Background: Ligand receptor ligation regulates immune-inflammatory cell chemotaxis.
Results: Vasoactive intestinal peptide and prostaglandin D2 share the CRTH2 receptor in inducing eosinophil chemotaxis.
Conclusion: There is a strong association between VIP and CRTH2 in eosinophil chemotaxis.
Significance: This is the first evidence that may indicate that CRTH2 could modulate the neuroimmunoregulatory axis in allergic eosinophil inflammation.
Keywords: Allergy, Cell Migration, Cell Surface Receptor, Eosinophils, Signal Transduction
Abstract
We explored the relation between vasoactive intestinal peptide (VIP), CRTH2, and eosinophil recruitment. It is shown that CRTH2 expression by eosinophils from allergic rhinitis (AR) patients and eosinophil cell line (Eol-1 cells) was up-regulated by VIP treatment. This was functional and resulted in exaggerated migratory response of cells against PGD2. Nasal challenge of AR patients resulted in a significant increase of VIP contents in nasal secretion (ELISA), and the immunohistochemical studies of allergic nasal tissues showed significant expression of VIP in association with intense eosinophil recruitment. Biochemical assays showed that VIP-induced eosinophil chemotaxis from AR patients and Eol-1 cells was mediated through the CRTH2 receptor. Cell migration against VIP was sensitive to protein kinase C (PKC) and protein kinase A (PKA) inhibition but not to tyrosine kinase or p38 MAPK inhibition or calcium chelation. Western blot demonstrated a novel CRTH2-mediated cytosol-to-membrane translocation of PKC-ϵ, PKC-δ, and PKA-α, -γ, and -IIαreg in Eol-1 cells upon stimulation with VIP. Confocal images and FACS demonstrated a strong association and co-localization between VIP peptide and CRTH2 molecules. Further, VIP induced PGD2 secretion from eosinophils. Our results demonstrate the first evidence of association between VIP and CRTH2 in recruiting eosinophils.
Introduction
The allergic inflamed airway contains a pool of mediators that compete as chemoattractive signals to eosinophils. Neuropeptides secreted from the sensory neurons have been reported as chemoattractants for eosinophils (1–4). Accumulating evidence indicates an important neuroimmune interaction between the rich expression of VIP3 on the allergic nasal tissue and bronchial smooth muscle bundle and inflammatory cell recruitment (5–7). Furthermore, eosinophils from intestinal mucosa store and secrete VIP (8). All of this evidence indicates an important neuroimmunoinflammatory axis between VIP and eosinophils.
VIP is a 28-amino acid polypeptide that exists in the parasympathetic nerves and to a lesser extent in the sensory fibers and is one of the most abundant of the neuropeptides found in the upper and lower airways (9, 10). Although VIP induces its biological activity through its specific receptors (11), we earlier failed to demonstrate a VPAC1 receptor on human eosinophils when compared with lymphocytes (4). Therefore, at the time, we proposed that VIP may activate human eosinophils through nonspecific phospholipids receptors. Recently, the novel chemoattractant receptor-homologous molecule expressed by TH2 cells, basophils, and eosinophils (CRTH2) gained a lot of attention as a promoter for PGD2-induced eosinophilia in allergic airway diseases (12–16). In an allergic rhinitis murine model, it has been demonstrated that the PGD2-CRTH2 interaction is elevated following pollen sensitization. This resulted in specific IgE and IgG1 production, nasal eosinophilia, and IL-4 and IL-5 production by submandibular lymph node cells. Additionally, CRTH2 mRNA in nasal mucosa was significantly elevated in Cry j 1-sensitized mice (17). Moreover, in nasal tissue, ligation of PGD2 to CRTH2 appeared to be selectively involved in eosinophil recruitment (18).
In addition to its expression on leukocytes, CRTH2 is also richly expressed in the different parts of the brain (19), which may further indicate a relation of this receptor to neuropeptides. Molecularly, CRTH2 is a seven-transmembrane G-protein-coupled receptor that is composed of 395 amino acids residues with lower homology to other protanoid receptors (13, 20), but to date no other agent is reported to utilize this receptor for eosinophil chemotaxis except for its ligand PGD2.
Accordingly, the current study was designed to explore the relation between CRTH2 and VIP in airway eosinophilic inflammation and to investigate the molecular events involved in this scenario. The Eol-1 cell line, which is ideal to study cellular proteins and has the ability to differentiate to mature eosinophils by n-butyrate, allowed us to explore these aims. Immunohistochemical analysis of nasal tissue from allergic chronic rhinosinusitis (ACRS) patients and nasal provocation challenges allowed us to validate our biochemical results and to have an applied in vivo correlation.
EXPERIMENTAL PROCEDURES
VIP Contents in Nasal Secretions, ELISA
The content of VIP was measured in 10 patients with AR and seven control healthy subjects after nasal provocation with the aeroallergen. Aeroallergens were chosen according to the results of skin test sensitivity and radioallergosorbant test of the patients. The control subjects were challenged with histamine. None of them were taking antihistamines or nasal/systemic cortisone therapy. After obtaining their consent, the purified and standardized allergen dilutions (Stallorgenes, 100 Index of reactivity/ml) were introduced into the nose. After 1–2 min, the patients started to blow their nose and were asked to continue collecting the secretion for 15 min. Saline nasal irrigation was then done 2–3 times, and the patients' vital signs were monitored for at least 30 min after challenge before they were discharged from the clinic. VIP levels in collected nasal secretion were measured using a VIP enzyme immunoassay kit (Phoenix Pharmaceuticals, Inc.) according to the manufacturer's recommendations. The sensitivity of our assay was 0.04 ng/ml. All nasal secretions were used at a dilution of 1:50 for the enzyme immunoassay.
Eosinophil Purification
Eosinophils were purified by Percoll solution separation from patients suffering from AR. Briefly, 60 ml of heparin-anti-coagulated peripheral blood were obtained by venopuncture. The blood was diluted with phosphate-buffered saline (PBS) containing 2% FCS in a ratio of 1:1. The Percoll solution at concentration of 66% was then placed carefully by a pipette in the bottom of the tube. After centrifugation for 30 min at 20 °C and 1500 rpm, a band and a pellet were obtained. The band is composed of mononuclear cells, whereas the pellet is a mixture of eosinophils and neutrophils. Sedimented red blood cells were removed by hypodense lysis. Eosinophils were further purified by immunomagnetic cell separation (Miltenyi Biotec, Bergisch Gladbach, Germany), using anti-CD16 as described previously (4). Eosinophil purity was >98%.
Eol-1 Cell Line
The human eosinophilic leukemia (Eol-1) cell line (Riken BioResource Center, Japan) was used in parts of the current biochemical study. Cells differentiation into mature eosinophils was induced by the histone deacetylase inhibitor n-butyrate precisely as described earlier (21).
Flow Cytometry Analysis (FACS)
CRTH2 surface expression on eosinophils and Eol-1 cells was analyzed by FACS (FACS CANTO II BD Systems). Briefly, after 30 min, stimulated (VIP) or not stimulated (buffer) cells were fixed with 4% paraformaldehyde for 15 min. The cells were then washed and incubated with the CRTH2 antibody (BD Pharmingen) for 60 min in the dark, on ice. After two additional washes, cells (105 cells/FACS plot) were conserved in paraformaldehyde 1% and then analyzed for their fluorescence intensity.
RT-PCR
Reverse transcription products were PCR-amplified with specific primers for HPRT, VIP, VPAC1, and VPAC2, as shown in Table 1.
TABLE 1.
