Abstract
Background
Intracavernous injection of cultured adipose tissue-derived stem cells (ADSCs) effectively restores erectile function in cavernous nerve (CN) injured rats when administered at the time of injury. However, culturing exposes ADSCs to risks of contamination and dedifferentiation. Furthermore, the acute treatment paradigm precludes selecting the patient subset benefitting from the treatment the most.
Objectives
To explore the effect of uncultured autologous adipose-derived stromal vascular fraction (SVF) on improving erectile function in a rat model of CN injury when administered at the time of injury or four weeks post-injury.
Design, setting, and interventions
Sixty-three male Sprague-Dawley rats were randomly divided into three groups: intracavernous injection of saline immediately after CN crush (vehicle), intracavernous injection of SVF immediately after CN crush (immediate-treatment), intracavernous injection of SVF four weeks post-CN crush (delayed-treatment). Twenty-six animals underwent sham surgery (sham). Functional testing and histological analysis were performed 12 weeks post-CN crush or sham surgery.
Measurements
Intracavernous pressure (ICP) response to CN electrostimulation, histological examination of midpenile cross-sections.
Results
Intracavernous injection of SVF, either immediately, or four weeks post-CN injury, resulted in significantly increased ICP/mean arterial pressure (MAP) ratios compared with the vehicle-treated group. Both immediate and delayed treatment with SVF significantly increased expression of neuronal nitric oxide synthase (nNOS) and neurofilament (NF) in dorsal penile nerves compared to the vehicle group. Furthermore, smooth muscle content and smooth muscle/collagen ratio within corpus cavernosum were significantly improved in both SVF treatment groups compared to vehicle-treated rats.
Conclusions
Uncultured autologous SVF injected immediately or 4 weeks post-CN crush improved erectile function, promoted nerve regeneration and prevented fibrosis of the corpus cavernosum following CN injury. The clinical availability of routine SVF isolation devices merits testing autologous SVF therapy in penile rehabilitation studies following radical prostatectomy in the near future.
Keywords: Cavernous nerve injury, erectile dysfunction, adipose tissue-derived stem cells, adipose-derived stromal vascular fraction, nerve regeneration
INTRODUCTION
Radical prostatectomy (RP) is considered the gold standard for the treatment of clinically localized prostate cancers, with excellent long-term oncological results [1]. Despite the advantages of nerve sparing techniques, laparoscopic and robot-assisted surgery, erectile dysfunction (ED) remains a frequent complication of RP [2, 3]. Phosphodiesterase type 5 inhibitors (PDE5i) are commonly used to treat ED after RP. However, the efficacy of PDE5i has been disappointing for many ED patients after RP [4, 5]. Therefore, there is an urgent need to develop novel and effective strategies for the treatment of ED after RP.
Adipose tissue has been suggested as an attractive and abundant stem cell source [6]. Similar to bone marrow mesenchymal stem cells, adipose tissue-derived stem cells (ADSCs) are multipotent, self-renewing cells with potential to differentiate into several cell types [7–9]. Additionally, it has been demonstrated that ADSCs are capable of secreting multiple growth factors and cytokines [10]. Intracavernous injection of cultured ADSCs has shown improvement of erectile function and preservation of corpus cavernosum microstructure in rat models of ED related to hyperlipidemia [11], diabetes mellitus [12] and cavernous nerve injury [13].
