Abstract
While the discovery of microRNAs has exponentially expanded our understanding of the regulatory mechanisms governing gene networks in many biological processes, the study of these tiny RNA powerhouses in cardiovascular disease is in its infancy. To date, there have been over 1200 human microRNAs identified, and they are estimated to affect the expression of over half of the protein-coding portion of the human genome. In this review, we will discuss miRNAs that are integral players in processes affecting risk factors for CVD, as well as miRNAs that act at the level of the vessel wall to affect atherogenesis. We will discuss how microRNAs are not only advancing the field of cardiovascular biology, but how some miRNAs are at the forefront of drug development and may be soon advancing into the clinic.
Keywords: microRNA, Atherosclerosis, Lipid metabolism, Vessel wall, Therapeutic, Lipoprotein
Introduction
MicroRNAs represent a layer of complexity in addition to transcriptional control of gene expression that can have profound effects on biological pathways. These non-coding RNAs are found either within intergenic or intronic regions of host genes, and make up approximately 10 % of the human genome [1]. Since their discovery in Caenorhabditis elegans, our understanding of how miRNAs can influence gene expression in humans has broadened considerably. Over half of the human transcriptome is now predicted to be under the control of miRNAs, many of which have been highly conserved throughout evolution [2]. It is estimated that over 1200 miRNAs are present in the human genome, and these are transcribed by RNA polymerase II as large, >50 nucleotide pri-miRNA containing the canonical hairpin structure, which renders them an ideal substrate for processing by the Drosha/DGCR8 complex. These precursor miRNA (pre-miRNA) molecules, approximately 70 nucleotides (nt) in length, are exported out of the nucleus by the nuclear transport protein Exportin 5 and undergo further processing in the cytoplasm by the enzyme Dicer, resulting in a 20–22 nt miRNA duplex (miRNA/miRNA*, or miRNA-5p/miRNA-3p). From this duplex, one mature miRNA molecule is loaded into the miRNA-induced silencing complex (miRISC), composed of Argonaute 2 and other RNA binding proteins essential for the miRNA:mRNA interaction [3]. In this multicomponent complex, the mature miRNA binds by partial base pairing of the seed region to complementary target sites in the 3′UTR of mRNAs, reducing protein expression through either mRNA degradation and/or translational repression [2]. Notably, the 3′UTR of a single mRNA molecule can be targeted by hundreds of miRNAs; similarly, one miRNA can simultaneously bind and repress multiple target genes, thereby providing a mechanism to synchronize the regulation of entire networks of genes. miRNAs thus represent a new class of targets for therapeutic intervention, and offer a unique approach to treating disease by modulating entire biological pathways.
Although our understanding of how miRNAs function in both normal physiology and pathophysiology is burgeoning, the fields of lipid metabolism and atherosclerosis have somewhat lagged behind other fields in uncovering novel roles for miRNAs in previously well-studied pathways. Here we will give an overview of the miRNAs that are best understood and their functions in atherogenesis and risk for developing coronary artery disease (CAD) (Fig. 1).
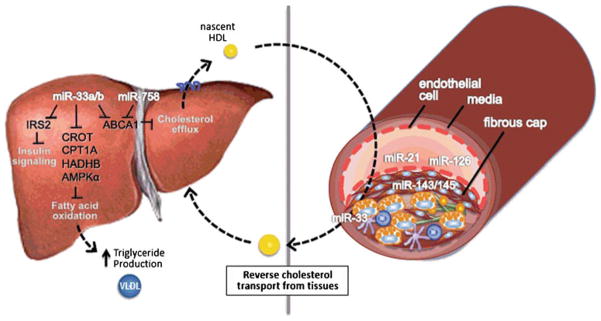
Fig. 1.