Primers for HPRT, VIP, VPAC1, and VPAC2
| Primer | Sequence | Size |
|---|---|---|
| bp | ||
| HPRT forward | 5′-GTT GGA TAT AAG CCA GAC TTT GTT G-3′ | 177 |
| HPRT reverse | 5′-CAG ATG TTT CCA AAC TCA ACT TGA A-3′ | |
| VIP forward | 5′-CCA GGC ATG CTG ATG GAG TTT TC-3′ | 227 |
| VIP reverse | 5′-CCT CTT TCC ATT CAG AAT TGA GTT-3′ | |
| VPAC1 forward | 5′-CTT CTG GTC GCC ACA GCT ATC CTG-3′ | 534 |
| VPAC1 reverse | 5′-ACT GCT GTC ACT CTT CCT GAT ATC-3′ | |
| VPAC2 forward | 5′-CGT CAC GGT GCC CTG CCC AAA AGT-3′ | 462 |
| VPAC2 reverse | 5′-CCC TCC ACC AGC AGC CAG AAG A-3′ |
The PCR conditions for HPRT were initial denaturation at 95 °C for 5 min followed by 35 cycles of 45 s at 95 °C, 45 s at 60 °C, and 45 s at 72 °C. The PCR conditions for VIP were denaturation at 94 °C for 5 min followed by 40 cycles of 30 s at 94 °C, 30 s at 57 °C, and 45 s at 72 °C and then a final cycle of 5 min at 72 °C. The PCR conditions for VPAC1 were similar to those for VIP: 30 s at 94 °C, 30 s at 60 °C, and 45 s at 72 °C. In the case of VIPAC2, the PCR conditions were denaturation at 94 °C for 5 min and 40 cycles of 30 s at 94 °C, 30 s at 58 °C, and 45 s at 72 °C and then a final cycle of 5 min at 72 °C. RT-PCR products were assayed on 1.8% agarose gel electrophoresis and visualized by staining with ethidium bromide.
Immunohistochemistry
Sections of nasal specimens underwent immunoperoxidase staining using antibodies directed against VIP (1:50) (ab8556, Abcam). The sections were deparaffinized in xylene and rehydrated in methanol. Endogenous peroxidases were blocked by 5% H2O2 treatment. Samples were then washed with PBS. A second treatment for 5 min with H2O2 (Dako) was then applied. The samples were washed again in PBS before being blocked for 10 min with Dako blocking reagent. Samples were then incubated with the primary antibody at room temperature overnight. After washings, the revelation was performed with the use of appropriate secondary antibodies and the LSAB2 system (VIP; Dako A/S) according to the supplier's recommendations. Immunoreactivity was visualized by a treatment with diaminobenzidine (Sigma-Aldrich), and the slides were counterstained with Mayer's hematoxylin. For staining intensity, − represents samples in which the staining was undetectable, whereas + and ++ denote samples with weak and strong staining, respectively.
Chemotaxis Assays
Chemotaxis assays were performed in triplicate in a 48-well microchemotaxis Boyden chamber incubated in 5% CO2 at 37 °C for 90 min. Aliquots of 29 μl of the chemotactic agent eotaxin (R&D Systems, Minneapolis, MN), VIP (Phoenix Pharmaceuticals), or PGD2 (Cayman Chemical) were placed in the lower wells, and 50 μl of either peripheral purified eosinophils or Eol-1 suspension (106 cells/ml) were placed in the upper wells. The two chambers were separated by a 5.0-μm pore polycarbonate membrane (Nuclepore, Whatman, Middlesex, UK). The controls consisted of a solution of Hanks' balanced salt solution. After a 90-min incubation at 37 °C, the membrane was removed, fixed in methanol, and stained with Diff-Quick (Baxter Scientific, Miami, FL). Migrated cells adherent to the lower surface were counted in 5 selected high power fields/well under a light microscope (magnification ×400). As for the blocking experiments, the cells were pretreated with VIP-R1 (Sigma) (the VIP receptor antagonist), antihuman CRTH2 receptor antibody (R&D Systems), H-89 dihydrochloride (VWR-Calbiochem) (a PKA inhibitor), bisindolylmaleimide (VWR-Calbiochem) (a PKC inhibitor), SB203580 (VWR-Calbiochem) (a p38 MAPK inhibitor), or Genistein (Sigma) (a tyrosine kinase inhibitor) for 60 min at 37 °C. Cells were then washed twice and resuspended in buffer medium, and their chemotaxis was checked as stated above.
Ca2+-depleted Cells
Ca2+-depleted cells were obtained by incubating 107 cells/ml with 30 μmol/liter of the calcium-chelating agent BAPTA-AM (Calbiochem) in test medium (130 mmol/liter NaCl, 5 mmol/liter NaHCO3, 4.6 mmol/liter KCl, 5 mmol/liter glucose, 2 mmol/liter EGTA, and 20 mmol/liter HEPES) for 30 min at 37 °C.
Assessment of Actin Reorganization with Phalloidin-FITC and the Cytoskeleton Changes
30 μl of cell suspensions at 106 cells/ml were placed in a μ-Slide VI coated (collagen IV) cell microscopy chamber (Ibidi Integrated BioDiagnostics, Munich, Germany) and left to adhere for 30 min. Stimulation with buffer, eotaxin, or VIP was then performed for 15 min. After two washes with PBS, the cells were fixed in 4% formaldehyde for 20 min and permeabilized with 0.1% saponin for another 30 min. The cells were then stained with Alexa Fluor 488®-phalloidin diluted 40× (Invitrogen) for 30 min, in the dark, on ice. After two washes with PBS, the cells were conserved in Prolong® gold antifade with DAPI (Invitrogen) and were then analyzed by confocal microscopy (Leica).
Preparation of Cell Extracts for Western Blotting
For preparation of whole cell extracts, cells were pelleted by centrifugation and washed twice with PBS. The cell pellets were resuspended in cold RIPA lysis buffer (25 mm Tris-HCl, pH 7.6, 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS; Pierce) supplemented with protease inhibitors (Complete, Roche Applied Science) and subsequently swirled for 10 min on ice. The extracts were then centrifuged at 14,000 × g for 15 min at 4 °C. The supernatants were analyzed for protein content by the Bio-Rad protein assay based on the Bradford method (Bio-Rad), whereas for preparation of membrane extracts, the Mem-PER eukaryotic membrane protein extraction reagent kit was used (Pierce). In accordance with the manufacturer's protocol, cells were pelleted by centrifugation and washed twice with PBS. The cell pellets were resuspended in reagent A supplemented with protease inhibitors (Complete, Roche Applied Science) and subsequently incubated for 10 min at room temperature. The suspensions were placed on ice, and diluted reagent C was added for 30 min. After centrifugation at 10,000 × g for 3 min at 4 °C, supernatants were incubated 10 min at 37 °C, and after a second centrifugation at 10,000 × g for 2 min at room temperature, membrane proteins were isolated. The supernatants were analyzed for protein concentration by the Bradford method (Bio-Rad).
Antibodies
The antibodies used for Western blot were rabbit anti-PKCδ antibody (C-17, sc-213), rabbit anti-PKCϵ antibody (C-15, sc-214), rabbit anti-PKAα cat antibody (C-20, sc-903), rabbit anti-PKAγ cat antibody (C-20, sc-905), and rabbit anti-PKA IIαreg antibody (C-20, sc-908); all were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-CRTH2 rabbit polyclonal antibody was obtained from Abcam (ab59382). The blocking antibody used was anti-CRTH2 rat monoclonal antibody (BM16) purchased from BD Biosciences.
