Background: Secretins are outer membrane dodecameric translocation channels in bacterial type II secretion systems (T2SS).
Results: The basic assembly unit of XcpQ, the T2SS secretin of the human pathogen Pseudomonas aeruginosa, is a dimer.
Conclusion: Functional secretin likely results from hexameric assembly of secretin subunit dimers.
Significance: This work is a conceptual advancement in understanding the assembly principles and dynamic function of bacterial secretins.
Keywords: Bacterial Pathogenesis, Protein Assembly, Protein Structure, Protein Translocation, Secretion, Pseudomonas aeruginosa, Dodecamer, Hexamer of Dimers, Secretin, Type II Secretion System
Abstract
The type II secretion system is a multiprotein assembly spanning the inner and outer membranes in Gram-negative bacteria. It is found in almost all pathogenic bacteria where it contributes to virulence, host tissue colonization, and infection. The exoproteins are secreted across the outer membrane via a large translocation channel, the secretin, which typically adopts a dodecameric structure. These secretin channels have large periplasmic N-terminal domains that reach out into the periplasm for communication with the inner membrane platform and with a pseudopilus structure that spans the periplasm. Here we report the crystal structure of the N-terminal periplasmic domain of the secretin XcpQ from Pseudomonas aeruginosa, revealing a two-lobe dimeric assembly featuring parallel subunits engaging in well defined interactions at the tips of each lobe. We have employed structure-based engineering of disulfide bridges and native mass spectrometry to show that the periplasmic domain of XcpQ dimerizes in a concentration-dependent manner. Validation of these insights in the context of cellular full-length XcpQ and further evaluation of the functionality of disulfide-linked XcpQ establishes that the basic oligomerization unit of XcpQ is a dimer. This is consistent with the notion that the dodecameric secretin assembles as a hexamer of dimers to ensure correct projection of the N-terminal domains into the periplasm. Therefore, our studies provide a key conceptual advancement in understanding the assembly principles and dynamic function of type II secretion system secretins and challenge recent studies reporting monomers as the basic subunit of the secretin oligomer.
Introduction
The opportunistic Gram-negative pathogen Pseudomonas aeruginosa is responsible for a wide range of human diseases, causing significant morbidity and mortality among immune-compromised humans, such as cystic fibrosis patients (1). The ability of P. aeruginosa to colonize host tissue, often persistently, and to establish infections relies to a large extent on its ability to secrete diverse virulence factors, such as exotoxins and proteolytic enzymes, across the cellular envelope defined by the inner and outer membranes (2–4).
One of the molecular weapons in the P. aeruginosa arsenal is the type II secretion system (T2SS),4 which is one of six secretion machineries encoded by the P. aeruginosa genome (4, 5). The T2SS encoded by the xcp genes of P. aeruginosa consists of 12 different oligomeric proteins (6) distributed over three subassemblies: a periplasmic filamentous pseudopilus, the inner membrane platform, and the outer membrane secretin (7–9). The latter, called XcpQ, is typically assembled via homo-oligomerization of 12 subunits and forms a large translocation channel in the outer membrane. Each subunit contains a canonical secretin motif at or near the C terminus (9–11). The N-terminal part of the protein shows more sequence variation and is organized into four subdomains N0, N1, N2, and N3 (12). This N-terminal quartet of domains is thought to protrude deep into the periplasm, where it interacts with other components of the T2SS system (i.e., XcpP and the pseudopilus) and exoproteins (13–15).
Recent structural studies of the periplasmic moiety of the XcpQ homolog GspD, called peri-GspD, from enterotoxigenic Escherichia coli in complex with a camelid antibody fragment (nanobody) allowed delineation of the structural domain organization of three of its four periplasmic subdomains (i.e., N0, N1, and N2; Protein Data Bank code 3EZJ) (12). Interestingly, the first two subdomains, N0 and N1 from the type III secretion system (T3SS) secretin EscC of enteropathogenic E. coli, show structural similarity with the N0 and N1 subdomains of GspD. However, in EscC the N0 domain is flipped by ∼180° relative to the N1 domain (Protein Data Bank code 3GR5) (16). In addition, cryo-electron microscopy studies on single particles of full-length GspD from Vibrio cholerae (13) provided the first three-dimensional view of a T2SS secretin as a pore formed by 12 GspD subunits. An important consensus from these studies has been the proposal that monomeric GspD secretins assemble under C12 symmetry to construct the functional GspD dodecamer. However, several recent studies (17, 18) suggested that the assembly of secretin dodecamers might proceed via oligomerization of dimers of secretin subunits.
Here we report the crystal structure of the periplasmic domain of the XcpQ secretin from P. aeruginosa. We complement our structural findings with an integrated series of studies using structure-based disulfide engineering and native mass spectrometry, as well as a functional assay, to establish that the basic oligomerization in a cellular context of XcpQ is a dimer. Taken together, our data suggest that the dodecameric assemblages of T2SS secretins are hexameric arrangements of dimers.
EXPERIMENTAL PROCEDURES
Expression and Purification of Recombinant Proteins
A DNA fragment coding for residues 35–325 of XcpQ (numbering according to UniProt #P35818), covering subdomains N0, N1, and N2 and the first 47 residues from N3, was amplified by PCR from genomic DNA using appropriate primers (supplemental Table S1). The PCR product was ligated into a pET15b+ expression vector (Invitrogen) using the NdeI and BamHI restriction sites included in the primers to give pET-N0N3′. For expression, E. coli BL21(DE3) transformed with pET-N0N3′ was grown in LB medium supplemented with carbenicillin (100 μg/ml) at 37 °C. Protein expression was induced when an optical density at 600 nm of 0.6–1.0 was reached with 1 mm isopropyl β-d-1-thiogalactopyranoside (Duchefa Biochemie) followed by growth for 5 h and harvesting by centrifugation. The ensuing cell pellet was resuspended (500 mm NaCl, 20 mm Tris, pH 8.0) in the presence of protease inhibitors (Complete®; Roche Applied Science), and the cells were lysed by sonication. The cell debris was pelleted by centrifugation at 75,000 × g for 30 min, and the supernatant was filtered using a syringe filter cap (0.22 μm). The clarified lysate was loaded onto a nickel-nitrilotriacetic acid column (Qiagen) pre-equilibrated with buffer A (20 mm Tris-HCl, pH 8, 500 mm NaCl) containing 10 mm imidazole, washed with buffer A containing 50 mm imidazole, and eluted with buffer A containing 250 mm imidazole. To remove the His6 tag, fractions of the purified protein were pooled and concentrated on a Vivaspin 15R column 10,000 molecular weight cutoff (Sartorius Stedim) to 1–2 ml before diluting the sample 10-fold with thrombin digestion buffer (150 mm NaCl, 20 mm Tris, pH 8, and 2.5 mm CaCl2). One unit of biotinylated thrombin (Novagen) was added per ml of diluted sample, and the cleavage reaction was allowed to continue for 12–40 h in the dark at room temperature until the protein was cleaved completely as evaluated by SDS-PAGE. Biotinylated thrombin was removed from the solution by adding streptavidin-agarose (Novagen) followed by centrifugation (10 min, 4000 × g) and filtration using a syringe filter cap (0.22 μm). Next the sample was subjected to size exclusion chromatography (SEC) on a Superdex 75 column (GE Healthcare) equilibrated with 150 mm NaCl, 20 mm HEPES, pH 7.5. Fractions containing pure protein (>95% purity as judged by SDS-PAGE) were pooled and concentrated to 10–15 mg/ml for crystallization trials. The purified protein, which was shorter than expected and only covered amino acid residues 35–277 of XcpQ (see further), was designated peri-XcpQ.
