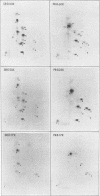
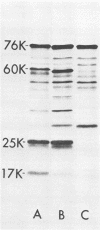
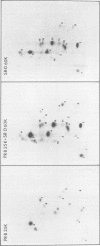
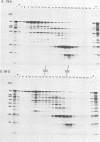
Abstract
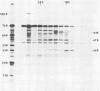
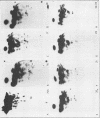
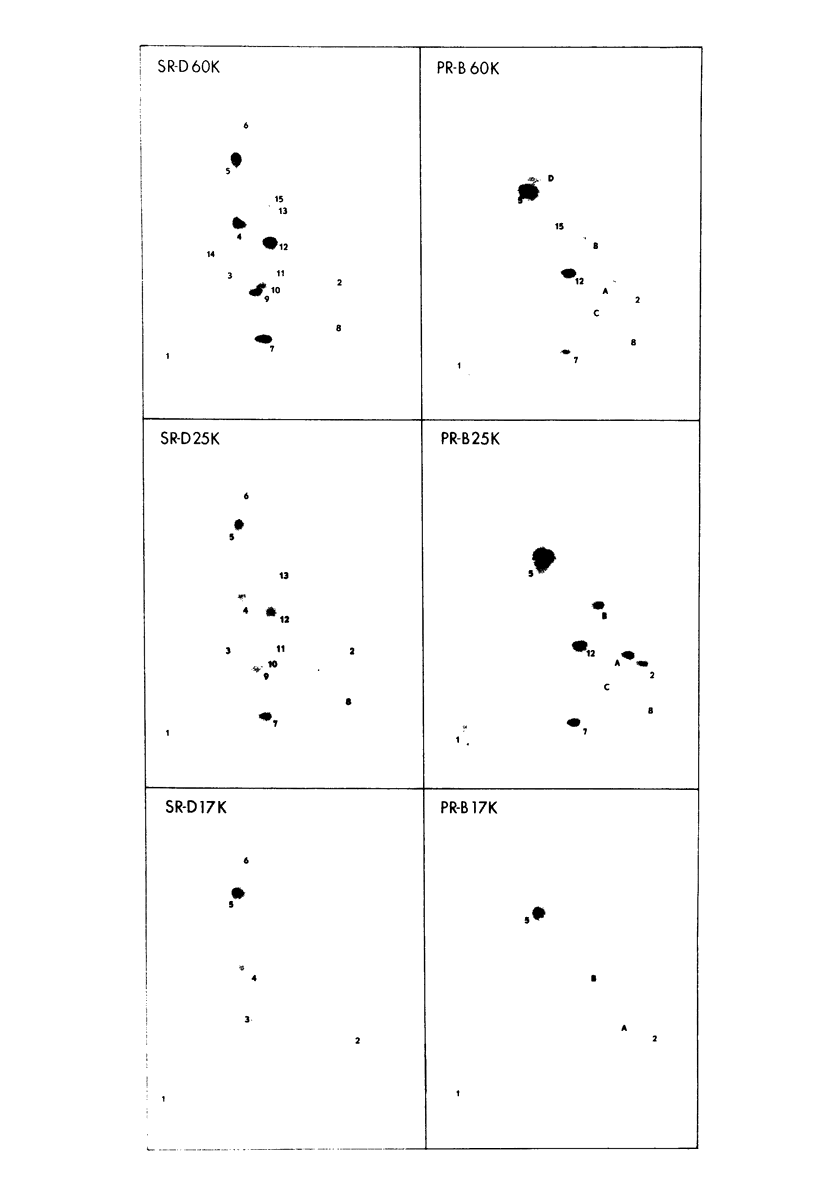
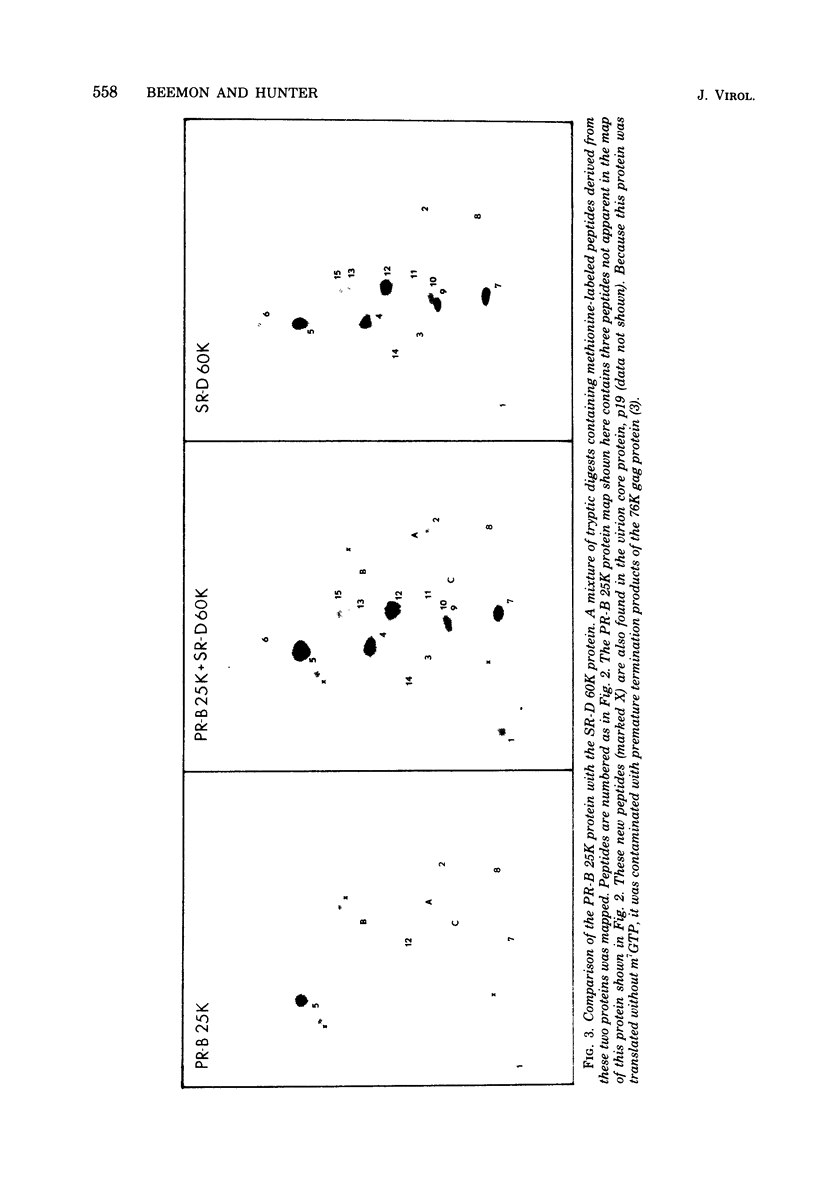
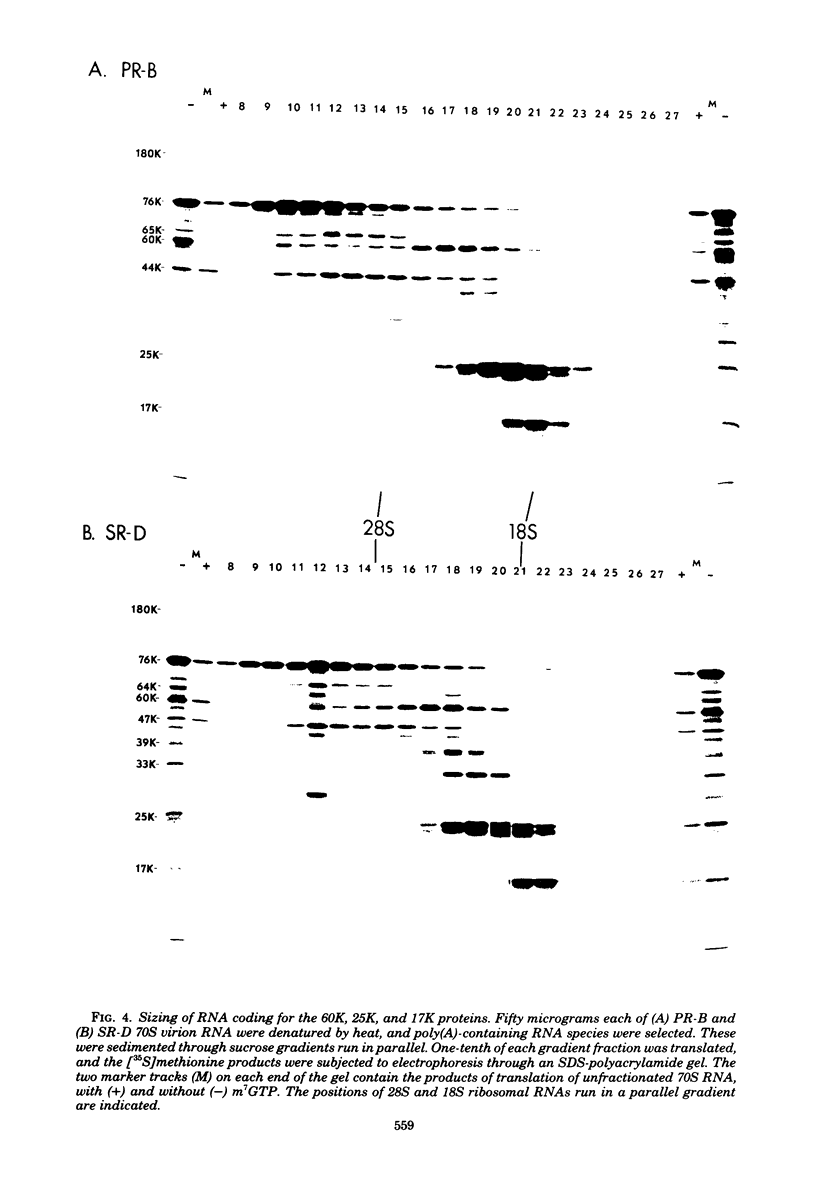
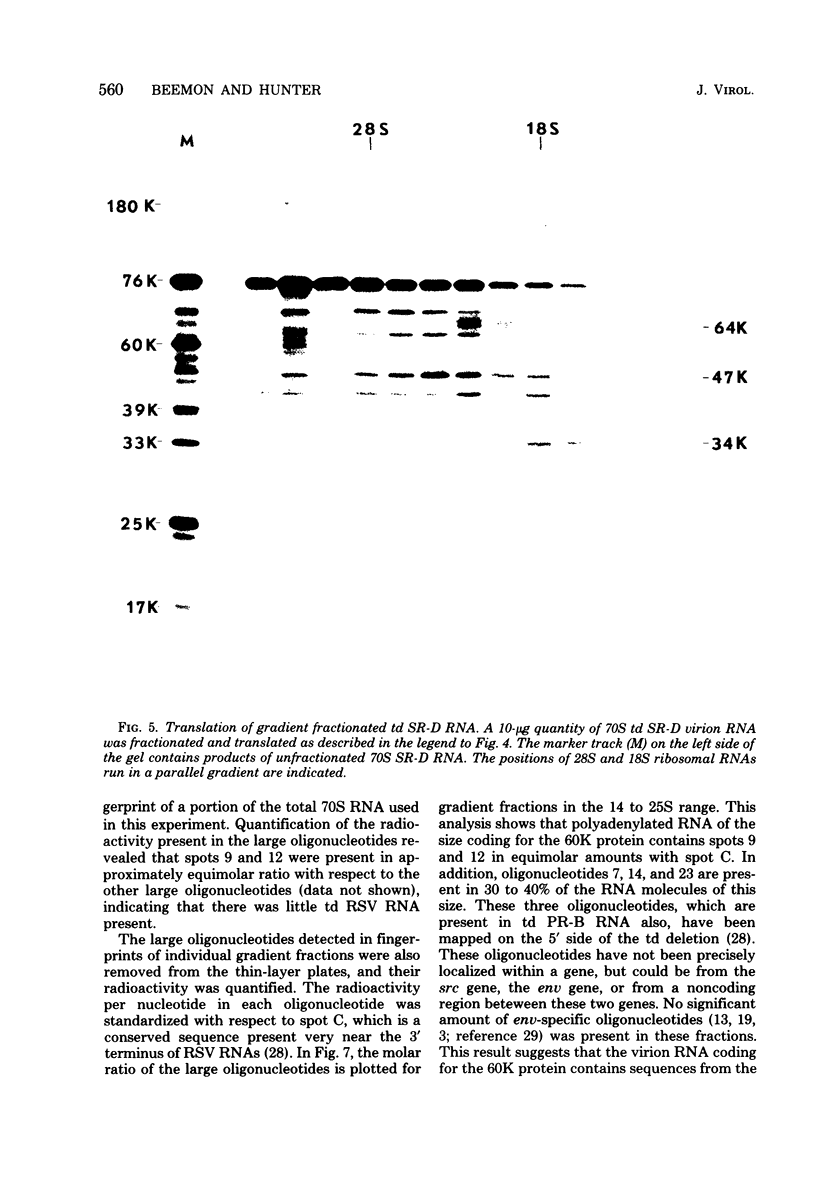
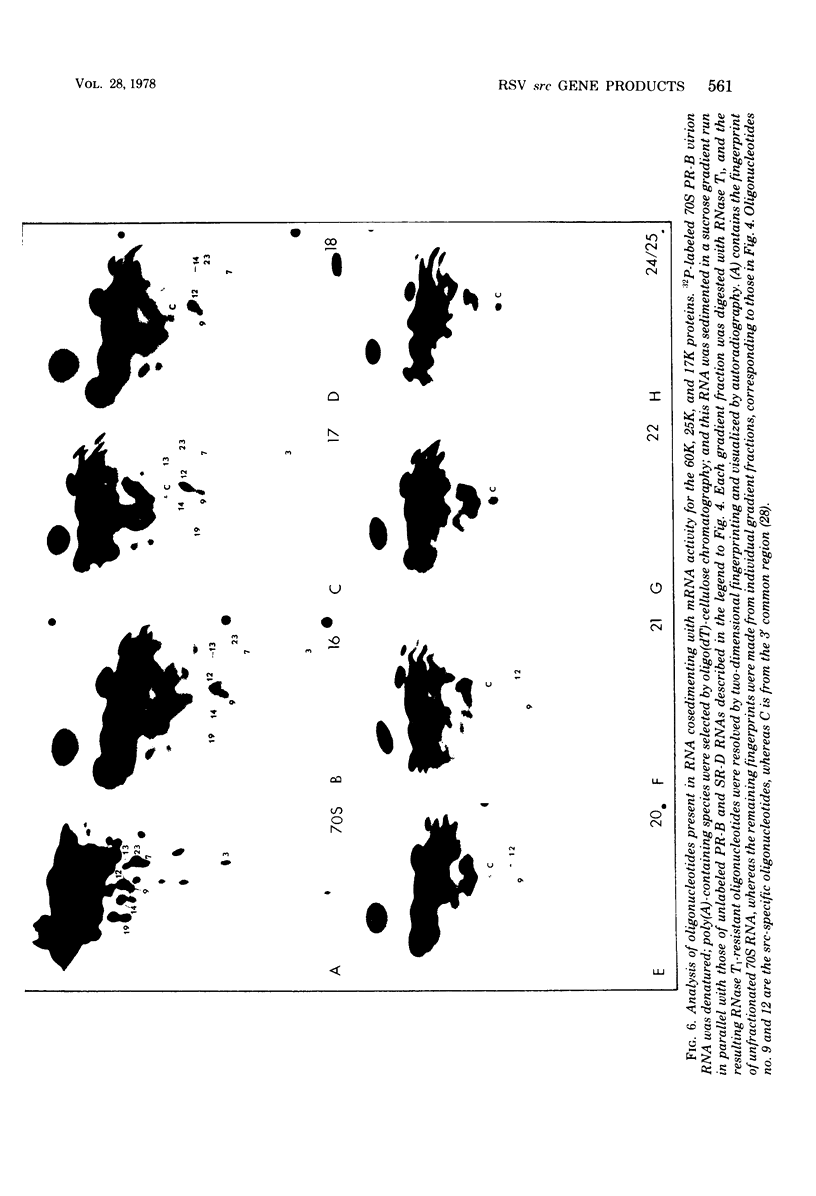
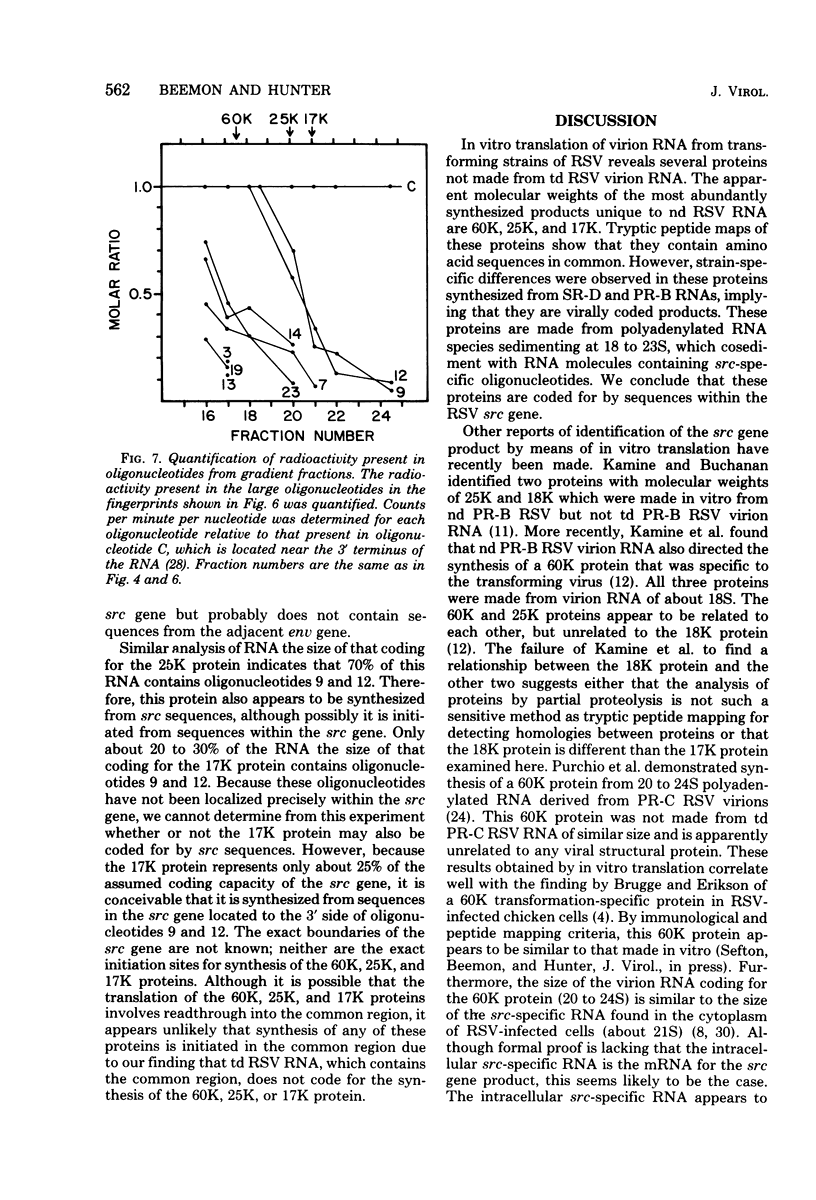
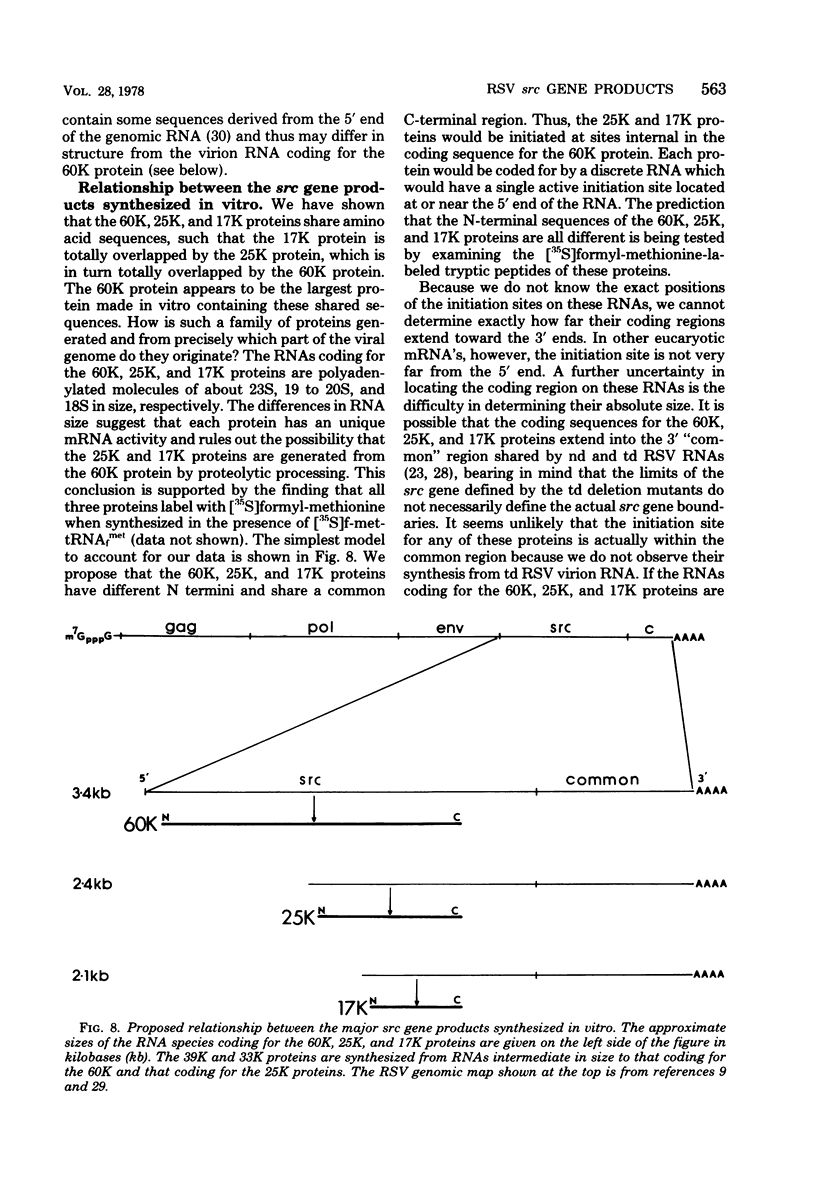
The cell-free synthesis of three major proteins from virion RNA of nondefective Rous sarcoma virus (RSV), but not from RNA of transformation-defective deletion mutants, has been observed. The apparent molecular weights of these transformation-specific proteins are approximately 60,000 (60K), 