Most types of adult stem cells require isolation and expansion in vitro to obtain cell numbers adequate for achieving beneficial effects [14]. ADSCs are isolated from the stromal vascular fraction (SVF) of adipose tissue and culture-expanded in various conditions [15]. The risks of xenogenic nutritional sources, microbial contamination and tumorigenesis during cell culture raise concerns regarding implanting these cultured stem cells in human subjects [16]. To avoid these risks, SVF isolation and administration without culturing steps has been proposed as an easier and safer way to utilize stem and progenitor cells in adipose tissue for treatment. Human clinical trials have recently been initiated to employ injection of SVF during breast reconstruction surgery and to treat myocardial infarction, traumatic calvaria defects, lipodystrophy, and type I and II diabetes [17]. Another caveat of previous studies is that ADSCs were administered at the time of CN injury. However, to serve as a remedy for nerve regeneration in humans, it would be more desirable to administer the agent at a later time point post-injury when it becomes more clear which patient would benefit most from the treatment, i.e. those developing ED after nerve-sparing prostatectomy. We hypothesized that SVF is effective in improving erectile function following cavernous nerve (CN) injury. The aim of this study was to examine the therapeutic effects of immediate and delayed treatment with intracavernous injection of uncultured autologous adipose tissue-derived SVF on erectile function and corpus cavernosum microstructure in a rat model of CN injury.
MATERIALS AND METHODS
Study design
Eighty-nine Sprague-Dawley rats (12 weeks old) were obtained from Charles River Laboratories (Wilmington, MA, USA). Sixty-three rats underwent bilateral CN crush injury and were randomly divided into three groups: intracavernous injection of saline (vehicle, n=23); intracavernous injection of SVF immediately after CN crush (immediate-treatment, n=17); intracavernous injection of SVF 4 weeks after CN crush (delayed-treatment, n=23). The remaining rats underwent sham surgery (sham, n=26). Twelve weeks after CN crush or sham surgery, all rats underwent erectile function measurement. Thereafter, they were sacrificed and penile tissues were harvested for histological analysis. All procedures were approved by the Institutional Animal Care and Use Committee at University of California, San Francisco. All functional tests and histological assessments were performed in a strictly blinded fashion.
Cavernous nerve injury
Under isoflurane anesthesia, the major pelvic ganglion (MPG) and CN were exposed on both sides of the prostate via a midline laparotomy. No further manipulation occurred in the sham group, while bilateral CN crush injury was performed as described previously in all other groups [13].
Isolation of SVF
During the same surgery or 4 weeks after CN injury, perigonadal adipose tissue was harvested in all rats. Adipose tissue was washed with sterile saline and minced followed by enzymatic digestion with Adipase™ (Tissue Genesis, Honolulu, HI, USA) at 37°C for 30 minutes. The cells were then washed, centrifugated, and the pellet was resuspended in saline. The nucleated cells from the pellet were counted with an automated cell counter and diluted to 5000 cells per μl in saline.
Intracavernous injection of SVF
Under isoflurane anesthesia, a skin incision was made to expose the penis and either 400μl SVF solution (5000 cell/μl) or 400μl saline was injected into the corpus cavernosum with a 25-G needle. An elastic band was placed at the base of the penis immediately before injection and was removed 3 min after injection.
Erectile function evaluation
Intracavernous pressure (ICP) response to electrostimulation of the CN was used to evaluate erectile function [18]. Briefly, under ketamine (100mg/kg) and midazolam (5mg/kg) anesthesia, the CN were exposed via midline laparotomy. The corpus cavernosum was cannulated with a heparinized (200U/ml) 25-G needle connected to a pressure transducer (Utah Medical Products, Midvale, UT, USA). The stimulus parameters were 1.5mA, 20Hz, pulse width of 0.2 ms, and duration of 50s. The maximum increase of ICP of three stimuli per side was selected for statistical analysis in each animal. Mean arterial blood pressure (MAP) was recorded using a 25-G needle inserted into the aortic bifurcation [13, 18].
Histology
The penile mid-shaft was harvested and fixed with 2% formaldehyde and 0.002% picric acid in 0.1M phosphate buffered saline (PBS) for 4 hours, followed by immersion in 30% sucrose in PBS overnight at 4°C. Tissues were embedded into optimal cutting temperature compound (OCT, Sakura Finetek, Torrance, CA, USA). Sections were cut at a thickness of 5 μm.
