Background: The bc1 complex catalyzes electron transfer from ubiquinol to cytochrome c and translocates protons across the membrane.
Results: Substitution of Arg-94 decreased the electron transfer activity and proton pumping capability and increased the superoxide production.
Conclusion: Arg-94 is an important amino acid for the proton pumping capability of the bc1 complex.
Significance: The result is significant for understanding the catalytic mechanism of the bc1 complex.
Keywords: Cytochrome b, Mutant, Protein Chemistry, Proton Pumps, Superoxide Ion, bc1 Complex
Abstract
Proton transfer involving internal water molecules that provide hydrogen bonds and facilitate proton diffusion has been identified in some membrane proteins. Arg-94 in cytochrome b of the Rhodobacter sphaeroides bc1 complex is fully conserved and is hydrogen-bonded to the heme propionate and a chain of water molecules. To further elucidate the role of Arg-94, we generated the mutations R94A, R94D, and R94N. The wild-type and mutant bc1 complexes were purified and then characterized. The results show that substitution of Arg-94 decreased electron transfer activity and proton pumping capability and increased O2˙̄ production, suggesting the importance of Arg-94 in the catalytic mechanism of the bc1 complex in R. sphaeroides. This also suggests that the transport of H+, O2, and O2˙̄ in the bc1 complex may occur by the same pathway.
Introduction
The cytochrome bc1 complex (also known as ubiquinol-cytochrome c oxidoreductase or complex III) is a multisubunit dimeric integral membrane protein complex. It is an essential segment of cellular energy-conserving electron transport chains in animals, plants, and bacteria (1, 2). This complex catalyzes electron transfer from ubiquinol to cytochrome c (or cytochrome c2 in bacteria) and concomitantly translocates protons across the membrane to generate a membrane potential and pH gradient for ATP synthesis. The cytochrome bc1 complexes from all species contain three core subunits, cytochrome b, cytochrome c1, and the Rieske iron-sulfur protein, which house two b-type cytochromes (bH and bL), one c-type cytochrome (c1), and a high potential Rieske [2Fe-2S] cluster, respectively. Depending on the source, the cytochrome bc1 complexes contain various numbers (zero to eight) of non-redox group-containing subunits, also called supernumerary subunits (3–5).
Intensive biochemical and biophysical studies on this complex (6–8) have led to the formulation of the “protonmotive Q-cycle” mechanism for electron and proton transfer in this complex (9–11). In the Q-cycle mechanism, it is postulated that the first electron of ubiquinol is transferred to the “high potential chain,” consisting of the Rieske iron-sulfur protein and cytochrome c1. Then, the second electron of ubiquinol, via a transient semiquinone, is passed through the “low potential chain,” consisting of cytochromes bL and bH, to reduce ubiquinone or ubisemiquinone bound at the ubiquinol reduction site (QN). One drawback of this sequential scheme is the lack of a “functional” semiquinone at the ubiquinol oxidation site (QP) (12–14), even though some radicals have been reported under abnormal conditions (15, 16). Recently, pre-steady-state kinetic analysis of the reduction of cytochrome bL and the Rieske iron-sulfur protein in the same sample using fast quenching coupled with EPR (17) indicated that both the iron-sulfur cluster and heme bL are reduced by ubiquinol at the same rate, suggesting a concerted scheme for the bifurcated oxidation of ubiquinol at the QP site (18, 19).
It has been established that for every electron transferred through the bc1 complex, two protons are translocated across the membrane. This 2H+/e− stoichiometry has been verified in a wide variety of species through in vitro studies of bc1 complexes in phospholipid (PL)2 vesicles.
Arg-79 in cytochrome b of the yeast bc1 complex is hydrogen-bonded to the heme propionate and provides a proton exit pathway to the bulk solvent mediated by a chain of hydrogen-bonded water molecules. Arg-79 is fully conserved in cytochrome b, indicating its importance for the catalytic mechanism (20). Arg-94 in cytochrome b of Rhodobacter sphaeroides corresponds to Arg-79 in yeast. To further elucidate the role of this important amino acid in cytochrome b of R. sphaeroides, we report herein detailed procedures for generating mutations of Arg-94 in cytochrome b and characterize the electron transfer activity, proton pumping capability, pre-steady-state reduction kinetics of hemes (b and c1), and superoxide anion production in purified bc1 complexes. We found that substitution of Arg-94 decreased electron transfer activity and proton pumping capability and increased superoxide anion production. The transport of H+, O2, and O2˙̄ may follow the same pathway.
EXPERIMENTAL PROCEDURES
Materials
Cytochrome c (horse heart, type III), stigmatellin, antimycin A, carbonyl cyanide m-chlorophenylhydrazone (CCCP), and valinomycin were purchased from Sigma. 8-Hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (pyranine) was from Acros Organics. Asolectin was obtained from Associated Concentrates and purified according to the method of Kagawa and Racker (21). n-Dodecyl-β-d-maltopyranoside and n-octyl-β-d-glucopyranoside were from Anatrace. Nickel-nitrilotriacetic acid gel, a QIAprep spin miniprep kit, and a PCR purification kit were obtained from Qiagen. 2-Methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo[1,2-α]pyrazin-3-onehydrochloride (MCLA) was from Molecular Probes. 2,3-Dimethoxy-5-methyl-6-(10-bromodecyl)-1,4-benzoquinol (Q0C10BrH2) was prepared in our laboratory as reported previously (22). All other chemicals were of the highest purity commercially available.
Generation of R. sphaeroides Cytochrome bc1 Mutants
Mutations were constructed using the QuikChange site-directed mutagenesis kit (Stratagene) with supercoiled double-stranded pGEM7Zf(+)-fbcB as a template. Forward and reverse primers were used for PCR amplification (Table 1). The pGEM7Zf(+)-fbcB plasmid (23) was constructed by ligating the NsiI-XbaI fragment from pRKD418-fbcFBC6HQ into the NsiI and XbaI sites of the pGEM7Zf(+) plasmid. The NsiI-XbaI fragment from the pGEM7Zf(+)-fbcBm plasmid was ligated into the NsiI and XbaI sites of the pRKD418-fbcFBKmC6HQ plasmid to generate the pRKD418-fbcFBmC6HQ plasmid. A plate-mating procedure (24) was used to mobilize the pRKD418-fbcFBmC6HQ plasmid in Escherichia coli S17 cells into R. sphaeroides BC17 cells. The presence of the engineered mutations was confirmed by DNA sequencing before and after semi-aerobic growth of the cells. The expression plasmid pRKDfbcFmBCHQ was purified from an aliquot of semi-aerobically grown culture using the Qiagen plasmid miniprep kit. Because R. sphaeroides cells contain four types of endogenous plasmids, the isolated plasmids lacked the purity and concentration needed for direct sequencing. Therefore, a 2-kilobase pair DNA segment containing the mutation sequence was amplified from the isolated plasmids by PCR. The PCR products were purified with an extraction kit from Sigma and then sequenced. DNA primers were purchased from Invitrogen. DNA sequencing was carried out at the Recombinant DNA/Protein Core Facility of Oklahoma State University.
TABLE 1.
