Background: Azipropofol is a photoactive analog of the general anesthetic propofol.
Results: In vivo photolabeling of tadpoles results in covalent ligand binding to neuronal proteins and prolongation of anesthesia.
Conclusion: Reconciling time-resolved gel proteomics with behavioral state allows identification of potential anesthetic targets.
Significance: In vivo activation of efficacious photolabels provides a novel approach to investigate mechanisms of general anesthesia.
Keywords: Anesthesia, Neurobiology, Photoaffinity Labeling, Protein Chemical Modification, Xenopus, Xenopus laevis, Azipropofol, Propofol, Tadpole
Abstract
General anesthetic photolabels have been instrumental in discovering and confirming protein binding partners and binding sites of these promiscuous ligands. We report the in vivo photoactivation of meta-azipropofol, a potent analog of propofol, in Xenopus laevis tadpoles. Covalent adduction of meta-azipropofol in vivo prolongs the primary pharmacologic effect of general anesthetics in a behavioral phenotype we termed “optoanesthesia.” Coupling this behavior with a tritiated probe, we performed unbiased, time-resolved gel proteomics to identify neuronal targets of meta-azipropofol in vivo. We have identified synaptic binding partners, such as synaptosomal-associated protein 25, as well as voltage-dependent anion channels as potential facilitators of the general anesthetic state. Pairing behavioral phenotypes elicited by the activation of efficacious photolabels in vivo with time-resolved proteomics provides a novel approach to investigate molecular mechanisms of general anesthetics.
Introduction
General anesthetics bind many proteins with low affinities (μm KD values), hindering analyses of ligand-target interactions. Photoactive analogs of clinically used general anesthetics have been developed to aid molecular studies (1–5). These probes share physicochemical properties with their parent molecules, retain anesthetic activity, and undergo photolysis under long wave ultraviolet light (UVA) (315–400 nm), a feature that limits damage to cellular macromolecules upon irradiation following equilibration with the ligands. With these compounds, anesthetic binding sites have been mapped on integrin lymphocyte function-associated antigen (3, 6), Torpedo nicotinic receptors (7, 8), β-tubulin (9), PKC (10), and GABAA receptors (11, 12), among others. Direct identification of anesthetic substrates from complex homogenates has proceeded with a neurosteroid analog (2) and halothane (13), the latter an unaltered general anesthetic containing a carbon-bromine bond broken by shorter UV wavelengths to create reactive carbon-centered radicals (14).
Despite anesthetic efficacy in vivo, the use of these probes has been limited to in vitro preparations. Electrophysiological evidence supports the concept that covalent incorporation of photoactive anesthetics to binding sites can result in prolonged modulation of functional proteins (15). Although alkylphenol anesthetics are thought to act in part through GABAA receptors, genetic studies prove that other “on-pathway” targets exist (16, 17). Thus, we tested the feasibility of activating the photoaffinity probe meta-azipropofol (AziPm)2 in vivo as a tool to identify novel molecular substrates that contribute to alkylphenol general anesthesia. AziPm is an analog of propofol (2,6-diisopropylphenol) that contains an alkyl diazirinyl group in the meta position of the phenol ring (see Fig. 1) (4). In vivo photolabeling of Xenopus laevis tadpoles equilibrated with AziPm results in a previously unreported behavioral phenotype that we call “optoanesthesia.” We describe this in conjunction with unbiased, time-resolved gel proteomics employing a tritiated version of the photolabel.
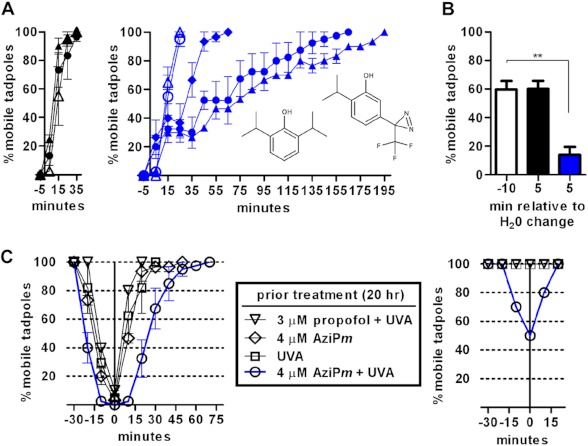
FIGURE 1.
