Abstract
Mice deficient for the adaptor Ndfip1 develop inflammation at sites of environmental antigen exposure. We show here that these animals contain fewer inducible regulatory (iTreg) cells. In vitro, Ndfip1-deficient T cells express normal levels of the transcription factor Foxp3 during the first 48 hours of iTreg cell differentiation, however this cannot be sustained. Abortive Foxp3 expression is because Ndfip1–/– cells produce interleukin 4 (IL-4). We demonstrate that Ndfip1 is transiently unregulated during iTreg cell differentiation in a transforming growth factor-β (TGF-β) dependent manner. Once expressed Ndfip1 promotes Itch-mediated degradation of the transcription factor JunB, thus preventing IL-4 production. Based on these data, we propose that TGF-β signaling induces Ndfip1 expression to silence IL-4 production, thus permitting iTreg cell differentiation.
Once in peripheral lymphoid compartments, T cells are poised to activate the immune system in an effort to destroy invading pathogens. These responses play essential roles in pathogen clearance; however, mechanisms exist to ensure that T cell responses are directed towards harmful pathogens while remaining tolerant to self. Furthermore, T cells must remain tolerant not only to self, but also to non-pathogenic (or harmless) environmental antigens. One mechanism that prevents T cells from directing immune responses towards self or environmental antigens is suppression by T regulatory (Treg) cells 1,2.
Treg cells are a specialized subset of T cell that can be generated in one of two ways. Natural Treg cells (nTregs) develop in the thymus and bear T cell receptors (TCR) that primarily recognize self-peptides, whereas iTreg cells, also known as adaptive Treg cells) differentiate from naïve T cell precursors in peripheral lymphoid tissues such as mesenteric lymph nodes that drain the gastrointestinal (GI) tract1,2. These two Treg cell subsets differ in their expression of certain genes and their plasticity, but both subsets express a transcription factor known as Foxp31,2. The essential role of Foxp3 in Treg cell development was revealed by genetic mutations leading to the loss of Foxp3 function. The spontaneous Scurfy (sf) mutation in mice results in a loss of function mutation in Foxp3 and death of the mice by 3-4 weeks of age3, while the loss of Foxp3 function in humans leads to IPEX (immunodysregulation, polyendocrinopathy and enteropathy, X-linked syndrome) 4,5. In these cases, the result of the genetic mutation is loss of Foxp3 expression and a consequent lack of functional Treg cells6-8.
Foxp3 expression in Treg cells relies on both TGF-β and IL-2 receptor (IL-2R) signaling9-11. Treg cells constitutively express CD25, the IL-2Rα component of the high affinity IL-2 receptor complex12. Signaling by IL-2 is important for Treg cell differentiation and maintenance9,10. In addition to IL-2, both nTreg and iTreg cells need TGF-β to induce Foxp3 expression9,11. Stimulation of naïve T cells by TGF-β promotes the induction of Foxp3 expression and iTreg cell differentiation13-18. Additionally, TGF-β dampens IL-4 production and thus suppresses TH2 differentiation19,20. Both of these TGF-β mediated outcomes depend on Smad proteins. For example, Smad3 binds to the Foxp3 gene and activate its transcription21. In addition to directly regulating Foxp3 transcription, Smad activation downstream of TGF-β signaling also induces the expression of TGF-β induced early gene 1 (TIEG1)22. TIEG1 is a transcription factor that binds the Foxp3 gene and induces its transcription23,24. Thus, Smad proteins induce Foxp3 expression by both direct and indirect mechanisms. Following TGF-β signaling, TIEG1 is monoubiquitylated by the E3 ubiquitin ligase known as Itch23. This monoubiquitylation allows TIEG1 to induce Foxp3 transcription23 and is proposed to explain why Itch-deficient T cells are defective at differentiating into iTreg cells in vitro23.
We have identified an adaptor protein, known as Ndfip1, that is required for Itch polyubiquitylation of transcription factors of the Jun family25. Jun family members can act with NFAT to induce expression of IL-426,27. Thus, in the absence of either Itch or Ndfip1, levels of Jun family members, such as JunB and c-Jun, accumulate and promote the transcription of IL-4 and TH2 polarization25,28 leading to TH2-mediated inflammation in the skin, lung, and GI tract25,28,29 in these mice. Knowing that Ndfip1 is required for Itch polyubiquitylation of JunB, we hypothesized Ndfip1 may also promote Itch mono-ubiquitylation of TIEG1. Indeed, T cells lacking Ndfip1 were much less likely to become iTreg cells in vitro than their wild-type (WT) counterparts. However, we did not see a defect in TIEG1 binding to the Foxp3 promoter in either Ndfip1–/– or Itch-deficient T cells within the first 48 hours of iTreg cell induction. Rather our results demonstrate that their defect in iTreg cell induction was due to overproduction of IL-4. Our data indicate that Ndfip1 is highly expressed in a TGF-β-dependent manner, peaking after 24 hours of iTreg cell induction, to prevent the accumulation of JunB and IL-4 production. Based on these results, we propose that Ndfip1 and Itch dampen IL-4 production and thus provide a window of opportunity for iTreg cell lineage commitment.
RESULTS
Mice lacking Ndfip1 have fewer iTregs in vivo
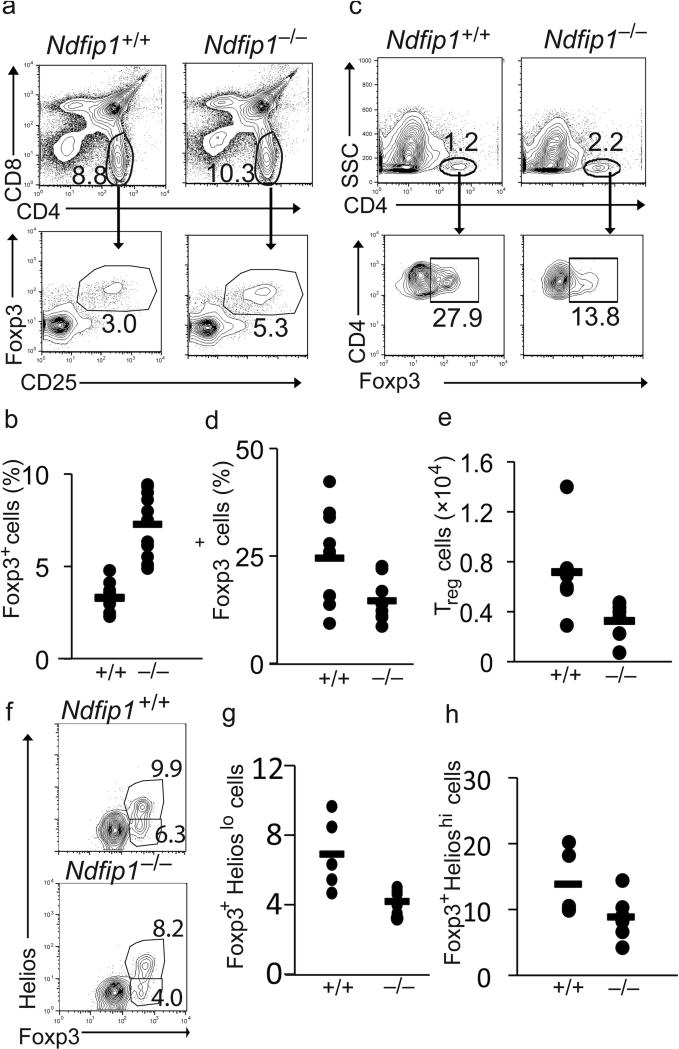
Ndfip1-deficient mice develop a severe atopic inflammatory disease, characterized by hyperproliferative T cells that produce TH2 cytokines and eosinophilia25,29. The disease is reminiscent of the TH2 aspects of the pathology that occurs in Scurfy mice3 and IPEX patients4,5, suggesting that Ndfip1–/– mice might have defects in Foxp3+ Treg cells. Thus, we sought to determine whether Ndfip1–/– mice had a block in the development of Foxp3+ nTreg cells in the thymus. We first analyzed numbers and percentages of CD4+CD25+Foxp3+ cells in the thymi of 4-6 week old mice (Figure 1a, b and Supplementary Figure 1a). This revealed a significant increase in the percentages of Foxp3+ Treg cells in Ndfip1–/– thymi. In contrast, no difference was seen when comparing the total numbers of these cells, possibly due to a decrease in the total number of thymocytes harvested from Ndfip1–/– mice (data not shown). Inflammation can cause both an increase in thymic Foxp3+ cells30 as well as thymic involution. To test whether the increased percentages were due to inflammation or increased nTreg cell differentiation, we analyzed 9-day old neonatal mice, when Treg cell numbers are increasing31, and 2.5-week old mice, prior to histologic evidence of inflammation29. Flow cytometric analysis of Foxp3+ T cells in the thymi of 9-day old mice revealed, based on both percentages and total numbers, that nTreg cells in mice lacking Ndfip1 are similar to their Ndfip1+/+ littermates (Supplementary Fig. 1b, c). Analysis of 2.5-week old Ndfip1-deficient mice showed slightly increased numbers and percentages of these cells (Supplementary Fig. 1d, e). From these data, we conclude that the increase in the percentages of nTreg cells in Ndfip1–/– mice at 4-6 weeks is consistent with changes caused by inflammation. Supporting this, when we analyzed thymi from mixed bone marrow chimeras, the percentages of WT nTreg cells were increased to amounts comparable with Ndfip1–/– cells (Supplementary Fig 1f). Moreover, while Foxp3 staining of the lymph nodes and spleens showed similar percentages (data not shown), there was an increase in the absolute numbers of Foxp3+ Treg cells in Ndfip1–/– over Ndfip1+/+ littermates (Supplementary Fig. 1g). Taken together, these results demonstrate that there is an age-dependent increase in the percentages of nTreg cells in the thymi of Ndfip1–/– mice due to inflammation. Additionally, since nTreg cells still develop in the absence of Ndfip1, Ndfip1 is not required for the development of Foxp3+ cells in the thymus.
