Background: Activation of TAK1 is an essential step in TNF- and IL-1-induced NF-κB activation pathways.
Results: DUSP14 dephosphorylates TAK1 at Thr-187 and negatively regulates TNF- and IL-1-induced NF-κB activation.
Conclusion: DUSP14 is a negative regulator of TNF- and IL-1-induced NF-κB activation.
Significance: Our study reveals a new post-translational regulatory mechanism of NF-κB activation triggered by the proinflammatory cytokines.
Keywords: Interleukin, NF-κB, Phosphorylation, Signal Transduction, Tumor Necrosis Factor (TNF), DUSP14, Dephosphorylation, TAK1
Abstract
The transcription factor NF-κB is critically involved in the inflammatory response triggered by the proinflammatory cytokines TNF and IL-1. Various studies have demonstrated that activation of TAK1 (TGF-β-activated kinase 1) is an essential step in TNF- and IL-1-induced NF-κB activation pathways. In this study, we identified a member of the dual-specificity phosphatase family, DUSP14, as a negative regulator of TNF- and IL-1-triggered NF-κB activation by expression screens. We found that DUSP14 interacted with TAK1 and that this interaction was enhanced by TNF or IL-1 stimulation. Overexpression of DUSP14 dephosphorylated TAK1 at Thr-187, a residue in the activation loop critically involved in TAK1 activation. Knockdown of DUSP14 increased basal as well as TNF- and IL-1-induced TAK1 phosphorylation at Thr-187. Overexpression of DUSP14, but not its phosphatase-deficient mutant, inhibited TNF- and IL-1-induced as well as TAK1-mediated NF-κB activation, whereas knockdown of DUSP14 had opposite effects. These findings suggest that DUSP14 negatively regulates TNF- or IL-1-induced NF-κB activation by dephosphorylating TAK1 at Thr-187. Our study reveals a new post-translational regulatory mechanism of NF-κB activation triggered by the proinflammatory cytokines.
Introduction
The transcription factor NF-κB plays critical roles in cell proliferation, inflammation, anti-apoptosis, and innate immunity (1, 2). In unstimulated cells, NF-κB is sequestered in the cytoplasm by its inhibitory proteins, which are members of the IκB family. Stimulation of various cell surface receptors, including receptors for proinflammatory cytokines such as TNF and IL-1, Toll-like receptors, and G protein-coupled receptors, results in phosphorylation and subsequently rapid ubiquitination and degradation of IκB proteins. Degradation of the IκB proteins liberates NF-κB and allows its translocation to the nucleus, where it controls the expression of downstream target genes (2, 3).
TAK1 (TGF-β-activated kinase 1) is a member of the MAPKKK family, which is activated not only by TGF-β but also by the proinflammatory cytokines TNF and IL-1. Upon TNF and IL-1 stimulation, TAK1 phosphorylates and activates the IκB kinase (IKK)2 kinase complex, leading to phosphorylation of IκBα and activation of NF-κB (4–6). Genetic studies using TAK1-deficient cells have demonstrated that TAK1 is an indispensable signaling intermediate in the TNF and IL-1 signaling pathways (6–8). In the proinflammatory signaling pathways, TAK1 is activated through ligand-dependent assembly of a TAK1 signaling complex containing TNF receptor-associated factor (TRAF) and TAK1-binding proteins TAB1–TAB3 (8–14).
The kinase activation loop of TAK1 contains phosphorylation sites at Thr-184, Thr-187, and Ser-192 (6, 15). Among the phosphorylation sites in the TAK1 activation loop, it has been demonstrated that phosphorylation at Thr-187 correlates with activation of TAK1 (15, 16), and stimulation by proinflammatory cytokines increases phosphorylation of TAK1 within the activation loop. The catalytic activity of TAK1 is required for this phosphorylation, suggesting that TAK1 autophosphorylates its activation loop (8, 15, 16). TAK1 is activated in a transient manner. Both TNF and IL-1 activate TAK1 within 1–2 min, and the activation peaks at 3–5 min and declines to the basal level within 15–30 min after stimulation (5–8).
Reversible protein phosphorylation, mediated by a plethora of protein kinases and phosphatases, is an important regulatory mechanism in a wide variety of biological processes such as cell development, differentiation, and transformation in higher eukaryotes (17). Previous studies suggest that phosphorylation and dephosphorylation are critically involved in regulation of several key components of the TNF- or IL-1-triggered NF-κB signaling pathways (17, 18).