A, time course of recovery for tadpoles following anesthetic equilibration and (left) sham treatment or (right) UVA exposure. 3 μm propofol (open symbols; left structure in right panel) or 4 μm AziPm (closed symbols; right structure in right panel) was used. Treatment times were 3 min (diamonds), 10 min (circles), or 20 min (triangles), and the water was changed at time 0. Data shown is the mean ± S.E. from 3–4 experiments per group. B, in vivo photolabeling for 10 min after equilibration with a sub-EC99 dose of 3 μm AziPm increased the immobile fraction of tadpoles. The water was changed at time 0, with photolabeling from −10 to 0 min. A one-way ANOVA found a significant difference between the three means (p < 0.01), and Bonferroni's post hoc test found a significant decrease in the percentage of mobile tadpoles after lamp exposure and water change (blue bar, p < 0.01). Data represent the mean ± S.E. from three experiments per treatment (± UVA). After equilibration, the tadpoles were randomly assigned to sham or UVA treatment, with the data at −10 min representing both groups and with sham-treated animals represented by the black bar. C, induction and recovery of tadpoles treated with (left) 2 μm propofol or (right) 0.8 μm propofol 20 h after the indicated treatments. Error bars represent S.E.
EXPERIMENTAL PROCEDURES
Materials
2,6-Diisopropylphenol was acquired from Sigma-Aldrich, and AziPm was synthesized by W. P. Dailey (University of Pennsylvania) through published methods (4). AziPm was radiolabeled by AmBios Labs (Boston, MA) by iodinating the ring and reducing with tritium under catalytic conditions. The final product was purified with HPLC. EcoLite(+) liquid scintillation mixture (MP Biomedicals) was used with a PerkinElmer Life Sciences Tri-Carb 2800TR instrument; a Varian Cary 300 Bio UV-visible spectrophotometer was used for spectroscopy. First and second dimension gels, electrophoresis apparatuses, and molecular weight markers were from Bio-Rad. UVA was generated by filtering a 100-watt arc mercury lamp through colored glass UV-visible broadband (∼340–615-nm) and UV band-pass (∼250–375-nm) filters (lamp and filters from Newport, Stratford, CT). Light intensities (measured with an optical power meter (Thorlabs, Newton, NJ)) were 28.1 microwatts/mm2 and 27.7 microwatts/mm2 at 350 and 375 nm, respectively. Albino X. laevis tadpoles (stage 45–47) were purchased from Nasco (Fort Atkinson, WI) and housed in supplied pond water for at least 24 h prior to experiments. Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania.
Tadpole Immobility Studies
Tadpoles were placed in Petri dishes with propofol or AziPm dissolved in pond water. The same physicochemical parameters determine anesthetic passage across the gills and skin as does passage across the blood brain barrier (18, 19). In some experiments, after a 30-min equilibration, tadpoles were transferred to fresh water; in others, after equilibration, tadpoles remained on the bench for a sham control or were exposed to UVA (photolabeled in vivo) before transfer to fresh water. Immobility was defined (and scored) as the percentage of tadpoles that did not swim, twitch, or right themselves throughout a 30-s time window preceding every 10-min interval. Tadpole assays are established measures of anesthetic potency (18, 19), and reversible immobility is the most commonly used general anesthetic end point. Alternative causes of immobility in our study (e.g. muscular toxicity) were not ruled out, but should have had additional and toxic features that would have been observed (e.g. cardiac muscle dysfunction, etc). The water temperature was 21–22 °C for experiments and changed <0.5 °C throughout any experiment.
In Vivo Photolabeling and Isolation of Neuronal Membranes
Tadpoles were incubated for 30 min with 4 μm [3H]AziPm and treated ± UVA for 10 min. After transfer to fresh water, tricaine methanesulfonate (500 mg/liter) was added immediately for the zero time point or at 165 min for the emergence time point, and the tadpoles were placed on ice. After decapitation, brains and spinal cords were removed with forceps under a dissecting microscope and placed in ice-cold 0.32 m sucrose, 5 mm Tris, pH 7.4, supplemented with protease inhibitors. Tissue isolation required less than 15 min following each time point; central nervous system (CNS) tissue was homogenized every 3–5 min using a Teflon/glass homogenizer.
CNS homogenates were centrifuged at 100,000 × g for 10 min, washed with isolation buffer, and recentrifuged. The pellet was homogenized in 5 mm Tris, pH 7.4, and centrifuged at 100,000 × g for 10 min, washed, and centrifuged again before resuspension in 2 mm Tris, pH 7.4. An aliquot was removed for a protein assay prior to freezing at −80 °C.
In Vitro Photolabeling
Unexposed tadpoles were anesthetized with tricaine methanesulfonate, and neuronal tissue, dissected as above, was homogenized in sucrose buffer, centrifuged at 100,000 × g for 10 min, washed, and recentrifuged. The pellet was suspended in isolation buffer, and the protein concentration was determined and then diluted to 1 mg/ml in a microcentrifuge tube. 4 μm [3H]AziPm ± 400 μm propofol was added and, after a brief vortex, the tissue was incubated at 21 °C in the dark for 10 min. After transfer to a quartz cuvette (path length, 1 mm), the tissue was photolabeled for 10 min using the same light source as above. The homogenates were then centrifuged at 100,000 × g, homogenized in 5 mm Tris, recentrifuged at 100,000 × g, washed, and stored at −80 °C in 2 mm Tris.