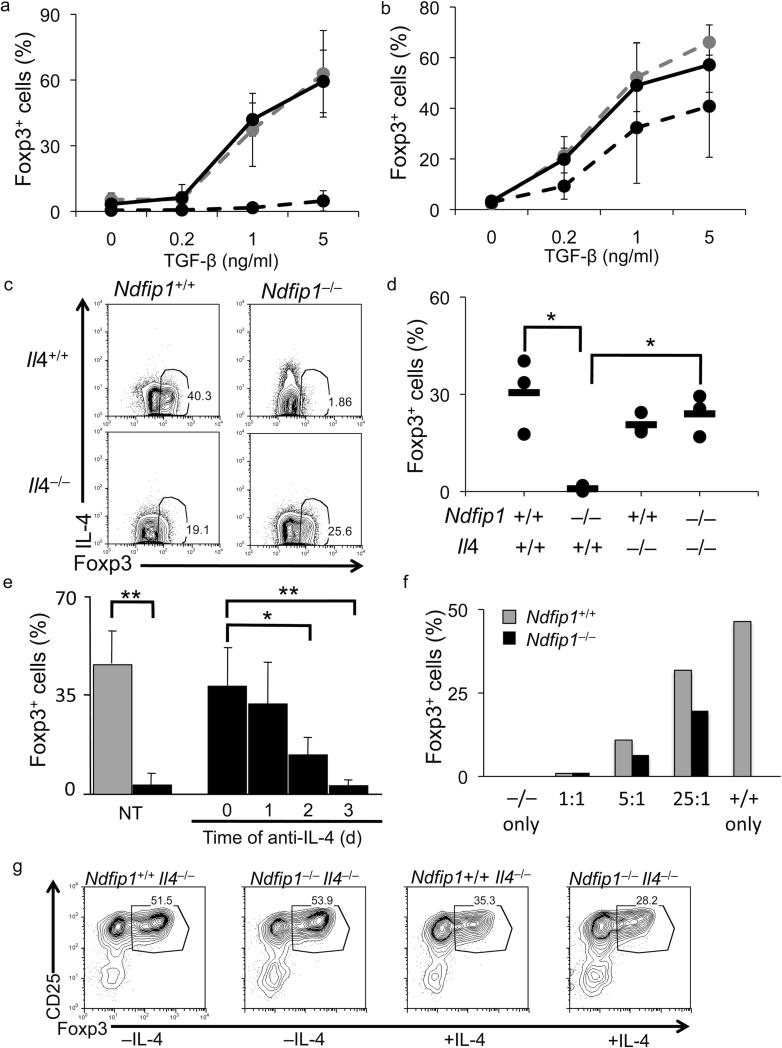
Figure 1. Reduced frequency of iTreg cells in Ndfip1–/– mice.
(a-e) Thymus and small bowel samples from 4 to 8 week old Ndfip1+/+ and Ndfip1–/– mice were analyzed for percentages of Foxp3+ regulatory T cells by flow cytometry. Representative contour and dot plots of Treg cells from the thymi and small bowels are depicted in a and c, respectively. (b) Percentages of CD25+ Foxp3+ Treg cells among the CD4 SP population from the thymus and (d) the percentages of Foxp3+ cells among the CD4+ population in the small bowel. n≥10 (b and d). (e) The number of CD4+ Foxp3+ T cells in the small bowels of Ndfip1–/– or Ndfip1+/+ mice. n=6. (f-h) The small bowels from 4 to 8 week old Ndfip1+/+ and Ndfip1–/– mice were analyzed for the percentage of iTreg cells (Foxp3+Helioslo) by flow cytometry. Representative plots are depicted in f and the percentage of Foxp3+ cells in individual mice is plotted in g and h. *P<0.05 **P<0.01 (two-tailed student T-test). Each dot represents one mouse, n≥5. The bar indicates the mean.
The small bowel is a major site of iTreg cell accumulation32-34. Importantly, the small bowel is a major site of inflammation in Ndfip1–/– mice29, suggesting that iTreg cell differentiation in the GI tract may be defective. Flow cytometry analysis of cells isolated from the small bowel of Ndfip1+/+ and Ndfip1–/– littermates revealed a significant decrease in the percentages and numbers of Foxp3+ Treg cells at this site (Fig. 1c-e). Recently, the transcription factor Helios was described as a marker to differentiate thymically derived nTreg cells from peripherally induced iTreg cells35. However, the use of Helios as a marker for iTreg cells remains controversial. While Helios expression is not a useful marker for iTreg cells differentiated in vitro36, most in vivo models support the original report37. Therefore, we sought to determine whether the decrease in Foxp3+ Treg cell in the small bowel was due to a decrease of the Helioslo iTreg cell population. Helios staining of the cells described in Fig 1c, showed a significant decrease in the percentages of Helioslo Foxp3+ population (Fig. 1f and g) while the percentages of Helioshi cells were lower but not statistically different from those in Ndfip1+/+ littermates (Fig. 1h). These results suggest that in the absence of Ndfip1 there is a defect in iTreg cell differentiation in vivo.
Ndfip1–/– T cells are defective in iTreg cell differentiation
To test whether Ndfip1 is required for iTreg cell differentiation, we next sought to determine whether Ndfip1–/– T cells could differentiate into Foxp3+ iTreg cells in vitro. To do this, we first needed to eliminate TH2-effector T cells from our cultures as these cells can inhibit iTreg cell differentiation19,38,39. Thus, we sorted naïve (CD25–, CD44low, CD62Lhi) CD4+ T cells from 5-7 week old Ndfip1–/– and Ndfip1+/+ littermates. To ensure that IL-4-producing effectors were removed, we tested the cells before and after sorting for IL-4 production by ELISA. Although IL-4 was not detectable in cultures of Ndfip1+/+ cells, prior to sorting Ndfip1–/– cells produced 4.1 ng/ml IL-4 after overnight stimulation. Sorting reduced IL-4 production by Ndfip1–/– cells to 0.02 ng/ml. Knowing this, we cultured naïve Ndfip1+/+ and Ndfip1–/– T cells under iTreg cell differentiation conditions for 5 days and then assessed their expression of CD25 and Foxp3. We found that Ndfip1-deficient T cells were severely impaired in their ability to induce Foxp3 even when given a concentration TGF-β sufficient to induce Foxp3 expression in nearly all of the WT T cells (Fig. 2a).
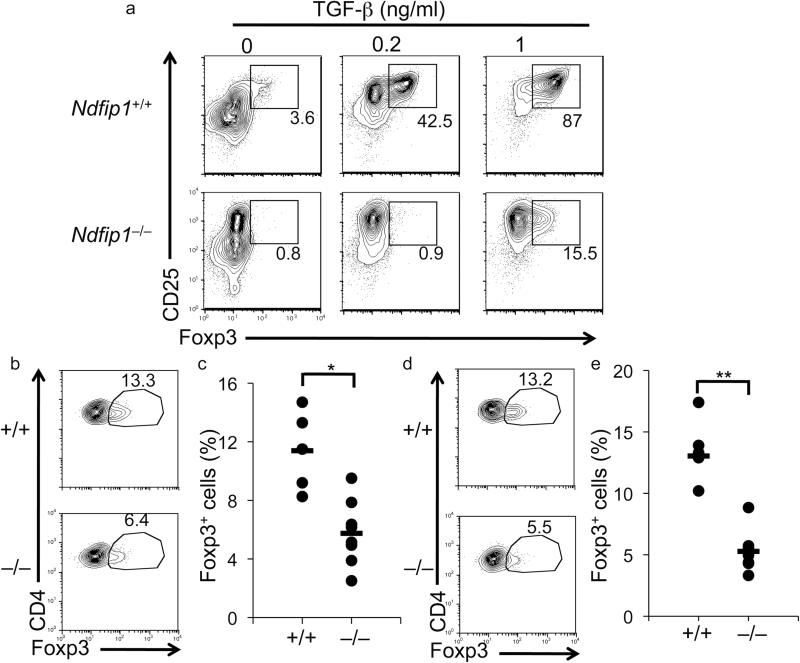
Figure 2. Impaired iTreg cell conversion in naïve T cells lacking Ndfip1.
(a) Naïve (CD25–CD62LhiCD44lo) CD4+ T cells were cultured for 5 days with IL-2, anti-CD3, anti-CD28 and varying concentrations of TGF-β. Cells were then analyzed by flow cytometry. Contour and dot plots of Ndfip1–/– and Ndfip1+/+ (littermate control) T cells are representative of 6 independent experiments. (b-e) 2×106 CD4+ T cells (CD45.2+) from either Ndfip1–/–Rag1–/–OTII+ or Ndfip1+/+Rag1–/–OTII+ mice were transferred intravenously into WT CD45.1+ recipient mice. Recipient mice were then fed Ova for 5 days. Analyses of iTreg cells in the mLN (b and c) and Peyer's Patches (d and e) was analyzed by flow cytometry. Representative dot plots are shown in b and d, while percentages of CD25+Foxp3+ cells among the CD45.2+ T cells are indicated (c and e). Ndfip1+/+ n≥5, Ndfip1–/– n=8. Data are representative of two experiments. *P=0.002 and **P=0.00002 (two-tailed student T-test).
