Abstract
Background and objective: Hepatic sinusoidal obstruction syndrome (HSOS) is characterized by painful hepatomegaly, ascites, increased body weight, and jaundice. Gynura segetum (Compositae), a plant widely used in Chinese traditional medicine, often leads to the development of HSOS. However, the mechanism is unclear. The aim was to study the role of matrix metalloproteinase-9 (MMP-9) in the onset of HSOS induced by Gynura segetum. Methods: Twenty-five male Sprague-Dawley rats were randomly divided into two groups. Twenty were exposed to 600 mg/kg daily Gynura segetum extract solution for three weeks; five control rats were exposed to tap water alone. Liver sections were evaluated by light microscopy with a modified scoring system. Routine transmission electron microscopy (TEM) methods were used to evaluate the ultrastructual features of fixed liver tissue, and blood samples were collected to determine liver enzyme concentrations. MMP-9 expression was assessed by both immunohistochemical staining and enzyme-linked immunosorbent assay (ELISA) methods. Results: A stable and reproducible rat model of HSOS was achieved by long-term exposure to Gynura segetum extract. The treated rats presented clinical symptoms and the histopathological manifestation of HSOS, including abnormal liver enzyme concentrations (alanine aminotransferase (ALT): (84.8±13.62) vs. (167.0±72.63) U/L, P<0.05; aspartate aminotransferase (AST): (27.6±6.31) vs. (232.8±108.58) U/L, P<0.05). Hematoxylin and eosin (H&E) staining and TEM together revealed deposition of red blood cells, the damage and destruction of hepatic sinusoidal endothelial cells, collapse of hepatic sinusoids, hemorrhage of subendothelial cells, atrophy and destruction of hepatocytes, etc. Compared with controls, the expression of MMP-9 in the blood sample, the lung and liver tissues of HSOS rats was increased. Conclusions: MMP-9 may have an important role in early pathological changes of HSOS, and thus the onset of the disease.
Keywords: Hepatic sinusoidal obstruction syndrome, Gynura segetum (Compositae), Sinusoidal endothelial cells, Matrix metalloproteinase-9
1. Introduction
Hepatic sinusoidal obstruction syndrome (HSOS), also called hepatic veno-occlusive disease (HVOD), is characterized by painful hepatomegaly, ascites, increased body weight, and jaundice (Wadleigh et al., 2003). Willmot and Robertson (1920) first reported that HSOS is caused by herbal tea which contained pyrrolizidine alkaloids (PAs). Since then, more scientists have reported that some other plant medicines, including Gynura segetum, will induce HSOS or hepatic injury (Chojkier, 2003). Gynura segetum (Compositae), which is the leaf of Sedum aizoon L., is a traditional Chinese herbal remedy extensively used to treat hemorrhage, trauma, neurosis, and hypertension (Chen et al., 2007). Patients who use Gynura segetum have been diagnosed with HSOS by physicians and pathologists in China (Lin et al., 2011). Scientists consider the PAs in Gynura segetum to be the cause of HSOS, though the pathogenesis is still unclear. To investigate HSOS pathogenesis, a stable animal model must be established. The classic HSOS rat model was established using monocrotaline (a single large dose of 160 mg/kg) (DeLeve et al., 1999), despite the narrow therapeutic window, and lack of detectable of sinusoidal fibrosis. Therefore this classic HSOS model still needs to be improved.
Matrix metalloproteinases (MMPs) can degrade most of the constituents of the extracellular matrix (ECM) (Hiller et al., 2000; Nagase et al., 2006). Twenty-three different MMPs are expressed in the human body; MMP-9 belongs to the gelatinases (Visse and Nagase, 2003). Disturbing the well-balanced equilibrium of MMPs results in many diseases, such as arthritis, nephritis, cancer, encephalomyelitis, chronic ulcers, and fibrosis. (Patterson et al., 2001). Tissue inhibitors of metalloproteinases (TIMPs) and MMPs combine to coordinate the degradation and remodeling of the ECM (Stolow et al., 1996; Chen et al., 2008). During the early stage of HSOS, the hepatic sinusoidal endothelial lining is damaged in a manner associated with increased MMP expression, which degrades the ECM surrounding the sinusoidal endothelial cells (SECs) and destroys the continuity of hepatic SECs. Herein, we establish a stable rat model of HSOS by exposure to Gynura segetum, and observe the expression of MMP-9 to detect the disease in its early phases.
