Background: Four Rab7 GTPase missense mutants cause autosomal dominant peripheral neuropathy Charcot-Marie-Tooth type 2B (CMT2B) disease.
Results: Rab7 CMT2B mutants impair epidermal growth factor receptor degradation, alter endosomal MAPK signaling, and down-regulate c-fos and Egr-1 expression.
Conclusion: Impaired endosomal trafficking alters transcriptional regulation that is important for axonal survival.
Significance: Rab7 CMT2B mutants affect a common pathway in CMT2B disease pathogenesis.
Keywords: Egr-1, Epidermal Growth Factor Receptor (EGFR), ERK, MAP Kinases (MAPKs), Membrane Trafficking, Neurological Diseases, p38 MAPK, Signaling, Rab7, c-fos
Abstract
Rab7 belongs to the Ras superfamily of small GTPases and is a master regulator of early to late endocytic membrane transport. Four missense mutations in the late endosomal Rab7 GTPase (L129F, K157N, N161T, and V162M) cause the autosomal dominant peripheral neuropathy Charcot-Marie-Tooth type 2B (CMT2B) disease. As yet, the pathological mechanisms connecting mutant Rab7 protein expression to altered neuronal function are undefined. Here, we analyze the effects of Rab7 CMT2B mutants on epidermal growth factor (EGF)-dependent intracellular signaling and trafficking. Three different cell lines expressing Rab7 CMT2B mutants and stimulated with EGF exhibited delayed trafficking of EGF to LAMP1-positive late endosomes and lysosomes and slowed EGF receptor (EGFR) degradation. Expression of all Rab7 CMT2B mutants altered the Rab7 activation cycle, leading to enhanced and prolonged EGFR signaling as well as variable increases in p38 and ERK1/2 activation. However, due to reduced nuclear translocation of p38 and ERK1/2, the downstream nuclear activation of Elk-1 was decreased along with the expression of immediate early genes like c-fos and Egr-1 by the disease mutants. In conclusion, our results demonstrate that Rab7 CMT2B mutants impair growth factor receptor trafficking and, in turn, alter p38 and ERK1/2 signaling from perinuclear, clustered signaling endosomes. The resulting down-regulation of EGFR-dependent nuclear transcription that is crucial for normal axon outgrowth and peripheral innervation offers a crucial new mechanistic insight into disease pathogenesis that is relevant to other neurodegenerative diseases.
Introduction
Charcot-Marie-Tooth (CMT)3 diseases are a clinically heterogenous group of inherited neuropathies that typically affect the peripheral nervous system with a prevalence of 40 in 100,000 individuals (1, 2). CMT neuropathies affect both the sensory and motor neurons and hence are also known as hereditary motor and sensory neuropathies (3). The similarities in CMT disease manifestations result from mutations in over 20 known genes and are ascribed to the extensive interdependence of Schwann cells and neurons and to the fact that the gene products are operative on common pathways (4). Multiple CMT disease-associated genes affect proteins that regulate endocytic membrane transport and thereby impact neurons and Schwann cells in the maintenance of axon viability. Therefore, greater insight into disease mechanisms and prospective treatments depends on a careful elucidation of the affected endocytic pathways.
In the context of altered endocytosis and CMT diseases, it is notable that mutation of the gene encoding the late endocytic Rab7 GTPase causes CMT2B. Although Rab7 is a well characterized regulator of protein and lipid flux to lysosomes, the molecular consequences of CMT2B mutant Rab7 protein expression are incompletely characterized. The mechanistic details of CMT2B pathogenesis are likely to be linked to the close coupling of Rab7 function in the regulation of growth factor receptor endocytosis and signaling. In neurons, Rab7 normally governs the internalization and degradation of EGFR, TrkA, and TrkB (5–8). Rab7 interacts with TrkA on late endosomes and functions to control lysosomal delivery in part by regulating the movement of late endosomes along microtubules through an association with the RILP effector and dynein-dynactin motor complex recruitment (9, 10) to regulate endosomal signaling crucial for neuronal survival (11). In contrast, mimicking the CMT2B autosomal dominant disease state through expression of Rab7 CMT2B mutant proteins results in impairment of lysosomal degradation of TrkA receptors and a parallel enhancement in TrkA signaling (12). Although these studies offered the first evidence that CMT may result from coupled alterations in trafficking and signaling, the effects on other growth factor receptors and transcriptional outcomes remained incompletely understood.
Rab7 is an integral component of the signaling and trafficking scaffolds that assemble on the endosomal membrane and has been studied in the context of EGFR trafficking and down-regulation. Peripheral innervation is critically dependent on proper EGFR signaling (13). Upon EGF stimulation, K-Ras is endocytosed and sorted to late endosomes, where it colocalizes with Rab7 and the p14-MP1 scaffolding protein complex that activates ERK (14). The p14-MP1-p18 complex is involved in late endosome biogenesis and growth factor receptor and ligand transport to the perinuclear region of the cell. The complex is also responsible for ERK1/2 activation by scaffolding MEK1, ERK1, and ERK2 following EGF stimulation (15–17). A second protein complex consisting of Rab7 and Dyn2-CIN85 on late endosomes regulates endosomal trafficking of EGFR to lysosomes for degradation and hence also impacts signaling (5, 6, 18–21). A recent study proposed the importance of subcellular localization of perinuclear late endosomes in regulating EGFR signaling and in turn influencing the signal strength of MAPKs, such as p38 and ERK1/2. Mislocalization of the signaling late endosomes to the cell periphery causes sustained ERK1/2 and p38 signaling that leads to enhanced Elk-1-driven nuclear signaling. However, increased clustering of the endosomes in the perinuclear region through overexpression of the constitutively active Rab7Q67L mutant leads to enhanced ERK1/2 signaling but not p38 activation and consequently decreases Elk-1-driven nuclear signaling (21). Thus, Rab7 as a pivotal integrator of neuronal signaling and trafficking is an important target for further investigation.
To date four missense mutants of Rab7, L129F, K157N, N161T, and V162M, have been reported to cause CMT2B disease (22–24). All of these substitutions occur outside the nucleotide-binding pocket and alter highly conserved amino acids present in all Rab7 proteins, suggesting that they are critical to GTPase function. In contrast, point mutations in or near the nucleotide binding pocket of Rab7 have been well characterized and have been shown to preferentially stabilize Rab7 in the active (GTP-bound, Rab7Q67L) or inactive (GDP-bound, Rab7T22N) states. In particular, inactivating mutants have significant inhibitory effect on endocytic trafficking and signaling (8, 25, 26). In this study, we analyzed the effect of the Rab7 CMT2B mutants on EGFR trafficking and the associated impact on endosomal and nuclear signaling. Because endocytic trafficking and signaling are closely intertwined, a careful correlation of changes in endocytic transport with changes in endosomal signaling will help clarify the mechanisms underlying CMT2B disease pathogenesis.
EXPERIMENTAL PROCEDURES
Cells and Reagents
HeLa, PC12, A431, and BHK-21 cells were from the American Type Culture Collection (Manassas, VA). HeLa cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS), 2 mm glutamine, 1× non-essential amino acids, 50 units/ml penicillin, and 50 μg/ml streptomycin. PC12 cells were grown in DMEM supplemented with 10% horse serum, 2.5% FBS, 2 mm glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. A431 cells were grown in DMEM supplemented with 10% FBS, 2 mm glutamine, 50 units/ml penicillin, and 50 mg/ml streptomycin. BHK-21 cells were grown in complete G-MEM (10% fetal calf serum, 2 mm glutamine, 50 units/ml penicillin, 50 mg/ml streptomycin, and 2.6 mg/ml tryptose phosphate broth). Cell culture reagents were purchased from Invitrogen. Restriction enzymes were from New England Biolabs (Ipswich, MA), and all chemicals used were from Sigma-Aldrich unless otherwise specified. Recombinant EGF was from Invitrogen. Stable cell lines expressing GFP-Rab7 were generated as detailed under “Mutagenesis and Plasmid Construction.”
Mutagenesis and Plasmid Construction
Rab7a used for the plasmid constructs in transient transfections, immunoprecipitation, and confocal studies was Canis lupus familiaris (NM_001003316) (27). GFP-tagged Rab7 CMT2B mutants (L129F, K157N, N161T, and V162M) were constructed by site-directed mutagenesis of wild-type GFP-Rab7 in the pEGFP-C3 vector. The plasmids were used as templates for PCR-based mutagenesis. All amino acid substitutions were generated by a one-step reverse cyclic PCR method using the appropriate base changes in the synthetic oligonucleotides (28). Details of mutagenesis have been described earlier (12). Stable PC12 cell lines were established using these canine Rab7 constructs with a G418 resistance marker. The Rab7 construct used to generate stable HeLa cells was of murine origin, and mutagenesis was performed on Rab7 in pEGFP-C1. The constructs were subcloned into pIRESneo2 and transfected to generate stable HeLa cell lines expressing GFP-Rab7 (29). Details of XAPC7-DsRed plasmid are given in earlier reports (30, 31).
Transient Transfection
Cell lines were cultured as described above and passed on consecutive days to maintain them in logarithmic growth phase immediately prior to transfection. Transfections of HeLa, BHK-21, and A431 cells were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Rab7 expression was maximal 16–24 h post-transfection, and experiments were conducted during this time frame. The colocalization studies of EGF with EEA1 and Lamp1 were done with transiently transfected HeLa cells.
Antibodies
A rabbit polyclonal antibody directed against Rab7 was used for immunoblotting and immunoprecipitation assays as described (30–32). The following commercial antibodies were used: mouse monoclonal antibody (mAb) directed against ERK1/2, mouse mAb directed against phospho-ERK1/2, and β-actin rabbit mAb HRP conjugate, all from Cell Signaling Technologies (Beverly, MA); rabbit polyclonal anti-EGFR and mouse mAb directed against GFP from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); mouse mAb directed against Rab7 from Sigma; and mouse mAb directed against XAPC7 from Affiniti Research Products Ltd. (Mamhead, UK).
Fluorescence Recovery after Photobleaching (FRAP) Assay
BHK-21 cells were seeded and grown to 50–60% confluence on coverslips. GFP-tagged wild-type Rab7, dominant negative Rab7T22N, constitutively active Rab7Q67L, and individual CMT2B mutants were overexpressed in BHK-21 cells using transient transfection. FRAP experiments to monitor GTPase activation were performed based on published procedures at 37 °C and using cells on glass coverslips mounted in a chamber suited for inverted microscopic imaging (10). Live cell images were collected using a Bio-Rad Radiance 2100 mounted on a Nikon TE2000 inverted microscope. A subset of GFP-Rab7 vesicles were bleached for 10 s by a high intensity light illumination at 488 nm, and the fluorescence recovery in the bleached spot was quantified. Fluorescence recovery was measured every 20 s for a total of 620 s for each sample. The FRAP measurements were performed on n = 30 cells for each Rab7 mutant and repeated a total of n = 3. FRAP measurements were made both near the nucleus and on peripheral vesicles with no significant differences. The recovery curves were corrected for loss of total fluorescence due to bleaching induced by repeated imaging.
