Abstract
Study Objectives:
Suvorexant (MK-4305) is an orexin receptor antagonist being developed for the treatment of insomnia. This report describes the effects of nighttime administration of suvorexant on polysomnography (PSG) sleep parameters in healthy young men.
Design:
Randomized, double-blind, placebo-controlled, 4-period crossover PSG study, followed by an additional 5th period to assess pharmacokinetics.
Setting:
Sleep laboratory.
Participants:
Healthy young men between 18 and 45 years of age (22 enrolled, 19 completed).
Interventions:
Periods 1–4: suvorexant (10 mg, 50 mg, or 100 mg) or placebo 1 h before nighttime PSG recording. Period 5: suvorexant 10 mg, 50 mg, or 100 mg.
Measurements and Results:
In Periods 1–4, overnight sleep parameters were recorded by PSG and next-morning residual effects were assessed by psychomotor performance tests and subjective assessments. Statistically significant sleep-promoting effects were observed with all doses of suvorexant compared to placebo. Suvorexant 50 mg and 100 mg significantly decreased latency to persistent sleep and wake after sleep onset time, and increased sleep efficiency. Suvorexant 10 mg significantly decreased wake after sleep onset time. There were no statistically significant effects of suvorexant on EEG frequency bands including delta (slow wave) activity based on power spectral analysis. Suvorexant was well tolerated. There was no evidence of next-day residual effects for suvorexant 10 mg. Suvorexant 50 mg statistically significantly reduced subjective alertness, and suvorexant 100 mg significantly increased reaction time and reduced subjective alertness. There were no statistically significant effects of any suvorexant dose on digit symbol substitution test performance. In Period 5, plasma samples of suvorexant were collected for pharmacokinetic evaluation. The median Tmax was 3 hours and apparent terminal t½ was 9–13 hours.
Conclusions:
In healthy young men without sleep disorders, suvorexant promoted sleep with some evidence of residual effects at the highest doses.
Citation:
Sun H; Kennedy WP; Wilbraham D; Lewis N; Calder N; Li X; Ma J; Yee KL; Ermlich S; Mangin E; Lines C; Rosen L; Chodakewitz J; Murphy GM. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. SLEEP 2013;36(2):259–267.
Keywords: Suvorexant, MK-4305, orexin, orexin receptor antagonist, insomnia, polysomnography, randomized trial
INTRODUCTION
Extensive research has demonstrated that the orexin system plays a critical role in the regulation of the transition between sleep and arousal.1–7 Orexinergic neurons are primarily localized to the lateral hypothalamus and have ascending projections to the cerebral cortex and descending projections to the wakefulness-promoting cell groups of the arousal system, including the monoaminergic and cholinergic cell groups.8 The actions of orexin neuropeptides are mediated by two G protein-coupled ligand receptors, orexin R1 and R2 (OX1R and OX2R).9,10 Orexin knockout/mutations have been linked to narcolepsy in human and animals.11,12 Antagonism of orexin receptors is hypothesized to facilitate sleep by transiently blocking orexinergic activity from the lateral hypothalamus and interconnected lower brainstem nuclei that sustain arousal/vigilance. Recently developed investigational orexin receptor antagonists, such as almorexant (ACT-078573) and GW-649868 (SB-649868), have been shown to promote sleep in animals and humans.13–17
Orexin receptor antagonists have a distinctly different mechanism from the benzodiazepine receptor agonists, which are the most common drugs prescribed for the treatment of insomnia. The benzodiazepine receptor agonists target GABA receptors located diffusely in the brain and are associated with side effects such as next-day sedation, memory disturbances, hallucinations, rebound insomnia, and physical and psychological dependence.18 It is possible that orexin receptor antagonists, which have focused effects on a small group of neurons mediating the transition between arousal and sleep, may have clinical advantages compared to currently available sedative-hypnotics.
Suvorexant (MK-4305) is a novel, orally active, potent orexin receptor antagonist19,20 that is currently in phase 3 clinical development for treatment of insomnia. In rodents, dogs, and rhesus monkeys, suvorexant reduced active wake time and increased rapid eye movement (REM) sleep and delta (slow wave) activity (SWA).19,20 Increase in SWA was also observed in healthy volunteers after morning administration of suvorexant as measured by quantitative EEG in a first-in-man study (unpublished data).
The primary objective of the present study was to assess the pharmacological effects of evening administration of suvorexant on sleep parameters and EEG power spectral measures using polysomnography (PSG) recording in healthy young men. Plasma pharmacokinetics, safety and tolerability, and next-day residual effects were also evaluated. Single doses of 10 mg, 50 mg, and 100 mg were selected for evaluation based on preliminary pharmacodynamic effects observed during daytime dosing with suvorexant in a first-in-man study (unpublished data).
METHODS
For full details of the study methods, see the study protocol in the supplemental material.
Subjects
The study (Merck Protocol 002) was conducted from May to August 2008, at Quintiles Drug Research Unit at Guy's Hospital, London, United Kingdom. Healthy young men between 18 and 45 years of age, with a body mass index ≤ 33 kg/m2, who were judged to be in good health based on medical history, physical examination, and laboratory safety evaluation were eligible. Subjects had to have a usual bedtime between 20:00 and 00:00 (in practice all had bedtimes between 22:00 and 00:00), with a self-report of total sleep duration of ≥ 6.5 h and ≤ 9 h per night during the 4 weeks prior to the study. Subjects could not be shift-workers, not have recently (within 1 week prior to treatment period) traveled across ≥ 3 time zones, and not have any other unusual changes in sleeping routine during the study. Subjects were asked not to take any medications during the study, including those that could affect sleep.