Western Blotting Experiments
Western blot analysis was performed on proteins extracted after 30 min or 24 h of treatment with VIP at 10−7 m (Phoenix Pharmaceuticals) as indicated and with or without pretreatment for 1 h with the blocking antibody. Samples were separated on a 12% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Roche Applied Science). The primary antibodies were used at a 1:200 dilution. The secondary anti-rabbit antibodies coupled with horseradish peroxidase (Amersham Biosciences) at a 1:3000 dilution were detected by chemiluminescence with the ECL system (Pierce). For Figs. 1D and 4, blots were scanned and quantified using ImageJ software, using GAPDH or Coomassie Blue staining as loading control, respectively.
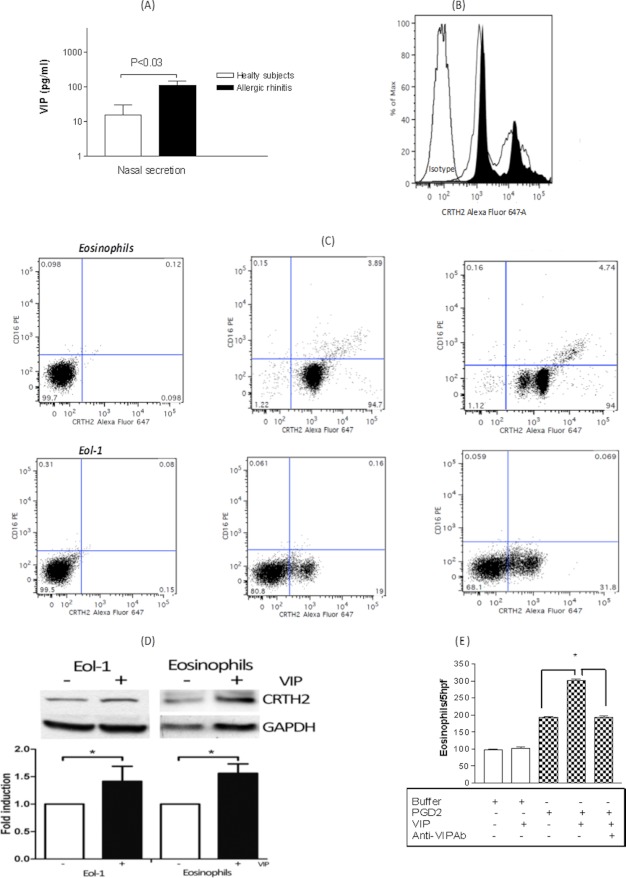
FIGURE 1.
A, quantity of VIP recovered from nasal secretion of AR patients following allergen challenge (n = 17; 7 healthy subjects and 10 patients with AR). *, p < 0.05. B, FACS analysis of CRTH2 receptor surface expression from peripheral blood eosinophils. The white histogram represents CRTH2 expression, whereas the black histogram represents CRTH2 expression after stimulation of cells by 10−7 m VIP for 24 h. Histograms are representative of 12 patients showing similar results. C, expression of CRTH2 and CD16 in eosinophils and Eol-1 cells. Left, control markers; middle, CRTH2 and CD16 expression in the absence of VIP treatment; right, CRTH2 and CD16 expression after VIP treatment. D, expression of CRTH2 in Eol-1 cells and in eosinophils with (+) or without (−) a 24-h VIP treatment. Western blots were performed on whole cell extracts; GAPDH was used as control. The graph represents quantification of three experiments. *, p < 0.05. E, priming effect of 10−7 m VIP on 10−9 m PGD2-induced eosinophils chemotaxis. Results are the mean ± S.E. (error bars) of three independent experiments performed in triplicate.
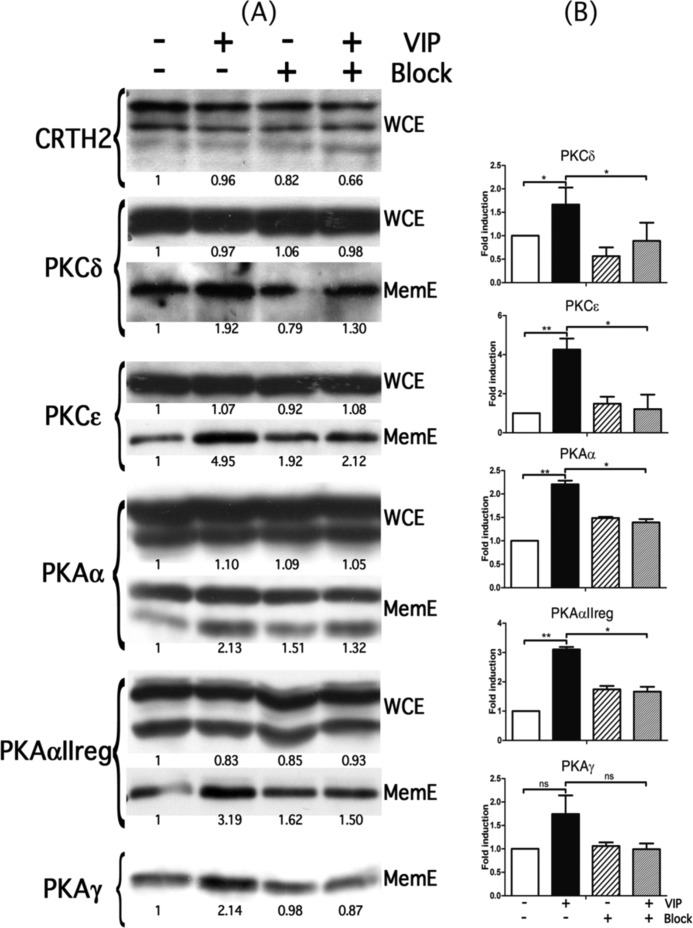
FIGURE 4.
Expression of CRTH2 and different PKA and PKC in Eol-1 cells after VIP treatment. A, Western blots were performed on either whole cell extracts (WCE) or membranous extracts (MemE) of Eol-1 cells treated (+) or not (−) with VIP for 30 min, with (+) or without (−) pretreatment with CRTH2 blocking antibody. B, graphs representing the quantification of Western blot experiments on membranous extracts. Coomassie Blue staining was used as a loading control. *, p < 0.05. Error bars, S.E.
Fluorescence-labeled VIP and CRTH2 Binding Studies
A nonradioactive technique utilizing a 1 μm concentration of Cy3-Ahx-VIP (PiCHEM), Cy3-Ahx-HSDAVFTDNYTRLRKQMAVKKYLNSILN-NH2, was used. Eol-1 cells were incubated with Cy3-Ahx-VIP for 30 min in the presence or absence of 10−7 m anti-VIP (Phoenix Pharmaceuticals), 10 μg/ml anti-CRTH2 blocking antibody, or 10−5 m anti-VIP receptor antagonist (VIP-R1). After two washes, cells were fixed and subjected immediately to confocal laser-scanning microscopy and FACS analysis. As for the co-localization experiments, cells were first stained with anti-CRTH2 Alexa Fluor-conjugated Ab (BD Pharmingen) for 30 min, washed, and then stained with the labeled Cy3-Ahx-VIP as described above. To control for peptide-unrelated staining, the cells were incubated with Cy3 alone (PiCHEM).
PGD2 Secretion by Eosinophils, ELISA
PGD2 level in the supernatant of cultured eosinophils from three allergic patients, to polyaeroallergens, was checked utilizing an ELISA kit (Cayman Chemical), according to the manufacturer's recommendations. The stimulation of eosinophils was for 30 min and for 24 h with either buffer only, 10−7 m VIP only, or 10−7 m VIP in the presence of 0.1 μg/ml anti-VIP.
Statistical Analysis
Results are expressed as the mean ± S.E. Statistical significance was analyzed by paired Student's t test and analysis of variance. A p value of <0.05 was considered to be statistically significant.