Oligonucleotides used for site-directed mutagenesis
The underlined bases correspond to the genetic codes for the amino acid(s) to be mutated.
| Primer | Sequence |
|---|---|
| R94A | |
| Forward | GCTTCATGCTGGCCTACCTGCATGCG |
| Reverse | CGCATGCAGGTAGGCCAGCATGAAGC |
| R94N | |
| Forward | GCTTCATGCTGAACTACCTGCATGCG |
| Reverse | CGCATGCAGGTAGTTCAGCATGAAGC |
| R94D | |
| Forward | GCTTCATGCTGGACTACCTGCATGCG |
| Reverse | CGCATGCAGGTAGTCCAGCATGAAGC |
Growth of Bacteria
E. coli cells were grown at 37 °C in LB medium. R. sphaeroides BC17 cells (24) were grown photosynthetically at 30 °C in enriched Siström's medium containing 5 mm glutamate and 0.2% casamino acids. Photosynthetic growth conditions for R. sphaeroides were essential as described previously (25). The concentrations and antibiotics used were as follows: ampicillin, 125 μg/ml; kanamycin sulfate, 30 μg/ml; tetracycline, 10 μg/ml for E. coli and 1 μg/ml for R. sphaeroides; and trimethoprim, 100 μg/ml for E. coli and 30 μg/ml for R. sphaeroides.
Enzyme Preparations and Specific Electron Transfer Activity Assay
Chromatophore membranes were prepared from frozen cell paste, and cytochrome bc1 complexes with a His6 tag placed at the C terminus of cytochrome c1 were purified from chromatophores as described previously (25) and stored at −80 °C in the presence of 10% glycerol. Protein concentrations were determined by absorbance at 280 nm using a converting factor of 1 A280 = 0.56 mg/ml. The concentrations of cytochromes b and c1 were determined spectrophotometrically using published molar extinction coefficients (26–28).
To assay ubiquinol-cytochrome c reductase activity, purified cytochrome bc1 complexes were diluted with 50 mm Tris-Cl (pH 8.0) containing 200 mm NaCl and 0.01% n-dodecyl-β-d-maltopyranoside to a final cytochrome b concentration of 1 μm unless specified otherwise. Appropriate amounts of the diluted samples were added to 1 ml of assay mixture containing 100 mm Na+/K+ phosphate buffer (pH 7.4), 0.3 mm EDTA, 100 μm cytochrome c, and 25 μm Q0C10BrH2. The specific electron transfer activities were determined by measuring the reduction of cytochrome c (the increase in the absorbance at a wavelength of 550 nm) with a Shimadzu UV-2401 PC spectrophotometer at 23 °C using a millimolar extinction coefficient of 18.5 for calculation. The non-enzymatic oxidation of Q0C10BrH2, determined under the same conditions in the absence of enzyme, was subtracted during specific activity calculations. Although the chemical properties of Q0C10BrH2 are comparable with those of Q0C10H2, the former is a better substrate for the cytochrome bc1 complex (22).
Preparation of Electron Transfer Complex-inlaid PL Vesicles
Protein-PL vesicles were prepared by the cholate dialysis method of Kagawa and Racker (21). The wild-type or mutant bc1 complex was mixed with 1 ml of asolectin micellar solution to give an asolectin (milligrams)/protein (milligrams) ratio of 40. The asolectin micellar solution was prepared by sonicating 200 mg of acetone-washed asolectin in 4 ml of 50 mm sodium phosphate buffer (pH 7.4) containing 2% sodium cholate and 100 mm KCl in an ice-water bath. Sonication was performed in an anaerobic environment by continually passing argon into the vessel. The bc1 complex/PL mixtures were incubated at 0 °C for 30 min before overnight dialysis at 4 °C against 100 volumes of 50 mm sodium phosphate buffer (pH 7.4) containing 100 mm KCl with three changes of buffer. The mixture was then dialyzed against 100 volumes of 150 mm KCl for 3–4 h.
Determination of Proton Translocation Activity of bc1 Complex-PL Vesicles
Proton translocation coupled to electron flow through the bc1 complex-PL vesicles was measured at room temperature using an Accumet Model 10 pH meter and Model 13-620-96 combination pH electrode. 25 nmol of Q0C10BrH2 was added to the 1.6-ml reaction mixture containing 150 mm KCl, 4 μm ferricytochrome c, 1 μm valinomycin, and an appropriate amount of bc1 complex-PL vesicles (30–50 μl). Electron flow was initiated by the addition of 5 nmol of ferricyanide, which oxidizes the cytochrome c and thus provides an electron acceptor for the complex. Electron flow under conditions in which no transmembrane ΔpH formed was measured in an identical manner except that the protonophore CCCP was present at a concentration of 2 μm to make the vesicles permeable to protons. Proton pumping (H+/e−) was calculated as the ratio of the decrease in pH upon ferricyanide addition to bc1 complex-PL vesicles before and after treatment with CCCP.
Fast Kinetics Study
Measurements were performed in an Applied Photophysics SX.18MV stopped-flow spectrometer with a photodiode array scan between 600 and 500 nm. The reaction was started by mixing equal volumes of solutions A and B at room temperature. For the experiment to determine electron transfer rates between ubiquinol and heme b or c1, solution A contained 50 mm Tris-Cl (pH 8.0) at 4 °C, 200 mm NaCl, 0.01% n-dodecyl-β-d-maltopyranoside, and 12.0 μm cytochrome bc1 complex (based on cytochrome c1). Solution B was the same as solution A except that the bc1 complex was replaced with 240 μm Q0C10BrH2. Reductions of cytochromes b and c1 were determined from the absorption changes at 560–580 nm and at 551–539 nm, respectively. When an inhibitor was used, the cytochrome bc1 complex was treated with a 5-fold molar excess of inhibitor over heme c1 for 5 min at 4 °C prior to the experiment.
Because the concentration of ubiquinol used was much higher than that of the cytochrome bc1 complex, the reactions between bc1 and ubiquinol were treated as pseudo first-order reactions. Time traces of the reaction were fitted using a first-order rate equation to obtain the pseudo first-order rate constants (k1) by KaleidaGraph.
For the experiment to determine proton production rates, solution A contained 150 mm KCl, 30 μm Q0C10BrH2, 2 μm valinomycin, 100 μm pyranine, and bc1 complex-PL vesicles (35 μl/ml of solution A). Solution B contained 150 mm KCl and 8 μm ferricytochrome c. Solutions A and B were both adjusted carefully to pH 7.0 with 2 mm KOH. Proton pumping was determined from the absorption changes at 457 nm.
Measurement of Superoxide Anion Generation
Superoxide anion generation by the cytochrome bc1 complex was determined by measuring the chemiluminescence of the MCLA-O2˙̄ adduct (29) in an Applied Photophysics SX.18MV stopped-flow spectrometer by leaving the excitation light off and registering the light emission (30). Reactions were carried out at 23 °C by mixing solutions A and B at 1:1. Solution A contained 100 mm Na+/K+ phosphate buffer (pH 7.4), 1 mm EDTA, 1 mm KCN, 1 mm NaN3, 0.1% bovine serum albumin, 0.01% n-dodecyl-β-d-maltopyranoside, and 5.0 μm wild-type or mutant bc1 complex. Solution B was the same as solution A except that the bc1 complex was replaced with 150 μm Q0C10BrH2 and 4 μm MCLA.