The study was conducted in accordance with principles of Good Clinical Practice and was approved by Guy's Research Ethics Committee and the Medicines and Healthcare Regulatory Agency of the United Kingdom. After explanation of the study procedures, risks, and benefits, written informed consent was obtained from all subjects. Subjects were compensated for their participation.
Study Design and Procedure
This was a randomized, double-blind, placebo-controlled, 4-period crossover PSG study, followed by an additional 5th period to assess pharmacokinetics. In Periods 1–4, subjects received the following 4 treatments in a randomized order according to a balanced crossover design: suvorexant 10 mg, 50 mg, 100 mg, and placebo. Subjects stayed overnight in a sleep laboratory for two 8-h PSG recording sessions for each treatment period. The first night was a habituation night (Night 1) without any medication administration. On the second night, subjects received one of the 4 treatments (suvorexant 10 mg, 50 mg, or 100 mg, or placebo) after ~4 h fasting and ~1 h before the PSG recording. The PSG recording was fixed to 8 h from lights-off at night to lights-on in the morning and commenced at the subject's usual bedtime. Visual scoring of PSG data was performed by blinded personnel at Quintiles Drug Research Unit at Guy's Hospital in 30-sec epochs according to Rechstaffen and Kales criteria.21 Measures of sleep onset (latency to persistent sleep [LPS]), sleep maintenance (wake after sleep onset [WASO]), sleep efficiency (SE), and total sleep time (TST) were assessed. The amount of time spent in each sleep stage (REM, NREM, SWS and Stages 1, 2, 3, and 4) and the number of stage shifts (NSS) were also assessed. Power spectral analysis of EEG data was performed to determine SWA (delta power), as well as activity in the alpha, theta, sigma, beta, and gamma frequency bands.
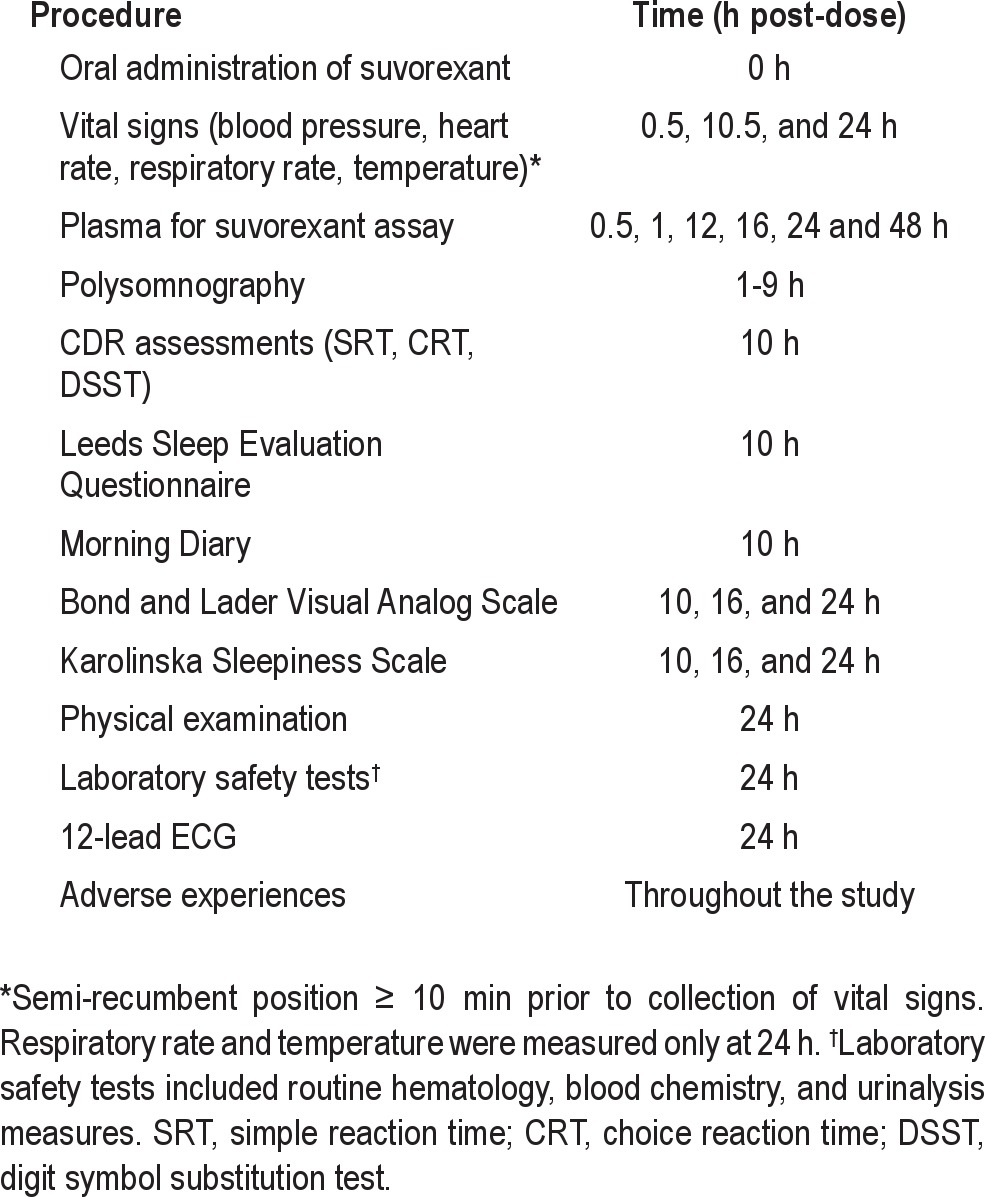
Next-day residual effects were assessed with psychomotor performance and subjective measurements in the morning. Psychomotor performance was assessed pre-dose and 10 h post-dose using simple reaction time (SRT), choice reaction time (CRT), and digit symbol substitution tests (DSST) from the Cognitive Drug Research test battery.22 These tests had a duration of approximately 2 min each. Subjective sleep effects (e.g., getting to sleep, quality of sleep) and next-day residual effects (e.g., pattern of awakening from sleep and behavior following waking) were also evaluated 10 h post-dose using the Leeds Sleep Evaluation Questionnaire (LSEQ).23 An exploratory assessment of subjective next-day residual effects was also performed using the Bond-Lader Visual Analogue Scale (VAS)24 and Karolinska Sleepiness Scale (KSS).25 The schedule of events after dosing in Periods 1–4 is summarized in Table 1.