Dual-specificity phosphatases are a heterogeneous group of protein phosphatases that participate in the regulation of divergent biological and pathological processes. This group of phosphatases can remove phosphate groups from critical phosphothreonine, phosphoserine, and phosphotyrosine residues on kinases, which generally leads to their deactivation. The phosphate-binding pocket of the DUSP family is formed by a loop containing the active-site motif HCXXGXXRS(T), where X is a variable residue, and catalysis is initiated by a conserved cysteine (19).
DUSP14 is a member of the DUSP family; it is 198 amino acid residues in length and is composed of a single catalytic phosphatase domain. The phosphate-binding loop in DUSP14 is composed of the sequence H110CAAGVSR117, in which cysteine 111 is the catalytic site. Previous studies suggest that conversion of cysteine 111 to serine (C111S) abrogates the ability of DUSP14 to dephosphorylate its substrates (19, 20).
In this study, we identified DUSP14 as an inhibitor of TNF- and IL-1-induced NF-κB activation pathways by expression screens. We also found that DUSP14 dephosphorylated TAK1 at Thr-187 within its kinase activation loop. Our findings suggest that DUSP14 acts as a TAK1 phosphatase to negatively regulate TNF- and IL-1-induced NF-κB activation.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Recombinant TNF-α and IL-1β (R&D Systems); horseradish peroxidase-conjugated anti-mouse IgG and anti-rabbit IgG (Thermo Fisher Scientific); mouse monoclonal antibodies against FLAG (Sigma), HA (OriGene), β-actin (Sigma), and Thr-187-phosphorylated TAK1, phospho-IκBα, phospho-IKKα/β, and phospho-TBK1 (Cell Signaling); and rabbit polyclonal antibody against DUSP14 (Abcam) were purchased from the indicated companies. Mouse antisera against TAK1 and IκBα were raised against the respective recombinant human full-length proteins.
Cell Culture and Transfection
The 293 and HeLa cells were cultured in DMEM supplemented with 10% fetal bovine serum (HyClone). Transfection of 293 cells was carried out by the standard calcium phosphate precipitation method.
Constructs
NF-κB- and IFN-β promoter-luciferase reporter plasmids and mammalian expression plasmids for HA-tagged TAK1, TAB1–TAB3, IKKα/β/γ, TRAF2, and TRAF6 were described previously (21). Mammalian expression plasmid for FLAG-tagged DUSP14 was constructed by standard molecular biology techniques. Mammalian expression plasmid for FLAG-tagged DUSP14(C111S) was constructed by standard site-directed mutagenesis.
Expression Cloning
The cDNA expression clones encoding 3000 mouse proteins were obtained from OriGene. The clones were transfected together with the NF-κB-luciferase reporter plasmid into 293 cells. Sixteen hours after transfection, cells were treated with TNF (10 ng/ml) or IL-1 (10 ng/ml) or left untreated for 8 h. The clones that inhibited TNF- or IL-1-triggered NF-κB activation were identified for further study.
Luciferase Reporter Assays
The 293 or HeLa cells (1 × 105) were seeded on 24-well plates and transfected 16 h later. In these experiments, empty control plasmid was added to ensure that the same amount of total DNA was transfected into each well. To normalize for transfection efficiency, 0.05 μg of pRL-TK Renilla luciferase reporter plasmid was added to each transfection. Luciferase assays were performed using a Dual-Luciferase assay kit (Promega). Firefly luciferase activities were normalized basal on Renilla luciferase activities.
Co-immunoprecipitation and Immunoblot Analysis
For transient transfection and co-immunoprecipitation experiments, 293 cells (1 × 106) were transfected for 24 h. The transfected cells were lysed in 1 ml of lysis buffer (20 mm Tris (pH 7.5), 150 mm NaCl, 1% Triton, 1 mm EDTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mm phenylmethylsulfonyl fluoride). For each immunoprecipitation, a 0.9-ml aliquot of lysate was incubated with 0.5 μg of the indicated antibody and 25 μl of a 1:1 slurry of GammaBind G Plus-Sepharose (Amersham Biosciences) for 4 h. The Sepharose beads were washed three times with 1 ml of lysis buffer containing 500 mm NaCl. The precipitates were analyzed by standard immunoblot analysis.
For endogenous immunoprecipitation experiments, 293 cells (5 × 107) were stimulated with TNF (10 ng/ml) or IL-1 (10 ng/ml) for the indicated times or left untreated. The subsequent procedures were carried out as described above.