EGFR Degradation Assays
For degradation assays, stable HeLa, stable PC12 cells, and A431 cells grown on 6-well plates were serum-starved for 5 h in DMEM with 25 μg/ml cycloheximide and stimulated with serum-free medium containing 100 ng/ml EGF (Invitrogen) and 25 μg/ml cycloheximide. At time points (0–4 h), cells were lysed with 80 μl of SDS lysis buffer (10 mm Tris, pH 7.5, 140 mm NaCl, 1% (w/v) SDS, 5 mm EDTA, 2 mm EGTA, 1 mm PMSF, 1 mm Na3VO4,10 mm NaF, 30 mm sodium β-glycerophosphate, and protease inhibitor mixture CLAP (10 μg/ml of chymostatin, leupeptin, antipain, and pepstatin A)) and brief sonication to shear DNA. Cellular debris was removed by centrifugation, and total protein concentration was quantified using a BCA protein assay (Pierce). For siRNA knockdown experiments, our previously reported protocol for endogenous Rab7 ablation was followed (29). Human Rab7 siRNA (Gene ID 7879) was purchased from Dharmacon Technologies.
Immunofluorescence Microscopy
Cells transfected with GFP-tagged Rab7 wild-type and CMT2B mutant plasmids were starved for 14 h and incubated with Alexa555-tagged EGF for 1 h at 4 °C. Cells were kept at 37 °C for 10 min followed by wash in DMEM and reincubated at 37 °C for 20, 60, and 120 min. Cells were fixed with 3% paraformaldehyde and permeabilized for 5 min in 0.1% (v/v) Triton X-100. Blocking and antibody incubations included 0.4% fish skin gelatin (Sigma-Aldrich). Coverslips were viewed on a Zeiss LSM 510 confocal microscope using plan-Neofluor ×40/1.30 oil or plan-Neofluor ×63/1.30 numerical aperture oil objectives, taking 0.4-μm optical sections. All images were exported as tiff files and compiled in Adobe Photoshop (San Jose, CA). For comparative analyses, cells were imaged under identical parameters, and fluorescence intensity was analyzed using Slidebook 4.1 software (Intelligent Imaging Innovations, Denver, CO). The percentage of colocalization was analyzed using Slidebook 4.1 software. Masks were created for each channel for all of the z-stacks acquired for each image, and the overlap was assessed with AND functions to determine the percentage of colocalization of the two channels.
Immunoprecipitation
BHK-21 cells were grown on 100-mm dishes, and GFP-Rab7 wild-type or individual CMT2B mutants were cotransfected with XAPC7-DsRed plasmid using Lipofectamine 2000 (Invitrogen). Mock transfections were performed using a suitable vector lacking an insert. Cell homogenates were prepared in homogenization buffer (20 mm Hepes, pH 7.4, and 2 mm CaCl2) by passage through a 27-gauge needle 10–20 times. Nuclei, cell debris, and large organelles were removed by low speed centrifugation at 1000 rpm for 5 min in an Eppendorf microcentrifuge at 4 °C. Mitochondria were removed by centrifugation at 1950 × g for 20 min, and a crude membrane fraction was collected by centrifugation at 105,000 × g for 2 h. The pellet was resuspended in RIPA buffer (1% (v/v) Nonidet P-40, 0.5% (w/v) deoxycholate, 0.1% (w/v) SDS, 50 mm Tris, pH 7.4, and 150 mm NaCl) containing a protease inhibitor mixture (1 mm phenylmethylsulfonyl fluoride, 1 mm benzamidine, 1 μg/ml CLAP), and immunoprecipitation of XAPC7 was performed using polyclonal rabbit anti-Rab7 as described (26, 30, 31). Immunoprecipitates were recovered with Protein A-Sepharose (Sigma-Aldrich) and resolved on 12% gels and transferred to a Hybond-C membrane (Amersham Biosciences). Immunoblot analysis was performed with a mAb directed against XAPC7 or with our rabbit polyclonal antibody directed against Rab7 (NW112) (32).
Gel Electrophoresis and Immunoblot Analyses
Samples were resolved by SDS-PAGE on 8% gels for EGFR degradation assays and 12% gels for p38 and ERK1/2, transferred to nitrocellulose, and blocked with 3% newborn calf serum in TBST. Samples comparing wild-type and mutant lysates were resolved on a single gel for each experiment. Actin, phosphoproteins, total p38, and ERK1/2 were analyzed on a single blot.
Luciferase Assay
Luciferase assays were used to measure Elk-1-driven gene activity. Assays were performed using the stable Path Detect Trans-Reporter plasmid constructs (Stratagene, La Jolla, CA) according to the manufacturer's instructions. After transfection, HeLa cells stably expressing Rab7 wild-type and CMT2B mutants were serum-starved for 14 h in DMEM, and then they were stimulated for 0, 15, 60, 120, 180, and 240 min with DMEM containing 100 ng/ml EGF. After stimulation with EGF, samples were washed twice with DMEM. Cells were harvested and lysed in luciferase lysis buffer (20 mm glycyl-glycine, pH 7.8, 50 mm NaCl, 2 mm EDTA,1 mm MgSO4, 5 mm dithiothreitol, 1% Triton X-100, and proteinase inhibitors (aprotinin, pepstatin, leupeptin, and Pefabloc SC)) 5 h after stimulation. One aliquot of the cell extract was used to measure the luciferase activity. The other aliquot was used to control protein expression and total protein amounts.
RT-PCR
HeLa cells stably expressing GFP-tagged Rab7 wild-type and CMT2B mutants were serum-starved and followed by EGF stimulation for 1 h. Cells were processed for RNA isolation using the Mini RNeasy isolation kit (Qiagen, Valencia, CA). The cDNA was synthesized using a first-strand cDNA synthesis kit (Invitrogen), and Taqman probe-based quantitative RT-PCR used Taqman Universal PCR Master Mix (Applied Biosystems/Invitrogen), according to the supplied protocols. Primers specific for c-fos and Egr-1 were obtained from Taqman gene expression assays (Applied Biosystems).
Subcellular Fractionation
Nuclear and cytosolic fractions were prepared as described previously with minor modifications (33). HeLa cells and PC12 cells stably expressing GFP-tagged Rab7 wild-type and CMT2B mutants were plated on 60-mm dishes. To separate the nuclear and cytosolic pools of ERK1/2, the cells were serum-starved for 3 h and stimulated with EGF for 15 or 30 min at 37 °C. The cells were washed three times in ice-cold PBS and scraped in 750 μl of lysis buffer (10 mm Tris-HCl, pH 7.4, 10 mm NaCl, 3 mm MgCl2, 0.3% (v/v) Nonidet P-40, 1 mm PMSF, 1 mm Na3VO4, 10 mm NaF, 30 mm sodium β-glycerophosphate, 10 μg/ml chymostatin, 10 μg/ml leupeptin, 10 μg/ml antipain, and 10 μg/ml pepstatin). Cells were incubated on ice for 5 min followed by centrifugation at 500 × g for 5 min to pellet the nuclei. The supernatant was considered the cytosolic fraction. Nuclear pellets were washed in the same lysis buffer without Nonidet P-40. The cytosolic and nuclear fractions were boiled in 2× Laemmli sample buffer and resolved on a 12% SDS-polyacrylamide gel and probed with a phospho-ERK1/2 antibody and phospho-p38 antibody. The purity of the nuclear and cytosolic fractions was checked with antibodies directed against nuclear lamin B and actin, respectively.
Statistical Analysis
Data are represented as means ± S.E. of at least three independent experiments. One-way ANOVA with Dunnett's post hoc test was conducted to compare differences between the means of each group relative to the control wild-type group for all assays. Statistical significance was accepted at p < 0.05 and denoted by an asterisk in the figures. GraphPad Prism version 5.0 was used to perform statistical analyses.
RESULTS
Rab7 Mutants Display Altered Membrane Association Kinetics
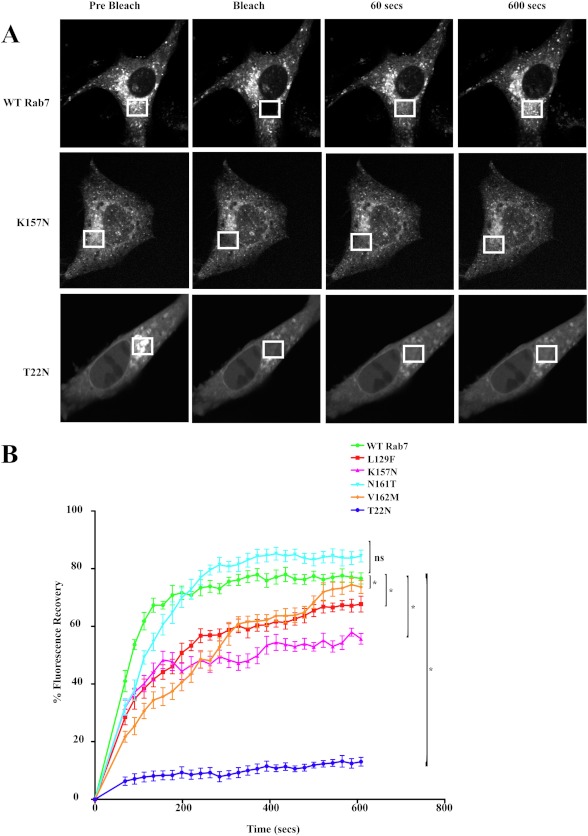
Rab7, like other Rab GTPases, exists in an active, GTP-bound state that is membrane-bound and an inactive, GDP-bound state that is cytosolic. To investigate the membrane association and cytosolic cycling of the Rab7 CMT2B mutants, BHK-21 cells were transiently transfected with GFP tagged wild-type Rab7 and CMT2B mutants, and FRAP analyses were conducted as described previously (10). Regions of the transfected cell were photobleached by high intensity laser, and the recovery of fluorescence was tracked over time (Fig. 1A). The time of recovery of 50% of the fluorescence measured for Rab7 wild-type (t½ = 40.6 s) was comparable with published literature (t½ = 52 s) (10). Three of the Rab7 CMT2B mutants showed a slower rate of fluorescence recovery compared with wild-type Rab7 (Fig. 1B and Table 1). The L129F and V162M showed similarly slow recoveries compared with the wild-type (t½ = 134.3 s and t½ = 127.6 s). Our finding is consistent with a previous report (34), although recovery rates for the two mutants were not explicitly stated. Among the previously untested mutants, K157N also showed a dramatically decreased fluorescence recovery (t½ = 130.7 s), whereas the half-time of fluorescence recovery of N161T (57.7 s) was similar to that of the wild type (40.6 s). As expected, the predominantly GDP-bound, cytosolic dominant negative Rab7T22N mutant showed little or no fluorescence recovery. The differences were statistically significant for Rab7V162M and Rab7L129F, in agreement with previous studies (34), and also for the previously untested Rab7 K157N and dominant negative Rab7T22N mutants. The slower recovery rates are indicative of increased membrane residence times for three of the four Rab7 CMT2B mutants, which would be expected to impact endocytic trafficking.
