Background: The heretofore unknown mechanism for acquisition of chick yolk sac function is described.
Results: Receptor expression, lipoprotein secretion, and lipid droplet metabolism in endodermal epithelial cells (EECs) change drastically upon vascularization.
Conclusion: Vascularization and gain in function of the developing yolk sac are coordinated processes.
Significance: We demonstrate that changes in gene expression during differentiation of EECs are critical for yolk sac maturation.
Keywords: Development, Differentiation, Epithelium, Lipoprotein Receptor, Lipoprotein Secretion, Nutrient Transport, Yolk Sac
Abstract
During chicken yolk sac (YS) growth, mesodermal cells in the area vasculosa follow the migrating endodermal epithelial cell (EEC) layer in the area vitellina. Ultimately, these cells form the vascularized YS that functions in nutrient transfer to the embryo. How and when EECs, with their apical aspect directly contacting the oocytic yolk, acquire the ability to take up yolk macromolecules during the vitellina-to-vasculosa transition has not been investigated. In addressing these questions, we found that with progressive vascularization, the expression level in EECs of the nutrient receptor triad, LRP2-cubilin-amnionless, changes significantly. The receptor complex, competent for uptake of yolk proteins, is produced by EECs in the area vasculosa but not in the area vitellina. Yolk components endocytosed by LRP2-cubilin-amnionless, preformed and newly formed lipid droplets, and yolk-derived very low density lipoprotein, shown to be efficiently endocytosed and lysosomally processed by EECs, probably provide substrates for resynthesis and secretion of nutrients, such as lipoproteins. In fact, as directly demonstrated by pulse-chase experiments, EECs in the vascularized, but not in the avascular, region efficiently produce and secrete lipoproteins containing apolipoprotein A-I (apoA-I), apoB, and/or apoA-V. In contrast, perilipin 2, a lipid droplet-stabilizing protein, is produced exclusively by the EECs of the area vitellina. These data suggest a differentiation process that orchestrates the vascularization of the developing YS with the induction of yolk uptake and lipoprotein secretion by EECs to ensure embryo nutrition.
Introduction
One of the most important requirements for normal development of the embryo is the adequate supply with nutrients from maternal sources. In mammals, the placenta provides the active maternal-fetal interface mediating nutrient transfer between the maternal and embryonic circulation. However, before placental circulation is established, the mammalian visceral yolk sac provides nutrition to the developing embryo during neural tube closure (reviewed in Ref. 1). In egg-laying (oviparous) species, such as the chicken (Gallus gallus), the functionally equivalent interface is provided by the yolk sac (YS)3 enclosing the yolk. The yolk, deposited into oocytes via receptor-mediated endocytosis of precursor macromolecules synthesized by the liver of the hen (2), is the almost exclusive source of nutrients for the developing embryo, because it contains macromolecular complexes comprising lipids, proteins, vitamins, minerals, and other essential micronutrients. The formation of the mammalian as well as the chicken YS, which when completed are composed of cells derived from all three germ layers, is initiated at the onset of gastrulation (3, 4). Progression of avian YS formation is a highly coordinated sequence of events, in which rapidly dividing ectodermal cells start to spread from the embryo to ultimately fully cover the yolk compartment (5). Closely behind the migrating front of the ectodermal layer and between the yolk surface and the ectoderm, the proliferating endodermal epithelial cells (EECs) of the YS follow the ectodermal cells and form a tight epithelial layer in close contact with the yolk (5, 6). Finally, from the so-called splanchnic mesoderm, which is tightly associated with the basal aspect of the EECs, cells migrate into the space between the ectodermal and endodermal layers and form a network of capillaries in tight contact with the other two cell layers (7). Thus, the three cell types in concert generate the fully functional YS capable of mediating the targeted transfer of yolk-derived nutrient components to the embryonic circulation.
The growing chicken YS consists of three morphologically and functionally different regions termed (i) area pellucida, the central area of the early blastoderm, which can be considered part of the embryo proper; (ii) area vasculosa, which forms the major, vascularized part of the nascent YS; and (iii) area vitellina, the most distal, non-vascularized, portion of the YS (7, 8). Ultrastructural studies have provided information on the dynamics of the morphological transformations of the YS, particularly on the phenotypic changes of the EECs upon transition of the area vitellina to the area vasculosa (5, 8). During this transition, the apical aspect of EECs acquires the typical characteristics of polarized epithelial cells with numerous villi, invaginations, and coated pits (5, 6, 8, 9). Taken together, these and other observations are in agreement with the EECs in the area vasculosa being the most active yolk-absorbing cells in the entire YS (5, 8, 10).
In contrast to the well studied morphological aspects of differentiation of YS cell types and the acquisition of functional features in the course of YS maturation, very little is known about the components and regulation of the molecular machinery that accomplishes the complex process of nutrient uptake from yolk into the EECs and subsequent transfer to the embryo. Although gene expression profiling of area vasculosa EECs from embryonic day 2–4 chick embryos showed that several transcripts for enzymes probably involved in lipid metabolism and for proteins important to embryonic growth are enriched in these cells, candidate genes whose products may be involved in yolk nutrient uptake did not emerge from this analysis (9). Thus, we have exploited the phenotypic differentiation of EECs upon vascularization of the YS (i.e. the area vitellina-to-area vasculosa transition) to directly identify and characterize proteins involved in the uptake into the EECs of lipoprotein particles and other nutrient precursors from the yolk. We show that following uptake and degradation of lipoproteins and other macromolecules from the yolk, the EECs de novo synthesize and secrete lipoproteins that differ in composition from those in yolk and are targeted to the embryonic circulation. Importantly, we reveal that the expression patterns of key molecules for nutrient transfer, including endocytic receptor complexes, apolipoproteins, and lipid droplet-associated proteins, differ between EECs in the area vasculosa and the area vitellina. The expression profiles are entirely compatible with the vascularized portion of the YS being its functionally active region, whereas the area vitellina EECs, a tissue comparable with the extraembryonic endoderm in early rodent embryos (11, 12), appears to have primarily lipid storage functions. These results support the notion that a cross-talk between the processes of EEC differentiation and of vasculogenesis is essential to YS function and, thus, for embryo development.
EXPERIMENTAL PROCEDURES
Animals
Sexually mature Derco brown (TETRA-SL) laying hens were purchased from Diglas Co. (Feuersbrunn, Austria). Animals were maintained on open floor space with free access to water and feed with a daily light period of 14 h. Fertilized eggs (available in house or purchased from Schropper GmbH, Schottwien, Austria) were incubated at 38 °C to maintain normal embryonic development.
Antibodies
Polyclonal antisera were raised in adult female New Zealand White rabbits against the indicated antigens as described previously (13). The following available rabbit polyclonal antisera against chicken (Gallus gallus, gg) proteins were used: anti-ggApoA-V antiserum (14); anti-ggApoA-I antiserum (15); anti-ggLRP2 antiserum raised against the intracellular domain of chicken LRP2 (16); and anti-ggMTP antiserum, directed against a C-terminal fragment comprising amino acids 879–893 of the chicken preprotein (NCBI accession number NP_001103254.1). Furthermore, we raised rabbit and mouse polyclonal anti-ggCubilin antisera against a peptide comprising amino acids 131–226 of chicken cubilin (NCBI accession number XP_001235156.2); rabbit polyclonal anti-ggAmnionless antiserum against a peptide comprising amino acids 240–342 of ggAmnionless (NCBI accession number XP_421379.2); and rabbit polyclonal anti-ggMesd antiserum against a full-length ggMesd-GST construct (NCBI accession number NP_001025722.1). The IgG fraction of an anti-ggApoB antiserum was purified by affinity chromatography as described (15, 17) and designated anti-ggApoB antibody. A rabbit polyclonal antibody against human ADFP (Plin2), with cross-reactivity to ggPlin2, was purchased from Abcam (ab52355). Monoclonal murine anti-rabbit GAPDH antibody (clone GAPDH-71.1), cross-reactive with ggGAPDH, was purchased from Sigma-Aldrich, and secondary HRP-coupled goat-anti rabbit IgG (A0545) and HRP-coupled goat-anti mouse IgG (W4021) were purchased from Sigma-Aldrich and Promega, respectively.
Preparation and Dissection of Yolk Sac Layers
Yolk sac tissue fragments from chicken embryos at day 5 of development were dissected mechanically with forceps to separate the EECs from the associated ectodermal and mesodermal cell layers. Separation was performed starting at the sinus terminalis. By firmly holding the endodermal cell sheet with one pair of tweezers and holding the sinus terminalis and associated cells with another one, the layers were carefully pulled apart in the direction toward the embryo. After separation of the cell sheets, they were washed extensively in PBS to remove adherent yolk and processed immediately for further experiments.