Immunohistochemical staining was performed according to a previously described protocol [18]. The primary antibodies included: rabbit anti-neuronal nitric oxide synthase (nNOS) (1:100, Santa Cruz Biotechnologies, Santa Cruz, CA, USA), and mouse anti-neurofilament (NF) (1:500, this antibody was obtained from the developmental Studies Hybridoma Bank (developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA, USA)). Actin was stained by incubation with Alexa-488-conjugated phalloidin (1:400, Invitrogen, Carlsbad, CA, USA) for 20 min at room temperature. The secondary antibodies used included Alexa-488 and Alexa-594 conjugated antibodies (1:500, Invitrogen). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen).
Masson’s trichome staining procedures were performed according to a previously described protocol [19].
Image analysis was performed by computerized densitometry using Image-Pro Plus 5.1 (Media Cybernetics, Silver Spring, MD, USA). To quantify smooth muscle, the percentage of f-actin bright positive area within the corpus cavernosum was analyzed. Masson’s trichrome staining was used to quantify the ratio between smooth muscle and collagen within the corpus cavernosum. For nNOS and neurofilament staining, the ratio between nNOS or NF positive area and total dorsal nerve area was calculated.
Statistical analysis
The results were analyzed using Prism 4 (GraphPad Software, San Diego, CA, USA) and expressed as mean ± standard deviation (SD). Multiple groups were compared using one-way analysis of variance followed by the Tukey-Kramer test for post-hoc comparisons. Statistical significance was set at P< 0.05.
RESULTS
SVF treatment improves erectile function after CN injury
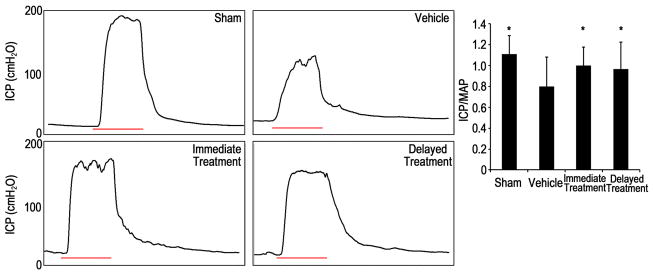
CN injury consistently resulted in decreased erectile function as compared to sham animals (Fig. 1). Recovery of erectile function was observed in both SVF treated groups. This was reflected by significantly increased ICP/MAP responses to cavernous nerve electrostimulation compared to the vehicle group. There was no significant difference between the immediate-treatment group and the delayed-treatment group.
Fig. 1.
Electrostimulation of cavernous nerves at 12 wk.
(A): representative intracavernous pressure (ICP) recording of each experimental group. The black curve represents ICP values in response to cavernous nerve stimulation. The red bar represents 50 second-electrical stimulation of the cavernous nerve. (B): the effect of SVF treatment in improving ICP/mean arterial pressure (MAP) ratio. * p<0.05 compared with vehicle group.
Histological data
SVF treatment promotes nerve regeneration
