Abstract
Brain regional analyses of total GluA1 and GluA1-pSer845 were used to delineate plasticity of the AMPA receptor in conjunction with cocaine-cue extinction learning. Rats were trained to self-administer cocaine paired with a 2-sec light cue and later underwent a single 2hr extinction session for which cocaine was withheld but response-contingent cues were presented. Control groups received yoked-saline sessions or received cocaine self-administration training without undergoing extinction training. Extinction-related increases and decreases, respectively, in total GluA1 were observed in the ventromedial prefrontal cortex (vmPFC) and basolateral amygdala (BLA). Phosphorylation of GluA1 at Ser845 was increased in the vmPFC and nucleus accumbens (NAc). Though total GluA1 did not change in NAc, there was a positive association between the number of responses during extinction training and the magnitude of total GluA1 in NAc. No significant changes were evident in the dorsal hippocampus. We conclude that the BLA and vmPFC, in particular, appear to be loci for the inhibition of learned behavior induced via extinction training, but each site may have different signaling functions for cocaine-cue extinction learning.
Keywords: Basolateral amygdala, Cocaine, Cocaine-cue extinction learning, GluA1 receptor, Self-administration, Ventromedial prefrontal cortex
Reactivity to drug cues plays an important role in relapse to drug use. Attenuating cue reactivity may aid in maintaining abstinence and preventing a return to drug taking. Progress in understanding the brain circuits involved in extinction of drug cue reactivity has been achieved by studying cue extinction in experimental animals. Employing an animal model that explicitly extinguishes responses only in the presence of discrete drug-paired cues, our recent findings using c-Fos protein expression as a molecular correlate of neural activity in 11 brain areas suggest that sites within amygdala, prefrontal cortex, hippocampus and striatum are actively engaged during cocaine-cue extinction training [1]. We found that c-Fos protein expression was selectively increased within basolateral amygdala (BLA) and prelimbic cortex (PL) by cocaine-cue extinction learning, while the pattern of c-Fos expression within the dorsal hippocampus (DH) and infralimbic cortex (IL) implicated these sites in processing the significance of cues (whether cocaine or saline) that were present during extinction training. Within the caudateputamen and nucleus accumbens (NAc), c-Fos expression did not differ amongst treatment groups but did positively correlate with rate of responding at the end of extinction training, suggesting that these sites may mediate motor output during extinction training. Expression of GluA2, the predominant subunit of the AMPA receptor throughout the adult brain [2], was not altered in any site examined after extinction training. In the current investigation, we focused on another key signal in synaptic plasticity underlying learning and memory, the GluA1 receptor subunit [3]. Specifically, we examined the impact of cocaine-cue extinction on total GluA1 protein expression in the brain sites we previously identified as being relevant during cocaine-cue extinction training [1]. Since phosphorylation of the GluA1 subunit at serine 845 (GluA1-pSer845) modulates the membrane expression of these receptors, various channel properties, and synaptic plasticity [4], we examined GluA1-pSer845 as well.
