Background: TLR4 contributes to pulmonary hypertension, a disease of endothelial dysfunction. The role of HMGB1, one endogenous ligand of TLR4, remains unexplored.
Results: HMGB1 inhibited pulmonary artery endothelial cell migration, which was reversed by TLR4 or IRF3 siRNA.
Conclusion: HMGB1 inhibits pulmonary artery endothelial cell migration via TLR4- and IRF3-dependent mechanism(s).
Significance: HMGB1, via TLR4, inhibits a critical process for pulmonary vascular regeneration.
Keywords: Cell Migration, Endothelial Cell, MyD88, Pulmonary Hypertension, Toll-like Receptors (TLRs), High Mobility Group Box 1 (HMGB1), Interferon Response Factor 3, Pulmonary Endothelium
Abstract
In pulmonary hypertension the loss of precapillary arterioles results from vascular injury causing endothelial dysfunction. Endothelial cell migration and proliferation are critical for vascular regeneration. This study focused on the effect of high mobility group box 1 protein (HMGB1) on these critical processes. HMGB1 had no effect on human pulmonary artery endothelial cell (HPAEC) proliferation. In contrast, treatment of HPAECs with HMGB1 dose-dependently inhibited VEGF-stimulated HPAEC migration. The effect of HMGB1 on HPAEC migration was TLR4-dependent because it was reversed by TLR4 siRNA or TLR4-neutralizing antibody. Exposure of HPAECs to hypoxia caused translocation and release of HMGB1 and inhibition of HPAEC migration. The effect of hypoxia on HPAEC migration was mediated by HMGB1 because HMGB1-neutralizing antibody but not control IgG restored HPAEC migration. Likewise, TLR4 siRNA but not control siRNA reversed the inhibitory effect of hypoxia in HPAECs. The canonical TLR4 signaling pathway requires the adaptor protein MyD88 and leads to downstream NFκB activation. Interestingly, HMGB1 failed to stimulate NFκB translocation to the nucleus, but instead activated an alternative pathway characterized by activation of interferon response factor 3 (IRF3). This was in contrast to human umbilical vein endothelial cells in which HMGB1 stimulated nuclear translocation of NFκB but not IRF3. IRF3 siRNA, but not MyD88 siRNA, reversed the inhibitory effect of HMGB1 on HPAEC migration. These data demonstrate that HMGB1 inhibits HPAEC migration, a critical process for vascular regeneration, via TLR4- and IRF3-dependent mechanisms.
Introduction
High mobility group box 1 (HMGB1)2 is a ubiquitously expressed nuclear protein involved in stabilization of nucleosomes. However, in response to cellular stress, HMGB1 translocates from the nucleus (1) into the extracellular environment, where it has been shown to lead to activation of pattern recognition receptors Toll-like receptor 4 (TLR4), Toll-like receptor 2 (TLR2), and the receptor of advanced glycation end products (RAGE) (2, 3). The importance of HMGB1 as a proinflammatory cytokine has been shown in diseases of chronic inflammation and exaggerated immune responses, such as atherosclerosis, and sepsis (4). HMGB1 has also been identified as a proangiogenic cytokine involved in tumor- and ischemia-induced angiogenesis (5–8).
Activation of TLR4 by HMGB1 triggers downstream signaling cascades leading to cytokine release and tissue damage. Which specific pathways are activated depends on the interaction of adaptor proteins with TLR4. The cytoplasmic domain of TLR4 contains a Toll-IL-1 receptor domain (TIR) that can interact with the adaptor molecule myeloid differentiation primary response gene (MyD88). MyD88-dependent signaling occurs via TRAF6 and the IKB complex leading to downstream NFκB activation. MyD88-independent signaling involves the TIR domain-containing, adaptor-inducing IFN-β protein (TRIF) and subsequent activation of interferon regulatory factor 3 (IRF3) (9–11).
Pulmonary hypertension (PH) is a fatal disease characterized by increased pulmonary vascular resistance leading to right heart failure and death. In the mouse model of chronic hypoxia-induced PH, it was recently shown that the development of the disease was significantly attenuated in mice containing a point mutation in TLR4 rendering the receptor non-functional (12, 13). Although nonnuclear HMGB1 has been shown to play a negative role in inflammatory/respiratory diseases of the lung, including asthma and acute lung injury (14–19), there are no studies on HMGB1 in pulmonary vascular disease. In this study we explored the effect of HMGB1 on human pulmonary artery endothelial cell (HPAEC) proliferation and migration, features necessary for vascular regeneration after injury.
EXPERIMENTAL PROCEDURES
Cell Culture
Human pulmonary artery endothelial cells (HPAECs) and human umbilical vein endothelial cells (HUVECs) were from Lonza and were cultured in EBM-2 medium (Lonza). Human dermal microvascular endothelial cells were from VEC Technologies and were cultured in MCDB-131 complete medium (VEC Technologies). Prior to each experiment cells were serum-starved overnight in minimal medium. Cells were used between passages 4 and 9.
Materials
Mouse anti-human TLR4 neutralizing antibody and nonimmune mouse IgG were from eBioscience (San Diego, CA). Polyclonal antibody against HMGB1 (provided by Dr. Kevin Tracey, North Shore-LIJ Health System Feinstein Institute for Medical Research) was prepared as described previously (20). Neutralizing activity of anti-HMGB1 was confirmed in HMGB1-stimulated macrophage cultures by assay of TNF release. In the presence of anti-HMGB1 antibody, neutralizing antibody was defined as inhibiting >80% of HMGB1-induced TNF release. Nonimmune rabbit IgG was purchased from Sigma-Aldrich. Antibodies against phospho-p38 (9211), total p38 (9212), HMGB1 (3935), β-actin (4967), IRF3 (4302), and NFκB-p65 (8242) were from Cell Signaling Technology (Danvers, MA). Antibodies against NUMA (610561) and HSP90 (610418) were from BD Biosciences.
HMGB1 Isolation
Full-length cDNA for human HMGB1 subcloned into the secretion signal of the FLAG expression vector, YEpFLAG (Sigma) was transformed into the yeast strain BJ3505. Full-length cDNA for human HMGB1 was subcloned in-frame to the secretion signal of the FLAG expression vector, YEpFLAG, modified to eliminate the FLAG cassette. This vector was transformed into the protease-deficient yeast strain BJ3505. The transformed yeast were grown at 30 ºC for 3 days on a rotary shaker at 75 rpm in 500 ml of Expression medium (1% glucose, 3% glycerol, 1% yeast extract, 2% peptone, 100 mm potassium phosphate, pH 6.4) according to Ngamkitidechakul and Twining (48).
Purification
The culture was chilled at 4 ºC and centrifuged for 10 min at 1000 × g. The supernatant was recentrifuged at 10,000 × g for 15 min. While on ice, sufficient ammonium sulfate was added slowly to obtain a concentration of 65%. After a 30-min incubation at 4 ºC, the mixture was centrifuged for 15 min at 10,000 × g. To the supernatant, sufficient ammonium sulfate was added to bring the final concentration to 80% and again incubated 30 min on ice and centrifuged for 15 min at 10,000 × g discarding the supernatant. The pellet was dialyzed extensively versus 50 mm NaPO4, 150 mm NaCl, pH 7.5. The dialysate was applied to Talon Resin (Clontech). The resin was washed with 50 mm NaPO4, 150 mm NaCl + 10 mm imidazole, pH 7.5. The protein was eluted with 50 mm NaPO4, 150 mm NaCl + 150 mm imidazole, pH 7.5. After purification, the protein was dialyzed versus 25 mm Tris, 150 mm KCl, pH 8.0, aliquoted, and snap frozen at −80 ºC.