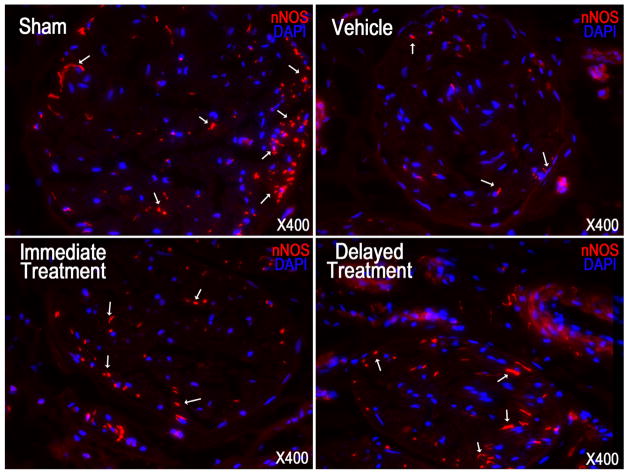
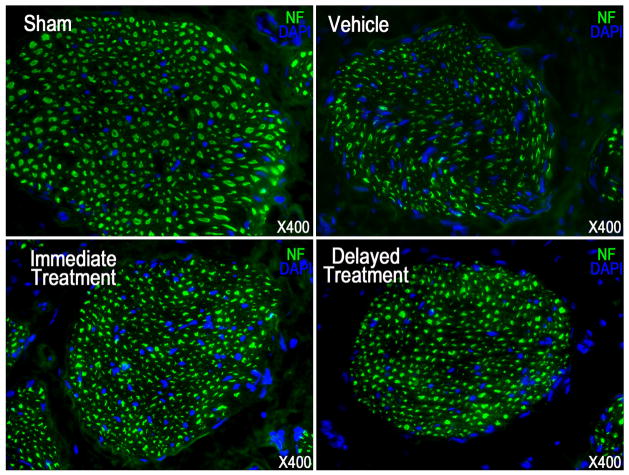
Examination of nNOS and neurofilament contents revealed nerve injury in the vehicle group as illustrated by a significant decrease of positive immunostaining compared to the sham group (Fig. 2–3, Table 1). Following SVF treatment immediately or 4 weeks after CN crush, the number of nNOS containing fibers, as well as the total axonal content (neurofilaments) was significantly higher in both groups compared with the vehicle group. There was no significant difference between the immediate and delayed treatments.
Fig. 2.
Neuronal nitric oxide synthase (nNOS) staining in a penile midshaft specimen.
Representative images of nNOS positive fibers in penile dorsal nerve of each experimental group are presented. nNOS positive fibers were stained red (arrow) in dorsal nerve. Original magnification is X 400.
Fig. 3.
Neurofilament (NF) staining in a penile midshaft specimen.
Representative images are presented for NF positive fibers in penile dorsal nerve of each experimental group. Original magnification is X 400.
Tab. 1.
nNOS, NF, actin and Masson’s trichrome staining data in each experimental group
| Groups | nNOS content in dorsal nerve, % | NF content in dorsal nerve, % | SM content in corpus cavernosum, % | Ratio between SM and collagen in corpus cavernosum |
|---|---|---|---|---|
| Sham | 1.46±0.39* | 14.64±2.36* | 5.67±1.05* | 0.089±0.0224* |
| Vehicle | 0.77±0.24 | 8.4±2.08 | 3.57±0.69 | 0.0445±0.0182 |
| Immediate | 1.19±0.25* | 12.62±2.62* | 4.72±.061* | 0.0714±0.0098* |
| Delayed | 1.08±0.25* | 13.12±2.21* | 4.51±0.67* | 0.0701±0.0166* |
Histomorphometric digital analysis was performed on tissue section of the rat penis. nNOS and NF were expressed as percentage of nNOS or NF positive area in dorsal nerve area. SM was expressed as percentage of actin positive area within corpus cavernosum. Ratio between smooth muscle and collagen within corpus cavernosum was analyzed for Masson’s trichrome staining.
P<0.05 compared with vehicle group.
Abbreviation: nNOS=neuronal nitric oxide synthase; NF=neurofilament; SM=smooth muscle.
SVF treatment improves cavernosal smooth muscle content
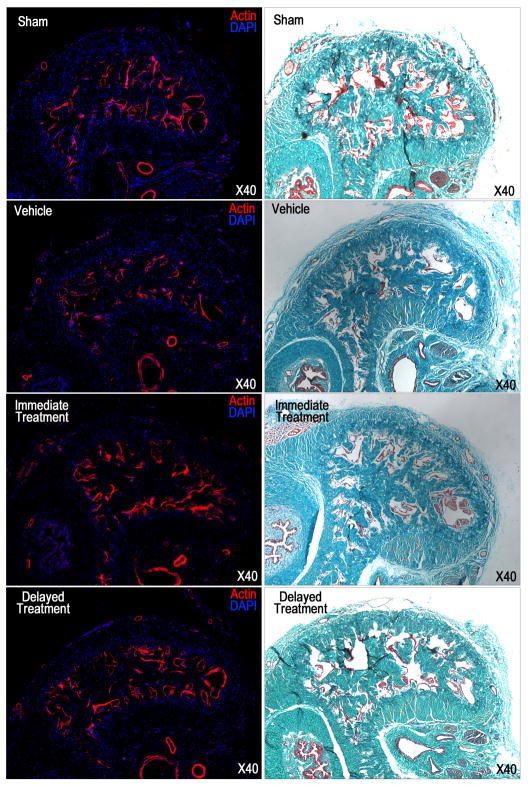
A significantly decreased smooth muscle content and smooth muscle/collagen ratio within the corpus cavernosum in the vehicle group compared with the sham group revealed the development of corpus cavernosum fibrosis following CN injury (Fig. 4, Table 1). Both SVF treatments partially, but significantly, restored the smooth muscle content and reduced fibrosis. Again, no significant difference was noted when SVF was administered immediately versus delayed.