Male Wistar rats (Crl(WI)BR; 275-300 g) were housed individually (08:00 h lights on, 20.00 h lights off) and maintained in accordance with the NIH Guide for Care and Use of Laboratory Animals and the Boston University Institutional Animal Care and Use Committee. Intravenous catheters were implanted under anesthesia as described previously [5]. Following recovery, rats were randomly assigned to either cocaine or yoked-saline groups and underwent 30 days of self-administration training. Cocaine HCl (NIDA, Bethesda, MD) was self-administered in 2hr daily sessions under a fixed ratio 5 (FR5) schedule of reinforcement, whereby each completion of 5 responses on the active lever produced an i.v. injection of 0.3 mg/kg cocaine (0.03 ml/sec of a 1.6 mg/ml solution). Cocaine was infused via motor-driven syringe pumps located inside the sound-attenuating cubicle, over an approximately 2-sec period and was simultaneous with a 2-sec onset of the white stimulus light located over the active lever (cocaine-paired stimulus). Each rat passively receiving saline was paired to a rat self-administering cocaine. Independent of lever-press behavior, each yoked-saline rat received the same number and temporal pattern of injections and cue light presentations as the cocaine self-administering rat to which it was paired. Following self-administration training, and subsequent to a 24hr abstinence period, rats with a history of cocaine self-administration were divided into 2 groups: those receiving an extinction session (COC EXT) and those receiving an abstinence session (COC No-EXT). During the single 2hr extinction training session, lever pressing was extinguished by substituting saline for cocaine injections delivered via motor-driven syringe pumps, while maintaining response-contingent presentations of the 2-sec light cue under the FR5 schedule. During the abstinence session, rats were placed in operant chambers for which levers were retracted, and without presentation of either the 2-sec light cue or the delivery of cocaine. One set of yoked-saline rats received a yoked-extinction test session (SAL EXT) for which conditions used were identical to those used during training. The other set of yoked-saline rats received an abstinence session on test day (SAL No-EXT). This experimental design allowed us to determine if changes in total GluA1 and GluA1-pSer845 in extinguished cocaine-trained rats resulted specifically from extinction learning or from some other factor (e.g., cue exposure per se during testing or a prior history of cocaine self-administration).
Rats were sacrificed by guillotine immediately after the 2hr test session. Key brain areas were rapidly dissected using a coronal rodent brain matrix (RBM-4000C, ASI Instruments, Warren, MI) according to the method described previously [6]. The DH, NAc, BLA and ventromedial prefrontal cortex (vmPFC) were dissected immediately on ice-cooled plates from 1mm slices, flash frozen in isopentane and stored at −80°C. Western blot analysis was performed as previously described [7]. The assay used antibodies against GluA1ct diluted at 1:1000 (described in [7]), GluA1pSer845 diluted at 1:500 (Millipore, Billerica, MA) and tubulin diluted at 1:1000 (Sigma, St. Louis, MO). Membranes were visualized using ECL (Amersham, Piscataway, NJ) and immunointensity was measured using Image J (http://rsbweb.nih.gov/ij/download.html) by a reviewer blinded to the status of the animal. To control for methodologically induced variability, samples from each behavioral group were processed at the same time, such that each western batch contained tissue from each experimental group. For GluA1 or GluA1-pSer845 blots, a band at 110 kD was measured and quantified. All membranes were reprobed for tubulin to indicate protein loading. GluA1 values were normalized to tubulin prior to analysis. Outliers (>2 SD) were omitted from the molecular analysis. A total of four outlying values (two for GluA1 and two for GluA1-pSer845) were obtained in the entire dataset.
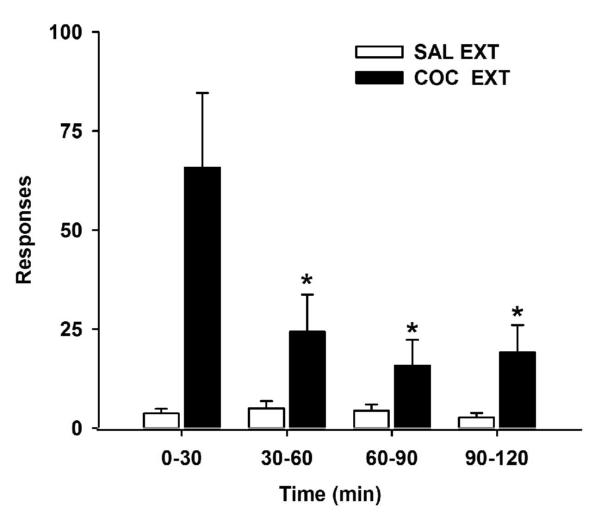
The last five cocaine self-administration or yoked-saline sessions were used to establish baseline behavior (Table I). Using 1-way ANOVA and post-hoc Tukey analyses, significant group differences were observed for active lever responding [F(3,30)=22.9, p = 0.001]. As expected, groups receiving cocaine (Groups 1 and 3) exhibited significantly greater levels of responding (p ≤ 0.001) than groups receiving yoked-saline (Groups 2 and 4). Infusions were not significantly different between the cocaine groups, and their inactive lever responses were ≤ 10% of active lever responses. Cocaine-trained rats learned to attenuate lever pressing for the motivationally salient response-contingent cues over the course of the 2hr extinction session (Fig. 1). Based on 2-way ANOVA and post-hoc Tukey analyses, active lever responding was significantly altered across the four 30-min bins [F(3,48)=4.0, p ≤ 0.01], an effect that was dependent on cocaine vs. saline history [F(3,48)=4.1, p ≤ 0.01]. The COC EXT group exhibited significantly more active lever responding during the first 30-min bin (0-30 min) compared to the remaining bins (60-120min; p ≤ 0.05). The SAL EXT group had low levels of responding during the entire 2hr session. During extinction training, inactive lever responses were ≤ 8% of active lever responses for animals with a history of cocaine self-administration.