Transfection with siRNA
All siRNA was purchased from Dharmacon. Cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Nontargeting siRNA was used as a negative control. Transfection efficiency was optimized by trying a range of siRNA and Lipofectamine 2000 concentrations.
Immunofluorescent Staining
Cells grown on coverslips were fixed in 2% paraformaldehyde and blocked in 2% BSA. Cells were then incubated in primary antibodies overnight followed by incubation for 60 min with fluorescently labeled secondary antibodies (Alexa Fluor 488). After nuclear staining for 40 s with DAPI, slides were covered using gelvatol. Images were taken using an Olympus Fluoview 1000 confocal microscope in the Center for Biological Imaging at the University of Pittsburgh.
Nuclear/Cytoplasmic Fractionation
Cells were grown on 10-cm dishes and treated as indicated. After treatment cells were washed with PBS, scraped, snap frozen, and centrifuged for 5 min at 4 °C at 3500 rpm. The pellet was resuspended in 0.5 ml of buffer A (500 μl of 1 m Tris, pH 7.5, 75 μl of 1 m MgCl2, 250 μl of 2 m KCl, 500 μl of Nonidet P-40 + protease inhibitor mixture), rotated for 1 h at 4 °C, and centrifuged for 5 min at 3500 rpm. The supernatant was collected as cytoplasmic fraction. The pellet was resuspended in 0.5 ml of buffer A and incubated 40 min on ice then centrifuged for 5 min at 3500 rpm. The pellet was resuspended in 40 μl of buffer C (1 ml of 1 m Tris, pH 7.5, 75 μl of 1 m MgCl2, 625 μl of 2 m KCl, 50 μl of Nonidet P-40, 4.2 ml of 5 m NaCl2, 20 μl of 0.5 m EDTA, 7.5 ml of glycerol with four times as much protease inhibitors), kept on ice for 30 min, then centrifuged 15 min (13,000 rpm at 4 ºC). The nuclear extract (supernatant) was collected, and 120 μl of buffer D (1 ml of 1 m Tris, pH 7.5, 50 μl of Nonidet P-40, 20 μl of 0.5 m EDTA, 10 ml of glycerol) was added. The fractions were stored for later determination of protein content and Western blotting.
Western Blotting
30 μg of cell lysate was separated by SDS-PAGE and transferred to nitrocellulose membranes. For Western analysis of cell culture media, equal volumes of cell culture media were loaded onto gels after centrifugation to remove floating cells and debris. Membranes were blocked in TBST (Tris-buffered saline, 0.1% Tween 20), 5% nonfat dry milk for 30 min, followed by incubation in primary antibody overnight. Membranes were washed in TBST before incubation for 1 h with horseradish peroxidase-conjugated secondary antibodies. Membranes were washed and developed using enhanced chemiluminescence substrate (Pierce).
Wound Healing Migration Assay
Endothelial cells were seeded onto 6-well plates. Cells were grown to confluence, serum-starved overnight, and treated with VEGF (50 ng/ml) alone or in combination with HMGB1 (between 10 and 1000 ng/ml). The monolayer of cells was then wounded by scratching lengthwise with a sterile pipette tip (three scratches per well with duplicate wells). Images were obtained at time 0 and 10 h after treatments. The wound area was determined using Photoshop CS3 extended software (Adobe), and migration was calculated as -fold change in wound area.
Cell Proliferation Assay Using [3H]Thymidine Incorporation
Serum-starved HPAECs were treated for 24 h either with VEGF, HMGB1, VEGF+HMGB1, or untreated control media, in the presence of 0.15 μCi of [methyl-3H]thymidine (6.7 Ci/mmol; NEN) per well of a 12-well plate at 37 °C in a total volume of 1 ml. After treatment, cells were rinsed twice with PBS, and 1 ml of ice-cold 10% trichloroacetic acid was added to each well for 30 min at 4 °C. After an additional rinse with the 10% TCA solution cells were lysed in 0.5 ml of 0.12 n NaOH containing 0.1% SDS for 1 h at room temperature. Samples were added to EcoLite (ICN) scintillation mixture and counted in a liquid scintillation spectrometer.
Reverse Transcription-PCR
RNA from cells was isolated using the RNeasy Mini kit (Qiagen) and reverse-transcribed into cDNA using the Superscript III First Strand synthesis system for RT-PCR kit (Invitrogen). For traditional PCR primers for inducible nitric-oxide synthase (iNOS), IFNβ, and IFN-stimulated gene 20 (ISG20) were designed using the Invitrogen Oligo PerfectTM Designer program. PCRs were carried out using GoTaq Green Master Mix. The number of PCR cycles was optimized for each primer set. For quantitative real-time PCR TaqMan primers for human IRF3, MyD88, TLR4, HPRT from Applied Biosystems were used. The reaction was run on an Applied Biosystems 7500 Real-time PCR system using the TaqMan Gene Expression Master Mix.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software. Data were analyzed by one-way ANOVA and Bonferroni post hoc tests. p values of < 0.05 were considered significant.
RESULTS
Hypoxia Induces HMGB1 Release from HPAECs
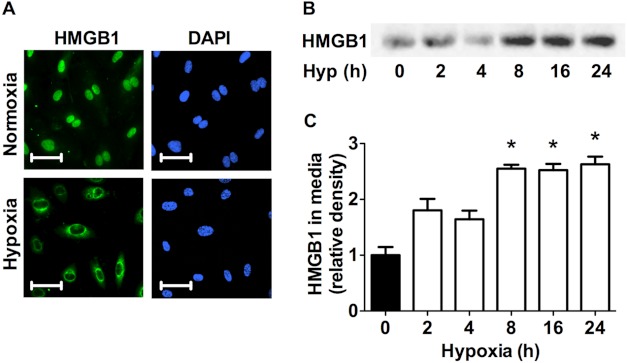
Several groups have previously shown that exposure to acute hypoxia leads to the release of HMGB1 from a variety of cell types, including systemic endothelial cells (6, 21). It has, however, not been explored in pulmonary endothelial cells. Fig. 1A shows representative images of HPAECs stained with αHMGB1 antibody (green) and DAPI (blue). Consistent with the observation that all cells express HMGB1 in the nuclear compartment under nonstress conditions HMGB1 (green) was located strictly within the nucleus in normoxia as indicated by its colocalization with nuclear DAPI staining. Exposure of HPAECs to 1% O2 for 8 h led to decreased nuclear HMGB1 staining with a concomitant increase in cytoplasmic HMGB1 staining. Furthermore, Western blot analysis performed on cell media of HPAECs exposed to hypoxia for the indicated time points revealed a significant increase of HMGB1 accumulation in the media (Fig. 1, B and C). These findings establish that hypoxia stimulates HMGB1 release from HPAECs.
FIGURE 1.
Hypoxia induces HMGB1 release in HPAECs. A, HPAECs exposed to 8 h of hypoxia (1%) were assessed for HMGB1 release by immunofluorescent staining (HPAECs, green; DAPI, blue; scale bars, 25 μm). Photomicrographs are representative of three independent experiments. B and C, Western blot analysis (B) and quantitative densitometry (C) of HMGB1 accumulation in the cell media of HPAECs exposed to hypoxia for the indicated times are shown. Blots are representative of four independent experiments. *, p < 0.05; error bars, S.E.
