K., Ikeda H., Hartley J. W., Stockert E., Rowe W. P., Old L. J. Changes in expression of murine leukemia virus antigens and production of xenotropic virus in the late preleukemic period in AKR mice. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4680–4684. doi: 10.1073/pnas.73.12.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MOLONEY J. B. Biological studies on a lymphoid-leukemia virus extracted from sarcoma 37. I. Origin and introductory investigations. J Natl Cancer Inst. 1960 Apr;24:933–951. [PubMed] [Google Scholar]
- Rapp U. R., Todaro G. J. Generation of oncogenic type C viruses: rapidly leukemogenic viruses derived from C3H mouse cells in vivo and in vitro. Proc Natl Acad Sci U S A. 1978 May;75(5):2468–2472. doi: 10.1073/pnas.75.5.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. K., Stephenson J. R. Intracistronic mapping of the murine type C viral gag gene by use of conditional lethal replication mutants. Virology. 1977 Sep;81(2):328–340. doi: 10.1016/0042-6822(77)90149-0. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Reynolds R. K., Devare S. G., Reynolds F. H. Biochemical and immunological properties of gag genecoded structural proteins of endogenous tyep C RNA tumor viruses of diverse mammalian species. J Biol Chem. 1977 Nov 10;252(21):7818–7825. [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]