Table 1.
Schedule of post-treatment procedures in Periods 1–4
In Treatment Period 5, subjects randomly received suvorexant 10 mg, 50 mg, or 100 mg and stayed overnight for pharmacokinetic blood sampling throughout the night. PSG recording was not performed in Period 5. There was a minimum of 96-h (> 5 half-life periods) washout period between treatment periods. Plasma samples for pharmacokinetic determinations were taken pre-dose, 0.5, 1, 2, 3, 4, 6, 8, 12, 16, 24, and 48 h post-dose. The plasma pharmacokinetic parameters (e.g., AUC0-∞, AUC0–24h, Cmax, Tmax, apparent terminal t½) of suvorexant following oral evening administration were determined. Sparser plasma sampling for pharmacokinetic analysis was also performed during Periods 1–4 (see Table 1, data not reported).
Suvorexant 10 mg and 50 mg tablets and matching placebos were used in the study. In order to maintain blinding, a double-dummy design was used, which required each subject to take 4 tablets. Participants were assigned to treatment using a computer-generated randomized allocation schedule prepared by a statistician at Merck. Study investigators, site staff, subjects, PSG scorers, psychomotor test scorers, and Merck monitoring staff remained blinded to treatment allocation throughout the study. Safety was monitored throughout the study by repeated clinical and laboratory evaluations. Safety evaluations included vital signs, physical examination, 12-lead ECGs, routine laboratory safety assessments (hematology, chemistry, and urinalysis) and adverse event reporting.
Pharmacokinetic Analysis
The analytical method for the determination of suvorexant concentrations was based on a liquid-liquid extraction of drug from human plasma. The drug and internal standard were separated using reversed phase HPLC and detected with tandem mass spectrometry. The lower limit of quantitation for this method was 1 ng/mL with a linear calibration range from 1 to 1,000 ng/mL. Suvorexant plasma concentrations were converted from units of ng/mL to μM using the molecular weight of suvorexant (MW = 450.932 g/mol). The pharmacokinetic parameters of suvorexant were calculated using the software WinNonlin (version 5.2.1). The apparent terminal rate constant (λ) was estimated by regression of the terminal log-linear portion of the plasma concentration-time profile; apparent terminal t½ was calculated as the quotient of ln(2) and λ. AUC0-∞ was estimated as the sum of AUC0-last and the extrapolated area given by the quotient of the last quantifiable plasma concentration and λ. AUC to the last time point with a quantifiable plasma concentration (AUC0-last), and AUC to 24 h (AUC0–24h) were calculated using the linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations up to the last quantifiable plasma concentration or the nominal sampling times at 24 h post-dose for AUC0–24h. Cmax and Tmax were obtained by inspection of the plasma concentration data.
Statistical Analysis
The analysis of pharmacodynamic endpoints was based on the per-protocol population excluding protocol violators according to prespecified criteria. The primary hypothesis was that at least one dose of suvorexant would be superior to placebo in enhancing SWA in healthy young men during the first half of the sleep period (animal data suggested that the increase in SWA was most marked during this time). A mixed model appropriate for a 4-period, randomized, crossover study was used to evaluate SWA data. Prior to statistical analysis, individual values of SWA during the first half of the sleep period were natural log-transformed and evaluated with a model that included fixed factors for period, treatment, and a random factor for subject. A test for first order carryover was assessed. A step-down approach was used to address the primary hypothesis, based on the assumption that the doses administered are associated with monotonically increasing SWA. To complete the first step of this test, the mean log treatment difference of the highest suvorexant dose versus placebo (suvorexant - placebo) and 90% confidence interval for the treatment difference were computed using the mean square error from the above mixed model and referencing a t-distribution. This difference and confidence interval were back-transformed to obtain the 90% confidence interval for the SWA treatment ratio (suvorexant/placebo). The data in other frequency bands were analyzed in a similar fashion.
The data on PSG sleep parameters (LPS, WASO, SE, TST) and sleep architecture (REM, NREM stages 1, 2, 3 and 4, SWS, NSS) were evaluated with a model that included fixed factors for period, treatment, and a random factor for subject. Prior to statistical analysis, individual values of all PSG parameters were natural log-transformed. There were several PSG endpoints having zero values for some observations. So that log-transformation could be applied, a value of 1 second was added to all observations for these endpoints. No multiplicity adjustment was made. The data on psychomotor performance (Cognitive Drug Research tests) and subjective residual effects (LSEQ) were analyzed using similar models. For the exploratory Bond-Lader VAS and KSS assessments, only summary data were provided and these results are not reported here.
The safety analysis included all subjects who took treatment. Summary statistics and counts were performed for safety measures.
Power and Sample Size
The sample size was based on the SWA endpoint. Assuming a total of 20 subjects completing the crossover study, and a one-sided test at α = 0.05, the study had 86% power to detect that suvorexant is superior to placebo in enhancing SWA during the first half of the sleep period, if the true increase was 20%. There was 80% power to detect an increase of 18%. A 20% enhancement of SWA was expected based on preclinical data with suvorexant. These calculations were based on an assumption of a log-scale within-subject standard deviation of 0.2, as seen in a previous unpublished study of an investigational hypnotic.