Selenomethionine-labeled peri-XcpQ was produced in the methionine-auxotrophic E. coli strain B834 grown in SelenoMetTM medium (Molecular Dimensions Limited). Purification and subsequent handling of the protein were carried out as described above.
Engineering and Production of Cysteine Mutants
Two single serine to cysteine substitution mutants of peri-XcpQ (S109C and S210C) were engineered using overlap extension PCR (19) starting with the wild-type pET-N0N3′ construct as template. For each mutation, three separate PCRs were performed based on appropriate forward and reverse primers (supplemental Table S1). In the first reaction the forward primer of the pET-N0N3′ construct and a reverse primer carrying the point mutation were used. In the second reaction, the reverse primer of the pET-N0N3′ construct and the forward primer carrying the target point mutation were employed. Finally, a third PCR was performed using the two purified PCR products as template and the terminal forward and reverse primers of the pET-N0N3′ construct.
All of the mutant constructs were subsequently introduced into a pET15b+ (Invitrogen) expression vector, and the proteins were produced in E. coli BL21(DE3). Purification of the cysteine containing mutant proteins was carried out as described above. To increase the yield of disulfide-linked, recombinant peri-XcpQ(S210C) for crystallization purposes, the sample was incubated with 0.3% (v/v) of H2O2 for 30 min before SEC. Expression constructs pB28_S109C and pB28_S210C, resulting in the substitutions S109C and S210C in full-length XcpQ, respectively, were produced starting with the pB28 vector coding for full-length XcpQ (9).
Analysis of Wild-type and Cysteine Mutants in Full-length XcpQ in Vivo
To investigate the disulfide bridge propensity of S109C and S210C in vivo, P. aeruginosa PAN1 (9), was transformed with pB28, pB28_S109C, pB28_S210C, and the pMMB67EH vector without insert, and transformants were grown on solid LB-agar medium supplemented with carbenicillin (100 μg/ml) and gentamicin (15 μg/ml). After 24 h, individual colonies from each plate were picked and grown overnight in liquid LB medium at 37 °C with antibiotic selection. One ml of this preculture was used to inoculate 20 ml fresh LB medium. The cultures were induced at an A600 nm of 0.6 with 0.1 mm isopropyl β-d-1-thiogalactopyranoside and allowed to grow further for 4 h. Next cells from 1 ml of each culture were pelleted by centrifugation. The pellets were dissolved in 1 ml of resuspension buffer (150 mm NaCl, 50 mm Tris-HCl, pH 8, 1% Nonidet P-40, 10 mm of EDTA, and 20 mm iodoacetamide to block any available cysteine sulfhydryls) supplemented with a mixture of protease inhibitors (Complete®; Roche Applied Science). The cells were disrupted by sonication followed by centrifugation at 20,000 × g for 15 min. Cell debris were dissolved in Laemmli loading buffer (2% SDS (w/v), 10% glycerol (v/v), 0.002% bromphenol blue (w/v), and 125 mm Tris-HCl, pH 6.8) according to the A600 nm (i.e., 100 μl/A600 nm of 1.00) and heated for 10 min at 95 °C. After 5 min, 3 μl of β-mercaptoethanol or distilled H2O was added to 30 μl of sample and heated again for 5 min. Finally, 20 μl was loaded on a 4–15% nonreducing acrylamide gel (Bio-Rad). Wild-type and mutant full-length XcpQ were visualized (Odyssey Imaging System (Li-Cor)) via immunoblotting on a PVDF membrane using primary antibodies directed against the N-terminal part of XcpQ (amino acid residues 12–100 of the mature protein) (9) and secondary antibodies with IRDye® 800CW goat anti-rabbit IgG (H+L) (Li-Cor). For analysis of multimer formation of full-length XcpQ, the boiling steps from the above protocol were omitted.
Crystallization and Structure Determination
Crystallization screens using purified and monodisperse preparations of peri-XcpQ, selenomethionine-labeled peri-XcpQ, and dimeric peri-XcpQ(S210C) were set up in sitting drop geometry (droplet: 0.1 μl of protein sample + 0.1 μl of reservoir solution; reservoir of 40 μl) using a Mosquito crystallization robot (TTP LabTech). This led to several hits featuring a variety of polyethylene glycols, buffers at near or above physiological pH, with magnesium or calcium ions. Several rounds of crystal optimization yielded diffraction quality crystals as follows: peri-XcpQ: 0.2 m MgCl2, 0.1 m Tris-HCl, pH 8.5, and 25% PEG 3350 (w/v); selenomethionine-labeled peri-XcpQ: 0.2 m MgCl2, 0.1 m Tris-HCl, pH 6.5, and 30% PEG 3350 (w/v); peri-XcpQ(S210C): 0.2 m calcium acetate, 0.1 m HEPES, pH 7.5, 14% PEG 8000 (w/v). For data collection under cryogenic conditions, crystals of peri-XcpQ were transferred gradually to crystal stabilization buffer containing 37.5% (w/v) of PEG 3350. Crystals of selenomethionine-labeled peri-XcpQ were cryo-cooled in their native droplet, and crystals of peri-XcpQ(S210C) were cryo-protected by gradually transferring the crystal to stabilization buffer containing 18% (w/v) of PEG 8000 and 5% (w/v) of PEG 3350. X-ray diffraction data were collected at several Synchrotron beamlines and were processed using XDS (Ref. 20; see also Table 1). The structure of peri-XcpQ was determined by single wavelength anomalous diffraction using data collected at the selenium x-ray absorption peak from selenomethione-labeled peri-XcpQ crystals. Determination of the selenium substructure for eight sites and the ensuing calculation of single wavelength anomalous diffraction phases were carried out in Phaser (21) as embedded in Phenix (22). The resultant electron density map to 2.8 Å resolution was improved via density modification protocols combining solvent flattering and proper 2-fold noncrystallographic symmetry averaging as implemented in RESOLVE (from Phenix). Model building was performed via alternating rounds of manual model building in Coot (23) and automized model building via Autobuild (from Phenix). Maximum-likelihood crystallographic refinement combining simulated annealing, conjugate gradient minimization, and optimization of atomic displacement parameter was performed with Phenix (22) and Buster (24) against the 2.0 Å resolution data set of the native protein. Finally, the refined model was validated using Coot (23) and MolProbity (25). The figures and structural comparisons (root mean square deviation (RMSD) calculation) were made using PyMOL (26), and analysis of dimerization interface was done using the protein interfaces, surfaces, and assemblies service of the Protein Data Bank (PDBe PISA) (27). The structure of peri-XcpQ(S210C) was determined by molecular replacement using the refined model peri-XcpQ carrying a S210A mutation as search model and was refined and validated as described above.