RNAi Experiments
Double-stranded oligonucleotides corresponding to the target sequences were cloned into the pSUPER.retro RNAi plasmid (Oligoengine). The following sequences were targeted for human DUSP14 mRNA: 1) 5′-CCATTGAGATCCCTAATTT-3′; 2) 5′-CCATTGGACTGTACTTTGA-3; and 3) 5′-GATTTCCGAGGGAGACATA-3.
RNAi-transduced Stable HeLa Cells
The 293 cells were transfected with two packaging plasmids (pGag-Pol and pVSV-G) and the GFP control RNAi or DUSP14 RNAi plasmid at a ratio of 3:1:3 by the standard calcium phosphate precipitation method. Cells were washed 12 h after transfection, and new medium without antibiotics was added. After 48 h, the recombinant virus-containing medium was filtered and used to infect HeLa cells in the presence of Polybrene (4 μg/ml). After 24 h, the medium was replaced with complete medium. The infected HeLa cells were selected using puromycin (0.5 μg/ml) before additional experiments were performed.
RESULTS
Identification of DUSP14 as a Negative Regulator of TNF- and IL-1-induced NF-κB Activation
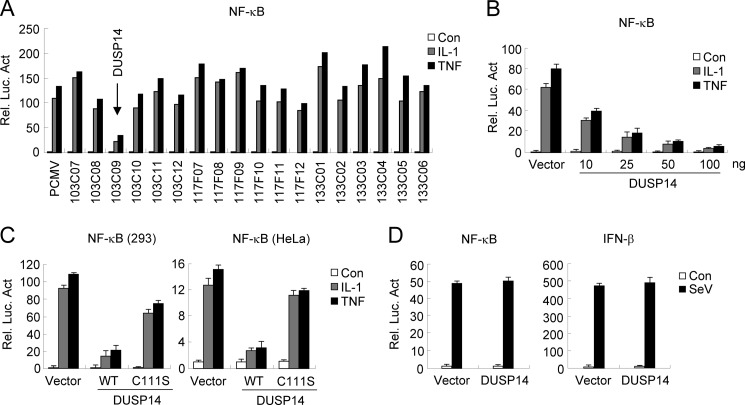
TNF and IL-1 are critically involved in inflammation by activating the transcription factor NF-κB. To better understand how TNF- and IL-1-induced NF-κB activation pathways are regulated at the molecular level, we screened a mouse cDNA library containing 3000 individual expression clones using NF-κB reporter assays. In the screening experiments, TNF- and IL-1-induced NF-κB activation was markedly inhibited by overexpression of DUSP14 (clone 103C09) (Fig. 1A). The ability of DUSP14 to inhibit TNF- and IL-1-induced NF-κB activation was dose-dependent in reporter assays in 293 cells (Fig. 1B). To determine whether the phosphatase activity of DUSP14 is required for its ability to inhibit TNF- and IL-1-induced NF-κB signaling, we examined the effects of the DUSP14 enzyme-inactive mutant C111S on TNF- and IL-1-induced NF-κB activation. As shown in Fig. 1C, DUSP14(C111S) lost the ability to inhibit TNF- and IL-1-induced NF-κB activation compared with wild-type DUSP14. Similarly, DUSP14, but not DUSP14(C111S), also inhibited TNF- and IL-1-induced NF-κB activation in HeLa cells (Fig. 1C). In reporter assays, DUSP14 did not inhibit Sendai virus (SeV)-induced activation of NF-κB and the IFN-β promoter (Fig. 1D). These results suggest that DUSP14 specifically down-regulates TNF- and IL-1-induced NF-κB activation.
FIGURE 1.
Identification of DUSP14 as a negative regulator of TNF- and IL-1-induced NF-κB activation. A, DUSP14 inhibits TNF- and IL-1-induced NF-κB activation. We screened 3000 individual mouse cDNA clones using reporter assays in 293 cells for their ability to regulate TNF- or IL-1-induced NF-κB activation. A fraction of the data are shown. Clone 103C09 encodes DUSP14. B, overexpression of DUSP14 inhibits TNF- and IL-1-induced NF-κB activation in a dose-dependent manner. The 293 cells (1 × 105) were transfected with NF-κB-luciferase reporter plasmid (20 ng) and increased amounts of DUSP14 expression plasmid. Sixteen hours after transfection, cells were left untreated or treated with TNF (10 ng/ml) or IL-1 (10 ng/ml) for 8 h before luciferase assays were performed. Graphs show means ± S.D. (n = 3). C, DUSP14, but not DUSP14(C111S), inhibits TNF- and IL-1-induced NF-κB activation in both 293 and HeLa cells. Experiments were performed as described for B. Graphs show means ± S.D. (n = 3). D, DUSP14 has no effect on SeV-induced activation of NF-κB and the IFN-β promoter. The 293 cells (1 × 105) were transfected with the indicated reporter and DUSP14 expression plasmids for 16 h. The cells were then infected with SeV (multiplicity of infection = 0.1) or left untreated for 8 h before luciferase assays. Graphs show means ± S.D. (n = 3). Rel. Luc. Act., relative luciferase activity; Con, control.