We next assessed whether defective iTreg conversion in Ndfip1–/– T cells could also be observed in vivo. For this, we adopted a recently described model of Ovalbumin (Ova) -induced iTreg cell conversion of Ova-specific (OTII transgenic) T cells33. To generate Ndfip1–/– Ova-specific T cells, we crossed Ndfip1–/– mice to Rag1–/– OTII. As with Ndfip1+/+Rag1–/–OTII+ T cells, T cells from Ndfip1–/–Rag1–/– OTII+ mice were naïve and Foxp3– when isolated and analyzed directly ex vivo (Supplementary Fig 2a,b). To test iTreg cell conversion in vivo, we transferred Ova-specific T cells into congenic recipients and fed animals a low dose of Ovalbumin (Ova) for 5 consecutive days. We found that approximately 13% of transferred WT T cells isolated from the Peyer's Patches, and the mesenteric lymph nodes (mLN) had differentiated into Foxp3+ iTreg cells in response to oral antigen (Fig. 2b-e). In contrast, fewer Ndfip1–/– T cells became Foxp3+ during this period, resulting in slightly reduced percentages in the small bowel (Supplementary Fig 2c) and significantly reduced percentages of Ndfip1–/– iTreg cells in the mLN and Peyer's Patches (Fig. 2b-e). These results demonstrate that Ndfip1–/– T cells are defective at converting into iTreg cells both in vitro and in vivo.
Impaired conversion by Ndfip1- and Itch-deficient cells
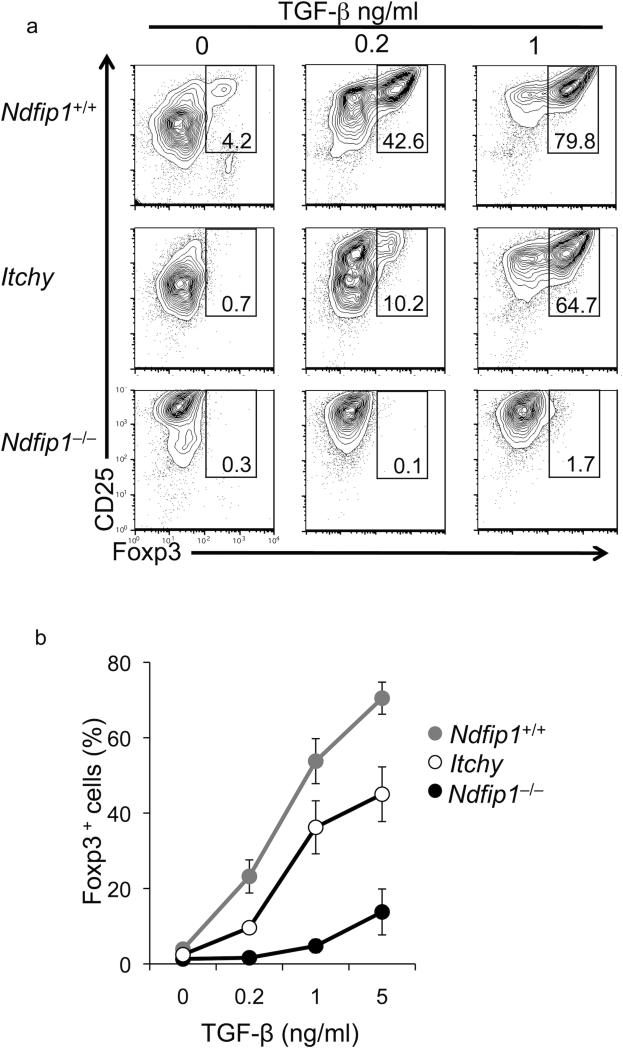
Ndfip1 is an adaptor protein that promotes the Itch-mediated ubiquitylation and consequent degradation of JunB and cJun25–transcription factors involved in TH2 development. Thus both Itchy mutant and Ndfip1–/– T cells are TH2 biased. Itchy mutant T cells are also impaired in iTreg cell conversion23. Considering this, we sought to test whether the defect in iTreg cell differentiation in Ndfip1–/– T cells was due to Ndfip1 regulation of Itch function. We thus compared the iTreg cell differentiation capacity of Ndfip1- and Itch-deficient T cells, using the same sorting and in vitro culture conditions described above. Consistent with what was shown previously23, Itchy mutant T cells are impaired at converting into iTreg cells (Fig. 3 a, b). We found that Ndfip1–/– T cells are even less likely to differentiate into iTregs in vitro than Itch-deficient counterparts (Fig. 3a, b). Combining data from these experiments, we calculated that Ndfip1–/– T cells would need approximately 29 fold more TGF-β for a half-max conversion to Foxp3+ iTreg cells than WT cells, whereas Itchy mutant T cells would need about 2 fold more TGF-β (Fig. 3b). This is unlikely to be due to background differences between the two strains as both have been backcrossed more than 9 generations onto C57BL6. Nonetheless, both Itchy mutant and Ndfip1–/– T cells are defective in iTreg cell conversion.
Figure 3. Both Ndfip1–/– and Itchy mutant T cells are defective in iTreg cell conversion.
(a) Naïve T cells were stimulated with various concentrations of TGF-β as described in Figure 2. After incubation, cells were analyzed for iTreg cell conversion by flow cytometry. Analyses of WT, Ndfip1–/–, and Itchy mutant T cells are shown. Data are representative of at least 6 independent experiments. (b) The mean frequency of Foxp3+ cells in the cultures of WT (gray circles), Itchy mutant (open circles), and Ndfip1–/– (black circles) cells is plotted over the indicated concentrations of TGF-β. (mean + SEM from 8-12 mice).
It has been suggested that Itch promotes iTreg cell differentiation via monoubiquitylation of TIEG123, a transcription factor that promotes Foxp3 expression. Monoubiquitylation of TIEG1 appeared to promote the association of TIEG1 with DNA elements in the Foxp3 locus23. TIEG1 binds two sites in the Foxp3 locus, one within the Foxp3 proximal promoter region24, and the other in an enhancer region known as CNS223. In Itchy mutant T cells, TIEG1 did not bind to the CNS2 enhancer region23, but binding of TIEG1 to the proximal promoter region was not described. However, the CNS2 region was recently shown to be irrelevant for iTreg cell differentiation40. Thus, to test whether Ndfip1 regulates TIEG1 binding to Foxp3 sequences, we used chromatin immunoprecipitation (ChIP) to analyze TIEG1 association with the Foxp3 proximal promoter region in T cells lacking either Ndfip1 or Itch. For this analysis, cells were analyzed for binding after both 18 and 42 hours of iTreg cell conversion. This was based on data that TGF-β signaling is particularly important during this period41. The location of the primers used to detect Foxp3 DNA bound to TIEG1 is illustrated in Supplementary Fig. 3a. TIEG1 associated with the Foxp3 proximal promoter region in WT, Itchy mutant and Ndfip1–/– T cells (Supplementary Fig. 3b). Supporting these results, TIEG1 was also bound to the CNS2 region as determined using previously published primers (data not shown). These results show that impaired iTreg cell differentiation in Ndfip1- and Itch- deficient T cells cannot be explained by a lack of TIEG1 binding to the Foxp3 locus at early time points during iTreg differentiation cell.
Abortive Foxp3 expression in T cells lacking Ndfip1
Based on our results thus far, TIEG1 is bound to the Foxp3 promoter 48 hours after iTreg cell induction. However, these cells do not express Foxp3 after 5 days in these same culture conditions. To resolve this apparent contradiction, we decided to test whether Ndfip1–/– T cells express Foxp3 during the time points tested by ChIP, namely two days after stimulation. Using the same protocol described in Fig. 2a, we tested Foxp3 expression by flow cytometry analysis at day 2 and again at day 5 during iTreg cell differentiation. Using this approach, we found that on day 2, Ndfip1–/– T cells express comparable levels of Foxp3 to those in WT cells (Fig. 4a, b). In contrast, but consistent with our previous results, Foxp3 expression is diminished by day 5 in Ndfip1–/– T cells, while it continues to increase in WT T cells. In addition, we see a similar trend in Foxp3 expression with Itch-deficient T cells (Supplementary Fig. 4a). Recently, it was shown that IL-2 can stabilize Foxp3 expression42. Thus, we sought to determine whether increased amounts of IL-2 can rescue the loss of Foxp3 expression in Ndfip1–/– T cells that occurred between day 2 and day 5. However, increasing the concentration of IL-2 in our cultures to 100U/ml did not rescue the defect (Supplementary Fig. 4b). Another possible explanation for the decline in Foxp3+ T cells from day 2 to day 5 could be that Foxp3+ T cells lacking Ndfip1 die during this culture. Thus, we assessed the percentage of 7AAD+ cells at day 2 and day 5 of iTreg cell differentiation. While we observed a slight increase in the percentage of Ndfip1–/– T cells that are 7AAD+ at day 2, at day 5 the percentages of 7AAD+ cells are reduced compared to controls (Supplementary Fig. 4c). These data suggest that other mechanisms must account for the loss of Foxp3+ cells in the Ndfip1–/– cultures. Consistent with Foxp3 protein expression, Foxp3 mRNA was induced, albeit to a lesser extent, in cells lacking Ndfip1 (Supplementary Fig. 5a). Ndfip1–/– T cells showed reduced Foxp3 mRNA levels beginning at day one while Itch-deficient T cells began to show a reduction in mRNA induction after 2 days of iTreg cell induction (Supplementary Fig. 5b). These data indicate that Foxp3 expression, and by inference iTreg cell induction, is initiated in Ndfip1- and Itch-deficient T cells, but then is aborted. Knowing that IL-4 can block Foxp3 expression and that Ndfip1–/– T cells are prone to produce IL-4 under other culture conditions, we hypothesized that IL-4 production by Ndfip1-deficient T cells could be aborting the iTreg cell differentiation process.