TABLE 1.
Crystallographic data collection and refinement statistics
The values in parentheses correspond to the highest resolution shell. All of the data were processed and scaled with the XDS package (20). Native x-ray data were collected at Beamline Proxima I at the Soleil Synchrotron. Selenomethionine data and S210C data were collected at Beamlines ID29 and ID23-1 at the European Synchrotron Radiation Facility, respectively.
| Native | Selenomethionine | S210C | |
|---|---|---|---|
| Data collection | |||
| Resolution range (Å), space group | 45–2.0, C2 | 50.0–2.8, C2 | 50.0–2.2, C2 |
| Unit cell | a = 119.0 Å, b = 39.5 Å, c = 92.9 Å, β = 99.8° | a = 119.0 Å, b = 39.8 Å, c = 94.8 Å, β = 99.7° | a = 120.4, b = 40.2 Å, c = 93.8 Å, β = 99.7° |
| Unique reflections, redundancy | 28,407 (2065), 3.07 (2.9) | 20,984 (1518), 3.2 (2.7) | 22,248 (1528), 2.9 (2.8) |
| Completeness (%) | 97.2 (96.9) | 98.7 (94.3) | 97.4 (93.8) |
| Average I/σ(I) | 13.31 (2.27) | 11.81 (1.72) | 10.87 (2.16) |
| Rmeasa | 0.069 (0.661) | 0.095 (0.809) | 0.117 (0.773) |
| Wilson B-factor (Å2) | 38.82 | 59.84 | 38.43 |
| Refinement | |||
| Resolution range | 24.78–2.03 | 46.02–2.20 | |
| Protein atoms | 6127 | 6279 | |
| Water molecules, hetero-atoms | 203, 1 | 141, 1 | |
| Rwork, Rfree | 0.2104, 0.2489 | 0.2130, 0.2338 | |
| Average ADP (Å2) | 36.80 | 33.39 | |
| RMSDbonds (Å), RMSDangles (°) | 0.0137, 1.764 | 0.0067, 1.165 | |
| Protein Data Bank code | 4E9J | 4EC5 | |
a Rmeas = Σh√nh/(nh − 1) ΣhΣi|I(h,i) − <I(h)>|/ΣhΣiI(h,i), where nh is the multiplicity, I(h,i) is the intensity of the ith measurement of reflection h, and <I(h)> is the average value over multiple measurements.
Protein Analysis by SDS-PAGE and Mass Spectrometry
To monitor cleavage of the His6 tag by thrombin and sample purity, protein samples were dissolved in Laemmli loading buffer with or without 5% (v/v) β-mercaptoethanol, heated for 5 min at 95 °C, and separated by electrophoresis in 15% polyacrylamide gels using Tris-glycine-SDS as running buffer. For identification of protein bands by peptide mass fingerprinting, appropriate bands were cut out and digested with trypsin overnight at 37 °C. The peptides were subsequently extracted, dried, and dissolved in 10 μl 0.1% formic acid. One microliter of the digestion mixture was mixed with an equal volume of matrix solution (3 mg/ml α-cyano-hydroxycinnamic acid (Sigma), 50% (v/v) acetronitrile, and 0.1% (v/v) trifluoroacetic acid) and subsequently subjected to mass spectrometric analyses on a 4800 plus TOF/TOF analyzer (Applied Biosystems).
Native and denaturing electrospray ionization-MS measurements were performed on a Synapt G1 (Waters) coupled with an Advion Nanomate source. For measurements under native conditions, protein samples at 0.2, 1.8, and 5.4 mg/ml were exchanged to a buffer containing 50–200 mm ammonium acetate, pH 6.8, with a Biospin 6 column (Bio-Rad). For measurements under denaturing conditions, the samples were exchanged to a buffer containing 50% (v/v) acetonitrile and 0.1% (v/v) formic acid. External calibration of mass spectra was carried out using 5 mg/ml cesium iodide, and experimental parameters for native mass spectrometry were optimized under the ion mobility spectrometry model as described before (28). Data analysis was processed with Masslynx V4.1 and Driftscopt V2.3.
Small Angle X-ray Scattering of Peri-XcpQ
Small angle x-ray scattering (SAXS) measurements on purified peri-XcpQ at concentrations of 0.5, 3, 5, 10, and 12 mg/ml were carried out at Beamline X33 of the EMBL (Deutsches Elektronen-Synchrotron, Hamburg, Germany).
Elastase Activity Screen
To investigate whether PAN1 cells transformed with pB28, pB28_S109C, pB28_S210C, or pMMB67EH were able to secrete elastase, 1 μl samples of overnight cultures, normalized to the same A600 nm, were spotted on LB-agar plates supplemented with carbenicillin (100 μg/ml) and gentamicin (15 μg/ml) and elastin as the substrate. Cells were grown at 37 °C for 48–72 h before evaluating halo formation. Elastin-containing plates were made as described (29). Briefly, 1 ml of sterilized water was added to 0.08 g of elastin from bovine neck ligament (Sigma) and pulverized well using a hand mortar and pestle. The resulting suspension was added to 50 ml of melted LB agar, to which carbenicillin (100 μg/ml) and gentamicin (15 μg/ml) were added, and inverted several times before pouring.
RESULTS
The Crystal Structure of Peri-XcpQ Reveals a Dimeric Periplasmic Domain
The pET-N0N3′ construct encodes residues 35–325 of XcpQ, covering subdomains N0, N1, N2, and the first 47 residues from N3. Surprisingly, upon treatment of the purified protein with thrombin to remove the N-terminal His6 tag, the protein migrated as a band corresponding to an apparent molecular mass of ∼25 kDa, deviating significantly from its expected molecular mass of 31.5 kDa. In-gel analysis of the band using peptide mass fingerprinting revealed that, in addition to the removal of the His6 tag, the protein was cleaved after Arg-277 to yield a protein covering amino acid residues 35–277 of XcpQ, hereafter termed peri-XcpQ. The mass of peri-XcpQ was subsequently determined by denaturing electrospray ionization-MS as 26548.21 ± 0.72 Da. Interestingly, the unexpected cleavage site after Arg-277 is close to the C terminus of the N2 subdomain as predicted from structure-based sequence alignment with GspD (12).
