Background: Src kinase-associated phosphoprotein 2 (SKAP2) is a substrate of Src family kinases.
Results: SKAP2 suppresses actin polymerization and cell migration by inhibiting the interaction between WAVE2 and cortactin.
Conclusion: SKAP2 is a negative regulator of actin assembly and tumor invasion.
Significance: The novel function of SKAP2 was found as a candidate for the suppressor of tumor invasion.
Keywords: Actin, Invasion, Migration, Src, Tumor, SKAP2, WAVE2, Cortactin
Abstract
In our attempt to screen for substrates of Src family kinases in glioblastoma, Src kinase-associated phosphoprotein 2 (SKAP2) was identified. Although SKAP2 has been suggested to be associated with integrin-mediated adhesion of hematopoietic cells, little is known about its molecular function and the effects in other types of cells and tumors. Here, we demonstrate that SKAP2 physically associates with actin assembly factors WAVE2 and cortactin and inhibits their interaction. Cortactin is required for the membrane localization of WAVE2, and SKAP2 suppresses actin polymerization mediated by WAVE2 and cortactin in vitro. Knockdown of SKAP2 in NIH3T3 accelerated cell migration and enhanced translocation of WAVE2 to the cell membrane, and those effects of SKAP2 depend on the binding activity of SKAP2 to WAVE2. Furthermore, reduction of SKAP2 in the glioblastoma promoted tumor invasion both in ex vivo organotypic rat brain slices and immune-deficient mouse brains. These results suggest that SKAP2 negatively regulates cell migration and tumor invasion in fibroblasts and glioblastoma cells by suppressing actin assembly induced by the WAVE2-cortactin complex, indicating that SKAP2 may be a novel candidate for the suppressor of tumor progression.
Introduction
Glioblastoma is the most malignant form of glioma (World Health Organization, grade IV). Despite recent advances in surgery, radiology, and chemotherapy, the prognosis of patients with glioblastoma has not improved substantially because of its invasive phenotype. Therefore, the discovery of novel therapeutic targets to control the invasiveness of glioblastoma is urgently needed.
Src family kinases (SFKs)2 receive different stimuli at the cell membrane and transmit signals to their substrates, which regulate different cellular functions, including proliferation, adhesion, migration, and stress responses (1). SFK-related pathways are likely to be involved in the progression of different malignancies (2). We previously reported the roles of different SFK substrates, including C9orf10/Ossa, CDCP1, and ARAP3, in the development of scirrhous gastric carcinoma (3–5).
In a screen for substrates of SFKs affecting invasion in glioblastoma, Src kinase-associated phosphoprotein 2 (SKAP2, also known as SCAP2, SKAP-HOM, SKAP55R, RA70, and PRAP) was identified. Previous reports showed that SKAP2 is a substrate of SFKs and highly homologous to SKAP55 (SKAP1) (6, 7). SKAP2 is expressed widely in lymphohematopoietic cells (6–8). SKAP2 and SKAP1 have been suggested to be involved in the cell adhesion of hematopoietic cells and immune cells through their association with integrin and filamentous actin (F-actin) (9–14). Both SKAP1 and SKAP2 have a pleckstrin homology (PH) domain for interaction with lipids at the membrane (12) and a carboxyl-terminal Src homology (SH) 3 domain (Fig. 1C) (15). In addition, SKAP2, but not SKAP1, has a coiled-coil domain at its amino terminus for self-dimerization (12) (Fig. 1C). However, the molecular functions of SKAP2 are not well understood, and little is known about the role of SKAP2 in tumors. Unexpectedly, we found that SKAP2 has negative effects on cell migration and tumor progression using glioblastoma cells and mouse fibroblasts. SKAP2 inhibits actin polymerization by interacting with the actin assembly factors WAVE2 (Wiskott-Aldrich syndrome protein (WASP) family VErprolin-homologous protein 2) (16, 17) and cortactin (18). These results suggest that SKAP2 may affect the invasiveness of tumors by modulating actin assembly at the cell membrane. Unlike other SFK substrates that promote tumor progression obtained from our similar approach (3, 4), SKAP2 may be a unique SFK substrate that negatively regulates the invasiveness of certain types of tumor cells.
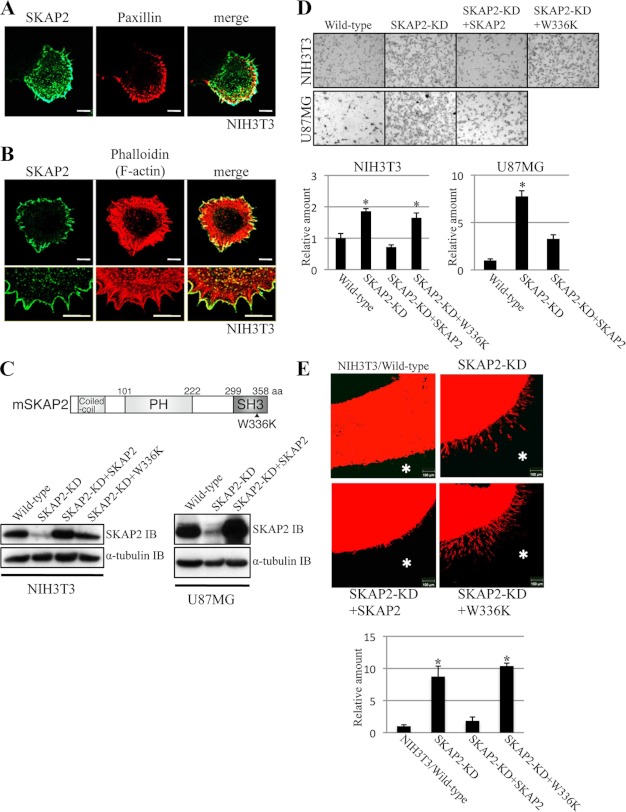
FIGURE 1.
SKAP2 localizes at the cell membrane and attenuates cell migration and invasion. A and B, NIH3T3 cells were immunostained using anti-SKAP2 (green) and anti-paxillin (A, red) antibodies or phalloidin (B, red). Bottom panels in B are enlarged images of the upper panels. Bar, 10 μm. C, top, structure of mouse SKAP2. SKAP2 consists of a coiled-coil domain, a PH domain, and an SH3 domain. The W336K SKAP2 mutation is represented. Bottom, SKAP2 was stably knocked down (KD) in NIH3T3 and U87MG cells using miRNA, and wild-type or W336K mutant SKAP2 was stably added back to the SKAP-KD cells. The expression level of SKAP2 was examined by immunoblotting (IB) using an anti-SKAP2 antibody. aa, amino acids. D, motility of NIH3T3 and U87MG cell lines was examined by the transwell assay. Top, representative pictures of migrated cells on the bottom surface of the transwell membrane. Bottom, number of migrated cells was counted. E, top, invasion of NIH3T3 cell lines into a matrix containing type I-collagen and Matrigel. The indicated cells were labeled with DiI (red), overlaid on top of the gel in the transwells, and incubated for 72 h. The gels were fixed and sliced and observed using confocal microscopy. Representative pictures are shown. Bar, 100 μm. *, position of the gel. Bottom, invading chains of cells were quantified by measuring the area of protrusion into the gel from the arc of cell clumps. D, bottom, and E, bottom, the amount of wild-type cells was defined as 1. The results from three independent experiments are shown as the means ± S.D. *, p < 0.01.