RESULTS
Subject Demographics
Twenty-two healthy young men were enrolled and 19 completed the study per protocol. Two subjects discontinued due to a protocol deviation (use of illicit drugs) following Period 1. One subject discontinued due to a protocol deviation (use of a concomitant therapy without permission from the investigator) after completing Period 3; this subject received suvorexant 50 mg, 100 mg, and placebo. All 22 subjects were included in the evaluation of safety. The 19 subjects who completed the study and the 1 subject who completed 3 periods were included in the primary analysis of pharmacodynamics, resulting in sample sizes of 20 for all treatments except suvorexant 10 mg (N = 19). The 19 subjects who completed the study were included in the analysis of pharmacokinetics, with sample sizes of 7 for each of suvorexant 50 mg and 100 mg, and a sample size of 5 for suvorexant 10 mg. Of the 22 enrolled subjects, 17 were white, the mean age was 29.6 years (range: 18 to 44), the mean height was 176.6 cm (range: 166 to 193) and the mean weight was 80.2 kg (range: 63.0 to 109.9).
PSG Assessments
Results for the PSG assessments are summarized in Table 1. Power spectral analysis showed that there was no statistically significant effect of suvorexant on SWA during the first half of the night (Table 2). Exploratory analyses for SWA by each hour of the night showed a similar pattern of findings across the night. In addition, suvorexant had no statistically significant effect on the alpha, theta, sigma, beta, and gamma frequency bands (data not shown).
Table 2.
Effect of suvorexant on SWA, PSG sleep measures, and PSG sleep architecture in healthy young men
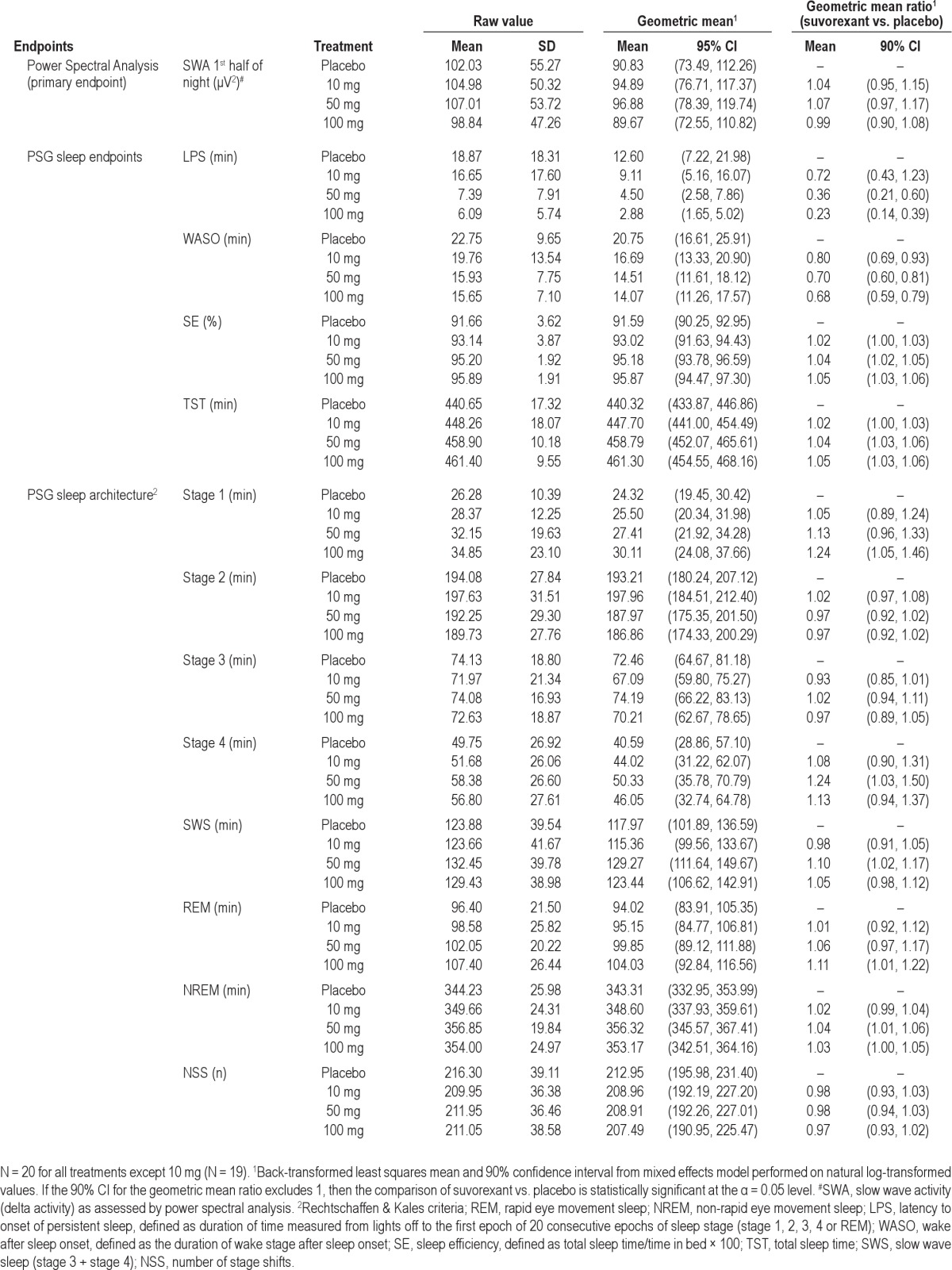
For PSG sleep measures (Table 2), suvorexant 50 mg and 100 mg statistically significantly decreased LPS and WASO. A corresponding statistically significant increase in SE and TST was observed. A statistically significant decrease in WASO was observed for suvorexant 10 mg. There were no consistent statistically significant effects of suvorexant on PSG sleep architecture stages over the entire 8-h PSG recording session (Table 2). There was no statistically significant effect of suvorexant on NSS compared to placebo suggesting that suvorexant does not lead to an imbalance in sleep stage changes or cause sleep fragmentation.