HMGB1 Inhibits VEGF-induced HPAEC Migration
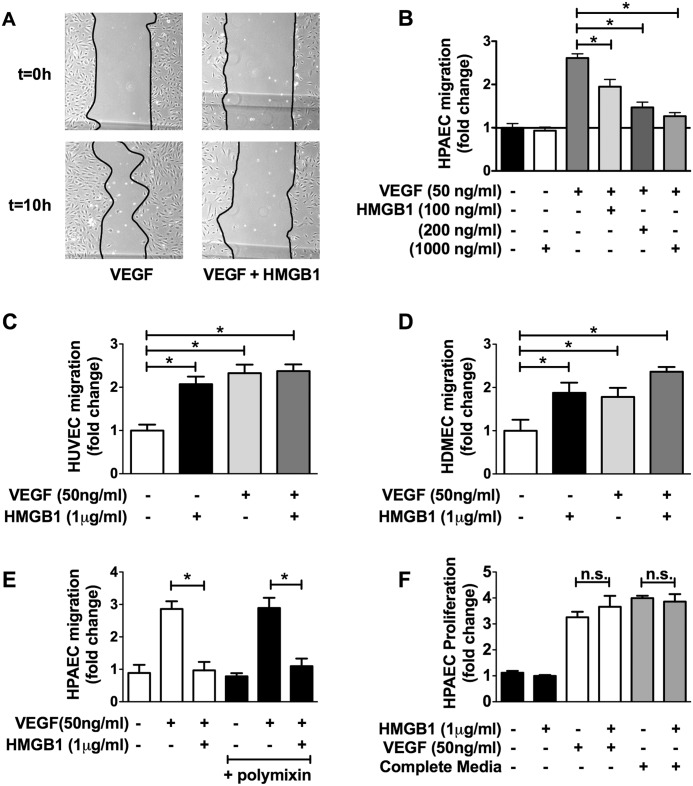
To study the effect of HMGB1 on HPAECs we picked end points of cell migration and proliferation. Fig. 2A shows representative images of monolayer wounds to HPAECs stimulated with VEGF (50 ng/ml) with or without HMGB1. Stimulation of HPAECs with VEGF led to a robust increase in cell migration over a 10-h period whereas addition of HMGB1 (100–1000 ng/ml) over the same time period blocked VEGF-induced migration in a dose-dependent fashion (Fig. 2B).
FIGURE 2.
HMGB1 inhibits VEGF-induced HPAEC migration. A, representative images of cell migration assay of HPAECs treated for 10 h with VEGF (50 ng/ml) or VEGF + HMGB1 (1 μg/ml). B, serum-starved HPAECs stimulated with VEGF (50 ng/ml) in the absence/presence of HMGB1 (100 ng/ml–1 μg/ml) and assessed for cellular migration using the monolayer wound assay. C and D, effect of HMGB1 (1 μg/ml) on VEGF (50 ng/ml)-stimulated HUVEC migration (C) and HDMEC migration (D). E, effect of polymyxin on HMGB1-inhibited HPAEC migration (1000 ng/ml). F, effect of HMGB1 on HPAEC proliferation using [3H]thymidine incorporation. Data represent the mean ± S.E. (error bars) of three independent experiments. Analysis of variance; *, p < 0.05.
Our results demonstrating that HMGB1 inhibits migration are contradictory to what has been reported in nonpulmonary endothelial cells. In particular, HMGB1 has been shown to stimulate cell migration and angiogenesis in HUVECs (6, 22–24). We were, therefore, curious to know whether we could recapitulate these results. Indeed, HMGB1 stimulated the migration of serum-starved HUVECs to the same extent as VEGF (Fig. 2C). To assure that this was not a general difference between arterial versus venous endothelial cells we also assayed the effect of HMGB1 on the migration of human dermal microvascular endothelial cells (HDMECs). Similar to HUVECs, HMGB1 stimulated, rather than inhibited, HDMEC migration (Fig. 2D).
Because bacterial lipopolysaccharide (LPS) also binds to and activates TLR4 (25) we tested whether HMGB1 could inhibit HPAEC migration in the presence or absence of polymyxin B, which binds LPS, preventing LPS-dependent TLR4 activation. Polymyxin B had no effect on the ability of HMGB1 to inhibit VEGF-stimulated HPAEC migration, demonstrating that the effect of HMGB1 on HPAECs is not mediated by LPS contamination (Fig. 2E).
We also examined whether HMGB1 could affect HPAEC proliferation. HPAECs were stimulated with HMGB1 with or without growth factor stimulation. HMGB1 had no effect on HPAEC proliferation alone or in conjunction with growth factors, demonstrating that HMGB1 does not affect proliferation in this cell type (Fig. 2F).
TLR4 Mediates the Effect of HMGB1 in HPAECs
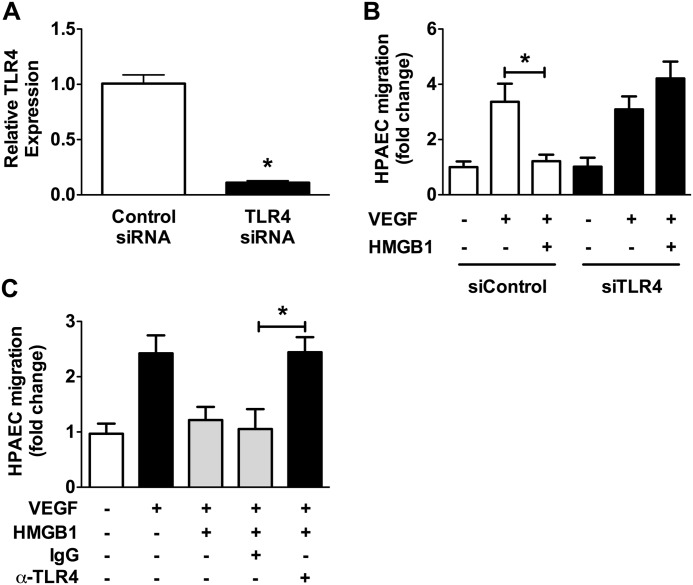
To establish a role for TLR4 in the inhibition of HPAEC migration we utilized small interference RNA (siRNA) against TLR4. Transfection of HPAECs with siRNA against TLR4 suppressed TLR4 mRNA levels by ∼90% compared with control siRNA (Fig. 3A). Stimulation of control siRNA or TLR4 siRNA-transfected cells with VEGF in the presence or absence of HMGB1 revealed that knockdown of TLR4 prevents the effect of HMGB1 on HPAEC migration (Fig. 3B). As an alternative method of assessing the role of TLR4 in mediating this effect, cells were pretreated with TLR4-blocking antibody or control IgG and then stimulated with VEGF in the presence and absence of HMGB1. Similar to the previous experiment with TLR4 siRNA, the TLR4-blocking antibody prevented the inhibitory effect of HMGB1 on HPAEC migration (Fig. 3C).
FIGURE 3.
TLR4 mediates the effects of HMGB1 of HPAEC migration. A, quantitative PCR for TLR4 in control and TLR4 siRNA-treated HPAECs. B, HPAECs were transfected with TLR4 siRNA (25 nm) or control siRNA (25 nm) and VEGF-induced cell migration assessed in the presence/absence of HMGB1 (1 μg/ml). (C) HPAECs were treated with TLR4 blocking antibody (20 μg/ml) or control IgG (20 μg/ml) and VEGF-induced cell migration assessed in the presence/absence of HMGB1 (1 μg/ml). Data represent the mean ± S.E. (error bars) of three independent experiments. Analysis of variance; *, p < 0.05.