25K, and 17K. Tryptic maps of methionine-containing peptides revealed the 17K, 25K, and 60K proteins to be overlapping in sequence. However, only partial homology was observed between the 17K, 25K and 60K proteins synthesized from Schmidt-Ruppin strain, subgroup D, RSV RNA and those synthesized from Prague strain, subgroup B, RSV, RNA. About half of the methionine peptides in the Schmidt-Ruppin strain, subgroup D, 60K protein were shared with the Prague strain, subgroup D, 60K protein, and the rest were distinct to each. The virion RNAs coding for the 60K, 25K, and 17K proteins were found to be polyadenylated and to sediment with maximal mRNA activity at about 23, 19 to 20, and 18S, respectively. In addition, transformation-specific proteins with molecular weights of 39K and 33K were observed by in vitro synthesis. These proteins are also related to the 60K, 25K, and 17K proteins and were synthesized from polyadenylated RSV RNA of approximately 21 to 22S. RNase T1-resistant oligonucleotides were analyzed in parallel, and the src-specific oligonucleotides were found to be first present in equimolar amounts in those gradient fractions sedimenting at 21 to 22S. Our data suggest that synthesis of the 60K protein is initiated near the 5' terminus of the src gene, whereas the 39K, 33K, 25K, and 17K proteins are initiated internally in the src gene. All of these proteins appear to be initiated independently, but they may have a common termination site.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Ray D. A., Brown N. R. Accumulation of water during transformation of cells by an avian sarcoma virus. Cell. 1974 Nov;3(3):307–313. doi: 10.1016/0092-8674(74)90146-9. [DOI] [PubMed] [Google Scholar]