Next-Day Residual Effects Evaluation
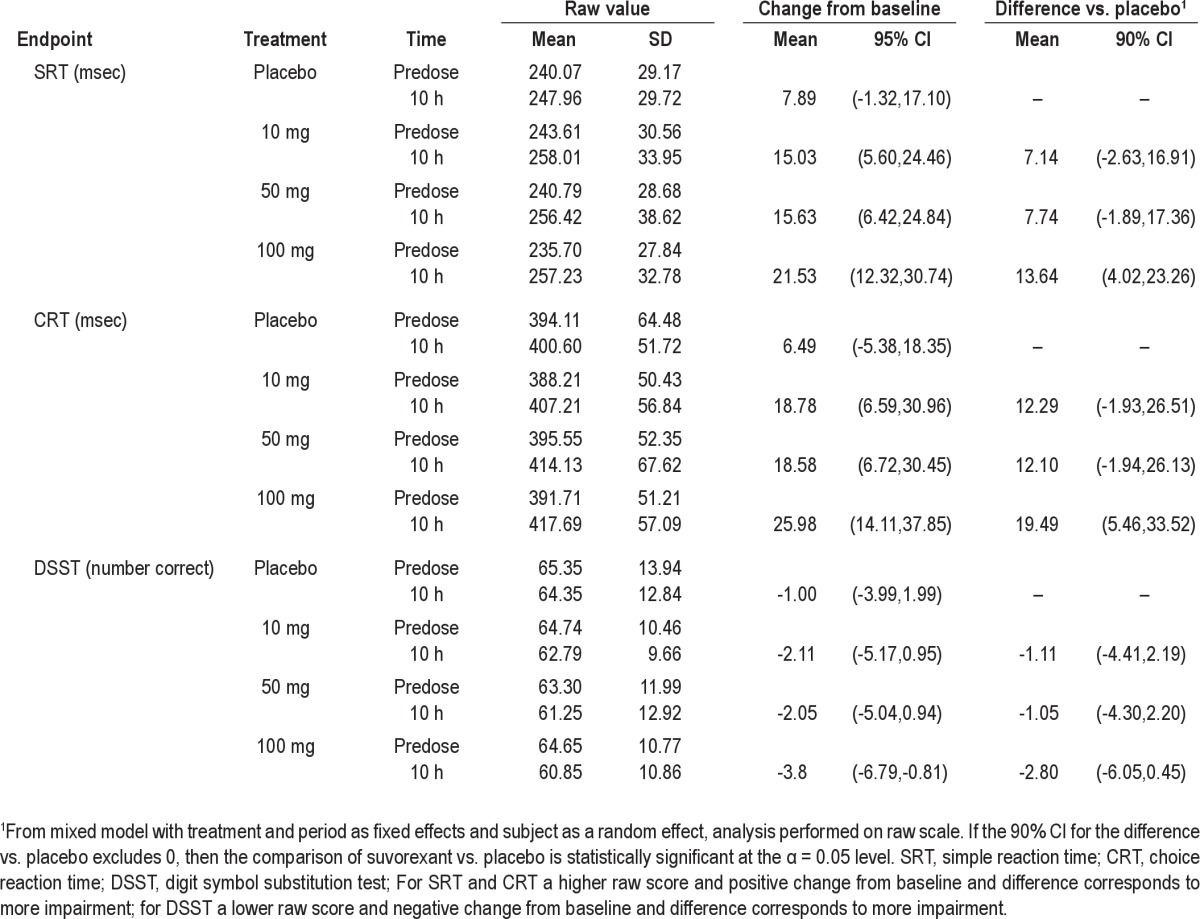
The effects of suvorexant on psychomotor performance were evaluated at 10 h post dose as exploratory measurement of next-day residual effect (Table 3). At 100 mg, there was a statistically significant increase in reaction time for both SRT and CRT, but no statistically significant effect on the DSST. There were no statistically significant changes on SRT, CRT, and DSST at 10 and 50 mg.
Table 3.