FIGURE 1.
Rab7 CMT2B mutants show differential membrane cycling. A, BHK-21 cells transfected with GFP-Rab7 WT and CMT2B mutants were used for FRAP studies. Regions of transfected cells were photobleached with a high intensity laser, and recovery of fluorescence was tracked over time. Representative time-lapse images of GFP-Rab7 vesicles before and after photobleaching are shown. At least 30 cells were analyzed for each cell condition in each trial, and results are representative of three independent experiments. B, FRAP analyses show a decreased rate of fluorescence recovery of all of the Rab7 CMT2B mutants compared with the wild type. Rab7T22N mutant showed little or no fluorescence recovery. The differences in fluorescence recovery from three independent trials and 30 cells per trial are plotted ± S.E. (error bars). *, p < 0.05 based on one-way ANOVA with Dunnett's post-test and relative to Rab7 wild-type control.
TABLE 1.
FRAP measurements of Rab7 wild type and other mutants
| Rab7 | t½ | Mobile fraction |
|---|---|---|
| s | % | |
| WT | 40.62 ± 19.4 | 73.46 ± 10.45 |
| L129F | 134.3 ± 9.7 | 56.59 ± 9.79 |
| K157N | 130.7 ± 6.1 | 53.27 ± 5.75 |
| N161T | 57.7 ± 20.5 | 81.71 ± 4.52 |
| V162M | 127.6 ± 13.6 | 48.8 ± 8.34 |
| T22N | 59.5 ± 9.6 | 11.18 ± 2.32 |
Rab7 CMT2B Mutants Delay EGFR Degradation
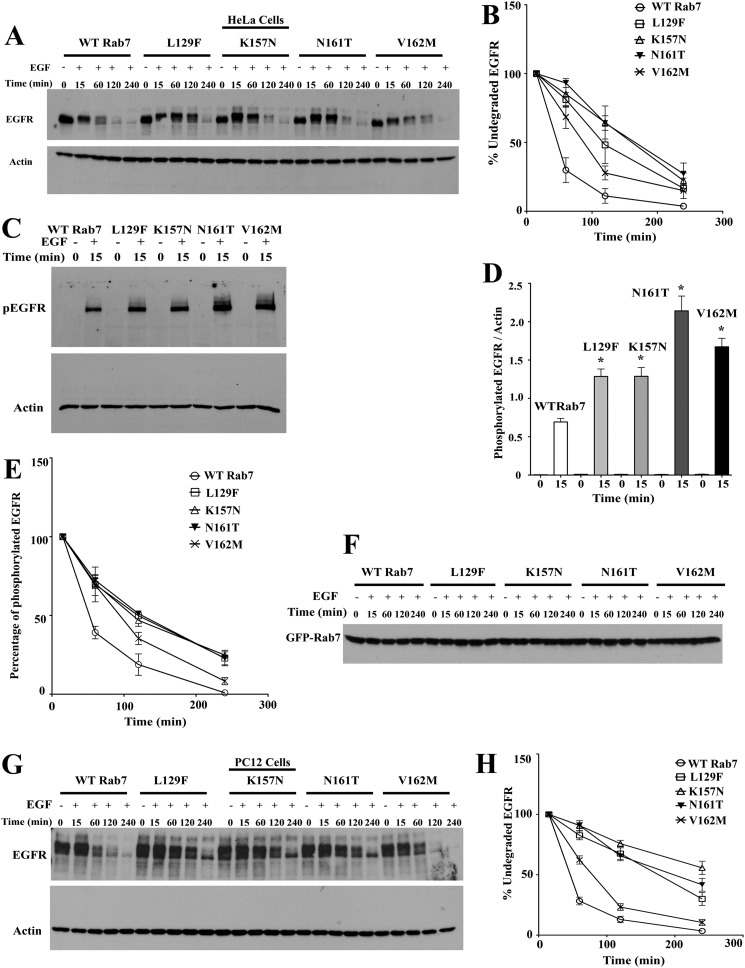
The effect of the Rab7 CMT2B mutants on endocytosis and transport of EGFR was monitored in HeLa cells and PC12 cells stably expressing GFP-tagged wild-type Rab7 and CMT2B mutants. Cells were serum-starved and EGF-stimulated, and EGFR degradation was traced over time as a measure of transport to lysosomes (Fig. 2, A–H). The inclusion of cycloheximide precluded further synthesis of EGFR upon stimulation. The levels of EGFR at 15 min poststimulation were considered to be 100%, and subsequent levels at each time point were calculated accordingly. Expression of all Rab7 CMT2B mutants kinetically delayed EGFR degradation in HeLa cells compared with the wild-type control (Fig. 2A). The Rab7N161T mutant-expressing cells showed the slowest EGFR degradation kinetics, whereas the Rab7V162M mutant exhibited the fastest degradation kinetics among the mutants (Fig. 2B).
FIGURE 2.
Rab7 CMT2B mutants delay EGFR degradation. A, HeLa cells stably transfected with GFP-tagged WT Rab7 and CMT2B disease mutants were starved in DMEM for 5 h and pretreated with cycloheximide for 1 h followed by EGF stimulation (100 ng/ml) in a time-dependent manner as indicated. Representative blots from one of three independent experiments are shown. Subsequently, cells were lysed, and equal amounts of protein were immunoblotted and probed for EGFR with actin as the loading control. B, films from three independent experiments were quantified by ImageJ analysis. The line graphs show levels of undegraded EGFR in GFP-WT Rab7- and CMT2B mutant-expressing cells. In each case, the amount of EGFR was normalized to the amount of actin. C, samples from EGFR degradation assays were probed for phospho-EGFR with actin as the loading control. D, films from at least three independent experiments were quantified by ImageJ analysis. The bar graphs show levels of phospho-EGFR in GFP-Rab7 wild type- and CMT2B mutant-expressing cells. In each case, the amount of phospho-EGFR was normalized to the amount of total actin. E, samples from EGFR degradation assays were probed with antibody against tyrosine 1045 of EGFR to analyze the rate of dephosphorylation of the EGFR at the given time points. The line graphs represent the levels of phosphorylated EGFR normalized to actin. F, samples from EGFR degradation assays were probed with antibody directed against GFP to assess the GFP-Rab7 expression levels for all the samples. The levels of GFP Rab7 were found to be similar for all of the samples. G, neuronal PC12 cells stably transfected with GFP-tagged WT Rab7 and CMT2B disease mutants were starved in DMEM for 5 h and pretreated with cycloheximide for 1 h, followed by EGF stimulation (100 ng/ml) in a time-dependent manner as indicated. Representative blots from one of three independent experiments are shown. Subsequently, cells were lysed, and equal amounts of protein were immunoblotted and probed for EGFR with actin as the loading control. H, films from at least three independent experiments were quantified by ImageJ analysis. The line graphs show levels of undegraded EGFR in GFP-WT Rab7- and CMT2B mutant-expressing cells. In each case, the amount of EGFR was normalized to the amount of actin. Values in all cases are from three independent experiments plotted ± S.E. (error bars). *, p < 0.05 based on one-way ANOVA with Dunnett's post-test and relative to Rab7 wild-type control. Non-linear curve fits were used to analyze degradation rates and obtain half-lives in GraphPad Prism version 5.0 (Table 2).
The activation status of the EGFR was assessed by probing the time-dependent phosphorylation using a phospho-EGFR antibody directed against the tyrosine 1045 residue that is phosphorylated on EGF stimulation. The levels of phosphorylated EGFR were normalized to the levels of total actin. The Rab7 CMT2B mutants showed 2–3-fold higher levels of phosphorylated EGFR within 15 min of EGF stimulation compared with the wild type (Fig. 2, C and D). The Rab7N161T mutant showed the highest level of EGFR phosphorylation followed by V162M, L129F, and K157N. The rate of dephosphorylation of EGFR measured over 240 min was found to be up to 3-fold slower in the disease mutants WT > K157N > V162M > L129F > N161T (Fig. 2E and Table 2). The levels of overexpressed GFP-Rab7 were similar in all of the cell lines under the given experimental conditions (Fig. 2F).
TABLE 2.
Half-lives of activated EGFR, p38, and ERK1/2 in stable HeLa cells expressing GFP Rab7 wild type and CMT2B mutants upon EGF stimulation
| Rab7 | EGFR dephosphorylation t½ | p-p38 dephosphorylation t½ | pERK1/2 dephosphorylation t½ |
|---|---|---|---|
| s | s | s | |
| WT | 35.14 ± 8.68 | 43.76 ± 17.37 | 69.39 ± 7.08 |
| L129F | 87.57 ± 8.47 | 187.9 ± 9.1 | 94.59 ± 3.28 |
| K157N | 74.41 ± 3.59 | 188.4 ± 4.17 | 222.7 ± 4.41 |
| N161T | 103 ± 3.70 | 177.9 ± 4.02 | 236.3 ± 2.62 |
| V162M | 86.42 ± 5.71 | 72.29 ± 10.62 | 138.4 ± 6.58 |
To confirm the results in additional cell lines, we tested the effects of Rab7 CMT2B mutants on EGFR degradation in stable PC12 cells (Fig. 2, G and H) and transiently transfected A431 keratinocytes (supplemental Fig. 1). A delay in EGFR degradation was found in both cell types overexpressing the Rab7 CMT2B mutants. The K157N, N161T, and L129F mutants showed significantly delayed EGFR degradation rates compared with wild type, with the V162M mutant exhibiting degradation rates most similar to wild-type (Fig. 2H). The cumulative data strongly suggest that the disease mutants impair endosomal trafficking and degradation of EGFR and that the effect is also observed in a cell model commonly used for studying neuronal differentiation.