EXPERIMENTAL PROCEDURES
Plasmids and siRNA
The cDNA construct of mouse SKAP2 was a kind gift of Dr. Takashi Momoi. Mutations in SKAP2 were introduced using site-directed mutagenesis. The fragments of the SKAP2-SH3 domain and PH domain were generated by PCR-based techniques. The cDNAs of WAVE2 and cortactin were amplified using RT-PCR in U87MG cells. For stable knockdown, the sequence for miRNA of SKAP2 (5′-TGCTGTTCAACATCTGCCAACAGGTTGTTTTGGCCACTGACTGACAACCTGTTCAGATGTTGAA-3′) was subcloned into the retroviral vector pDON-AI. For stable add-back, SKAP2-HA cDNA with silent mutations (wild-type SKAP, 142AAC CTG TTG GCA GAT GTT GAA162; SKAP2 add-back, 142AAC TTA CTA GCA GAT GTG GAG162; mutagenized nucleic acids are represented by the underlined type) was subcloned into the retroviral vector pBabe-puro for the infection of NIH3T3 and into the lentiviral vector pCSII-puro for the infection of U87MG cells. The stealth siRNA sequence for the knockdown of cortactin was 5′-GAGUACCAGUCGAAGCUUUTT-3′.
Transfection and Virus Infection
Cortactin was knocked down and added back in NIH3T3 cells by electroporation using a microporator MP-100 (Digital Bio) with 1 × 105 cells, 50 pmol of siRNA, and 0.5 μg of DNA. COS-1 cells were transfected using FuGENE 6 (Roche Applied Science), and in other experiments, cells were transfected using Lipofectamine 2000 (Invitrogen), according to each procedure. Recombinant retroviral or lentiviral plasmids were cotransfected with packaging vectors into 293 cells to allow the production of those viral particles. U87MG and NIH3T3 cells stably expressing SKAP2 miRNA were established after viral infection through selection in medium containing G418. Stable add-back of SKAP2 in SKAP2-KD cells and U87F4 or C6 cells stably overexpressing SKAP2-HA were established through puromycin selection.
Antibodies and Reagents
Purchased antibodies were as follows: actin (mouse monoclonal, Millipore, MAB1501; cortactin (mouse monoclonal, Millipore, 05-180); CXCR4 (rabbit polyclonal, Abcam, ab2074); EGFP (rabbit polyclonal, Abnova, PAB8931); ERK (rabbit polyclonal, Cell Signaling Technology, 9102S); FLAG (mouse monoclonal, Sigma, F3165); GST (POD-conjugated, mouse monoclonal, Nakarai, 04559-74); HA (mouse monoclonal, Babco, 16B12); paxillin (mouse monoclonal, Invitrogen, 03-6100); phosphotyrosine (mouse monoclonal, Millipore, 4G10); rhodamine (mouse monoclonal, Rockland, 200-301-246); SKAP2 (rabbit polyclonal, ProteinTech Group, 12926-1-AP); α-tubulin (mouse monoclonal, Sigma, T5168); and WAVE2 (rabbit polyclonal, Cell Signaling Technology, 3659S). F-actin was stained using phalloidin 546 (Invitrogen, A22283) or phalloidin 670 (Cytoskeleton, PHDN1).
Immunoprecipitation
Cell lysates were prepared with protease inhibitors in PLC buffer (50 mm Hepes (pH 7.5), 150 mm NaCl, 1.5 mm MgCl2, 1 mm EGTA, 10% glycerol, 100 mm NaF, 1 mm Na3VO4, and 1% Triton X-100). To precipitate the proteins, 1 μg of monoclonal or affinity-purified polyclonal antibody was incubated with 500 μg of cell lysate for 2 h at 4 °C and then precipitated with protein G beads for 1 h at 4 °C. Immunoprecipitates were extensively washed with PLC buffer and immunoblotted.
Immunofluorescence
Immunofluorescence was performed as described previously (19). The cover glasses were coated with fibronectin (Biological Industries), and a total of 1.0 × 104 cells were plated on a glass. The cells were fixed using 4% paraformaldehyde 3 h after plating if there were no instructions. The cells were stained and visualized using a confocal microscopic system (Zeiss). The quantification of signal intensities was conducted using ImageJ software (National Institutes of Health).
Subcellular Fractionation
NIH3T3 cells were plated on cell culture dishes and incubated until the cells adhered to the dish, then immediately harvested, and lysed. The cell lysates were divided into cytoplasm and plasma membrane fractions using the plasma membrane protein extraction kit (Biovision). Each fraction was immunoblotted.
GST Pulldown Assay
GST-SKAP2-SH3 domain, GST-SKAP2-PH domain, and GST alone were expressed in competent cells BL21 (DE3), using isopropyl 1-thio-β-d-galactopyranoside induction, and purified using glutathione-Sepharose beads (GE Healthcare). Beads coated with 10 μg of each GST fusion protein were incubated with the protein extract of COS-1 transfected with WAVE2-HA or cortactin-HA for 4 h at 4 °C. The beads were washed and immunoblotted using an anti-HA antibody.
Transwell Assay (Monolayer)
The transwell assay was performed using transwell chambers with a polycarbonate nucleopore membrane (Falcon). Filters (8-μm pore size) were rehydrated with 100 μl of medium. Then 1 × 104 cells in 200 μl of serum-free medium were seeded onto the upper part of each chamber, and the lower compartment was filled with 700 μl of the same medium with 10% FBS. After incubation for 8 h at 37 °C, nonmigrated cells on the upper surface of the filter were wiped off with a cotton swab, and the migrated cells on the lower surface of the filter were fixed and stained with Giemsa stain solution. The total number of migrated cells was determined by counting cells in five microscopic fields per well, and the extent of migration was expressed as the average number of cells per microscopic field.
Three-dimensional Gel Invasion Assay
The transwell migration assay conditions were described under “Transwell Assay (Monolayer)” and have been described previously (20). The gel (serum-free medium containing 0.2 mg/ml type I-collagen (Nitta Gelatin) and 2.5 mg/ml Matrigel matrix (BD Biosciences)) was laid onto the upper chamber of transwells. Then 3 × 104 cells labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbacyanine perchlorate (Invitrogen) in 200 μl of serum-free medium were overlaid on the gel. The lower compartment of the transwell was filled with 700 μl of the same medium with 10% FBS. After incubation for 72 h at 37 °C, the gel was fixed using 4% paraformaldehyde and cut into 200-μm thick slices. 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbacyanine perchlorate-labeled cells in the slices were visualized using a confocal microscopic system (Zeiss). The amount of invading cell chains was quantified using ImageJ.
Actin Polymerization Assay
The actin polymerization assay was performed by modifying a previously reported procedure (21, 22). Briefly, protein G beads were coated with anti-EGFP antibody and EGFP-cortactin or EGFP-WAVE2. The beads were incubated at room temperature for 1 h with HeLa cell extracts, the extracts of U87MG cells retrovirally expressing WAVE2, 130 ng/μl recombinant GST-SKAP2, 2.5 μm rhodamine-conjugated monomeric G-actin (Cytoskeleton), and 1 mm ATP. Wild-type U87MG extracts and GST alone were used as the negative controls of WAVE2 and GST-SKAP2, respectively. After incubation, the beads were washed, denatured by SDS-sample buffer, and subjected to immunoblotting. The quantification of signal intensities was conducted using ImageJ. Instead of rhodamine-actin, pyrene-actin (Cytoskeleton) was also used in this actin polymerization assay, and signal intensities of polymerized pyrene-actin were measured using a micro plate reader Infinite M200 (Tecan).