miRNAs regulating vessel wall homeostasis and lipoprotein metabolism in the liver. In the liver, miR-33a/b act to repress genes involved in fatty acid b- oxidation [CROT HADHB, CPT1A and PRKAA1 (AMPKa)], and miR-33a/b and miR-758 inhibit ABCA1 –a key transport that effluxes cholesterol to generate nascent HDL particles. This HDL can travel to the artery wall to promote reverse cholesterol transport from the plaque, where miR-33a/b also functions to upregulate ABCA1 in macrophage foam cells. Roles for miR-21 and miR-126 have been reported in endothelial cells, whereas miR-143/145 act in smooth muscle cells to regulate vascular function
MicroRNA Regulation of Lipids and Lipoproteins
One of the most common risk factors for the development of cardiovascular disease is an imbalance between circulating lipoproteins. The plasma level of apoB-containing lipoproteins [low density lipoprotein (LDL), and its precursor, very low density lipoprotein (VLDL)] is among the strongest risk factors for CAD. Excessive LDL cholesterol (LDL-C) in the circulation results in these particles becoming trapped in the vessel wall, where they can become modified (either aggregated and/or oxidized), eliciting an immune response from resident and recruited immune cells. If circulating LDL-C remains high, macrophages in the arterial intima become engorged with lipid and are unable to egress out of the atheroma, resulting in a chronically inflamed environment that promotes plaque formation. In an opposing force, high density lipoproteins (HDL) promote the efflux of cholesterol from these overloaded macrophages and ferries excess cholesterol from peripheral tissues (like the vessel wall) to the liver for excretion. Thus, the imbalance between high circulating LDL-C and low HDL-C sets the stage for the development and persistence of inflammatory atherosclerosis. In particular, even in the setting of managed LDL-C levels, HDL-C is an independent predictor of CAD, with an increase of just 1 mg/dL in HDL-C carrying with it a 2–3 % decrease in risk [4].
Despite optimum medical therapy to reduce LDL-C, there remains a substantial burden of CAD and other metabolic diseases that needs to be addressed. As low HDL remains an independent risk factor in patients treated with statins to reduce LDL-C, there has been tremendous interest in therapeutically targeting HDL-C for the treatment of atherosclerosis. The identification of the gene defect in Tangier disease (in which individuals have remarkably low HDL-C) as the ATP-binding cassette transporter A1 (ABCA1) brought to light a key player in HDL pathway, and there has been tremendous focus on targeting this protein for therapeutic benefit ever since. Through its ability to efflux cholesterol to apolipoprotein A1, the main protein component of HDL, ABCA1 has two important functions: In the liver, this action of ABCA1 loads cholesterol onto lipid-poor apoA1 to generate nascent HDL particles, while in peripheral tissues such as the macrophages trapped in atherosclerotic plaques, ABCA1 and its related family member ABCG1 export excess cellular cholesterol onto lipid-poor apoA1 and mature HDL particles, respectively. This process, termed reverse cholesterol transport (RCT) is thought to be critical to the protective effects of HDL. However HDL also has anti-inflammatory and anti-oxidant properties, which can ameliorate the cholesterol-rich, pro-inflammatory plaque macrophages. One of the most exciting discoveries in this area in recent years has been the identification of microRNAs that target ABCA1 and the demonstration that these can be manipulated to alter HDL levels in mice and non-human primates [5••, 6••, 7, 8]. As discussed below, several microRNAs have emerged as potent regulators of cholesterol metabolism, and these discoveries have broadened our understanding of the pathways regulating lipoprotein metabolism, as well as the potential new opportunities for therapeutic intervention.
miR-122 Identified as the First Cholesterol-Regulatory miRNA
Nearly a decade ago, Chang et al. first reported the presence of an abundantly expressed miRNA in the liver, miR-122, and noted that it was highly conserved across species [9]. A multitude of studies in the following years demonstrated that miR-122 is indeed one of the most highly expressed hepatic miRNAs, accounting for nearly 70 % of all liver miRNAs [10]. In seminal work by Esau et al. and Elmen et al., inhibition of miR-122 using modified anti-sense oligonucelotides in both normal and obese mice resulted in an ~30 % decrease in total plasma cholesterol, seemingly through regulation of genes involved in the cholesterol biosynthesis pathway [11],[12]. Of note, the cholesterol metabolism genes altered by anti-miR-122 treatment were not direct targets of miR-122 (i.e., they did not contain sites complementary to the miR-122 seed sequence in their 3′UTR), but rather their regulation resulted from secondary effects on miR-122 targets in the liver. Antagonism of miR-122 in fat-fed mice resulted in a significant improvement in liver steatosis, with decreased liver triglyceride content and increased oxidation of fatty acids, thus highlighting its potential as a therapeutic target [11]. These studies in mice paved the way for the use of modified anti-sense oligonucleotides to be employed in a model highly relevant to humans: African green monkeys treated with locked-nucleic acid (LNA) anti-miR122 had up to 30 % lower total plasma cholesterol levels than their control anti-miR counterparts, without showing evidence of toxicity or histopathological changes in the liver [13]. This generated considerable excitement and highlighted the promise of anti-miR122 as a therapy in humans to potentially ameliorate lipid-related disorders. However, given the broad number of miR-122 target genes altered by anti-miR122 (potentially >100), it remains to be seen whether antagonism of miR-122 is a viable therapeutic option for hypercholesterolemia and/or hepatosteatosis [12]. Notably, miR-122 also binds the 5′UTR of the hepatitis C virus (HCV), and is necessary for viral replication and propagation [14, 15]. Pre-clinical data in non-human primates showed a profound effect on HCV viral infection and overall pathological improvement upon treatment with anti-miR122 oligonucleotides, with prolonged effects beyond the treatment period [16••]. This has lead to the development of antisense miR-122 as the first miRNA therapeutic to enter clinical trials, lead by Santaris Pharma A/S. Indeed, anti-miR122 therapy, termed miraversen, has completed phase 2a trials and shows a significant reduction in HCV RNA in all doses used without additional safety or efficacy concerns. Thus anti-miR122 has the potential to replace or be used in addition to traditional interferon therapy to treat HCV disease progression.