RESULTS AND DISCUSSION
Photosynthetic Growth Behaviors of the Wild-type and Mutant bc1 Complexes
Because the cytochrome bc1 complex is absolutely necessary for photosynthetic growth of R. sphaeroides, a mutant with a substitution at a critical position will not grow photosynthetically, whereas mutants with substitutions at noncritical positions will grow. Thus, observing the photosynthetic growth behavior, one can determine whether substitutions are at critical positions. In mid-log phase, semi-aerobically dark-grown wild-type and mutant cells were inoculated into enriched Siström's medium and subjected to anaerobic photosynthetic growth conditions. Table 2 shows that mutants could grow, indicating that substitutions of Arg-94 are noncritical to the complex. These mutants grew at a decreased rate compared with the wild-type bc1 complex, suggesting that substitutions of Arg-94 affect the function of the cytochrome bc1 complex and thus retard the growth of R. sphaeroides.
TABLE 2.
Characterization of mutants
The cytochrome bc1 complexes were in 50 mm Tris-Cl (pH 8.0) containing 200 mm NaCl, 200 mm histidine, 0.5% octyl glucoside, and 10% glycerol.
| Strain | Photosynthetic growth | Activitya |
|---|---|---|
| WT | ++++ | 4.58 (100%) |
| R94A | + | 0.18 (4%) |
| R94D | ++ | 1.61 (35%) |
| R94N | +++ | 2.34 (51%) |
a The enzyme-specific electron transfer activity of the purified bc1 complexes is expressed as micromoles of cytochrome c reduced per min/nmol of cytochrome b at room temperature.
Proton Translocation Activity of the Wild-type and Mutant bc1 Complexes
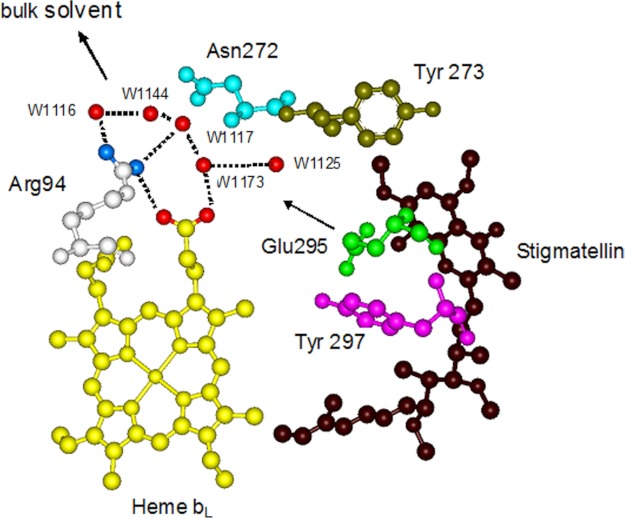
The bc1 complex catalyzes electron transfer from ubiquinol to cytochrome c and concomitantly translocates protons of ubiquinol across the membrane. The two protons of ubiquinol are released via two pathways. The ejection of the first proton is controlled by the protonation and deprotonation of the histidine ligands of the [2Fe-2S] cluster. The histidine ligands take up a proton from the substrate, ubiquinol, upon reduction of the [2Fe-2S] cluster and release it to the intermembrane space when oxidized by cytochrome c1, as was suggested recently (31). Glu-295 in cytochrome b is important for the release of the second proton, as proposed previously (31–33). Glu-295 is completely conserved in mitochondrial cytochrome b (34), and the importance of the residue in proton transfer is indicated by mutagenesis studies because alteration of glutamine abolishes ubiquinol oxidation in R. sphaeroides (35). Additionally, recent kinetic studies showed that protonation of a group with a pKa of 5.7 blocked catalysis, and this effect was attributed to Glu-295 (36). The second proton of ubiquinol is first transferred to Glu-295 to form the neutral acid and is then released and delivered to heme propionate A by rotation of the side chain of Glu-295. The subsequent proton release is mediated by a hydrogen-bonded water chain stabilized by cytochrome b residues (Fig. 1) (20, 31).
FIGURE 1.
Proton exit pathway formed by a chain of hydrogen-bonded water molecules at the QP site with the stigmatellin bound. Blue dots on Arg-94 indicate the NH1 and NH2 groups. Red dots on heme bL indicate O1A and O2A. Other red dots indicate water (W) molecules. The arrow shows the proton exit pathway from Glu-295 to bulk solvent.
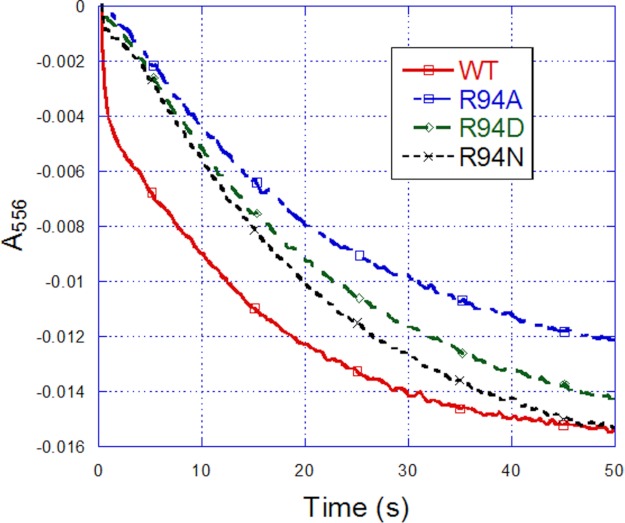
The proton pumping property has been well demonstrated in PL vesicles embedded with the purified mitochondrial bc1 complex (37–40). Fig. 2 shows a typical proton translocation activity assay for PL vesicles embedded with the R. sphaeroides bc1 complex. After pH equilibrium was reached for the mixture containing Q0C10BrH2, valinomycin, ferricytochrome c, and bc1 complex-PL vesicles, an aliquot of ferricyanide solution (arrow 1) was added to initiate electron flow from Q0C10BrH2 to cytochrome c. After pH equilibrium was attained, the protonophore CCCP was added (arrow 2) to render the liposome membrane freely permeable to protons, and then a second aliquot of ferricyanide was added (arrow 3). The ratio of the pH changes produced by the addition of equal amounts of ferricyanide before (x) and after (y) the addition of CCCP was taken as a measure of the H+/e− ratio for the proton translocation activity of the bc1 complex. The protons released after CCCP addition were the “scalar” protons only, as no contribution from the accumulation of “vectorially” translocated protons was possible.
FIGURE 2.