Quantitative PCR
Total RNA from chicken YS or its dissected layers was isolated using TRI reagent (MRC) following the manufacturer's protocol. One microgram of RNA was used to synthesize single-stranded cDNA using SuperScriptII reverse transcriptase (Invitrogen) and oligo(dT) primer. The obtained cDNA was subjected to quantitative PCR (qPCR) using the Eppendorf Mastercycler system and the KAPA SYBR FAST qPCR Master Mix (Peqlab) following the manufacturer's instructions. The primer sets were as follows: ggβ-actin forward, 5′-AGC TAT GAA CTC CCT GAT GG-3′; ggβ-actin reverse, 5′-ATC TCC TTC TGC ATC CTG TC-3′; ggApoA-I forward, 5′-GAC CGC ATT CGG GAT ATG GT-3′; ggApoA-I reverse, 5′-ATC TCG CGC ACC TCC TTG TA-3′; ggApoB forward, 5′-CTC CAT CAG AGC AGT CGA GT-3′; ggApoB reverse, 5′-ATT AGG CCT CAG GGA CAG TG-3′; ggApoA-V forward, 5′-CGC TGA TAA GAT CGC CTT CC-3′; ggApoA-V reverse, 5′-TGG ATG TTG CGG TGC AGA TC-3′; ggMTP forward, 5′-GCA GAC GCC AGC ATC TCT-3′; ggMTP reverse, 5′-TAC AAC AGC CGG AAG CTT GC-3′; ggCubilin forward, 5′-TGA ACT CTC TGG ATG GCT TC-3′; ggCubilin reverse, 5′-CTC GTT CTG CAT CAA CAC AG-3′; ggLRP2 forward, 5′-GGA GTG TTA GCG ATT GGA GGC-3′; ggLRP2 reverse, 5′-CCA CAC TAC CAG CTC CTG TTA-3′; ggAmnionless forward, 5′-TCT GGC TCT GGG TTC ACA GC-3′; ggAmnionless reverse, 5′-GAA CAG GGA TCA CTC GCC G-3′. The authenticity of all amplified PCR products was confirmed by DNA sequencing. Diluted cDNA samples were used for all qPCRs. Serial dilutions of 10−1 to 10−9 of the target PCR products were freshly prepared. All samples were analyzed in duplicates and compared with the serial dilutions serving as internal standard. The levels of chicken β-actin mRNA as housekeeping gene were measured and used for normalization. Values are expressed in arbitrary units relative to β-actin, as the average of triplicate measurements ± S.E.
Preparation of Total and Membrane Protein Extracts
All operations were performed at 4 °C. For total protein extracts, fresh chicken tissues were homogenized in buffer containing 50 mm Tris/HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, and complete protease inhibitor mixture (Roche Applied Science; catalogue no. 11836170001) (4 ml/g wet weight) with an Ultra Turrax homogenizer. Homogenates were centrifuged at 620 × g for 10 min to sediment debris. The supernatant was incubated on ice for 30 min with intermittent vortexing. A supernatant (designated total tissue extract) was obtained by centrifugation at 300,000 × g for 60 min, quickly frozen in liquid N2, and stored at −80 °C until use. Membrane fractions and detergent extracts were prepared from fresh yolk sac tissue as described (18), except that the extraction buffer contained 1% Triton X-100.
Western Blotting
For Western blotting, protein extracts were analyzed by one-dimensional SDS-PAGE and electrophoretically transferred to nitrocellulose membranes (Hybond-C Extra, Amersham Biosciences) using semidry blotting. Nonspecific binding sites were blocked with TBS (20 mm Tris-HCl, pH 7.4, 140 mm NaCl) containing 5% (w/v) nonfat dry milk and 0.1% Tween 20 (blocking buffer) for 1 h at room temperature. Proteins of interest were detected with the indicated specific primary antisera/antibodies, followed by incubation with HRP-conjugated secondary antibodies and development with the enhanced chemiluminescence protocol (Pierce). The sizes of proteins were estimated using a broad range molecular mass standard (20–250 kDa) from Bio-Rad.
Pulse-Chase Radiolabeling Experiments and Immunoprecipitation
Yolk sac tissue fragments were washed extensively in PBS and incubated in starvation medium (RPMI 1640 without l-methionine, l-cysteine, and l-cystine; MP Biochemicals) for 1 h at 37 °C and 5% CO2. Subsequently, the medium was removed, and the tissue was pulse-labeled in starvation medium containing 250 μCi/ml [35S]methionine/cysteine (Easytag Express protein labeling mix from PerkinElmer Life Sciences, NEG-722) for 1 h. After the pulse phase, the labeling medium was removed, and the cells were incubated in Dulbecco's modified Eagle's medium F-12 (DMEM F-12, Sigma) containing 10% fetal calf serum (FCS), 2 mm l-glutamine, 5 mm l-methionine, 2 mm l-cysteine, 0.1 mg/ml streptomycin, and 100 units/ml penicillin. Supernatants and tissue were collected after the indicated chase times and processed as follows. Supernatants were centrifuged for 10 min at 8000 rpm and 4 °C to remove cell debris. After the addition of complete proteinase inhibitor mixture (Roche Applied Science), aliquots of the supernatants were subjected to immunoprecipitation by the addition of the indicated antisera/antibodies followed by incubation for 16 h with Protein A-Sepharose beads (Invitrogen) that had previously been washed extensively with TBS. Where indicated, the medium supernatants obtained after 1-h pulse/3-h chase were subjected to serial ultracentrifugation steps to obtain fractions with different buoyant densities for immunoprecipitation as follows. All centrifugation steps were carried out at 4 °C and 40,000 × g in a Beckman L-70 ultracentrifuge using a 50.2Ti rotor. The d < 1.006 fraction was floated by two consecutive centrifugation steps for 24 h each. After adjusting the density of the infranatant to 1.063 g/ml with solid KBr, the 1.006 < d < 1.063 fraction was obtained by a 24-h centrifugation step. Finally, the density of the infranatant was adjusted to 1.210 g/ml, and the HDL fraction (1.063 < d < 1.210) was separated from the lipid-free bottom fraction (d > 1.210) by centrifugation for 48 h. The separated floating fractions and the bottom fraction were subjected to immunoprecipitation in the absence of detergent as described above for radiolabeled unfractionated supernatants. When yolk sac EEC layers were subjected to pulse-chase radiolabeling, cells from the indicated chase time points were lysed and homogenized by adding 300 μl of lysis buffer (25 mm Tris/HCl, pH 7.4, 95 mm NaCl, 3 mm EDTA, 1% SDS, and complete proteinase inhibitor mixture) and sonication for 15 s. Samples were centrifuged at 4 °C for 10 min at 6000 × g to remove cell debris, and the supernatants were subjected to immunoprecipitation with the antisera/antibodies indicated in the Figs. 5, 6, and 8 legends, followed by binding to Protein A-Sepharose beads as described above. After washing the beads five times extensively with PBS, samples were mixed with Laemmli buffer (19) containing 50 mm DTT and heated for 5 min at 95 °C. Samples were centrifuged for 2 min at 8000 rpm, and the supernatants were subjected to SDS-PAGE and subsequent autoradiography.
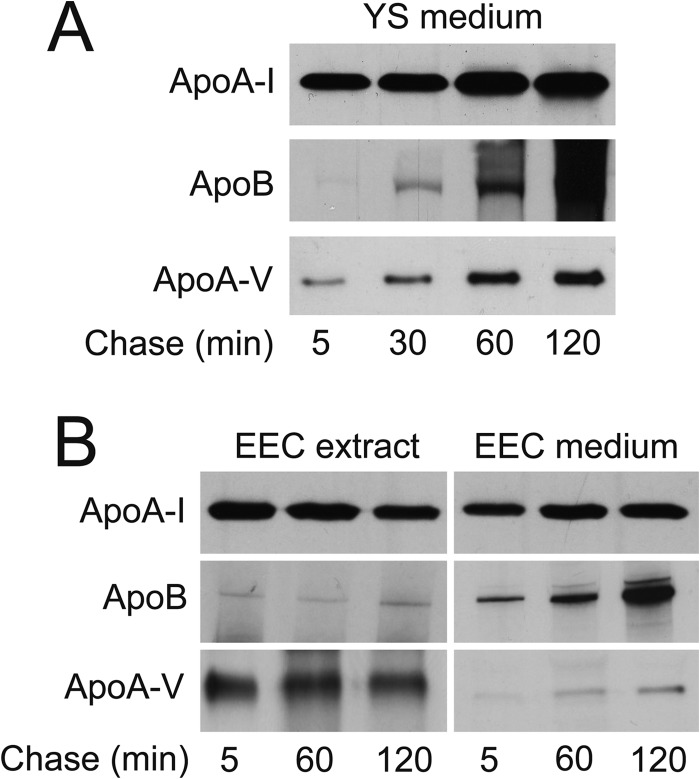
FIGURE 5.
Endodermal epithelial cells of the yolk sac synthesize and secrete apolipoproteins. Whole yolk sac tissue explants (A) and EECs from the area vasculosa (B) were pulse-labeled with [35S]methionine/cysteine for 1 h and chased for the indicated time periods. Radiolabeled secreted apolipoproteins were immunoprecipitated with specific antisera/antibodies directed against apoA-I, apoB, and apoA-V from the conditioned media (A and B) and cellular apolipoproteins from cell extracts (B) as described under “Experimental Procedures.”
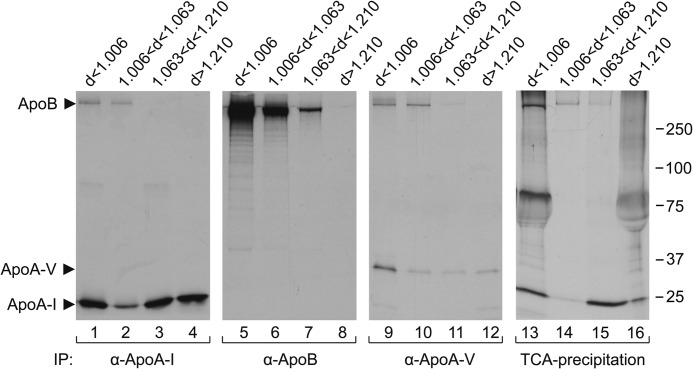
FIGURE 6.