miR-33: Regulation of HDL Biosynthesis, Triglyceride Metabolism and Atherosclerosis
A recent flurry of studies by a number of groups, including our own, uncovered a novel pathway involving cooperative regulation of metabolic networks by the sterol-response element binding protein (SREBP) genes that code for transcription factors that regulate sterol control, and a family of microRNAs miR-33a/b embedded within these loci. We reported that encoded within intron 16 of the SREBP-2 gene is miR-33a, a highly conserved microRNA that is conserved in humans through frogs, and is even present in Drosophila. A closely related microRNA, miR-33b, is also embedded in intron 17 of the SREBP-1 gene, however miR-33b is present in a more restricted number species (humans, non-human primates, pigs, but not rodents or rabbits) [6••]. miR-33a/b bind the 3′UTR, and thus controls the expression, of a number of cholesterol transport proteins, including ABCA1, ABCG1 and NPC1—all of which are essential players in the removal of cholesterol from the cell and into the RCT pathway [5••, 6••, 7, 17, 18]. Interestingly, these genes provide an opposing force to SREBP2, which initiates the transcription of genes involved in cholesterol synthesis and uptake, and thus their repression by miR-33a would help to enforce the action of its host gene. This cooperativity represents the first such example of a transcription factor/miRNA regulatory circuit. Using a variety of approaches to inhibit miR-33a in mice, from lentiviral delivery, LNA silencing and even a gene knock-out strategy, the silencing of this microRNA was shown to cause an increase in circulating HDL-cholesterol, ranging from 25 % to 40 %
In a related example, miR-33a/b also repress the expression of genes involved in fatty acid oxidation (CPT1A, CROT, HADHB, PRKAA1) pathways, and inhibition of miR-33b results in an increased rate of fatty-acid β oxidation [17, 19]. As in the example of miR-33a/SREBP2, miR-33b appears to reinforce the actions of SREBP-1 in inducing fatty acid synthesis by blocking the oxidation of the newly synthesized fatty acids, thereby preventing futile cycling. However, studies of the miR-33b/SREBP1 axis in animals models were somewhat hampered by the lack of miR-33b in rodents and rabbits. To overcome this, we treated African green monkeys with anti-miR33 oligonucleotides that effectively inhibit both miR-33a and miR-33b, which differ by only 2 of 19 nucleotides in their mature form. In this study, systemic delivery of an anti-miR-33 oligonucleotide for 12 weeks recapitulated the 40 % increase in HDL-cholesterol observed previously in mice, but also resulted in a 50 % decrease in serum VLDL-associated triglyceride [8]. These alterations in plasma lipoproteins were apparent as early as 4 weeks of treatment, and reached a maximum reduction of 50 % after 12 weeks, during which no adverse effects of anti-miR treatment were reported. Fractionation of plasma lipoproteins revealed that the decrease in plasma triglycerides derived from reduced VLDL-associated triglycerides, primarily the large VLDL particles that are newly secreted from the liver. At a molecular level, inhibition of endogenous miR-33 in the liver resulted in de-repression of ABCA1 mRNA and protein, as well as key members of the fatty acid oxidation pathway, CPT1A, CROT and HADHB. In addition, there was also a marked decrease of SREBP-1 at both the mRNA and protein level. The reduction of this key regulator of fatty acid synthesis, and host gene of miR-33b, was surprising as it is not a direct target of miR-33. However, further analyses identified an increase in hepatic expression of AMPK1 alpha (encoded by PRKAA1), which is a negative regulator of the SREBP-1 pathway and also a direct target of miR-33. This secondary targeting of SREBP-1 resulted in a decrease in expression of key genes involved in fatty acid synthesis downstream of this transcription factor, including fatty acid synthase (FASN), ATP citrate lyase (ACLY) and acetyl-CoA carboxylase alpha (ACACA) in the livers of anti-miR33 treated monkeys. Thus, by simultaneously increasing fatty acid oxidation via derepression of HADHB, CPT1A and CROT, and decreasing fatty acid synthesis via inhibition of the SREBP-1 pathway (SREBF1, FASN, ACLY, ACACA), anti-miR-33 treatment results in a pronounced reduction in plasma VLDL triglyceride. These findings established, in a model highly relevant to humans, that pharmacological inhibition of miR-33a and b is a promising therapeutic strategy to raise plasma HDL and lower VLDL triglycerides for the treatment of dyslipidemias that increase cardiovascular disease risk. As low HDL and high VLDL triglycerides are commonly associated with metabolic syndrome [20], miR-33 inhibitors may have clinical utility for the treatment of this growing health concern.