Table 1.
Experimental design and number of responses and infusions (mean ± SEM) during the 2hr cocaine self-administration or yoked-saline sessions at baseline.
| Group | N | Experimental Design | Baseline Responses | Infusions | ||
|---|---|---|---|---|---|---|
| Training | Test | Active | Inactive | |||
| 1 | 12 | Cocaine | EXT | 291.9 ± 31.8 | 25.1 ± 9.7 | 54.2 ± 5.8 |
| 2 | 6 | Saline | EXT | 18.5 ± 4.1 | 5.7 ± 1.3 | N/A |
| 3 | 11 | Cocaine | No-EXT | 273.3 ± 29.2 | 50.7 ± 12.1 | 48.6 ± 5.9 |
| 4 | 5 | Saline | No-EXT | 18.4 ± 1.7 | 8.2 ± 1.7 | N/A |
Figure 1.
Time course of cocaine-cue extinction learning in animals previously trained to self-administer cocaine (n= 12) or receiving yoked-saline (n=6). Values are the mean ! SEM active lever responses for sequential 30min bins over the 2hr extinction training session. * p<0.05 compared to the first 30min of extinction training.
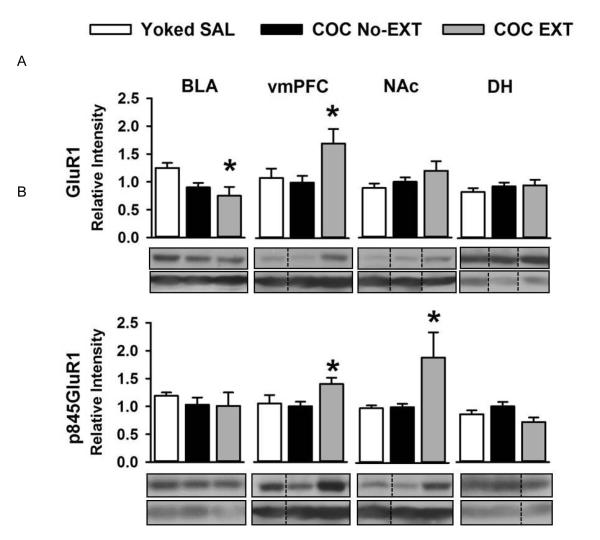
Against this background of extinction vs. control training and cocaine vs. saline history, differences in GluA1 or GluA1-pSer845 were determined using the Dunnett t-test procedure for multiple comparisons against a control group. This procedure controls for type 1 error [8]. Representative images of Western blots for GluR1, GluA1-pSer845 and tubulin for each brain region are shown in Fig. 2A and 2B. For each of the four brain regions examined, values were statistically similar in the two yoked-saline groups for GluA1 (t(9) = −1.215 to 2.128, p > 0.05) and GluA1-pSer845(t(9) = −0.12 to 0.750, p > 0.05). Therefore, data for the two yoked-saline groups were combined (separately for GluA1 and GluA1-pSer845 in each brain region) to represent the saline control values (Yoked SAL) used in the Dunnett t-test analyses. For each brain region, GluA1 and GluA1-pSer845 in the COC No-EXT group did not differ significantly from the respective Yoked SAL control group (Fig. 2A and 2B; p >0.05). Importantly, cocaine-cue extinction training produced a significant decrease in total GluA1 in the BLA (Fig. 2A; COC EXT < Yoked SAL, p ≤ 0.02), whereas in the vmPFC (ventral PL + IL combined), cocaine-cue extinction training produced significant increases in both total GluA1 (Fig. 2A; COC EXT > Yoked SAL, p ≤ 0.03) and GluA1-pSer845 (Fig. 2B; COC EXT > Yoked SAL, p ≤ 0.03). Analysis between the number of active lever responses during the last 30 min of the extinction session