Abstract
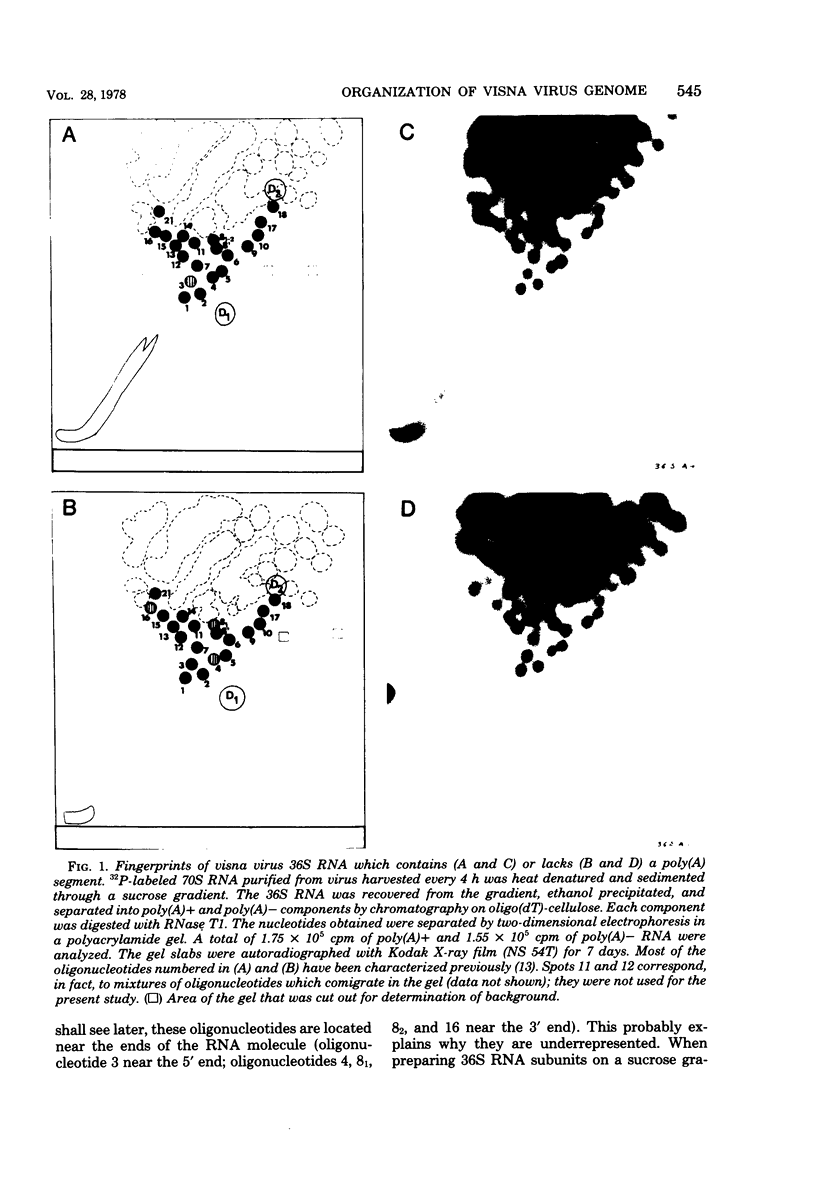
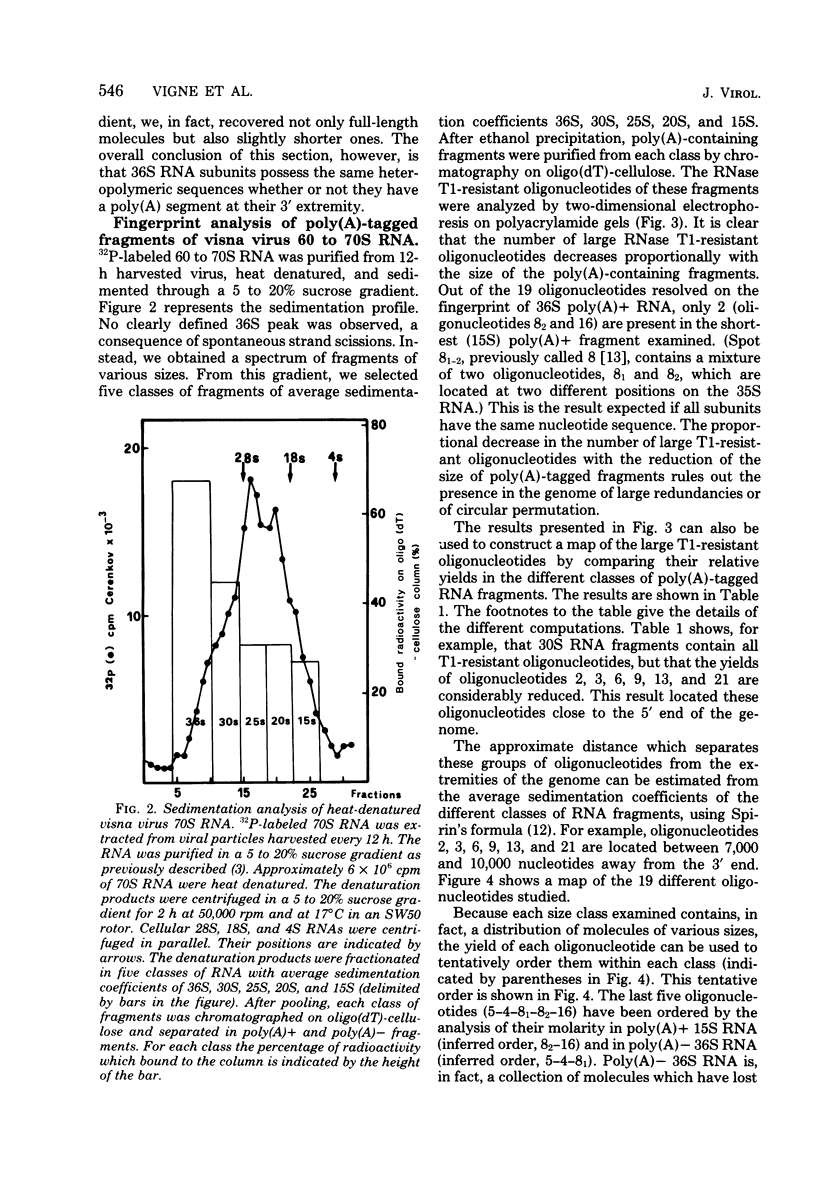
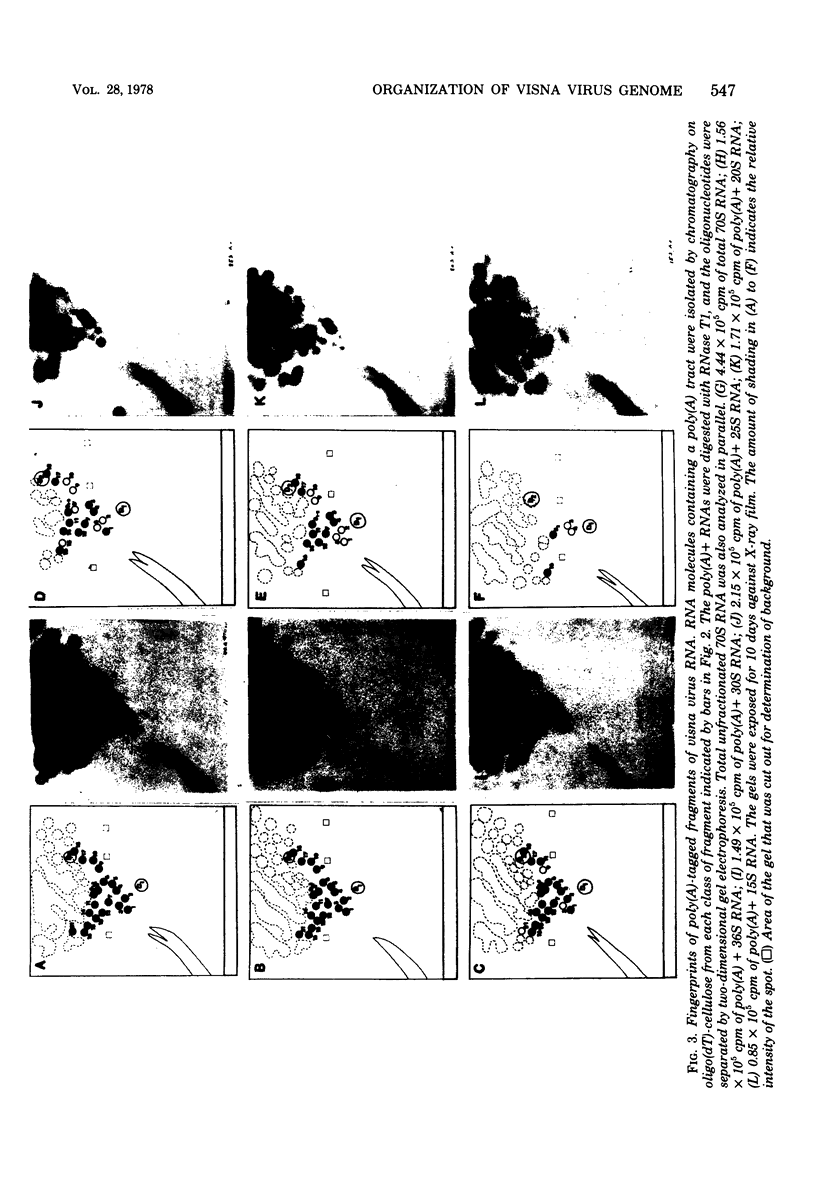
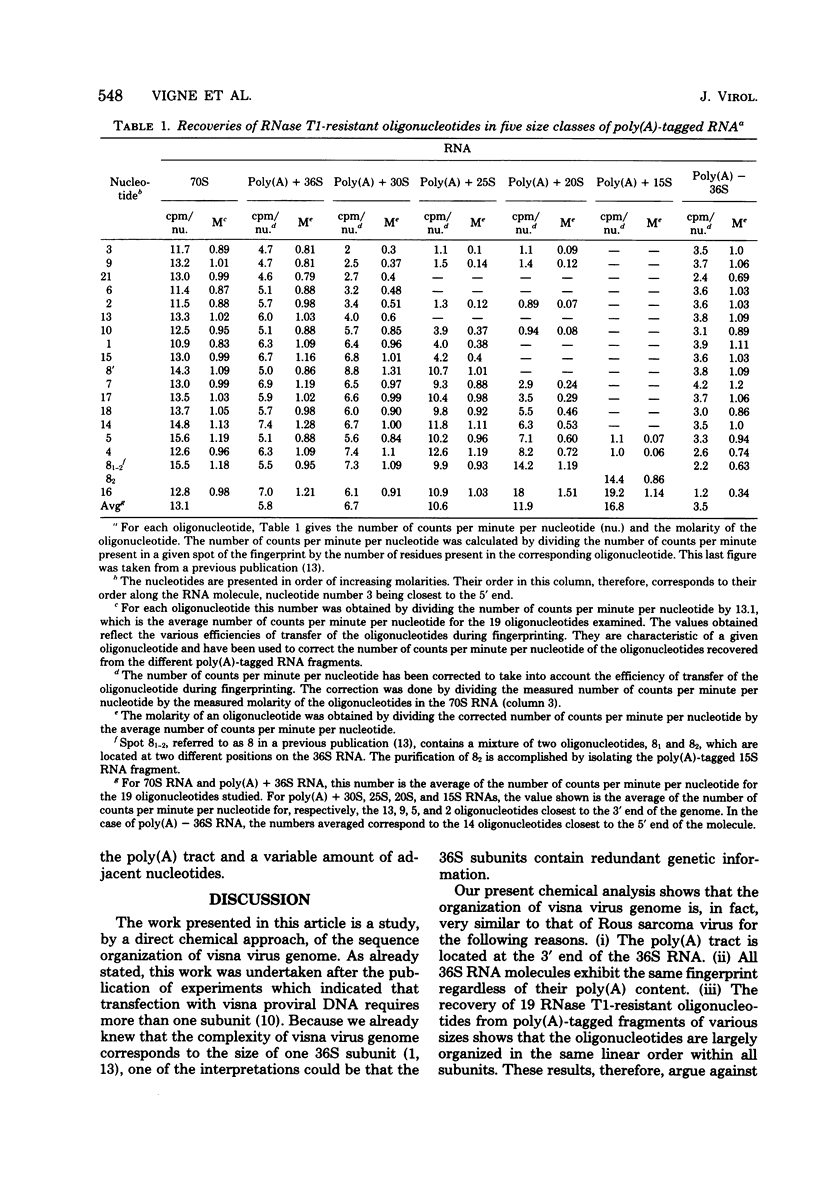
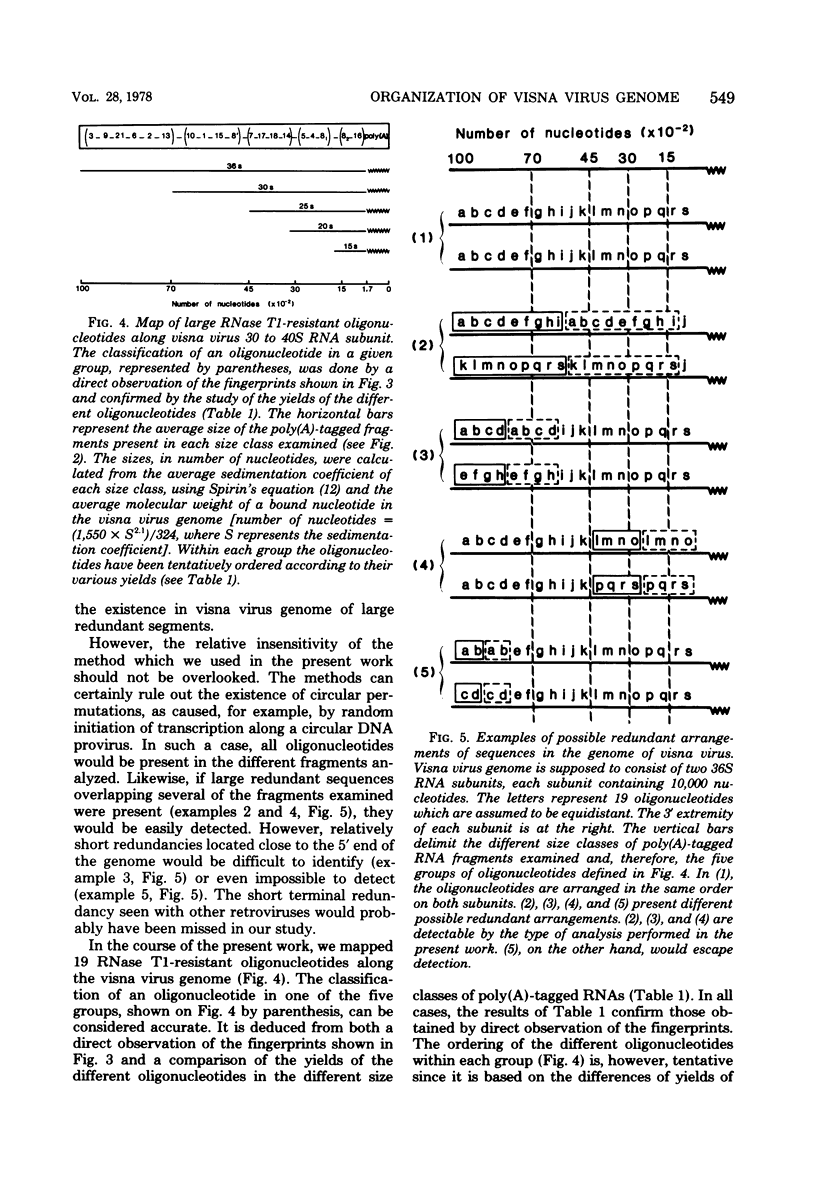
A previous study of the infectivity of visna virus proviral DNA suggested that the genetic information of the virus is distributed over at least two of the RNA subunits. Because the genetic complexity of visna virus corresponds to the size of one subunit, this result may imply that sequence redundancies exist within each subunit. In the present article we have examined this question by constructing a map of the large RNase T1-resistant oligonucleotides of the viral