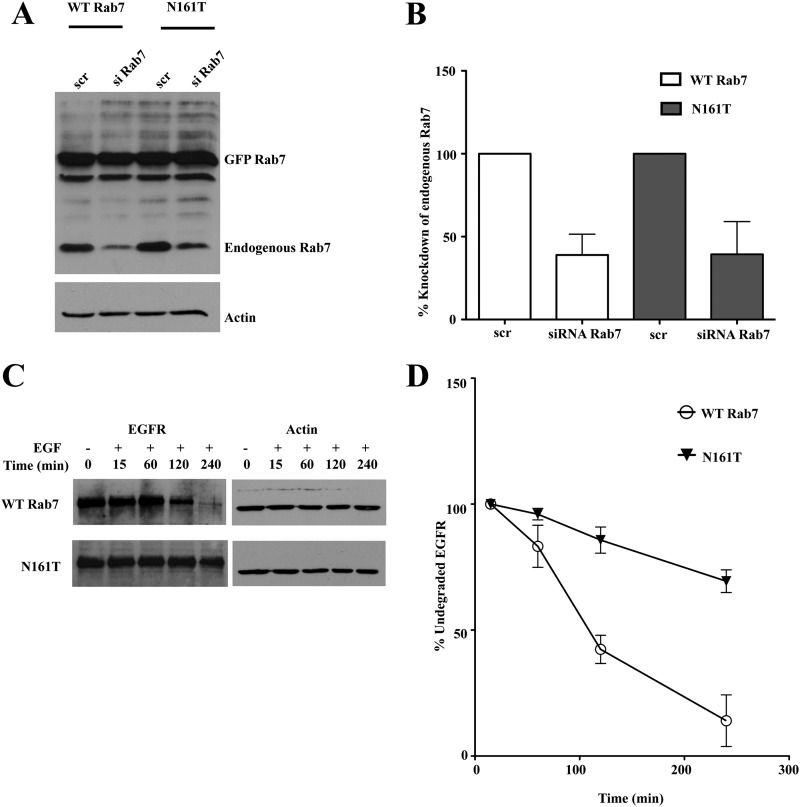
The milder impairment in EGFR trafficking caused by the disease mutants in the HeLa cells relative to PC12 cells or A431 keratinocytes might be attributed to differences in EGFR levels or the presence of endogenous wild-type Rab7 that can still serve to facilitate normal endocytic trafficking in the cells stably overexpressing the Rab7 CMT2B mutants. Coexpression of mutant allele and wild-type alleles is the expected genotype in autosomal dominant CMT2B. Nevertheless, we wanted to further confirm that the slowed EGFR transport and degradation were directly attributable to the Rab7 CMT2B mutants. Therefore, HeLa cells overexpressing mutant GFP-tagged Rab7N161T protein of murine origin were treated with siRNA to specifically deplete the endogenous human wild-type Rab7 or with a scrambled siRNA control. Knockdown efficiency was close to 60% using siRab7 relative to the scrambled (scr) siRNA control (Fig. 3, A and B). Following siRNA treatment, the cells were stimulated with EGF (100 ng/ml) in a time-dependent manner. Knockdown of endogenous human Rab7 in cells overexpressing Rab7N161T showed a more pronounced delay in EGFR degradation than was seen in the presence of endogenous wild-type Rab7 protein (Fig. 3, C and D). It was somewhat surprising that there was also a mild slowdown of EGFR degradation in the cells where endogenous Rab7 was depleted but GFP-tagged Rab7 wild-type protein was overexpressed, suggesting that the GFP tag may have a mild effect on protein function, as has been noted for Arf GTPases (35). Overall, the siRNA data support the conclusion that the Rab7 CMT2B mutants directly impair the trafficking and degradation of EGFR.
FIGURE 3.
Knockdown of endogenous wild-type Rab7 showed a pronounced delay in EGFR degradation in cells overexpressing Rab7N161T mutant compared with the cells overexpressing Rab7 wild-type. A, HeLa cells stably expressing murine GFP-Rab7 WT and CMT2B mutants were transfected with scrambled siRNA (scr) and siRNA targeted against human Rab7a (siRab7) to knockdown the endogenous Rab7. Cells were kept for 48 h to bring about effective knockdown. A representative Western blot is shown, showing the unaltered expression of murine GFP-Rab7 (∼50 kDa) and knockdown of endogenous Rab7 (∼25 kDa). B, densitometric quantification of the Western blots showed ∼50% knockdown of endogenous Rab7 in both GFP-Rab7 wild type- and N161T-expressing cells. C, HeLa cells stably expressing murine GFP-Rab7 wild type and CMT2B mutants were transfected with siRNA targeted against endogenous Rab7. The cells were incubated for 48 h following transfection to bring about effective knockdown of endogenous Rab7, following which cells were starved for 5 h and pretreated with cycloheximide for 1 h and stimulated with EGF for the indicated times. Rab7N161T showed a pronounced delay in EGFR degradation compared with the wild type. D, densitometric quantitation of films from at least three independent experiments showed a faster kinetics of EGFR degradation in cells expressing wild-type Rab7 compared with those expressing Rab7N161T. Error bars, S.E.
Rab7 CMT2B Mutants Slow Endocytic Transport of EGF
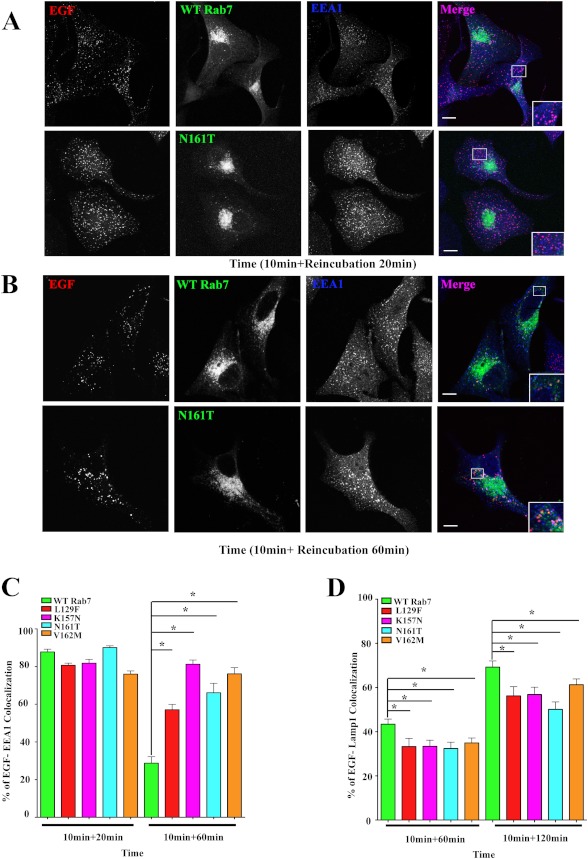
To assess the stage where growth factor transport was being delayed by the Rab7 CMT2B mutants, we tracked the time-dependent transit of internalized EGF from the early endosome to late endosomes/lysosomes. HeLa cells were transiently transfected with GFP-tagged Rab7 wild type or CMT2B mutants. Post-transfection, cells were serum-starved and stimulated with Alexa555-labeled EGF for 10 min, followed by washing and reincubation in serum-free medium for 20 or 60 min, respectively. At the 20 min time point, internalized EGF was significantly associated with EEA1-positive early endosomes in the Rab7 CMT2B mutant cells seen as purple punctate structures in the merged images (Fig. 4A). Quantitative analyses of the degree of colocalization of EGF with EEA1 at 20 min poststimulation were essentially identical in cells expressing wild-type and mutant proteins, suggesting no impairment in internalization or delivery to early endosomes. However, the degree of colocalization of EEA1 and EGF was significantly higher in the Rab7 CMT2B mutants at the longer time point (60 min) compared with the wild-type Rab7 cells, as can be seen from more purple punctuate structures in the Rab7N161T mutant compared with the wild-type (Fig. 4B). The Rab7 K157N mutant exhibited the greatest retention and accumulation of EGF in the EEA1-positive early endosomes, followed by V162M, N161T, and L129F (Fig. 4C). The data indicate a delay in the transit of the internalized EGF from early to late endosomes when the CMT2B Rab7 mutant proteins are expressed.
FIGURE 4.
Rab7 CMT2B mutants show a delay in EGF transport from early to late endosomes. HeLa cells transfected with GFP-tagged wild-type (WT) Rab7 and Rab7 CMT2B mutants were starved for 14 h and stimulated with Alexa555-tagged EGF for 10 min followed by reincubation in DMEM for 20 and 60 min (A and B) at 37 °C, permeabilized with Triton X-100, and stained with anti-EEA-1 antibody. C, bar graphs showing the degree of colocalization of EGF and EEA-1 at 20 and 60 min. At least 30 cells were scored for each construct of one experiment for a total of three independent experiments. The degree of colocalization of EGF with EEA-1 was analyzed by Slidebook 4.1 software, and the differences are represented as ±S.E. (error bars). Scale bar, 10 μm. D, bar graphs showing the degree of colocalization of EGF and Lamp1 at 60 and 120 min. At least 30 cells were scored for each construct of one experiment for a total of three independent experiments. The degree of colocalization of EGF with Lamp1 was analyzed by Slidebook 4.1 software, and the differences are represented as ±S.E. Values in all cases are from three independent experiments plotted ± S.E. *, p < 0.05 based on one-way ANOVA with Dunnett's post-test and relative to Rab7 wild-type control.
The transport of internalized EGF to late endosomes/lysosomes was measured by stimulating with Alexa555-labeled EGF for 10 min, followed by washing and reincubation in serum-free medium for 1–3 h and staining for Lamp1-positive structures. Quantitative analyses at two time points showed a marginally higher degree of colocalization of EGF and Lamp1 in cells expressing wild-type Rab7 compared with the disease mutants (Fig. 4D). This is consistent with the protracted localization of EGF in EEA1-positive early endosomes. At a longer time point (3 h), the Rab7 CMT2B mutants showed a higher degree of colocalization of EGF and Lamp1 (data not shown), consistent with a kinetic delay in progressing to lysosomal degradation. It is worth noting that the difference in the degree of colocalization between the wild-type and Rab7 CMT2B mutants is about 10–15% and is statistically significant. These data further support a delayed trafficking of EGF and its associated receptor to late endocytic compartments in the Rab7 CMT2B mutant-expressing cells compared with the wild type. Our results suggest that the endosomal traffic is not completely blocked in the presence of endogenous wild-type protein but instead is slowed. Such a phenotype is consistent with the slow but progressive nature of CMT2B disease. One possible mechanism for the delayed transport could be differential Rab7 effector protein interactions with the Rab7 CMT2B mutants and could account for the autosomal dominant phenotype (see, for example, supplemental Fig. 2).