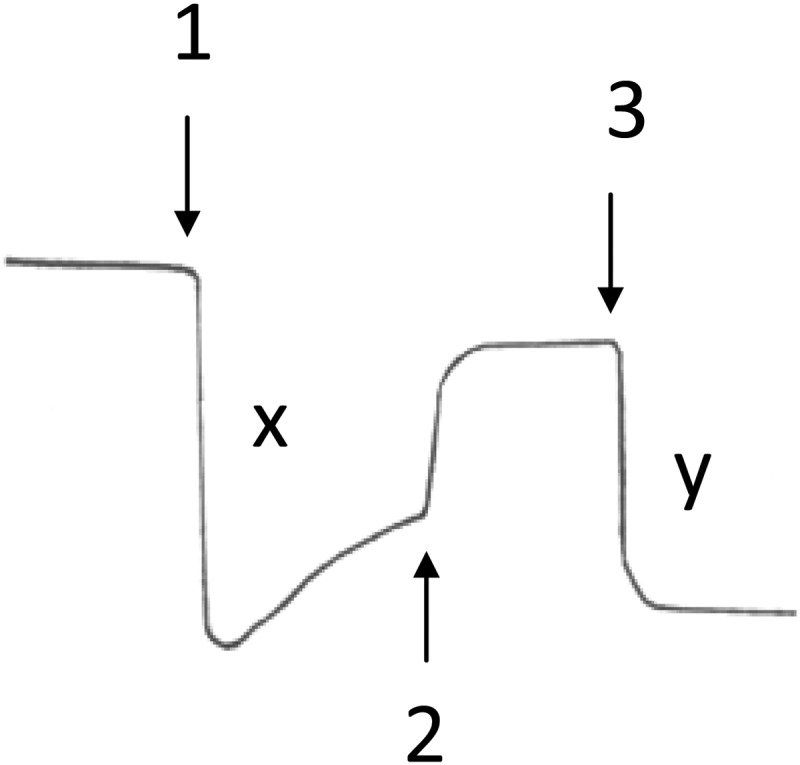
Proton pumping of R. sphaeroides cytochrome bc1 complexes embedded in PL vesicles. Arrows indicate the points of addition of 5 nmol of ferricyanide (arrow 1), 2 μm CCCP (arrow 2), and 5 nmol of ferricyanide (arrow 3). Note that the proton pumping ratio (H+/e−) = x/y.
Table 3 shows the proton translocation activity (H+/e−) of the wild-type and mutant bc1 complexes. The proton translocation activity of R94N showed a small decrease compared with the wild-type bc1 complex, whereas that of R94A and R94D exhibited a significant decrease. H+/e− reflects only the final ratio of proton translocation to electron transfer. To further study the proton translocation rate, a kind of dye, pyranine, was used in a stopped-flow experiment. Fig. 3 shows that the magnitude of proton translocation rate of the wild-type and mutant bc1 complexes can be ranked as WT > R94N > R94D > R94A. These results indicate that a positive correlation exists between the changes in proton translocation activity (H+/e−) and the proton translocation rate.
TABLE 3.
Proton pumping ratios (H+/e−) for PL vesicles harboring wild-type and mutant bc1 complexes
For the definition of H+/e− and experimental conditions, see “Experimental Procedures” and the legend to Fig. 2.
| Vesicle type | H+/e− |
|---|---|
| WT | 1.5 |
| R94A | 1.2 |
| R94D | 1.23 |
| R94N | 1.48 |
FIGURE 3.
Determination of proton translocation rate of PL vesicles embedded with wild-type or mutant bc1 complexes using a stopped-flow spectrophotometer. Reaction conditions were as described under “Experimental Procedures.” pH changes were monitored by following the absorption change of pyranine at 457 nm.
Proton transfer pathways involving internal water molecules that provide hydrogen bonds and facilitate proton diffusion have been identified in other membrane proteins such as bacteriorhodopsin (41) and cytochrome c oxidase (42). Although the mechanisms for proton translocations are different for different proteins, the underlying principle for bringing protons to the active sites is similar. For Arg-94, the nitrogen atom of the guanidyl easily forms a hydrogen bond with H2O, and the repulsion function of the positive charge to H+ is also favorable for H+ movement. For Ala, the carbon atom of the methyl does not easily form a hydrogen bond with H2O, and there is no positive charge on its side chain. In addition, as a hydrophobic amino acid, the hydrophobic microenvironment is also unfavorable for H+ movement. Thus, the proton translocation activity of the R94A mutant is inhibited. The structure of Asn is similar to that of Arg except for a small difference in molecular weight, so the proton translocation activity of the R94N mutant is similar to that of the wild type. The low proton translocation activity of R94D may be due to the negative charge of the side chain of Asp exerting an attractive force to H+, which is unfavorable for H+ movement. The low proton translocation activity of the mutants indicates that some of the “second H+” of Q0C10BrH2 cannot be pumped into the exterior of the vesicle and also suggests that Arg-94 in cytochrome b of the bc1 complex is an important amino acid for proton pumping.
Effect of Mutations on Cytochrome b Reduction by Ubiquinol
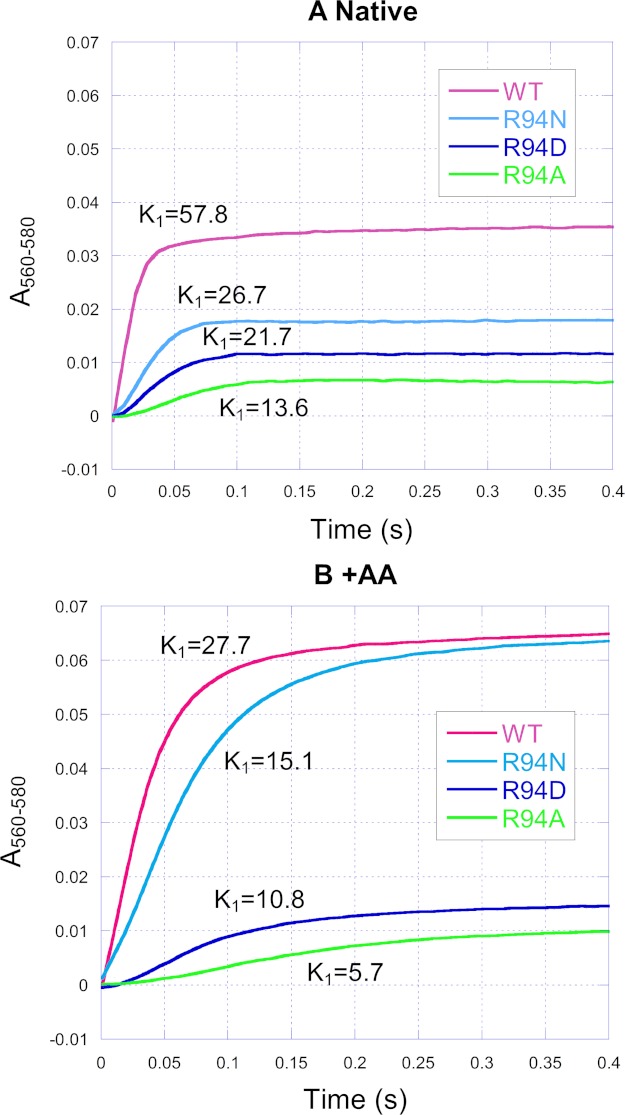
Fig. 4 shows the time course traces of heme b reduction by Q0C10BrH2 in the wild type and mutants in the absence (panel A) and presence (panel B) of antimycin A. Antimycin A is a QN site inhibitor that blocks electron transfer from heme bH to ubiquinone and prevents reduction of heme bH by Q0C10BrH2 through the QN site.