genome. Our principal results are as follows: (i) all 36S RNA subunits have the same genetic content regardless of their polyadenylic acid [poly(A)] content; (ii) the poly(A) tract is present at the 3' end of the molecule; and (iii) the recoveries of 19 large RNase T1-resistant oligonucleotides from poly(A)-tagged RNA fragments of various sizes demonstrate that the oligonucleotides are organized in the same linear order within all subunits. Our results, therefore, exclude the existence of large sequence redundancies in the genome of visna virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K. L., Faras A. J., Hasse A. T., Duesberg P. H., Maisel J. E. Genomic complexities of murine leukemia and sarcoma, reticuloendotheliosis, and visna viruses. J Virol. 1976 Feb;17(2):525–537. doi: 10.1128/jvi.17.2.525-537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Tamalet J., Filippi P., Delbecchi L. The high molecular weight RNA of Visna virus. Biochimie. 1973;55(8):885–891. doi: 10.1016/s0300-9084(73)80165-8. [DOI] [PubMed] [Google Scholar]
- Brahic M., Vigne R. Properties of visna virus particles harvested at short time intervals: RNA content, infectivity, and ultrastructure. J Virol. 1975 May;15(5):1222–1230. doi: 10.1128/jvi.15.5.1222-1230.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Infectious rous sarcoma virus and reticuloendotheliosis virus DNAs. J Virol. 