2. Materials and methods
2.1. Extraction and component analysis of alkaloids in Gynura segetum
The root of Gynura segetum from Zhejiang province was identified by Dr. Bing WU of the College of Pharmacy, Zhejiang University, Hangzhou, China. The Gynura segetum root (10 kg) was extracted with ethanol for 1 h thrice. The resulting solution was concentrated to 420 g of Gynura segetum extract. To analyze the components, the extract was extracted with 70% ethanol thrice and then heated to evaporate the ethanol. A total of 2 ml of the residue was acidified to pH 3 with hydrochloric acid (12 mol/L) and was extracted thrice with chloroform. This solution was then alkalized to pH 10–11 with ammonia (16.5 mol/L) and extracted with chloroform thrice. The extracted solution was dried and reconstituted in 1 ml methanol, which was centrifuged (10 000 r/min) for 10 min. The supernatant was subjected to gas chromatography using an Agilent SB C-18 column with a methanol mobile phase (A) or 10 mmol/L formic acid/10 mmol/L ammonium acetate (B). The sample was eluted using a 5%–95% gradient for 60 min with a flow rate of 1.0 ml/min. The resulting solutions were analyzed on an Agilent 1100 series LC/Finnigan LCQ Deca XP plus ion trap mass spectrometer (Thermo Finnigan, San Jose, CA) using electrode spray ionization (ESI), with cation detection, using an m/z quality scanning scope (100–1 200).
2.2. Rat HSOS model
Twenty-five male Sprague-Dawley rats weighing 200–250 g were provided by the Laboratory of the Department of Surgery, the First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China. The study protocol was according to the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the United States National Institutes of Health and was approved by the Animal Ethic Review Committees of Zhejiang University. Rats were randomly divided into two groups. Twenty were gavaged with 600 mg/kg Gynura segetum extract solution daily for three weeks. The five rats in the control group were gavaged with tap water alone. Two of the rats in the treatment group died (on Days 14 and 16, respectively); their livers were excised and histopathological slides prepared as described below. The other 23 rats in the treatment group were killed after three weeks. Blood samples were taken to determine the levels of liver enzyme activity. Their livers and lungs were excised; parts of both organs were frozen at −80 °C for enzyme-linked immunosorbent assay (ELISA) analysis, and the rest was immersed in formaldehyde for sectioning.
2.3. Histopathology
Liver tissues were fixed in 10% buffered formalin and embedded in paraffin for light microscopy. Liver sections were evaluated by light microscopy by experienced pathologists who worked at Department of Surgery, the First Affiliated Hospital Medical School, Zhejiang University, Hangzhou, China, with a modified scoring evaluation system based on DeLeve et al. (1999)’s which includes six parameters: endothelial damage of the central venules, coagulative necrosis of hepatocytes; subendothelial hemorrhage of central venules, sinusoidal hemorrhage, subendothelial fibrosis of the central venules, and sinusoidal fibrosis. For transmission electron microscopy (TEM), the fresh rat livers were cut into 1 mm3 sections and immersed in 2.5% glutaraldehyde at 4 °C overnight. Routine TEM (JEM-1230, JEOL Ltd., Japan) methods were used to evaluate the ultrastructual features of the tissue samples.