Distribution of apolipoproteins A-I, B, and A-V among yolk sac-derived lipoprotein fractions. 35S-Labeled lipoproteins secreted by YS tissue were isolated and fractionated as described under “Experimental Procedures” and subsequently were immunoprecipitated (IP) with the indicated respective antisera/antibodies under detergent-free conditions. Equal amounts of isolated lipoprotein fractions were precipitated with TCA as described under “Experimental Procedures.” Autoradiography was performed after 4–18% SDS-PAGE of the precipitates.
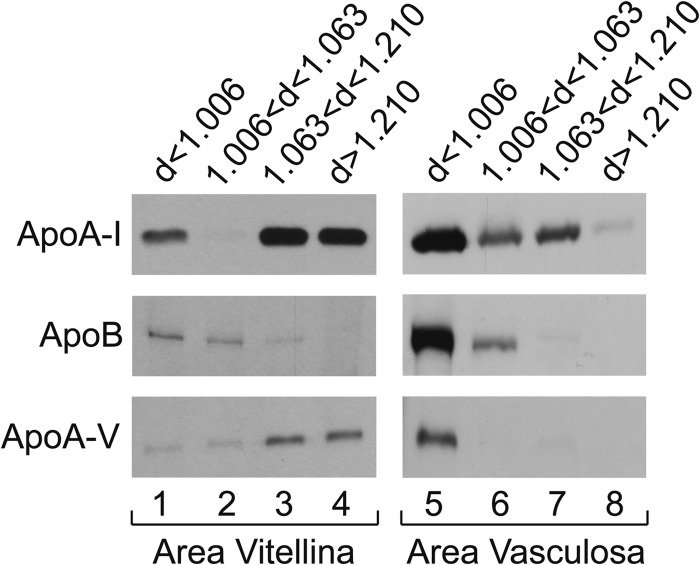
FIGURE 8.
EECs in the area vitellina and in the area vasculosa show different apolipoprotein secretion patterns. Biosynthetically radiolabeled lipoproteins, secreted into the media of EEC explants from either the area vitellina or the area vasculosa, were isolated and fractionated as described under “Experimental Procedures” and immunoprecipitated with specific antisera/antibodies directed against apoA-I, apoB, or apoA-V. Autoradiography was performed after separation of the immunoprecipitated proteins by SDS-PAGE as described under “Experimental Procedures.” Exposure times for apoA-I immunoprecipitates were 16 h for lipoproteins derived from both YS areas. For apoB and apoA-V immunoprecipitates, exposure times were 21 days for both apolipoproteins derived from the area vitellina and 16 h and 10 days, respectively, for lipoprotein fractions derived from the area vasculosa.
Trichloroacetic Acid (TCA) Precipitations
To 200 μl of lipoprotein fraction, 50 μl of a TCA stock solution (100% (w/v) TCA) was added and incubated for 10 min at 4 °C. After centrifugation of the samples for 5 min at 14,000 × g, the supernatant was removed, and the pellets were washed two times with ice-cold acetone. Finally, the pellets were dried on a heating block at 95 °C for 5 min to remove remaining acetone. The pellets were resuspended in 4× Laemmli buffer, boiled for 10 min at 95 °C, and subjected to SDS-PAGE and subsequent autoradiography.
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine Perchlorate (DiI) Labeling of yVLDL
The yolk was carefully extracted from a fertilized egg at day 5 of embryonic development and mixed with 5 volumes of ice-cold lipoprotein isolation buffer (20 mm Tris/HCl, 150 mm NaCl, 0.2 mm EDTA) by end-over-end rotation until a homogeneous solution was obtained. The diluted yolk was subjected to ultracentrifugation at 40,000 × g for 24 h at 4 °C in a Beckman L-70 Ultracentrifuge using a 50.2Ti rotor. The yVLDL fraction was recovered from the top of the tube and diluted with lipoprotein isolation buffer to a protein concentration of 3–5 mg/ml. To 1 mg of yVLDL, 50 μl of DiI solution (Sigma-Aldrich; 3 mg/ml in dimethyl sulfoxide) were added and incubated for 16 h at 37 °C in the dark under gentle mixing. After ultracentrifugation for 24 h, the DiI-labeled yVLDL fraction was recovered from the top of the tube, dialyzed against 150 mm NaCl, 0.02 mm EDTA for 24 h, and stored light-protected at 4 °C until use.
Culture of Endodermal Epithelial Cell Explants and DiI-yVLDL Uptake Assay
EEC explants were incubated in DMEM F-12 containing 10% FCS, 2 mm l-glutamine, 0.1 mg/ml streptomycin, and 100 units/ml penicillin. After 48 h in culture, the proliferating EECs, forming a monolayer around the seed explant, were washed two times with PBS and then transferred to serum-free DMEM F-12. DiI-labeled yVLDL was added to the medium (final concentration, 50 μg/ml), and the cells were incubated as described in the Fig. 1 legend. Subsequently, the cells were washed three times in PBS and fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. After an additional wash, the cells were incubated for 30 min in PBS with BODIPY 493/503 (Molecular Probes; 10 μg/ml final concentration) for the visualization of intracellular lipid droplets, and with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear counterstaining, washed, and mounted in fluorescence mounting medium (Dako). Images were obtained with a Zeiss LSM Meta 510 confocal microscope.
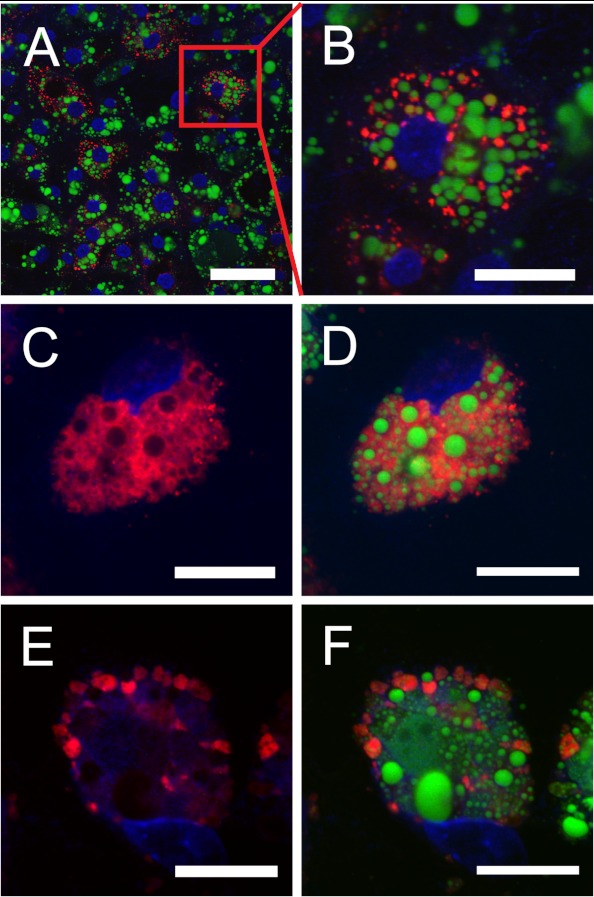
FIGURE 1.
Endodermal epithelial cells take up yVLDL particles. Explants from YS were transferred to Lab Tek Permanox 4-well imaging chambers. After 48 h of EEC outgrowth, the medium was changed to serum-free conditions, and 50 μg/ml DiI-labeled yVLDL (red fluorescence) was added. In A and B, the EECs were incubated with the labeled lipoprotein for 4 h at 37 °C; B shows the magnification of a cell from A. In C–F, the cells were incubated with 50 μg/ml DiI-yVLDL for 4 h, the medium was removed and replaced with medium without DiI-yVLDL, and cells were further incubated for 20 h. In E and F, the incubation medium contained 200 μm chloroquine, an inhibitor of lysosomal degradation. After the incubations, the cells were washed two times with PBS, fixed in 4% PFA in PBS, pH 7.4, for 30 min at room temperature, and washed two times with PBS. After counterstaining with BODIPY 493/503 (Molecular Probes) for the visualization of lipid droplets (green fluorescence) and DAPI for the nuclei, respectively, cells were mounted in Fluorescent Mounting Medium (Dako). Images were obtained with a Zeiss LSM510 confocal microscope. Scale bar, 50 μm (A) or 20 μm (B–F).
Immunohistochemistry
Frozen YS tissue was embedded in O.C.T. compound (Tissue-Tek), and sections of 4 μm thickness were prepared. The sections were fixed for 30 min in 4% paraformaldehyde in PBS and washed three times in PBS. Slides were air-dried at room temperature and stored at −20 °C until use. Slides were blocked in TBS/HCl, pH 7.4, containing 5% (w/v) nonfat dry milk (blocking solution) for 1 h at room temperature and subsequently incubated for 1 h at room temperature with primary antiserum diluted in blocking solution. After washing three times in TBS, secondary goat anti-rabbit Alexa Fluor 488-labeled antibody or rabbit anti-mouse Texas Red labeled antibody (both from Molecular Probes) diluted in blocking solution were applied to the slides. Nuclei were counterstained with DAPI. After washing, slides were mounted in fluorescence mounting medium (Dako). Images were obtained with a Zeiss LSM Meta 510 confocal microscope.