FIGURE 4.
Time traces of cytochrome b reduction by Q0C10BrH2 in the wild-type and mutant complexes in the absence (A) and presence (B) of antimycin A. Reactions and measurements were performed as described under “Experimental Procedures.” The reaction was monitored by photodiode array scanning for 0.4 s. Cytochrome b was determined from the increase in A560–580. AA, antimycin A.
In the case of the native complex (Fig. 4A), the extent of heme b reduction of mutants decreased relative to the wild type. It should be noted that heme b reduction is mostly heme bH (43). The heme bH reduction by ubiquinol in the bc1 complex, according to the Q-cycle mechanism, is affected by (i) the forward reduction through the QP site to heme bL and then to bH, (ii) the reoxidation of reduced heme bH by ubiquinone, and (iii) the “back door” reduction through the QN site. Therefore, it is difficult to explain the differences in the extent of heme b reduction among the wild type and mutants.
In the presence of antimycin A (Fig. 4B), heme b reduction by Q0C10BrH2 occurred only through the QP site via heme bL, so this case is simplified. Relative to the wild type, the heme b reduction extent of R94N, R94D, and R94A decreased by 2.4, 77.71, and 84.93%, respectively. This decrease implies that some electrons may deviate from the low potential electron transfer chain and leak from the reduced heme bL or ubisemiquinone at the QP site during bc1 catalysis. The results also indicate that the extent of the electron leak is WT < R94N < R94D < R94A.
Whether in the absence or presence of antimycin A, the rate constants of heme b reduction of the mutants all decreased relative to the wild type, and the extent of the decrease can be described as R94N < R94D < R94A (Fig. 4). The decrease in the rate constants of heme b reduction suggests that the transfer rate of the second electron of Q0C10BrH2 is decreased for mutants.
The addition of antimycin A (Fig. 4) increased the extent and decreased the rate of heme b reduction in the wild type and mutants, and these results are consistent with a previous report (44). However, the degree of this effect varied in these complexes. Relative to the native case (Fig. 4A), the rate of heme b reduction of the wild type, R94N, R94D, and R94A was decreased by 52, 43, 50, and 58%, respectively. Relative to the native case (Fig. 4A), the extent of heme b reduction of the wild type, R94N, R94D, and R94A was increased by 83, 255, 25, and 56%, respectively.
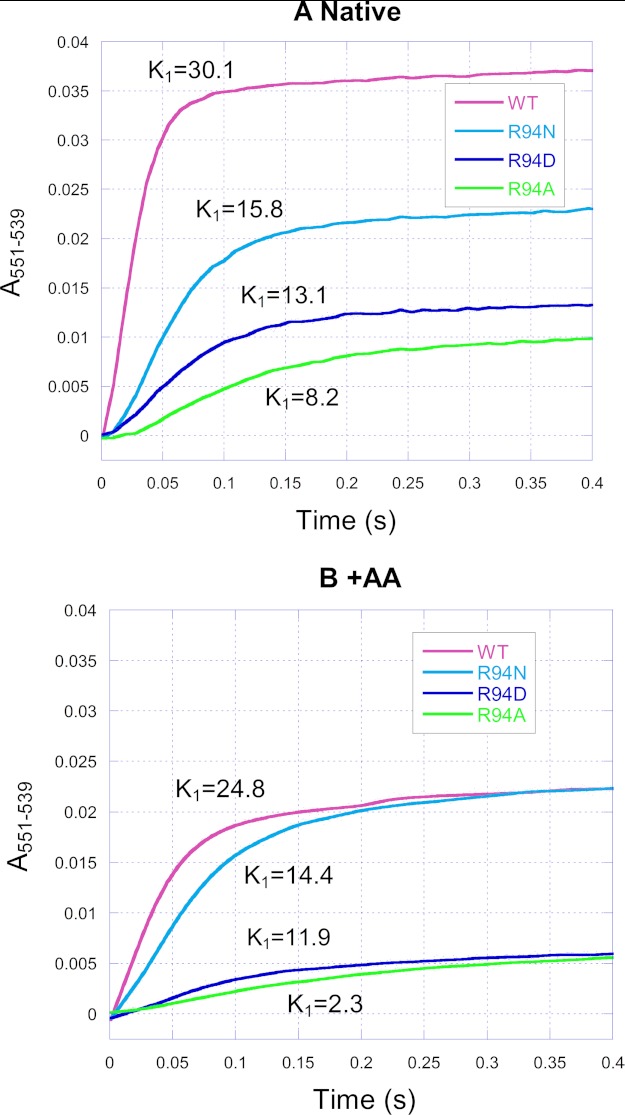
Effect of Mutations on Cytochrome c1 Reduction by Ubiquinol
Fig. 5 shows the time course traces of heme c1 reduction by Q0C10BrH2 in the wild type and mutants in the absence (panel A) and presence (panel B) of antimycin A. In both the absence and presence of antimycin A, the rate constants of heme c1 reduction of all mutants decreased relative to the wild type, and the extent of the decrease is R94N < R94D < R94A. The decrease in the rate constants of heme c1 reduction suggests that the transfer rate of the first electron of Q0C10BrH2 is decreased for mutants.
FIGURE 5.
Time traces of cytochrome c1 reduction by Q0C10BrH2 in the wild-type and mutant complexes in the absence (A) and presence (B) of antimycin A. Reactions and measurements were performed as described under “Experimental Procedures.” The reaction was monitored by photodiode array scanning for 0.4 s. Cytochrome c1 was determined from the increase in A551–539. AA, antimycin A.
Relative to the values for the native case, the addition of antimycin A decreased the rate of heme c1 reduction by 18, 9, 9, and 73% in the wild type, R94N, R94D, and R94A, respectively (Fig. 5). These results are consistent with previous reports (18, 43) that antimycin A has a significant effect on the reduction rate of heme c1 in cytochrome bc1 complexes. This inhibitive effect has been attributed to the long-range effect of antimycin A on the QP site when binding to the QN site (18, 43). In other words, the effect of antimycin A on the heme c1 reduction rate does not occur through the low potential redox component but through the cytochrome b protein subunit.
In the Q-cycle mechanism, the oxidation of ubiquinol and reduction of ubiquinone or ubisemiquinone occur mainly in the QP and QN sites of cytochrome b. In the mutant bc1 complexes, the QP and QN sites are functional because all of the mutant complexes were sensitive to antimycin A (Figs. 4B and 5B) and stigmatellin (a QP site inhibitor) (data not shown). The changes in electron transfer activity or the changes in heme b and c1 reduction should all be related to the mutation at Arg-94, but they are not related to the impairment of the QP or QN site. Substitution of Arg-94 decreased the proton translocation rate, slowed down the decomposition rate of Q0C10BrH2 by feedback inhibition, and then slowed down the transfer of the two electrons of ubiquinol. A decrease in the first electron transfer induced the heme c1 reduction rate decrease and the electron transfer activity decrease (Table 2). A decrease in the second electron transfer induced the heme b reduction rate decrease (Fig. 4).