2.4. MMP-9 immunohistochemistry (IHC)
Tissue sections were prepared as described for the hematoxylin and eosin (H&E) staining, then deparaffinized and dehydrated. Staining was performed using the Goat Histostain-Plus kits (Tianjin Haoyang Biologics, China). Following dehydration, the liver sections were treated with 3% H2O2 for 5–10 min to block endogenous peroxidase activity, and then washed and immersed in phosphate-buffered saline (PBS) for 5 min. Antigen retrieval was performed by microwaving the samples for 15 min. Non-specific protein binding was blocked with rabbit serum at room temperature for 15 min prior to incubation with an MMP-9 sheep anti-human polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA; 1:200 dilution) at 4 °C overnight. The next morning, the samples were washed thrice with PBS for 3 min, and then incubated with the secondary antibody at room temperature for 10–15 min and washed thrice with PBS for 3 min. Diaminobenzamidine (DAB) was used to detect the secondary antibody colorimetrically, resulting in a dark brown color. The tissue samples were washed with tap water, then stained with H&E and dehydrated. Coverslips were mounted using resin. As a staining control, PBS was used in place of the primary antibody. Ten fields were observed for each slide under high power magnification (400×) by observers who were unaware of the treatment.
2.5. ELISA determination of MMP-9
To determine serum concentrations of MMP-9, the tails were cut off, and 2 ml of blood was removed from each rat and centrifuged (10 000 r/min) for 15 min. The resulting supernatant was instantly frozen at −20 °C. To determine liver and lung tissue concentrations of MMP-9, fresh liver tissue and lung tissue taken by celiotomy were cut into small pieces. Cold saline was added to the liver and lung tissues in test tubes. ELISA was performed using a kit from Shanghai Xitang Biologics (China) according to the manufacturer’s instructions. The sensitivity of the assays of MMP-9 was 15 pg/ml.
2.6. Statistical analysis
All the data were analyzed by the statistical software package SPSS 16.0. The data obtained is presented as the mean±standard deviation (SD). Correlations were assessed using the Student’s t-test. A P-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Alkaloids in Gynura segetum
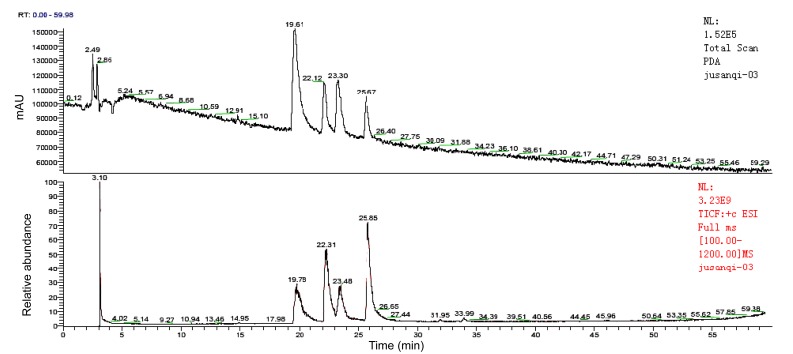
Results of analysis of mass spectrometry (Fig. 1) revealed four kinds of alkaloids: seneciphylline, seneciphylline-N-oxide, senecionine, and senecionine-N-oxide (Table 1).
Fig. 1.
Mass spectrometry of Gynura segetum extract
Table 1.