Whole-mount in Situ Hybridization
Antisense digoxigenin-labeled in situ probes directed against a region of chicken cubilin (nucleotides 716–1627 of NCBI number XM_001235155.2) were generated by in vitro transcription of PCR products containing the T7 RNA polymerase promoter sequence at their 3′-ends. In situ hybridization analysis was carried out exactly as described at the GEISHA (Gallus expression in Situ Hybridization Analysis) Web site (“whole mount in situ hybridization protocol for mRNA detection”). This protocol represents a slight modification of those published (20, 21).
RESULTS
Based on our previous demonstration of expression of the major lipoprotein transport receptor termed LR8 (LDL receptor relative with eight ligand binding repeats) in the chicken yolk sac (22), we now analyzed the endocytosis of yVLDL particles (23, 24) by cultured YS EECs in detail. To this end, we isolated the lipoprotein from yolk and labeled the particles with the lipophilic dye, DiI. As shown in Fig. 1, upon incubation of cultured EECs obtained from YS explants with DiI-yVLDL at 37 °C for 4 h, the lipoprotein particles were internalized and delivered to structures probably representing the endosomal-lysosomal system (Fig. 1, A and B). However, the DiI-yVLDL-loaded compartments (red fluorescence) remained in coexistence with the large neutral lipid-rich lipid droplets (LDs; green fluorescence) (Fig. 1B). Diffusion of DiI fluorescence after incubation of the cells for 20 h following the 4-h uptake period suggested degradation of yVLDL accompanied by release of lipids (Fig. 1, C and D); a transfer of a portion of yVLDL-derived lipids to the LDs may also occur. However, when the lysosomal inhibitor chloroquine was added to the cells during the 20-h incubation period, diffusion of the DiI stain was not observed. Rather, DiI-yVLDL-positive intracellular compartments appeared to swell as a consequence of impaired lysosomal degradation (Fig. 1, E and F). Thus, EECs efficiently endocytose yVLDL particles and lysosomally process them, in turn providing components, particularly lipids, probably for use in biosynthesis of secretory products.
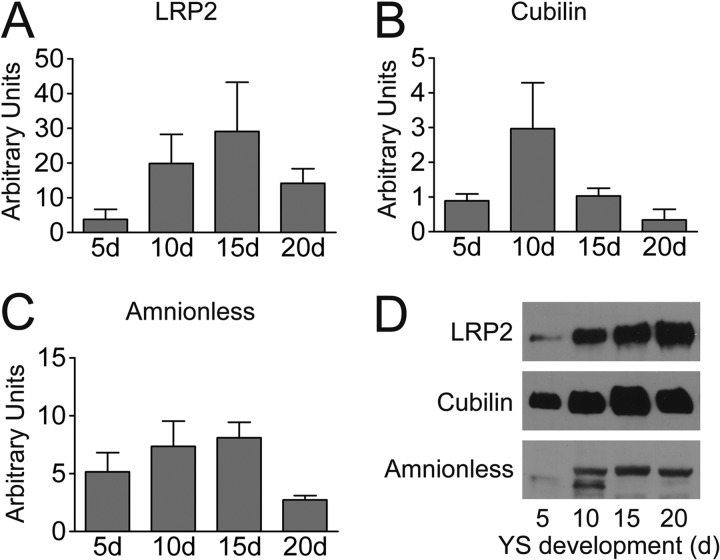
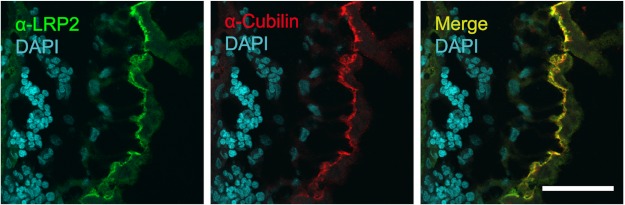
Before elucidating the further fate of yolk macromolecules internalized by LR8, which interacts with apoB (the major apolipoprotein of VLDL) and vitellogenin (25), we tested whether the YS produces endocytic receptors predicted to mediate the uptake of additional yolk components. Of interest regarding the chick YS, the transmembrane proteins LRP2 and amnionless as well as the peripheral membrane protein cubilin, which form a trimeric complex with significant endocytic activity for a large variety of macromolecules, have been demonstrated in the mammalian visceral YS (1, 26–29), a tissue essential to embryo nutrition prior to the development of the placenta (see Introduction). Furthermore, cubilin-mediated uptake of apoA-I and HDL-cholesterol by the rat visceral YS has been demonstrated (26). Thus, to gain insight into receptor expression patterns in the chick YS, we tested for the expression of the LRP2-cubilin-amnionless receptor tricomplex. To this end, we obtained sequence information for galline LRP2 (16), cubilin, and amnionless and produced rabbit polyclonal antibodies to each of the three proteins as described under “Experimental Procedures.” With these tools in hand, we demonstrated by qPCR (Fig. 2, A–C) and Western blotting (Fig. 2D) that all three components of the endocytic complex are indeed co-expressed in the chicken YS. Expression is observed already at day 5 of embryonic development; whereas mRNA levels of the three receptors varied during development of the YS (Fig. 2, A–C), the levels of the proteins remained high during days 10–20 (Fig. 2D). In order to serve as a platform for the uptake of yolk components into the YS, the three proteins have to be co-localized and positioned on the EEC surface immediately adjacent to the yolk. As Fig. 3 directly demonstrates by immunohistochemistry, LRP2 and cubilin (unfortunately, our anti-chicken amnionless antibody could only be used for Western blotting) clearly colocalize at the apical aspect of the large, polarized EECs. Taken together, these findings are compatible with a role of endocytic YS receptors, including LR8 and the LRP2-cubilin-amnionless receptor complex in taking up known yolk components such as VLDL and vitellogenin (25, 30, 31), vitamin-binding proteins (32, 33), and apoA-I/HDL (34).
FIGURE 2.
Expression of LRP2, cubilin, and amnionless in the chicken yolk sac at 5, 10, 15, and 20 days of development. mRNA levels in the yolk sac of LRP2 (A), cubilin (B), and amnionless (C) were determined using qPCR as described under “Experimental Procedures.” Transcript levels are expressed as arbitrary units relative to chicken β-actin and represent the average of at least triplicate measurements ± S.E. (error bars). D, analysis of LRP2, cubilin, and amnionless protein levels by Western blotting. Fifty micrograms of membrane protein per lane from yolk sacs of the indicated developmental stages were separated either by 6% (LRP2 and cubilin) or 12% (amnionless) SDS-PAGE under non-reducing conditions, blotted to nitrocellulose membranes, and probed with antisera directed against ggLRP2, ggCubilin, and ggAmnionless, followed by an HRP-coupled anti-rabbit secondary antibody as described under “Experimental Procedures.”
FIGURE 3.
The multiligand receptors LRP2 and cubilin colocalize at the apical surface of the endodermal epithelial cells of the yolk sac. Cryosections (4 μm) of the area vasculosa of yolk sac tissue from 10-day-old chicken embryos were prepared as described under “Experimental Procedures.” The sections were stained with our rabbit anti-ggLRP2 antiserum (green staining) and our mouse anti-ggCubilin antiserum (red staining). The merged image is shown on the right. For the visualization of cell nuclei, the sections were counterstained with DAPI. Images were acquired using a Zeiss LSM 510 confocal microscope. Scale bar, 50 μm.
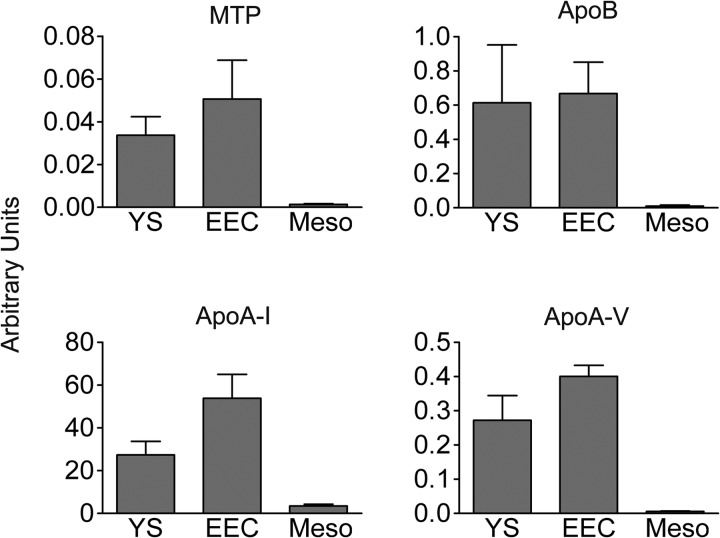
Next, we tested whether the deposition of yolk components into the EECs is linked to a function of these cells in providing substrates for resynthesis and secretion of nutrients (e.g. lipoproteins) for targeting to the embryo. Although a participation of the YS EECs in the synthesis of lipoproteins has been implied previously (35), proteins required for lipoprotein production have not yet been directly demonstrated in these cells. Thus, we investigated by qPCR whether EECs express genes whose products are crucial for assembly of lipoproteins. To this end, we separated the EEC layer from the mesodermal tissue of a YS fragment derived from the area vasculosa. As shown in Fig. 4, the large subunit of microsomal triglyceride transfer protein (MTP), considered to be essential for lipid loading of apoB during lipoprotein assembly (36, 37), is expressed in the YS. Significantly, the MTP transcripts are found almost exclusively in the EECs and are virtually absent from the mesodermal layer (Fig. 4). As observed for MTP, the EEC layer, but not the mesoderm, expresses apoB, apoA-I, and apoA-V, which are apolipoprotein components of major lipoprotein classes. The quantitative analysis also revealed that the transcript levels of apoA-I were very high in comparison with those of apoB and apoA-V (Fig. 4).