The decreases in the proton translocation rate, the heme b and c1 reduction rate, and the electron transfer activity all changed in the same order of R94N < R94D < R94A for the three mutants. This positive correlation also supports the relationship discussed above. In addition, the decreases in the heme b reduction rate and the electron transfer activity are also related to the production of superoxide anion, as discussed below.
Effect of Mutations on Superoxide Anion Production by the Cytochrome bc1 Complex
The above results show that some electrons may leak from the low potential electron transfer chain during bc1 catalysis for mutants. What is the fate of these leaked electrons? One of the possible pathways is a reaction with molecular oxygen to form superoxide anions. If this is the pathway, one should see more superoxide anion production by mutants than the wild-type complex because more electrons leak from the mutant complexes.
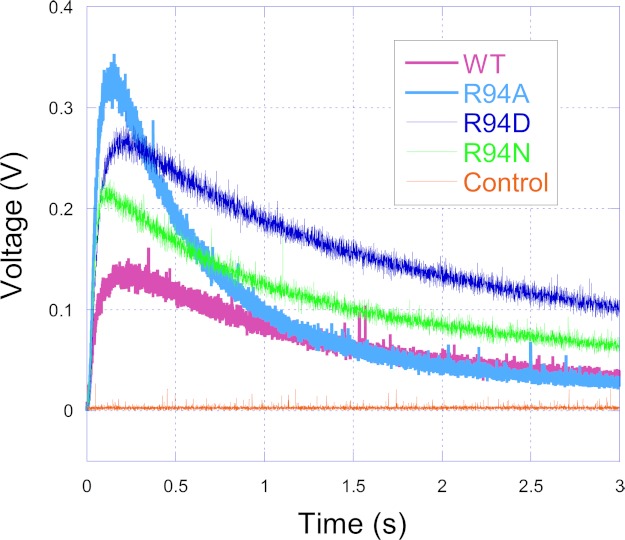
Fig. 6 shows the tracings of O2˙̄ generation by the wild type and mutants. MCLA-O2˙̄ chemiluminescence induced by the bc1 complex reached peak intensity after ∼0.1 s at room temperature and then decayed. Relative to the wild type, O2˙̄ generation increased by 18, 36, and 61% for R94N, R94D, and R94A, respectively. No luminescence was detected when the bc1 complex was omitted from the enzyme-containing solution. This result suggests that some electrons do leak from the low potential electron transfer chain to O2 and then form O2˙̄ during bc1 catalysis. The result also indicates that the extent of the electron leak is WT < R94N < R94D < R94A, which is the same as the result from the heme b reduction analysis above. There is an inverse relationship between O2˙̄ production and the heme b reduction rate (Figs. 4 and 6). The heme b reduction rate indicates the second electron transfer rate. It can therefore be deduced that another reason for the decreased second electron transfer rate in the hydrophobic QP pocket for mutants is the increased O2˙̄ production. More superoxide anions released means a lower electron transfer rate.
FIGURE 6.
Time traces of superoxide anion generation. The superoxide anion generation reactions were carried out at 23 °C in an Applied Photophysics SX.18MV stopped-flow reaction analyzer by mixing solutions A and B (1:1) containing enzyme complexes and substrate as described under “Experimental Procedures.” The orange trace is for control experiments, when no bc1 complexes were present in the system.
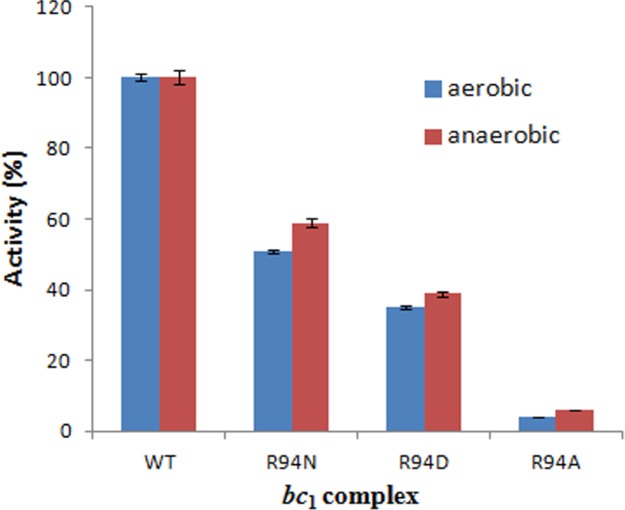
In addition, the generation of more O2˙̄ implies that more electrons are consumed by O2 in the bc1 complex, which would result in decreased electron transfer activity. Thus, the decreased activity of mutants is ascribed to the increased O2˙̄ generation as well as to the decreased proton pumping (discussed above). Under anaerobic conditions, there is no O2˙̄ generation. Thus, one can deduce that the difference between activities in a mutant and the wild type under anaerobic conditions should be less than that under aerobic conditions. This is indeed the case. Fig. 7 shows that, compared with the wild type, the electron transfer activities of R94N, R94D, and R94A decreased by 49, 65, and 96%, respectively, under aerobic conditions, whereas they decreased by 41, 61, and 94%, respectively, under anaerobic conditions. The difference is significant (p < 0.05, Student's t test) between aerobic and anaerobic conditions for every mutant complex. These data also show that the effect of the superoxide anion generation on the decrease in the electron transfer activity of mutants is R94N > R94D > R94A, and the superoxide anion generation has a smaller effect on the decrease in the electron transfer activity of mutants compared with the proton pumping decrease.
FIGURE 7.
Electron transfer activity changes in the wild-type and mutant complexes under aerobic and anaerobic conditions. The activity of the wild type is regarded as 100%, and that of mutants is described as the percentage of the wild type. Data represent an average of three experiments. Error bars indicate S.D. The difference is significant (p < 0.05, Student's t test) between aerobic and anaerobic conditions for every mutant complex.
Transport Pathway of H+, O2, and O2˙̄ in the bc1 Complex
It is known that O2˙̄ is generated inside the bc1 complex, perhaps in the hydrophobic environment of the QP pocket through bifurcated oxidation of ubiquinol by transfer of its two electrons to a high potential electron acceptor, an iron-sulfur cluster, and a low potential heme bL or molecular oxygen. The protein subunits, at least those surrounding the QP pocket, play a role either in preventing the release of O2˙̄ from its production site to aqueous environments or in preventing O2 from getting access to the hydrophobic QP pocket (45). The increase in superoxide anion production by mutants (Fig. 6) may result from the rapid transport of O2 and O2˙̄ between the hydrophobic QP pocket and the aqueous phase because the O2 concentrations in the aqueous phase of the wild type and mutants are the same.