and the magnitude of GluA1 and GluA1-pSer845 in both these areas showed no significant associations. Cocaine-cue extinction training also produced significant increases in GluA1-pSer845 in the NAc (Fig. 2B; COC EXT > Yoked SAL, p ≤ 0.03). In addition, analysis between the number of active lever responses during the last 30 min of the extinction session and the magnitude of total GluA1 expression in NAc indicated a positive association (Pearson’s r = 0.612; n=18; p ≤ 0.01). For the DH, GluA1 and GluA1-pSer845 in the COC EXT group did not differ significantly from the respective Yoked SAL control group (Fig. 2A and 2B), and no significant associations were found.
Figure 2.
Panels show (A) total GluA1 and (B) GluA1-pSer845 after cocaine-cue extinction [n=10-12] or control training [n=10-11]. Values are the mean ! SEM relative optical intensity measurements. * p<0.05 compared to the Yoked SAL control group [n=10-11]. Photomicrographs of representative images for western blots of total GluR1, GluR1-pSer845 and ubulin are shown below each data plot.
The findings from the present study provide correlative evidence of a role for the GluA1 AMPA receptor subunit within the BLA and vmPFC during cocaine-cue extinction learning. These results are consistent with accumulating evidence indicating the importance of these memory systems for extinction of both drug- and fear-conditioned responses [9]. We speculate that these sites may have different signaling functions, given the opposing influence of cocaine-cue extinction training on total GluA1 protein expression. In BLA, total GluA1 protein expression was decreased in rats undergoing a single 2hr session of cocaine-cue extinction training. This change may be transient as total GluA1 protein expression is not altered in BLA after multiple sessions of cocaine-cue extinction training [11]. Past research on fear extinction learning [12] has shown a loss of calcium permeable AMPA receptors (GluA1-containing) in BLA in the absence of a change in GluA1-pSer845, similar to our results. Others show a reduction in the surface expression and a rapid internalization of GluA1-containing AMPA receptors in BLA during fear extinction learning [13]. Receptor trafficking studies are needed to determine whether or not there is an internalization of GluA1-containing AMPA receptors in BLA during the early stages of cocaine-cue extinction learning.