FIGURE 4.
The yolk sac endodermal epithelial cells, but not mesodermal cells, express genes for lipoprotein assembly. Transcript levels of apoA-I, apoB, apoA-V, and MTP were analyzed with qPCR in the YS and, after dissection, in the EECs and in cells of the mesodermal layer (Meso). Transcript levels are expressed as arbitrary units relative to chicken β-actin and represent the average of at least triplicate measurements ± S.E. (error bars).
To shed light on this aspect at the protein level, we directly investigated the biosynthesis of lipoproteins and their apoproteins by the YS and EECs, respectively. Pulse-chase radiolabeling experiments (Fig. 5) revealed that the undissected YS tissue (Fig. 5A) and, specifically, the EECs (Fig. 5B) indeed constitutively synthesize and secrete each of the three apolipoproteins. The data also show, in agreement with those obtained by qPCR, that apoA-I is the predominant apolipoprotein produced, particularly by the EEC layer. The production and secretion by the YS EECs of apoA-V, which in mammals is produced exclusively in the liver, indicates that this apolipoprotein, hitherto unknown, is a component of lipid metabolism in the chicken YS and/or embryo. Because the apolipoproteins are secreted from the EECs, we tested whether they are components of newly synthesized lipoprotein particles. Thus, following biosynthetic radiolabeling of the products of YS tissue, the conditioned media were fractionated according to their buoyant densities (d < 1.006; 1.006 < d < 1.063; 1.063 < d < 1.21; and d > 1.210), and aliquots of each fraction, including the bottom fraction, were immunoprecipitated in the absence of detergents with antibodies against chicken apoA-I, apoB, or apoA-V (Fig. 6, lanes 1–12). In addition, precipitation with TCA of the secreted radiolabeled proteins revealed that the apolipoproteins and unidentified protein(s) of 65–80 kDa were the major proteins secreted from YS EECs (lanes 13–16). Taken together, these data demonstrate that the secreted apolipoproteins are indeed components of lipoproteins, because they were mainly present at densities of <1.210 g/ml. Unexpectedly, chicken apoA-I, the major apolipoprotein of mammalian HDL particles (1.063 < d < 1.210), was immunoprecipitable in all fractions (Fig. 6, lanes 1–4). Immunoprecipitation with anti-apoB (Fig. 6, lanes 5–8) showed the vast majority of apoB in the two top fractions (lanes 5 and 6) and a minor amount in the fraction with 1.063 < d < 1.210 (lane 7). ApoA-I was absent from all immunoprecipitates obtained with anti-apoB (lanes 5–8), and thus apoA-I was, if at all, associated only with a minor population of particles also containing apoB (i.e. those seen in lanes 1 and 2). Particles immunoprecipitated with anti-apoA-V predominated at d < 1.006 (lanes 9–12), in agreement with a previous report (14), did not contain significant amounts of apoA-I and appeared to be a minor fraction of the apoB-containing lipoproteins, albeit different from the apoA-I/apoB particles (cf. lanes 1 and 2). These results demonstrate the production by the YS of lipoproteins with low and very low density harboring apoA-I and imply that lipoprotein assembly pathway(s) in the EECs may differ from those in other systems of lipoprotein secretion.
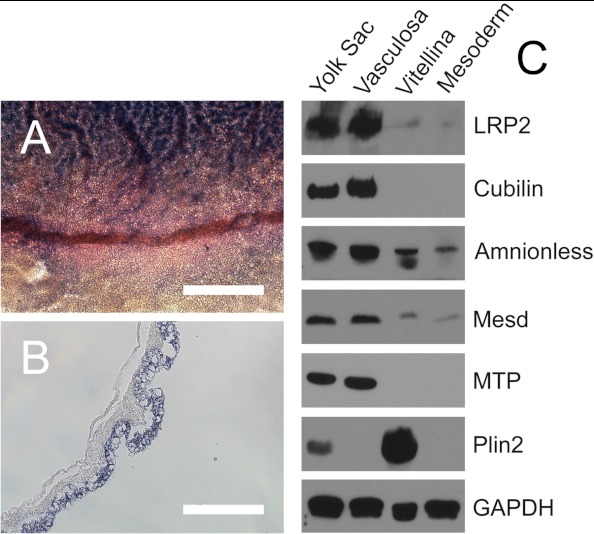
In our efforts to delineate the mechanism for the acquisition of transport function by the EECs during YS development, we observed significant changes in gene expression levels in the EEC layer upon YS vascularization. In order to provide an illustrative example for these regional differences, we performed whole-mount in situ hybridization analysis of cubilin expression (Fig. 7A) in a YS fragment containing the sinus terminalis, a blood vessel constituting the boundary between area vitellina and area vasculosa (seen below and above the vessel, respectively, in Fig. 7A). Hybridization analysis of a yolk sac section derived from the area vasculosa showed that the cubilin transcript is exclusively present in the EECs (Fig. 7B). Next, because yolk sac EECs are the source of newly synthesized and secreted lipoproteins and express LRP2, cubilin, apoA-I, apoB, apoA-V, and MTP, we became interested in the profiles of proteins produced by EECs located in the nonvascular and the vascularized region of the YS, respectively. Therefore, the levels of these proteins in the unfractionated YS, the EECs from the area vasculosa and area vitellina, and in the mesoderm were compared by Western blotting (Fig. 7C). We discovered that LRP2, cubilin, and amnionless, as well as the LRP chaperone mesodermal differentiation factor (Mesd (38)) and MTP are clearly detected in EECs derived from the area vasculosa but absent in EECs in the area vitellina (Fig. 7C). In striking contrast, perilipin 2 (Plin2; formerly called adipocyte differentiation-related protein, or adipophilin), a protein important for formation and protection of LDs from lipolysis (39), is expressed exclusively in the area vitellina of the YS (Fig. 7C). This finding is compatible with a role of LDs in storage of triacylglycerides in EECs of the area vitellina and as source of lipids for lipoprotein synthesis by EECs in the area vasculosa. Finally, we revealed that among the apolipoproteins analyzed, only apoA-I is produced in significant amounts in and secreted from both regions of the YS, albeit with higher levels in the area vasculosa, particularly in the d < 1.006 fraction (Fig. 8, lanes 1 and 5, and cf. Fig. 6, lane 1). In agreement with the absence of MTP in the area vitellina (Fig. 7C), apoB levels were very low to undetectable in all lipoprotein fractions secreted by the area vitellina and negligible in comparison with the levels in area vasculosa-derived lipoproteins (Fig. 8; note that the autoradiography exposure time for lanes 1–4 was ∼30-fold longer than for lanes 5–8). Interestingly, secreted apoA-V was associated mainly with lipoproteins of d > 1.063 produced in the area vitellina (Fig. 8, lanes 1–4) but was present exclusively in the d < 1.006 fraction of the lipoproteins produced in the area vasculosa (Fig. 8, lanes 5–8). Taken together, these findings suggest that during transformation of the area vitellina into the area vasculosa, a coordinated change in gene expression and lipoprotein synthesis in EECs endows the vascularized area of the YS with the function of mediating nutrient transfer from the yolk to the embryo.
FIGURE 7.
EECs in the area vasculosa and area vitellina display opposite levels of proteins involved in yolk uptake and resynthesis of lipoproteins. Whole-mount in situ hybridization was performed with YS tissue at day 5 of embryonic development using digoxigenin-labeled antisense probes for chicken cubilin. A shows the transition zone from the area vasculosa (top) to the area vitellina (bottom). B, a YS section through the cubilin-positive area vasculosa. Scale bar, 800 μm in A and 200 μm in B. In C, fresh tissue samples of either the whole YS, the EECs of the area vasculosa (Vasculosa), the EECs of the area vitellina (Vitellina), or the blood vessel-containing mesodermal cell layer (Mesoderm) from 5-day chicken embryos were isolated, and total protein fractions were prepared as described under “Experimental Procedures.” Fifty micrograms of total protein lysate per lane from the indicated sources were separated either by 6% (LRP2 and cubilin) or 12% (amnionless, Mesd, MTP, Plin2, and GAPDH for loading control) SDS-PAGE under non-reducing conditions, blotted to nitrocellulose membranes, and probed with the indicated antisera/antibodies. For visualization of bound primary antibodies, corresponding HRP-coupled secondary antibodies were used as described under “Experimental Procedures.”
DISCUSSION
In the current study, we have used the growing chicken YS to delineate the processes and molecules that provide this organ with the ability to transfer nutrients from the yolk to the embryo. The rationale for our investigations is based on the questions (i) whether the mature chicken YS resembles the mammalian visceral YS, a tissue essential to embryo nutrition prior to the onset of placental circulation, and (ii) at what point of YS development the tissue becomes competent for nutrient transport. Because the epithelium of the mature mammalian visceral YS expresses certain genes that have hitherto not been studied in the chicken, we initially established that these and other key components are indeed expressed in the fully developed avian YS. Importantly, we found that only the EEC layer of the area vasculosa produces the multiligand receptors cubilin and LRP2, as well as amnionless, which form a trimeric endocytosis-competent complex (40, 41). This triad has been established as a key player in nutrient transport by the mammalian visceral YS (1, 26). Mutations in any of the three genes or blocking of their function by antibodies compromises normal embryonic development in rodents, especially affecting neural tube and brain formation (29, 42). The ligand binding domains of cubilin and LRP2 recognize a vast number of diverse proteins, many of which are yolk constituents, such as protein-bound vitamins, hormones, and a range of lipoproteins (28). Furthermore, we have previously shown that the YS EECs also express LR8 (the galline homologue of the mammalian VLDL receptor), which is capable of internalizing apoB-containing lipoproteins, such as VLDL (22), expanding the list of receptors involved in the uptake of yolk precursor macromolecules into the EECs.