Scintillation Counting of Neuronal Tissue
Dissected CNS tissue from tadpoles treated with 4 μm [3H]AziPm ± UVA was placed in 1 ml of ice-cold 2% SDS, 1% Triton X-100, 5 mm Tris, pH 7.4, supplemented with protease inhibitors. Following homogenization, the protein concentration was determined. 5 and 10 μl of the homogenates were added to separate vials in scintillation fluid. The disintegrations per minute (dpm) from each vial were normalized to the corresponding protein amount, and the mean of the two values was used for a single experimental measurement.
IEF/SDS-PAGE
After thawing, 100 μg of neuronal membrane protein was centrifuged for 15 min at 15,000 × g. Following removal of the supernatant, the pellet was dissolved in 125 μl of 7 m urea, 2 m thiourea, 20 mm dithiothreitol, and 0.2% carrier ampholytes. Isoelectric focusing and SDS-PAGE proceeded according to the manufacturer's instructions, with 3–10 nonlinear pH strips (7 cm) and 4–15% SDS-PAGE. Tissue from ∼25 tadpoles sufficed for a single gel.
Spot Intensity Quantitation and Liquid Scintillation Counting
Gels were washed with water and fixed overnight in 15% trichloroacetic acid before staining with Coomassie Blue G-250. After destaining, the gels were scanned on a Bio-Rad GS-800 calibrated densitometer with quantitation performed using the accompanying Quantity One software. Background was subtracted with a box drawn between the 50- and 75- kDa molecular mass markers, and mean optical density multiplied by spot area was recorded from contoured spots.
Spots were excised with a 1.5-mm cylindrical hole punch and placed into scintillation vials. 400 μl of 30% hydrogen peroxide was added, and the sealed vials were incubated overnight at 65 °C to dissolve the polyacrylamide. These were cooled to room temperature before adding scintillation fluid.
Mass Spectrometry Analysis
Trypsin-digested samples were separated on a nanoLC column before online electrospray into a Thermo LTQ linear ion trap. Raw data were acquired with Xcalibur. The National Center for Biotechnology Information (NCBI) online protein database was searched with the term “Xenopus,” and the FASTA formatted sequences of the results were downloaded. This downloaded database was searched with SEQUEST for protein identification. Parameters were 1 atomic mass unit of parent ion tolerance, 1 atomic mass unit of fragment ion tolerance, and 1 missed cleavage. The search result files were combined with Scaffold 3 and filtered with the following criteria: Xcorr scores (+1 ion) 1.7, (+2) 2.3, (+3) 2.8; protein identification confidence 99.9%; peptide identification confidence 95%; two peptide minimum. Spectra were manually inspected to ensure quality and confidence.
Statistics
GraphPad Prism 5 was used for figure preparation and data analysis. Student's t test, one-way ANOVA, two-way ANOVA, and Bonferroni's correction were calculated within the GraphPad software. Significance is expressed as *, p < 0.05 and **, p < 0.01.
RESULTS
Optoanesthesia in Xenopus Tadpoles
Albino tadpoles anesthetized with 3 μm propofol or 4 μm AziPm (approximate EC99 doses) recovered on similar time scales following transfer to fresh pond water (Fig. 1A, left). The tadpoles are equilibrated with the alkylphenol, and recovery under these conditions is largely a function of drug diffusion back into the water.
In this study, we hypothesized that covalent occupation of ligand binding sites in vivo would result in prolonged anesthetic effects following washout of unadducted compound. The diazirine of AziPm has a peak absorbance of ∼370 nm, undergoing photolysis to form a reactive carbene, whereas propofol absorbs wavelengths less than 300 nm. Albino tadpoles immobilized with AziPm and exposed to UVA before transfer to fresh water exhibited prolonged immobility not observed with propofol control groups (Fig. 1A, right). Further, a relationship between lamp exposure time and recovery time was evident, suggesting progressive occupancy of functionally relevant sites. No toxicity (premature death) or differences in body mass were observed between tadpoles treated with either alkylphenol anesthetic or alkylphenol anesthetic with UVA following emergence (measured up to 10 days).
Covalent adduction would concentrate ligand into protein sites with infinitely low off-rates, increasing the apparent potency of the molecule. In vivo photolabeling for 10 min after equilibration with a sub-EC99 AziPm dose markedly increased the population of immobilized tadpoles (Fig. 1B). Further, we hypothesized that retained attachment of AziPm in functionally relevant targets after washout and emergence would manifest as a decrease in the effective concentration of propofol for immobility. Thus, 20 h after emergence, tadpoles treated as above were exposed to 2 or 0.8 μm propofol. Animals photolabeled in vivo displayed increased sensitivity (more rapid induction, slower emergence, and induction with a lower dose) relative to controls (Fig. 1C).