1974 Nov;14(5):1132–1141. doi: 10.1128/jvi.14.5.1132-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F. Classification and nomenclature of viruses. Second report of the International Committee on Taxonomy of Viruses. Intervirology. 1976;7(1-2):1–115. doi: 10.1159/000149938. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Baringer J. R. The structural polypeptides of RNA slow viruses. Virology. 1974 Jan;57(1):238–250. doi: 10.1016/0042-6822(74)90124-x. [DOI] [PubMed] [Google Scholar]
- Haase A. T. The slow infection caused by visna virus. Curr Top Microbiol Immunol. 1975;72:101–156. doi: 10.1007/978-3-642-66289-8_4. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Traynor B. L., Ventura P. E., Alling D. W. Infectivity of visna virus DNA. Virology. 1976 Mar;70(1):65–79. doi: 10.1016/0042-6822(76)90236-1. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Vigne R., Brahic M., Filippi P., Tamalet J. Complexity and polyadenylic acid content of visna virus 60-70S RNA. J Virol. 1977 Jan;21(1):386–395. doi: 10.1128/jvi.21.1.386-395.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C., Parsons J. T., Coffin J. W., Rymo L., Billeter M. A., Hofstetter H. Studies on the structure and synthesis of Rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1043–1056. doi: 10.1101/sqb.1974.039.01.120. [DOI] [PubMed] [Google Scholar]