During very early yolk sac development, the spreading EECs have been reported to engulf yolk in a nonspecific phagocytic process (8, 43, 44), leading to the incorporation into the EECs of yolk-derived LDs, which are stored in EECs for lipid utilization at later phases of YS growth (5, 6, 8). Our observation that the degradation of LR8-endocytosed yVLDL, as well as of other yolk components imported via LRP2-cubilin-amnionless, probably contributes to the growth of these preexisting cytosolic LDs in the EECs (Fig. 1) supports previous suggestions that receptor-mediated uptake prevails in later stages (i.e. upon vascularization) (5, 8). In regard to lysosomal degradation of yolk precursors, the activities of lysosomal proteases cathepsin B and D have been shown to be high in the endodermal cells of the quail yolk sac (45). Furthermore, inhibition of the rat visceral YS lysosomal proteinases cathepsin B and L result in a decrease in embryonic growth and an increase in fetal abnormalities, including neural tube defects (46).
The newly gained knowledge about yolk component uptake by the YS prompted us to investigate whether the functions of EECs in the area vitellina change upon vascularization of the adjacent mesoderm, as indicated by changes in expression of these proteins. Morphologically, when the transition from the nonvascularized area vitellina to the area vasculosa is complete, the EECs have acquired a uniform columnar character (8). Furthermore, during this transition, the shape and size of intracellular LDs have been reported to change (8).
The morphological changes during vascularization of the mesoderm may be an adaptive consequence of EEC function(s) to progressive vascularization. Indeed, the fact that the endocytic receptor complex LRP2-cubilin-amnionless and the chaperone Mesd are present practically only in the EECs of the area vasculosa, and not in the area vitellina (Fig. 7C), strongly supports this notion. Furthermore, in functional terms, this finding complements data from electron microscopy studies showing that the EECs from the area vasculosa, but not from the area vitellina, contain numerous coated pits and canaliculi at their apical plasma membrane (8). Recently, based on gene expression profiling (9) and ultrastructural studies (35), secretion of serum proteins and lipoproteins by the chicken YS endoderm has been proposed but was not analyzed by biochemical means. Studies by Farese et al. (47), using embryonic-lethal apoB knock-out mice, concluded that apoB plays an essential role in the transport of lipids via secretion of apoB-containing lipoproteins; however, again, lipoprotein synthesis and secretion were not documented by direct evidence. EECs in the area vitellina lack the precursors derived from receptor-mediated uptake of yolk macromolecules and produce only traces of apoB (Fig. 8), and therefore the area vitellina cells may have a limited capacity to secrete lipoproteins. On the other hand, receptor-mediated uptake of yolk components by EECs of the area vasculosa leads to metabolic requirements different from those in EECs of the area vitellina (e.g. they must cope with the large amounts of lipids taken up from the yolk). An explanation for how the area vasculosa EECs manage this metabolic challenge is provided by our findings (Figs. 4–6 and 8) that they produce MTP and secrete apoA-I, apoB, and apoA-V as protein moieties of newly synthesized lipoproteins targeted for ultimate uptake and utilization by the embryo.
The protein composition of the isolated lipoproteins secreted into the media of cultured chick YS EECs (Fig. 6), particularly the high levels of apoA-I, raises several questions and speculations. The presence of apoA-I, the prime apolipoprotein of HDL particles in mammals, male chickens, and immature hens (48), in YS-derived lipoprotein fractions with a density of d < 1.006 g/ml may indicate that the avian yolk sac can produce unusual lipidated macromolecules. Chickens synthesize only apoB100 and not apoB48, which is the main component of mammalian triglyceride-rich chylomicrons, and they also do not produce apoE (48–50), an apolipoprotein that is acquired by chylomicrons in the mammalian circulation. Of interest is the previous suggestion by the group of the late D.L. Williams (51) that in nerve regeneration, avian peripheral apoA-I and mammalian peripheral apoE may perform common functions in these two classes of animals. Chylomicron secretion by mammalian intestinal enterocytes (52) and production by the EECs of the area vasculosa of lipoproteins with very low density containing apoA-I and apoB may represent analogous processes. Furthermore, MTP, essential for the synthesis of both VLDL and chylomicrons in mammals (53, 54), is only expressed in area vasculosa EECs.
To date, the exact nature of chylomicron-like lipoproteins, if they exist, in chickens remains enigmatic. Chicken lipoproteins reported to be derived from the intestine have been called portomicrons because they are believed to be directly secreted into the portal vein, a fact that probably has precluded their isolation and biochemical characterization (48, 49, 55–57). Thus, the apoA-I-harboring EEC-derived, possibly chylomicron-like particles appear to represent a unique chicken-specific lipoprotein class. Significantly, apoA-I is a major ligand recognized by cubilin, a component of the endocytic LRP2-cubilin-amnionless receptor complex (26, 28). Furthermore, apoB and apoA-V, present in the same lipoprotein fraction as apoA-I, are recognized by members of the LDL receptor family (14, 23). Therefore, these triglyceride-rich particles would constitute an ideal nutrient for the developing embryo via uptake and utilization by specific receptors. The secretion of lipid-bound apoA-V by EECs is an intriguing aspect of our novel findings, because the functions of apoA-V include an enhancement of lipoprotein binding to members of the chicken LDL receptor family (14) and the stimulation of proteoglycan-bound lipoprotein lipase (58) at the endothelium of embryonic capillaries. These properties of apoA-V would accelerate delivery to and utilization of lipids by embryonic tissues. Efforts to isolate sufficient amounts of these YS-specific particles for detailed functional analyses are now under way.
Together, these data support a model for the coordinated functional differentiation of the developing chicken YS as outlined in Fig. 9. Key features of this process at the molecular level are that the EECs switch from a storage type to a metabolically highly active type of cells with the acquisition of vascularization. The conversion is characterized by the onset of and/or increase in the production of endocytic yolk protein receptors, of accessory proteins, and of apolipoproteins in addition to apoA-I, as well as by the secretion of lipoproteins by EECs in the neovascularized region of the growing YS. Consequently, in contrast to the EECs of the area vasculosa, which synthesize and secrete (apo)lipoproteins at high rates, the requirement for LD lipids in the EECs of the area vitellina (with very low levels of lipoprotein production; Fig. 8) is expected to be low. This difference might be regulated by proteins that play a role in the stability of LDs (i.e. affect the sensitivity of LDs to lipolysis and/or availability of substrate for lipoprotein synthesis). One of the known proteins with such a function is Plin2, which promotes the accumulation and growth of LDs in cells by compromising lipolysis (39, 59). Plin2 knock-out mice show elevated VLDL secretion rates and a decrease in hepatic lipid content (60), and knockdown of Plin2 in McA-RH7777 hepatoma cells resulted primarily in increased VLDL secretion (61). The fact that Plin2 is absent from the EECs in the area vasculosa but present in the area vitellina (Fig. 7C), where lipoproteins containing apoB are hardly produced at all, indicates that Plin2 performs a so far unknown regulatory function in lipid metabolism of the developing chicken YS.
FIGURE 9.
The developing chicken YS: acquisition of nutrient transport function by differentiation of the EECs is linked to induction of vascularization. Three regions of the growing YS can be distinguished, each schematically represented by an EEC and associated cells. The three regions are the area vitellina (left), a transition zone (center), and the area vasculosa (right). The area vitellina EECs lack endocytic receptors and nonspecifically phagocytose yolk components while they migrate along the yolk surface underneath the ectoderm. Due to the presence of Plin2 on the area vitellina LD surface (red circle around LDs in area vitellina), the lipids of the LDs, particularly triglycerides, are not available for extensive lipidation of apoA-I, and therefore the predominant secreted lipoprotein particles have a high density. In the transition zone, characterized by migration of mesenchymal progenitor cells into the interstitium between ecto- and endoderm, receptors begin to be produced, phagocytotic yolk uptake continues, and as Plin2 levels drop, some LD lipids become available for increased lipoprotein synthesis and secretion. In the area vasculosa, blood vessel formation is coordinated with the enhanced production and localization of endocytic receptors with specificity for selected yolk components to the apical aspect of EECs, the disappearance of Plin2, and the expression of genes specifying MTP and additional apolipoproteins. The LDs are lipolyzed, and the lipids liberated from LDs plus components derived from uptake and lysosomal processing of lipoproteins, such as yVLDL, and of other yolk macromolecules are utilized for the assembly of lipoproteins containing newly synthesized apoA-I, apoB, and apoA-V (i.e. VLDL-like and HDL-like particles that become efficiently secreted for transfer into the adjacent blood vessels, thereby supplying the embryo with nutrients generated by transformation of oocytic yolk in the EECs).