Analysis results of Gynura segetum extract
| Alkaloid | Retention time in mass spectrometer (min) | m/z |
| Seneciphylline | 19.78 | 333 |
| Seneciphylline-N-oxide | 22.31 | 349 |
| Senecionine | 23.48 | 335 |
| Senecionine-N-oxide | 25.85 | 351 |
3.2. Rat model of HSOS
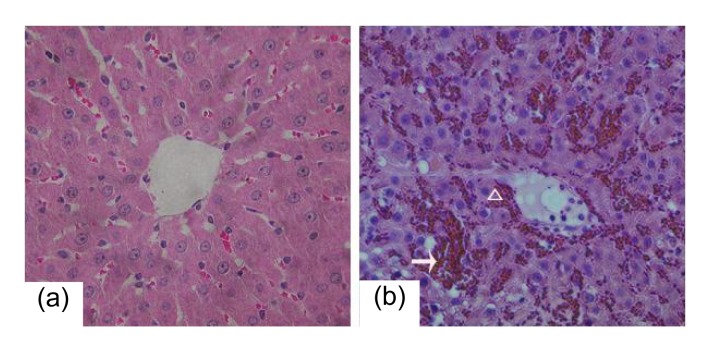
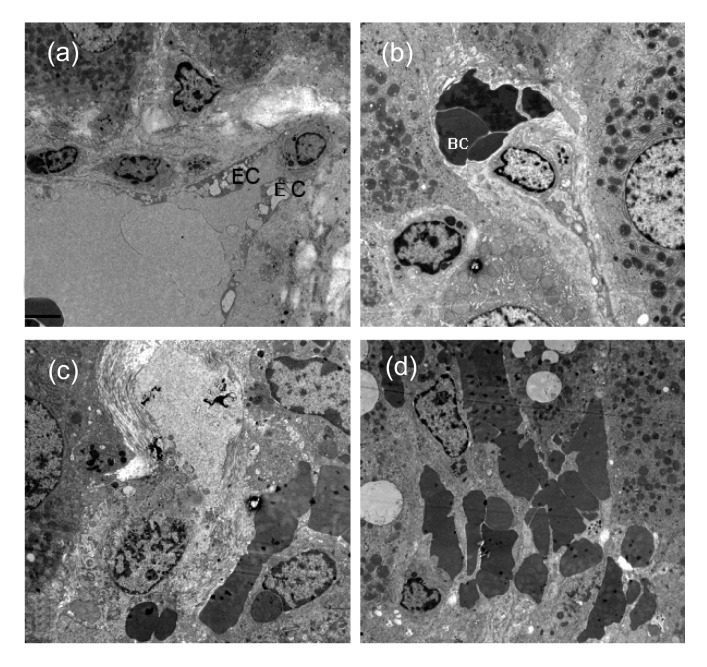
A group of 20 rats were exposed to Gynura segetum extract (600 mg/kg daily) for three weeks. Ascites occurred in 10 rats (50%). Two rats died, one on Day 14 and the other on Day 16. All 20 rats had piloerection, jaundice of the skin and mucosa, and decreased movement. Analysis of the liver tissue of these rats revealed obvious hemostasis, hepatomegaly, and cirrhotic nodules in the livers. H&E staining revealed hepatic sinusoidal hemostasis, deposition of red blood cells, endothelial injury of central venules, hemorrhage of subendothelial cells, coagulative necrosis, atrophy, and destruction of hepatocytes, and inflammatory cells infiltration in hepatic lobules (Fig. 2). The damage and destruction of hepatic sinusoidal endothelial cells, collapse of hepatic sinusoids, deposition of red blood cells, infiltration of macrophages and monocytes in hepatic sinusoids, fibrosis and necrosis of hepatic parenchymal cells, and venular fibrosis were not prominent in central venules of hepatic lobules as determined by TEM (Fig. 3). In concordance with these results, blood work revealed that alanine aminotransferase (ALT), total bilirubin (TB), and aspartate aminotransferase (AST) levels were distinctly higher in the treated rats than in the control rats (Table 2).
Fig. 2.
Damage in hepatic tissue of the the rats exposed to Gynura segetum extract
(a) Liver tissue from a control rat. (b) Liver tissue from an HSOS rat reveals hemostasis, deposition of red blood cells (arrow), injured central venular endothelial cells in the hepatic lobules, subendothelium hemorrhage (triangle), and coagulative necrosis, atrophy and destruction of hepatic cells. Magnification: 400×
Fig. 3.
Rat liver tissues from the HSOS group
(a) Incomplete hepatic sinusoidal endothelial lining (EC: endothelial cell); (b) Disrupted structure of hepatic sinusoids (BC: blood cell); (c) Fibrosis of hepatic sinusoids, entrance of red blood cells into the space of Disse; (d) Red blood cells deposition in damaged hepatic sinusoids with disrupted structures. Magnification: (a) 4000×, (b) 5000×, (c) 6000×, (d) 4000×
Table 2.