Fig. 4.
Masson’s trichrome and phalloidin staining in a penile midshaft specimen.
(A): representative images of actin staining of each experimental group. Smooth muscle in corpus cavernosum was stained red. Original magnification is X 40. (B): representative images of Masson’s trichrome staining of each experimental group. Smooth muscle and connective tissue are stained red and green respectively. Original magnification is X 40.
DISCUSSION
Cell culture expansion of stem cells is often required to reach sufficient cell numbers for treatment purposes. However, current major restrictions regarding the use of cultured cells in human subjects are contamination, unexpected cell differentiation, and tumorigenesis [20]. Unlike other stem cells that are present at low density in their native tissues, ADSCs have been shown to be abundantly present in adipose tissue [21]. Most importantly, compared to other stem cell sources such as the bone marrow, large amounts of adipose tissue can be harvested with minimal or no side effects from human subjects. This provides a new perspective to use adipose tissue-derived cells in a clinical setting. Adipose tissue is composed of two main cell populations, mature adipocytes and the SVF. SVF is a heterogeneous fraction containing preadipocytes, mature endothelial cells, vascular smooth muscle cells, macrophages, fibroblasts, and a large population of stem cells and (endothelial and smooth muscle) progenitor cells [15, 22].
In the present study, SVF was isolated from adipose tissue and intracavernously injected into rats after CN injury. Compared to previous studies in this setting, there was a notable spontaneous nerve regeneration in untreated animals [18, 23]. This is likely due to the long time-frame between nerve injury and evaluation. However, this nerve regeneration was significantly improved by SVF injection. It can be speculated that the stem/progenitor cells in the SVF provided the therapeutic effect and preserved erectile function of CN injured rats by producing pro-survival, anti-apoptotic and neurotrophic factors [24, 25]. We have confirmed these neuroregenerative processes of ADSC in previous studies in which early migration of ADSC towards the MPG was observed [19].
This is the first study to examine the effects of stem cells treatment for ED in a chronic state after nerve trauma. Functional recovery and penile histological improvement were observed when treatment was delayed by four weeks at time when neurodegeneration and end-organ damage, including fibrosis, have occurred. The effects of the delayed administration of SVF are in agreement with results from stem cells studies in the central nervous system. Direct injection of bone marrow stromal cells in the brain 2 months after traumatic brain injury resulted in neuroregeneration [26], and systemic injection of umbilical tissue-derived cells in a similar brain-injury model resulted in locally increased vascular density and decreased apoptosis, thus supporting functional recovery [27]. In the present study, we found decreased fibrosis in the corpus cavernosum. Whether this effect is secondary to re-innervation and re-oxygenation of the corpus cavernosum, or due to local interaction between stem cells and the extracellular matrix remains undetermined. It has previously been shown that ADSC produce matrix-metalloproteinases that may aid in reducing deposited collagen following corpus cavernosum denervation [28]. On the other hand, it has been shown that ADSC migrate away from the corpus cavernosum early after injection [19].