Effect of suvorexant on psychomotor tests performed the morning after dosing (10 h post-dose) in healthy young men
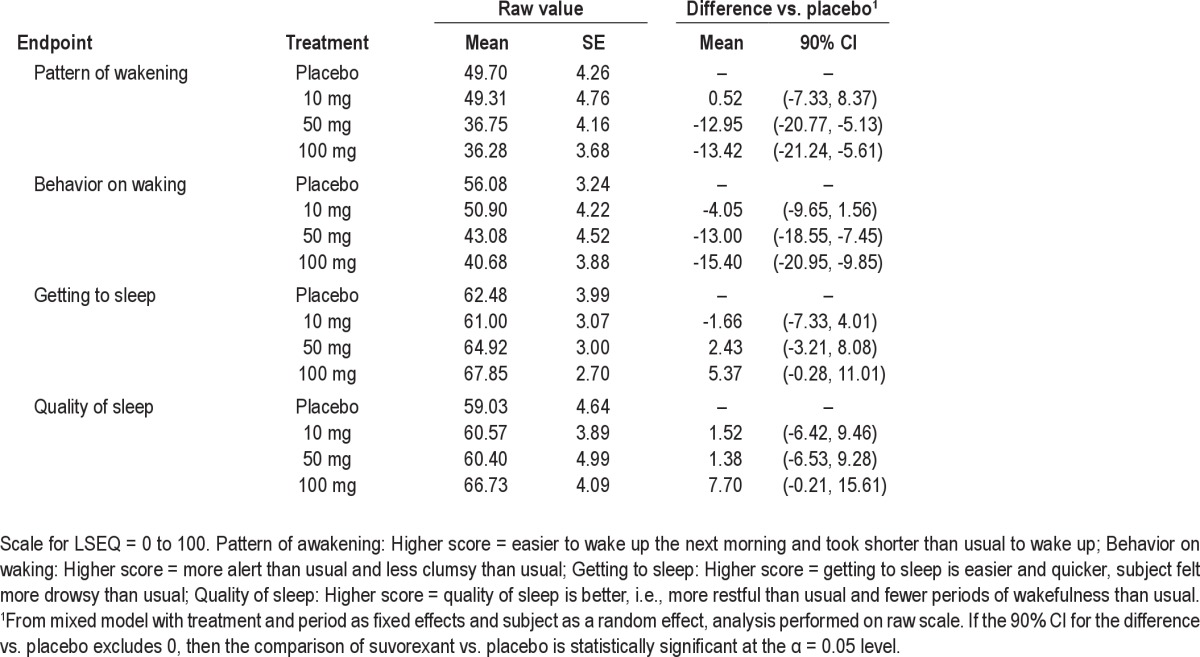
With regard to subjective assessments, on the LSEQ (Table 4), suvorexant 50 mg and 100 mg showed a statistically significant effect compared to placebo on “pattern of wakening” and “behavior on waking” at 10 h post-dose, suggesting less ease in waking up and less alertness following waking compared to placebo.
Table 4.
Effect of suvorexant on LSEQ ratings performed the morning after dosing (10 h post-dose) in healthy young men
Pharmacokinetics
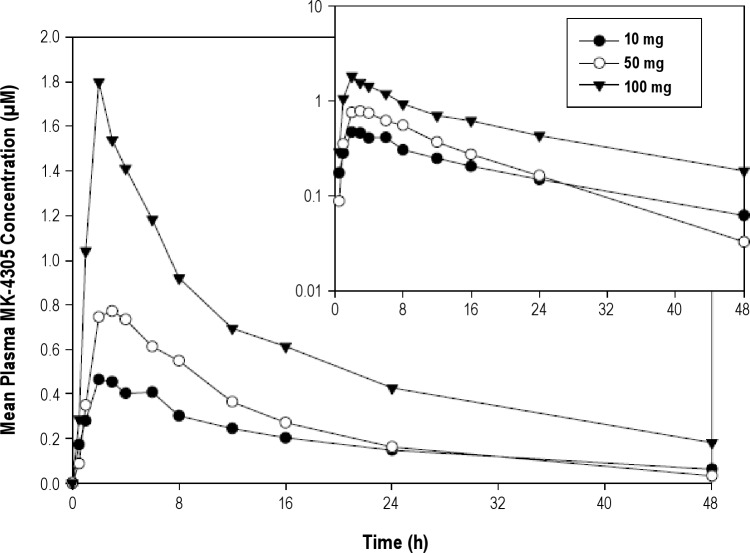
A plot of mean suvorexant plasma concentrations over time following a single evening dose administration of 10 mg, 50 mg, and 100 mg in healthy young men in Period 5 is shown in Figure 1. The pharmacokinetics of suvorexant are summarized in Table 5. Median Tmax was 3 h and mean apparent terminal t½ was 9 to 13 h. As shown in Table 5 and Figure 1, plasma concentrations of suvorexant increased with dose. In a plot of plasma concentrations over time (Figure 1), it can be seen that the curve for the 50 mg exposure unexpectedly crosses that for the 10 mg exposure at 24 h. In Period 5, 2 subjects had unusual suvorexant plasma concentrations at 10 mg and 50 mg doses, respectively, that were not consistent with the exposures observed in other subjects at the same dose levels nor with the exposures observed for the same subjects in Periods 1–4 at the same doses. These observations suggest the possibility that the Period 5 plasma concentrations for these 2 subjects may not be representative of their respective intended doses.
Figure 1.
Mean suvorexant (MK-4305) plasma concentrations (μM) versus time (h) following a single dose administration of 10 mg, 50 mg, or 100 mg in healthy young men in Period 5 (N = 5 for 10 mg, N = 7 for 50 mg and 100 mg). Inset shows data on semi-log scale.
Table 5.
Pharmacokinetics of suvorexant in healthy young men

Tolerability and Safety
The numbers of subjects with adverse events are summarized in Table 6. Suvorexant was generally well tolerated in healthy young men. No serious clinical adverse events were reported and no subjects discontinued suvorexant due to an adverse event. There were no reports of sleep paralysis, hallucinations, or cataplexy. The most frequently reported adverse events were somnolence and headache. Somnolence was reported in the morning after evening administration, and was more frequently reported by subjects receiving 100 mg (20%) than by subjects receiving 50 mg, 10 mg, or placebo (< 5%). All reported adverse events were rated as mild in intensity by the investigator. One subject reported a rash on 2 occasions following suvorexant; the rash was rated as mild in intensity and was considered to be probably not related to study drug by the investigator. No laboratory adverse events were reported. No clinically meaningful treatment related changes were observed for vital signs, physical examinations, ECGs, or laboratory values.
Table 6.