RESULTS
Relationship between VIP, Eosinophil, and CRTH2 in AR and ACRS
To investigate the in vivo amount of VIP secreted by the healthy and allergic airway in response to nasal provocation, we measured VIP amounts by ELISA in the nasal secretions from AR patients and compared them to controls. As seen in Fig. 1A, there was significantly higher content of VIP in nasal secretions from allergic subjects when compared with the controls. Nasal cytology from AR patients' nasal secretions showed eosinophilia (data not shown). We have recently reported, by immunohistochemical studies of nasal tissue obtained from the middle turbinate as a part of the surgical procedure from patients undergoing endoscopic sinus surgery for ACRS and nonallergic chronic rhinosinusitis, a positive expression of CRTH2 in a population of infiltrating eosinophils and lymphocytes, respectively, when compared with controls operated for reduction of the inferior turbinates, highlighting the importance of CRTH2 in inflammatory cell recruitment to the inflamed nose (22). Therefore, we next investigated whether VIP could modulate the expression of CRTH2 on human eosinophils from AR patients. As demonstrated in Fig 1B, eosinophil treatment with 10−7 m VIP for 24 h resulted in up-regulation of the expression of CRTH2. The mean fluorescence intensity of CRTH2 was 56 ± 10 and 69 ± 5 for the spontaneous expression from AR patients, after a 24-h culture in buffer medium alone or in the presence of 10−7 m VIP, respectively.
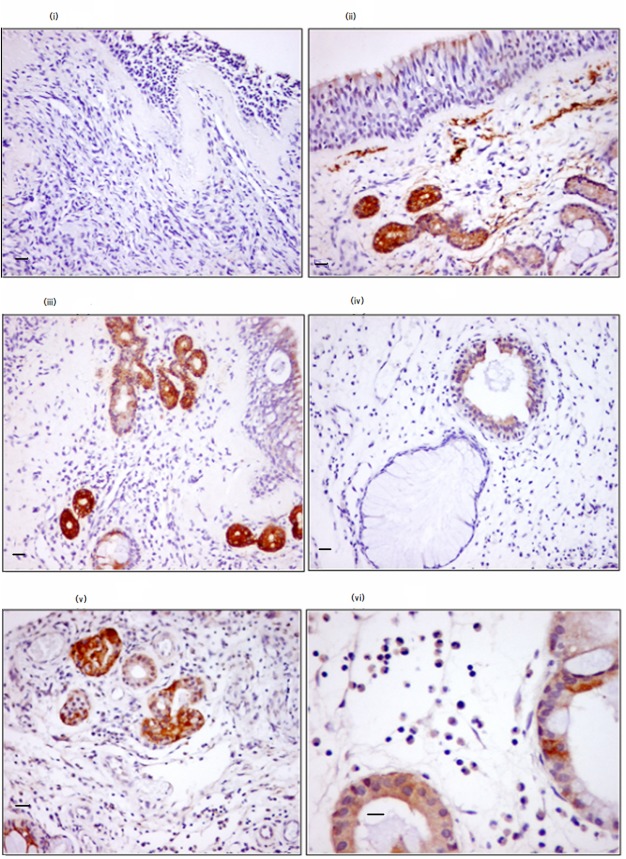
Interestingly, all histograms demonstrated a double or triple population of CRTH2 expression in eosinophils and Eol-1 cells, as seen in Fig. 1B. To further investigate these heterogeneous populations of CRTH2 expression, we double-stained the cells with anti-CRTH2 and anti-CD16. As seen in Fig. 1C, less than 3% of peripheral blood eosinophils expressed CD16, and none of the Eol-1 cells did. The VIP treatment of eosinophils and Eol-1 cells with VIP did not modulate the percentage of CD16-positive cells but increased the expression and total protein content of CRTH2 (Fig. 1D). This up-regulation was functional with exaggerated eosinophil chemotaxis against a suboptimal dose of 10−9 m PGD2 (Fig. 1E). Further, VIP immunohistochemical analysis from nasal middle turbinate mucosa of patients with ACRS demonstrated significant expression by the epithelial layer and lamina propria (Fig. 2, ii–v) over controls (nasal tissue obtained from the inferior turbinate for turbinate reduction). The expression of VIP was associated with intense eosinophil infiltration (Fig. 2, vi). All of the above data point to a possible in vivo association between VIP, eosinophil, and CRTH2 in the pathophysiology of allergy of the upper airway.
FIGURE 2.
Relationship between eosinophils infiltration and VIP expression within nasal tissue. The brown color represents VIP expression. i, control subject; ii–vi, patients with ACRS. Note the expression of VIP by the epithelial cells (ii), some stromal cells (ii–vi), and the infiltrating eosinophils (vi). Staining intensity was as follows: − for the control, in the epithelium and subepithelium layer; ++ for ACRS in the epithelium and subepithelium layer. Magnification was ×200 for i–v images and ×400 for vi. Scale bars, 25 μm (i–v) and 50 μm (vi).
Eosinophilotactic Activity of VIP
To investigate whether VIP attracts human eosinophils from AR patients in a pattern different from what we reported earlier from normal peripheral blood eosinophils (4), the following chemotaxis assays were performed. As can be seen from Fig. 3A, VIP at a wide range of doses (10−5 to 10−9 m) significantly chemoattracted eosinophils from AR patients and lost its significant chemotactic activity at 10−10 m. The efficacy of VIP eosinophilotactic activity was comparable with that in the positive control, eotaxin, but the chemotaxis index was less than that for eotaxin (confidence interval = 4.7 for eotaxin and 3 for VIP). Also, the VIP dose-response curve was different from that of classical chemokines, showing neither dose dependence nor the classical bell-shaped curve of chemokines. Checkerboard analysis confirmed a mainly chemotactic effect with a lesser chemokinetic effect (data not shown). These results indicate the ability of VIP to attract normal eosinophils and eosinophils from AR patients in a similar fashion.
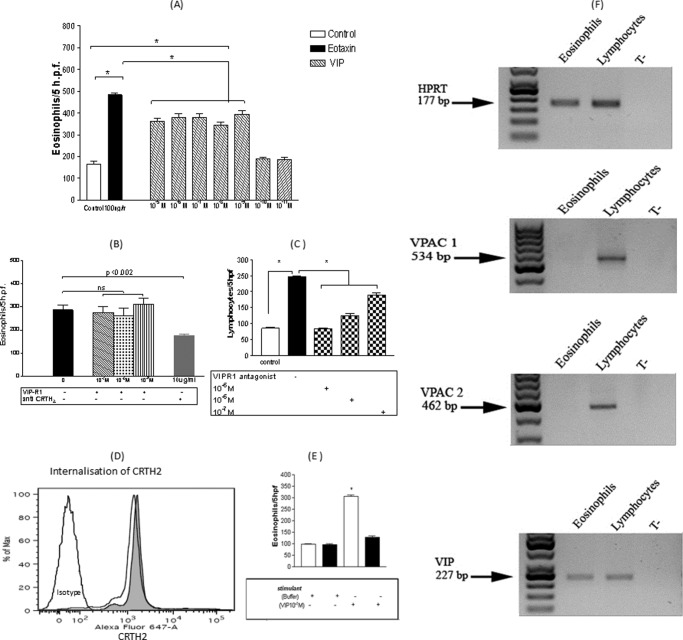
FIGURE 3.