As to the coordination of the switch in the metabolic status of the EECs during vitellina-to-vasculosa differentiation with the process of vascularization (Fig. 9), we can only speculate about the mechanism that orchestrates these events. If the differentiation process were the default pathway for vitellina EECs, it may be suppressed, requiring release of the block; and if not, their differentiation might simply be induced. In both settings, yet unknown factor(s) would have to be invoked. The key to answering this question may lie in the mesodermal vascularization process that occurs in parallel to EEC differentiation. Interestingly, the first YS blood vessels form within mesodermal tissue at sites with close contact to the endodermal cell sheet (62–64). A signal from the endoderm, probably triggered by proteins of the hedgehog family, is crucial for vascular tube formation from mesodermal angioblasts (65–67). In fact, the expression of bone morphogenetic protein 4 (BMP-4) in the mesoderm, which is induced by hedgehog signals from the adjacent endoderm, has been shown to be essential for the proper formation of endothelial tubes (65). Interestingly, an involvement of BMP-4 in the differentiation of murine primitive endodermal cells to visceral endoderm-like cells, which come to lie adjacent to extraembryonic mesoderm during YS formation, has been reported (68, 69). Preliminary experiments have revealed BMP-4 as a potential inducer of the differentiation process for chick YS endodermal cells from the area vitellina to gain an area vasculosa-like appearance. This observation will form the basis for future studies to elucidate the molecular mechanisms underlying the vitellina-to-vasculosa differentiation, which, as shown here, provides the chicken YS with the competence for nutrient transfer.
This work was supported by Austrian Science Fund (FWF) Grants P20218-B11 (to W. J. S.) and P19680-B11 (to M. H.), by the Austrian Ministry of Science and Research GEN-AU Grant GOLD-Genomics of Lipid-associated Disorders (to W. J. S.), and by a DOC-fFORTE-fellowship of the Austrian Academy of Sciences (to J. A. P.).
- YS
- yolk sac
- EEC
- endodermal epithelial cell
- qPCR
- quantitative PCR
- DiI
- 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- yVLDL
- yolk VLDL
- LD
- lipid droplet
- MTP
- microsomal triglyceride transfer protein
- apo
- apolipoprotein
- ggApo
- G. gallus apolipoprotein
- ggLRP2
- ggMTP, ggβ-actin, ggCubilin, ggAmnionless, ggMesd, ggPlin2, and ggGAPDH, G. gallus LRP2, MTP, β-actin, cubilin, amnionless, Mesd, Plin2, and GAPDH, respectively.
REFERENCES
- 1. Zohn I. E., Sarkar A. A. (2010) The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation. Birth Defects Res. A Clin. Mol. Teratol. 88, 593–600 [DOI] [PubMed] [Google Scholar]
- 2. Schneider W. J., Osanger A., Waclawek M., Nimpf J. (1998) Oocyte growth in the chicken. Receptors and more. Biol. Chem. 379, 965–971 [PubMed] [Google Scholar]
- 3. Palis J., McGrath K. E., Kingsley P. D. (1995) Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood 86, 156–163 [PubMed] [Google Scholar]
- 4. Stern C. D., Downs K. M. (2012) The hypoblast (visceral endoderm). An evo-devo perspective. Development 139, 1059–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoshizaki N., Soga M., Ito Y., Mao K. M., Sultana F., Yonezawa S. (2004) Two-step consumption of yolk granules during the development of quail embryos. Dev. Growth Differ. 46, 229–238 [DOI] [PubMed] [Google Scholar]
- 6. Mobbs I. G., McMillan D. B. (1981) Transport across endodermal cells of the chick yolk sac during early stages of development. Am. J. Anat. 160, 285–308 [DOI] [PubMed] [Google Scholar]
- 7. Sheng G. (2010) Primitive and definitive erythropoiesis in the yolk sac. A bird's eye view. Int. J. Dev. Biol. 54, 1033–1043 [DOI] [PubMed] [Google Scholar]
- 8. Mobbs I. G., McMillan D. B. (1979) Structure of the endodermal epithelium of the chick yolk sac during early stages of development. Am. J. Anat. 155, 287–309 [DOI] [PubMed] [Google Scholar]
- 9. Nakazawa F., Alev C., Jakt L. M., Sheng G. (2011) Yolk sac endoderm is the major source of serum proteins and lipids and is involved in the regulation of vascular integrity in early chick development. Dev. Dyn. 240, 2002–2010 [DOI] [PubMed] [Google Scholar]
- 10. Sheng G., Foley A. C. (2012) Diversification and conservation of the extraembryonic tissues in mediating nutrient uptake during amniote development. Ann. N.Y. Acad. Sci. 1271, 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunn N. R., Hogan B. L. (2001) How does the mouse get its trunk? Nat. Genet. 27, 351–352 [DOI] [PubMed] [Google Scholar]
- 12. Kwon G. S., Fraser S. T., Eakin G. S., Mangano M., Isern J., Sahr K. E., Hadjantonakis A. K., Baron M. H. (2006) Tg(Afp-GFP) expression marks primitive and definitive endoderm lineages during mouse development. Dev. Dyn. 235, 2549–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hummel S., Lynn E. G., Osanger A., Hirayama S., Nimpf J., Schneider W. J. (2003) Molecular characterization of the first avian LDL receptor. Role in sterol metabolism of ovarian follicular cells. J. Lipid Res. 44, 1633–1642 [DOI] [PubMed] [Google Scholar]
- 14. Dichlberger A., Cogburn L. A., Nimpf J., Schneider W. J. (2007) Avian apolipoprotein A-V binds to LDL receptor gene family members. J. Lipid Res. 48, 1451–1456 [DOI] [PubMed] [Google Scholar]
- 15. Nimpf J., George R., Schneider W. J. (1988) Apolipoprotein specificity of the chicken oocyte receptor for low and very low density lipoproteins. Lack of recognition of apolipoprotein VLDL-II. J. Lipid Res. 29, 657–667 [PubMed] [Google Scholar]
- 16. Plieschnig J. A., Gensberger E. T., Bajari T. M., Schneider W. J., Hermann M. (2012) Renal LRP2 expression in man and chicken is estrogen-responsive. Gene 508, 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hermann M., Seif F., Schneider W. J., Ivessa N. E. (1997) Estrogen dependence of synthesis and secretion of apolipoprotein B-containing lipoproteins in the chicken hepatoma cell line, LMH-2A. J. Lipid Res. 38, 1308–1317 [PubMed] [Google Scholar]
- 18. Stifani S., George R., Schneider W. J. (1988) Solubilization and characterization of the chicken oocyte vitellogenin receptor. Biochem. J. 250, 467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laemmli U. K., Quittner S. F. (1974) Maturation of the head of bacteriophage T4. IV. The proteins of the core of the tubular polyheads and in vitro cleavage of the head proteins. Virology 62, 483–499 [DOI] [PubMed] [Google Scholar]
- 20. Acloque H., Wilkinson D. G., Nieto M. A. (2008) In situ hybridization analysis of chick embryos in whole-mount and tissue sections. Methods Cell Biol. 87, 169–185 [DOI] [PubMed] [Google Scholar]
- 21. Streit A., Stern C. D. (2001) Combined whole-mount in situ hybridization and immunohistochemistry in avian embryos. Methods 23, 339–344 [DOI] [PubMed] [Google Scholar]
- 22. Hermann M., Mahon M. G., Lindstedt K. A., Nimpf J., Schneider W. J. (2000) Lipoprotein receptors in extraembryonic tissues of the chicken. J. Biol. Chem. 275, 16837–16844 [DOI] [PubMed] [Google Scholar]
- 23. Schneider W. J. (2009) Receptor-mediated mechanisms in ovarian follicle and oocyte development. Gen. Comp. Endocrinol. 163, 18–23 [DOI] [PubMed] [Google Scholar]
- 24. Walzem R. L., Hansen R. J., Williams D. L., Hamilton R. L. (1999) Estrogen induction of VLDLy assembly in egg-laying hens. J. Nutr. 129, 467S–472S [DOI] [PubMed] [Google Scholar]
- 25. Stifani S., Barber D. L., Nimpf J., Schneider W. J. (1990) A single chicken oocyte plasma membrane protein mediates uptake of very low density lipoprotein and vitellogenin. Proc. Natl. Acad. Sci. U.S.A. 87, 1955–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Assémat E., Vinot S., Gofflot F., Linsel-Nitschke P., Illien F., Châtelet F., Verroust P., Louvet-Vallée S., Rinninger F., Kozyraki R. (2005) Expression and role of cubilin in the internalization of nutrients during the peri-implantation development of the rodent embryo. Biol Reprod 72, 1079–1086 [DOI] [PubMed] [Google Scholar]
- 27. Kalantry S., Manning S., Haub O., Tomihara-Newberger C., Lee H. G., Fangman J., Disteche C. M., Manova K., Lacy E. (2001) The amnionless gene, essential for mouse gastrulation, encodes a visceral-endoderm-specific protein with an extracellular cysteine-rich domain. Nat. Genet. 27, 412–416 [DOI] [PubMed] [Google Scholar]
- 28. Kozyraki R., Gofflot F. (2007) Multiligand endocytosis and congenital defects. Roles of cubilin, megalin and amnionless. Curr. Pharm. Des. 13, 3038–3046 [DOI] [PubMed] [Google Scholar]