The analysis of vmPFC showed a significant increase in total GluA1 protein expression in rats undergoing the 2hr session of cocaine-cue extinction training. The concomitant increase in GluA1-pSer845 in vmPFC suggests that these changes are of functional significance for cocaine-cue extinction learning. Phosphorylation of GluA1-S845 is mediated by PKA and regulates AMPA receptor channel conductance [14], as well as AMPA receptor membrane insertion [15] and the expression of long-term potentiation (LTP) [16]. Dephosphorylation of GluA1-S845 is mediated by NMDAR signaling and causes AMPA receptor endocytosis [4] and long-term depression [17]. Thus, changes in GluA1-S845 phosphorylation will alter synaptic strength and regulate cellular processes in learning and memory. Since GluA1-Ser845 phosphorylation is necessary for membrane trafficking of AMPA receptors and synaptic plasticity [4,15], the parallel increase of total and phosphorylated GluA1 in the vmPFC suggests that cocaine-cue extinction learning may be associated with trafficking of GluA1-containing AMPA receptors to surface membranes in vmPFC. It is important to note that AMPA receptor trafficking to surface membranes plays a key role in synaptic plasticity, given that surface trafficking occurs in an activity-dependent manner during LTP [18]. LTP-like changes have been observed in mPFC in relation to fear extinction learning and are a good index of maintenance of extinction [19-21]. While receptor trafficking is efficient for transient changes of AMPA receptor synaptic localization, alterations in total receptor amount, which is determined by a balance of protein synthesis and degradation, may regulate long-term modulation of synaptic efficacy. An increase in GluA1 abundance following cocaine-cue extinction training in the vmPFC could result from enhanced transcription and translation, and/or suppressed protein degradation. The opposite mechanisms may underlie the observed downregulation of GluA1 in BLA. Additionally, GluA1 is subject to ubiquitination and proteasome-mediated degradation [7]; whether or not these processes are involved in cocaine-cue extinction learning remains to be determined.
In past cocaine self-administration studies, Zavala and colleagues [10] reported no change in tissue level of GluA1 protein in either IL or PL following multiple sessions of cocaine-cue extinction training, similar to what others report in vmPFC and dmPFC following a single 6hr session [22]. This latter group did, however, observe increased expression of GluA1 protein in the post-synaptic density fraction of the dmPFC. It remains to be determined if the increase in GluA1 and GluA1-pSer845 observed in vmPFC of the current study is restricted to the PL vs. IL subregion, but previous research examining c-Fos protein expression suggests that the PL rather than the IL, may be involved specifically in cocaine-cue extinction learning [1].
In contrast to BLA and vmPFC, GluA1 receptor mechanisms in the NAc may mediate motor output or motivation for cocaine during cocaine-cue extinction training rather than extinction learning. This is based on the fact that a positive, rather than negative, association between total GluA1 and response output was observed, as previously reported for c-Fos protein expression [1]. However, it is possible that the observed increase in GluA1-pSer845 in the NAc of cocaine-extinguished rats relative to the other groups of rats may reflect differences in the consolidation of extinction learning as the amount of learning may have been different when more unreinforced responses were made during the test session. Previously, it was suggested that the effects of post-session administration of D-cycloserine (DCS) into the NAc shell for facilitating control over cocaine seeking during extinction training could arise from DCS-mediated actions at NMDA receptors in the NAc that subsequently alter glutamate receptor trafficking [10]. This idea is consistent with the observed increase in GluA1-pSer845 in the NAc of cocaine-extinguished rats in the present study. Additional studies are needed to understand the nature of the relationship between NAc plasticity and response output during cocaine-cue extinction training.
Overall, our findings here and elsewhere [1] provide converging correlative evidence that the BLA and sites within the vmPFC are important neurosubstrates for cocaine-cue extinction learning. To close the loop between these correlative measures of behavior and neuroplasticity, local manipulation of GluA1 levels in select brain regions would be needed to establish if the GluA1-containing AMPA receptors located within the BLA and vmPFC are critically involved in mediating cocaine-cue extinction learning. It is important to note that in fear extinction learning, there is a dynamic interaction between the mPFC and BLA [23]. It is hypothesized that the mPFC may integrate input from the BLA to gate the expression of fear via projections to inhibitory or excitatory circuits within the amygdala [21, 24-25]. Our current findings would support such a relationship for cocaine-cue extinction learning, and research directly examining the way by which the BLA and vmPFC interact during cocaine-cue extinction learning is warranted. Future studies that more precisely assess the time course and persistence of changes in GluA1 are needed as well. Furthermore, future studies directly assessing the behavioral significance of altered corticolimbic GluA1 receptors may open new avenues to differentially manipulate neural circuitries and signaling critical for the cocaine-cue extinction process to further our understanding of the underlying mechanisms in extinction learning in cocaine-addicted populations.