Knockdown of DUSP14 Enhances TNF- and IL-1-induced NF-κB Activation
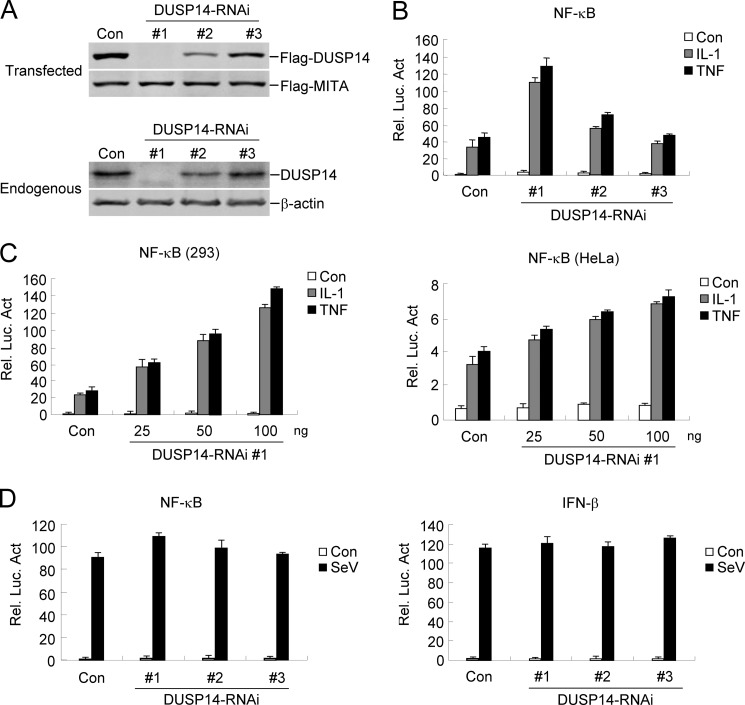
To determine whether DUSP14 regulates TNF- and IL-1-induced signaling under physiological conditions, we investigated the effects of knockdown of DUSP14 on TNF- and IL-1-triggered NF-κB activation. We constructed three human RNAi plasmids that target different sites of DUSP14 mRNA. Immunoblot analysis indicated that DUSP14 RNAi plasmid 1 markedly inhibited the expression of transfected and endogenous DUSP14 in 293 cells, whereas DUSP14 RNAi plasmids 2 and 3 had weak or no marked inhibitory effects on DUSP14 expression (Fig. 2A). In reporter assays, knockdown of DUSP14 expression potentiated TNF- and IL-1-induced NF-κB activation (Fig. 2B). The degrees of potentiation correlated with the efficiencies of knockdown of DUSP14 by each RNAi plasmid (Fig. 2, A and B). We chose DUSP14 RNAi plasmid 1 for additional experiments. In reporter assays, DUSP14 RNAi potentiated TNF- and IL-1-induced NF-κB activation in a dose-dependent manner in both 293 and HeLa cells (Fig. 2C). However, knockdown of DUSP14 did not potentiate SeV-induced activation of NF-κB or the IFN-β promoter (Fig. 2D). Collectively, these results suggest that DUSP14 is a negative regulator of TNF- and IL-1-induced NF-κB activation.
FIGURE 2.