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T. In vitro translation yields a possible Rous sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3302–3306. doi: 10.1073/pnas.74.8.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Hu S., Knight C. A., Davidson N. Heteroduplex analysis of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):477–481. doi: 10.1073/pnas.74.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamine J., Buchanan J. M. Cell-free synthesis of two proteins unique to RNA of transforming virions of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1977 May;74(5):2011–2015. doi: 10.1073/pnas.74.5.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamine J., Burr J. G., Buchanan J. M. Multiple forms of sarc gene proteins from Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):366–370. doi: 10.1073/pnas.75.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Duesberg P. H., Hanafusa H. Transformation-defective mutants of Rous sarcoma virus with src gene deletions of varying length. J Virol. 1977 Dec;24(3):910–914. doi: 10.1128/jvi.24.3.910-914.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Hu S. S., Vogt P. K. Occurrence of partial deletion and substitution of the src gene in the RNA genome of avian sarcoma virus. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4781–4785. doi: 10.1073/pnas.74.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Marciani D. J., Papas T. S. Cell-free synthesis of the precursor polypeptide for avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4951–4954. doi: 10.1073/pnas.74.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Harvey R., Smith A. E. The size of Rous sarcoma virus mRNAs active in cell-free translation. Nature. 1977 Aug 4;268(5619):416–420. doi: 10.1038/268416a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Bercoff R., Billeter M. A. Characterization of the 3'-terminal region of the large molecular weight RNA subunits from normal and transformation-defective Rous sarcoma virus. Biochim Biophys Acta. 1976 Dec 1;454(2):383–388. doi: 10.1016/0005-2787(76)90240-9. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Mellon P., Vogt P. K. Distribution of envelope-specific and sarcoma-specific nucleotide sequences from different parents in the RNAs of avian tumor virus recombinants. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1073–1077. doi: 10.1073/pnas.73.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]