Bioinformatic analyses predict that miR-33a and miR-33b largely repress the same subset of genes, and to date, there have been no genes identified that are specifically targeted by miR-33a versus miR-33b. However, as these microRNAs are co-transcribed with their host genes, the relative abundance of miR-33a and b is likely to be regulated by conditions that induce SREBP-2 and SREBP-1, and thus may be quite different. Interestingly, the relative expression of SREBP-2 mRNA in the liver is significantly less than that of SREBP-1 [21]; and thus, it would be predicted that miR-33a would also be less abundant than miR-33b. This was confirmed to be true in non-human primates, where levels of miR-33b were approximately three-fold greater than miR-33a [8]. Furthermore, under conditions that cause SREBP-1 transcription to be elevated, such as hyperinsulinemia, miR-33b levels may be dramatically increased, and lead to strong repression of its target genes. Of note, two of the hallmarks of the metabolic syndrome are high plasma insulin levels and low levels of HDL-C [20], and it is interesting to speculate that this may be due in part to increased hepatic transcription of SREBP-1/miR-33b, and a resulting decrease in ABCA1 expression. While this provocative hypothesis remains to be tested, it is illustrative of how a single miRNA might have profound consequences on both normal physiology and pathological states.
Plasma HDL cholesterol levels bear a strong inverse correlation with cardiovascular disease risk, and previous studies have shown that direct infusion of HDL in apolipoprotein E–deficient mice, cholesterol-fed rabbits, or human subjects with established atherosclerosis reduces both plaque size and complexity. Given the clinical desirability of raising HDL to both prevent and regress cardiovascular disease, we tested whether anti-miR33 treatment could ameliorate pre-existing atherosclerosis in mice. Ldlr−/− mice with large, complex existing atherosclerotic lesions were treated with saline, control anti-miR or anti-miR33 oligonucleotides for 4 weeks. In this mouse model of atherosclerosis, miR-33 inhibition increased hepatic ABCA1 and plasma HDL-C by 35 % as previously seen in wild type mice [22••]. However, as there is growing evidence that the absolute levels of plasma HDL-C are less important than the ability of the HDL to promote removal of cholesterol from peripheral tissues (such as macrophages in atherosclerotic plaques) into the feces for excretion- a process known as reverse cholesterol transport—it was important to also measure HDL functionality in these mice. Using an in vivo assay to measure the efficiency of reverse cholesterol transport, we established that the HDL generated by miR-33 inhibition increased the transport of radiolabeled cholesterol from macrophage foam cells to the plasma, liver, and feces by up to 80 %. Furthermore, the anti-miR33 generated HDL retained its anti-inflammatory properties, particularly its ability to promote macrophage cholesterol efflux and to protect endothelial cells from cytokine induced inflammation. Likely as a result of these positive attributes, anti-miR-33 treatment caused a marked regression of atherosclerosis in just 4 weeks that was characterized by a 35 % reduction in plaque size, decreased lipid and macrophage content, and a transition to a more stable plaque phenotype. Of particularly interest, immunohistochemical detection of the modified anti-miRs showed that the oligonucleotides penetrated macrophages within the atherosclerotic plaque, where they directly increased ABCA1 mRNA expression, likely increasing cholesterol efflux from these cells and reducing overall plaque cholesterol content. Further analyses of plaque macrophages isolated by laser capture microdissection showed a reduction in inflammatory gene expression in anti-miR-33 treated mice. Thus, the benefit of anti-miR33 therapy is two-fold: it increases hepatic ABCA1 expression, circulating HDL and reverse cholesterol transport, and simultaneously increases macrophage cholesterol efflux from the plaque, resulting in lesion regression and a less inflammatory plaque milieu. Together, these studies suggested that miR-33 inhibition may be a promising therapeutic modality for the treatment of atherosclerosis.