Ex Vivo and in Vivo Tumor Implantation
All animal experimental protocols were approved by the Committee for Ethics of Animal Experimentation, and the experiments were conducted in accordance with the guidelines for Animal Experiments of Akita University. Cerebral organotypic cultures were prepared from Wistar rats (CLEA, Japan) and cultured according to the standard interface method with some modifications (23). Briefly, the brain was removed, dissected, and transversely sliced at the putamen into slices with a thickness of 400 μm on a microslicer (Dosaka EM). The isolated slices were incubated on ice for at least 15–20 min in DMEM and were transferred onto a polycarbonate nucleopore membrane (Falcon). Tumor cells labeled with 3,3′-dioctadecyloxacarbocyanine (Invitrogen) were spotted (0.5 ml containing 2 × 104 cells) on the putamen of sliced rat brain. The slices were maintained in a humidified incubator at 37 °C in a 5% CO2 atmosphere, and invasion of tumor cells was visualized through a fluorescence dissecting scope (Olympus). Intracranial dissemination of tumors was tested by intracranial injection of 2 × 105 tumor cells suspended in 30 ml of DMEM into 6-week-old BALB/c nude mice (n = 5, each). The mice were sacrificed 14 days after injection. The brains of nude mice were fixed and embedded in paraffin. Paraffin blocks were sectioned into slices and subjected to H&E staining.
RESULTS
SKAP2 Localizes to the Leading Edge of the Cell Membrane
To identify SFK substrates expressed specifically in malignant tumors, we established a subline of human glioblastoma U87MG cells possessing a high potential for intracranial invasion. Through repeated selection, i.e. repeated cycles of intracranial implantation of U87MG cells into nude mice, removal of tumor nodules, and culture, we obtained clone U87F4 (supplemental Fig. S1A). Phosphotyrosine-containing proteins bound to SFKs were isolated from the extracts of U87F4 tumor nodules by sequential purification using c-YES SH2 domain and anti-phosphorylated tyrosine antibody affinity columns (supplemental Fig. S1B). A band of ∼55 kDa was cut out and analyzed using mass spectrometry as described previously (3, 24), which resulted in the identification of SKAP2.
The expression profile of SKAP2 in tumors was obtained by immunoblotting cell extracts of various tumor cell lines with an anti-SKAP2 antibody (supplemental Fig. S2A). Although SKAP2 was detected in several cancer cells, a very high level of SKAP2 expression was observed in mouse fibroblasts NIH3T3 cells. SKAP2 expression was compared between NIH3T3 and RAW 264.7 mouse macrophages that were reported to express SKAP2 at a high level (10). Because α-tubulin level was very low in RAW 264.7, ERK (MAPK) was analyzed as a loading control. Expression of SKAP2 in NIH3T3 was at a significant level, compared with RAW 264.7 (supplemental Fig. S2B).
Examination of the intracellular localization of endogenous SKAP2 in NIH3T3 cells showed that SKAP2 localized mainly to the leading edge of the cell membrane (Fig. 1, A and B). Although SKAP2 was also detected in the cytoplasm, it was rarely present in focal adhesions where paxillin is localized or actin bundles of microspikes (Fig. 1, A and B).
SKAP2 Attenuates Cell Migration and Invasion
The localization of SKAP2 at the cell periphery led us to investigate whether SKAP2 contributes to the construction of the leading edge of the cell membrane and cell migration. SKAP2 was knocked down (KD) in NIH3T3 and U87MG cells by miRNA and then added back (Fig. 1C). To generate miRNA-resistant SKAP2, silent mutations were introduced into mouse SKAP2 cDNA. The migration of these cells was examined by transwell assay (20). SKAP2-KD cells migrated more rapidly than wild-type cells both in the U87MG and NIH3T3 cell lines, whereas restoration of wild-type SKAP2 significantly suppressed cell migration (Fig. 1D). The invasiveness of NIH3T3 cells was assessed by three-dimensional gel invasion assay. Wild-type NIH3T3 cells did not invade into the matrix when they were overlaid on the matrix containing type I collagen and Matrigel. However, the invasion of SKAP2-KD NIH3T3 cells was significant and was detected as chains of cells invading the gel (Fig. 1E). Invasion of NIH3T3 cells was suppressed after wild-type SKAP2 was restored in SKAP2-KD NIH3T3 cells (Fig. 1E). SKAP2 has an SH3 domain, and the SH3 domain interacts with many proline-rich proteins (15). We investigated whether the SH3 domain of SKAP2 is involved in the regulation of cell migration and invasion. The W336K SKAP2 mutant has a point mutation in the double tryptophan of the SH3 domain that impairs its binding to proline-rich proteins (15). The W336K SKAP2 mutant did not rescue the migration and invasion of SKAP2-KD NIH3T3 cells (Fig. 1, D and E). These results indicated that SKAP2 has a negative effect on cell migration and invasion and that its SH3 domain is important for this effect.
To confirm the negative effect of SKAP2 on cell migration and invasion, SKAP2 was overexpressed in U87F4 and a rat glioblastoma C6 that are highly invasive cell lines (Fig. 2A). SKAP2 overexpression inhibited the migration and invasion of these cells (Fig. 2, B and C).
FIGURE 2.
SKAP2 overexpression inhibits cell migration and invasion. A, SKAP2 was stably overexpressed in U87F4 and C6 cells using lentivirus. The expression level of SKAP2 was examined by immunoblotting (IB) using an anti-SKAP2 antibody. B, motility of U87F4 and C6 was examined by the transwell assay as in Fig. 1D. C, invasion of U87F4 and C6 into a matrix containing type I collagen and Matrigel was analyzed as in Fig. 1E. Bar, 100 μm. *, position of the gel.
Because SKAP2 was identified as a tyrosine-phosphorylated protein in U87F4, the effect of phosphorylation of SKAP2 on cell migration and invasion was examined. For this purpose, the known phosphorylation site tyrosine 260 of SKAP2 was substituted to phenylalanine (8). SKAP2 Y260F mutant suppressed the migration and invasion of U87F4 more than the wild-type SKAP2 (Fig. 2, B and C). Invasion of C6 was also suppressed more effectively by SKAP2 Y260F (Fig. 2C). These results suggest that the inhibitory effect of SKAP2 on cell migration and invasion might be attenuated by its phosphorylation.
SKAP2 Interacts with WAVE2 and Affects Its Localization
The localization of SKAP2 to the edge of the cell membrane and the effects of the SKAP2-SH3 domain on cell migration suggested that SKAP2 may interact with proline-rich proteins that coexist at the cell membrane and contribute to the reorganization of the actin cytoskeleton. Among several molecules, we focused on WAVE2, a well known actin assembly factor containing a proline-rich domain that regulates actin assembly at the cell membrane (16, 17). When SKAP2 and WAVE2 were coexpressed in COS-1 cells, WAVE2 was coimmunoprecipitated with SKAP2 but at a significantly reduced level by the W336K SKAP2 mutant (Fig. 3A). Reciprocally, wild-type but not W336K SKAP2 was coimmunoprecipitated with WAVE2 (Fig. 3B). The association of SKAP2 and WAVE2 was also observed in physiological conditions of NIH3T3 (Fig. 3C). In addition, WAVE2 expressed in COS-1 was pulled down by the GST-SKAP2-SH3 domain but not by the GST-SKAP2-PH domain or GST alone (Fig. 3D). These results indicated that the SH3 domain of SKAP2 is the binding site for WAVE2. Furthermore, the effect of SKAP2 phosphorylation on the SKAP2-WAVE2 interaction was investigated. SKAP2 was less bound to WAVE2 when it was phosphorylated by Fyn (Fig. 3E).