A, VIP eosinophilotactic activity compared with eotaxin. Results are ± S.E. of 10 independent experiments performed in triplicate. *, p < 0.05. B, modulatory effects of VIP-R1 and anti-CRTH2 blocking antibody on 10−7 m VIP eosinophilotactic activity. Results are ± S.E. (error bars) of six independent experiments performed in triplicate. The culture medium consisted of a solution of Hanks' balanced salt solution for all treatments (buffer only, VIP-R1, and anti-CRTH2) during the chemotaxis assays. C, blocking effect of VIP-R1 on 10−7 m VIP-induced lymphocyte chemotaxis. Results are the mean ± S.E. (error bars) of five independent experiments performed in triplicate. *, p < 0.05. D, internalization of surface CRTH2 receptors in human eosinophils after a 5-min stimulation with 10−7 m PGD2. The gray histogram represents CRTH2 spontaneous expression, whereas the white histogram represents CRTH2 expression after stimulation with 10−7 m PGD2 for 5 min. E, effect of the treatment of eosinophils with 10−7 m PGD2, 5 min before and during the chemotaxis assay, against 10−7 m VIP (mean ± S.E. (error bars) of five independent experiments performed in triplicate). White bars represent migration in the absence of PGD2, whereas black bars represent migration in the presence of PGD2. F, RT-PCR showing expression of VPAC1 and VPAC2 by human lymphocytes but not human eosinophils, whereas VIP protein was expressed by both cell types.
In agreement with our earlier report from normal subjects (4), eosinophil chemotaxis from AR patients against VIP was not VIP receptor-mediated, as seen in Fig 3B. Intriguingly, pretreatment of eosinophils from AR patients with 10 μg/ml CRTH2 receptor antibody before inducing eosinophils chemotaxis against VIP did significantly inhibit VIP-induced eosinophil chemotaxis (Fig. 3B). These results pointed to a possible association between VIP and CRTH2 in mediating eosinophil chemotaxis and the lack of expression of VPAC1 by human eosinophils. To further explore this possibility, a search for VIP receptors in eosinophils was performed. As demonstrated by RT-PCR in Fig. 3F, eosinophils expressed VIP protein but did not express VPAC1 or VPAC2. Further, control experiments on human lymphocytes demonstrated the ability of VPAC1 to dose dependently mediate VIP-induced lymphocytes chemotaxis, as shown in Fig. 3C. Next we performed a competition assay between VIP and PGD2, the only known ligand to date for CRTH2. A 5-min stimulation of eosinophils with PGD2 resulted in quick internalization of CRTH2, as shown in Fig. 3D, and significantly reduced VIP-induced eosinophil chemotaxis, as shown in Fig. 3E.
Involvement of Ca2+-independent PKC and PKA in the Signal Transduction of VIP-induced Eosinophil Chemotaxis
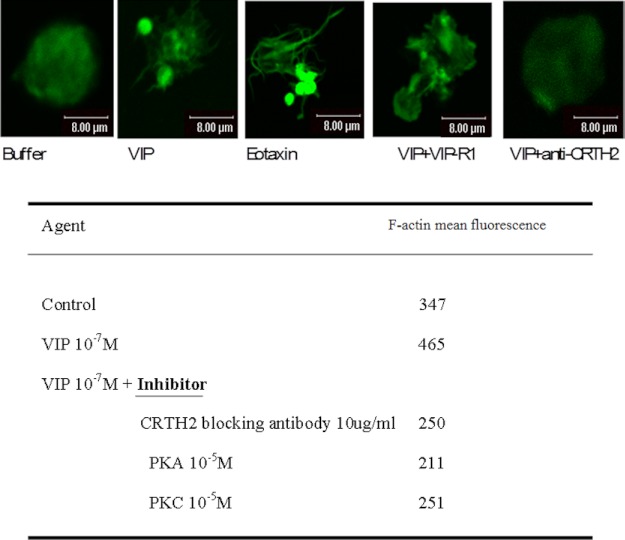
First we tested several protein kinase inhibitors for their ability to modify VIP-induced eosinophil chemotaxis. As seen in Table 2, PKC (bisindolylmaleimide) and PKA (H-89) inhibitors did block VIP-induced eosinophil chemotaxis, but neither p38-MAPK (SB203580) nor tyrosine kinase (genistein) inhibitors blocked VIP-induced eosinophil chemotaxis. The latter rather increased the VIP-induced eosinophil chemotaxis through up-regulation of CRTH2 surface expression (supplemental Fig. 3). Under Ca2+-intact or Ca2+-depleted conditions, VIP-induced eosinophil chemotaxis was equal (Table 2). Our results indicate the involvement of Ca2+-independent PKC and PKA activity in eosinophil's CRTH2 stimulation by VIP. There are two recognized isoforms of PKC that are Ca2+-independent, namely PKCδ and PKCϵ. To further study the signal transduction mechanism(s) involved in VIP stimulation of CRTH2, we utilized an eosinophil cell line (Eol-1) that expresses the CRTH2 receptor (22) and thus was ideal for the signal transduction studies. We performed Western blot experiments to confirm the involvement of PKC and PKA residue activation in Eol-1 cells following stimulation by VIP.
TABLE 2.
Effect of different protein kinase inhibitors on VIP-induced eosinophil chemotaxis
| Inhibitor | With Ca2+ |
p value | Without Ca2+ |
p value | ||
|---|---|---|---|---|---|---|
| −Inhibitor | +Inhibitor | −Inhibitor | +Inhibitor | |||
| H-89 dihydrochloride (10−5 m) | 329 ± 14 | 193 ± 21 | 0.0005 | 316 ± 12 | 169 ± 5 | 0.022 |
| Bisindolylmaleimide (10−5 m) | 329 ± 14 | 174 ± 9 | <0.0001 | 316 ± 12 | 150 ± 3 | 0.022 |
| SB203580 (10−5 m) | 329 ± 14 | 341 ± 16 | NSa | 316 ± 12 | 304 ± 4 | NS |
| Genistein (10−5 m) | 329 ± 14 | 381 ± 9 | 0.041 | 316 ± 12 | 321 ± 7 | NS |
a NS, not significant.
VIP Increases the PKCδ, PKCϵ, PKAα, PKAαIIreg, and PKAγ Membranous Level through CRTH2
We performed Western blots on whole cell extracts and membranous extracts of Eol-1 cells at low and high passages treated or not with VIP for 30 min. Data obtained on Eol-1 cells at high passages are represented in Fig. 4. Results showed that VIP did not modulate the CRTH2 level in the whole cell extracts after 30 min of stimulation. Similar results were observed in the Eol-1 cells at low passages (data not shown). However, the level of CRTH2 was higher in Eol-1 at high passages in comparison with Eol-1 at low passages (data not shown). The presence of CRTH2 was also studied in membranous extracts, but the protein could not be detected. As observed in Fig. 4A, VIP did not modify the absolute level of the different PKCs and PKAs studied (see whole cell extracts (WCE)). However, VIP clearly increased the level of these proteins in membranous extracts, particularly PKCϵ, PKAα, and PKAαIIreg (Fig. 4A, compare lane 2 with lane 1). These increases are significant, as shown in the graphs in Fig. 4B (quantification of membranous extracts). These effects seemed to be transient because at 24 h of treatment with VIP, the level of the different PKCs and PKAs stayed similar to that in the untreated cells (data not shown). Thus, it appears that VIP is able to induce the recruitment of the different PKCs and PKAs to the membrane after a short time of treatment of Eol-1 cells at high passages. Interestingly, in Eol-1 cells at low passages, the membranous localization of the different PKCs and PKAs was not modulated by the VIP treatment. This could be correlated with a lower level of CRTH2 protein in these cells in comparison with Eol-1 cells at high passages.