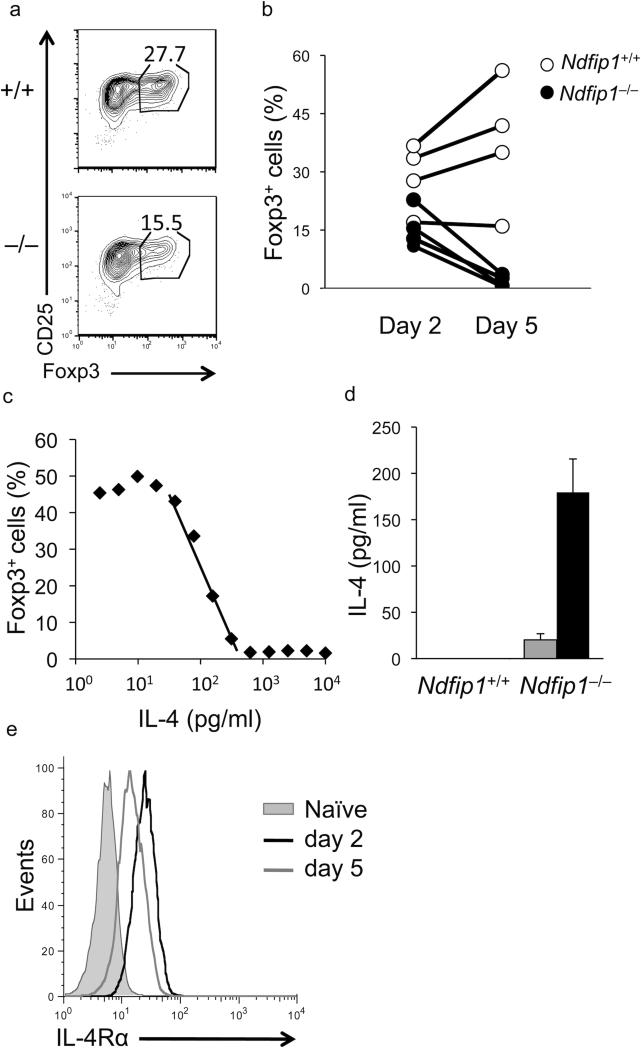
Figure 4. Foxp3 expression declines in Ndfip1–/– T cells after day 2.
(a and b) Foxp3 expression was assessed in Ndfip1+/+ and Ndfip1–/– T cells at day 2 and day 5 during iTreg cell differentiation. (a) Representative dot plots from Ndfip1+/+ and Ndfip1–/– T cells after 2 days of stimulation in the presence of 1ng/ml TGF-β are shown. (b) Graph depicts the combined data from 4 Ndfip1+/+ (open circles) and 4 Ndfip1–/– (closed circles) mice. Each dot represents a single mouse and samples from the same mouse are connected by a line. (c) The amount of IL-4 needed to inhibit iTreg cell differentiation was assessed by adding IL-4 to Ndfip1+/+ cells in iTreg cell cultures. The percentage of cells that acquire Foxp3+ during culture is plotted on log scale against the amount of IL-4 added. Data are representative of three independent experiments. An IC50 of 190 pg/ml was calculated by performing regression analysis of the linear region of the plot (average value of the three experiments). (d) ELISA analysis of IL-4 concentrations in the supernatants of T cells stimulated for 1 (gray bars) or 2 (black bars) days under iTreg cell conditions (1ng/ml TGF-β) (mean + S.D. of three mice) (e) Analysis of IL-4Rα expression on naïve Ndfip1+/+ T cells (gray filled) and T cells that were activated for 2 (black line) or 5 (gray line) days in the presence of 1ng/ml TGF-β. Data are representative of 2 independent experiments.
To begin to test this, we first wanted to determine the amount of IL-4 that inhibits iTreg cell differentiation by adding IL-4 into cultures of WT cells undergoing iTreg cell conversion. Using this approach, we found that iTreg conversion was inhibited by small amounts of IL-4. Graphing this on a logarithmic scale, we could quantify the half maximal inhibitory concentration of IL-4 as 190 pg/ml (Fig. 4c). Knowing this, we next sought to determine whether Ndfip1–/– T cells were producing amounts of IL-4 that would block iTreg cell differentiation. To do this, we measured the amount of IL-4 in cultures of Ndfip1–/– and Ndfip1+/+ cells undergoing iTreg cell differentiation using ELISA. While we saw little IL-4 produced from sorted naïve Ndfip1–/– T cells cultured for 24 hours, we found that the amount of IL-4 detected in supernatants increased after 48 hours of stimulation to levels sufficient to inhibit iTreg cell differentiation (Fig. 4d). Interestingly, while the amount of IL-4 produced by Ndfip1–/– T cells at 24 hours was not different regardless of whether the cells were stimulated in the presence or absence of TGF-β (data not shown), the amount of IL-4 produced by Ndfip1–/– T cells at 48 hours of iTreg cell culture was lower than Ndfip1–/– T cells stimulated in the absence of TGF-β (Supplementary Fig. 5c, 5d). This is consistent with previous data showing TGF-β can attenuate IL-4 production19,20. Furthermore, in agreement with the less severe defect in Itchy mutant T cells undergoing iTreg cell differentiation (Fig. 3), T cells lacking Itch produced much less IL-4 than Ndfip1-deficient counterparts (Supplementary Fig. 5c, 5e).
To test whether cells were able to detect IL-4 from their environment, we used flow cytometry to analyze levels of the IL-4 receptor (IL-4R). After day 1 in culture IL-4R expression was only slightly elevated compared to levels on naïve T cells (data not shown). In contrast, by day 2 of iTreg cell differentiation, IL-4R had increased (Fig. 4e). This elevated expression of IL-4R at day 2 was seen in cells stimulated in the presence or absence of TGF-β, likely due to IL-2R signaling43. This implies that there is a ‘window of opportunity’ in iTreg cell differentiation during which T cells express IL-4R to sense cues from their environment. Signals they receive through these receptors likely impact how they proceed in the differentiation process. Furthermore, these data suggest that the impaired iTreg cell differentiation in Ndfip1- and Itch-deficient T cells may be due to IL-4 produced by these cells.
Knowing that Ndfip1–/– T cells cultured under iTreg cell differentiation conditions in vitro produced high levels of IL-4 and were defective in iTreg cell differentiation we tested IL-4 production in Ndfip1–/– T cells using the in vivo model described in Fig. 2a. Consistent with our in vitro results (Supplementary Fig. 5c, 5d), Ova-specific Ndfip1–/– T cells induced to become iTreg cells in vivo also produced IL-4, suggesting that the in vivo and in vitro iTreg cell defects worked via a similar mechanism (Supplementary Fig. 6).
IL-4 blocks iTreg differentiation in Ndfip1-/- T cells
To determine whether IL-4 was inhibiting iTreg cell differentiation in Ndfip1- and Itch-deficient T cells, we performed iTreg cell conversion assays in the presence or absence of antibodies that block the binding of IL-4 to its receptor. While addition of anti-IL-4 had no impact on WT cells (data not shown), when IL-4 blocking antibodies were added to the Ndfip1- and Itch-deficient T cells, iTreg cell differentiation was restored to that seen in the WT (Fig. 5a, b). Thus, production of IL-4 is sufficient to explain why both Ndfip1–/– and Itchy mutant T cells are poor at differentiating into iTreg cells in vitro. Additionally, if the Ndfip1–/– T cells were converted into iTreg cells in the presence of anti-IL-4 blocking antibodies, the cells could suppress just as well as WT iTreg cells (Supplementary Fig. 7).
Figure 5. Depletion of IL-4 restores iTreg cell conversion in Ndfip1- and Itch-deficient T cells.
(a-g) iTreg cell conversion assays were performed in the presence of 1ng/ml TGF-β (unless otherwise noted) and Foxp3 expression was analyzed on day 5. (a and b) The percentage of Foxp3+ cells was analyzed following iTreg cell conversion with (solid black line) and without (dashed black line) anti-IL-4 using Ndfip1–/– (a) and Itchy mutant (b) T cells. Conversion of Ndfip1+/+ cells is shown (gray dashed line). (mean + s.d.; n≥4 mice; 2-4 independent experiments) (c and d) iTreg cell conversion of naïve T cells from mice of indicated genotypes. Data are representative of 3 mice of each genotype. (d) Analyses of experiments described in c are shown (dot represents each mouse; dash shows the mean). *P<0.05 (two-tailed student T-test). (e) Percentages of Foxp3+ cells were analyzed among Ndfip1+/+ (gray bar) or Ndfip1–/– (black bars) T cells cultured under iTreg cell conditions either without (NT) or with the addition of anti-IL-4 blocking antibodies at time points indicated. The percentage of Foxp3+ Ndfip1+/+ T cells did not change with addition of anti-IL-4 (not shown). Bars show the mean + s.d. at least 3 mice. *P=0.01, **P<0.005 (two-tailed student T-test). (f) Ndfip1+/+ CD45.1+ or Ndfip1–/– CD45.2+ naïve T cells were stimulated alone or in mixed cultures at the indicated ratios (Ndfip1–/–=1 at all ratios). The graph depicts one representative experiment of two. (g) Ndfip1+/+ IL4–/– or Ndfip1–/– Il4–/– T cells were differentiated into iTreg cells with or without the addition of 200pg/ml IL-4 (the half maximal inhibitory concentration of IL-4).