Highlights.
Cocaine-cue extinction increased and decreased, respectively, total GluA1 in vmPFC and BLA
Cocaine-cue extinction increased GluA1-pSer845 in vmPFC and NAc
Lever responding positively correlated with total GluA1 in NAc
The BLA and vmPFC appear to be loci for cocaine-cue extinction learning
Understanding extinction mechanisms may improve exposure therapy in cocaine addicts
Acknowledgements
This study was supported by seed funding from the Boston University Center for Neuroscience and DA024315. We thank Ms. Jamie Gauthier for assistance with data collection.
Footnotes
Author contribution BND, GK, KL-M, HM and KK were responsible for the study concept and design. BND and AL collected behavioral data, conducted the Western blot analyses, and analyzed data. BND drafted the manuscript, and all authors provided important intellectual content, critically reviewed the manuscript and approved the final version for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nic Dhonnchadha BÁ, Lovascio BF, Shrestha N, Lin A, Leite-Morris KA, Man HY, Kaplan GB, Kantak KM. Changes in expression of c-Fos protein following cocaine-cue extinction learning. Behav Brain Res. 2012;234:100–6. doi: 10.1016/j.bbr.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30(3):126–34. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- [3].Santos SD, Carvalho AL, Caldeira MV, Duarte CB. Regulation of AMPA receptors and synaptic plasticity. Neuroscience. 2009;158:105–25. doi: 10.1016/j.neuroscience.2008.02.037. [DOI] [PubMed] [Google Scholar]
- [4].Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci USA. 2007;104:3579–84. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nic Dhonnchadha BÁ, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–67. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Heffner TG, Hartman JA, Seiden LS. Rapid method for the regional dissection of the rat brain. Pharmacol Biochem Behav. 1980;13:453–6. doi: 10.1016/0091-3057(80)90254-3. [DOI] [PubMed] [Google Scholar]
- [7].Lin A, Hou Q, Jarzylo L, Amato S, Gilbert J, Shang F, Man HY. Nedd4-mediated AMPA receptor ubiquitination regulates receptor turnover and trafficking. J Neurochem. 2011;119:27–39. doi: 10.1111/j.1471-4159.2011.07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Winer B. Statistical Principles in Experimental Design. McGraw-Hill; New York: 1971. [Google Scholar]
- [9].Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–88. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Torregrossa MM, Sanchez H, Taylor JR. D-cycloserine reduces the context specificity of pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci. 2010;30(31):10526–33. doi: 10.1523/JNEUROSCI.2523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–52. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–12. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mao SC, Hsiao YH, Gean PW. Extinction training in conjunction with a partial agonist of the glycine site on the NMDA receptor erases memory trace. J Neurosci. 2006;26:8892–9. doi: 10.1523/JNEUROSCI.0365-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20(1):89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6(2):136–43. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- [16].Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281(2):752–8. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- [17].Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21(5):1151–62. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- [18].Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Ann Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- [19].Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- [20].Hugues S, Chessel A, Lena I, Marsault R, Garcia R. Prefrontal infusion of PD098059 immediately after fear extinction training blocks extinction-associated prefrontal synaptic plasticity and decreases prefrontal ERK2 phosphorylation. Synapse. 2006;60:280–7. doi: 10.1002/syn.20291. [DOI] [PubMed] [Google Scholar]
- [21].Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- [22].Ghasemzadeh MB, Vasudevan P, Giles C, Purgianto A, Seubert C, Mantsch JR. Glutamatergic plasticity in medial prefrontal cortex and ventral tegmental area following extended-access cocaine self-administration. Brain Res. 2011;1413:60–71. doi: 10.1016/j.brainres.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vouimba RM, Maroun M. Learning-induced changes in mPFC-BLA connections after fear conditioning, extinction, and reinstatement of fear. Neuropsychopharmacology. 2011;36:2276–85. doi: 10.1038/npp.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–37. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]