We believe that this study bridges an important gap in translating the results of previous ADSCs studies towards the clinical setting and application in human subjects. In the current age, SVF is being used in various clinical trials and has proven safe and effective in various settings [17]. With the observation of beneficial effects of SVF replicating those of ADSC, a novel approach that is directly applicable in the clinical setting has been identified, and initial clinical studies can now be expected within a short time-frame. Liposuction and injection of SVF during the same procedure as radical prostatectomy may be a reasonable treatment option for pre-operatively motivated patients. Furthermore, the positive results of delayed treatment imply that treatment can also be given several weeks following prostatectomy at the time patients begin attempting sexual intercourse.
There are some important limitations to this study. First, ICP/MAP values exceeding 1 were noted in few rats, which may appear supraphysiological. However, ICP can be raised to several hundred mmHg if both the cavernous and pudendal nerves are stimulated [29]. This may happen during stimulation when the current spreads to the pudendal efferent nerve fibers due to the scars around the MPG and cavernous nerves. The prolonged observation time after injury may have allowed for more extensive scar development. Second, the exact composition of the rat SVF was not examined in this study. However, it is likely that the cell elements in the SVF of the rat are comparable to those in human SVF [30]. Third, we have not determined whether the decreased fibrosis in the corpus cavernosum is the result of a direct interaction between ADSC and the extracellular matrix or whether this is the result of increased innervation of the corpus cavernosum. Further research is ongoing to determine these interactions and to further understand the effects of cellular therapies in ED following CN injury.
CONCLUSION
Autologous SVF injected immediately or 4 weeks post-CN crush improved erectile function, promoted nerve regeneration and prevented fibrosis of the corpus cavernosum following CN injury. The clinical availability of routine SVF isolation devices merits testing autologous SVF therapy in penile rehabilitation studies following radical prostatectomy in the near future.
Take home message.
Intracavernous injection of uncultured autologous adipose-derived stromal vascular fraction (SVF)enhances erectile recovery in a rat model of cavernous nerve crush injury by improving nerve regeneration and ameliorating architectural changes in the corpus cavernosum. SVF is effective in the acute and chronic setting of cavernous nerve injury.
Acknowledgments
We thank Robert Lund and Linda Mummah-Schendel for their contributions to this study. MA is a fellow of the Research Foundation - Flanders (FWO).
Footnotes
Financial disclosures: This study was partially supported by a grant from American Medical Systems.
References
- 1.Heidenreich A, Fau, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. 2011:1873–7560. doi: 10.1016/j.eururo.2010.10.039. (Electronic) [DOI] [PubMed] [Google Scholar]
- 2.Dubbelman Yd, Fau, Dohle GR, Dohle Gr, Fau, Schroder FH, Schroder FH. Sexual function before and after radical retropubic prostatectomy: A systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006:0302–2838. doi: 10.1016/j.eururo.2006.06.009. (Print) [DOI] [PubMed] [Google Scholar]
- 3.Walz J, Fau, Burnett AL, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010:1873–7560. doi: 10.1016/j.eururo.2009.11.009. (Electronic) [DOI] [PubMed] [Google Scholar]
- 4.Kendirci M, Hellstrom WJ. Current concepts in the management of erectile dysfunction in men with prostate cancer. Clin Prostate Cancer. 2004;3(2):87–92. doi: 10.3816/cgc.2004.n.017. [DOI] [PubMed] [Google Scholar]
- 5.Hatzimouratidis K, Fau, Burnett AL, et al. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. 2009:1873–7560. doi: 10.1016/j.eururo.2008.10.028. (Electronic) [DOI] [PubMed] [Google Scholar]