Rab7 CMT2B Mutants Enhance p38 Activation
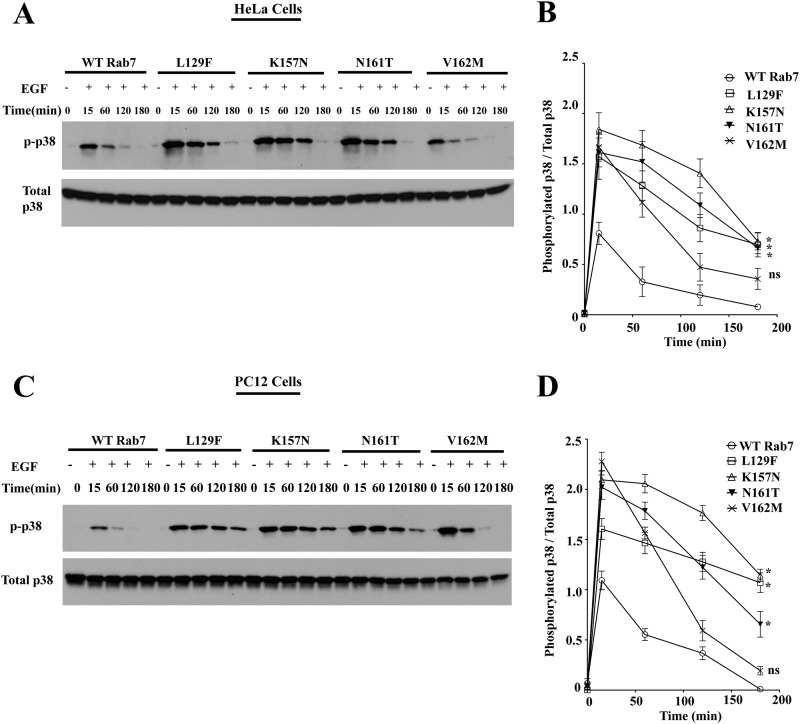
Previous studies have shown that overexpression of the GDP-locked Rab7 T22N mutant leads to impaired degradation of Trk receptors with enhanced endosomal signaling (9). Although the Rab7 CMT2B mutants are competent to bind GTP, their phenotype in the EGFR trafficking assays was reminiscent of dominant negative Rab7. To further study the effect of overexpression of Rab7 CMT2B mutants on EGF-stimulated endosomal signaling, HeLa cells and neuronal PC12 cells stably expressing Rab7 wild-type or CMT2B mutants were starved and stimulated with EGF in a time-dependent manner. Rab7 CMT2B mutants showed enhanced activation of p38 compared with the wild type in both the stable HeLa and PC12 cells overexpressing Rab7 CMT2B mutants (Fig. 5, A–D). Quantification showed higher levels of phosphorylated p38 at all time points in the cells expressing Rab7 CMT2B mutants compared with the wild-type Rab7 (Fig. 5, B and D). The rates of dephosphorylation of activated p38 were up to 4.3-fold slower in the CMT2B mutants as compared with Rab7 wild-type (Table 2).
FIGURE 5.
Rab7 CMT2B mutants show enhanced p38 activation. A, HeLa cells stably expressing GFP-WT Rab7, GFP-Rab7L129F, GFP-Rab7K157N, GFP-Rab7N161T, and GFP-Rab7V162M were starved with DMEM for 5 h and stimulated with EGF (100 ng/ml) for the indicated times. Subsequently, cells were lysed, and equal amounts of protein were immunoblotted and probed for total and phosphorylated p38. B, films from three independent experiments were quantified densitometrically. The line graph shows levels of p-p38 in GFP-Rab7 WT- and CMT2B mutant-expressing cells. In each case, the amount of p-p38 was normalized to the total p38 protein expressed. Differences are represented as ±S.E. (error bars). C, PC12 cells stably expressing GFP-WT Rab7, GFP-Rab7L129F, GFP-Rab7K157N, GFP-Rab7N161T, and GFP-Rab7V162M were starved in DMEM for 5 h and stimulated with EGF (100 ng/ml) for the indicated times. Subsequently, cells were lysed, and equal amounts of protein were immunoblotted and probed for total and phosphorylated p38. D, films from three independent experiments were quantified densitometrically. The line graph shows levels of phosphorylated p38 in GFP-WT Rab7- and CMT2B mutant-expressing cells. In each case, the amount of phosphorylated p38 was normalized to the total p38 protein expressed. Differences are represented as ±S.E. *, p < 0.05 based on one-way ANOVA with Dunnett's post-test and relative to Rab7 wild-type control.
Rab7 CMT2B Mutants Enhance ERK1/2 Activation
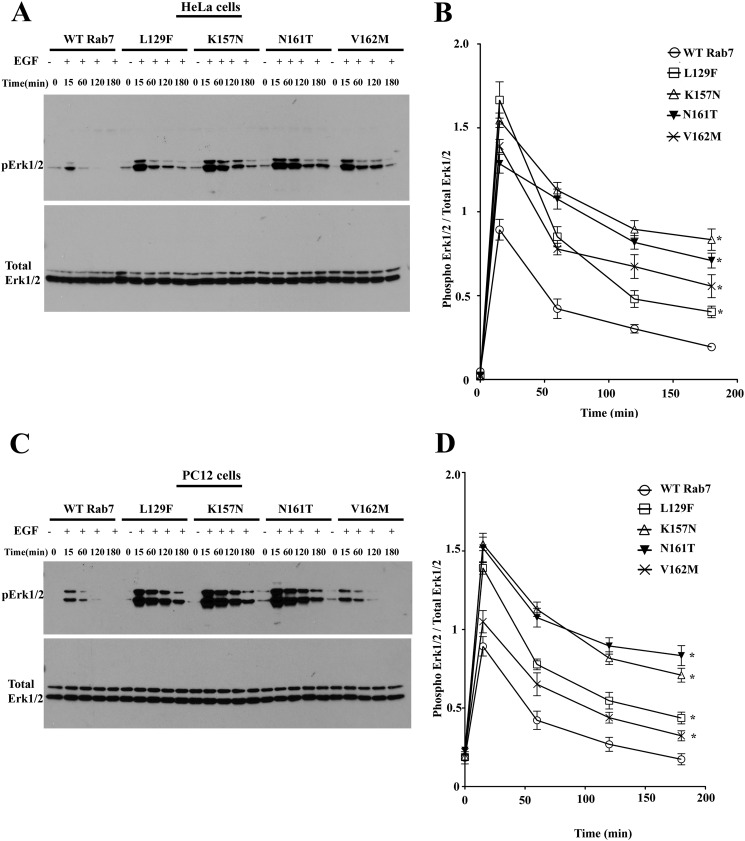
To further study the effect of overexpression of Rab7 CMT2B mutants on EGF-stimulated ERK1/2 signaling, HeLa cells and PC12 cells stably expressing the Rab7 CMT2B mutants were starved and stimulated with EGF in a time-dependent manner. Rab7 CMT2B mutants showed enhanced activation of p38 compared with the wild type in both the stable HeLa and PC12 cells overexpressing Rab7 CMT2B mutants (Fig. 6, A–D). Quantification showed higher levels of phosphorylated ERK1/2 at all time points in the cells expressing Rab7 CMT2B mutants compared with the wild-type Rab7 (Fig. 6, B and D). The rates of dephosphorylation of activated ERK1/2 were up to 3.4-fold slower in the CMT2B mutants as compared with the Rab7 wild type (Table 2).
FIGURE 6.
Rab7 CMT2B mutants show enhanced ERK1/2 activation. A, HeLa cells stably expressing GFP-WT Rab7, GFP-Rab7L129F, GFP-Rab7K157N, GFP-Rab7N161T, and GFP-Rab7V162M were starved with DMEM for 5 h and stimulated with EGF (100 ng/ml) for the indicated times. Subsequently, cells were lysed, and equal amounts of protein were immunoblotted and probed for total and phosphorylated ERK1/2. B, films from three independent experiments were quantified densitometrically. The line graph shows levels of pERK1/2 in GFP-Rab7 WT- and CMT2B mutant-expressing cells. In each case, the amount of pERK1/2 was normalized to the total ERK1/2 protein expressed. Differences are represented as ±S.E. (error bars). C, PC12 cells stably expressing GFP-WT Rab7, GFP-Rab7L129F, GFP-Rab7K157N, GFP-Rab7N161T, and GFP-Rab7V162M were starved in DMEM for 5 h and stimulated with EGF (100 ng/ml) for the indicated times. Subsequently, cells were lysed and equal amounts of protein were immunoblotted and probed for total and phosphorylated ERK1/2. D, films from three independent experiments were quantified densitometrically. The line graph shows levels of pERK1/2 in GFP-WT Rab7- and CMT2B mutant-expressing cells. In each case, the amount of pERK1/2 was normalized to the total ERK1/2 protein expressed. Differences are represented as ±S.E. *, p < 0.05 based on one-way ANOVA with Dunnett's post-test and relative to Rab7 wild-type control.