FIGURE 3.
SKAP2 physically interacts with WAVE2. A, top, structure of human WAVE2. WHD, WAVE homology domain; VCA, verprolin homology, cofilin homology and acidic region; aa, amino acids. Bottom, COS-1 cells were transfected with wild-type or the W336K SKAP2 mutant tagged with FLAG at the amino terminus (FLAG-SKAP2) and carboxyl-terminally HA-tagged WAVE2 (WAVE2-HA). FLAG-SKAP2 was immunoprecipitated (IP) using an anti-FLAG antibody. WAVE2 and SKAP2 were detected using anti-HA and anti-SKAP2 antibodies, respectively. The arrow indicates WAVE2-HA. B, COS-1 cells were transfected as described in A. WAVE2-HA was immunoprecipitated using an anti-HA antibody, and SKAP2 and WAVE2 were detected. C, endogenous WAVE2 in NIH3T3 cells was immunoprecipitated using an anti-WAVE2 antibody or the control normal rabbit IgG. SKAP2 and WAVE2 were detected using anti-SKAP2 and anti-WAVE2 antibodies, respectively. The asterisk represents nonspecific bands. IB, immunoblot. D, top, diagram of the recombinant GST fusion SKAP2 fragments used in this study. Middle, protein extract of COS-1 transfected with WAVE2-HA was pulled down by GST alone, the GST-PH domain, or the SH3 domain of SKAP2, and immunoblotted using an anti-HA antibody. Bottom, GST fusion proteins bound to glutathione-Sepharose beads were electrophoresed and analyzed using an anti-GST antibody. E, COS-1 cells were transfected with FLAG-SKAP2, WAVE2-HA, and Fyn tyrosine kinase and analyzed as in A. The phosphorylation of SKAP2 in FLAG IP fractions was detected using an anti-phosphotyrosine antibody.
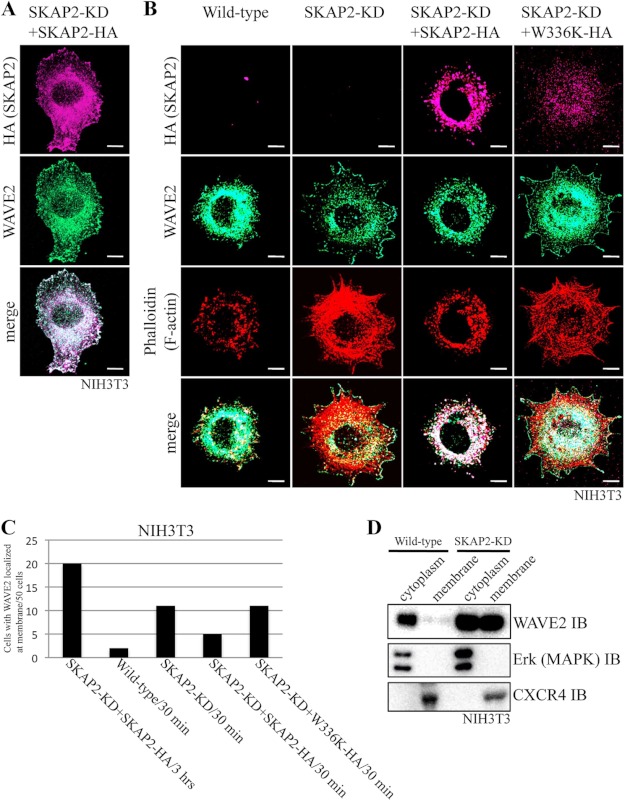
Next, we examined the significance of the interaction between SKAP2 and WAVE2 in the regulation of the actin cytoskeleton. To examine the localization of SKAP2 and WAVE2, SKAP2-KD NIH3T3 cells rescued with HA-tagged wild-type SKAP2 was used because of the technical limitation of the antibodies to simultaneously detect both proteins. WAVE2 was colocalized with SKAP2-HA at both the cell membrane and cytoplasm (Fig. 4A). We confirmed that staining with an anti-HA antibody correctly represents the localization of SKAP2 by the separate staining with an anti-SKAP2 antibody (supplemental Fig. S3). Changes in the distribution of WAVE2 were further analyzed in NIH3T3 cells under different conditions with regard to SKAP2. When NIH3T3 cells were attached to a fibronectin matrix, WAVE2 was initially located in the cytoplasm 30 min after plating (Fig. 4B, wild-type) and then translocated to the cell membrane 3 h after plating (Fig. 4A). Conversely, in SKAP2-KD NIH3T3 cells, the membrane translocation of WAVE2 was rapid, and higher amounts of cortical actin were observed than in wild-type NIH3T3 cells 30 min after plating (Fig. 4B). Increased stress fiber formation was also observed in SKAP2-KD cells as expected (14). Reconstitution of wild type, but not the W336K mutant of SKAP2 into SKAP2-KD NIH3T3 cells, suppressed the rapid recruitment of WAVE2 to the cell membrane, and consistent with this, the formation of cortical actin was also reduced (Fig. 4B). Because a large amount of wild-type SKAP2 was located in the cytoplasm and rarely observed at the cell membrane 30 min after plating, WAVE2 might be retained in the cytoplasm through its association with SKAP2. In the W336K SKAP2 mutant that does not interact with WAVE2, the localization of WAVE2 was not affected.
FIGURE 4.
SKAP2 colocalizes with WAVE2 and affects its localization. A, SKAP2-KD NIH3T3 cells reconstituted with SKAP2-HA were cultured on fibronectin-coated coverslips for 3 h and immunostained using anti-HA (magenta) and anti-WAVE2 (green) antibodies. Bar, 10 μm. B, NIH3T3 cell lines were cultured on fibronectin for 30 min and immunostained using anti-HA (magenta) and anti-WAVE2 (green) antibodies and phalloidin (red). W336K, W336K SKAP2 mutant. Bar, 10 μm. C, number of cells bearing membrane-localized WAVE2 was counted in a total of 50 cells for each in A and B. D, wild-type and SKAP2-KD NIH3T3 cells were subjected to subcellular fractionation as described under “Experimental Procedures.” WAVE2 in each fraction was analyzed using an anti-WAVE2 antibody. ERK (MAPK) and CXCR4 were analyzed using anti-ERK and anti-CXCR4 antibodies as controls of proteins in cytoplasm and cell membrane, respectively. IB, immunoblot.
To obtain the biochemical evidence in addition to immunofluorescence as to the effect of SKAP2 on the membrane translocation of WAVE2, NIH3T3 was fractionated in cytoplasm and plasma membrane. As expected, SKAP2-knockdown increased WAVE2 in the fraction of the plasma membrane (Fig. 4D). These results further confirmed the interaction between SKAP2 and WAVE2 and suggested that SKAP2 suppresses the translocation of WAVE2 and causes changes in the cell membrane cytoskeleton.