Furthermore, it is unlikely that other TH2 cytokines, such as IL-5, that are also detectable in the supernatants of Ndfip1- and Itch-deficient T cells undergoing iTreg cell differentiation (data not shown) lead to impaired iTreg cell differentiation since the addition of IL-5 to WT T cells undergoing iTreg cell conversion had no effect on their ability to become Foxp3+ (Supplementary Fig. 8).
To confirm that IL-4 production by Ndfip1–/– T cells was inhibiting iTreg cell differentiation, we generated mice lacking both Ndfip1 and IL-4. As shown (Fig. 5c, d), iTreg cell differentiation in T cells from Ndfip1–/–Il4–/– mice was similar to T cells from Ndfip1+/+Il4–/– littermates. These data show that IL-4 produced by Ndfip1- or Itch-deficient T cells prevents iTreg cell differentiation, since blocking either IL-4 production or the binding of IL-4 to its receptor restores iTreg cell differentiation in these cells in vitro.
As described above, IL-4 production increases in Ndfip1–/– T cells during iTreg cell differentiation between 24-48 hours at a time when the cells are upregulating IL-4R expression. This suggests that there is an early ‘window’ during Foxp3 induction following the initial stimulation when cells are sensing their environment and that IL-4 signaling during this time would lead to abortive iTreg cell differentiation. To test whether IL-4 was indeed mediating the abrogation of Foxp3 expression during this ‘window’, we repeated iTreg cell conversion assays, blocking IL-4 signaling at various times following the initial stimulation. The delayed addition of IL-4 blocking antibodies after 24 hours restored iTreg cell differentiation in Ndfip1–/– T cells (Fig. 5e) and this did not occur if the antibodies were added after 48 or 72 hours. These data show that IL-4 can abrogate Foxp3 expression during this early stage of iTreg cell differentiation and that there is a ‘window’ between 24-48 hours following initial stimulation where Foxp3 expression is unable to be rescued by neutralization of IL-4.
IL-4 produced by the Ndfip1-deficient T cells could act preferentially on the cells producing the cytokine, and/or it could act on neighboring cells, preventing their expression of Foxp3. To test which of these occurred in iTreg cell differentiation cultures, we mixed Ndfip1–/– and congenic WT cells together at various ratios prior to initiating iTreg cell differentiation. Using these mixed cultures, we found that IL-4 produced by the Ndfip1–/– T cells was able to inhibit iTreg cell differentiation of WT cells at all ratios tested (Fig. 5f). Thus, IL-4 can prevent iTreg cell differentiation in trans. However, Ndfip1–/– T cells were more defective at iTreg cell differentiation than their WT counterparts, particularly when co-cultured at low ratios (25:1) (Fig. 5f). This indicates that while IL-4 can act in both an autocrine and paracrine manner, it has a more profound effect on Ndfip1–/– cells. This implies that the there is something intrinsic to T cells lacking Ndfip1 that makes them more sensitive to IL-4 than their WT counterparts. This could be due to enhanced IL-4 receptor signaling in T cells lacking Ndfip1. To test this, we added IL-4, at the half maximal inhibitory concentration (based on Fig. 4c) to Ndfip1+/+Il4–/– and Ndfip1–/–Il4–/– T cells undergoing iTreg cell differentiation (to eliminate the confounding effects of IL-4 production by the cells). As predicted, we saw approximately 50% inhibition of iTreg differentiation in Il4–/– cells and a similar level of inhibition in Ndfip1–/–Il4–/– cells (Fig. 5g). Interestingly, the Ndfip1–/– cells showed a modest (but not statistically significant) increase in sensitivity to IL-4. This may be due to the slight increase in IL-4R levels we observe in Ndfip1–/– T cells (data not shown). This might also explain why the Ndfip1–/– cells were more inhibited than their WT counterparts in the co-culture experiments. Nonetheless, this appears to play a minor role in the defective iTreg cell differentiation as this difference is not as profound as their difference in IL-4 production.
Normal frequency of iTreg cells in Ndfip1–/– Il4–/–
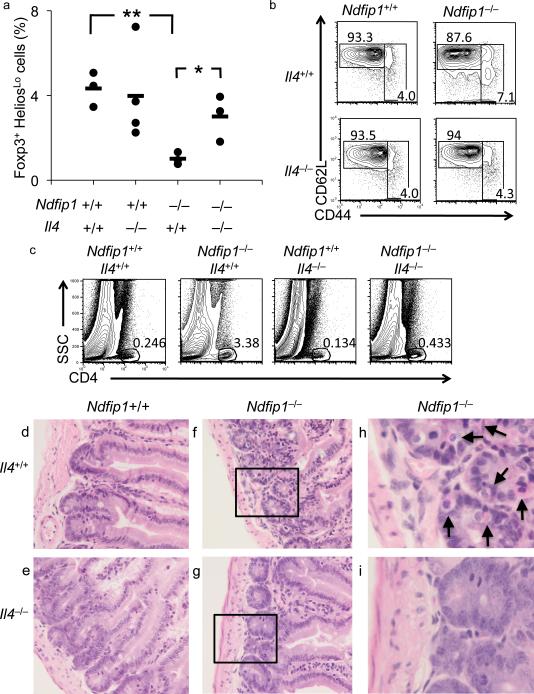
To test whether IL-4 production accounts for the reduced numbers of iTreg cells in Ndfip1–/– animals, we analyzed the percentages of Foxp3+Helioslo cells in the small bowel from mice lacking both Ndfip1 and IL-4. Whereas mice lacking Ndfip1 have reduced percentages of iTreg cells (Foxp3+Helioslo) in the small bowel, mice lacking both Ndfip1 and IL-4 showed percentages comparable to WT and IL-4-deficient mice (Fig. 6a). Thus, similar to what we observed with iTreg cell differentiation in vitro, iTreg cell differentiation in Ndfip1–/– mice in vivo appears to be due to overproduction of IL-4.
Figure 6. Reduced inflammatory disease in Ndfip1–/– mice lacking IL-4.
(a) Small bowels from Ndfip1+/+, Ndfip1–/–, Ndfip1+/+Il4–/–, and Ndfip1–/–Il4–/– mice were analyzed for the frequency of iTreg cells (Foxp3+Helioslo) by flow cytometry. The frequency of iTreg cells among the CD4+ population from the small bowel is shown. Each dot represents a single mouse and the mean is shown by lines. *P=0.04, **P=0.003. (b) CD4+ T cells from the lymph nodes were analyzed for activation by staining with anti-CD62L and anti-CD44. Representative plots from mice 6-7 weeks are shown. (c) The percentage of CD4+ T cells in the small bowel was assessed by flow cytometry. The plots shown are representative of 3-4 mice of each genotype. (d-i) H&E staining of 40X magnified samples from the small bowel of Ndfip1+/+ (d), Ndfip1–/– (f), Ndfip1+/+ Il4–/– (e), and Ndfip1–/– Il4–/–(g) mice are shown. Images are typical of 3-4 mice from each genotype. (h and i) Inset of the boxed areas in f and g, respectively. Arrows in h indicate representative eosinophils in the field. Note the disorganized architecture of the Ndfip1–/– bowel that is not observed in Ndfip1–/– Il4–/– mice.
Mice lacking Ndfip1 have increased percentages of activated T cells in their peripheral lymphoid organs25, 29. These activated T cells could be the direct or indirect consequence of aborted iTreg cell differentiation in vivo. Thus, having shown that iTreg cell induction was restored in mice lacking both Ndfip1 and IL-4, we next wanted to determine whether the percentages of activated T cells were reduced in mice lacking both Ndfip1 and IL-4. While mice lacking Ndfip1 had twice as many CD44hi cells as Ndfip1+/+ controls, the percentages of these cells in mice lacking both Ndfip1 and IL-4 were comparable to controls (Fig. 6b). Supporting this, fewer CD4+ T cells were found in the small bowel of mice lacking both Ndfip1 and IL-4 than in mice lacking only Ndfip1 (Fig. 6c). Furthermore, mice lacking both Ndfip1 and IL-4 had reduced GI pathology, as evidenced by reduced eosinophil infiltration, compared to mice lacking only Ndfip1 (Fig. 6d-i). Additionally, mice lacking both Ndfip1 and IL-4 have longer life-spans than their Ndfip1–/– counterparts and have reduced inflammation in their lungs, as evidenced by decreased infiltrating leukocytes (manuscript in preparation). This would be expected, since eosinophil infiltration is likely the result of TH2 cytokine production in this model29. Nonetheless, these in vivo data suggest that when Ndfip1–/– T cells cannot make IL-4, more T cells differentiate into iTreg cells, fewer T cells have an activated phenotype, and GI pathology is reduced.