Rab7 CMT2B Mutants Decrease Elk-1-driven Gene Activation and Downstream c-fos and Egr-1 Expression
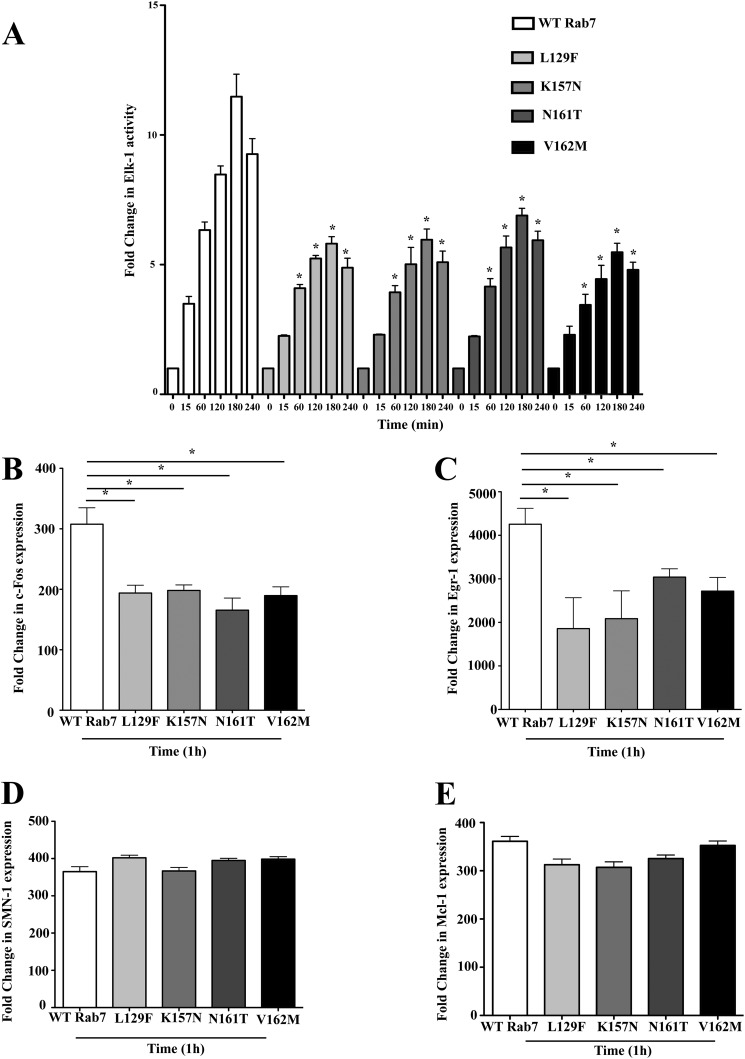
Activated ERK1/2 translocates to the nucleus to activate the transcription factor Elk-1 to drive gene activity (36). To investigate the impact of the enhanced endosomal activation of p38 and ERK1/2 on nuclear signaling, HeLa cells stably expressing the Rab7 CMT2B mutants were co-transfected with plasmids encoding the Elk-1 and luciferase gene controlled by the Elk-1-driven promoter. Following a 14-h starvation, cells were stimulated with EGF for 15–240 min, and luciferase activity was measured. Cells overexpressing the Rab7 CMT2B mutants showed lower Elk-1-driven gene activity at all time points compared with the cells overexpressing wild-type Rab7 (Fig. 7A). Although disease mutants exhibited enhanced ERK activation, the downstream Elk-1 target showed decreased activation. The altered Elk-1-driven gene activity in Rab7 CMT2B-expressing cells would be expected to influence downstream genes under the transcriptional control of Elk-1. Therefore, we also analyzed the expression of two genes directly targeted by active Elk-1, c-fos and Egr-1. Quantitative real-time PCR analyses showed that all of the Rab7 CMT2B mutants have significantly lower levels of c-fos and Egr-1 gene expression compared with the wild type following 1 h of EGF stimulation (Fig. 7, B and C). However, not all genes that have important neuronal functions and have been reported to be subject to Elk-1 regulation were uniformly affected. There was no apparent decrease in SMN1 (survival-of-motor neuron 1), and decreases in Mcl-1 (myeloid cell leukemia-1) transcription were too small to reach statistical significance (Fig. 7, D and E). This suggests that the CMT2B mutants cause selective changes in Elk-1-regulated transcription.
FIGURE 7.
Rab7 CMT2B mutants show lower Elk-1-driven gene activation and c-fos and Egr-1 expression on EGF stimulation. A, HeLa cells stably expressing GFP-tagged WT Rab7 and CMT2B mutants were transfected with plasmids pFR Luc and pFA2 Elk-1 to study Elk-1-driven gene activity. Cells were starved in DMEM for 14 h, followed by EGF stimulation (100 ng/ml) in a time-dependent manner. Following stimulation, cells were kept in serum-free medium for 6 h and lysed, and luciferase activity was determined in a luminometer. Each time point was performed twice and measured in triplicates. Relative light units are normalized to the protein amounts. Disease mutants showed lower Elk-1-driven gene activity. Rab7 CMT2B mutants were compared with the wild-type Rab7 at respective time points. Differences are represented as ±S.E. (error bars). B, HeLa cells stably expressing GFP-Rab7 wild type and CMT2B mutants were serum-starved, EGF-stimulated for 1 h, and processed for RT-PCR as detailed under “Experimental Procedures.” The bar graphs show the lower expression of c-fos upon EGF stimulation in cells expressing GFP-Rab7 CMT2B mutants compared with the wild type. Differences are represented as ±S.E. C, bar graphs showing the lower expression of Egr-1 upon 1-h EGF stimulation in cells expressing GFP-Rab7 CMT2B mutants compared with the wild type. Differences are represented as ±S.E. D and E, bar graphs showing no difference in expression levels of SMN1 and Mcl-1 upon 1-h EGF stimulation in cells expressing GFP-Rab7 CMT2B mutants compared with the wild type. *, p < 0.05 based on one-way ANOVA with Dunnett's post-test and relative to Rab7 wild-type control.
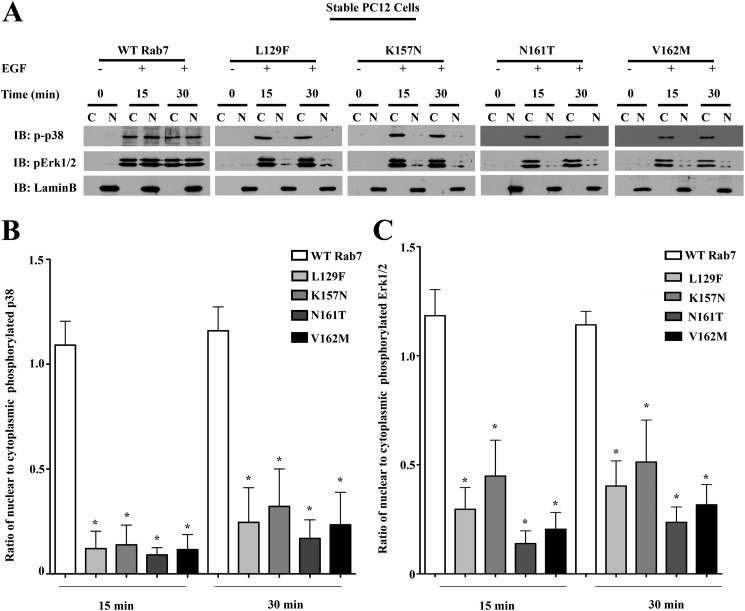
Rab7 CMT2B Mutants Cause Delayed Nuclear Shuttling of Activated MAPKs
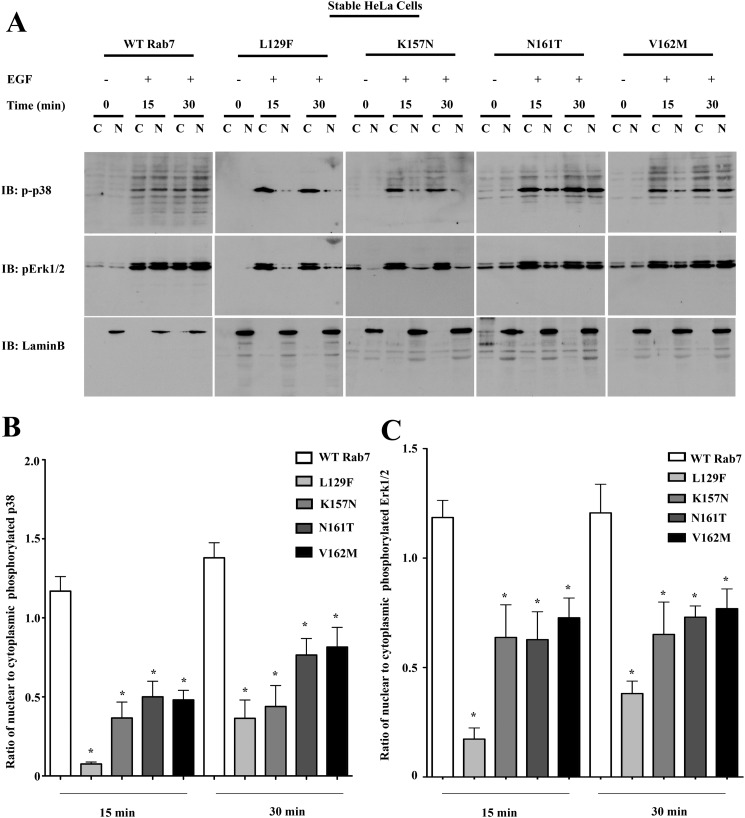
The apparently contradictory observation that Rab7 CMT2B mutants show an enhanced p38 and ERK1/2 activation at the signaling endosomes whereas the Elk-1-driven gene activity and expression of its downstream target genes are impaired was subjected to further study. We previously observed an altered nuclear translocation of activated ERK1/2 in the Rab7 CMT2B mutants compared with the wild type following NGF stimulation of PC12 cells (12). We therefore tested if a similar delay in nuclear translocation of activated p38 and ERK1/2 exists in response to EGF signaling and might account for the observed decrease in Elk-1-driven gene activity in the disease mutants. HeLa cells and neuronal PC12 cells stably expressing the GFP-tagged Rab7 CMT2B mutants were serum-starved and stimulated with EGF for 15 and 30 min prior to subcellular fractionation to separate the nuclear and cytosolic fractions. ERK1/2 nuclear translocation was impaired most significantly in the L129F and K157N mutants and to a lesser extent in the N161T and V162M mutants. Activated p38 nuclear translocation was markedly delayed in cells expressing all of the Rab7 CMT2B mutants. The nuclear marker Lamin B and actin (not shown) served as a control for purity of the nuclear and cytosolic fractions. In both the HeLa (Fig. 8) and PC12 (Fig. 9) cells stably expressing the Rab7 CMT2B mutants, the nuclear/cytoplasmic ratio of activated p38 distribution was significantly lower than in cells expressing Rab7 wild type (Figs. 8B and 9B). Furthermore, in HeLa cells, Tukey's post hoc test showed that the nuclear/cytoplasmic ratio of activated p38 was significantly lower in the L129F mutant compared with N161T and V162M mutants. In PC12 cells, no statistically significant differences were observed between the individual CMT2B mutants. Similar trends were observed for the distribution of activated ERK1/2 (Figs. 8C and 9C). These results indicate that the Rab7 CMT2B mutants have similar effects in different cell lines in impairing nuclear signaling.
FIGURE 8.
Rab7 CMT2B mutants show delayed nuclear shuttling of activated MAPKs in HeLa cells. A, stable HeLa cells expressing GFP-tagged WT and Rab7 CMT2B mutants were serum-starved for 5 h and stimulated with EGF (100 ng/ml) for 15 and 30 min, respectively. Subsequently, cells were lysed, subjected to subcellular fractionation to separate the cytosolic (C) and nuclear (N) fractions, and immunoblotted with antibodies directed against phosphorylated p38 and phosphorylated ERK1/2. The purity of fractions was confirmed by probing for the nuclear marker lamin B. Rab7 CMT2B mutants showed an impaired nuclear translocation of p-p38. Rab7L129F- and Rab7K157N-expressing cells showed an impaired translocation of pERK1/2, which was also observed in Rab7N161T- and Rab7V162M-expressing cells but to a lesser extent. B and C, bar graphs showing the differences in the nuclear/cytoplasmic ratio of activated p38 and ERK1/2 following 15 and 30 min of EGF stimulation. Differences are represented as ±S.E. (error bars). *, p < 0.05 based on one-way ANOVA with Dunnett's post-test and relative to Rab7 wild-type control. Comparison between CMT2B mutants was based on one-way ANOVA with Tukey's post-test and showed L129F significantly different from N161T and V162M for p38 activation at 15 min of EGF stimulation (p < 0.05).