Hypoxia-induced HMGB1 Release Leads to Inhibition of HPAEC Migration via TLR4
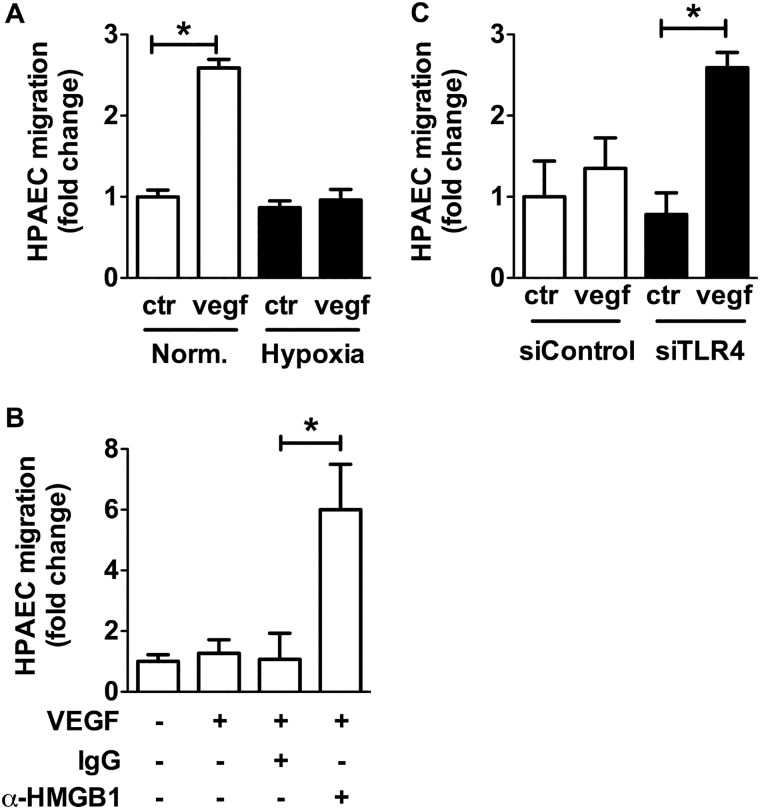
Based on the findings of HMGB1 release in hypoxia, we assessed the effect of hypoxia (1% O2) on HPAEC migration. Again, in contrast to the reported literature on HUVECs (18, 19), hypoxia inhibited VEGF induced-HPAEC migration (Fig. 4A). This effect of hypoxia was mediated by HMGB1 because neutralization of HMGB1 with a αHMGB1 antibody restored the ability of HPAECs to migrate under hypoxic conditions (Fig. 4B). Likewise, TLR4 siRNA but not control siRNA reversed the effect of hypoxia on HPAECs (Fig. 4C). These data demonstrate that HMGB1 inhibits VEGF-induced HPAEC migration in normoxia as well as in hypoxia in a TLR4-dependent fashion.
FIGURE 4.
Hypoxia-induced HMGB1 release leads to inhibition of HPAEC migration via TLR4. VEGF-stimulated cell migration was assessed in HPAECs exposed to (A) hypoxia (1% O2), (B) αHMGB1-neutralizing antibody (10 μg/ml) or IgG control antibody, (C) hypoxia when transfected with siRNA against TLR4 (25 nm) or control siRNA (25 nm). Data represent the mean ± S.E. (error bars) of three to four independent experiments. Analysis of variance; *, p < 0.05.
HMGB1 Stimulates IRF3 Nuclear Translocation in HPAECs but Not in HUVECs
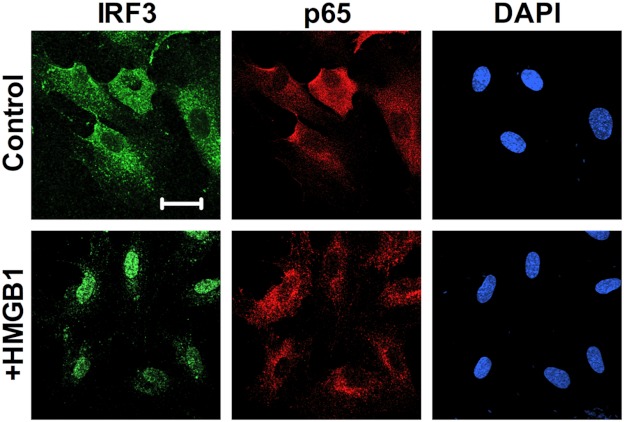
Downstream signaling of TLR4 activation commonly involves the adaptor protein MyD88 followed by NFκB activation via translocation of the p65 subunit of NFκB into the nucleus. MyD88-independent signaling involves the adaptor protein TRIF leading to downstream IRF3 phosphorylation and translocation into the nucleus (20). To test which signaling pathway is activated by HMGB1, HPAECs were treated with HMGB1 for various times and assayed for nuclear translocation of IRF3 and the NFκB-p65. Immunofluorescent staining for IRF3 and p65 showed that treatment of HPAECs with HMGB1 for 2 h resulted in nuclear accumulation of IRF3 but not NFκB-p65 (Fig. 5).
FIGURE 5.
HMGB1 stimulates nuclear translocation of IRF3 in HPAECs. A, HPAECs exposed for 2 h to HMGB1 (1 μg/ml) were assessed for IRF3 nuclear translocation by immunofluorescent staining against IRF3 (green; nuclear stain DAPI, blue; scale bar, 50 μm). Images are representative of three independent experiments.
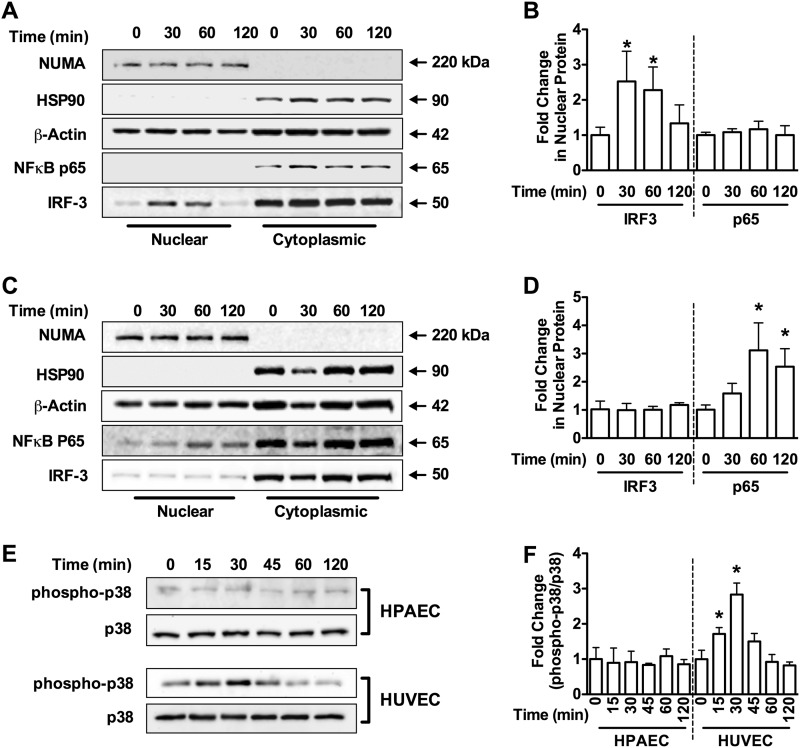
To confirm these findings, we prepared cytoplasmic and nuclear fractions from HPAECs after HMGB1 stimulation and analyzed the fractions for IRF3 and NFκB-p65 by Western blotting. HMGB1 failed to cause nuclear translocation of NFκB-p65 in HPAECs. However, we observed a robust increase in nuclear IRF3 in response to HMGB1 (Fig. 6, A and B). Consistent with our earlier findings of opposite effects on cell migration, treatment of HUVECs with HMGB1 stimulated nuclear translocation of NFκB-p65, but not IRF3 (Fig. 6, C and D).