Knockdown of DUSP14 enhances TNF- and IL-1-induced NF-κB activation. A, effects of DUSP14 RNAi plasmids on expression of DUSP14. Upper panels, the 293 cells (2 × 105) were transfected with FLAG-DUSP14 (0.2 μg each), FLAG-MITA (0.05 μg), and the indicated RNAi plasmids (1 μg). Thirty-six hours later, immunoblot analysis was performed with anti-FLAG antibody. Lower panels, the 293 cells (2 × 105) were transfected with the indicated RNAi plasmids (1 μg) for 48 h, and immunoblot analysis was then performed with the indicated antibodies. B, effects of DUSP14 RNAi plasmids on TNF- and IL-1-induced NF-κB activation. The 293 cells (1 × 105) were transfected with the NF-κB reporter (0.01 μg) and the indicated RNAi plasmids (0.5 μg). Thirty-six hours after transfection, cells were left untreated or treated with TNF (10 ng/ml) or IL-1 (10 ng/ml) for 8 h before luciferase assays were performed. The graph shows means ± S.D. (n = 3). C, dose-dependent effects of DUSP14 RNAi on TNF- and IL-1-induced activation of NF-κB in 293 (left panel) and HeLa (right panel) cells. The experiments were performed as described for B. Graphs show means ± S.D. (n = 3). D, effects of DUSP14 RNAi on SeV-triggered activation of NF-κB and the IFN-β promoter. The 293 cells (1 × 105) were transfected with the indicated reporter (0.05 μg) and DUSP14 RNAi plasmids (0.5 μg) for 36 h. Cells were then infected with SeV or left uninfected for 8 h before luciferase assays were performed. Graphs show means ± S.D. (n = 3). Rel. Luc. Act., relative luciferase activity; Con, control.
DUSP14 Interacts with TAK1
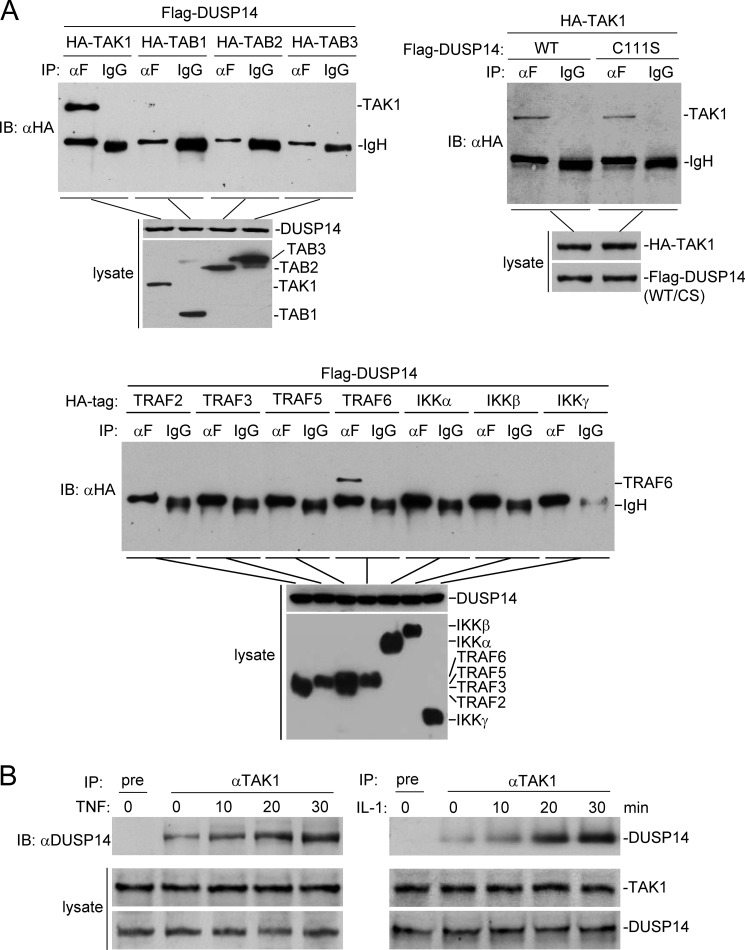
To investigate the regulatory mechanisms of DUSP14 in TNF- and IL-1-induced signaling, we examined the ability of DUSP14 to interact with known components in the TNF- and IL-1-induced NF-κB activation pathways. Transient transfection and co-immunoprecipitation experiments indicated that DUSP14 interacted strongly with TAK1 and weakly with TRAF6, but not with the other examined components in the TNF- and IL-1-induced NF-κB activation pathways, such as TAB1–TAB3 and IKKα/β/γ (Fig. 3A). Co-immunoprecipitation experiments also indicated that DUSP14 did not interact with some other members of the TRAF family, such as TRAF2, TRAF3, and TRAF5 (Fig. 3A). Compared with the DUSP14-TAK1 interaction, the DUSP1-TRAF6 interaction is much weaker. It is possible that DUSP14 interacts with TRAF6 indirectly via TAK1. The interaction between DUSP14 and TAK1 does not depend on the enzymatic activity of DUSP14 because DUSP14(C111S) was still able to interact with TAK1 (Fig. 3A). In untransfected cells, endogenous DUSP14 interacted with TAK1 weakly, and this interaction was increased upon TNF and IL-1 stimulation (Fig. 3B). These results suggest that DUSP14 interacts with TAK1 and that their interaction is enhanced following TNF or IL-1 stimulation.