Crystallization trials using purified recombinant peri-XcpQ after cleavage of the His6 tag led to x-ray data of high quality to 2.0 Å resolution (Table 1). Because attempts to determine the crystal structure by molecular replacement using search models derived from the structure of the homologous GspD (12) (25% sequence identity, Protein Data Bank code 3EZJ) were unsuccessful, we resorted to structure determination by single wavelength anomalous diffraction using selenomethione-labeled peri-XcpQ (Table 1). Phase improvement by density modification exploiting the apparent 2-fold noncrystallographic symmetry yielded readily traceable electron density for two molecules of peri-XcpQ in the crystal asymmetric unit.
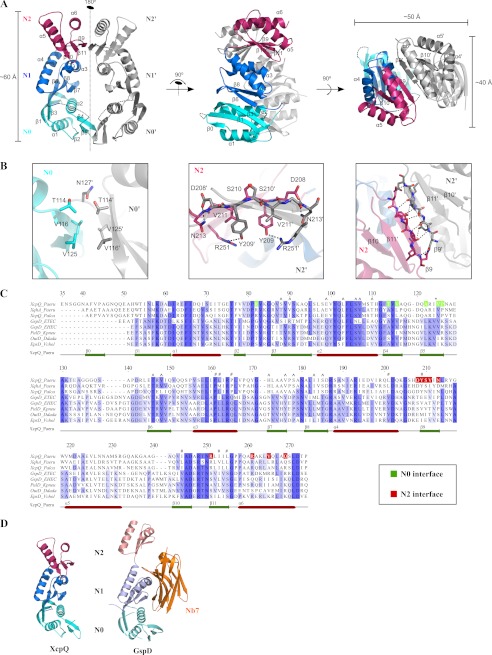
The crystal structure of peri-XcpQ (Protein Data Bank code 4E9J) unveiled a dimeric assembly in which the N0, N1, and N2 domains in each subunit run in parallel along a 2-fold axis. Both monomers make contacts at the N0 and N2 subdomains (Fig. 1, A and B). The backbone of both molecules could be traced completely except for the loop connecting subdomains N0 and N1 (residues 129–139 in chain A and residues 128–142 in chain B) and the first 16 N-terminal residues and the last three C-terminal residues. However, extra electron density was observed at the N terminus of both peri-XcpQ molecules, corresponding to an additional β-strand (hereafter called β0), notably absent in the homologous structure of peri-GspD. Unfortunately, this electron density could not be interpreted unambiguously, except for five residues (residues 41–45) in chain A.
FIGURE 1.
Structure of peri-XcpQ. A, three views of the homodimer of peri-XcpQ. Note the 2-fold symmetry axis and the extended β-sheet of the N2 interface. B, detail of the interaction interface between N0:N0′ (left panel) and extended β-sheet between N2:N2′ (middle and right panels). C, alignment of peri-XcpQ sequences against peri-GspD sequences from different species, annotated by secondary structure elements of peri-XcpQ. Residues participating at the N0:N0′ and N2:N2′ interfaces are colored in green and red, respectively. Residues that are involved via side chain interactions in the N0:N1 and N1:N2 interfaces are annotated with ^ and #, respectively (see also supplemental Table S2). The figure was made using Jalview 2 (39). D, comparison of the peri-XcpQ monomer model and peri-GspD monomer (Protein Data Bank code 3EZJ) in complex with a Nb7 nanobody. Note that the bound nanobody occupies a site that would not be compatible with dimerization as observed in peri-XcpQ.
The N0 subdomain consists of two central helices flanked by a mixed three-stranded β-sheet (including β2, β4, and β5) on one side and a three-stranded antiparallel β-sheet (including β0, β1, and β3) on the other side. Except for β0, the observed topology and fold are almost identical to the N0 subdomain of peri-GspD.
The N1 and N2 subdomains share the same fold, albeit with a RMSD of 9.071 Å, and consist of two α-helices flanked on one side by an antiparallel three-stranded β-sheet. The 310 helix 3 observed in subdomain N1 of peri-GspD was less well defined in peri-XcpQ. Consequently, helix 3 and 4 are merged into one α-helix (noted in Fig. 1A as helix α3). However, two short loops connecting β-strands 7 and 8 of the N1 subdomain and β-strands 10 and 11 of the N2 subdomain do have 310 character, whereas this is not the case in the peri-GspD structure.
The three subdomains in the peri-XcpQ subunit pack compactly against their adjacent subdomains, such that the N0:N1 and N1:N2 interfaces bury ∼700 and ∼250 Å2, respectively. The N0 and N1 interaction includes a β-sheet extension involving β3 from N0 and β6 from N1 and a cluster of hydrophobic residues, including Leu-95 and Leu-103 from N0 and Thr-146, Val-148, Val-176, and Ile-183 from N1 (supplemental Table S2). In addition, the N2 subdomain is folded back onto and interacts with subdomain N1, burying hydrophobic residues including Pro-161, Leu-162, Pro-165, and Leu-201 from N1 and Tyr-209, Ile-244, Ile-253, and Leu-255 from the N2 subdomain (supplemental Table S2). Sequence alignments between different T2SS secretin proteins (Fig. 1C) showed that the hydrophobic cores of the N0:N1 and N1:N2 interaction interfaces are conserved.
In the context of dimeric peri-XcpQ, the two subunits interact via their corresponding N0 and N2 subdomains (supplemental Table S2). The N0:N0′ interaction site buries ∼350 Å2 of surface area and features a hydrophobic core created by a β-sandwich involving Val-125, Val-116, and Thr-114 (contributing its methyl group) from β4 and β5 and their counterparts (Fig. 1B, left panel). Notably, these amino acids are well conserved across the T2SS secretin family (Fig. 1C), suggesting that similar N0:N0′ interfaces are possible in homologous secretins.
Contrasting the interaction mode of the N0:N0′ interface, the N2:N2′ interface is more extensive (∼550 Å2 buried surface area) and features an extended six-stranded antiparallel β-sheet, which is mediated by β9 of each of the participating monomers (Fig. 1B, middle and right panels). The side chains protruding from the central strands (β9 and β9′) contact each other via complementary interactions (Fig. 1B, middle panel). Here the hydrophobic core of Tyr-209 contacts with Val-211′ from the partner monomer. Furthermore, Asp-208 can form hydrogen bonds with both the backbone and the side chain from residue Asn-213′. Finally, Ser-210 and Ser-210′ interact via a hydrogen bond, and Arg-251 on the neighboring β11 hydrogen bonds to the hydroxyl group of Tyr-209′ on β9′. Mapping the N2 interface onto the sequence alignment indicated that none of the involved residues are conserved among members the secretins of the T2SS (Fig. 1C). In addition to the extended β-sheet formed upon peri-XcpQ dimerization, three additional residues: Arg-261, Val-265, and Gln-269 all residing on α6 contribute to the N2:N2′ interface. These residues make interactions in an antiparallel fashion because of the dimer 2-fold axis, such that Arg-261 interacts with Gln-269′. Val-265 and Val-265′ contribute to the weak hydrophobic interface between both α6 helices.