Altered liver enzyme activity in Blood panel results
| Group | ALT (U/L) | AST (U/L) | TB (μmol/L) |
| Control (n=5) | 84.8±13.62 | 27.6±6.31 | 0.6±0.89 |
| HSOS (n=20) | 167.0±72.63* | 232.8±108.58* | 26.8±19.50* |
P<0.05, compared to the control group
ALT: alanine aminotransferase; TB: total bilirubin; AST: aspartate aminotransferase
3.3. Expression of MMP-9 in the HSOS rats
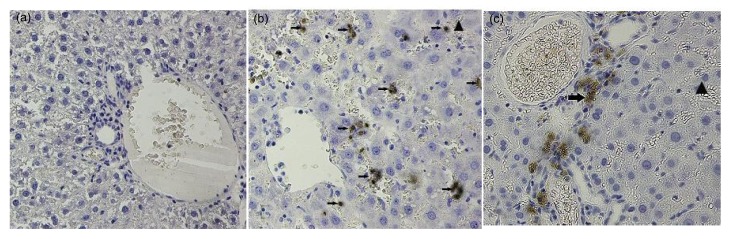
IHC revealed few MMP-9 positive cells in liver tissue from the control group, especially compared to the tissue from the treated rats. MMP-9 positive cells in these tissues were found primarily in the portal area and in hepatic sinusoids with significant red blood cell deposition (Fig. 4). ELISA analysis of the plasma, liver, and lung tissues of treated rats revealed significantly higher MMP-9 expression than that of the control group (P<0.05) (Table 3).
Fig. 4.
Differential MMP-9 expression and localization in the livers from control rats and HSOS model rats
(a) Control liver tissue has very few MMP-9 positive cells, and the tissue appears normal. (b, c) Liver tissue from treated rats revealed many MMP-9 positive cells (arrows). These positive cells were found primarily in portal areas and in hepatic sinusoids with red blood cells (triangles). The hepatic cells were not organized, and the hepatic sinusoids had a significant loss of structural integrity. Magnification: 400×
Table 3.
ELISA determination of MMP-9 concentration
| Group | Blood (μg/ml) | Liver (μg/ml) | Lung (μg/ml) |
| Control | 1.144±0.006 | 1.145±0.007 | 0.839±0.011 |
| HSOS | 1.836±0.175* | 1.454±0.264# | 0.996±0.139Δ |
P<0.005, compared to control group
P<0.005, compared to control group
P<0.05, compared to control group
4. Discussion
Herein, to understand the mechanism by which Gynura segetum extract induces HSOS, we examined the expression of MMP-9, which was elevated in the liver, as well as in the lung tissue. We established a rat model of HSOS using long-term exposure to Gynura segetum root extract, to correlate with patients with HSOS caused by Gynura segetum treatment. Component analysis of Gynura segetum root revealed four kinds of alkaloids: seneciphylline, seneciphylline-N-oxide, senecionine, and senecionine-N-oxide, all of which are PAs. In accordance with preliminary experiments, we chose a dosage of 600 mg/kg daily for three weeks. The higher dosage necessary for an effect is most likely the result of a different extraction method of the Gynura segetum preparation. The HSOS rat model was established successfully, as demonstrated by histopathology and blood work. While the dosage we used for the gavage was larger than that used by other researchers, we performed our extraction by a different method, which would explain the differing efficacies of the preparations. Finally, based on the stable HSOS rat model, we could investigate more unknown mechanism of Gynura segetum-induced HSOS.