FIGURE 6.
Differential signaling in HPAECs versus HUVECs. Western blot analysis for NUMA (150 kDa, nuclear marker), HSP90 (90 kDa, cytoplasmic marker), β-actin (42 kDa), NFκB-p65 (65 kDa), and IRF3 (45 kDa) in cytoplasmic and nuclear fractions from HPAECs (A) and HUVECs (C) treated with HMGB1 (1 μg/ml) for the indicated times. Blots are representative of three independent experiments and are quantified in B and D, respectively. E, Western blot analysis for phospho-p38 MAPK and total p38 MAPK in HPAECs and HUVECs for the indicated times. Blots are representative of three independent experiments and are quantified in F. Graphs represent the mean ± S.E. (error bars) of three independent experiments. Analysis of variance; *, p < 0.05 versus unstimulated control.
In addition to activation of NFκB, MyD88-dependent TLR4 signaling leads to activation of p38 MAPK. Treatment of HPAECs with HMGB1 failed to stimulate phosphorylation p38 MAPK, whereas HMGB1 stimulated robust phosphorylation of p38 MAPK in HUVECs. These provide further evidence of MyD88-independent TLR4 signaling in HPAECs (Fig. 6, E and F).
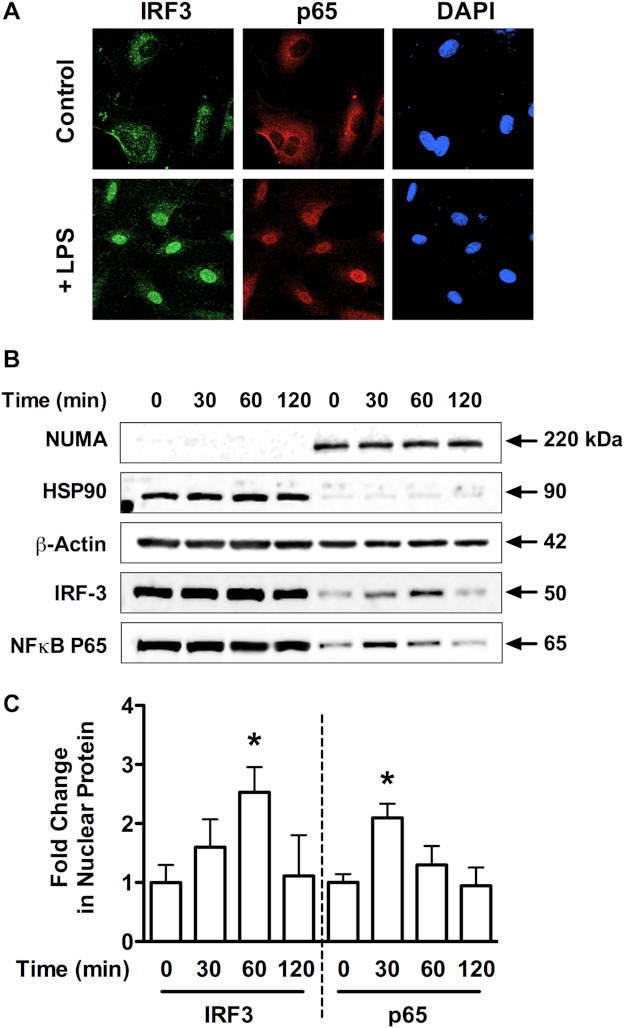
The lack of NFκB activation in the HPAECs was not due to a defect in the MyD88-NFκB pathway in these cells because the TLR4 agonist LPS was able to stimulate NFκB activation in these cells. Treatment of HPAECs with LPS lead to the nuclear translocation of both IRF3 and p65 as assessed by immunofluorescent staining (Fig. 7A) and nuclear fractionation experiments (Fig. 7, B and C). These data further suggest different mechanisms of TLR4 activation between HMGB1 and LPS, which has been observed in other cell types (2, 26).
FIGURE 7.
LPS stimulates MyD88-dependent and -independent TLR4 signaling in HPAECs. A, HPAECs exposed for 1 h to LPS (1 μg/ml) were assessed for IRF3 nuclear translocation by immunofluorescent staining against IRF3 (green; nuclear stain DAPI, blue; scale bar, 25 μm). Images are representative of three independent experiments. B, Western blot analysis is shown of NUMA (150 kDa, nuclear marker), HSP90 (90 kDa, cytoplasmic marker), β-actin (42 kDa), NFκB-p65 (65 kDa), and IRF3 (50 kDa) in cytoplasmic and nuclear fractions from HPAECs treated with LPS (1 μg/ml) for the indicated times. Blots are representative of three independent experiments and are quantified in C. Data represent the mean ± S.E. (error bars) of three independent experiments. Analysis of variance; *, p < 0.05 versus unstimulated control.
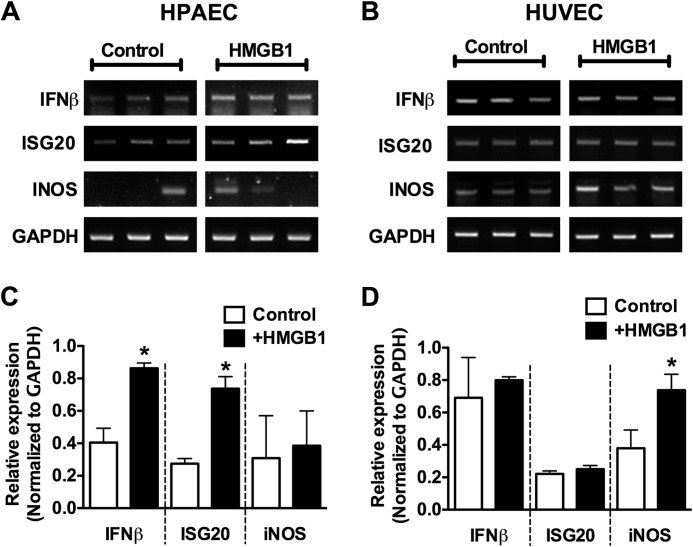
As a final method to examine MyD88-dependent versus MyD88-independent signaling in HPAECs we examined downstream induction of MyD88/NFkB-dependent (iNOS) (27, 28) and IRF3-dependent (IFNβ and ISG20) (29, 30) genes by RT-PCR. As expected, HMGB1 stimulated the induction of IFNβ and ISG20 but not iNOS in HPAECs (Fig. 8, A and B). Similarly, HMGB1 stimulated iNOS, but not IFNβ or ISG20 in HUVECs (Fig. 8, C and D). Together these data provide strong evidence that HMGB1 stimulates MyD88-independent TLR4 signaling in HPAECs.