Peri-XcpQ Forms Dimers in Solution in a Concentration-dependent Manner
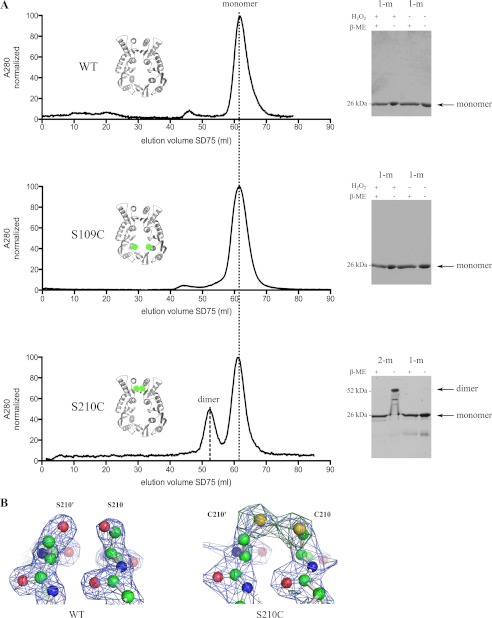
To probe the dimerization propensity of peri-XcpQ and to cross-validate the crystallographically observed interfaces in solution, we resorted to structure-based engineering of cysteine point mutants. Our hypothesis was that native dimerization interfaces would become covalently linked via a disulfide bridge between engineered cysteines at the dimer interfaces. Thus, based on the crystal structure of peri-XcpQ, we deemed it opportune to mutate Ser-210 at the N2 dimer interface to cysteine (S210C). Ser-210 resides in the middle of β9, which mediates the antiparallel extended β-sheet between N2 domains and interacts via a hydrogen bond with Ser-210′ (Fig. 1B, middle and right panels). As a negative control, we engineered a second variant featuring a S109C point mutation. Ser-109 resides on the inside face of N0 in the peri-XcpQ dimer and would not be expected to form an intersubunit disulfide bond because of its prohibitively large distance from S109C′ (∼15 Å).
Purification of wild-type peri-XcpQ and the two mutant variants by SEC followed by SDS-PAGE in the presence or absence of reducing agent revealed that peri-XcpQ(S210C) migrated as a mixture of disulfide-linked and noncovalently linked monomers (Fig. 2A). However, the efficiency of formation of these disulfide bridges appeared to be rather low, most likely because of strict geometric requirements for forming such bonds (30) and the fact that the two residues involved are solvent-exposed. Nevertheless, the yield of dimeric peri-XcpQ(S210C) could be increased 10-fold when the protein sample was incubated with 0.3% (v/v) H2O2 for 30 min before SEC (data not shown). On the other hand, peri-XcpQ(S109C) retained the chromatographic and electrophoretic behavior of wild-type peri-XcpQ. As expected, no disulfide bridges could be induced when H2O2 was added before SEC or to purified samples of wild-type peri-XcpQ or the S109C variant, respectively (Fig. 2A).
FIGURE 2.
Cysteine disulfide-bridge engineering in peri-XcpQ. A, elution profiles of wild-type, S109C, and S210C peri-XcpQ in size exclusion chromatography and the corresponding SDS-PAGE analyses. For both wild type and S109C, the major peak (monomer, 1-m) was selected and submitted to oxidation by H2O2 or water as control prior the SDS-PAGE analysis. The elution profile of S210C peri-XcpQ mutant shows an extra peak that corresponds with a dimer (2-m) of peri-XcpQ as shown on SDS-PAGE. The positions of both substituted amino acids are denoted in green in the model of peri-XcpQ. B, detail of electron density map (2Fo − Fc (1.5 σ) and Fo − Fc (3.3 σ); αc,MR) of the disulfide bridge of the S210C mutant of peri-XcpQ in comparison with the map for the wild-type protein structure. β-ME, β-mercaptoethanol.
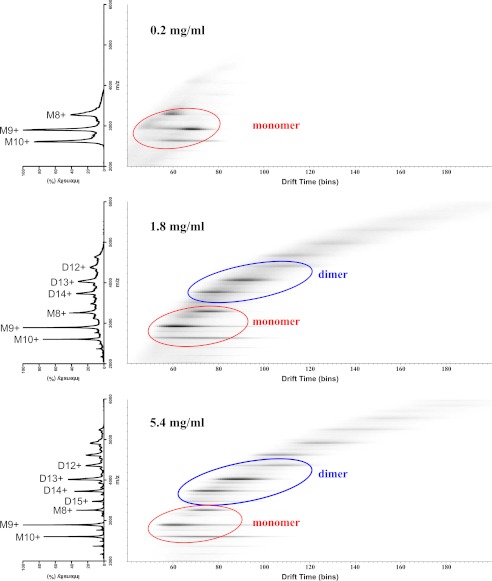
To further explore the dimerization properties, increasing concentrations of purified wild-type peri-XcpQ were analyzed via native electrospray ionization ion mobility mass spectrometry. Our data show that peri-XcpQ at the lowest concentration (0.2 mg/ml) is predominantly monomeric, whereas at higher concentrations (1.8 and 5.4 mg/ml), peri-XcpQ forms considerably more dimeric species (Fig. 3). This transition was observed in our SAXS experiments as well. Interestingly, at concentrations higher than 5 mg/ml, peri-XcpQ has a propensity to form higher order oligomers, a behavior that was additionally observed during our SAXS measurements. However, we have been unable to model the oligomerization behavior of XcpQ via SAXS, most likely because of the complexity of the oligomerization mixtures.
FIGURE 3.
Peri-XcpQ forms dimers in solution in a concentration-dependent manner. Ion mobility drift time chromatograms obtained from native mass spectrometry on wild-type peri-XcpQ at three different concentrations. The clusters highlighted with ovals correspond to particular oligomeric states. Each state exhibits different m/z species. M, monomeric; D, dimeric. The numbers denote the charge of the protein.
To confirm the presence of a disulfide bond in peri-XcpQ(S210C) in the context of the dimeric configuration observed in our crystal structure of wild-type peri-XcpQ, we grew crystals from pure disulfide-linked peri-XcpQ(S210C) protein fractions and determined its structure at 2.2 Å resolution by molecular replacement using the refined model for peri-XcpQ carrying an alanine at position 210 (Table 1). Indeed, peri-XcpQ(S210C) retains the dimeric assembly of peri-XcpQ and positive difference electron density contoured at 3 σ in a map calculated with Fourier coefficients Fo − Fc; αc,MR revealed clear evidence for the presence of a disulfide bridge at the interface of peri-XcpQ(S210C) (Fig. 2B). Crystallographic refinement of the model and observation of difference electron density residuals suggested that the disulfide bridge is not fully occupied in peri-XcpQ(S210C). As we started with pure dimeric protein, the observed disulfide bond break is likely due to radiation damage, which is an inherent problem in x-ray crystallography using synchrotron radiation (31). To estimate the ratio of oxidized to reduced Cys-210, we carried out test refinements probing pairwise combinations of occupancies of the two oxidation states. Thus, in the final structure (Protein Data Bank code 4EC5), two alternative conformations for Cys-210 and Cys-210′ are given, resembling the reduced (open) and oxidized (closed) form of the disulfide bridge.