Ndfip1 limits JunB levels during iTreg cell commitment
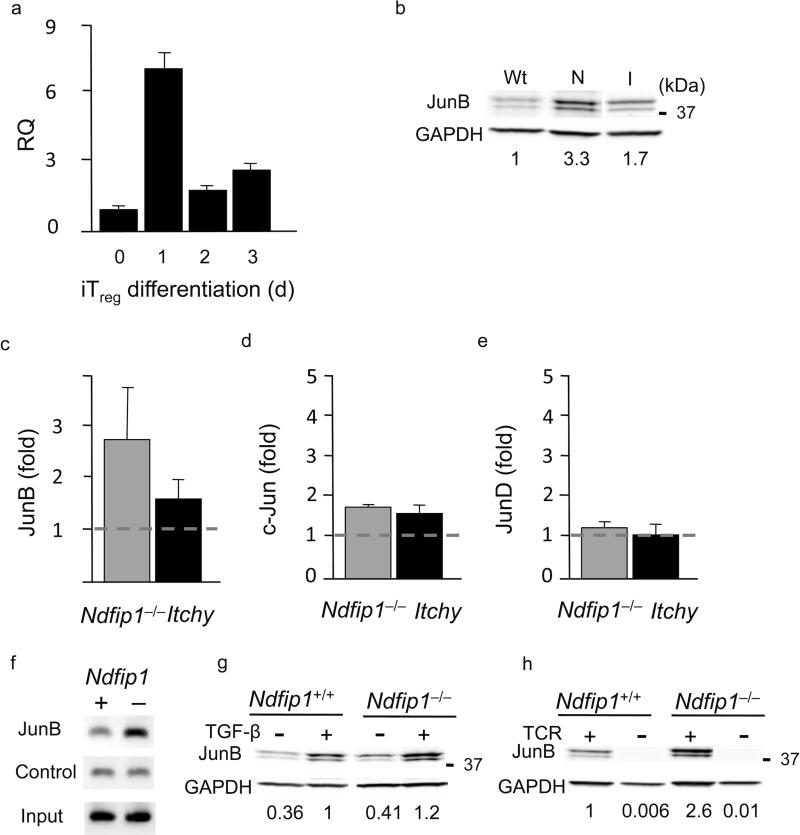
Taken together, our data show that as WT cells begin to differentiate into iTreg, cells they upregulate both Foxp3 and their IL-4R. This implies that during this time they are acutely sensitive to cues from their environment, such as the presence of IL-4. It seems likely that Ndfip1 is acting at this stage since Ndfip1–/– T cells express Foxp3 early during iTreg cell differentiation and then fail to fully differentiate into iTreg cells. Thus, we analyzed the expression of Ndfip1 at different time points during iTreg cell differentiation. To do this, we cultured naïve T cells under iTreg cell differentiation conditions and extracted mRNA on days 1, 2 and 3, and expression of Ndfip1 was determined using Quantitative real-time PCR (qRT-PCR). Ndfip1 mRNA expression peaked in cells cultured 1 day in the presence of TGF-β (Fig. 7a). Expression of Ndfip1 peaks at approximately the time when cells are expressing both Foxp3 and IL-4R, and committing to the iTreg cell lineage. This may explain why Foxp3 expression fails between day 2 and day 5 in Ndfip1–/– T cells. It is worth noting that the induction of Ndfip1 expression was TGF-β-dependent since stimulation without TGF-β showed lower levels of Ndfip1 expression (data not shown). Taken together these data indicate that, in the first 24-48 hours of iTreg cell differentiation, T cells upregulate Ndfip1 in an effort to dampen IL-4 and allow iTreg cell differentiation.
Figure 7. Ndfip1 expression peaks early during iTreg cell differentiation to attenuate JunB expression.
(a) qRT-PCR analysis of Ndfip1 expression. Results presented are relative to the expression of Ndfip1 in naïve T cells (RQ) and show one representative plot (n=7, 3 independent experiments) (mean + s.d. of triplicate samples). (b and c) JunB protein (normalized to GAPDH) at day 3 of iTreg cell conversion. Values show fold expression over WT (set to one and indicated by the dashed gray line) (b) Representative blot and (c) graph of cumulative data from Ndfip1+/+ (Wt), Ndfip1–/– (N) and Itchy mutant (I) iTreg cells (mean + s.d., n≥3). (d and e) c-Jun (d) and JunD (e) are shown as described above for JunB. (f) ChIP analysis of T cells containing (+) or lacking (-) Ndfip1. PCR using primers specific for the Il4 promoter was performed on chromatin DNA obtained before (Input) and after immunoprecipitation (IP) with anti-JunB or control IP. (n=5 Ndfip1+/+ and n=4 Ndfip1–/–. 2 independent experiments) (g-h) Representative blot of JunB expression in T cells containing (+/+) or lacking (–/–) Ndfip1 after approximately 2 days of stimulation (g) under normal iTreg conditions (+) or in the absence (-) of TGF-β and (h) under normal iTreg cell conditions (TCR+) or under TCR withdrawal conditions (TCR-) described in supplementary materials. Representative blot of JunB expression is shown. Values shown are adjusted for GAPDH and normalized to Ndfip1+/+ T cells (set to 1) stimulated +TGF-β (g) or normal iTreg cell conditions (+TCR) (h).
To identify a transcription factor that could account for the increased IL-4 production in Ndfip1- and Itch-deficient T cells we assessed the expression levels of factors that are known to promote early IL-4 production, namely Gata3 and Jun family members. Whereas Gata3 mRNA expression is increased at day 5 after iTreg cell induction, Gata3 expression is comparable to Ndfip1+/+ T cells at day 2 in both Ndfip1- and Itch-deficient T cells (Supplementary Fig. 9a). We next looked at JunB, c-Jun, and JunD levels during iTreg cell differentiation (Fig. 7b-7e). We found a considerable increase in JunB protein in Ndfip1–/– T cells (Fig. 7b, c). Itchy mutant T cells also show elevated JunB but Ndfip1-deficient T cells had higher JunB than both Itch-deficient and Ndfip1+/+ T cells (Fig. 7b, c). This is consistent with the increased production of IL-4 by Ndfip1–/– T cells during iTreg cell conversion (Supplementary Fig. 5c, e). Elevated JunB protein expression in Ndfip1–/– T cells was evident as early as day 2 following iTreg cell induction and increased further over that seen in the control cells at day 3 (Supplementary Fig. 9b). In Ndfip1+/+ cells, JunB protein increased from day 1 to day 2 but then stayed relatively constant at day 3 (Supplementary Fig. 9c) when Ndfip1-deficient T cells had increased amounts (Supplementary Fig. 9b). Taken together, these data show that the elevated amounts of IL-4 produced by Ndfip1- and Itch-deficient T cells during iTreg cell differentiation is likely due to the accumulation of JunB in these cells.
To determine whether the elevated amounts of JunB in Ndfip1–/– T cells could be a cause or consequence of the IL-4 production during iTreg cell differentiation, we next tested if there was an increase in JunB levels in Ndfip1–/– T cells in the presence or absence of anti-IL-4. We found that JunB protein was still elevated in Ndfip1–/– T cells undergoing iTreg cell differentiation in the presence of anti-IL-4, suggesting elevated JunB was not a consequence of IL-4R signaling (Supplementary Fig. 9d). Thus, we next tested whether JunB might be the cause of IL-4 production. Supporting that JunB causes IL-4 production, we detected JunB binding to the IL-4 promoter in Ndfip1–/– T cells (Fig. 7f). However, we did not observe any binding of JunD to this region (Supplementary Fig. 9e).
Knowing that JunB could bind the IL-4 promoter and was not downstream of IL-4 production, we next sought to determine the signals leading to JunB expression in T cells undergoing iTreg cell differentiation. To do this, we cultured cells under iTreg cell conditions in the presence or absence of TGF-β or after removal of TCR stimulation. TGF-β induced an increase in JunB protein in both Ndfip1+/+ and Ndfip1–/– T cells (Fig. 7g). Thus, as has been seen previously in other cell types, TGF-β signaling can induce JunB expression. Furthermore, consistent with previously published data44, we found that TCR signals are necessary for JunB expression (Fig. 7h). When we cultured Ndfip1+/+ and Ndfip1–/– T cells for 24 hours under normal iTreg cell conditions and then removed TCR signaling for the duration of the culture, JunB protein was undetectable. Having found a scenario under which JunB was not expressed, we next tested whether TCR withdrawal affected iTreg cell differentiation in Ndfip1+/+ and Ndfip1–/– T cells. We found that the withdrawal of TCR signals during iTreg cell differentiation had no discernible impact on Ndfip1+/+ T cells. Importantly, TCR signal withdrawal resulted in a loss of IL-4 production (data not shown) and restored iTreg cell differentiation in Ndfip1–/– cells (Supplementary Fig. 9f). Thus, iTreg cell differentiation can be rescued by removal of initial TCR signals concomitant with loss of JunB expression. Taken together these results show that overexpression of JunB is not a consequence of IL-4R signaling and that JunB is likely an active participant leading to IL-4 overproduction in Ndfip1–/– T cells during iTreg cell differentiation.
Previous data has shown that JunB levels are increased in TH2 cells lacking Ndfip1 and that this was due to impaired degradation of JunB25. To test whether the elevated levels of JunB were the result of impaired degradation or increased production, we assessed JunB mRNA levels and protein stability during iTreg cell differentiation. JunB mRNA expression in Ndfip1–/– T cells was comparable to that in WT T cells (Supplementary Fig. 10a). In contrast, and consistent with it's known role as an adaptor for E3 ubiquitin ligases, Ndfip1–/– T cells showed impaired degradation of JunB (Supplementary Fig. 10b). Thus, the increased levels of JunB in T cells lacking Ndfip1 were a result of increased stability of JunB. Given these results, we propose a model in which TGF-β induces expression of Ndfip1 to dampen IL-4 production during iTreg cell differentiation (Supplementary Fig. 11).