Lastly, 4 μm AziPm in pond water was photolyzed for a period corresponding to twice the diazirine half-life (i.e. to a final concentration of ∼1 μm (measured by absorption spectroscopy) plus whatever the product(s) of photolysis are). Tadpoles were then placed in this solution, and after 30 min, immobility was not observed, ruling out the possibility that a more potent, “caged” anesthetic with slower washout kinetics was released with light (data not shown). Together, these data suggest prolonged anesthetic influence due to photoadduction of ligand in vivo, a phenomenon we termed optoanesthesia.
[3H]AziPm in Tadpole Neuronal Tissue
Because general anesthetics are assumed to exert their effects through CNS targets, retention of photoactivated AziPm in neural tissue was measured following optoanesthesia. Brains and spinal cords from control tadpoles and those photolabeled in vivo with [3H]AziPm were isolated to quantify radioactivity after recovery in fresh water (Fig. 2A). Following [3H]AziPm induction, without washout, no difference is seen between groups treated ± UVA. However, 8-fold more radioactivity was noted in the neuronal tissue of photolabeled animals at 165 min, the point of emergence for all tadpoles exposed to 4 μm AziPm and 10 min of UVA.
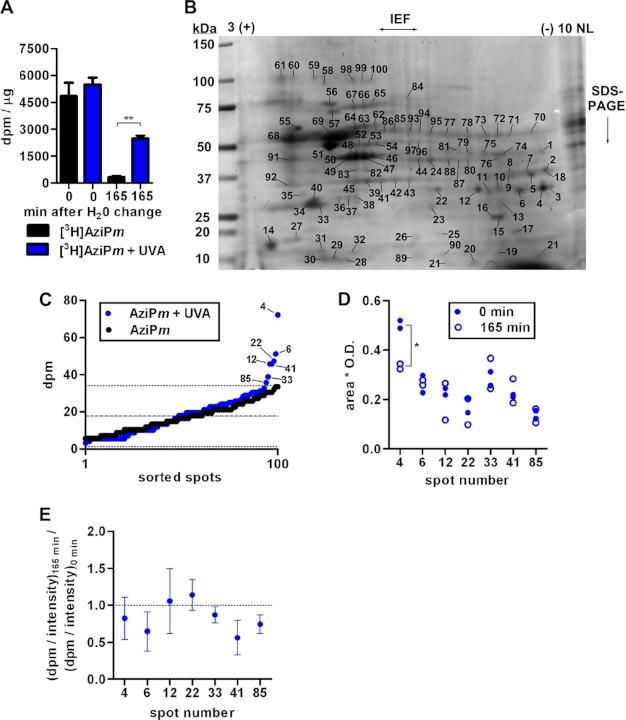
FIGURE 2.
A, quantitation of dpm normalized to protein amount in CNS tissue of tadpoles treated with AziPm ± UVA for 10 min. Data are from 3 experiments per treatment (10 tadpoles per experiment). Mean ± S.E. is shown, and data were analyzed by one-way ANOVA with Bonferroni's post hoc test comparing dpm within each time point (p < 0.01). B, representative Coomassie Blue-stained gel of tadpole neuronal membrane protein separated first by isoelectric focusing on a 3–10 non-linear (NL) pH gradient strip followed by SDS-PAGE. C, mean dpm of spots excised from gels of tissue isolated immediately after in vivo [3H]AziPm ± UVA treatment. dpm values were arranged in ascending order, with measurements from select spots indicated. The dashed line indicates background mean from + UVA gels with the dotted line indicating two standard deviations. D, Coomassie Blue stain intensity quantified from in vivo gel spots. Spot 4 was found to decrease with a two-tailed Student's t test (p < 0.05). E, the ratio (dpm/intensity)165 min divided by (dpm/intensity)0 min shows the change in the fraction of photolabeled protein over the emergence period. S.D. is shown, and a ratio of 1 would indicate no change.
Identification of Photolabeled Proteins
Optoanesthesia indicates that neuronal substrates photolabeled in vivo are relevant targets of AziPm and possibly propofol anesthesia. For protein identifications, neuronal membrane protein from tadpoles equilibrated with [3H]AziPm and photolabeled for 10 min was subjected to IEF/SDS-PAGE. Duplicate gels were stained, and 100 random spots were excised for scintillation counting (Fig. 2B). Mean background radiation (from three spots excised from each gel containing no detectable Coomassie Blue staining in a region through which the SDS-PAGE separated proteins migrated) was 18.0 dpm, and the mean for all 100 spots was 18.8 dpm. Seven spots contained dpm greater than two standard deviations from the background mean (Fig. 2C). No protein spots from control tadpoles incubated with [3H]AziPm but not exposed to the lamp contained counts exceeding this background threshold.