Full-length XcpQ Forms Dimers in Vivo
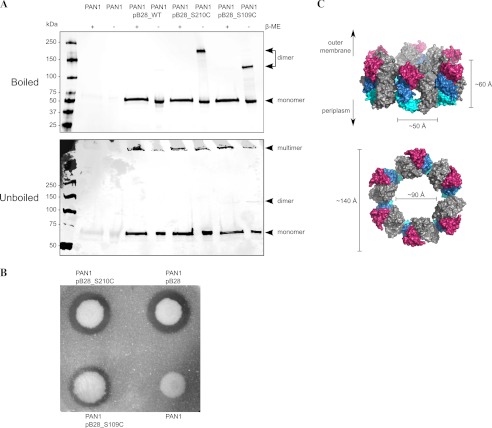
To extrapolate our structural and biochemical findings on dimeric peri-XcpQ to full-length XcpQ in vivo, PAN1 cells were transformed with constructs pB28, pB28_S109C, and pB28_S210C encoding for wild-type full-length XcpQ and its S109C and S210C mutant variants, respectively. Strain PAN1 is a xcpQ deletion mutant with a gentamicin resistance cassette inserted at the position of xcpQ on the chromosome (32). Western blot analysis of wild-type and mutant XcpQ under reducing and nonreducing conditions revealed that both mutants XcpQ(S210C) and XcpQ(S109C) migrated as two distinct species on SDS-PAGE (Fig. 4A), consistent with approximately a 50:50 mixture of covalent dimers and monomers, probably because of the low efficiency in disulfide bridge formation. In contrast, the wild-type protein migrated in both conditions at the molecular mass level of monomeric XcpQ (∼66 kDa).
FIGURE 4.
Dodecameric full-length XcpQ is a hexamer of dimers in vivo. A, Western blot analysis of boiled and unboiled samples of PAN1 cells under reducing and nonreducing conditions. PAN1 cells were transformed with constructs pB28, pB28_S109C, and pB28_S210C encoding for wild-type full-length XcpQ and its S109C and S210C variants, respectively. B, elastase activity assay on PAN1 transformants. Except for the parental strain, all transformants expressing XcpQ or its mutant variants show the formation of halos around the colonies as a result of proteolysis of elastin substrate in the plate. C, hexameric model of peri-XcpQ dimers obtained by manual modeling based on the molecular envelope of full-length EpsD as obtained by cryo-electron microscopy (Electron Microscopy Data Bank code 1763) (37).
The protein bands corresponding to the covalently associated dimers in both mutants showed intriguing differences in electrophoretic behavior, whereas all of the monomeric protein bands migrate at the same level as wild-type XcpQ. Such discrepancies have also been recently observed for cysteine mutants in the N1 or N2 subdomains of OutD (18) and appear to be common in membrane proteins solubilized with SDS for electrophoretic separation (33).
To investigate whether recombinant wild-type and mutant full-length XcpQ are still assembled in the outer membrane as functional secretins, PAN1 cells were subjected to an elastase activity assay by growing them on LB-agar plates supplemented with elastin. Elastase is one of the major exoproteins exported by the T2SS in P. aeruginosa (34–36). All transformed PAN1 strains, except for the negative control transformed with empty vector, showed formation of a halo around the colonies after 48–72 h of growth. However, the halo around the S109C variant appeared consistently smaller than those of the WT and S210C variant in several replicas (Fig. 4B). In addition, PAN1 samples were loaded on gel without first heating them and subsequently analyzed by Western blotting (Fig. 4A). Except for the negative control, two bands were observed in all samples, including wild type, with a similar ratio between both. The lowest band corresponds to the monomeric species, whereas the second band, which did not enter the gradient gel completely, corresponds to multimeric XcpQ (most likely the XcpQ dodecamer with a molecular mass of ∼0.8 MDa). It is noteworthy that a faint band corresponding to the dimeric species could be observed for the S109C variant in our analysis by Western blot analysis. However, the majority of covalently linked XcpQ(S109C) is incorporated in the presumed dodecameric species.
DISCUSSION
Despite nearly two decades of continuous developments in our understanding of the structural and mechanistic determinants of bacterial type II secretion systems (7, 8), several key questions remain unanswered. In particular, the assembly principles underlying the formation of outer and inner membrane complexes and their communication between both have remained elusive.
In this contribution, we present direct structural, biochemical, and cellular evidence that XcpQ, the secretin of the T2SS from P. aeruginosa, is a dimeric protein that oligomerizes into a functional dodecameric assembly via C6 symmetry of dimeric building blocks. Prior to our study, the working paradigm for secretin structure has been that secretin monomers assemble under C12 symmetry to construct the dodecameric rings as visualized by electron microscopy (11, 15, 37). In contrast, our study supports a hexameric assembly of dimers, a model that was also recently suggested by other studies (17, 18).
Arguably, the most intriguing finding from our studies has been the discovery that peri-XcpQ adopts a dimeric structure with two well defined interaction interfaces at the tips of the three-domain structure. This is because a crystallographic study of the periplasmic secretin domain GspD from enterotoxigenic E. coli in complex with a nanobody had revealed a monomeric structure (12). According to this study, the development of such a nanobody binder was deemed necessary to improve the stability and crystallizability of peri-GspD constructs that proved recalcitrant to crystallization trials (12). Furthermore, this structure subsequently set the stage for modeling of the electron density envelope of full-length GspD from V. cholerae as a dodecameric assembly of GspD monomers (8, 37).
Structural comparisons between peri-XcpQ and peri-GspD show that despite the low sequence identity between the two proteins (i.e., 25%), their individual subdomains are structurally very similar with RMSD values of 0.64, 1.66, and 1.01 Å for subdomains N0, N1, and N2, respectively. However, in contrast to peri-GspD, the N2 subdomain of peri-XcpQ is rotated backwards to interact with the preceding N1 subdomain. The ensuing N1:N2 interface buries hydrophobic residues that are conserved or replaced by similar residues throughout all members of the T2SS family, indicating that the N1:N2 interface might be a conserved structural feature. In this regard, we note that part of the binding epitope (i.e., framework contacts) of the Nb7 nanobody to GspD targets the N1:N2 interdomain interface (Fig. 1D), prying open a possible N1:N2 interaction. Even more striking is the overlap of the nanobody epitope with surfaces in peri-GspD, of which the counterparts in peri-XcpQ establish the dimerization interfaces.