FIGURE 9.
Rab7 CMT2B mutants show delayed nuclear shuttling of activated MAPKs in neuronal PC12 cells. A, Stable PC12 cells expressing GFP-tagged WT and Rab7 CMT2B mutants were serum-starved for 5 h and stimulated with EGF (100 ng/ml) for 15 and 30 min, respectively. Subsequently, cells were lysed, subjected to subcellular fractionation to separate the cytosolic (C) and nuclear (N) fractions, and immunoblotted with antibodies directed against phosphorylated p38 and phosphorylated ERK1/2. The purity of fractions was confirmed by probing for the nuclear marker lamin B. Rab7 CMT2B mutants showed an impaired nuclear translocation of p-p38 and pERK1/2. B and C, bar graphs showing the differences in the nuclear/cytoplasmic ratio of activated p38 and ERK1/2 following 15 and 30 min of EGF stimulation. Differences are represented as ±S.E. (error bars). *, p < 0.05 based on one-way ANOVA with Dunnett's post-test and relative to Rab7 wild-type control. Comparison between CMT2B mutants based on one-way ANOVA with Tukey's post-test showed no significant differences between the mutants.
DISCUSSION
Charcot-Marie-Tooth type 2B disease occurs due to L129F, K157N, N161T, and V162M substitutions in the ubiquitously expressed Rab7 protein. A perplexing mystery of the Rab7 mutations is that only the peripheral nerves are affected. This has been attributed to the presence of one or more specific effectors of Rab7 that affect unique pathways in the peripheral neurons to cause disease (37). The Rab7 CMT2B mutants being predominantly in the GTP bound state have been suggested to cause more rapid degradation of the endocytosed growth factor receptor (38). However, our results suggest slowed membrane cycling of Rab7 CMT2B mutants, which is directly linked to slowed trafficking and degradation of endocytosed growth factor receptors, leading to enhanced and prolonged endosomal signaling. A possible explanation for slowed transport is a differential interaction of Rab7 CMT2B mutants with downstream effector proteins. Previous studies have indicated enhanced interaction of Rab7L129F and Rab7V162M mutants with cholesterol sensor ORP1L, Vps13C, and clathrin heavy chain (34). Another study showed that Rab7K157N does not interact with the retromer complex, Vps35/29/26. This was the first report of a loss of function for the Rab7K157N disease mutant and led to the postulate that neuronal degeneration might result from altered retromer regulation of endosomal membrane protein sorting. The Rab7 CMT2B mutants, including the K157N mutant, are autosomal dominant mutants; CMT2B patients harboring the K157N mutation would still possess the wild-type Rab7, but the disease mutant would affect the kinetics of VPS35/29/26 recruitment, leading to reduced efficiency of endosomal protein sorting, which could be detrimental in the very long axons that are affected in CMT2B patients (29). In fact, our studies also show a loss of function of Rab7V162M mutant that has diminished interaction with the Rab7 interactor α proteasomal subunit, XAPC7. XAPC7 has been shown to be a negative regulator of endocytic transport (30), and the impaired interaction with Rab7V162M mutant caused a relatively faster degradation of internalized growth factor receptor compared with the other disease mutants (supplemental Fig. 2). In cytototoxic T-lymphocytes, Rab7V162M mutant showed more tightly clustered lytic granules around the centrosome compared with those of the wild type, although increased dynein recruitment was not observed (39). We think that such allele-specific differences may account for kinetic differences between the mutants and help to reveal mechanistic information about Rab7 effector interactions. However, allele-specific differences in the ability of the Rab7 CMT mutants to bind various effectors cannot solely be at the root of the common pathology seen in all Rab7 CMT2B mutations. The effects that are common to the different alleles (e.g. altered EGFR signaling/degradation) are most likely to be informative regarding the pathologic mechanisms.
Previous studies have shown that overexpression of the constitutively active Rab7Q67L mutant causes a perinuclear accumulation of endosomes that delays the delivery of EGFR to the late endosomes and subsequent EGFR degradation. Additionally, ERK signaling was enhanced, whereas p38 signaling decreased (21). We have also seen the Rab7 CMT2B-positive endosomes to be more concentrated in the perinuclear region although not quite as tightly clustered as the Rab7Q67L-positive endosomes, which might partially explain the enhanced p38 and ERK activation and slower EGFR degradation. Enhanced endosomal p38 and ERK1/2 activation may reflect slowed membrane sequestration of the internalized activated growth factor receptor into intralumenal vesicles. The relatively higher membrane residence time of Rab7 CMT2B mutants suggested by FRAP experiments accounts for an increased residence time of the MAPK scaffold, in turn accounting for the enhanced ERK1/2 and p38 activation following growth factor stimulation of Rab7 CMT2B mutant-expressing cells. In PC12 cells, EGF stimulation results in transient MAPK activation in contrast to NGF signaling, where sustained activation of MAPK continues signaling by phosphorylating transcription factors. These differential consequences of signaling determine the proliferative (EGF-induced) and differentiation (NGF-induced) capacity of the cell (40). Interestingly, EGFR family members have also been shown to function in concert, autonomously or non-autonomously, to regulate the development of the peripheral nervous system (13, 41–43). Hence, impaired trafficking of internalized epidermal growth factor receptors can reduce nuclear signaling and expression of immediate early genes to cause an overall developmental defect. Our previous study showed a similar defect in nuclear shuttling of activated ERK1/2 upon NGF stimulation in PC12 cells expressing the Rab7 CMT2B mutants (12). All four Rab7 CMT2B mutants showed differential nuclear to cytoplasmic distribution of activated ERK upon NGF stimulation of TrkA receptors compared with the wild type. In the present study, we analyzed the effects of EGF stimulation in non-neuronal HeLa and neuronal PC12 cells. Indeed, nuclear distribution of the signaling MAPKs was reduced, as previously observed for TrkA signaling. The data are consistent with a prolonged half-life of activated pEGFR due to delayed trafficking to lysosomes, linked to the prolonged membrane residence time of the mutant Rab7 proteins and a concomitant increased membrane residence time of the MAPK scaffold.
Our study also showed a reduced nuclear signaling with lower Elk-1-driven gene activity and lower transcription of c-fos and Egr-1 in the disease mutants, most likely due to the impaired nuclear shuttling of activated p38 and ERK1/2 in the disease mutants. Elk-1 uses different DNA binding modes to regulate distinct classes of target genes involved in cellular processes ranging from cytoskeletal regulation to apoptosis (44). For example, transcription of SMN1 is dependent on ETS-domains in Elk-1 rather than on serum response factor element recognition, as well as on glycogen synthase kinase 3 (45, 46). This may explain why we did not observe any impact of the CMT2B mutants on SMN1 transcription. Mcl-1 is a prosurvival factor that regulates autophagy in mature neurons via a Rab7-Beclin-dependent pathway (45, 47). Although Mcl-1 is also subject to transcriptional regulation by Elk-1, reductions in Mcl-1 transcription were too slight to reach statistical significance in our experimental system but might be more critical in a neuronal context. Overall, our results suggest an impaired trafficking and altered endosomal and nuclear signaling in cells expressing the Rab7 CMT2B mutants and lay the foundation for a better understanding of the disease pathology.
Rab7, although a ubiquitous protein, affects principally the peripheral nervous system in CMT2B disease. The more pronounced effect of the CMT2B mutants in reducing nuclear translocation of p38 and ERK1/2 in PC12 cells might be attributed to neuronal cell-specific effectors and/or a greater sensitivity to defects in endocytic trafficking, as suggested previously (37, 48). Charcot-Marie-Tooth diseases typically manifest in the second or third decade of life. Impaired trafficking of growth factor receptors may over time alter the balance between proliferative and differentiation signals that cause axonal shortening and eventual loss of contacts necessary for viability. Peripheral axons can grow as long as 1 meter, and impairment in long distance transport from the axonal tip to the cell body through differential interaction of Rab7 with specific effector proteins may impair endosomal traffic and in turn affect endosomal and nuclear signaling to cause progressive neurodegeneration. The similarities between CMT diseases and hereditary spastic paraplegia, together with the recent identification of SNPs in Rab7 that are linked to late onset Alzheimer disease (49), suggest that our findings may be more broadly relevant to other neurodegenerative diseases.
Supplementary Material
Acknowledgments
Images in this paper were generated in the University of New Mexico and Cancer Center Fluorescence Microscopy Shared Resource (funded as detailed on the Web site). We thank Dr. Edward Bedrick for helpful discussions regarding the statistical analyses. We thank the University of New Mexico flow cytometry facility for the selection of GFP-Rab7-expressing stable PC12 cell lines. We are grateful to Jamie Padilla and Genevieve Phillips for excellent technical support and Janet Kelly and Lauren Thal for outstanding administrative support.
This work was supported by National Science Foundation Grant 425 (MCB0956027) (to A. W. N.) and Medical Research Council Grant G0701444 (to M. N. J. S.).

This article contains supplemental Figs. 1 and 2.
- CMT
- Charcot-Marie-Tooth
- EGFR
- EGF receptor
- FRAP
- fluorescence recovery after photobleaching
- ANOVA
- analysis of variance
- pERK and p-p38
- phosphorylated ERK and p38, respectively.