FIGURE 3.
DUSP14 interacts with TAK1. A, DUSP14 interacts with TAK1 in the mammalian overexpression system. The 293 cells (2 × 106) were transfected with the indicated expression plasmids. Twenty-four hours after transfection, co-immunoprecipitation (IP) and immunoblot analysis (IB) were performed with the indicated antibodies (upper panels). Expression of the transfected proteins was analyzed by immunoblotting with anti-FLAG and anti-HA antibodies (lower panels). IgH, immunoglobulin heavy chain; CS, DUSP14(C111S). B, TNF and IL-1 induce association of DUSP14 and TAK1 in untransfected cells. The 293 cells (2 × 107) were left untreated or treated with TNF (10 ng/ml) or IL-1 (10 ng/ml) for the indicated times. Co-immunoprecipitation and immunoblot analysis were performed with the indicated antibodies. The levels of endogenous TAK1 and DUSP14 were detected by immunoblotting with mouse anti-TAK1 and rabbit anti-DUSP14 antibodies, respectively.
DUSP14 Dephosphorylates TAK1
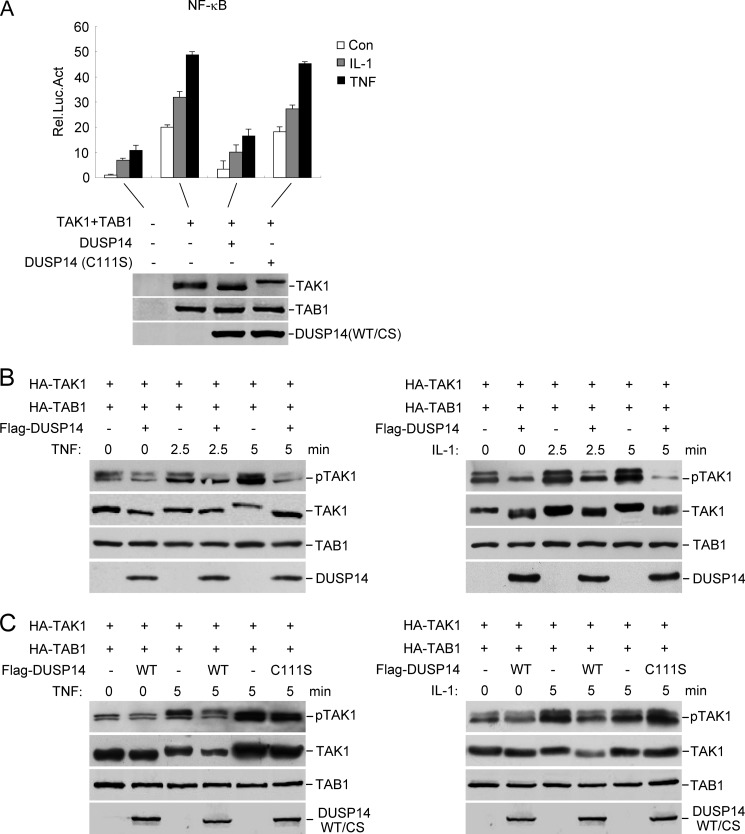
Because DUSP14 interacts with TAK1 and negatively regulates TNF- and IL-1-induced NF-κB activation, we reasoned that DUSP14 functions by dephosphorylating TAK1. Previously, it was reported that TAB1 is an activation subunit of TAK1 by enhancing phosphorylation of TAK1 at the activation loop and that overexpression of TAB1 enables TAK1 to activate NF-κB (9–11, 15, 16). As shown in Fig. 4A, wild-type DUSP14, but not the phosphatase-deficient mutant DUSP14(C111S), inhibited TAK1-mediated and TNF- and IL-1-induced NF-κB activation.
FIGURE 4.