SKAP2 Interacts with Cortactin
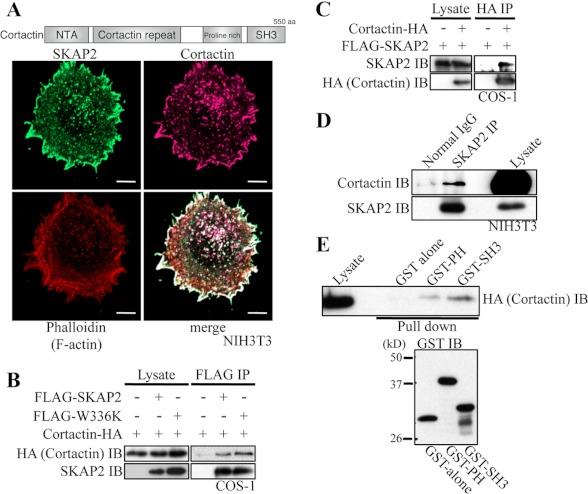
WAVE2 is known to conduct actin polymerization at the cell membrane (16, 17). Among molecules known to be involved in actin assembly, we found that cortactin associates with SKAP2. Cortactin is a scaffolding protein for different regulators and coordinates actin assembly at the cell membrane (18). Cortactin was colocalized with SKAP2 at the edge of the cell membrane in NIH3T3 cells (Fig. 5A). Association of SKAP2 with cortactin was reciprocally detected by immunoprecipitation in the lysates of COS-1 cells expressing these proteins (Fig. 5, B and C). Moreover, SKAP2-cortactin interaction was also detected in NIH3T3 cells (Fig. 5D). In contrast to WAVE2, cortactin bound also to the W336K SKAP2 mutant (Fig. 5B). GST pulldown assays showed that cortactin binds to the SH3 domain of SKAP2 and weakly to the PH domain of SKAP2 (Fig. 5E). The membrane localization of cortactin itself was not affected by SKAP2, as the same level of cortactin was detected at the cell membrane in SKAP2-KD NIH3T3 as in wild-type NIH3T3 cells 30 min after plating on fibronectin (supplemental Fig. S4).
FIGURE 5.
SKAP2 interacts with cortactin. A, top, structure of human cortactin. NTA, amino-terminal acidic region; aa, amino acids. Bottom, NIH3T3 cells were immunostained using anti-SKAP2 (green) and anti-cortactin (magenta) antibodies and phalloidin (red). Bar, 10 μm. B, COS-1 cells were transfected with cortactin-HA with or without FLAG-SKAP2 or W336K mutant. FLAG-SKAP2 was immunoprecipitated using an anti-FLAG antibody. Cortactin and SKAP2 were detected using anti-HA and anti-SKAP2 antibodies, respectively. C, COS-1 cells were transfected as indicated. Cortactin-HA was immunoprecipitated (IP) using an anti-HA antibody, and SKAP2 was detected. D, endogenous SKAP2 in NIH3T3 cells was immunoprecipitated using an anti-SKAP2 antibody. Cortactin and SKAP2 were detected using anti-cortactin and anti-SKAP2 antibodies, respectively. E, top, protein extracts from COS-1 cells transfected with cortactin-HA were pulled down by GST alone, the GST-PH domain, or the SH3 domain of SKAP2 and immunoblotted (IB) with an anti-HA antibody. Bottom, immunoblot of GST fusion proteins was shown.
SKAP2 Inhibits Actin Assembly Induced by the WAVE2-Cortactin Complex
As SKAP2 interacted with WAVE2 and cortactin, we next examined how SKAP2 contributes to the functions of these proteins. The interaction between WAVE2 and cortactin in breast cancer cells was suggested using immunofluorescence and a FRET-based approach (25). We observed the interaction between WAVE2-cortactin in COS1 cells expressing these proteins and in NIH3T3 cells by immunoprecipitation (Fig. 6, A and B). Although the significance of the WAVE2-cortactin interaction is not fully understood, we observed that the membrane localization of WAVE2 and the peripheral spreading of cells were suppressed by siRNA against cortactin in NIH3T3 cells and partially restored by re-expression of wild-type cortactin (Fig. 6C). These results suggest the significance of cortactin for the recruitment of WAVE2 to the cell membrane.
FIGURE 6.
Cortactin recruits WAVE2 to the cell membrane and SKAP2 inhibits their interaction. A, COS-1 cells were cotransfected with EGFP-WAVE2 and cortactin-HA. Cortactin-HA was immunoprecipitated (IP) using an anti-HA antibody. WAVE2 and cortactin were detected using anti-EGFP and anti-HA antibodies, respectively. B, endogenous WAVE2 in NIH3T3 cells were immunoprecipitated using an anti-WAVE2 antibody. Cortactin and WAVE2 were detected using anti-cortactin and anti-WAVE2 antibodies, respectively. C, left, control, cortactin-KD and human cortactin added-back NIH3T3 cells were immunostained using anti-cortactin (magenta) and anti-WAVE2 (green) antibodies. Bar, 10 μm. Top right, cortactin was transiently knocked down in NIH3T3 cells using siRNA, and human cortactin was transiently added back. The expression level of cortactin was examined by immunoblotting (IB) with an anti-cortactin antibody. Bottom right, percentages of signal intensities of WAVE2 at the cell periphery compared with those in the whole cell were quantified using ImageJ. D, FLAG-SKAP2 and Fyn in addition to cortactin-HA and EGFP-WAVE2 were expressed in COS-1 cells, and the interaction between WAVE2 and cortactin was analyzed (top panels). To confirm the phosphorylation of SKAP2 by Fyn, SKAP2 was immunoprecipitated using an anti-FLAG antibody, and detected by anti-FLAG and anti-phosphotyrosine antibodies (bottom panels).
The molecular interaction between WAVE2 and cortactin and the effect of SKAP2 on their interaction were analyzed using COS-1 cells cotransfected with EGFP-WAVE2, cortactin-HA, and FLAG-SKAP2. WAVE2 was coimmunoprecipitated with cortactin but to a lesser extent in the presence of coexpressed SKAP2 (Fig. 6D). Furthermore, SKAP2 phosphorylation by cotransfected Fyn restored the interaction between WAVE2-cortactin (Fig. 6D). Therefore, SKAP2 inhibits the physical interaction between WAVE2 and cortactin, and SKAP2 phosphorylation attenuates its inhibitory effect.
We further examined whether SKAP2 affects actin assembly induced by WAVE2 and cortactin using an in vitro actin polymerization assay (21, 22). Cortactin-coated protein G beads were incubated with WAVE2 expressed in U87MG cells using adenovirus and rhodamine-conjugated monomeric G-actin together with or without GST-tagged SKAP2 (Fig. 7A). The Arp2/3 protein complex, an essential actin polymerization factor (26), was supplied from HeLa cell extracts. For quantitative analyses of polymerized actin, the beads were washed and immunoblotted using an anti-rhodamine antibody. Without the addition of WAVE2, polymerized actin was little detected by cortactin alone (Fig. 7A). However, actin polymerization was clearly detected in the presence of cortactin with WAVE2 but not when SKAP2 was present (Fig. 7A). We also detected WAVE2 in the bead fractions, which was reduced by the addition of SKAP2, indicating that the formation of the WAVE2-cortactin complex was disturbed by SKAP2.