Number (%) of healthy young men with clinical adverse events by treatment

DISCUSSION
In this study we evaluated the effects of nighttime administration of suvorexant, an orexin receptor antagonist, on PSG sleep parameters and sleep architecture, as well as EEG power spectral profile, in healthy young men. Based on data from animal EEG studies19,20 and daytime EEG studies in healthy young men following morning administration of suvorexant (unpublished) and another orexin receptor antagonist, almorexant,16 we expected that suvorexant would increase SWA in healthy young men during nighttime sleep following evening administration. SWA is quantified as the power spectral density for the high-amplitude low-frequency delta activity. SWA usually increases during sleep, especially during deep sleep (stages 3 and 4). Contrary to our prediction, suvorexant did not increase SWA in healthy men during nighttime sleep following evening administration in this study. This discrepancy from previous studies could be due to different dosing (evening versus morning) and EEG recording schedules (nighttime versus daytime). It has been shown that circadian variations affect sleep drive propensity for SWA.26 When EEG data was collected during nighttime sleep after evening dosing in the present study, the baseline SWA was already high due to sleep, leaving a minimal window for further increment in non–sleep-deprived healthy subjects.
In addition to the absence of effects on delta/SWA, the power spectral analysis for other frequency bands (i.e., alpha, theta, sigma, beta, and gamma) did not reveal any difference between suvorexant treatment and placebo. This absence of a power spectral “signature” is unique compared to marketed hypnotics such as benzodiazepines and zolpidem which produce consistent and predictable changes in the power spectra.27–29
The effect of suvorexant on PSG sleep parameters and sleep architecture were explored in this study. Suvorexant 50 mg and 100 mg decreased LPS and WASO and increased SE and TST in healthy subjects without sleep disorders, while suvorexant 10 mg decreased WASO. These are key efficacy endpoints used to evaluate the effects of sleep-promoting agents in clinical trials with primary insomnia patients. These data demonstrate that significant sleep-promoting effects of suvorexant, both on sleep onset and sleep maintenance, can occur in a population of healthy young men in a sleep laboratory setting. Analysis of sleep architecture endpoints showed no consistent pattern of change on any sleep stages following suvorexant. The NSS analysis suggested no significant change on sleep stage transitions after treatment with suvorexant, and therefore no sleep fragmentation. Similar findings with suvorexant have been observed in primary insomnia patients.30
The residual effects of suvorexant were evaluated at 10 h post evening administration of suvorexant via exploratory objective and subjective measurements. At 100 mg, suvorexant showed statistically significant increases on reaction time tasks as compared to placebo, but no statistically significant effect on a digit symbol substitution test. At 10 mg and 50 mg, suvorexant did not show a statistically significant effect on any of the objective evaluations. However, increased tiredness upon waking was observed at 50 mg and 100 mg suvorexant on the LSEQ. Thus suvorexant 100 mg showed statistically significant residual effects on both objective and subjective measures (but not all measures); suvorexant 50 mg showed statistically significant residual effects on some subjective measures only; and suvorexant 10 mg did not show statistically significant residual effects on either objective or subjective measures. It is uncertain whether the magnitude of the observed statistically significant residual effects at the 50 and 100 mg doses of suvorexant is clinically meaningful. These subjective and objective evaluations of residual effects were generally consistent with adverse event reports. Four subjects (out of 20 [20%]) reported somnolence in the morning after receiving 100 mg suvorexant, whereas only one subject (< 5%) reported next-day somnolence at 10 mg and 50 mg. Additional studies are planned to further characterize the residual effect profile of suvorexant at clinically relevant doses.
The pharmacokinetics of suvorexant following single evening dosing with 10 mg, 50 mg and 100 mg was evaluated in the current study. Plasma concentrations of suvorexant increased with dose and the half-life varied from 9 to 13 h. As a sleep-promoting agent, the half-life of suvorexant may be considered relatively long compared to some of the commonly prescribed benzodiazepine agonists. However, there did not appear to be significant next-day residual effects as assessed by objective psychomotor performance tests at the lower doses (i.e., 10 mg and 50 mg) where significant sleep promoting effects were demonstrated. It is possible that circadian variation of orexin levels may play a role in determining residual effects.31 Naturally increasing orexin levels in the morning may counteract possible residual effects resulting from suvorexant.
Suvorexant was generally well tolerated in healthy young men in this study. The most frequently reported adverse events were somnolence and headache. As noted above, there was an increase in somnolence adverse events (20%) at the 100 mg dose. All adverse events were mild. There were no reports of sleep paralysis, hallucinations, or cataplexy.
The results of the current study suggest that an orexin receptor antagonist, suvorexant, has sleep-promoting effects in healthy subjects without known sleep impairments. As this study was performed in healthy young men in a sleep laboratory setting, the generalizability of the observed sleep-promoting effects require further evaluation in insomnia patients and in settings similar to the home environment. Another limitation of this study is the relatively small sample size. This limits the ability to assess pharmacodynamic dose-response relationships due to insufficient power. Nevertheless, results from the present study helped inform dose selection for the subsequent phase 2 clinical program of suvorexant. A phase 2b proof-of-concept study in primary insomnia patients found that doses of 10 mg to 80 mg of suvorexant showed significant dose-related improvements for sleep induction and maintenance and were generally well tolerated.30
DISCLOSURE STATEMENT
This study was supported by Merck. Drs. Sun, Kennedy, Lewis, Calder, Li, Ma, Yee, Ermlich, Mangin, Lines, Rosen, Chodakewitz, and Murphy are current or former employees of Merck and own stock/stock options in Merck. Dr. Wilbraham is an employee of Quintiles and the company was paid by Merck to perform the study.
ACKNOWLEDGMENTS – AUTHOR AFFILIATION UPDATES
Drs. Sun and Kennedy contributed equally to the work and are joint first authors. The funding organization (Merck Sharp – Dohme Corp.) was involved in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. All authors are responsible for the work described in this paper. All authors were involved in at least one of the following: conception, design, acquisition, analysis, statistical analysis, interpretation of data, and drafting the manuscript and/or revising the manuscript for important intellectual content. All authors provided final approval of the version to be published. The authors thank Jaqueline McCrea (Merck) and Rachel Schembri (Quintiles) for assistance with study administration, and Sheila Erespe (Merck) for assistance in formatting the manuscript. Dr. Sun is currently affiliated with Amgen in Thousand Oaks, CA. Dr. Kennedy is currently affiliated with Genentech, Inc., in South San Francisco, CA. Dr. Calder is currently affiliated with Novartis Institutes for Biomedical Research in Horsham, United Kingdom.