Response to in Vivo Photolabeling
We hypothesized that for tadpoles to regain movement (“emerge”), the cellular components contributing to mobility must adapt by removing photolabeled proteins whose activity is altered and/or by replacing these photolabeled macromolecules with newly synthesized proteins. To test this, neuronal membranes were isolated 165 min after tadpoles were photolabeled (when all had emerged) as above for IEF/SDS-PAGE, with the previously identified spots from duplicate gels assayed for dpm. The mean from three spots contained counts within 10% of the initial value (12, 22, and 33), whereas decreases of 46, 35, 42, and 28% were noted in spots 4, 6, 41, and 85, respectively. Coomassie Blue intensity was quantified to assess changes in protein expression, and with the exception of spot 4, little variation was observed (Fig. 2D).
Spot dpm was normalized to corresponding Coomassie Blue intensities for the in vivo experiments. We proposed that proteins with decreased radioactivity content coincident with emergence gain additional credibility as functionally important targets. Thus, we calculated the ratio of normalized photolabel incorporation at 165 min to that at the zero time point for each spot (Fig. 2E). A ratio of 1 would indicate that the fraction of adducted protein did not change over the 165 min. We found that the ratios from spots 6, 33, 41, and 85 are significantly less than 1, suggesting potential relevance in emergence from optoanesthesia.
Conserved Specificity and Target Identification
In vitro photolabeling of neuronal homogenates with 4 μm [3H]AziPm ± 400 μm propofol was performed to investigate the conserved, saturable specificity of protein sites. Protein spots identified as photolabeled in vivo were analyzed, and all contained dpm above background. A significant effect of propofol on normalized dpm was revealed with decreased photolabel incorporation ranging from 2 to 75% in each spot (p < 0.05, two-way ANOVA; n = 3 and n = 2 for each spot (−) and (+) propofol, respectively) (Table 1). A separate gel was run for protein identification. Six spots were unambiguously identified as containing a single protein, whereas two high confidence identifications were possible in the seventh (Table 1).
TABLE 1.
Protein spot analysis and LC-MS/MS identification
| Spot | % of displacementa | Protein ID | NCBI accession number | Theoreticalb molecular mass | Observedc molecular mass | Theoreticalb pI | Observedc pI | % of sequence coverage | Spectra count |
|---|---|---|---|---|---|---|---|---|---|
| Da | Da | ||||||||
| 4 | 40.1 | VDAC-2 | gi 62826006 | 30,183 | 27,937 | 8.36 | 8.99 | 29 | 18 |
| 6 | 52.0 | VDAC-2 | gi 62826006 | 30,183 | 27,448 | 8.36 | 8.27 | 23 | 14 |
| 12 | 46.5 | VDAC-1 | gi 28302268 | 30,627 | 28,671 | 6.85 | 6.71 | 20 | 11 |
| 22 | 74.5 | VDAC-1 | gi 28302268 | 30,627 | 29,160 | 6.85 | 6.21 | 26 | 16 |
| 33 | 3.4 | SNAP-25 | gi 33416802 | 23,172 | 26,468 | 4.74 | 4.89 | 30 | 15 |
| 41 | 1.7 | Gβ4 | gi 49257618 | 37,504 | 33,084 | 5.70 | 5.78 | 20 | 11 |
| 85 | 26.6 | PDIA3 | gi 28302197 | 56,086 | 54,992 | 5.72 | 5.91 | 30 | 25 |
| 26.6 | VHA-55 | gi 28436920 | 56,411 | 54,992 | 5.56 | 5.91 | 20 | 21 |
a [3H]AziPm displacement by propofol from in vitro photolabeling experiments.
b Theoretical values were computed with the ExPASy Compute pI/Mw tool. Monoisotopic molecular weights are shown.
c Observed values were estimated from molecular weight markers and IEF-resolving estimations published by the manufacturer of the gels.
DISCUSSION
We describe optoanesthesia, a light-induced anesthetic potentiation, and present a method through which novel general anesthetic targets can be discovered. Reconciling proteomic data with a behavioral phenotype provides a powerful means to assign relevance to identified binding partners. The mechanisms of recovery from optoanesthesia are likely to reside in adducted, relevant proteins being targeted for accelerated degradation and/or being replaced by newly synthesized protein. An alternative hypothesis, not tested here, is that the activities of proteins that are not targets of AziPm are altered to compensate for the covalent modification of the alkylphenol binding partners. Although emergence pathways may be distinct from induction pathways (20), emergence must still require an offloading of the anesthetic from induction targets; thus, we view our initial hypothesis, that label intensity should decrease in functionally relevant targets, as reasonable.
Performing photolabeling in live organisms assured that molecular targets were in a functional state, and because the primary effect of the anesthetic (immobility) was prolonged, confirmed that relevant targets were adducted. Further evidence for ligand incorporation to relevant general anesthetic sites was seen by the increased sensitivity to propofol after in vivo photolabeling. Despite label attachment to the identical proteins in vitro, substantial displacement of photoactive ligand by the parent propofol was most evident with VDACs. These mitochondrial porins with multiple phosphorylation states were also the most prominently labeled proteins in vivo. Protection of photolabeling by propofol suggests conserved and specific alkylphenol site(s). An alternative explanation for propofol competition is allosterism, which would require separate specific cavities for AziPm and for propofol, a possibility we view as unlikely.