REFERENCES
- 1. Barisic N., Claeys K. G., Sirotković-Skerlev M., Löfgren A., Nelis E., De Jonghe P., Timmerman V. (2008) Charcot-Marie-Tooth disease. A clinico-genetic confrontation. Ann. Hum. Genet. 72, 416–441 [DOI] [PubMed] [Google Scholar]
- 2. Shy M. E., Garbern J. Y., Kamholz J. (2002) Hereditary motor and sensory neuropathies. A biological perspective. Lancet Neurol. 1, 110–118 [DOI] [PubMed] [Google Scholar]
- 3. Young P., Suter U. (2003) The causes of Charcot-Marie-Tooth disease. Cell Mol. Life Sci. 60, 2547–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bienfait H. M., Baas F., Koelman J. H., de Haan R. J., van Engelen B. G., Gabreëls-Festen A. A., Ongerboer de Visser B. W., Meggouh F., Weterman M. A., De Jonghe P., Timmerman V., de Visser M. (2007) Phenotype of Charcot-Marie-Tooth disease Type 2. Neurology. 68, 1658–1667 [DOI] [PubMed] [Google Scholar]
- 5. Snider M. D. (2003) A role for rab7 GTPase in growth factor-regulated cell nutrition and apoptosis. Mol. Cell. 12, 796–797 [DOI] [PubMed] [Google Scholar]
- 6. Edinger A. L., Cinalli R. M., Thompson C. B. (2003) Rab7 prevents growth factor-independent survival by inhibiting cell-autonomous nutrient transporter expression. Dev. Cell 5, 571–582 [DOI] [PubMed] [Google Scholar]
- 7. Deinhardt K., Salinas S., Verastegui C., Watson R., Worth D., Hanrahan S., Bucci C., Schiavo G. (2006) Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 52, 293–305 [DOI] [PubMed] [Google Scholar]
- 8. Bucci C., Thomsen P., Nicoziani P., McCarthy J., van Deurs B. (2000) Rab7. A key to lysosome biogenesis. Mol. Biol. Cell 11, 467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saxena S., Bucci C., Weis J., Kruttgen A. (2005) The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J. Neurosci. 25, 10930–10940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jordens I., Fernandez-Borja M., Marsman M., Dusseljee S., Janssen L., Calafat J., Janssen H., Wubbolts R., Neefjes J. (2001) The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11, 1680–1685 [DOI] [PubMed] [Google Scholar]
- 11. Ascano M., Bodmer D., Kuruvilla R. (2012) Endocytic trafficking of neurotrophins in neural development. Trends Cell Biol. 22, 266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. BasuRay S., Mukherjee S., Romero E., Wilson M. C., Wandinger-Ness A. (2010) Rab7 mutants associated with Charcot-Marie-Tooth disease exhibit enhanced NGF-stimulated signaling. PLoS One 5, e15351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maklad A., Nicolai J. R., Bichsel K. J., Evenson J. E., Lee T. C., Threadgill D. W., Hansen L. A. (2009) The EGFR is required for proper innervation to the skin. J. Invest. Dermatol. 129, 690–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu A., Tebar F., Alvarez-Moya B., López-Alcalá C., Calvo M., Enrich C., Agell N., Nakamura T., Matsuda M., Bachs O. (2009) A clathrin-dependent pathway leads to KRas signaling on late endosomes en route to lysosomes. J. Cell Biol. 184, 863–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teis D., Wunderlich W., Huber L. A. (2002) Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell 3, 803–814 [DOI] [PubMed] [Google Scholar]
- 16. Nada S., Hondo A., Kasai A., Koike M., Saito K., Uchiyama Y., Okada M. (2009) The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J. 28, 477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teis D., Taub N., Kurzbauer R., Hilber D., de Araujo M. E., Erlacher M., Offterdinger M., Villunger A., Geley S., Bohn G., Klein C., Hess M. W., Huber L. A. (2006) p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J. Cell Biol. 175, 861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schroeder B., Weller S. G., Chen J., Billadeau D., McNiven M. A. (2010) A Dyn2-CIN85 complex mediates degradative traffic of the EGFR by regulation of late endosomal budding. EMBO J. 29, 3039–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sokolova I. P., Arnautov A. M., Nikol'skiï N. N., Kornilova E. S. (1998) [Studies of small GTPase Rab7 association with endosomes of cells expressing normal and mutant forms of epidermal growth factor receptors]. Tsitologiia 40, 862–868 [PubMed] [Google Scholar]
- 20. Ceresa B. P., Bahr S. J. (2006) rab7 activity affects epidermal growth factor:epidermal growth factor receptor degradation by regulating endocytic trafficking from the late endosome. J. Biol. Chem. 281, 1099–1106 [DOI] [PubMed] [Google Scholar]
- 21. Taub N., Teis D., Ebner H. L., Hess M. W., Huber L. A. (2007) Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling. Mol. Biol. Cell 18, 4698–4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verhoeven K., De Jonghe P., Coen K., Verpoorten N., Auer-Grumbach M., Kwon J. M., FitzPatrick D., Schmedding E., De Vriendt E., Jacobs A., Van Gerwen V., Wagner K., Hartung H. P., Timmerman V. (2003) Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am. J. Hum. Genet. 72, 722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meggouh F., Bienfait H. M., Weterman M. A., de Visser M., Baas F. (2006) Charcot-Marie-Tooth disease due to a de novo mutation of the RAB7 gene. Neurology 67, 1476–1478 [DOI] [PubMed] [Google Scholar]
- 24. Houlden H., King R. H., Muddle J. R., Warner T. T., Reilly M. M., Orrell R. W., Ginsberg L. (2004) A novel RAB7 mutation associated with ulcero-mutilating neuropathy. Ann. Neurol. 56, 586–590 [DOI] [PubMed] [Google Scholar]
- 25. Feng Y., Press B., Wandinger-Ness A. (1995) Rab 7. An important regulator of late endocytic membrane traffic. J. Cell Biol. 131, 1435–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Press B., Feng Y., Hoflack B., Wandinger-Ness A. (1998) Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J. Cell Biol. 140, 1075–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. (1990) Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62, 317–329 [DOI] [PubMed] [Google Scholar]
- 28. Gama L., Breitwieser G. E. (2002) Generation of epitope-tagged proteins by inverse polymerase chain reaction mutagenesis. Methods Mol. Biol. 182, 77–83 [DOI] [PubMed] [Google Scholar]
- 29. Seaman M. N., Harbour M. E., Tattersall D., Read E., Bright N. (2009) Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J. Cell Sci. 122, 2371–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dong J., Chen W., Welford A., Wandinger-Ness A. (2004) The proteasome α-subunit XAPC7 interacts specifically with Rab7 and late endosomes. J. Biol. Chem. 279, 21334–21342 [DOI] [PubMed] [Google Scholar]
- 31. Mukherjee S., Dong J., Heincelman C., Lenhart M., Welford A., Wandinger-Ness A. (2005) Functional analyses and interaction of the XAPC7 proteasome subunit with Rab7. Methods Enzymol. 403, 650–663 [DOI] [PubMed] [Google Scholar]
- 32. Stein M. P., Feng Y., Cooper K. L., Welford A. M., Wandinger-Ness A. (2003) Human VPS34 and p150 are Rab7 interacting partners. Traffic 4, 754–771 [DOI] [PubMed] [Google Scholar]
- 33. Tohgo A., Pierce K. L., Choy E. W., Lefkowitz R. J., Luttrell L. M. (2002) β-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J. Biol. Chem. 277, 9429–9436 [DOI] [PubMed] [Google Scholar]
- 34. McCray B. A., Skordalakes E., Taylor J. P. (2010) Disease mutations in Rab7 result in unregulated nucleotide exchange and inappropriate activation. Hum. Mol. Genet. 19, 1033–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jian X., Cavenagh M., Gruschus J. M., Randazzo P. A., Kahn R. A. (2010) Modifications to the C-terminus of Arf1 alter cell functions and protein interactions. Traffic 11, 732–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whitmarsh A. J., Shore P., Sharrocks A. D., Davis R. J. (1995) Integration of MAP kinase signal transduction pathways at the serum response element. Science 269, 403–407 [DOI] [PubMed] [Google Scholar]
- 37. Cogli L., Piro F., Bucci C. (2009) Rab7 and the CMT2B disease. Biochem. Soc. Trans. 37, 1027–1031 [DOI] [PubMed] [Google Scholar]
- 38. Cogli L., Progida C., Lecci R., Bramato R., Krüttgen A., Bucci C. (2010) CMT2B-associated Rab7 mutants inhibit neurite outgrowth. Acta Neuropathol. 120, 491–501 [DOI] [PubMed] [Google Scholar]
- 39. Daniele T., Hackmann Y., Ritter A. T., Wenham M., Booth S., Bossi G., Schintler M., Auer-Grumbach M., Griffiths G. M. (2011) A role for Rab7 in the movement of secretory granules in cytotoxic T lymphocytes. Traffic 12, 902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marshall C. J. (1995) Specificity of receptor tyrosine kinase signaling. Transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 [DOI] [PubMed] [Google Scholar]
- 41. Lin W., Sanchez H. B., Deerinck T., Morris J. K., Ellisman M., Lee K. F. (2000) Aberrant development of motor axons and neuromuscular synapses in erbB2-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 97, 1299–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Golding J. P., Trainor P., Krumlauf R., Gassmann M. (2000) Defects in pathfinding by cranial neural crest cells in mice lacking the neuregulin receptor ErbB4. Nat. Cell Biol. 2, 103–109 [DOI] [PubMed] [Google Scholar]
- 43. Riethmacher D., Sonnenberg-Riethmacher E., Brinkmann V., Yamaai T., Lewin G. R., Birchmeier C. (1997) Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature 389, 725–730 [DOI] [PubMed] [Google Scholar]
- 44. Odrowaz Z., Sharrocks A. D. (2012) ELK1 uses different DNA binding modes to regulate functionally distinct classes of target genes. PLoS Genet. 8, e1002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Demir O., Aysit N., Onder Z., Turkel N., Ozturk G., Sharrocks A. D., Kurnaz I. A. (2011) ETS-domain transcription factor Elk-1 mediates neuronal survival. SMN as a potential target. Biochim. Biophys. Acta 1812, 652–662 [DOI] [PubMed] [Google Scholar]
- 46. Makhortova N. R., Hayhurst M., Cerqueira A., Sinor-Anderson A. D., Zhao W. N., Heiser P. W., Arvanites A. C., Davidow L. S., Waldon Z. O., Steen J. A., Lam K., Ngo H. D., Rubin L. L. (2011) A screen for regulators of survival of motor neuron protein levels. Nat. Chem. Biol. 7, 544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Germain M., Nguyen A. P., Le Grand J. N., Arbour N., Vanderluit J. L., Park D. S., Opferman J. T., Slack R. S. (2011) MCL-1 is a stress sensor that regulates autophagy in a developmentally regulated manner. EMBO J. 30, 395–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agola J., Jim P., Ward H., Basuray S., Wandinger-Ness A. (2011) Rab GTPases as regulators of endocytosis, targets of disease and therapeutic opportunities. Clin Genet. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vardarajan B. N., Bruesegem S. Y., Harbour M. E., St George-Hyslop P., Seaman M. N., Farrer L. A. (2012) Identification of Alzheimer disease-associated variants in genes that regulate retromer function. Neurobiol. Aging. 33, 2231.e15–2231.e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.