SUPPLEMENTAL MATERIAL
REFERENCES
- 1.De Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagan JJ, Leslie RA, Patel S, et al. Orexin activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;14:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–65. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- 4.Baumann CR, Bassetti CL. Hypocretins (orexins) and sleep-wake disorders. Lancet Neurol. 2005;4:673–82. doi: 10.1016/S1474-4422(05)70196-4. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–81. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 6.Scammell TE, Saper CB. Orexins: looking forward to sleep, back at addiction. Nat Med. 2007;2:126–8. doi: 10.1038/nm0207-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohno K, Sakurai T. Orexin neuronal circuitry: Role in regulation of sleep and wakefulness. Front Neuroendocrinol. 2008;29:70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roecker AJ, Coleman PJ. Orexin receptor antagonists: medicinal chemistry and therapeutic potential. Curr Top Med Chem. 2008;8:977–87. doi: 10.2174/156802608784936746. [DOI] [PubMed] [Google Scholar]
- 10.Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–66. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeitzer JM, Nishino S, Mignot E. The neurobiology of hypocretins (orexins), narcolepsy and related therapeutic interventions. Trends Pharmacol Sci. 2006;27:368–74. doi: 10.1016/j.tips.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Siegel JM, Moore R, Thannickal T, Nienhuis R. A brief history of hypocretin/orexin and narcolepsy. Neuropsychopharmacology. 2001;25(Suppl):S14–20. doi: 10.1016/S0893-133X(01)00317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorffner G, Anderer P, Saletu B, et al. Effect of almorexant treatment on sleep variables in patients with primary insomnia compared with healthy controls. Eur Neuropsychopharm. 2010;20(Suppl):S252. [Google Scholar]
- 14.Bettica PU, Lichtenfeld U, Squassante L, et al. The orexin receptor antagonist SB-649868 promotes and maintains sleep in healthy volunteers and in patients with primary insomnia. Sleep. 2009;32(Abstract Suppl):A252. [Google Scholar]
- 15.Gerrard PA, Porter R, Holland V, et al. Preclinical pharmacology of SB-649868: a novel orexin OX1/OX2 receptor antagonist possessing potent hypnotic activity in rodents and primates. Sleep. 2009;32(Abstract Suppl):A42. [Google Scholar]
- 16.Brisbare-Roch C, Dingemanse J, Koberstein R, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–5. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 17.Dingemanse J, Dorffner G, Hajak G, et al. Proof-of-concept study in primary insomnia patients with almorexant (ACT-078573), a dual orexin receptor antagonist. Sleep Biol Rhythms. 2007;5:A194. [Google Scholar]
- 18.Roth T, Roehrs TA. Issues in the use of benzodiazepine therapy. J Clin Psychiatry. 1992;53(Suppl):14–8. [PubMed] [Google Scholar]
- 19.Cox CD, Breslin MJ, Whitman DB, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl] [5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl] methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53:5320–32. doi: 10.1021/jm100541c. [DOI] [PubMed] [Google Scholar]
- 20.Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by MK-4305 - a novel dual orexin receptor antagonist. J Neurogenetics. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 21.Rechtschaffen A, Kales A. Washington DC: National Institute of Health, Publication 204. Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subject. [Google Scholar]
- 22.Wesnes K, Simpson PM, Christmas L. Vol 1. Chichester, New York: Wiley; 1987. The assessment of human information processing abilities in psychopharmacology: measures and methods, [Google Scholar]
- 23.Parrott AC, Hindmarch I. The Leeds Sleep Evaluation Questionnaire in psychopharmacological investigations - a review. Psychopharmacology (Berl) 1980;71:173–9. doi: 10.1007/BF00434408. [DOI] [PubMed] [Google Scholar]
- 24.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Psychol. 1974;47:211–18. [Google Scholar]
- 25.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 26.Duncan WC, Barbato G, Fagioli I, Garcia-Borreguero D, Wehr TA. A biphasic daily pattern of slow wave activity during a two-day 90 minute sleep wake schedule. Arch Ital Biol. 2009;147:117–30. [PubMed] [Google Scholar]
- 27.Lundahl J, Deacon S, Maurice D, Staner L. EEG spectral power density profiles during NREM sleep for gaboxadol and zolpidem in patients with primary insomnia. J Psychopharmacol. 2012;26:1081–7. doi: 10.1177/0269881111424457. [DOI] [PubMed] [Google Scholar]
- 28.Feinberg I, Maloney T, Campbell IG. Effects of hypnotics on the sleep EEG of healthy young adults: new data and psychopharmacologic implications. J Psychiatr Res. 2000;34:423–38. doi: 10.1016/s0022-3956(00)00038-8. [DOI] [PubMed] [Google Scholar]
- 29.Trachsel L, Dijk DJ, Brunner DP, Klene C, Borbély AA. Effect of zopiclone and midazolam on sleep and EEG spectra in a phase-advanced sleep schedule. Neuropsychopharmacology. 1990;3:11–8. [PubMed] [Google Scholar]
- 30.Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79:2265–74. doi: 10.1212/WNL.0b013e31827688ee. [DOI] [PubMed] [Google Scholar]
- 31.Grady SP, Nishino S, Czeisler CA, Hepner D, Scammell TE. Diurnal variation in CSF orexin-A in healthy male subjects. Sleep. 2006;29:295–7. doi: 10.1093/sleep/29.3.295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.