Although VDACs are highly abundant proteins, this binding is not interpreted as ”nonspecific.“ Specificity can be viewed as specific to a particular physiological outcome or, on the molecular level (and favored here), high occupancy, saturable binding. Other general anesthetics have been shown to bind VDACs in vitro, but functional consequences have yet to be reported. VDACs bind GABAA receptors (21, 22), known propofol targets, but knock-outs of VDAC-1 and VDAC-3 do not appear to alter anesthetic sensitivity (22). Knock-out of VDAC-2 in mice is embryonic lethal, and interestingly, our time-resolved approach implicates VDAC-2 over VDAC-1 and VDAC-3 (Fig. 2E). However, this may reflect a general ”protective“ effect of VDAC-2 from cellular apoptosis (23).
Of the other identified proteins, SNAP-25 and Gβ4 exhibited decreases in the ratio of photolabeled to unmodified protein at the time of emergence. Published evidence has suggested that anesthetic interactions with SNAP-25 and/or Gβ4 might contribute to depressed neuronal signaling. For example, SNAP-25, a component of the ternary SNARE complex, binds volatile anesthetics at physiologically relevant concentrations (24), and isoflurane and propofol inhibit neurotransmitter release through interactions with SNAREs or associated proteins (25, 26). Mutagenesis in SNARE complex proteins (including SNAP-25) alters organism sensitivity to general anesthetics (27). Mammalian studies suggest that SNAP-25 may be predominantly expressed in excitatory neurons (28, 29), and this protein negatively regulates voltage-gated calcium channels independent of its role in exocytosis (30, 31). Gβ (as part of Gβγ) can also directly inhibit presynaptic voltage-gated calcium channels (32, 33) and binds to SNAP-25 and syntaxin to inhibit neuronal exocytosis (34, 35).
The lack of [3H]AziPm displacement by propofol on some proteins can be interpreted in several ways. For instance, anesthetic site(s) (e.g. on SNAREs) may not be conserved among ligands; isoflurane and halothane bind to the SNARE complex in a noncompetitive and nonsaturable manner (24). The hydrophobic interior of the coiled-coil complex likely harbors multiple sites of varying affinities, each capable of binding ligands with low occupancy. Alternatively, protein substrates (including Gβ) may be specific to AziPm but not propofol (propofol specificity was tested as the photolabel parent). Regardless, these interpretations do not preclude functional involvement in hypnosis or optoanesthesia, as we did not test competition with nontritiated AziPm, but also, little evidence suggests that saturable binding underlies these states.
With our approach in a model vertebrate organism, the tadpole, we provide in vivo evidence for the functional involvement of synaptic targets previously suspected only from in vitro or lower organism studies. Proving this involvement will require extensive genetic manipulations. Additionally, we have identified only a subset of targets bound by AziPm in vivo, not including ion channels (such as the GABAA receptor) that are the object of some general anesthetic hypotheses. Resolving low abundance proteins with multiple transmembrane domains is not feasible with IEF/SDS-PAGE (36, 37), and thus, a priori we did not anticipate their identification. The development of proteomic approaches complimentary to IEF/SDS-PAGE, and those that are capable of expanding the dynamic range of neuronal protein detection, will further the investigative power of optoanesthesia.
In conclusion, we anticipate the translation of in vivo photolabeling and behavior-paired proteomics to a wider variety of model organisms and photoactive molecules to investigate molecular mechanisms of general anesthetic pharmacology.
Acknowledgments
We thank Bill Dailey for synthesizing meta-azipropofol and Chao-Xing Yuan of the University of Pennsylvania Proteomics Core for assistance with mass spectrometry experiments.
This work was supported, in whole or in part, by National Institutes of Health Grants GM055876 (to R. G. E.), NS080519 (to B. P. W.), and GM088156 (to M. B. K.).
- AziPm
- meta-azipropofol
- IEF
- isoelectric focusing
- ANOVA
- analysis of variance
- VDAC
- voltage-dependent anion channel.