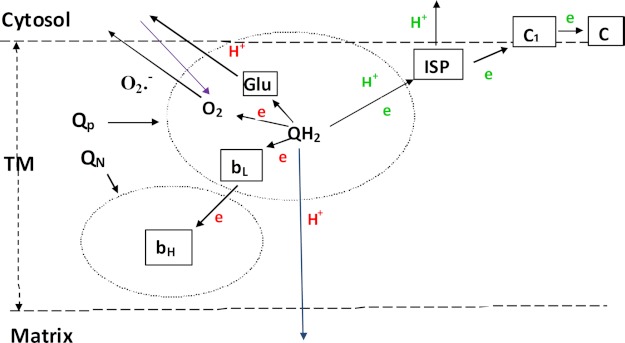
Substitution of Arg-94 changes the proton pumping and O2˙̄ generation simultaneously. This suggests that the transport of H+, O2, and O2˙̄ may occur by the same pathway as described in Figs. 1 and 8. Arg-94 of cytochrome b may function as a gate for the transport of H+, O2, and O2˙̄. However, the factors that influence the transport of H+, O2, and O2˙̄ may be different. H+ transport is related mainly to the hydrogen bond and charge as described above, whereas that of O2 and O2˙̄ may be affected mainly by the side chain size of Arg-94 or its substitute. The long side chain, e.g. that of Arg-94 in the wild type, is unfavorable for the movement of O2 and O2˙̄, and this results in low O2˙̄ production. However, a short side chain, e.g. that of Ala-94, is favorable for the movement of O2 and results in high O2˙̄ production. In addition, as a hydrophobic amino acid of Ala, the hydrophobic environment is also favorable to the solubility of O2 and generation of O2˙̄ (45). More O2˙̄ production by R94D than R94N may be related to the negative charge, which has a repellent force to O2˙̄, providing a favorable condition for O2˙̄ movement. In this study, only one residue was mutated and studied, so this suggestion remains to be further investigated.
FIGURE 8.
Schematic depiction of the transfer of the two electrons and two protons of ubiquinol at the QP pocket when it is oxidized in the bc1 complex. The first electron and proton are in green, and the second electron and proton are in red. The blue arrow indicates protons that return to the matrix for mutants; the purple arrow indicates oxygen molecules that are transferred to the QP quinol oxidation pocket from the cytosol solvent. QN is the quinone reduction pocket. QH2, ubiquinol; TM, transmembrane region; ISP, Rieske iron-sulfur protein.
This work was supported, in whole or in part, by National Institutes of Health Grant GM30721 (to C.-A. Y.). This work was also supported by National Science Foundation Grant MCB0077650 (to L. Y.) and Oklahoma Agricultural Experiment Station Projects 1819 and 2372 from Oklahoma State University.
- PL
- phospholipid
- CCCP
- carbonyl cyanide m-chlorophenylhydrazone
- MCLA
- 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo[1,2-α]pyrazin-3-one hydrochloride
- Q0C10BrH2
- 2,3-dimethoxy-5-methyl-6-(10-bromodecyl)-1,4-benzoquinol.
REFERENCES
- 1. Bartoschek S., Johansson M., Geierstanger B. H., Okun J. G., Lancaster C. R., Humpfer E., Yu L., Yu C.-A., Griesinger C., Brandt U. (2001) Three molecules of ubiquinone bind specifically to mitochondrial cytochrome bc1 complex. J. Biol. Chem. 276, 35231–35234 [DOI] [PubMed] [Google Scholar]
- 2. Trumpower B. L., Gennis R. B. (1994) Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu. Rev. Biochem. 63, 675–716 [DOI] [PubMed] [Google Scholar]
- 3. Xia D., Yu C.-A., Kim H., Xia J. Z., Kachurin A. M., Zhang L., Yu L., Deisenhofer J. (1997) Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science 277, 60–66 [DOI] [PubMed] [Google Scholar]
- 4. Iwata S., Lee J. W., Okada K., Lee J. K., Iwata M., Rasmussen B., Link T. A., Ramaswamy S., Jap B. K. (1998) Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science 281, 64–71 [DOI] [PubMed] [Google Scholar]
- 5. Zhang Z., Huang L., Shulmeister V. M., Chi Y. I., Kim K. K., Hung L. W., Crofts A. R., Berry E. A., Kim S. H. (1998) Electron transfer by domain movement in cytochrome bc1. Nature 392, 677–684 [DOI] [PubMed] [Google Scholar]
- 6. Erecińska M., Chance B., Wilson D. F., Dutton P. L. (1972) Aerobic reduction of cytochrome b566 in pigeon-heart mitochondria. Proc. Natl. Acad. Sci. U.S.A. 69, 50–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wikström M. K., Berden J. A. (1972) Oxidoreduction of cytochrome b in the presence of antimycin. Biochim. Biophys. Acta 283, 403–420 [DOI] [PubMed] [Google Scholar]
- 8. Alexandre A., Lehninger A. L. (1979) Stoichiometry of H+ translocation coupled to electron flow from succinate to cytochrome c in mitochondria. J. Biol. Chem. 254, 11555–11560 [PubMed] [Google Scholar]
- 9. Mitchell P. (1976) Possible molecular mechanisms of the protonmotive function of cytochrome systems. J. Theor. Biol. 62, 327–367 [DOI] [PubMed] [Google Scholar]
- 10. Brandt U., Trumpower B. (1994) The protonmotive Q cycle in mitochondria and bacteria. Crit. Rev. Biochem. Mol. Biol. 29, 165–197 [DOI] [PubMed] [Google Scholar]
- 11. Crofts A. R. (2004) The cytochrome bc1 complex: function in the context of structure. Annu. Rev. Physiol. 66, 689–733 [DOI] [PubMed] [Google Scholar]
- 12. Link T. A. (1997) The role of the 'Rieske' iron sulfur protein in the hydroquinone oxidation (QP) site of the cytochrome bc1 complex: the 'proton-gated affinity change' mechanism. FEBS Lett. 412, 257–264 [DOI] [PubMed] [Google Scholar]
- 13. Jünemann S., Heathcote P., Rich P. R. (1998) On the mechanism of quinol oxidation in the bc1 complex. J. Biol. Chem. 273, 21603–21607 [DOI] [PubMed] [Google Scholar]
- 14. Zhang H., Osyczka A., Dutton P. L., Moser C. C. (2007) Exposing the complex III Qo semiquinone radical. Biochim. Biophys. Acta 1767, 883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Vries S., Albracht S. P., Berden J. A., Slater E. C. (1981) A new species of bound ubisemiquinone anion in QH2:cytochrome c oxidoreductase. J. Biol. Chem. 256, 11996–11998 [PubMed] [Google Scholar]
- 16. Cape J. L., Bowman M. K., Kramer D. M. (2007) A semiquinone intermediate generated at the Qo site of the cytochrome bc1 complex: importance for the Q-cycle and superoxide production. Proc. Natl. Acad. Sci. U.S.A. 104, 7887–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu J., Egawa T., Yeh S.-R., Yu L., Yu C.-A. (2007) Simultaneous reduction of iron-sulfur protein and cytochrome bL during ubiquinol oxidation in cytochrome bc1 complex. Proc. Natl. Acad. Sci. U.S.A. 104, 4864–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Snyder C. H., Gutierrez-Cirlos E. B., Trumpower B. L. (2000) Evidence for a concerted mechanism of ubiquinol oxidation by the cytochrome bc1 complex. J. Biol. Chem. 275, 13535–13541 [DOI] [PubMed] [Google Scholar]