FIGURE 8.
Differential gene induction by HMGB1 in HPAECs versus HUVECs. A and B, HPAECs (A) and HUVECs (B) were stimulated with HMGB1 (1 μg/ml) for 4 h and then assessed for the induction of ISG20, IFNβ, or iNOS mRNA by RT-PCR. C and D, the relative expression of each gene is quantified in the histograms and normalized to GAPDH mRNA expression. Data represent the mean ± S.E. (error bars) of three independent experiments. Analysis of variance; *, p < 0.05 versus unstimulated control.
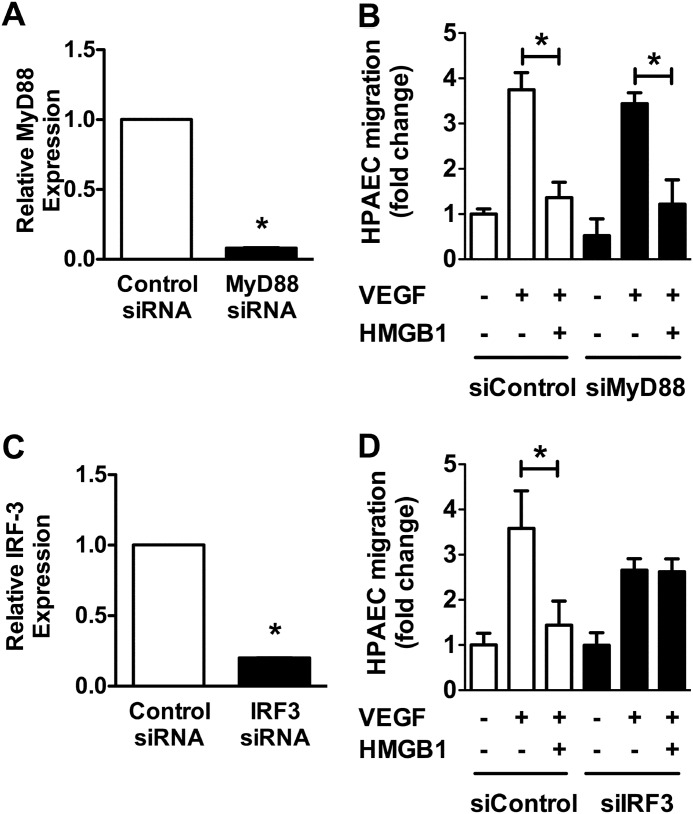
Inhibition of HPAEC Migration by HMGB1 Is IRF3-dependent and MyD88-independent
To determine the role of IRF3 and/or MyD88 in the inhibition of VEGF-induced HPAEC migration by HMGB1, HPAECs were transfected with siRNA against IRF3 or MyD88 and stimulated to migrate in the presence or absence of HMGB1. Silencing MyD88 did not reverse the inhibitory effect of HMGB1, providing further evidence that it does not participate in this signaling pathway (Fig. 9B). Knockdown of IRF3, on the other hand, prevented the inhibitory effect of HMGB1 on HPAEC migration (Fig. 9D). Both MyD88 siRNA and IRF3 siRNA decreased their respective mRNA expression by ∼90% (Fig. 9, A and C).
FIGURE 9.
Inhibition of HPAEC migration by HMGB1 is IRF3-dependent. A, relative MyD88 expression assessed by quantitative RT-PCR after treating HPAECs with control or MyD88siRNA. B, VEGF-stimulated cell migration assessed in HPAECs treated with siRNA against MyD88 (25 nm) or with control siRNA (25 nm) in the presence/absence of HMGB1 (1 μg/ml). C, relative IRF-3 expression using quantitative PCR after treating HPAECs with scramble or IRF-3 siRNA. D, VEGF-stimulated cell migration assessed in HPAECs treated with siRNA against IRF3 (25 nm) or with control siRNA (25 nm) in the presence/absence of HMGB1 (1 μg/ml). In all panels, data represent the mean ± S.E. (error bars) of three independent experiments. Analysis of variance; *, p < 0.05.
DISCUSSION
A prominent feature of pulmonary hypertension is the loss of small, amuscular arterioles in the periphery of the lung as a result of vascular injury. In the mouse model of chronic hypoxia-induced PH data suggest that induction of angiogenesis is protective by promoting endothelial regeneration in areas of vascular injury. This is exemplified by studies demonstrating that pharmacologic blockade of VEGF receptors aggravates (31), whereas VEGF overexpression in the lung attenuates chronic hypoxia-induced PH (32). Similarly, overexpression of the angiogenesis inhibitor angiostatin aggravates chronic hypoxia-induced PH (33).
A previous study demonstrated a role for TLR4 in the pathogenesis of chronic hypoxia-induced PH in mice. Here we explored the effect of the endogenous TLR4 agonist HMGB1 on pulmonary artery endothelial cell proliferation and migration, two features necessary for vascular regeneration.
A key facet of the innate immune response is self/nonself recognition. Through the use of pattern recognition receptors, the innate immune system is capable of detecting conserved pathogen motifs, termed pathogen-associated molecular patterns (PAMPs), thereby stimulating an immune response (34). In recent years, however, it has been acknowledged that the self/nonself paradigm fails to explain innate immune activation in diseases involving sterile inflammation (35). The discovery of danger-associated molecular patterns (DAMPs), endogenous molecules that have a function similar to PAMPs, has provided a context for understanding innate immune activation in the absence of foreign pathogens (36). HMGB1 fulfills the functions of a DAMP, being involved in inflammation caused by both infectious and noninfectious stimuli (37). Once released, HMGB1 signals through various pattern recognition receptors leading to cytokine release and tissue damage (2, 3, 38).
A role for HMGB1-TLR4 interaction in driving immunopathology was initially described in 2005. It was found that an HMGB1 neutralizing antibody protects WT mice (C3H/HeouJ) but not TLR4-defective (C3H/HEJ) mice from liver ischemia reperfusion injury (39). Since then, several studies have demonstrated a role for HMGB1-TLR4 interactions in the pathology of diseases including inflammation-induced seizures (40), inflammation-induced skin cancer (41), end organ injury following tissue trauma (42), and ischemic kidney injury (43).
Recently it was shown that HMGB1 mediates TLR4-dependent angiogenesis (44). Thus it was surprising that, whereas HMGB1 promotes migration of HUVECs, treatment of HPAECs with HMGB1 led to a TLR4-dependent decrease in migration. This differential effect on HPAECs versus HUVECs is likely due to a general difference in pulmonary versus systemic endothelial cells because HMGB1 also stimulated the migration of HDMEC. Similar to the effect of HMGB1, exposure of HPAECs to hypoxia inhibited migration in an HMGB1 and TLR4-dependent fashion whereas hypoxia is known to stimulate migration of systemic endothelial cells including HUVECs (45, 46). These data demonstrate that HMGB1 from either exogenous or endogenous sources is capable of inhibiting HPAEC migration.
The contradictory effects of HMGB1 in HPAECs versus HUVECs led us to consider whether there might be different TLR4-dependent signaling mechanism in systemic versus pulmonary endothelial cells. TLR4 signaling may be either MyD88-dependent or -independent. MyD88-dependent signaling leads to NFκB activation whereas MyD88-independent signaling involves TRIF and downstream IRF3 activation (9–11). We observed that addition of HMGB1 to HPAECs led to translocation of IRF3, but not the p65 subunit of NFκB, from the cytoplasm into the nucleus. In stark contrast, in HMGB1-stimulated HUVECs we observed NFκB, but not IRF3, nuclear translocation. Consistent with this observation, HMGB1 induced IRF3-dependent but not NFκB-dependent genes. Small interference RNA against IRF3 but not MyD88 prevented the inhibitory effect of HMGB1 on HPAEC migration. These data demonstrate a role for IRF3 in mediating the effects of HMGB1 in HPAECs and provide a basis for the differential effects of HMGB1 on HPAEC versus HUVEC migration.