In order to identify the potential implication of CRTH2 in these events, we pretreated cells with a blocking antibody against CRTH2 before treatment with VIP. The pretreatment with this blocking antibody clearly blocked the effect of VIP on all PKCs and PKAs (compare lane 4 with lane 2). Thus, these results indicate that CRTH2 seems to be partially involved in the membranous recruitment of PKCδ, PKCϵ, PKAα, PKAαIIreg, and PKAγ induced by VIP.
VIP-induced Eosinophil Cytoskeletal Changes
Cell migration following exposure to chemoattractants is preceded by many processes, including cytoskeletal reorganization and cell shape changes. Rapid and reversible polymerization of globular monomeric actin into filamentous polymeric actin (F-actin) initiates shape changes. Therefore, we performed further experiments to check these cellular events. As demonstrated in Fig. 5, cells adherent to the collagen type 4-coated slides changed their shape with F-actin reorganization and increased their content from mean fluorescence of 347 for the control stimulation to 465 in response to 10−7 m VIP stimulation, as judged by FACS analysis. Similar results were obtained with other tested doses of VIP (10−5 to 10−9 m). Blockage of CRTH2 receptor but not VIP-R1 inhibited eosinophil shape changes and F-action reorganization and reduced the F-actin contents to 250. A similar reduction of F-actin contents to near basal levels were also observed with blockage of PKA and PKC (Fig. 5). Taken collectively, our data further support a specific chemotaxis signal for VIP in eosinophil that is mediated through the CRTH2 receptor and involves the PKA and PKC pathways. To this end, the issue of VIP receptors is in continuous evolution, and recently another variant of the five-transmembrane isoform of VIP receptors was identified (23). To the best of our knowledge, this is the first article to show a signal transduction of chemoattractant other than PGD2 and its derivative that may utilize the CRTH2 receptor on human eosinophils. To further elaborate on the exact fashion of ligation between VIP and CRTH2, we explored in the following experiments the possibilities of direct physical co-localization and binding as well as the indirect possibility through liberation of PGD2 by eosinophils in response to VIP.
FIGURE 5.
Confocal images of peripheral blood eosinophil shape changes from AR patients. Green, F-actin. Stimulant concentrations were as follows: VIP, 10−7 m; VIP-R1, 10−5 m; anti-CRTH2 antibody, 10 μg/ml. Similar images were obtained with other VIP concentrations (10−5 to 10−9 m) and VIP-R1 (10−5 to 10−7 m). Images are from one experiment representative of three, all showing similar results.
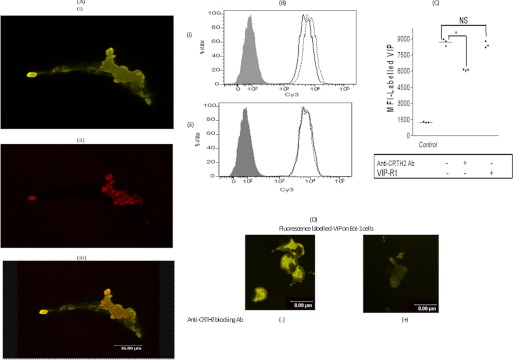
Strong Co-localization between VIP and CRTH2 with Reduction of VIP Binding to Eol-1 Cells by Anti-CRTH2 Blocking Ab
Confocal images in Fig. 6A demonstrated strong association and co-localization of VIP and CRTH2 molecules. This was further supported by FACS analysis (Fig. 6, B and C) and confocal images (Fig. 6D) that demonstrated significant reduction of VIP binding to Eol-1 in the presence of anti-CRTH2 receptor blocking Ab. Of note, no modulation of VIP binding was observed in the presence of VIP-R1 (Fig. 6, B and C). These results point to strong physical ligation between VIP and CRTH2 in eosinophils and may indicate specific binding of VIP to CRTH2 on human eosinophils.
FIGURE 6.
A, confocal images of one experiment representative of three, all showing similar images. i, Eol-1 stained with labeled-VIP (in green); ii, Eol-1 stained with anti-CRTH2 (in red); iii, both markers i and ii. Of note, the controls with unlabeled VIP and Cy3 dye alone did not show any fluorescence (supplemental Fig. 1). The Pearson's coefficient for the presented images was 0.2, as assessed by Microsoft co-localization software. B, histogram from one experiment representative of three, showing labeled VIP on the Eol-1 cell surface in the presence and absence of anti-CRTH2 Ab and anti-VIP receptor antagonist. i, gray, control; dotted line, VIP fluorescence; solid line, VIP fluorescence in the presence of anti-CRTH2 blocking Ab. ii, gray, control; dotted line, VIP fluorescence; solid line, VIP fluorescence in the presence of anti-VIP receptor antagonist (VIP-R1). C, mean fluorescence intensity (MFI) of labeled VIP on the surface of Eol-1 cells. Results are the mean ± S.E. of three independent experiments. D, confocal images of labeled VIP binding to Eol-1 cells in the absence (i) or presence (ii) of blocking anti-CRTH2 Ab. NS, not significant.
VIP Induces PGD2 Secretion by Eosinophils
Finally, to gain further insight about the possible indirect mechanisms by which VIP stimulates eosinophil chemotaxis through CRTH2, we cultured eosinophils from atopic subjects (n = 3) for 30 min and 24 h, with 10−7 m VIP. Supernatants were then collected, and the amount of PGD2 secreted was measured by ELISA. Interestingly, the mean percentage of PGD2 secreted by eosinophils increased upon stimulation with VIP for 30 min and 24 h from 100% to 105.19 and 140.17%, respectively (Table 3). These results may indicate the ability of VIP to stimulate CRTH2 through its PGD2 secretagouge activity in human eosinophils.
TABLE 3.
Time course of the percentage of PGD2 secreted in pg/ml by human eosinophils in response to VIP stimulation
| Stimulant |
|||
|---|---|---|---|
| Buffer | VIP | VIP + anti-VIP | |
| % | % | % | |
| 30 min | 100 | 105.2 | 96.03 |
| 24 h | 100 | 140.17 | 84.41 |
DISCUSSION
Eosinophilia is a hallmark in AR and is blamed for the chronicity of the disease. Therefore, the current interest in airway eosinophilic inflammation is to discover the molecular events resulting in eosinophil recruitment and hence to develop an effective mode of therapy. The biological effects of VIP presented herein on eosinophil chemotaxis and previous reports on its effect on mast cell chemotaxis, degranulation, and cytokine production (5, 24) indicate an important role for VIP in allergic inflammation of the airway. We showed that the allergic nasal tissue secretes VIP in doses that attracted human eosinophils in Boyden chambers. Further, the allergic nasal tissue was infiltrated with eosinophils, which significantly express VIP. Eosinophil treatment with VIP for 24 h up-regulated CRTH2 on human eosinophils and increased the amount of total CRTH2 protein. This was VPAC1- and VPAC2-independent and seems to be an autostimulation of CRTH2 in response to VIP ligation. This up-regulation was functional and resulted in exaggerated eosinophilotactic response against the suboptimal dose of PGD2 (22). This indicates that VIP is a potent primer for PGD2-induced eosinophil chemotaxis. This priming effect seems to be different from the priming effect of IFN-γ and TNF-α that we reported earlier (22), which was through the functional up-regulation of CRTH2 expression, because neither IFN-γ nor TNF-α modulated the total amount of CRTH2 protein.