Therapeutic targeting of ABCA1 by anti-miR-33 is also actively being studied in Alzheimer’s disease, where levels of ABCA1 have been shown to correlate inversely with amyloid load [23]. In the brain, ABCA1 effluxes cholesterol to apolipoprotein E, which is the major apolipoprotein in this tissue and an established genetic risk factor for Alzheimer’s disease [23]. There is also significant interest in identifying other microRNAs that target ABCA1 that may work in a complementary or synergistic manner with miR-33. A recent study showed that the 3′UTR of ABCA1 is also targeted by miR-758, and like miR-33, overexpression of miR-758 represses expression of ABCA1 in human and mouse macrophage and hepatic cell lines, thereby altering cellular cholesterol efflux to apoA1 [24]. miR-758 is an intergenic miRNA that like miR-33 is down-regulated after cholesterol loading in macrophages and in most tissues from mice fed with high cholesterol diet. Thus, both miR-33 and miR-758 may cooperate to down-regulate ABCA1 under low cholesterol conditions, however the presence of three miR-33 binding sites at the beginning of the ABCA1 3′UTR indicates that miR-33a/b may target ABCA1 with higher efficacy. The post-transcriptional regulation of ABCA1 expression will likely involve several different miRNAs and the physiological relevance of each miRNA may thus be determined by its relative tissue expression. However, one tissue in which miR-758 may predominate is the brain, where it is more highly expressed than miR-33 [24].
Another emerging area of interest in anti-miR-33 therapy is the role of this microRNA in glucose metabolism and insulin secretion. Elevated islet cholesterol levels have been shown to contribute to impaired β-cell function and glucose tolerance in mice and humans, and therapies that upregulate ABCA1 are thought to hold promise for the maintenance of normal islet function in diabetes. Recently, miR-33 was shown to be expressed in pancreatic islets and β-cells, where it upregulates ABCA1, and its inhibition reduced islet cholesterol levels and improved insulin secretion in Apoe−/− mice. Conversely, miR-33 overexpression decreased glucose-stimulated insulin secretion in human and mouse islets. These findings suggest that anti-miR33 upregulation of ABCA1 may be therapeutically beneficial for individuals with combined defects in β-cell function and cholesterol homeostasis. Furthermore, miR-33 has been shown to directly impact insulin signaling by targeting the insulin receptor substrate 2 (IRS2) and FSR-2 genes, which participate in the signaling cascade that mediates the effects of insulin[25], as well as other genes involved in glucose metabolism such as sirtuin-6 (SIRT6)[25]. miR-33a/b over-expression reduces IRS2 levels and inhibits the activation of downstream messenger cascades, including AKT[25], and in both mice and monkeys, inhibition of miR-33 increased hepatic expression of IRS2. While the animals used in studies to date were normoglycemic, future studies in animal models of obesity/diabetes will be important to fully understand the impact of miR-33 on insulin signaling and diabetes.
Vessel-Wall miRNAs and Their Therapeutic Potential
miRNAs in Vessel Wall Homeostasis
Our understanding of how miRNAs might be involved in the development of vascular dysfunctions come predominantly from studies of miRNAs that regulate vessel wall health and integrity. For example, SMCs are critical players in the pathological progression of atherosclerosis, and in a seminal discovery, Cordes et al. described how the miRNA cluster containing miR-143 and miR-145 control SMC phenotype [26••]. They showed that the cardiac- and smooth muscle-specific miR-143/miR-145 suppress the expression of Kruppel-like factor 4 (Klf4) and Elk-1 to maintain SMCs in a differentiated, non-proliferative quiescent state. Notably, miR-143/145 are downregulated in human atherosclerotic plaques, and gene rescue experiments using over-expression of miR-143/145 increased focal adhesion sites and contractility, thus strengthening the plaque [27]. It was recently postulated that miR-143/145 therapy might enhance SMC contractility and fibrous cap formation, while suppressing SMCs proliferation and synthetic function, ultimately leading to a more stable plaque [28]. In a provocative finding, miR-143/145 were also shown to be secreted into the extracellular space by endothelial cells and subsequently taken up by SMCs in vitro [29•]. Such a transfer in vivo would protect from atherosclerosis, and in support of this, miR-143/145-containing microvesicles from ECs in culture reduced disease progression when delivered to atherosclerosis-prone mice. These exciting findings established that functional miRNA-mediated transfer from the extracellular space could result in gene silencing in the recipient cell and altered plaque phenotype [29•].