There is some controversy as to whether the effects of HMGB1 are mediated by HMGB1 itself or contaminating bacterial LPS (47). In consideration of this and the fact that TLR4 is not only activated by HMGB1 but also by LPS, we provide several lines of evidence to exclude the possibility that LPS contamination accounts for the observation that HMGB1 inhibits HPAEC migration. HMGB1 was produced in yeast cells, excluding the possibility of bacteria-derived LPS contaminating the preparation. Experiments including the addition of the LPS-neutralizing agent polymyxin B had no effect on the ability of HMGB1 to inhibit HPAEC migration. Our HMGB1 preparation did not cause the activation of NFκB in HPAECs, whereas LPS did. Finally, the limulus assay did not detect any measurable endotoxin in our HMGB1 preparation (data not shown). Taken together, we are self-assured that the effects of HMGB1 on HPAEC migration represent a novel and direct inhibitory effect of HMGB1.
In summary, our experiments demonstrate a novel role for HMGB1 and TLR4 in the pulmonary endothelium. Whereas HMGB1 stimulates the canonical TLR4/MyD88/NFκB pathway in systemic endothelial cells, it activates a MyD88-independent but IRF3-dependent pathway in HPAECs. This contrast in signaling may be beneficial in selectively targeting the HMGB1/TLR4 pathway for the treatment of PH.
Acknowledgments
We thank Dr. Kevin Tracey (North Shore-LIJ Health System Feinstein Institute for Medical Research) for the HMGB1 neutralizing antibody.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-HL085134 and R03-HL110794 (to P. M. B.), P50-GM53789 (to T. R. B.), and T32-098036 (to E. M. B.).
- HMGB1
- high mobility group box 1
- HDMEC
- human dermal microvascular endothelial cell
- HPAEC
- human pulmonary artery endothelial cell
- HUVEC
- human umbilical vein endothelial cell
- iNOS
- inducible NOS
- IRF3
- interferon response factor 3
- ISG20
- IFN-stimulated gene 20
- MyD88
- myeloid differentiation primary response gene
- PH
- pulmonary hypertension
- TIR
- Toll-IL1 receptor domain
- TLR
- Toll-like receptor
- TRIF
- TIR domain-containing, adaptor-inducing IFN-β protein.
REFERENCES
- 1. Evankovich J., Cho S. W., Zhang R., Cardinal J., Dhupar R., Zhang L., Klune J. R., Zlotnicki J., Billiar T., Tsung A. (2010) High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J. Biol. Chem. 285, 39888–39897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park J. S., Svetkauskaite D., He Q., Kim J. Y., Strassheim D., Ishizaka A., Abraham E. (2004) Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 279, 7370–7377 [DOI] [PubMed] [Google Scholar]
- 3. Park J. S., Gamboni-Robertson F., He Q., Svetkauskaite D., Kim J. Y., Strassheim D., Sohn J. W., Yamada S., Maruyama I., Banerjee A., Ishizaka A., Abraham E. (2006) High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 290, C917–924 [DOI] [PubMed] [Google Scholar]
- 4. Huang W., Tang Y., Li L. (2010) HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine 51, 119–126 [DOI] [PubMed] [Google Scholar]
- 5. Ohmori H., Luo Y., Kuniyasu H. (2011) Non-histone nuclear factor HMGB1 as a therapeutic target in colorectal cancer. Expert Opin. Ther. Targets 15, 183–193 [DOI] [PubMed] [Google Scholar]
- 6. Sachdev U., Cui X., Hong G., Namkoong S., Karlsson J. M., Baty C. J., Tzeng E. (2012) High mobility group box 1 promotes endothelial cell angiogenic behavior in vitro and improves muscle perfusion in vivo in response to ischemic injury. J. Vasc. Surg. 55, 180–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schlueter C., Weber H., Meyer B., Rogalla P., Röser K., Hauke S., Bullerdiek J. (2005) Angiogenetic signaling through hypoxia. HMGB1: an angiogenetic switch molecule. Am. J. Pathol. 166, 1259–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Beijnum J. R., Nowak-Sliwinska P., van den Boezem E., Hautvast P., Buurman W. A., Griffioen A. W. (March 5, 2012) Tumor angiogenesis is enforced by autocrine regulation of high-motility group box 1. Oncogene 10.1038/onc.2012.49 [DOI] [PubMed] [Google Scholar]
- 9. Arancibia S. A., Beltrán C. J., Aguirre I. M., Silva P., Peralta A. L., Malinarich F., Hermoso M. A. (2007) Toll-like receptors are key participants in innate immune responses. Biol. Res. 40, 97–112 [DOI] [PubMed] [Google Scholar]
- 10. Fitzgerald K. A., Chen Z. J. (2006) Sorting out Toll signals. Cell 125, 834–836 [DOI] [PubMed] [Google Scholar]
- 11. McGettrick A. F., O'Neill L. A. (2010) Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr. Opin. Immunol. 22, 20–27 [DOI] [PubMed] [Google Scholar]
- 12. Young K. C., Hussein S. M., Dadiz R., deMello D., Devia C., Hehre D., Suguihara C. (2010) Toll-like receptor 4-deficient mice are resistant to chronic hypoxia-induced pulmonary hypertension. Exp. Lung Res. 36, 111–119 [DOI] [PubMed] [Google Scholar]
- 13. Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 14. Abraham E., Arcaroli J., Carmody A., Wang H., Tracey K. J. (2000) HMG-1 as a mediator of acute lung inflammation. J. Immunol. 165, 2950–2954 [DOI] [PubMed] [Google Scholar]
- 15. Angus D. C., Yang L., Kong L., Kellum J. A., Delude R. L., Tracey K. J., Weissfeld L. (2007) Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit. Care Med. 35, 1061–1067 [DOI] [PubMed] [Google Scholar]
- 16. Gong Q., Xu J. F., Yin H., Liu S. F., Duan L. H., Bian Z. L. (2009) Protective effect of antagonist of high-mobility group box 1 on lipopolysaccharide-induced acute lung injury in mice. Scand. J. Immunol. 69, 29–35 [DOI] [PubMed] [Google Scholar]
- 17. Ogawa E. N., Ishizaka A., Tasaka S., Koh H., Ueno H., Amaya F., Ebina M., Yamada S., Funakoshi Y., Soejima J., Moriyama K., Kotani T., Hashimoto S., Morisaki H., Abraham E., Takeda J. (2006) Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 174, 400–407 [DOI] [PubMed] [Google Scholar]
- 18. Reiss L. K., Uhlig U., Uhlig S. (2012) Models and mechanisms of acute lung injury caused by direct insults. Eur. J. Cell Biol. 91, 590–601 [DOI] [PubMed] [Google Scholar]
- 19. Ueno H., Matsuda T., Hashimoto S., Amaya F., Kitamura Y., Tanaka M., Kobayashi A., Maruyama I., Yamada S., Hasegawa N., Soejima J., Koh H., Ishizaka A. (2004) Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am. J. Respir. Crit. Care Med. 170, 1310–1316 [DOI] [PubMed] [Google Scholar]