We were thus left to wonder whether the targeting of peri-GspD by the Nb7 nanobody may have interfered with the dimerization potential of peri-GspD. Our crystallographic analysis of dimeric peri-XcpQ and further validation of the dimerization propensity of peri-XcpQ by diverse methods, including disulfide engineering followed by crystallographic validation thereof, certainly point in this direction. Moreover, the amino acids in the observed N0:N0′ interfaces are well conserved across the T2SS secretin family. On the other hand, the main structural feature of the N2:N2′ interface is an extended six-stranded antiparallel β-sheet, which is established via complementation of the main chain hydrogen-bonding network of β9 and β9′ and strengthened by side chain interactions above and below the plane of the β-sheet. Although the conservation of sequences coding for β9 in homologous secretins is not strong, the β-strand character of the interface residues is conserved as evidenced by the structure of the homologous peri-GspD and secondary structure predictions of other T2SS secretin members. We further note that the observed concentration-dependent dimerization of peri-XcpQ suggests that the oligomeric state of recombinant peri-GspD used to immunize animals for antibody development was likely a monomer because only low concentrations were employed (12).
To derive the biological relevance of the observed oligomerization behavior of peri-XcpQ, one needs to consider the protein in its cellular context. Secretins are large outer membrane proteins with periplasmic N-terminal and membrane-embedded C-terminal domains. These C-terminal secretin domains coupled with the constraints of membrane dimensionality are essentially poised to drive secretin oligomerization, resulting in a local high concentration of parallel oriented XcpQ molecules.
Thus, although peri-XcpQ exhibits a concentration-dependent dimerization in solution manifested at rather high protein concentrations, full-length XcpQ in a cellular context would be expected to adopt its functional oligomeric assembly much more readily. As such, the presence of the membrane-inserted C-terminal domains of XcpQ, accounting for approximately the half of the protein, and the geometric restrictions of the locally planar membrane, will essentially improve the Kd and kinetics of oligomerization by several orders of magnitude. Nonetheless, it is not straightforward to extrapolate the peri-XcpQ concentrations to in vivo concentrations, because the mechanism of biogenesis pathway for ∼50–100 copies of functional XcpQ dodecamers (11) is still unknown. We envisage that the insights we provide here may inspire a structure-based investigation of such a mechanistic pathway.
In this study we attempted to provide such insights via structure-based engineering of a cysteine disulfide at the N2:N2′ dimer interface and showed that full-length XcpQ does engage in dimeric subassemblies. This was also the case for our second cysteine mutant in N0, contrasting its inability to form a disulfide bridge in peri-XcpQ (Figs. 2A and 4A). This suggests that the periplasmic domain or at least the N0 and N1 subdomains in full-length XcpQ should be dynamic or flexible. In this regard, the very limited extent of the N1:N2 interface (∼250 Å2) could support this inherent flexibility. It is also known that the N-terminal periplasmic domain of XcpQ interacts with exoproteins and other T2SS components such as XcpP (13–15) and that these contacts might affect the conformational state of secretin domains, something that has been shown by studies on the OutC/OutD system (18). In this way the two Cys-109 residues might have come close enough to engage in disulfide bridge formation. In addition, the halo formed around the S109C variant in the elastase activity assay seems to be consistently smaller than the halo formed around the wild type and the halo of the S210C variant (Fig. 4B). This suggests that the effect of disulfide bridge formation of the S109C variant on the T2SS apparatus is more abortive compared with the S210C variant. However, we cannot rule out the possibility that during biogenesis of XcpQ to the final dodecameric assembly, covalently linked S109C variants are preformed and that, as such, they are impaired to assemble into functional dodecameric assemblies. In this regard, we note that the S210C variant does not seem to have interfered with the XcpQ biogenesis at all. This would also explain why a small fraction of dimeric species was observed for pB28_S109C but not for pB28_S210C (Fig. 4A).
The lateral proximity of periplasmic domains in secretin dodecamers is further supported by recent cysteine engineering in OutD from Dickeya dadantii (18, 38), which showed that homodimeric OutD species can be identified. Based on the crystal structure of peri-XcpQ, these disulfide bridges were introduced on the outside of OutD dimers. Such dimers would in turn be in lateral proximity to one another in the OutD dodecamer.
Taken together, the recent diverse structural, biochemical, and functional studies by us and others point to a new consensus for the assembly of secretin dodecamers. We propose that the basic assembly unit in secretins is a dimer, which in turn obeys 6-fold symmetry to generate the functional dodecamer (Fig. 4C). Such an assembly mode challenges the recently proposed dodecameric models of the N-terminal domain of the T2SS secretin based on the crystal structure of peri-GspD and the EM structure of EpsD from V. cholerae, which were both based on C12 symmetry as applied to GspD/EpsD monomers (12, 37). In fact, the observed features in reported image class averages for EpsD do not rule out the application of C6 symmetry (see supplemental figure S1 from Ref. 37) and are compatible with a hexamer of dimers assembly. Furthermore, negative stain EM images of pseudocrystals of XcpQ from Pseudomonas alcaligenes (11) clearly show hexagonal particles, consistent with our proposed model. In addition, a recent study by EM of the structure of BfpB, the secretin for type IV pili, proposed a dodecameric secretin that can be assembled as a set of six dimers (17). Finally, we note that the crystal structure of the periplasmic domain of EscC, the T3SS secretin from enteropathogenic E. coli, also forms dimers in the crystal lattice (16), but the possible relevance of dimers in the oligomeric assembly of T3SS with an even number of subunits has not been addressed.
Supplementary Material
Acknowledgments
We thank the European Synchrotron Radiation Facility (Grenoble, France) and SOLEIL (Gif-sur-Yvettes, France) for Synchrotron beam time allocation, and the staffs of Beamlines ID-23 (European Synchrotron Radiation Facility) and Proxima (SOLEIL) for technical support.
This work was supported by grants from Ghent University (Bijzonder Onderzoeksfonds-Geconcentreerde Onderzoeksactie instrument and co-funding to the Chinese Scholarship Council grant), Hercules Foundation Grant AUGE019, and BELSPO IAP Grant iPROS, P7/44, and Grant Agreement Number 226716 from the European Commission under the 7th Framework Programme: Research Infrastructures.

This article contains supplemental Tables S1 and S2.
The atomic coordinates and structure factors (codes 4E9J and 4EC5) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- T2SS
- type II secretion system
- T3SS
- type III secretion system
- RMSD
- root mean square deviation
- SAXS
- small angle x-ray scattering
- SEC
- size exclusion chromatography.