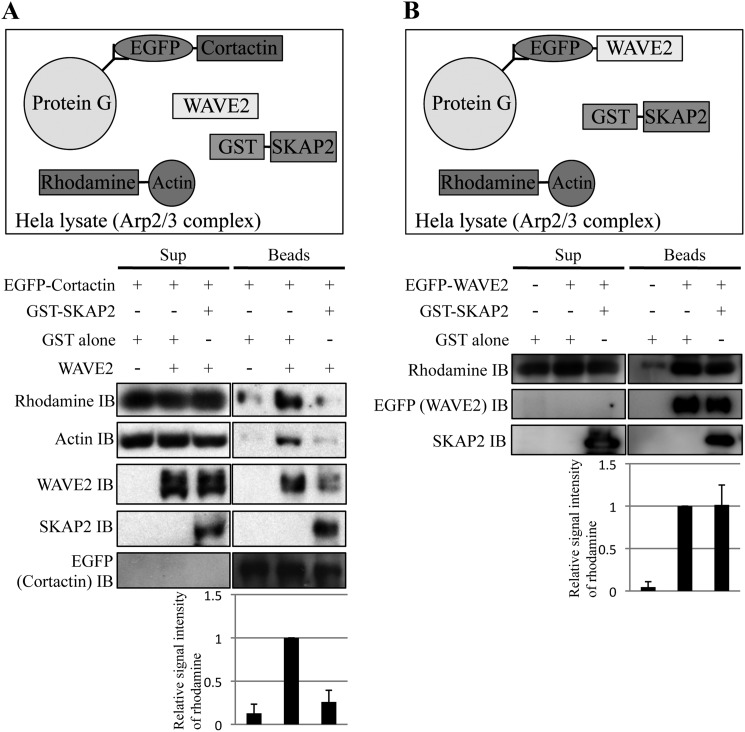
FIGURE 7.
SKAP2 directly inhibits actin polymerization induced by the WAVE2-cortactin complex. A, top, diagram of the actin polymerization assay. Rhodamine-conjugated monomeric G-actin, WAVE2, and GST-SKAP2 were added to cortactin-coated protein G beads. HeLa cell lysates were also added to supply the Arp2/3 protein complex. After incubation, protein G beads were separated from the reaction mixture, washed, and subjected to immunoblotting (IB). Bottom, polymerized actin, which was produced in the complex of cortactin and WAVE2, was detected using anti-rhodamine and anti-actin antibodies. WAVE2, SKAP2, and cortactin were detected using anti-WAVE2, anti-SKAP2, and anti-EGFP antibodies, respectively. Sup, reaction mixture; beads, beads fraction after incubation. B, top, diagram of the actin polymerization assay using EGFP-WAVE2-coated beads. Bottom, polymerized actin was analyzed as A. A and B, signal intensities of rhodamine-actin in the beads fraction were quantified. Signal intensities of lanes with WAVE2 without SKAP2 were defined as 1. The results from the three independent experiments are shown as the means ± S.D.
We also examined whether SKAP2 affects WAVE2-induced actin polymerization without the addition of recombinant cortactin. The amount of polymerized actin at WAVE-coated beads was not changed by the addition of SKAP2 (Fig. 7B). These results suggest that the negative effect of SKAP2 on actin assembly largely depends on its interference with WAVE2-cortactin interaction rather than its direct effect on WAVE2.
Actin polymerization at cortactin or WAVE2-coated beads was also analyzed using pyrene-actin instead of rhodamine-actin, and a similar result showing that SKAP2 inhibits cortactin-WAVE2 complex-induced actin polymerization was obtained (supplemental Fig. S5).
SKAP2 Suppresses Tumor Invasion
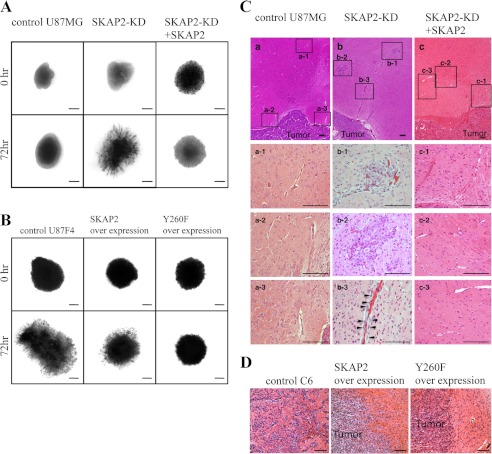
To evaluate the biological effects of SKAP2 on the invasion of tumor cells in brain tissue, U87MG was examined in an ex vivo organotypic rat brain slice. The tumors of control U87MG mice exclusively expanded in the brain, with a relatively clear margin (Fig. 8A). In contrast, SKAP2-KD displayed greater invasion into the organotypic rat brain slice, and the margins of the expanding SKAP2-KD tumors were irregularly shaped (Fig. 8A, 5/5 explants). Re-expression of SKAP2 suppressed the invasion of SKAP2-KD tumors (Fig. 8A, 5/5 explants). U87F4 cells widely invaded into the rat brain slices, which was partially inhibited by overexpression of wild-type SKAP2 and was more effectively suppressed by the Y260F SKAP2 mutant (Fig. 8B).
FIGURE 8.
SKAP2 suppresses the invasion of glioblastoma in the mouse brain. A and B, invasion of glioblastoma in ex vivo organotypic rat brain slices. 3,3′-Dioctadecyloxacarbocyanine-labeled cells were spotted on the putamen of sliced rat brains and cultured for 72 h. The areas of the tumor were visualized through a fluorescent dissecting scope. A total of five brain explants for each group were examined, and representative images are shown. Bar, 500 μm. A, control, SKAP2-KD and SKAP2-added back U87MG. B, control, wild-type, or Y260F SKAP2-overexpressed U87F4 cells. C and D, glioblastomas were injected into nude mice brains. Mice were sacrificed 14 days later, and the brains were sliced and stained by H&E. Five mice in each group were examined, and representative images are shown. Bar, 100 μm. C, control (panel a), SKAP2-KD (panel b), and SKAP2 added back (panel c) U87MG cells. Panel a, a-1 to a-3, panel b, b-1 to b-3, and panel c, c-1 to c-3: high magnification fields of the boxed areas in panels a–c, respectively. Arrowheads indicate tumor cells invading along the capillary. D, control, wild-type, or Y260F SKAP2-overexpressed C6 cells.
To further examine the effect of SKAP2 on tumor progression, U87MG cells were injected into the brain of nude mice. Tumor size was not significantly different between control wild-type U87MG and SKAP2-KD or SKAP2-added back U87MG (data not shown). However, a significant amount of microsatellites of tumor cells was detected around capillaries in the brain of mice injected with SKAP2-KD U87MG, whereas such microsatellites were rarely detected in the control or SKAP2-added back U87MG group (Fig. 8C, 4/5 versus 0/5 and 0/5, respectively). Consistently, SKAP2-KD U87MG was frequently observed in the perivascular space along the capillaries leading to the tumor satellites (Fig. 8C, panel b, b-3).