- 19. Crofts A. R., Holland J. T., Victoria D., Kolling D. R., Dikanov S. A., Gilbreth R., Lhee S., Kuras R., Kuras M. G. (2008) The Q-cycle reviewed: how well does a monomeric mechanism of the bc1 complex account for the function of a dimeric complex? Biochim. Biophys. Acta 1777, 1001–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palsdottir H., Lojero C. G., Trumpower B. L., Hunte C. (2003) Structure of the yeast cytochrome bc1 complex with a hydroxyquinone anion Qo site inhibitor bound. J. Biol. Chem. 278, 31303–31311 [DOI] [PubMed] [Google Scholar]
- 21. Kagawa Y., Racker E. (1971) Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXV. Reconstitution of vesicles catalyzing 32Pi-adenosine triphosphate exchange. J. Biol. Chem. 246, 5477–5487 [Google Scholar]
- 22. Yu C.-A., Yu L. (1982) Syntheses of biologically active ubiquinone derivatives. Biochemistry 21, 4096–4101 [DOI] [PubMed] [Google Scholar]
- 23. Xiao K., Liu X., Yu C.-A., Yu L. (2004) Evidence for electron equilibrium between the two hemes bL in the dimeric cytochrome bc1 complex. Biochemistry 43, 1488–149514769025 [Google Scholar]
- 24. Mather M. W., Yu L., Yu C.-A. (1995) The involvement of threonine 160 of cytochrome b of Rhodobacter sphaeroides cytochrome bc1 complex in quinone binding and interaction with subunit IV. J. Biol. Chem. 270, 28668–28675 [DOI] [PubMed] [Google Scholar]
- 25. Tian H., Yu L., Mather M. W., Yu C.-A. (1997) The involvement of serine 175 and alanine 185 of cytochrome b of Rhodobacter sphaeroides cytochrome bc1 complex in interaction with iron-sulfur protein. J. Biol. Chem. 272, 23722–23728 [DOI] [PubMed] [Google Scholar]
- 26. Berden J. A., Slater E. C. (1970) The reaction of antimycin with a cytochrome b preparation active in reconstitution of the respiratory chain. Biochim. Biophys. Acta 216, 237–249 [DOI] [PubMed] [Google Scholar]
- 27. Yu C.-A., Yu L., King T. E. (1972) Preparation and properties of cardiac cytochrome c1. J. Biol. Chem. 247, 1012–1019 [PubMed] [Google Scholar]
- 28. Yu L., Dong J. H., Yu C.-A. (1986) Characterization of purified cytochrome c1 from Rhodobacter sphaeroides R-26. Biochim. Biophys. Acta 852, 203–211 [DOI] [PubMed] [Google Scholar]
- 29. Nakano M. (1990) Determination of superoxide radical and singlet oxygen based on chemiluminescence of luciferin analogs. Methods Enzymol. 186, 585–591 [DOI] [PubMed] [Google Scholar]
- 30. Denicola A., Souza J. M., Gatti R. M., Augusto O., Radi R. (1995) Desferrioxamine inhibition of the hydroxyl radical-like reactivity of peroxynitrite: role of the hydroxamic groups. Free Radic. Biol. Med. 19, 11–19 [DOI] [PubMed] [Google Scholar]
- 31. Crofts A. R., Hong S., Ugulava N., Barquera B., Gennis R., Guergova-Kuras M., Berry E. A. (1999) Pathways for proton release during ubihydroquinone oxidation by the bc1 complex. Proc. Natl. Acad. Sci. U.S.A. 96, 10021–10026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hunte C., Koepke J., Lange C., Rossmanith T., Michel H. (2000) Structure at 2.3 Å resolution of the cytochrome bc1 complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure 8, 669–684 [DOI] [PubMed] [Google Scholar]
- 33. Crofts A. R., Barquera B., Gennis R. B., Kuras R., Guergova-Kuras M., Berry E. A. (1999) Mechanism of ubiquinol oxidation by the bc1 complex: different domains of the quinol binding pocket and their role in the mechanism and binding of inhibitors. Biochemistry 38, 15807–15826 [DOI] [PubMed] [Google Scholar]
- 34. Esposti M. D., De Vries S., Crimi M., Ghelli A., Patarnello T., Meyer A. (1993) Mitochondrial cytochrome b: evolution and structure of the protein. Biochim. Biophys. Acta 1143, 243–271 [DOI] [PubMed] [Google Scholar]
- 35. Brasseur G., Saribaş A. S., Daldal F. (1996) A compilation of mutations located in the cytochrome b subunit of the bacterial and mitochondrial bc1 complex. Biochim. Biophys. Acta 1275, 61–69 [DOI] [PubMed] [Google Scholar]
- 36. Covián R., Moreno-Sánchez R. (2001) Role of protonatable groups of bovine heart bc1 complex in ubiquinol binding and oxidation. Eur. J. Biochem. 268, 5783–5790 [DOI] [PubMed] [Google Scholar]
- 37. Leung K. H., Hinkle P. C. (1975) Reconstitution of ion transport and respiratory control in vesicles formed from reduced coenzyme Q-cytochrome c reductase and phospholipids. J. Biol. Chem. 250, 8467–8471 [PubMed] [Google Scholar]
- 38. Güner S., Robertson D. E., Yu L., Qui Z.-H., Yu C.-A., Knaff D. B. (1991) The Rhodospirillum rubrum cytochrome bc1 complex: redox properties, inhibitor sensitivity and proton pumping. Biochim. Biophys. Acta 1058, 269–279 [DOI] [PubMed] [Google Scholar]
- 39. Lorusso M., Gatti D., Boffoli D., Bellomo E., Papa S. (1983) Redox-linked proton translocation in the b-c1 complex from beef-heart mitochondria reconstituted into phospholipid vesicles. Eur. J. Biochem. 137, 413–420 [DOI] [PubMed] [Google Scholar]
- 40. Cocco T., Lorusso M., Di Paola M., Minuto M., Papa S. (1992) Characteristics of energy-linked proton translocation in liposome reconstituted bovine cytochrome bc1 complex. Eur. J. Biochem. 209, 475–481 [DOI] [PubMed] [Google Scholar]
- 41. Gennis R. B., Ebrey T. G. (1999) Proton pump caught in the act. Science 286, 252–253 [DOI] [PubMed] [Google Scholar]
- 42. Wikström M., Verkhovsky M. I., Hummer G. (2003) Water-gated mechanism of proton translocation by cytochrome c oxidase. Biochim. Biophys. Acta 1604, 61–65 [DOI] [PubMed] [Google Scholar]
- 43. Yang S., Ma H. W., Yu L., Yu C.-A. (2008) On the mechanism of quinol oxidation at the QP site in the cytochrome bc1 complex. J. Biol. Chem. 283, 28767–28776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin Y., Tso S. C., Yu C.-A., Yu L. (2009) Effect of subunit IV on superoxide generation by Rhodobacter sphaeroides cytochrome bc1 complex. Biochim. Biophys. Acta 1787, 913–919 [DOI] [PubMed] [Google Scholar]
- 45. Yin Y., Yang S., Yu L., Yu C.-A. (2010) Reaction mechanism of superoxide generation during ubiquinol oxidation by the cytochrome bc1 complex. J. Biol. Chem. 285, 17038–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]