The expression patterns of CRTH2 by human eosinophils showed heterogeneous populations, with some populations expressing higher amounts of the receptor than others (Fig. 1, B and C). To exclude the possibility of either contamination with other cell types or some cells being more activated than others, we double-stained eosinophils with CRTH2 and CD16. The latter can be induced in vitro by mediator stimulation and is temporarily expressed in asthmatics following allergen challenge. Our FACS results excluded any effect of CD16 acquired expression with this heterogeneous CRTH2 expression in the absence or presence of VIP. Further, the lack of expression of CD16 by Eol-1 cells also excluded such possibilities. We also excluded apoptosis/necrosis (data not shown) or cell aggregation as a reason for this heterogeneous pattern (supplemental Fig. 2C). We also induced the expression of CD48, a subtype of the CD2 Ig superfamily, by eotaxin stimulation. These molecules are up-regulated in allergy of the airways (25–28) and are inducible in eosinophils by eotaxin stimulation in vitro. The expression of CD48 molecules was clearly up-regulated in stimulated cells (supplemental Fig. 2A). Although both subpopulation of eosinophils expressed CD48 molecules, the fluorescence intensity of these molecules was higher in the subpopulation of eosinophils expressing greater amounts of CRTH2 (supplemental Fig. 2C). These results indicate that the eosinophil population expressing greater amounts of the CRTH2 receptor is more primed to subsequent stimuli. On the other hand, the ability of genistein to induce CRTH2 up-regulation, with three different heterogeneous populations in Eol-1 cells (supplemental Fig. 3), may indicate an interesting regulation of the rate of synthesis and/or recycling of CRTH2 by tyrosine kinase and/or phosphatase activity.
Chemokine-mediated signal transduction is believed to involve (i) Ca2+ mobilization, protein kinase C, and heterotrimeric GTP-binding proteins in a classical view (29) and (ii) kinases and phosphatases, adaptor proteins, and small GTP-binding proteins in an alternate view (30–33). Our data indicate that VIP-induced eosinophil chemotaxis was mediated through the CRTH2 receptor and that calcium per se was not involved as a second messenger in the signal transduction. This VIP-CRTH2 ligation involved a novel PKCϵ, PKCδ, PKAα, PKAαIIreg, and PKAγ cytosol-to-membrane translocation without altering the total contents of these proteins. To this end, the increased migration of eosinophils against VIP in the presence of genistein treatment is consistent with our earlier study that indicated modulation of eosinophil migration against different groups of chemoattractants by herbimycin A, erbstatin, and pervanadate (34). Further, genistein was able to increase the surface expression of CRTH2 on Eol-1 cells (supplemental Figs. 3 and 4), which may explain the increased migration of eosinophils against VIP in the presence of genistein and support our signal transduction results that pointed to association between VIP and CRTH2 in inducing eosinophil migration.
We next explored the possible mechanisms of association between VIP and CRTH2. Our confocal image and FACS analysis results indicate a strong physical association and co-localization between VIP and CRTH2 but not the VIP-R1 receptor. This is supported by the fact that eosinophils lack the expression of VPAC1 and -2 and that anti-CRTH2 Ab treatment of Eol-1 cells, but not VIP-R1, resulted in significant reduction of VIP binding. Further, PGD2 and VIP seemed to be competitive with human eosinophils in chemotaxis assays. On the other hand, VIP induced PGD2 secretion from human eosinophils. This effect was as early as 30 min and lasted up to 24 h and was blocked by anti-VIP Ab, indicating a specific secretagouge activity. This may further provide an indirect pathway of activating CRTH2 on human eosinophils by VIP and may explain the lack of the characteristic bell-shaped dose response of VIP-induced eosinophil chemotaxis shown in Fig. 3A.
In conclusion, to our best knowledge, this is the first article that links signaling of a neuropeptide (VIP) to CRTH2 on human eosinophils. Several separate lines of evidence point toward such an association between VIP and CRTH2 in inducing eosinophil chemotaxis as follows: (i) the ability of anti-CRTH2 blocking Ab to inhibit VIP-induced human eosinophil cytoskeletal changes and chemotaxis; (ii) the ability of anti-CRTH2 blocking Ab to block VIP-induced PKC and PKA membrane translocation in Eol-1 cells; (iii) the ability of anti-CRTH2 blocking Ab to significantly reduce VIP binding to eosinophils; (iv) the ability of VIP to induce protein synthesis of CRTH2 and its surface expression; and (v) the ability of VIP to induce PGD2 secretion by eosinophils.
Although it seems certain that CRTH2 modulates the biochemical events of VIP-induced eosinophils chemotaxis, the exact mode of VIP and CRTH2 interactions remains unclear. However, our results open channels for other researchers to further explore the possibility of specific binding of VIP to CRTH2 through further advanced binding assays, such as FRET, that are beyond the scope of this work.
Supplementary Material
Acknowledgments
We thank Patrick Roncarati (Department of Experimental Pathology, Liege University Hospital) for invaluable technical assistance with the RT-PCR. We also thank Dr. S. Ormenese (manager), R. Stephan (technician), and G. Moraes (imaging specialist) from the Platform GIGA-Cell Imaging and Flow Cytometry for support with flow cytometry and confocal microscopy. We also thank Dr. Jérôme Kroonen (Department of Human Genetics and GIGA Research Center (University of Liège) and Department of Neurosurgery and Integrated Cancer Center (University Medical Center of Utrecht)) for invaluable assistance and advice on the proximity ligation assay.
This work is supported by Centre Hospitalier Universitaire de Liege Grant 4717.

This article contains supplemental Figs. 1–4.
- VIP
- vasoactive intestinal peptide
- Ab
- antibody
- ACRS
- allergic chronic rhinosinusitis
- AR
- allergic rhinitis.