The effects of SKAP2 overexpression and phosphorylation were also investigated in in vivo tumor implantation. Tumors of C6 cells are irregularly shaped and significantly invaded into the mouse brain, although such invasion was not observed in mice bearing tumors overexpressing wild-type or Y260F SKAP2 (Fig. 8D, 5/5 versus 0/5 and 0/5, respectively). The overexpression of Y260F SKAP2 mutant in C6 cells suppressed tumor invasion to a greater extent than that of wild-type SKAP2 (Fig. 8D). Over all, these results suggest that SKAP2 suppresses the progression of glioblastoma by exerting a negative effect on cell invasion, which is attenuated by its phosphorylation.
DISCUSSION
SKAP2 is known as an adaptor protein for SFKs dominantly analyzed in immune cells. We identified SKAP2 in the U87F4 glioblastoma cell line (supplemental Fig. S1B), and we further detected its expression in NIH3T3 fibroblasts and some tumor cells of the epithelial origin (supplemental Fig. S2). Our analyses suggested that SKAP2 may be a negative regulator of cell migration in glioblastoma cells and fibroblasts. In response to the cell adhesion, cortactin localized to the cell membrane independently of SKAP2 (supplemental Fig. S4). The knockdown of cortactin suppressed the translocation of WAVE2 from the cytosol to the cell membrane, which suggests that cortactin is important as a scaffolding protein for WAVE2 at the cell membrane. SKAP2 binds both to WAVE2 and cortactin and suppresses cell migration by inhibiting actin assembly at the cell membrane through interference of the membrane translocation of WAVE2 and/or the physical interaction between WAVE2 and cortactin. Phosphorylation of SKAP2 by SFKs negatively regulates these inhibitory effects of SKAP2 (Fig. 9).
FIGURE 9.
Diagram showing the possible mechanism of the regulation of actin polymerization and cell migration by SKAP2. Left, WAVE2 is recruited to cortactin at the cell membrane, polymerizes actin through Arp2/3 complex, and cell migration occurs. Middle, SKAP2 interferes with the association between WAVE2 and cortactin, and inhibits actin polymerization, which suppresses cell migration and tumor invasion. Right, SKAP2-phosphorylation by SFKs attenuates its inhibitory effect on WAVE2-cortactin interaction, cell migration and invasion.
In contrast to this study, in response to T-cell receptor signaling, SKAP2 associates with adhesion- and degranulation-promoting adaptor protein (ADAP) (7, 27, 28), which is exclusively expressed in T-lymphocytes and other immune cells, and forms a complex with WASP and VASP (29, 30), which are partners that have been suggested to sustain actin polymerization. SKAP2 was also reported to promote ruffle generation in bone marrow-derived macrophages (12). Although it is not well known whether SKAP2 affects the motility of these cells, SKAP2 seems to have multiple functions in actin assembly. For example, in cells expressing ADAP such as T-cells and bone marrow-derived macrophages, SKAP2 may act as an enhancer of actin polymerization, whereas SKAP2 may negatively regulate actin assembly through association with WAVE2 and cortactin in other cells. The expression of ADAP was not detected or was detected at a very low level in NIH3T3 and U87MG cells by RT-PCR (data not shown). Further investigation is necessary using other tissues and cell lines to elucidate the mechanisms by which SKAP2 orchestrates actin polymerization and to determine in which tumors SKAP2 acts as a negative or positive regulator of cell invasion.
Precise mechanisms of the negative effect of SKAP2 on actin assembly are expected to be elucidated. In our results of the immunoprecipitation analysis and the actin polymerization assay, SKAP2 attenuated the physical association of cortactin with WAVE2. However, we observed the almost complete inhibition of actin polymerization by the addition of SKAP2, despite detectable amounts of WAVE2 still attached to the cortactin-coated beads (Fig. 7A). It is possible that the small amount of the ternary complex WAVE2-SKAP2-cortactin could be formed as cortactin binds both the SH3 and PH domains of SKAP2 (Fig. 5E). SKAP2 may functionally suppress the actin polymerization by interfering with the molecules required for the actin assembly in the cortactin-WAVE2 complex, which were included in the cell lysate used in this assay.
In the presence of SKAP2, the rapid translocation of WAVE2 from the cytosol to the cell membrane was disturbed after the cells attached to the matrix. However, the membrane localization of cortactin was not disturbed by SKAP2. The significance of cortactin for actin remodeling is suggested by the observation that cortactin-knockdown inhibits the cell migration and the formation of membrane protrusions (31). Our results suggest that the cortactin-WAVE2 complex at the leading edge of the cell membrane is required for the peripheral spreading of the cells in response to the cell adhesion, which is negatively regulated by SKAP2. It should be elucidated whether SKAP2 affects the membrane translocation of WAVE2 induced by other factors such as cytokines. As a physiological role, SKAP2 may contribute to stabilize the cell membrane in NIH3T3 cells. Removal of SKAP2 or modification of SKAP2 activity may switch the state of cells from static to actively mobile.
In this study, SKAP2 was identified as a tyrosine-phosphorylated protein from a more malignant and invasive glioblastoma (U87F4, supplemental Fig. S1B), although SKAP2 has a negative effect on the tumor invasion. From these facts, it was hypothesized that SKAP2 phosphorylation attenuates the negative effect of SKAP2 on the invasion. We suggested that SKAP2 phosphorylation suppressed its negative effects on the invasion by inhibiting its interaction with WAVE2 and restoring the interaction between WAVE2 and cortactin (Figs. 2, B and C, 3E, 6D, and 8, B and D). However, the significance of SKAP2 phosphorylation was not fully elucidated. For example, the effect of SKAP2 on actin polymerization could not be analyzed in this report. Tyrosine 260 of SKAP2 was identified as the major phosphorylation site by Fyn (8), which we also confirmed. However, the substantial level of phosphorylation remained in SKAP2 Y260F mutant when it was exogenously expressed (data not shown).
Our study indicates that SKAP2 plays an important role in the control of actin assembly and cell migration. At least in a glioblastoma, SKAP2 is suggested to have a negative effect on tumor invasion. Knockdown of SKAP2 caused many microsatellites of tumor nests in the mouse brain (Fig. 8C), which may induce the dissemination of glioblastoma. To investigate SKAP2 as the candidate for suppressor of tumor invasion, expression of SKAP2 should be analyzed in the pathological specimens of human brain tumors. Because we also detected various amounts of SKAP2 expression in other types of cancers (supplemental Fig. S2), it is important to collect the expression profiles of SKAP2 in the specimens of those cancers and evaluate the significance of SKAP2 as the indicator of tumor progression. In the future, functional peptides might be synthesized from SKAP2 and exert a suppressive effect on tumor invasion.
Supplementary Material
Acknowledgments
We thank Dr. Takashi Momoi for donating the plasmid-encoding mouse SKAP2 (RA70). The excellent secretarial work and experimental help by Namiko Aiba are also acknowledged.
This work was supported by Japan Society for the Promotion of Science Grants 22300324 and 23650590 (to M. T.) and 24700364 (to S. S.), National Cancer Center Research and Development Fund Grant 23-A-9 (to M. T.), the Uehara Memorial Foundation, the Naito Foundation, and the Yasuda Medical Foundation.

This article contains supplemental Figs. S1–S5.
- SFK
- Src family kinase
- SH
- Src homology
- PH
- pleckstrin homology
- EGFP
- enhanced GFP.