DUSP14 dephosphorylates TAK1. A, the phosphatase activity of DUSP14 is required for its inhibitory effect on TAK1-mediated NF-κB activation. The 293 cells were transfected with the indicated plasmids, and NF-κB reporter assays were performed (upper panel). Expression of the transfected proteins was analyzed by immunoblotting with anti-FLAG and anti-HA antibodies (lower panel). The graph shows means ± S.D. (n = 3). Rel. Luc. Act., relative luciferase activity; Con, control; CS, DUSP14(C111S). B, DUSP14 dephosphorylates TAK1 at Thr-187. The 293 cells (4 × 105) were transfected with the indicated expression plasmids. Twenty-four hours after transfection, cells were left untreated or treated with TNF (10 ng/ml) or IL-1 (10 ng/ml) for the indicated times. Cell lysates were analyzed by immunoblotting with anti-phospho-TAK1 (Thr-187), anti-HA, and anti-FLAG antibodies. C, the phosphatase activity of DUSP14 is required for its ability to dephosphorylate TAK1 at Thr-187. The experiments were performed as described for B.
Because phosphorylation of Thr-187 in the activation loop of TAK1 is essential for the activation of TAK1 by TNF and IL-1, we examined whether DUSP14 dephosphorylates TAK1 at Thr-187. When overexpressed, TAK1 was phosphorylated at Thr-187 as detected by a phosphoamino acid-specific antibody and its slower migration (Fig. 4B). TNF or IL-1 stimulation further enhanced the phosphorylation of TAK1 at Thr-187. Overexpression of DUSP14 diminished basal and TNF- or IL-1-induced phosphorylation of TAK1 at Thr-187 (Fig. 4B). In similar experiments, the phosphatase-deficient mutant DUSP14(C111S) lost the ability to dephosphorylate TAK1 (Fig. 4C). These results suggest that DUSP14 dephosphorylates TAK1 at Thr-187 and that this activity is required for its inhibition of TNF- and IL-1-induced NF-κB activation.
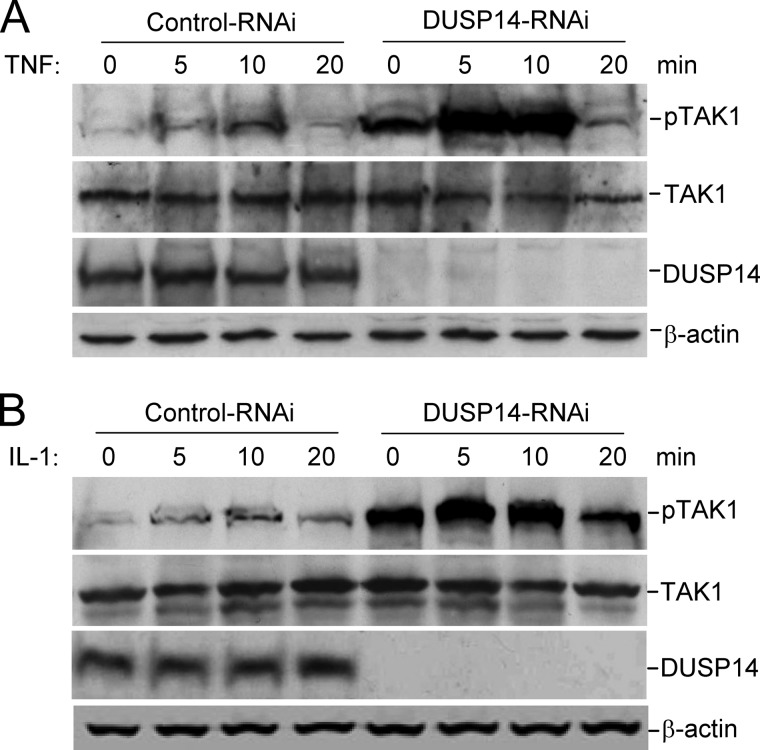
Knockdown of DUSP14 Potentiates Basal and TNF- or IL-1-induced Phosphorylation of Endogenous TAK1
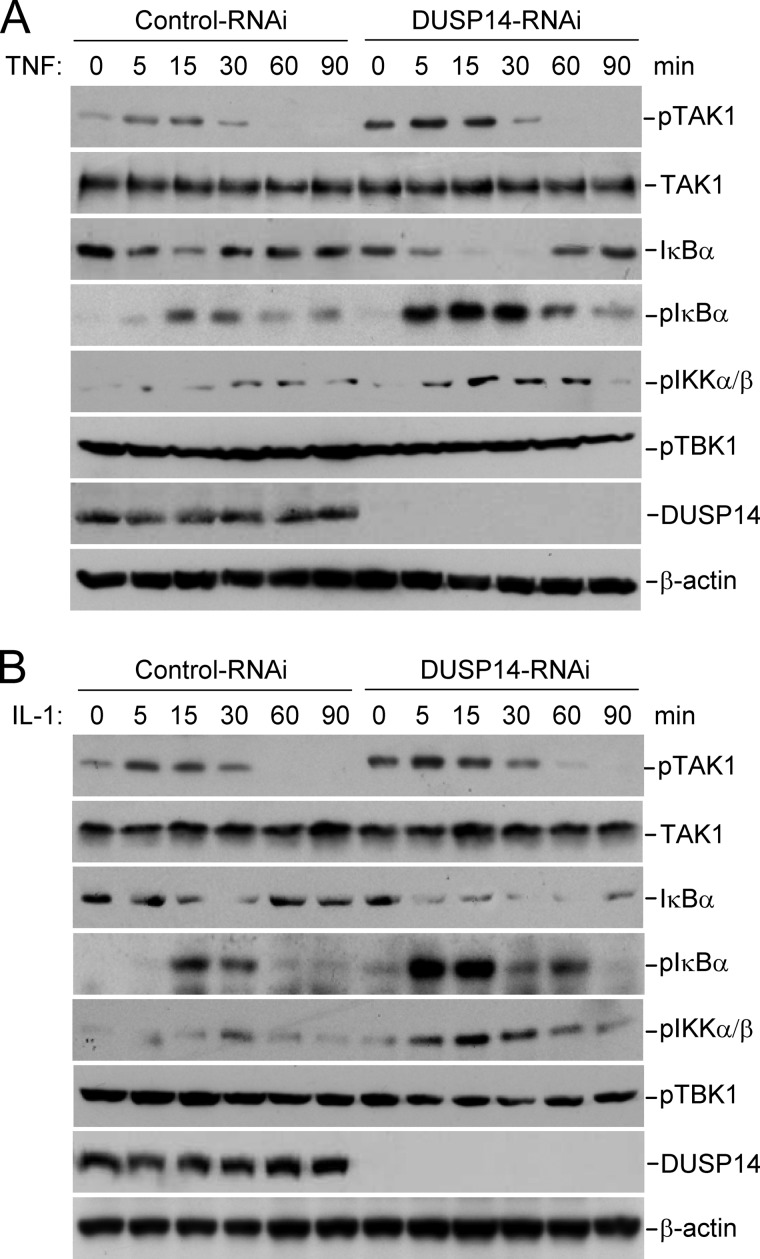