REFERENCES
- 1. Husain S. S., Ziebell M. R., Ruesch D., Hong F., Arevalo E., Kosterlitz J. A., Olsen R. W., Forman S. A., Cohen J. B., Miller K. W. (2003) 2-(3-Methyl-3H-diaziren-3-yl)ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate: a derivative of the stereoselective general anesthetic etomidate for photolabeling ligand-gated ion channels. J. Med. Chem. 46, 1257–1265 [DOI] [PubMed] [Google Scholar]
- 2. Darbandi-Tonkabon R., Hastings W. R., Zeng C.-M., Akk G., Manion B. D., Bracamontes J. R., Steinbach J. H., Mennerick S. J., Covey D. F., Evers A. S. (2003) Photoaffinity labeling with a neuroactive steroid analogue: 6-azi-pregnanolone labels voltage-dependent anion channel-1 in rat brain. J. Biol. Chem. 278, 13196–13206 [DOI] [PubMed] [Google Scholar]
- 3. Eckenhoff R. G., Xi J., Shimaoka M., Bhattacharji A., Covarrubias M., Dailey W. P. (2010) Azi-isoflurane, a photolabel analog of the commonly used inhaled general anesthetic isoflurane. ACS Chem. Neurosci. 1, 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall M. A., Xi J., Lor C., Dai S., Pearce R., Dailey W. P., Eckenhoff R. G. (2010) m-Azipropofol (AziPm) a photoactive analogue of the intravenous general anesthetic propofol. J. Med. Chem. 53, 5667–5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stewart D. S., Savechenkov P. Y., Dostalova Z., Chiara D. C., Ge R., Raines D. E., Cohen J. B., Forman S. A., Bruzik K. S., Miller K. W. (2011) p-(4-Azipentyl)propofol: a potent photoreactive general anesthetic derivative of propofol. J. Med. Chem. 54, 8124–8135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuki K., Bu W., Xi J., Sen M., Shimaoka M., Eckenhoff R. G. (2012) Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. FASEB J. 26, 4408–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ziebell M. R., Nirthanan S., Husain S. S., Miller K. W., Cohen J. B. (2004) Identification of binding sites in the nicotinic acetylcholine receptor for [3H]azietomidate, a photoactivatable general anesthetic. J. Biol. Chem. 279, 17640–17649 [DOI] [PubMed] [Google Scholar]
- 8. Cui T., Mowrey D., Bondarenko V., Tillman T., Ma D., Landrum E., Perez-Aguilar J. M., He J., Wang W., Saven J. G., Eckenhoff R. G., Tang P., Xu Y. (2012) NMR structure and dynamics of a designed water-soluble transmembrane domain of nicotinic acetylcholine receptor. Biochim. Biophys. Acta 1818, 617–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Z.-W., Chen L.-H., Akentieva N., Lichti C. F., Darbandi R., Hastings R., Covey D. F., Reichert D. E., Townsend R. R., Evers A. S. (2012) A neurosteroid analogue photolabeling reagent labels the colchicine-binding site on tubulin: a mass spectrometric analysis. Electrophoresis 33, 666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das J., Addona G. H., Sandberg W. S., Husain S. S., Stehle T., Miller K. W. (2004) Identification of a general anesthetic binding site in the diacylglycerol-binding domain of protein kinase Cδ. J. Biol. Chem. 279, 37964–37972 [DOI] [PubMed] [Google Scholar]
- 11. Li G.-D., Chiara D. C., Sawyer G. W., Husain S. S., Olsen R. W., Cohen J. B. (2006) Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J. Neurosci. 26, 11599–11605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Z.-W., Manion B., Townsend R. R., Reichert D. E., Covey D. F., Steinbach J. H., Sieghart W., Fuchs K., Evers A. S. (2012) Neurosteroid analogue photolabeling of a site in the TM3 domain of the β3 subunit of the GABAA receptor. Mol. Pharmacol. 82, 408–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xi J., Liu R., Asbury G. R., Eckenhoff M. F., Eckenhoff R. G. (2004) Inhalational anesthetic-binding proteins in rat neuronal membranes. J. Biol. Chem. 279, 19628–19633 [DOI] [PubMed] [Google Scholar]
- 14. Eckenhoff R. G., Shuman H. (1993) Halothane binding to soluble proteins determined by photoaffinity labeling. Anesthesiology 79, 96–106 [DOI] [PubMed] [Google Scholar]
- 15. Zhong H., Rüsch D., Forman S. A. (2008) Photo-activated azi-etomidate, a general anesthetic photolabel, irreversibly enhances gating and desensitization of γ-aminobutyric acid type A receptors. Anesthesiology 108, 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jurd R., Arras M., Lambert S., Drexler B., Siegwart R., Crestani F., Zaugg M., Vogt K. E., Ledermann B., Antkowiak B., Rudolph U. (2003) General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB J. 17, 250–252 [DOI] [PubMed] [Google Scholar]
- 17. Nguyen H. T., Li K. Y., daGraca R. L., Delphin E., Xiong M., Ye J. H. (2009) Behavior and cellular evidence for propofol-induced hypnosis involving brain glycine receptors. Anesthesiology 110, 326–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Downes H., Courogen P. M. (1996) Contrasting effects of anesthetics in tadpole bioassays. J. Pharmacol. Exp. Ther. 278, 284–296 [PubMed] [Google Scholar]