Endothelial cells are considered the gatekeepers for vascular inflammation and overall vessel wall health. When intact and healthy, they maintain vessel wall dilation and prevent inflammatory cell infiltration, which ultimately prevents atherosclerotic plaque progression. Although a variety of miRNAs have been shown to play a role in EC-mediated angiogenesis, there are a select few miRNAs that may play more relevant roles in pro- and anti-atherosclerotic functions. For example, miR-21 was recently shown by two groups to be induced by differential flow conditions in the vessel wall. The first report of miR-21 in maintaining vessel wall integrity came from a group showing that miR-21 was induced by laminar shear stress (i.e., protective flow) in vitro, which increased expression of the vasodilator eNos and reduced EC apoptosis [30]. More recently, miR-21 was also reported to be induced by oscillatory (i.e., athero-prone) shear stress and linked to endothelial cell inflammatory marker expression [31]. Although these findings appear to be contradictory, both suggest that miR-21 plays an important role in modulating EC responses to hemodynamic forces, perhaps through targeting of different gene expression pathways in different regions of the aorta.
In a series of papers in 2008, miR-126 was identified as an endothelial-specific miRNA embedded in the EGF-like 7 gene, that blunts the cellular response to vascular endothelial growth factor (VEGF) [32–34]. Deletion of miR-126 in mice [33] and zebrafish [34] caused leaky vessels and an overall loss of vascular EC function, due to miR-126 targeting of Sprouty-related protein SPRED1 and phosphoinositol-3 kinase pathway. This represented one of the first examples of a concerted mechanism of an intronic miRNA and its host gene: Egfl7 is expressed during vascular tube formation and is necessary for vasculogenesis, while miR-126 is simultaneously expressed to maintain vessel wall integrity during this process [35]. The importance of miR-126 in the pathogenesis of atherosclerosis lies in its ability to inhibit TNFα-induced vascular cell adhesion molecule-1 (VCAM-1) expression in ECs, reducing inflammatory cell adhesion and spreading [34]. Interestingly, apoptotic bodies within the atherosclerotic plaque have been shown to deliver miR-126 to neighboring cells, thereby increasing the expression of the anti-inflammatory chemokine CXCL12 and reducing atherosclerotic lesion development [36]. This study suggests that delivering miR-126 may be beneficial for the treatment or prevention of atherosclerosis, though this has not yet been investigated in humans.
The Vulnerable Plaque: Made Weaker by miRNA?
As atherosclerotic plaques progress, the vessel wall continues to undergo insults such as inflammatory cell accumulation, loss of SMC contractility and increased EC leakiness. The protective fibrous cap, primarily composed of extracellular matrix (ECM) proteins and SMC, becomes thinner over time, rendering it susceptible to rupture, thrombosis and ultimately, myocardial infarction. In addition to the miRNAs described above involved in vascular cell health and homeostasis that may become dysregulated, it is perhaps no surprise that miRNAs also contribute centrally to the initiation of the vulnerable plaque [37, 38]. In an attempt to gain insight into the age-dependent weakening of the vessel wall and the miRNAs that might modulate this process, Boon et al. identified the miR-29 family as among the most up-regulated miRNAs in old versus young aortic tissue [39•]. In a variety of animal models of aortic aneurysm, as well as human thoracic aneurysm tissue, miR-29 was found to be aberrantly expressed and inversely correlated with levels of stabilizing extracellular matrix proteins [39•]. Treatment with LNA-modified anti-miR-29 recovered ECM loss and protected against vessel-wall dilation, indicating that inhibitors targeting miR-29, or other similar as yet unidentified microRNAs, may hold promise for the stabilization of vulnerable plaques.