- 6.Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 7(2):269–91. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 7.Lin GT, et al. Potential of Adipose-Derived Stem Cells for Treatment of Erectile Dysfunction. Journal of Sexual Medicine. 2009;6:320–327. doi: 10.1111/j.1743-6109.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ning HX, et al. Fibroblast Growth Factor 2 Promotes Endothelial Differentiation of Adipose Tissue-Derived Stem Cells. Journal of Sexual Medicine. 2009;6(4):967–979. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuk PA, et al. Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salgado AJ, et al. Adipose tissue derived stem cells secretome: soluble factors and their rolesin regenerative medicine. Curr Stem Cell Res Ther. 5(2):103–10. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 11.Huang YC, et al. The Effect of Intracavernous Injection of Adipose Tissue-Derived Stem Cells on Hyperlipidemia-Associated Erectile Dysfunction in a Rat Model. Journal of Sexual Medicine. 7(4):1391–1400. doi: 10.1111/j.1743-6109.2009.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia MM, et al. Treatment of Erectile Dysfunction in the Obese Type 2 Diabetic ZDF Rat with Adipose Tissue-Derived Stem Cells. Journal of Sexual Medicine. 7(1):89–98. doi: 10.1111/j.1743-6109.2009.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albersen M, et al. Injections of AdiposeTissue-Derived Stem Cells and Stem Cell Lysate Improve Recovery of Erectile Function in a Rat Model of Cavernous Nerve Injury. Journal of Sexual Medicine. 2011;7(10):3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, et al. Stem cells: novel players in the treatment of erectile dysfunction. Asian J Androl. 2011 doi: 10.1038/aja.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin G, et al. Defining Stem and Progenitor Cells within Adipose Tissue. Stem Cells and Development. 2008;17(6):1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ning H, et al. Identification of an aberrant cell line among human adipose tissue-derived stem cell isolates. Differentiation. 2009;77(2):172–80. doi: 10.1016/j.diff.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casteilla L, et al. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3(4):25–33. doi: 10.4252/wjsc.v3.i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albersen M, et al. Pentoxifylline promotes recovery of erectile function in a rat model of postprostatectomy erectile dysfunction. Eur Urol. 2011;59(2):286–96. doi: 10.1016/j.eururo.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fandel TM, et al. Recruitment of Intracavernously Injected Adipose-Derived Stem Cells to the Major Pelvic Ganglion Improves Erectile Function in a Rat Model of Cavernous Nerve Injury. Eur Urol. 2011 doi: 10.1016/j.eururo.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gimble JM, et al. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: let’s not get lost in translation. Stem Cells. 2011;29(5):749–54. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- 21.Housman TS, et al. The safety of liposuction: results of a national survey. Dermatol Surg. 2002;28(11):971–8. doi: 10.1046/j.1524-4725.2002.02081.x. [DOI] [PubMed] [Google Scholar]
- 22.Fraser JK, et al. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends in Biotechnology. 2006;24(4):150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Kendirci M, et al. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerveinjury. J Urol. 2010;184(4):1560–6. doi: 10.1016/j.juro.2010.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei X, et al. Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neuroscience Letters. 2009;462(1):76–79. doi: 10.1016/j.neulet.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 25.Rehman J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 26.Xiong Y, Fau, Qu C, et al. Delayed transplantation of human marrow stromal cell-seeded scaffolds increases transcallosal neural fiber length, angiogenesis, and hippocampal neuronal survival and improves functional outcome after traumatic brain injury in rats. 2009:1872–6240. doi: 10.1016/j.brainres.2009.01.032. (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Fau, Li Y, et al. Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. 2011:1524–4628. doi: 10.1161/STROKEAHA.110.593129. (Electronic) [DOI] [PubMed] [Google Scholar]
- 28.Kachgal S, Fau, Putnam AJ, Putnam AJ. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. 2011:1573–7209. doi: 10.1007/s10456-010-9194-9. (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lue TF, et al. Hemodynamics of erection in the monkey. J Urol. 1983;130(6):1237–41. doi: 10.1016/s0022-5347(17)51768-1. [DOI] [PubMed] [Google Scholar]
- 30.Lin SD, et al. Engineering adipose tissue from uncultured human adipose stromal vascular fraction on collagen matrix and gelatin sponge scaffolds. Tissue Eng Part A. 2011;17(11–12):1489–98. doi: 10.1089/ten.TEA.2010.0688. [DOI] [PubMed] [Google Scholar]