- 29. Willnow T. E., Hilpert J., Armstrong S. A., Rohlmann A., Hammer R. E., Burns D. K., Herz J. (1996) Defective forebrain development in mice lacking gp330/megalin. Proc. Natl. Acad. Sci. U.S.A. 93, 8460–8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Retzek H., Steyrer E., Sanders E. J., Nimpf J., Schneider W. J. (1992) Molecular cloning and functional characterization of chicken cathepsin D, a key enzyme for yolk formation. DNA Cell Biol. 11, 661–672 [DOI] [PubMed] [Google Scholar]
- 31. Shen X., Steyrer E., Retzek H., Sanders E. J., Schneider W. J. (1993) Chicken oocyte growth. Receptor-mediated yolk deposition. Cell Tissue Res. 272, 459–471 [DOI] [PubMed] [Google Scholar]
- 32. Vieira A. V., Schneider W. J. (1993) Transport and uptake of retinol during chicken oocyte growth. Biochim. Biophys. Acta 1169, 250–256 [DOI] [PubMed] [Google Scholar]
- 33. Mac Lachlan I., Nimpf J., Schneider W. J. (1994) Avian riboflavin binding protein binds to lipoprotein receptors in association with vitellogenin. J. Biol. Chem. 269, 24127–24132 [PubMed] [Google Scholar]
- 34. Vieira P. M., Vieira A. V., Sanders E. J., Steyrer E., Nimpf J., Schneider W. J. (1995) Chicken yolk contains bona fide high density lipoprotein particles. J. Lipid Res. 36, 601–610 [PubMed] [Google Scholar]
- 35. Kanai M., Soji T., Sugawara E., Watari N., Oguchi H., Matsubara M., Herbert D. C. (1996) Participation of endodermal epithelial cells on the synthesis of plasma LDL and HDL in the chick yolk sac. Microsc. Res. Tech. 35, 340–348 [DOI] [PubMed] [Google Scholar]
- 36. Hussain M. M., Bakillah A. (2008) New approaches to target microsomal triglyceride transfer protein. Curr. Opin. Lipidol. 19, 572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shelness G. S., Ledford A. S. (2005) Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr. Opin. Lipidol. 16, 325–332 [DOI] [PubMed] [Google Scholar]
- 38. Hsieh J. C., Lee L., Zhang L., Wefer S., Brown K., DeRossi C., Wines M. E., Rosenquist T., Holdener B. C. (2003) Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell 112, 355–367 [DOI] [PubMed] [Google Scholar]
- 39. Listenberger L. L., Ostermeyer-Fay A. G., Goldberg E. B., Brown W. J., Brown D. A. (2007) Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J. Lipid Res. 48, 2751–2761 [DOI] [PubMed] [Google Scholar]
- 40. Ahuja R., Yammani R., Bauer J. A., Kalra S., Seetharam S., Seetharam B. (2008) Interactions of cubilin with megalin and the product of the amnionless gene (AMN). Effect on its stability. Biochem. J. 410, 301–308 [DOI] [PubMed] [Google Scholar]
- 41. Coudroy G., Gburek J., Kozyraki R., Madsen M., Trugnan G., Moestrup S. K., Verroust P. J., Maurice M. (2005) Contribution of cubilin and amnionless to processing and membrane targeting of cubilin-amnionless complex. J. Am. Soc. Nephrol. 16, 2330–2337 [DOI] [PubMed] [Google Scholar]
- 42. Fisher C. E., Howie S. E. (2006) The role of megalin (LRP-2/Gp330) during development. Dev. Biol. 296, 279–297 [DOI] [PubMed] [Google Scholar]
- 43. Bellairs R. (1963) Differentiation of the yolk sac of the chick studied by electron microscopy. J. Embryol. Exp. Morphol. 11, 201–225 [Google Scholar]
- 44. Lambson R. O. (1970) An electron microscopic study of the entodermal cells of the yolk sac of the chick during incubation and after hatching. Am. J. Anat. 129, 1–19 [DOI] [PubMed] [Google Scholar]
- 45. Gerhartz B., Auerswald E. A., Mentele R., Fritz H., Machleidt W., Kolb H. J., Wittmann J. (1997) Proteolytic enzymes in yolk-sac membrane of quail egg. Purification and enzymatic characterisation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 118, 159–166 [DOI] [PubMed] [Google Scholar]
- 46. Daston G. P., Baines D., Yonker J. E., Lehman-McKeeman L. D. (1991) Effects of lysosomal proteinase inhibition on the development of the rat embryo in vitro. Teratology 43, 253–261 [DOI] [PubMed] [Google Scholar]
- 47. Farese R. V., Jr., Cases S., Ruland S. L., Kayden H. J., Wong J. S., Young S. G., Hamilton R. L. (1996) A novel function for apolipoprotein B. Lipoprotein synthesis in the yolk sac is critical for maternal-fetal lipid transport in mice. J. Lipid Res. 37, 347–360 [PubMed] [Google Scholar]
- 48. Hermier D., Forgez P., Chapman M. J. (1985) A density gradient study of the lipoprotein and apolipoprotein distribution in the chicken, Gallus domesticus. Biochim. Biophys. Acta 836, 105–118 [DOI] [PubMed] [Google Scholar]
- 49. Barakat H. A., St Clair R. W. (1985) Characterization of plasma lipoproteins of grain- and cholesterol-fed White Carneau and Show Racer pigeons. J. Lipid Res. 26, 1252–1268 [PubMed] [Google Scholar]
- 50. Rajavashisth T. B., Dawson P. A., Williams D. L., Shackleford J. E., Lebherz H., Lusis A. J. (1987) Structure, evolution, and regulation of chicken apolipoprotein A-I. J. Biol. Chem. 262, 7058–7065 [PubMed] [Google Scholar]
- 51. Dawson P. A., Schechter N., Williams D. L. (1986) Induction of rat E and chicken A-I apolipoproteins and mRNAs during optic nerve degeneration. J. Biol. Chem. 261, 5681–5684 [PubMed] [Google Scholar]
- 52. van Greevenbroek M. M., de Bruin T. W. (1998) Chylomicron synthesis by intestinal cells in vitro and in vivo. Atherosclerosis 141, S9–S16 [DOI] [PubMed] [Google Scholar]
- 53. Sharp D., Blinderman L., Combs K. A., Kienzle B., Ricci B., Wager-Smith K., Gil C. M., Turck C. W., Bouma M. E., Rader D. J. (1993) Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinaemia. Nature 365, 65–69 [DOI] [PubMed] [Google Scholar]
- 54. Wetterau J. R., Aggerbeck L. P., Bouma M. E., Eisenberg C., Munck A., Hermier M., Schmitz J., Gay G., Rader D. J., Gregg R. E. (1992) Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258, 999–1001 [DOI] [PubMed] [Google Scholar]
- 55. Bensadoun A., Rothfeld A. (1972) The form of absorption of lipids in the chicken, Gallus domesticus. Proc. Soc. Exp. Biol. Med. 141, 814–817 [DOI] [PubMed] [Google Scholar]
- 56. Fraser R., Heslop V. R., Murray F. E., Day W. A. (1986) Ultrastructural studies of the portal transport of fat in chickens. Br. J. Exp. Pathol. 67, 783–791 [PMC free article] [PubMed] [Google Scholar]
- 57. Brown E. M., Dower H. J. (1990) Characterization of apolipoproteins from chicken plasma. J. Chromatogr. 512, 203–212 [DOI] [PubMed] [Google Scholar]
- 58. Nilsson S. K., Heeren J., Olivecrona G., Merkel M. (2011) Apolipoprotein A-V. A potent triglyceride reducer. Atherosclerosis 219, 15–21 [DOI] [PubMed] [Google Scholar]
- 59. Imamura M., Inoguchi T., Ikuyama S., Taniguchi S., Kobayashi K., Nakashima N., Nawata H. (2002) ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am. J. Physiol. Endocrinol. Metab. 283, E775–E783 [DOI] [PubMed] [Google Scholar]
- 60. Chang B. H., Li L., Saha P., Chan L. (2010) Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice. J. Lipid Res. 51, 2132–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Magnusson B., Asp L., Boström P., Ruiz M., Stillemark-Billton P., Lindén D., Borén J., Olofsson S. O. (2006) Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 26, 1566–1571 [DOI] [PubMed] [Google Scholar]
- 62. Mato M., Aikawa E., Kishi K. (1964) Some observations on interstice between mesoderm and endoderm in the area vasculosa of chick blastoderm. Exp. Cell Res. 35, 426–428 [DOI] [PubMed] [Google Scholar]
- 63. Pardanaud L., Yassine F., Dieterlen-Lievre F. (1989) Relationship between vasculogenesis, angiogenesis and haemopoiesis during avian ontogeny. Development 105, 473–485 [DOI] [PubMed] [Google Scholar]
- 64. Wilt F. H. (1965) Erythropoiesis in the chick embryo. The role of endoderm. Science 147, 1588–1590 [DOI] [PubMed] [Google Scholar]
- 65. Astorga J., Carlsson P. (2007) Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development 134, 3753–3761 [DOI] [PubMed] [Google Scholar]
- 66. Vokes S. A., Krieg P. A. (2002) Endoderm is required for vascular endothelial tube formation, but not for angioblast specification. Development 129, 775–785 [DOI] [PubMed] [Google Scholar]
- 67. Vokes S. A., Yatskievych T. A., Heimark R. L., McMahon J., McMahon A. P., Antin P. B., Krieg P. A. (2004) Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development 131, 4371–4380 [DOI] [PubMed] [Google Scholar]
- 68. Artus J., Douvaras P., Piliszek A., Isern J., Baron M. H., Hadjantonakis A. K. (2012) BMP4 signaling directs primitive endoderm-derived XEN cells to an extraembryonic visceral endoderm identity. Dev. Biol. 361, 245–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Paca A., Séguin C. A., Clements M., Ryczko M., Rossant J., Rodriguez T. A., Kunath T. (2012) BMP signaling induces visceral endoderm differentiation of XEN cells and parietal endoderm. Dev. Biol. 361, 90–102 [DOI] [PubMed] [Google Scholar]