Extracellular miRNA: Exponential Complexity
Recently, a number of studies have identified miRNAs in the circulation of patients suffering from a variety of symptomatic and asymptomatic cardiovascular diseases [40]. However, as is the case with any biomarker, it is difficult to reach a consensus among these studies as to which miRNAs (if any) would provide a clear indication of an underlying disease or event, and which may be causal of this pathology. In an interesting study, Vickers et al. demonstrated that HDL particles carry miRNA in the circulation, and that the profile of miRNAs carried on the lipoproteins from healthy versus diseased individuals varies [41]. By this mechanism, miRNAs may be used as a form of communication between the liver (the site of HDL biogenesis, and potentially miRNA incorporation) and distant tissues. While the exact pathophysiological consequences of this HDL-based miRNA targeting are unknown, it highlights the complexity of miRNA networks and how much is awaiting discovery.
Therapeutic Future for miRNAs in Cardiometabolic Diseases?
As we begin to understand more about how miRNAs function in the progression of atherosclerosis, the answers to some of the mechanistic questions that have plagued the field for decades may finally be in sight. miRNAs are predicted to regulate almost half of the human genome; given that atherosclerosis is a multi-factorial, genetically complex trait, miRNAs are likely to have a major impact on the many different processes driving this disease. As we have discussed, miRNAs regulate multiple pathways that contribute to cardiovascular disease risk, at times conferring resistance, and at other times offering protection. But as we foray into developing new therapeutics to treat this disease, how much do we truly understand? To date, the only miRNA-based therapeutic in the clinic has targeted miR-122 for the treatment of HCV infection, successfully and without safety concerns. However, given the multiple targets of miR-122, it is unlikely that this will be pursued as a therapeutic for preventing metabolic diseases that affect a broader population, despite its cholesterol-lowering capabilities. In contrast, anti-miR33 therapy is gaining considerable attention for its ability to simultaneously increase HDL-C and lower VLDL-triglycerides, without affecting LDL-C. It would be predicted that using anti-miR33 as a combination therapy with statins to increase HDL-C may be even more beneficial, as statins upregulate SREBP2 and hence miR-33, and may indirectly keep HDL levels low [6••]. Although miRNA therapies offer a unique approach to treating disease by modulating biological pathways, one concern may be understanding the complement of actual targets of a particular miRNA. There are some reports that miR-33 can function as a tumor suppressor by downregulating the oncogenic Pim-1 pathway [42], while others claim miR-33 increases stem cell proliferation via p53 inhibition [43]. Additionally, some reports link miR-33 to the cell cycle by its regulation of cyclin-dependent kinase 6 (CDK6) [44], while other studies have shown CDK6 is not targeted by miR-33 [42]. It is likely that miRNA function (both expression of the miRNA and its targets) has tissue specific contexts, and may very well be dysregulated in neoplasms [45]. Nevertheless, profiles from non-human primates treated with anti-miR33 show no upregulation of tumorigenic genes, including potential miR-33 targets like p53 or CDK6, nor any abnormal liver pathology [8]. In fact, there are relatively few gene expression changes after treatment with anti-miR33, suggesting that miR-33 targeting is quite specific, providing an optimistic outlook on the path ahead.
While the majority of examples discussed above have involved miRNAs whose functions it would be desirable to silence, some miRNA-based therapies to treat atherosclerosis may require miRNA over-expression for potential therapeutic benefit. To date, this has proved to be a logistical challenge: miRNA mimics are double-stranded RNA molecules, making them more recognizable to the immune system and more degradable by exo/endonucleases than their single-stranded anti-miRNA counterparts. This is in part an explanation of why anti-miR therapies have moved into the clinic with relative ease. Nonetheless, pharmaceutical companies are developing delivery strategies for double-stranded RNA and have achieved some success with siRNA-based therapies. In contrast to the more potent, one-target effects of siRNA, miRNAs are considered “fine-tuners” of multiple genes in a given regulatory network, without the one-for-all effect that characterizes siRNA. This may prove to be more effective at improving complex diseases like atherosclerosis, which involve many pathways and would benefit from a multi-modal therapeutic. Regardless, miRNA mimic therapy awaits the development of an effective delivery method, preferably one that can target cells within the atherosclerotic plaque as well as the liver, as a means to improve disease most efficiently (Fig. 1).