REFERENCES
- 1. Kung V. L., Ozer E. A., Hauser A. R. (2010) The accessory genome of Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 74, 621–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sandkvist M. (2001) Type II secretion and pathogenesis. Infect. Immun. 69, 3523–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerlach R. G., Hensel M. (2007) Protein secretion systems and adhesins. The molecular armory of Gram-negative pathogens. Int. J. Med. Microbiol. 297, 401–415 [DOI] [PubMed] [Google Scholar]
- 4. Bleves S., Viarre V., Salacha R., Michel G. P., Filloux A., Voulhoux R. (2010) Protein secretion systems in Pseudomonas aeruginosa. A wealth of pathogenic weapons. Int. J. Med. Microbiol. 300, 534–543 [DOI] [PubMed] [Google Scholar]
- 5. Filloux A. (2011) Protein secretion systems in Pseudomonas aeruginosa. An essay on diversity, evolution, and function. Front. Microbiol. 2, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wretlind B., Pavlovskis O. R. (1984) Genetic mapping and characterization of Pseudomonas aeruginosa mutants defective in the formation of extracellular proteins. J. Bacteriol. 158, 801–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McLaughlin L. S., Haft R. J., Forest K. T. (2012) Structural insights into the type II secretion nanomachine. Curr. Opin. Struct. Biol. 22, 208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korotkov K. V., Sandkvist M., Hol W. G. (2012) The type II secretion system. Biogenesis, molecular architecture and mechanism. Nat. Rev. Microbiol. 10, 336–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bitter W., Koster M., Latijnhouwers M., de Cock H., Tommassen J. (1998) Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 27, 209–219 [DOI] [PubMed] [Google Scholar]
- 10. Genin S., Boucher C. A. (1994) A superfamily of proteins involved in different secretion pathways in Gram-negative bacteria. Modular structure and specificity of the N-terminal domain. Mol. Gen. Genet. 243, 112–118 [DOI] [PubMed] [Google Scholar]
- 11. Brok R., Van Gelder P., Winterhalter M., Ziese U., Koster A. J., de Cock H., Koster M., Tommassen J., Bitter W. (1999) The C-terminal domain of the Pseudomonas secretin XcpQ forms oligomeric rings with pore activity. J. Mol. Biol. 294, 1169–1179 [DOI] [PubMed] [Google Scholar]
- 12. Korotkov K. V., Pardon E., Steyaert J., Hol W. G. (2009) Crystal structure of the N-terminal domain of the secretin GspD from ETEC determined with the assistance of a nanobody. Structure 17, 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reichow S. L., Korotkov K. V., Gonen M., Sun J., Delarosa J. R., Hol W. G., Gonen T. (2011) The binding of cholera toxin to the periplasmic vestibule of the type II secretion channel. Channels 5, 215–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Douzi B., Ball G., Cambillau C., Tegoni M., Voulhoux R. (2011) Deciphering the Xcp Pseudomonas aeruginosa type II secretion machinery through multiple interactions with substrates. J. Biol. Chem. 286, 40792–49801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korotkov K. V., Gonen T., Hol W. G. (2011) Secretins. Dynamic channels for protein transport across membranes. Trends Biochem. Sci. 36, 433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spreter T., Yip C. K., Sanowar S., André I., Kimbrough T. G., Vuckovic M., Pfuetzner R. A., Deng W., Yu A. C., Finlay B. B., Baker D., Miller S. I., Strynadka N. C. (2009) A conserved structural motif mediates formation of the periplasmic rings in the type III secretion system. Nat. Struct. Mol. Biol. 16, 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lieberman J. A., Frost N. A., Hoppert M., Fernandes P. J., Vogt S. L., Raivio T. L., Blanpied T. A., Donnenberg M. S. (2012) Outer membrane targeting, ultrastructure, and single molecule localization of the enteropathogenic Escherichia coli type IV pilus secretin BfpB. J. Bacteriol. 194, 1646–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X., Pineau C., Gu S., Guschinskaya N., Pickersgill R. W., Shevchik V. E. (2012) Cysteine scanning mutagenesis and disulfide mapping analysis of the arrangement of GspC and GspD protomers within the T2SS. J. Biol. Chem. 287, 19082–19093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 [DOI] [PubMed] [Google Scholar]
- 20. Kabsch W. (2010) XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX. A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Emsley P., Lohkamp B., Scott W. G., Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blanc E., Roversi P., Vonrhein C., Flensburg C., Lea S. M., Bricogne G. (2004) Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr. D Biol. Crystallogr. 60, 2210–2221 [DOI] [PubMed] [Google Scholar]
- 25. Chen V. B., Arendall W. B., 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., Richardson D. C. (2010) MolProbity. All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The PyMOL Molecular Graphics System, version 1.2r3pre, Schrödinger, LLC [Google Scholar]
- 27. Krissinel E. (2011) Macromolecular complexes in crystals and solutions. Acta Crystallogr. D Biol. Crystallogr. 67, 376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruotolo B. T., Benesch J. L., Sandercock A. M., Hyung S. J., Robinson C. V. (2008) Ion mobility-mass spectrometry analysis of large protein complexes. Nat. Protoc. 3, 1139–1152 [DOI] [PubMed] [Google Scholar]
- 29. Werb Z., Gordon S. (1975) Elastase secretion by stimulated macrophages. Characterization and regulation. J. Exp. Med. 142, 361–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Indu S., Kochat V., Thakurela S., Ramakrishnan C., Varadarajan R. (2011) Conformational analysis and design of cross-strand disulfides in antiparallel β-sheets. Proteins 79, 244–260 [DOI] [PubMed] [Google Scholar]
- 31. Weik M., Ravelli R. B., Kryger G., McSweeney S., Raves M. L., Harel M., Gros P., Silman I., Kroon J., Sussman J. L. (2000) Specific chemical and structural damage to proteins produced by Synchrotron radiation. Proc. Natl. Acad. Sci. U.S.A. 97, 623–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alexeyev M. F., Shokolenko I. N., Croughan T. P. (1995) Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene 160, 63–67 [DOI] [PubMed] [Google Scholar]
- 33. Rath A., Glibowicka M., Nadeau V. G., Chen G., Deber C. M. (2009) Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 106, 1760–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Braun P., Ockhuijsen C., Eppens E., Koster M., Bitter W., Tommassen J. (2001) Maturation of Pseudomonas aeruginosa elastase. Formation of the disulfide bonds. J. Biol. Chem. 276, 26030–26035 [DOI] [PubMed] [Google Scholar]
- 35. Kessler E., Safrin M., Gustin J. K., Ohman D. E. (1998) Elastase and the LasA protease of Pseudomonas aeruginosa are secreted with their propeptides. J. Biol. Chem. 273, 30225–30231 [DOI] [PubMed] [Google Scholar]
- 36. Lazdunski A., Guzzo J., Filloux A., Bally M., Murgier M. (1990) Secretion of extracellular proteins by Pseudomonas aeruginosa. Biochimie 72, 147–156 [DOI] [PubMed] [Google Scholar]
- 37. Reichow S. L., Korotkov K. V., Hol W. G., Gonen T. (2010) Structure of the cholera toxin secretion channel in its closed state. Nat. Struct. Mol. Biol. 17, 1226–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gu S., Kelly G., Wang X., Frenkiel T., Shevchik V. E., Pickersgill R. W. (2012) Solution structure of the HR domain of the type II secretion system. J. Biol. Chem. 287, 9072–9080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Waterhouse A. M., Procter J. B., Martin D. M., Clamp M., Barton G. J. (2009) Jalview version 2. A multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.