- 19. Krasowski M. D., Jenkins A., Flood P., Kung A. Y., Hopfinger A. J., Harrison N. L. (2001) General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of γ-aminobutyric acid (GABA) current at the GABAA receptor but not with lipid solubility. J. Pharmacol. Exp. Ther. 297, 338–351 [PubMed] [Google Scholar]
- 20. Friedman E. B., Sun Y., Moore J. T., Hung H.-T., Meng Q. C., Perera P., Joiner W. J., Thomas S. A., Eckenhoff R. G., Sehgal A., Kelz M. B. (2010) A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One 5, e11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bureau M. H., Khrestchatisky M., Heeren M. A., Zambrowicz E. B., Kim H., Grisar T. M., Colombini M., Tobin A. J., Olsen R. W. (1992) Isolation and cloning of a voltage-dependent anion channel-like Mr 36,000 polypeptide from mammalian brain. J. Biol. Chem. 267, 8679–8684 [PubMed] [Google Scholar]
- 22. Darbandi-Tonkabon R., Manion B. D., Hastings W. R., Craigen W. J., Akk G., Bracamontes J. R., He Y., Sheiko T. V., Steinbach J. H., Mennerick S. J., Covey D. F., Evers A. S. (2004) Neuroactive steroid interactions with voltage-dependent anion channels: lack of relationship to GABAA receptor modulation and anesthesia. J. Pharmacol. Exp. Ther. 308, 502–511 [DOI] [PubMed] [Google Scholar]
- 23. Cheng E. H. Y., Sheiko T. V., Fisher J. K., Craigen W. J., Korsmeyer S. J. (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301, 513–517 [DOI] [PubMed] [Google Scholar]
- 24. Nagele P., Mendel J. B., Placzek W. J., Scott B. A., D'Avignon D. A., Crowder C. M. (2005) Volatile anesthetics bind rat synaptic snare proteins. Anesthesiology 103, 768–778 [DOI] [PubMed] [Google Scholar]
- 25. Herring B. E., Xie Z., Marks J., Fox A. P. (2009) Isoflurane inhibits the neurotransmitter release machinery. J. Neurophysiol. 102, 1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herring B. E., McMillan K., Pike C. M., Marks J., Fox A. P., Xie Z. (2011) Etomidate and propofol inhibit the neurotransmitter release machinery at different sites. J. Physiol. 589, 1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Swinderen B., Saifee O., Shebester L., Roberson R., Nonet M. L., Crowder C. M. (1999) A neomorphic syntaxin mutation blocks volatile-anesthetic action in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 96, 2479–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verderio C., Pozzi D., Pravettoni E., Inverardi F., Schenk U., Coco S., Proux-Gillardeaux V., Galli T., Rossetto O., Frassoni C., Matteoli M. (2004) SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron 41, 599–610 [DOI] [PubMed] [Google Scholar]
- 29. Garbelli R., Inverardi F., Medici V., Amadeo A., Verderio C., Matteoli M., Frassoni C. (2008) Heterogeneous expression of SNAP-25 in rat and human brain. J. Comp. Neurol. 506, 373–386 [DOI] [PubMed] [Google Scholar]
- 30. Wiser O., Trus M., Hernández A., Renström E., Barg S., Rorsman P., Atlas D. (1999) The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc. Natl. Acad. Sci. U.S.A. 96, 248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Condliffe S. B., Corradini I., Pozzi D., Verderio C., Matteoli M. (2010) Endogenous SNAP-25 regulates native voltage-gated calcium channels in glutamatergic neurons. J. Biol. Chem. 285, 24968–24976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ikeda S. R. (1996) Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature 380, 255–258 [DOI] [PubMed] [Google Scholar]
- 33. Herlitze S., Garcia D. E., Mackie K., Hille B., Scheuer T., Catterall W. A. (1996) Modulation of Ca2+ channels by G-protein βγ subunits. Nature 380, 258–262 [DOI] [PubMed] [Google Scholar]
- 34. Gerachshenko T., Blackmer T., Yoon E.-J., Bartleson C., Hamm H. E., Alford S. (2005) Gβγ acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat. Neurosci. 8, 597–605 [DOI] [PubMed] [Google Scholar]
- 35. Blackmer T., Larsen E. C., Bartleson C., Kowalchyk J. A., Yoon E.-J., Preininger A. M., Alford S., Hamm H. E., Martin T. F. J. (2005) G protein βγ directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat. Neurosci. 8, 421–425 [DOI] [PubMed] [Google Scholar]
- 36. Bunai K., Yamane K. (2005) Effectiveness and limitation of two-dimensional gel electrophoresis in bacterial membrane protein proteomics and perspectives. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 815, 227–236 [DOI] [PubMed] [Google Scholar]
- 37. Issaq H., Veenstra T. (2008) Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE): advances and perspectives. BioTechniques 44, 697–698, 700 [DOI] [PubMed] [Google Scholar]