Footnotes
Disclosure K.J. Rayner: Consultancy for Regulus Therapeutics; K.J. Moore: Consultancy for Regulus Therapeutics
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Claverie J-M. Fewer genes, more noncoding RNA. Science. 2005;309(5740):1529–30. doi: 10.1126/science.1116800. [DOI] [PubMed] [Google Scholar]
- 2.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13(4):271–82. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 3.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 4.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62 (5):707–14. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 5••.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328 (5985):1566–9. doi: 10.1126/science.1189123. This study was among the first to uncover a role for miR-33 encoded within SREBF gene to regulate cholesterol transport and HDL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6••.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328(5985):1570–3. doi: 10.1126/science.1189862. This study was among the first to uncover a role for miR-33 encoded within SREBF gene to regulate cholesterol transport and HDL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107(27):12228–32. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478(7369):404–7. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12(9):735–9. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 10.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1(2):106–13. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 11.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo anti-sense targeting. Cell Metabolism. 2006;3(2):87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Elmen J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008;36(4):1153–62. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452(7189):896–9. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 14.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309(5740):1577–81. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 15.Jopling CL, Schutz S, Sarnow P. Position-dependent function for a tandem microRNA miR-122-binding site located in the hepatitis C virus RNA genome. Cell Host Microbe. 2008;4(1):77–85. doi: 10.1016/j.chom.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327(5962):198–201. doi: 10.1126/science.1178178. This was the first demonstration of anti-miRNA use in pre-clinical non-human primate models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, et al. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010;285 (44):33652–61. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci U S A. 2010;107(40):17321–6. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108(22):9232–7. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120 (16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 21.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121(7):2921–31. doi: 10.1172/JCI57275. The first demonstration that anti-microRNA oligonucleotides can directly act on atherosclerotic plaque macrophages and improve plaque phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan J, Donkin J, Wellington C. Greasing the wheels of Abeta clearance in Alzheimer’s disease: the role of lipids and apolipoprotein E. Biofactors. 2009;35(3):239–48. doi: 10.1002/biof.37. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez CM, Davalos A, Goedeke L, Salerno AG, Warrier N, Cirera-Salinas D, et al. MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011 doi: 10.1161/ATVBAHA.111.232066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 108(22):9232–7. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705–10. doi: 10.1038/nature08195. These authors uncovered an important role for miR-143 and miR-145 in maintaining smooth muscle cell contractility and regulating cell plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119(9):2634–47. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Sullivan JF, Martin K, Caplice NM. Microribonucleic acids for prevention of plaque rupture and in-stent restenosis: “a finger in the dam”. J Am Coll Cardiol. 2011;57(4):383–9. doi: 10.1016/j.jacc.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 29•.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14(3):249–56. doi: 10.1038/ncb2441. Very elegant study suggesting endothelial cells communicate with smooth muscle cells in the vessel wall via secreted miRNA. [DOI] [PubMed] [Google Scholar]
- 30.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. 2010;393 (4):643–8. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011;108 (25):10355–60. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–84. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–71. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105 (5):1516–21. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, et al. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428(6984):754–8. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- 36.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2(100):ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 37.Haver VG, Slart RH, Zeebregts CJ, Peppelenbosch MP, Tio RA. Rupture of vulnerable atherosclerotic plaques: microRNAs conducting the orchestra? Trends Cardiovasc Med. 2010;20(2):65–71. doi: 10.1016/j.tcm.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Martin K, O’Sullivan JF, Caplice NM. New therapeutic potential of microRNA treatment to target vulnerable atherosclerotic lesions and plaque rupture. Curr Opin Cardiol. 2011;26(6):569–75. doi: 10.1097/HCO.0b013e32834b7f95. [DOI] [PubMed] [Google Scholar]
- 39•.Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011;109(10):1115–9. doi: 10.1161/CIRCRESAHA.111.255737. The first demontration that anti-miRNA therapy could improve plaque vulnerability in an aneurysm mouse model. [DOI] [PubMed] [Google Scholar]
- 40.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110(3):483–95. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 41.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13 (4):423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas M, Lange-Grunweller K, Weirauch U, Gutsch D, Aigner A, Grunweller A, et al. The proto-oncogene Pim-1 is a target of miR-33a. Oncogene. 2012;31(7):918–28. doi: 10.1038/onc.2011.278. [DOI] [PubMed] [Google Scholar]
- 43.Herrera-Merchan A, Cerrato C, Luengo G, Dominguez O, Piris MA, Serrano M, et al. miR-33-mediated downregulation of p53 controls hematopoietic stem cell self-renewal. Cell Cycle. 2010;9 (16):3277–85. doi: 10.4161/cc.9.16.12598. [DOI] [PubMed] [Google Scholar]
- 44.Cirera-Salinas D, Pauta M, Allen RM, Salerno AG, Ramirez CM, Chamorro-Jorganes A, et al. Mir-33 regulates cell proliferation and cell cycle progression. Cell Cycle. 2012;11(5) doi: 10.4161/cc.11.5.19421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]