DISCUSSION
iTreg cells, generated from naïve T cell precursors in peripheral lymphoid compartments, can attenuate immune responses to either self or environmental antigens1,2. These regulatory T cells are characterized by expression of Foxp32. However, expression of Foxp3 is not sufficient to define a regulatory T cell, as activated T cells can transiently upregulate Foxp345 along with transcription factors that dictate other T cell fates. This has lead to the proposal that transcription factors compete in the early differentiation phase of T cells, potentially integrating environmental signals that ultimately allow cells to commit towards a particular T cell lineage. Here we report that Ndfip1 helps to regulate this process by dampening TH2 cytokine production during the decision making phase of iTreg cell differentiation.
In contrast to the apparent defect in iTreg cell differentiation in Ndfip1–/– mice, we find elevated percentages of nTreg cells in the thymi of Ndfip1–/– mice, thus nTreg cells do develop in the absence of Ndfip1. This increase in nTreg cells was likely a result of the inflammatory cytokines present in these mice. However, a more precise analysis of nTreg cells in mice 3 to 9 days old would be required to entirely rule out a role for Ndfip1 in nTreg cell development. TGF-β is important for both nTreg cell differentiation and iTreg cell differentiation 9,13,16,17,30. That we see defects only during iTreg cell differentiation could reflect a reduced capacity of developing thymocytes to produce or respond to IL-4 compared to peripheral naïve T cells. Also, whether IL-4 can affect nTreg cell development in Ndfip1–/– mice is not clear. We do not detect significant amounts of IL-4 in the serum of Ndfip1–/– even when they present with overt signs of inflammation when IL-4 production is detectable in splenocytes (unpublished observation). Thus, it will be important to determine whether IL-4 is produced locally by Ndfip1–/– thymocytes and whether developing nTreg cells respond to IL-4. While it is clear that there are circumstances under which CD4 -single positive (SP) cells in the thymus can make IL-4, it is possible that IL-4 is not produced in the thymi of young Ndfip1–/– mice since the majority of CD4 SP thymocytes in neonatal mice are not functionally competent and do not respond the same as peripheral T cells46. These will be the focus of future studies.
Preventing IL-4 production is a particular challenge for cells undergoing iTreg cell differentiation. While iTreg cells are dependent on IL-2R signaling9,10, these signals are known to promote both IL-4 production and IL-4R expression47. Thus, as iTreg cells differentiate, they receive IL-2R signals, increase expression of their IL-4R43, and inhibit their own IL-4 production to seek cues from their environment. If IL-4 production is not silenced during this period, it could prevent iTreg cell differentiation in both an autocrine and paracrine manner. This could result in enhanced and/or prolonged immune responses with damaging consequences.
Although it is known that iTreg cell differentiation is remarkably sensitive to effector cytokines such as IL-419,38,39, the mechanisms that prevent IL-4 production by T cells during iTreg cell differentiation are only partially understood. For example, it is known that TGF-β receptor signaling dampens IL-4 production in WT T cells19. In part, this is because TGF-β receptor signaling reduces Gata3 expression20. We show here that in the absence of Ndfip1, T cells produce IL-4 at levels that inhibit their own iTreg cell differentiation, and iTreg cell differentiation of other T cells in their vicinity.
Paradoxically, JunB was increased in a TGF-β-dependent manner. While this was surprising, data in other non-immune cell types has shown that TGF-β can induce JunB via a Smad-dependent pathway48. Why and how TGF-β induces JunB expression in T cells is not clear. However, JunB expression in WT cells plateaus at day 2 during iTreg cell differentiation. In contrast, in Ndfip1–/– T cells, JunB expression continues to increase and JunB was bound to the IL-4 promoter in Ndfip1–/– T cells undergoing iTreg cell differentiation, demonstrating a causal role for JunB in IL-4 production. Supporting this, in one scenario under which JunB is not expressed in Ndfip1–/– T cells, IL-4 is not produced and iTreg cell differentiation is restored.
TGF-β also induces increased expression of Ndfip1, an adaptor protein that promotes the ubiquitylation and degradation of Jun-family proteins by the E3 ligase Itch. Ndfip1 is particularly important in the first 24-48 hours of iTreg cell differentiation, as cells are increasing expression of Foxp3 and IL-4R. In the absence of Ndfip1, T cells initially increase Foxp3, but also aberrantly express IL-4. This ultimately aborts the iTreg cell differentiation process in these cells. Given these results, we suggest that Ndfip1 promotes Itch ubiquitylation and degradation of JunB to prevent IL-4 production and allow iTreg cell differentiation. While these data support a role for Ndfip1 regulation of Itch, it is also clear that Ndfip1 also regulates iTreg cell differentiation via an Itch-independent mechanism.
Interestingly, it seems that Ndfip1 is not needed once Foxp3+ Treg cells are fully differentiated since cells that had already committed to the Treg lineage have lower Ndfip1 expression than their naïve T cell counterparts (data not shown). Supporting this, Ndfip1–/– cells that differentiate into iTreg cells (in the presence of anti-IL-4) suppress proliferation as well as WT iTreg cells.
Here we define an early ‘window’ where Ndfip1 is expressed to dampen IL-4 production during iTreg cell differentiation. The kinetics of Ndfip1 expression and inhibition of iTreg cell differentiation by IL-4 are consistent with data showing that optimal iTreg cell conversion occurs when TGF-β was added within 1-2 days41. Thus, environmental cues received by the T cell early during this time can alter the ability of these cells to differentiate into Foxp3 expressing Treg cells. Supporting this, it is known that Foxp3 (induced by TGF-β receptor signaling) can bind directly to Gata3 (induced by IL-4R signaling) to prevent the induction of TH2 cytokines19. On the other hand, if Gata3 levels increase (due to IL-4R signaling) and outcompete Foxp3, iTreg cell differentiation is prevented 38,39. Based on the data we have presented, we propose that Ndfip1 dampens IL-4 production during TGF-β stimulation to provide a ‘window of opportunity’ for iTreg cell differentiation.
Supplementary Material
Acknowledgements
We thank Hilda Ramon (University of Pennsylvania) for discussions and for help with setting up iTreg cell culture conditions. We thank Dr. Avinash Bhandoola (University of Pennsylvania) for critical reading of the manuscript and Dr. Janis K. Burkhardt (University of Pennsylvania) for helpful discussions. We also thank Amy Laroche (Children's Hospital of Philadelphia) for technical assistance, as well as the staff of the flow cytometry core at the University of Pennsylvania. This work was supported by the NIH grants RO3 AR057144, 1F32AI085837 and R01AI093566.
Methods
Mice
CD45.1+ (C57BL6.SJL-Ptprca Pepcb/BoyJ), Il4–/– (B6.129P2-Il4tm1Cgn/J), OT-II (B6.Cg-Tg (TcraTcrb) 425Cbn/J) and Rag1–/– (B6.129S7-Rag1tm1Mom/J) mice were purchased from the Jackson Laboratory. T cell transgenic OT-II Rag1–/– mice were obtained by crossing OT-II transgenic mice with Rag1–/– mice. Ndfip1–/– and Itchy mutant (also referred to as Itch-deficient) mice were previously described25, 28 and have been backcrossed to C57BL/6 mice for more than 9 generations. Ndfip1–/– mice were bred from heterozygous parents since Ndfip1–/– mice die prematurely. Ndfip1+/+ littermates were used as controls. Ndfip1+/+ and Ndfip1–/– were 4-8 weeks of age unless otherwise noted. For data presented in figure 7e-h, T cells lacking Ndfip1 were derived from both Ndfip1–/– mice and Cd4-Cre Ndfip1fl/fl mice. T cells from these Cd4-Cre Ndfip1fl/fl mice lack Ndfip1 (data not shown) and respond similarly to T cells from Ndfip1–/– mice (for these data T cells from at least one Ndfip1–/– mouse were used for comparison). Cd4-Cre mice will be described elsewhere (manuscript in preparation). All mice used were maintained in a barrier facility at the Children's Hospital of Philadelphia and all animal experiments were approved and in accordance with guidelines established by the Children's Hospital of Philadelphia Institutional Animal Care and Use Committee.
Isolation of cells from the small bowel and thymus
The small bowel was dissected and the Peyer's Patches were excised. The lumen of the small bowel was cleaned by flushing with PBS. A section of the small bowel was then minced in DMEM media with 0.9mg/mL of collagenase A (Sigma), 0.8mg/mL collagenase 1A (Sigma), and 20μg/ml of DNase I (Sigma). Minced tissues were then incubated for 1hr at room temperature, with end over end mixing. The resulting cell suspension was passed through 100μm and then 40μm filters after which FBS was added to a final concentration of 10%. Thymi were harvested, and passed through 70μm filters to obtain cell suspensions.
Antibodies and Flow cytometry
Antibodies used for flow cytometry analysis include anti-CD4 (GK1.5, Biolegend) and anti-CD8 (53-6.7, Biolegend), anti-CD45.2 (104, Biolegend), anti-CD62L (MEL-14, eBioscience), anti-CD44 (IM7, Biolegend) anti-CD25 (PC61.5, eBioscience), anti-IL-4Rα/CD124 (mIL4R-M1, BD Biosciences), anti-Foxp3 (FJK-16s, eBioscience), or anti-Helios (22F6, Biolegend). Additionally, some experiments used streptavidin Alexa Fluor 647 or 488 conjugates (Invitrogen). Data was collected using a FACSCalibur (BD Biosciences) and analyzed by FlowJo (TreeStar).