The classical scoring system for HSOS histological sections (DeLeve et al., 1999) includes seven parameters: endothelial damage of the central venules, coagulative necrosis of hepatocytes, subendothelial hemorrhage of central venules, sinusoidal hemorrhage, subendothelial hemorrhage of the central venules, inflammation of the central venules, and lobular inflammation. Chen et al. (2008) published an improved HSOS scoring system, adding sinusoidal fibrosis. Observing paraffin sections under a light microscope and an electron microscope, revealed that the sinusoidal fibrosis was more distinct than the lobular central venular fibrosis in the treated rats. In accordance with recent research into the pathogenesis of HSOS, sinusoidal damage occurred at an early stage of HSOS (DeLeve et al., 2002), which resulted in sinusoidal fibrosis, sinusoidal stenosis or obstruction, blockage of blood flow, degeneration and necrosis of hepatocytes, and finally fibrosis, stenosis, and occlusion in central venules (Richardson and Guinan, 1999). Changes in the adventitia of lobular central venules occurred in later stages of HSOS. Therefore, adventitial fibrosis is not suitable to detect HSOS at an early stage. Our research will improve the evaluation of HSOS by scoring, which is beneficial to the diagnosis and treatment of HSOS.
The pathogenesis of HSOS is not clear, but it is associated with glutathione (GSH) depletion, nitric oxide (NO) depletion, increased MMP expression, and known factors involved in hepatic fibrosis. The role of GSH depletion in HSOS pathogenesis has been demonstrated in vitro and in vivo (DeLeve, 1994; 1996; DeLeve et al., 1996). GSH is markedly depleted in SECs, which precedes cell death, and is the most common biochemical change induced by drugs and toxins implicated in HSOS. Furthermore, maintaining GSH levels in the presence of such toxins prevents cell death, and continuous infusion of GSH or N-acetylcysteine prevents the development of HSOS in the monocrotaline model (DeLeve et al., 2003). GSH infusion 24 h after monocrotaline exposure only reduces the degree of hepatic sinusoidal injury, but to a lesser extent than prophylactic treatment with glutathione (DeLeve et al., 2003). In parallel with the decline in hepatic flow, NO levels in the hepatic vein decrease (DeLeve et al., 2003). Indeed, inhibition of NO synthesis in the rat HSOS model caused by a sub-toxic dose of monocrotaline aggravated the disease. In addition, infusion of a liver-specific NO precursor prevents the morphological changes associated with HSOS, as well as the clinical symptoms. This effect suggests involvement of vasoconstriction and NO depletion in the development of HSOS (DeLeve et al., 2003).
Hyaluronic acid (HA) is a polysaccharide, which is cleared by hepatic sinusoidal endothelial cells. The level of serum HA reflects damage to the SECs. Serum HA levels are increased in patients with HSOS, and correlate with the severity of the disease (Petaja et al., 2000; Fried et al., 2001). In addition, levels of N-terminal propeptide for type III procollagen (P-III-P), a sensitive index of hepatic fibrosis, increase in patients with HSOS. In fact, serum P-III-P levels prior to exposure might predict which patients are at risk for developing HSOS (Rio et al., 1993; Tanikawa et al., 2000). A recent study showed that the hemochromatosis C282Y allele is a risk factor for HSOS, and that polymorphisms in carbamyl-phosphate synthetase, a rate-limiting urea cycle enzyme, may counteract its adverse effects (Kallianpur et al., 2005).
The ECM assists in the maintenance of cell morphology and organ development. MMPs are a family of structurally and functionally related calcium and zinc endopeptidases that can degrade most ECM constituents (Hiller et al., 2000; Nagase et al., 2006). In the early stages of HSOS, damage to the integrity of the sinusoidal endothelial lining is associated with increased MMP expression, which degrades the ECM and destroys the continuity of hepatic sinusoidal endothelial cells. In vivo and in vitro SEC studies (DeLeve et al., 2003) have shown that monocrotaline causes F-actin depolymerization in SECs; blocking F-actin depolymerization decreased the expression of MMP-9. We found that MMP-9 was over-expressed in the livers of treated rats. IHC revealed that MMP-9 positive cells were primarily SECs and the vascular endothelial cells in portal areas, which suggests that hepatic SECs are the primary secretors of MMP-9.