- 20. Yang H., Ochani M., Li J., Qiang X., Tanovic M., Harris H. E., Susarla S. M., Ulloa L., Wang H., DiRaimo R., Czura C. J., Wang H., Roth J., Warren H. S., Fink M. P., Fenton M. J., Andersson U., Tracey K. J. (2004) Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. U.S.A. 101, 296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsung A., Klune J. R., Zhang X., Jeyabalan G., Cao Z., Peng X., Stolz D. B., Geller D. A., Rosengart M. R., Billiar T. R. (2007) HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 204, 2913–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chavakis E., Hain A., Vinci M., Carmona G., Bianchi M. E., Vajkoczy P., Zeiher A. M., Chavakis T., Dimmeler S. (2007) High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ. Res. 100, 204–212 [DOI] [PubMed] [Google Scholar]
- 23. Schlueter C., Hauke S., Loeschke S., Wenk H. H., Bullerdiek J. (2005) HMGA1 proteins in human atherosclerotic plaques. Pathol. Res. Pract. 201, 101–107 [DOI] [PubMed] [Google Scholar]
- 24. van Beijnum J. R., Dings R. P., van der Linden E., Zwaans B. M., Ramaekers F. C., Mayo K. H., Griffioen A. W. (2006) Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood 108, 2339–2348 [DOI] [PubMed] [Google Scholar]
- 25. Beutler B. (2000) Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12, 20–26 [DOI] [PubMed] [Google Scholar]
- 26. Park J. S., Arcaroli J., Yum H. K., Yang H., Wang H., Yang K. Y., Choe K. H., Strassheim D., Pitts T. M., Tracey K. J., Abraham E. (2003) Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am. J. Physiol. Cell Physiol. 284, C870–879 [DOI] [PubMed] [Google Scholar]
- 27. Wang E., Feng Y., Zhang M., Zou L., Li Y., Buys E. S., Huang P., Brouckaert P., Chao W. (2011) Toll-like receptor 4 signaling confers cardiac protection against ischemic injury via inducible nitric oxide synthase- and soluble guanylate cyclase-dependent mechanisms. Anesthesiology 114, 603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsai T. H., Chen S. F., Huang T. Y., Tzeng C. F., Chiang A. S., Kou Y. R., Lee T. S., Shyue S. K. (2011) Impaired Cd14 and Cd36 expression, bacterial clearance, and Toll-like receptor 4-MyD88 signaling in caveolin-1-deleted macrophages and mice. Shock 35, 92–99 [DOI] [PubMed] [Google Scholar]
- 29. DeFilippis V. R., Robinson B., Keck T. M., Hansen S. G., Nelson J. A., Früh K. J. (2006) Interferon regulatory factor 3 is necessary for induction of antiviral genes during human cytomegalovirus infection. J. Virol. 80, 1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doyle S., Vaidya S., O'Connell R., Dadgostar H., Dempsey P., Wu T., Rao G., Sun R., Haberland M., Modlin R., Cheng G. (2002) IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17, 251–263 [DOI] [PubMed] [Google Scholar]
- 31. Taraseviciene-Stewart L., Kasahara Y., Alger L., Hirth P., Mc Mahon G., Waltenberger J., Voelkel N. F., Tuder R. M. (2001) Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 15, 427–438 [DOI] [PubMed] [Google Scholar]
- 32. Partovian C., Adnot S., Raffestin B., Louzier V., Levame M., Mavier I. M., Lemarchand P., Eddahibi S. (2000) Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am. J. Respir. Cell Mol. Biol. 23, 762–771 [DOI] [PubMed] [Google Scholar]
- 33. Pascaud M. A., Griscelli F., Raoul W., Marcos E., Opolon P., Raffestin B., Perricaudet M., Adnot S., Eddahibi S. (2003) Lung overexpression of angiostatin aggravates pulmonary hypertension in chronically hypoxic mice. Am. J. Respir. Cell Mol. Biol. 29, 449–457 [DOI] [PubMed] [Google Scholar]
- 34. Kumar H., Kawai T., Akira S. (2011) Pathogen recognition by the innate immune system. Int. Rev. Immunol. 30, 16–34 [DOI] [PubMed] [Google Scholar]
- 35. Matzinger P. (1994) Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 [DOI] [PubMed] [Google Scholar]
- 36. Bianchi M. E. (2007) DAMPs, PAMPs, and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 [DOI] [PubMed] [Google Scholar]
- 37. Klune J. R., Dhupar R., Cardinal J., Billiar T. R., Tsung A. (2008) HMGB1: endogenous danger signaling. Mol. Med. 14, 476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hori O., Brett J., Slattery T., Cao R., Zhang J., Chen J. X., Nagashima M., Lundh E. R., Vijay S., Nitecki D. (1995) The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin: mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 270, 25752–25761 [DOI] [PubMed] [Google Scholar]
- 39. Tsung A., Sahai R., Tanaka H., Nakao A., Fink M. P., Lotze M. T., Yang H., Li J., Tracey K. J., Geller D. A., Billiar T. R. (2005) The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 201, 1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maroso M., Balosso S., Ravizza T., Liu J., Aronica E., Iyer A. M., Rossetti C., Molteni M., Casalgrandi M., Manfredi A. A., Bianchi M. E., Vezzani A. (2010) Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 16, 413–419 [DOI] [PubMed] [Google Scholar]
- 41. Mittal D., Saccheri F., Vénéreau E., Pusterla T., Bianchi M. E., Rescigno M. (2010) TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 29, 2242–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levy R. M., Mollen K. P., Prince J. M., Kaczorowski D. J., Vallabhaneni R., Liu S., Tracey K. J., Lotze M. T., Hackam D. J., Fink M. P., Vodovotz Y., Billiar T. R. (2007) Systemic inflammation and remote organ injury following trauma require HMGB1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1538–1544 [DOI] [PubMed] [Google Scholar]
- 43. Chen J., Hartono J. R., John R., Bennett M., Zhou X. J., Wang Y., Wu Q., Winterberg P. D., Nagami G. T., Lu C. Y. (2011) Early interleukin 6 production by leukocytes during ischemic acute kidney injury is regulated by TLR4. Kidney Int. 80, 504–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin Q., Yang X. P., Fang D., Ren X., Zhou H., Fang J., Liu X., Zhou S., Wen F., Yao X., Wang J. M., Su S. B. (2011) High-mobility group box-1 mediates Toll-like receptor 4-dependent angiogenesis. Arterioscler. Thromb. Vasc. Biol. 31, 1024–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nagata D., Mogi M., Walsh K. (2003) AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 278, 31000–31006 [DOI] [PubMed] [Google Scholar]
- 46. Zhu X. Y., Daghini E., Chade A. R., Lavi R., Napoli C., Lerman A., Lerman L. O. (2008) Disparate effects of simvastatin on angiogenesis during hypoxia and inflammation. Life Sci. 83, 801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bianchi M. E. (2009) HMGB1 loves company. J. Leukoc. Biol. 86, 573–576 [DOI] [PubMed] [Google Scholar]
- 48. Ngamkitidechakul C., Twining S. S. (2002) Buffered non-fermenter system for lab-scale production of secreted recombinant His-tagged proteins in Saccharomyces cerevisiae. BioTechniques 33, 1296–1300 [DOI] [PubMed] [Google Scholar]