In vitro iTreg cell cultures
Spleens and lymph node cells were sorted for naïve T cells (CD4+ CD25-CD62Lhi CD44lo) using a FACS Aria (BD biosciences) or MoFlo (Beckman Coulter). 0.5 -1×106 naïve T cells were stimulated with 5 μg/ml plate-bound anti-CD3 (145-2C11, BD biosciences) and anti-CD28 (37.51, BD biosciences) in complete media (DMEM, 10% FCS, 50 U/ml IL-2) with or without TGF-β (PeproTech) at the indicated concentrations. We noticed that Ndfip1+/+ cells stimulated without the addition of exogenous TGF-β displayed a small percentage of Foxp3+ cells on day 5 due to the presence of TGF-β in the media, therefore in some experiments we added anti-TGF-β antibodies (1D11, R and D systems). Where indicated, either 20μg/ml anti-IL-4 antibodies (11B11, Biolegend) were added to block IL-4 or exogenous murine IL-4 (PeproTech) was added at the indicated concentrations to the indicated cells. T cells were incubated at 37°C 5% CO2 and then analyzed for Foxp3 expression on day 2 and/or 5. While we noted variability in the percent converted cells even among WT mice from experiment to experiment, the trend was always the same between the samples from the various mouse strains.
In vivo iTreg cell model
2×106 OT-II T cells from either Ndfip1–/–Rag1–/–OTII+ or Ndfip1+/+Rag–/–OTII+ mice were enriched by either sorting for Thy1.2+ cells or depleting MHC class II+ cells and then transferred intravenously into CD45.1+ mice. Recipient mice were fed 1.5% OVA (grade III, Sigma) in the drinking water for 5 consecutive days33 after which the mesenteric lymph nodes, Peyer's Patches and small bowel were harvested and processed.
ELISA
ELISA was performed using supernatants from cultured cells as described in the supplementary methods.
RNA isolation and Q PCR
RNA was isolated and analyzed by Q PCR as described in the supplementary methods. Ndfip1 primer and probe sequences are as follows: forward-TCCACCATACAGCAGCATCACT; reverse-AGAGTGCAGCATATTT; and probe-TTTGGAAATCCAGATTCATCTTTG.
Immunoblot
T cells were stimulated under iTreg cell conditions as described above or under TCR withdrawal conditions (described in supplementary methods). Cells were then harvested after 2-3 days following the initial stimulation, counted and washed with cold Dulbecco's Phosphate-Buffered Saline. Harvested cells were lysed and prepared for SDS-PAGE as noted in the supplementary methods. For blotting, PVDF membranes were blocked for 1 hour at room temperature with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) and then immunoblotted with anti-JunB (mouse monoclonal antibody C-11, Santa Cruz), anti-c-Jun (rabbit monoclonal antibody, 60A8, Cell Signaling), anti-JunD (rabbit polyclonal antibodies, Santa Cruz), or anti-GAPDH (mouse monoclonal antibody 6C5, Millipore). Secondary antibodies were either Alexa Fluor 680 or IRdye 800 conjugated. Immunoblots were imaged using the Odyssey Imager system (LI-COR Biosciences, Lincoln, NE).
ChIP for JunB binding to the Il4 promoter
4.5 ×106 naïve T cells were stimulated as described for in vitro iTreg cell cultures in the presence of 1ng/ml TGF-β for the indicated times. Cells were then harvested and fixed as described in the supplementary materials. IP was performed with anti-JunB (mouse monoclonal antibody C-11, Santa Cruz) antibodies and protein-G beads blocked with sheared salmon sperm DNA (Millipore). Primers for the Il4 promoter were forward, 5’-GAGCCAGTGGCAACCCTACGCTGATAAG-3’ and reverse, 5’-CTGCCAGCATTGCATTGTTAGC-3’ 49 and surround the AP-1 site described in Li et al.50
Statistics
All statistical analysis was performed by student T-tests. A P value ≤ 0.05 was the threshold used to determine statistical significance. Error bars represent standard deviation of the mean unless otherwise noted.
Footnotes
Author Contributions
A.M.B. designed and performed experiments and wrote the manuscript; N.R.-H., C.R.R. and E.A.N. did experiments and contributed data; P.M.O designed experiments and wrote the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 2.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or division of labor. Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 4.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 5.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 6.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 7.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 8.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 9.Josefowicz SZ, Rudensky AY. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–65. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Kitani A, Strober W. Molecular mechanisms regulating TGF-beta-induced Foxp3 expression. Mucosal Immunol. 2010;3:230–238. doi: 10.1038/mi.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Wing K, Miyara M. Regulatory T cells-a brief history and perpective. Eur. J. Immunol. 2007;37:S116–123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 15.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fantini MC, et al. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 17.Park HB, Paik DJ, Jang E, Hong S, Youn J. Acquisition of anergic and suppressive activities in transforming growth factor-beta-costimulated CD4+CD25- T cells. Int. Immunol. 2004;16(8):1203. doi: 10.1093/intimm/dxh123. [DOI] [PubMed] [Google Scholar]
- 18.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dardalhon V, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells, and together with TGF-beta generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorelik L, Fields PE, Flavell RA. TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 2000;165:4773–4777. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 21.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 2007;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 22.Hefferan TE, et al. Overexpression of a nuclear protein, TIEG, mimics transforming growth factor-beta action in human osteoblast cells. J. Biol. Chem. 2000;275:20255–20259. doi: 10.1074/jbc.C000135200. [DOI] [PubMed] [Google Scholar]
- 23.Venuprasad K, et al. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat. Immunol. 2008;9:245–253. doi: 10.1038/niXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Z, et al. Kruppel-like factor KLF10 targets transforming growth factor-beta1 to regulate CD4(+)CD25(-) T cells and T regulatory cells. J. Biol. Chem. 2009;11:24914–24924. doi: 10.1074/jbc.M109.000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliver PM, et al. Ndfip1 protein promotes the function of Itch ubiquitin ligase to prevent T cell activation and T helper 2 cell-mediated inflammation. Immunity. 2006;25:929–940. doi: 10.1016/j.immuni.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rooney JW, Hoey T, Glimcher LH. Coordinate and cooperative roles for NF-AT and AP-1 in the regulation of murine IL-4 gene. Immunity. 1995;2:473–483. doi: 10.1016/1074-7613(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 27.Li-Weber M, Giasi M, Krammer PH. Involvement of Jun and Rel proteins in up-regulation of interleukin-4 gene activity by the T cell accessory molecule CD28. J. Biol. Chem. 1998;273:32460–32466. doi: 10.1074/jbc.273.49.32460. [DOI] [PubMed] [Google Scholar]
- 28.Fang D, et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. [DOI] [PubMed]
- 29.Ramon HE, et al. The ubiquitin ligase adaptor Ndfip1 regulates T cell-mediated gastrointestinal inflammation and inflammatory bowel disease susceptibility. Mucosal Immunol. 2011;4:314–324. doi: 10.1038/mi.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-β signaling in the development of natural CD4+ CD25+Foxp3+ regulatory T cells. Nat. Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 31.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui KR, Powrie F. CD103+ GALT DCs promote Foxp3+ regulatory T cells. Mucosal Immunol . 2008;1(Suppl 1):S34–S38. doi: 10.1038/mi.2008.43. [DOI] [PubMed] [Google Scholar]
- 35.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verhagen J, Wraith DC. Comment on “Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells”. J Immunol. 2010;185:7129. doi: 10.4049/jimmunol.1090105. [DOI] [PubMed] [Google Scholar]
- 37.Thornton AM, Shevach EM. Response to comment on “Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells”. J Immunol. 2010;185:7130. doi: 10.4049/jimmunol.1090105. [DOI] [PubMed] [Google Scholar]
- 38.Wei J, et al. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantel PY, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS. Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J. Immunol. 2007;178:7667–7677. doi: 10.4049/jimmunol.178.12.7667. [DOI] [PubMed] [Google Scholar]
- 42.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-β-induced Foxp3+ T cells in vivo. J Immunol. 2011;186:6329–6337. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao W, et al. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor α-chain expression. Nat. Immunol. 2008;9:1288–1296. doi: 10.1038/ni.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang Q, et al. IL-2 and IL-4 stimulate MEK1 expression and contribute to T cell resistance against suppression by TGF-β and IL-10 in Asthma. J Immunol. 2010;185:5704–5713. doi: 10.4049/jimmunol.1000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007;37:129–38. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 46.Ramsdell F, Jenkins M, Dinh Q, Fowlkes BJ. The majority of CD4+CD8-thymocytes are functionally immature. J Immunol. 1991;147:1779–1785. [PubMed] [Google Scholar]
- 47.Cote-Sierra J, et al. Interleukin 2 plays a central role in Th2 differentiation. Proc. Natl. Acad. Sci. USA. 2004;101:3880–5. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jonk LJ, Itoh S, Heldin CH, ten Dijke P, Kruijer W. Identification and functional characterization of a Smad binding element (SBE) in the JunB promoter that acts as a transforming growth factor-β, activin, and bone morphogenetic protein-inducible enhancer. J. Biol. Chem. 1998;273:21145–21152. doi: 10.1074/jbc.273.33.21145. [DOI] [PubMed] [Google Scholar]
- 49.Park J, Kim SH, Li Q, Chang YT, Kim TS. Inhibition of interleukin-4 production in activated T cells via the downregulation of AP-1/NF-AT activation by N-lauroyl-D-erythro-sphingosine and N-lauroyl-D-erythro-C20-sphingosine. Biochem. Pharmacol. 2006;71:1229–1239. doi: 10.1016/j.bcp.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 50.Li B, Tournier C, Davis RJ, Flavell RA. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. The EMBO Journal. 1999;18:420–432. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.