REFERENCES
- 1. Numao T., Agrawal D. K. (1992) Neuropeptides modulate human eosinophil chemotaxis. J. Immunol. 149, 3309–3315 [PubMed] [Google Scholar]
- 2. Dunzendorfer S., Meierhofer C., Wiedermann C. J. (1998) Signaling in neuropeptide-induced migration of human eosinophils. J. Leukoc. Biol. 64, 828–834 [DOI] [PubMed] [Google Scholar]
- 3. El-Shazly A. E., Masuyama K., Eura M., Ishikawa T. (1996) Immunoregulatory effect of substance P in human eosinophil migratory function. Immunol. Invest. 25, 191–201 [DOI] [PubMed] [Google Scholar]
- 4. El-Shazly A., Masuyama K., Tsunoda N., Eura M., Ishikawa T. (2000) Non-specific activation of human eosinophil functional responses by vasoactive intestinal peptide. Allergol. Int. 49, 19–26 [Google Scholar]
- 5. El-Shazly A., Berger P., Girodet P. O., Ousova O., Fayon M., Vernejoux J. M., Marthan R., Tunon-de-Lara J. M. (2006) Fraktalkine produced by airway smooth muscle cells contributes to mast cell recruitment in asthma. J. Immunol. 176, 1860–1868 [DOI] [PubMed] [Google Scholar]
- 6. Heppt W., Dinh Q. T., Cryer A., Zweng M., Noga O., Peiser C., Melvan M., Witt C., Fischer A., Groneberg D. A. (2004) Phenotypic alteration of neuropeptide-containing nerve fibres in seasonal intermittent allergic rhinitis. Clin. Exp. Allergy 34, 1105–1110 [DOI] [PubMed] [Google Scholar]
- 7. Fischer A., Wussow A., Cryer A., Schmeck B., Noga O., Zweng M., Peiser C., Dinh Q. T., Heppt W., Groneberg D. A. (2005) Neuronal plasticity in persistent perennial allergic rhinitis. J. Occup. Environ. Med. 47, 20–25 [DOI] [PubMed] [Google Scholar]
- 8. Metwali A., Blum A. M., Ferraris L., Klein J. S., Fiocchi C., Weinstock J. V. (1994) Eosinophils within the healthy or inflamed human intestine produce substance P and vasoactive intestinal peptide. J. Neuroimmunol. 52, 69–78 [DOI] [PubMed] [Google Scholar]
- 9. Ghatei M. A., Sheppard M. N., O'Shaughnessy D. J., Adrian T. E., McGregor G. P., Polak J. M., Bloom S. R. (1982) Regulatory peptides in the mammalian respiratory tract. Endocrinology 111, 1248–1254 [DOI] [PubMed] [Google Scholar]
- 10. Baraniuk J. N., Lundgren J. D., Okayama M., Mullol J., Merida M., Shelhamer J. H., Kaliner M. A. (1990) Vasoactive intestinal peptide in human nasal mucosa. J. Clin. Invest. 86, 825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harmar A. J., Arimura A., Gozes I., Journot L., Laburthe M., Pisegna J. R., Rawlings S. R., Robberecht P., Said S. I., Sreedharan S. P., Wank S. A., Waschek J. A. (1998) International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 50, 265–270 [PMC free article] [PubMed] [Google Scholar]
- 12. Nagata K., Tanaka K., Ogawa K., Kemmotsu K., Imai T., Yoshie O., Abe H., Tada K., Nakamura M., Sugamura K., Takano S. (1999) Selective expression of a novel surface molecule by human Th2 cells in vivo. J. Immunol. 162, 1278–1286 [PubMed] [Google Scholar]
- 13. Hirai H., Tanaka K., Yoshie O., Ogawa K., Kenmotsu K., Takamori Y., Ichimasa M., Sugamura K., Nakamura M., Takano S., Nagata K. (2001) Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 193, 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiraishi Y., Asano K., Nakajima T., Oguma T., Suzuki Y., Shiomi T., Sayama K., Niimi K., Wakaki M., Kagyo J., Ikeda E., Hirai H., Yamaguchi K., Ishizaka A. (2005) Prostaglandin D2-induced eosinophilic airway inflammation is mediated by CRTH2 receptor. J. Pharmacol. Exp. Ther. 312, 954–960 [DOI] [PubMed] [Google Scholar]
- 15. Royer J. F., Schratl P., Lorenz S., Kostenis E., Ulven T., Schuligoi R., Peskar B. A., Heinemann A. (2007) A novel antagonist of CRTH2 blocks eosinophil release from bone marrow, chemotaxis and respiratory burst. Allergy 62, 1401–1409 [DOI] [PubMed] [Google Scholar]
- 16. Uller L., Mathiesen J. M., Alenmyr L., Korsgren M., Ulven T., Högberg T., Andersson G., Persson C. G., Kostenis E. (2007) Antagonism of the prostaglandin D2 receptor CRTH2 attenuates asthma pathology in mouse eosinophilic airway inflammation. Respir. Res. 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nomiya R., Okano M., Fujiwara T., Maeda M., Kimura Y., Kino K., Yokoyama M., Hirai H., Nagata K., Hara T., Nishizaki K., Nakamura M. (2008) CRTH2 plays an essential role in the pathophysiology of Cry j 1-induced pollinosis in mice. J. Immunol. 180, 5680–5688 [DOI] [PubMed] [Google Scholar]
- 18. Okano M., Fujiwara T., Sugata Y., Gotoh D., Masaoka Y., Sogo M., Tanimoto W., Yamamoto M., Matsumoto R., Eguchi N., Kiniwa M., Isik A. U., Urade Y., Nishizaki K. (2006) Presence and characterization of prostaglandin D2-related molecules in nasal mucosa of patients with allergic rhinitis. Am. J. Rhinol. 20, 342–348 [DOI] [PubMed] [Google Scholar]
- 19. Nagata K., Hirai H. (2003) The second PGD2 receptor CRTH2. Structure, properties, and functions in leukocytes. Prostaglandins Leukot. Essent. Fatty Acids 69, 169–177 [DOI] [PubMed] [Google Scholar]
- 20. Narumiya S., Sugimoto Y., Ushikubi F. (1999) Prostanoid receptors. Structures, properties, and functions. Physiol. Rev. 79, 1193–1226 [DOI] [PubMed] [Google Scholar]
- 21. Ishihara K., Takahashi A., Kaneko M., Sugeno H., Hirasawa N., Hong J., Zee O., Ohuchi K. (2007) Differentiation of eosinophilic leukemia EoL-1 cells into eosinophils induced by histone deacetylase inhibitors. Life Sciences 80, 1213–1220 [DOI] [PubMed] [Google Scholar]
- 22. El-Shazly A., Moonen V., Mawet M., Begon D., Henket M., Arafa M., Louis R., Delvenne P., Lefebvre P. P. (2011) Inter. Immunopharmacol. 11, 1864–1870 [DOI] [PubMed] [Google Scholar]
- 23. Marchese A., Sawzdargo M., Nguyen T., Cheng R., Heng H. H., Nowak T., Im D. S., Lynch K. R., George S. R., O'dowd B. F. (1999) Discovery of three novel orphan G-protein-coupled receptors. Genomics 56, 12–21 [DOI] [PubMed] [Google Scholar]
- 24. Kulka M., Sheen C. H., Tancowny B. P., Grammer L. C., Schleimer R. P. (2008) Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 123, 398–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munitz A., Bachelet I., Eliashar R., Khodoun M., Finkelman F. D., Rothenberg M. E., Levi-Schaffer F. (2006) CD48 is an allergen and IL-3-induced activation molecule on eosinophils. J. Immunol. 177, 77–83 [DOI] [PubMed] [Google Scholar]
- 26. Muntiz A., Bachelet I., Finkelman F. D., Rothenberg M. E., Levi-Schaffer F. (2007) CD48 is critically involved in allergic eosinophilic airway inflammation. Am. J. Respir. Crit. Care. Med. 175, 911–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Munitz A., Bachelet I., Fraenkel S., Katz G., Mandelboim O., Simon H. U., Moretta L., Colonna M., Levi-Schaffer F. (2005) 2B4 (CD244) is expressed and functional on human eosinophils. J. Immunol. 174, 110–118 [DOI] [PubMed] [Google Scholar]
- 28. El-Shazly A. E., Henket M., Lefebvre P. P., Louis R. (2011) 2B4 (CD244) is involved in eosinophil adhesion and chemotaxis, and its surface expression is increased in allergic rhinitis after challenge. Int. J. Immunopathol. Pharmacol. 24, 949–960 [DOI] [PubMed] [Google Scholar]
- 29. Snyderman R., Pike M. C. (1984) Chemoattractant receptors on phagocytic cells. Annu. Rev. Immunol. 2, 257–281 [DOI] [PubMed] [Google Scholar]
- 30. Downey G. P. (1994) Mechanisms of leukocyte motility and chemotaxis. Curr. Opin. Immunol. 6, 113–124 [DOI] [PubMed] [Google Scholar]
- 31. Bokoch G. M. (1995) Chemoattractant signaling and leukocyte activation. Blood 86, 1649–1660 [PubMed] [Google Scholar]
- 32. Bacon K. B., Szabo M. C., Yssel H., Bolen J. B., Schall T. J. (1996) RANTES induces tyrosine kinase activity of stably complexed p125FAK and ZAP-70 in human T cells. J. Exp. Med. 184, 873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knall C., Worthen G. S., Johnson G. L. (1997) Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc. Natl. Acad. Sci. U.S.A. 94, 3052–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El-Shazly A., Masuyama K., Samejima Y., Eura M., Ishikawa T. (1998) Modulation of normal human eosinophil chemotaxis in vitro by herbimycin A, erbstatin and pervanadate. Int. Arch. Allergy Immunol. 117, 10–13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.