REFERENCES
- 1. Playford M. P., Schaller M. D. (2004) The interplay between Src and integrins in normal and tumor biology. Oncogene 23, 7928–7946 [DOI] [PubMed] [Google Scholar]
- 2. Wheeler D. L., Iida M., Dunn E. F. (2009) The role of Src in solid tumors. Oncologist 14, 667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanaka M., Sasaki K., Kamata R., Hoshino Y., Yanagihara K., Sakai R. (2009) A novel RNA-binding protein, Ossa/C9orf10, regulates activity of Src kinases to protect cells from oxidative stress-induced apoptosis. Mol. Cell. Biol. 29, 402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uekita T., Tanaka M., Takigahira M., Miyazawa Y., Nakanishi Y., Kanai Y., Yanagihara K., Sakai R. (2008) CUB-domain-containing protein 1 regulates peritoneal dissemination of gastric scirrhous carcinoma. Am. J. Pathol. 172, 1729–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yagi R., Tanaka M., Sasaki K., Kamata R., Nakanishi Y., Kanai Y., Sakai R. (2011) ARAP3 inhibits peritoneal dissemination of scirrhous gastric carcinoma cells by regulating cell adhesion and invasion. Oncogene 30, 1413–1421 [DOI] [PubMed] [Google Scholar]
- 6. Kouroku Y., Soyama A., Fujita E., Urase K., Tsukahara T., Momoi T. (1998) RA70 is an Src kinase-associated protein expressed ubiquitously. Biochem. Biophys. Res. Commun. 252, 738–742 [DOI] [PubMed] [Google Scholar]
- 7. Marie-Cardine A., Verhagen A. M., Eckerskorn C., Schraven B. (1998) SKAP-HOM, a novel adaptor protein homologous to the FYN-associated protein SKAP55. FEBS Lett. 435, 55–60 [DOI] [PubMed] [Google Scholar]
- 8. Curtis D. J., Jane S. M., Hilton D. J., Dougherty L., Bodine D. M., Begley C. G. (2000) Adaptor protein SKAP55R is associated with myeloid differentiation and growth arrest. Exp. Hematol. 28, 1250–1259 [DOI] [PubMed] [Google Scholar]
- 9. Black D. S., Marie-Cardine A., Schraven B., Bliska J. B. (2000) The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell. Microbiol. 2, 401–414 [DOI] [PubMed] [Google Scholar]
- 10. Bourette R. P., Thérier J., Mouchiroud G. (2005) Macrophage colony-stimulating factor receptor induces tyrosine phosphorylation of SKAP55R adaptor and its association with actin. Cell. Signal. 17, 941–949 [DOI] [PubMed] [Google Scholar]
- 11. Reinhold A., Reimann S., Reinhold D., Schraven B., Togni M. (2009) Expression of SKAP-HOM in DCs is required for an optimal immune response in vivo. J. Leukocyte Biol. 86, 61–71 [DOI] [PubMed] [Google Scholar]
- 12. Swanson K. D., Tang Y., Ceccarelli D. F., Poy F., Sliwa J. P., Neel B. G., Eck M. J. (2008) The Skap-Hom dimerization and PH domains comprise a 3′-phosphoinositide-gated molecular switch. Mol. Cell 32, 564–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Togni M., Swanson K. D., Reimann S., Kliche S., Pearce A. C., Simeoni L., Reinhold D., Wienands J., Neel B. G., Schraven B., Gerber A. (2005) Regulation of in vitro and in vivo immune functions by the cytosolic adaptor protein SKAP-HOM. Mol. Cell. Biol. 25, 8052–8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou L., Zhang Z., Zheng Y., Zhu Y., Wei Z., Xu H., Tang Q., Kong X., Hu L. (2011) SKAP2, a novel target of HSF4b, associates with NCK2/F-actin at membrane ruffles and regulates actin reorganization in lens cell. J. Cell Mol. Med. 15, 783–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macias M. J., Wiesner S., Sudol M. (2002) WW and SH3 domains, two different scaffolds to recognize proline-rich ligands. FEBS Lett. 513, 30–37 [DOI] [PubMed] [Google Scholar]
- 16. Derivery E., Gautreau A. (2010) Generation of branched actin networks. Assembly and regulation of the N-WASP and WAVE molecular machines. BioEssays 32, 119–131 [DOI] [PubMed] [Google Scholar]
- 17. Takenawa T., Miki H. (2001) WASP and WAVE family proteins. Key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 114, 1801–1809 [DOI] [PubMed] [Google Scholar]
- 18. Ren G., Crampton M. S., Yap A. S. (2009) Cortactin. Coordinating adhesion and the actin cytoskeleton at cellular protrusions. Cell. Motil. Cytoskeleton 66, 865–873 [DOI] [PubMed] [Google Scholar]
- 19. Tanaka M., Kamata R., Sakai R. (2005) EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J. Biol. Chem. 280, 42375–42382 [DOI] [PubMed] [Google Scholar]
- 20. Cavalcanti B. N., Rode Sde M., França C. M., Marques M. M. (2011) Pulp capping materials exert an effect on the secretion of IL-1β and IL-8 by migrating human neutrophils. Braz. Oral Res. 25, 13–18 [DOI] [PubMed] [Google Scholar]
- 21. Fradelizi J., Noireaux V., Plastino J., Menichi B., Louvard D., Sykes C., Golsteyn R. M., Friederich E. (2001) ActA and human zyxin harbour Arp2/3-independent actin-polymerization activity. Nat. Cell Biol. 3, 699–707 [DOI] [PubMed] [Google Scholar]
- 22. Kang H., Wang J., Longley S. J., Tang J. X., Shaw S. K. (2010) Relative actin nucleation promotion efficiency by WASP and WAVE proteins in endothelial cells. Biochem. Biophys. Res. Commun. 400, 661–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valster A., Tran N. L., Nakada M., Berens M. E., Chan A. Y., Symons M. (2005) Cell migration and invasion assays. Methods 37, 208–215 [DOI] [PubMed] [Google Scholar]
- 24. Tanaka M., Sasaki K., Kamata R., Sakai R. (2007) The C terminus of ephrin-B1 regulates metalloproteinase secretion and invasion of cancer cells. J. Cell Sci. 120, 2179–2189 [DOI] [PubMed] [Google Scholar]
- 25. Eiseler T., Hausser A., De Kimpe L., Van Lint J., Pfizenmaier K. (2010) Protein kinase D controls actin polymerization and cell motility through phosphorylation of cortactin. J. Biol. Chem. 285, 18672–18683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goley E. D., Welch M. D. (2006) The ARP2/3 complex. An actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7, 713–726 [DOI] [PubMed] [Google Scholar]
- 27. Liu J., Kang H., Raab M., da Silva A. J., Kraeft S. K., Rudd C. E. (1998) FYB (FYN-binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc. Natl. Acad. Sci. U.S.A. 95, 8779–8784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang H., Rudd C. E. (2008) SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol. 18, 486–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coppolino M. G., Krause M., Hagendorff P., Monner D. A., Trimble W., Grinstein S., Wehland J., Sechi A. S. (2001) Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP, and WASP that links the actin cytoskeleton to Fcγ receptor signalling during phagocytosis. J. Cell Sci. 114, 4307–4318 [DOI] [PubMed] [Google Scholar]
- 30. Krause M., Sechi A. S., Konradt M., Monner D., Gertler F. B., Wehland J. (2000) Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins, and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton. J. Cell Biol. 149, 181–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bryce N. S., Clark E. S., Leysath J. L., Currie J. D., Webb D. J., Weaver A. M. (2005) Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 15, 1276–1285 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.