Like Gynura segetum, monocrotaline is a PA, which has proven liver toxicity and can cause pulmonary injury (Chojkier, 2003). A rat model of pulmonary artery hypertension after peritoneal injection of monocrotaline has been successfully established (Kolettis et al., 2007; Maruyama et al., 2007). ELISA results revealed MMP-9 in the pulmonary tissues of treated rats was much higher than that of control rats. Gynura segetum has both liver and lung toxicity, and can cause MMP-9 over-expression in the liver. In addition, MMP-9 metabolites can reach the lungs, producing pathophysiological changes.
In conclusion, MMP-9 has an important role in the pathogenesis of HSOS, and the over-expression of MMP-9 in rat lungs could be associated with pulmonary artery hypertension. For further study of HSOS pathogenesis, we presented a stable rat model of HSOS. The events inducing increased MMP-9 expression could be explored further. The pathological changes in multiple organs of HSOS rats demonstrate that patients with HSOS caused by taking Gynura segetum must take steps to protect multiple organs.
Acknowledgments
We thank Dr. Bing WU of the College of Pharmacy, Zhejiang University, Hangzhou, China, for technical assistance on the extract and analysis of Gynura segetum.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30925033), and the Administration of Traditional Chinese Medicine of Zhejiang Province (No. 2007ZA016), China
References
- 1.Chen MY, Cai JT, Du Q. Hepatic veno-occlusive disease associated with the use of Gynura segetum . Europ J Intern Med. 2007;18(8):609. doi: 10.1016/j.ejim.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Chen MY, Cai JT, Du Q, Wang LJ, Chen JM, Shao LM. Reliable experimental model of hepatic veno-occlusive disease caused by monocrotaline. Hepatobiliary Pancreat Dis Int. 2008;7(4):395–400. [PubMed] [Google Scholar]
- 3.Chojkier M. Hepatic sinusoidal-obstruction syndrome: toxicity of pyrrolizidine alkaloids. J Hepatol. 2003;39(3):437–446. doi: 10.1016/S0168-8278(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 4.DeLeve LD. Dacarbazine toxicity in murine liver cells: a model of hepatic endothelial injury and glutathione defense. J Pharmacol Exp Ther. 1994;268(3):1261–1270. [PubMed] [Google Scholar]
- 5.DeLeve LD. Cellular target of cyclophosphamide toxicity in the murine liver: role of glutathione and site of metabolic activation. Hepatology. 1996;24(4):830–837. doi: 10.1002/hep.510240414. [DOI] [PubMed] [Google Scholar]
- 6.DeLeve LD, Wang X, Kuhlenkamp JF, Kaplowitz N. Toxicity of azathioprine and monocrotaline in murine sinusoidal endothelial cells and hepatocytes: the role of glutathione and relevance to hepatic venoocclusive disease. Hepatology. 1996;23(3):589–599. doi: 10.1002/hep.510230326. [DOI] [PubMed] [Google Scholar]
- 7.DeLeve LD, McCuskey RS, Wang X, Hu L, McCuskey MK, Epstein RB, Kanel GC. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology. 1999;29(6):1779–1791. doi: 10.1002/hep.510290615. [DOI] [PubMed] [Google Scholar]
- 8.DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22(1):27–42. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- 9.DeLeve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125(3):882–890. doi: 10.1016/S0016-5085(03)01056-4. [DOI] [PubMed] [Google Scholar]
- 10.Fried MW, Duncan A, Soroka S, Connaghan DG, Farrand A, Peter J, Strauss RM, Boyer TD, McDonald GB. Serum hyaluronic acid in patients with veno-occlusive disease following bone marrow transplantation. Bone Marrow Transplant. 2001;27(6):635–639. doi: 10.1038/sj.bmt.1702821. [DOI] [PubMed] [Google Scholar]
- 11.Hiller O, Lichte A, Oberpichler A, Kocourek A, Tschesche H. Matrix metalloproteinases, collagenase-2, macrophage elastase, collagenase-3, and membrane type 1-matrix metalloproteinase impair clotting by degradation of fibrinogen and factor XII. J Biol Chem. 2000;275(42):33008–33013. doi: 10.1074/jbc.M001836200. [DOI] [PubMed] [Google Scholar]
- 12.Kallianpur AR, Hall LD, Yadav M, Byrne DW, Speroff T, Dittus RS, Haines JL, Christman BW, Summar ML. The hemochromatosis C282Y allele: a risk factor for hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35(12):1155–1164. doi: 10.1038/sj.bmt.1704943. [DOI] [PubMed] [Google Scholar]
- 13.Kolettis T, Vlahos AP, Louka M, Hatzistergos KE, Baltogiannis GG, Agelaki MM, Mitsi A, Malamou-Mitsi V. Characterisation of a rat model of pulmonary arterial hypertension. Hellenic J Cardiol. 2007;48(4):206–210. [PubMed] [Google Scholar]
- 14.Lin G, Wang JY, Li N, Gao H, Ji Y, Zhang F, Wang H, Zhou Y, Ye Y, Xu HX, Zheng J. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum . J Hepatol. 2011;54(4):666–673. doi: 10.1016/j.jhep.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Maruyama H, Watanabe S, Kimura T, Liang J, Nagasawa T, Onodera M, Aonuma K, Yamaguchi I. Granulocyte colony-stimulating factor prevents progression of monocrotaline-induced pulmonary arterial hypertension in rats. Circ J. 2007;71(1):138–143. doi: 10.1253/circj.71.138. [DOI] [PubMed] [Google Scholar]
- 16.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Patterson ML, Atkinson SJ, Knäuper V, Murphy G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001;503(2-3):158–162. doi: 10.1016/S0014-5793(01)02723-5. [DOI] [PubMed] [Google Scholar]
- 18.Petaja J, Pitkanen S, Vettenranta K, Fasth A, Heikinheimo M. Serum tumor marker CA 125 is an early and sensitive indicator of veno-occlusive disease in children undergoing bone marrow transplantation. Clin Cancer Res. 2000;6:531–535. [PubMed] [Google Scholar]
- 19.Richardson P, Guinan E. The pathology, diagnosis, and treatment of hepatic veno-occlusive disease: current status and novel approaches. Br J Haematol. 1999;107(3):485–493. doi: 10.1046/j.1365-2141.1999.01680.x. [DOI] [PubMed] [Google Scholar]
- 20.Rio B, Bauduer F, Arrago JP, Zittoun R. N-terminal peptide of type III procollagen: a marker for the development of hepatic veno-occlusive disease after BMT and a basis for determining the timing of prophylactic heparin. Bone Marrow Transplant. 1993;11(6):471–472. [PubMed] [Google Scholar]
- 21.Stolow MA, Bauzon DD, Li J, Sedgwick T, Liang VC, Sang QA, Shi YB. Identification and characterization of a novel collagenase in Xenopus laevis: possible roles during frog development. Mol Biol Cell. 1996;7(10):1471–1483. doi: 10.1091/mbc.7.10.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanikawa S, Mori S, Ohhashi K, Akiyama H, Sasaki T, Kaku H, Hiruma K, Matsunaga T, Morita T, Sakamaki H. Predictive markers for hepatic veno-occlusive disease after hematopoietic stem cell transplantation in adults: a prospective single center study. Bone Marrow Transplant. 2000;26(8):881–886. doi: 10.1038/sj.bmt.1702624. [DOI] [PubMed] [Google Scholar]
- 23.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 24.Wadleigh M, Ho V, Momtaz P, Richardson P. Hepatic veno-occlusive disease: pathogenesis, diagnosis and treatment. Curr Opin Hematol. 2003;10(6):451–462. doi: 10.1097/00062752-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Willmot FC, Robertson GW. Senecio disease, or cirrhosis of the liver due to Senecio poisoning. Lancet. 1920;196(5069):848. doi: 10.1016/S0140-6736(01)00020-4. [DOI] [Google Scholar]