To determine whether DUSP14 plays a physiological role in dephosphorylating TAK1, we examined the effects of DUSP14 knockdown on TAK1 phosphorylation. We found that knockdown of DUSP14 enhanced basal and TNF- or IL-1-induced phosphorylation of endogenous TAK1 at Thr-187 in 293 cells (Fig. 5A) and HeLa cells (Fig. 6). Knockdown of DUSP14 consistently potentiated TNF- and IL-1-induced phosphorylation of IKK and IκBα and degradation of IκBα in HeLa cells (Fig. 6), which are hallmarks of TNF- and IL-1-induced downstream activation. In these experiments, knockdown of DUSP14 had no marked effect on TBK1 phosphorylation. These results suggest that DUSP14 specifically regulates TAK1 activation.
FIGURE 5.
Knockdown of DUSP14 enhances phosphorylation of TAK1 at Thr-187. The 293 cells were transfected with control or DUSP14 RNAi plasmid for 48 h. The cells were either left untreated or treated with TNF (10 ng/ml; A) or IL-1 (10 ng/ml; B) for the indicated times. Cell lysates were analyzed by immunoblotting with anti-phospho-TAK1 (Thr-187), anti-TAK1, anti-DUSP14, and anti-β-actin antibodies.
FIGURE 6.
Effects of DUSP14 knockdown on phosphorylation of TAK1, IKKα/β, and IκBα and degradation of IκBα in HeLa cells. HeLa cells stably transduced with control or DUSP14 RNAi plasmid were either left untreated or treated with TNF (10 ng/ml; A) or IL-1 (10 ng/ml; B) for the indicated times and then subsequently analyzed by immunoblotting with the indicated antibodies.
DISCUSSION
The transcription factor NF-κB plays critical roles in a broad range of physiological and pathological processes such as cell proliferation, immune regulation, inflammation, and anti-apoptosis (1–3). TNF and IL-1 are two cytokines that induce inflammation through activation of NF-κB (4, 5). Phosphorylation and dephosphorylation, mediated by many protein kinases and phosphatases, respectively, play important roles in regulating TNF- and IL-1-triggered NF-κB activation (3, 17, 18). In this study, we identified the phosphatase DUSP14 as a modulator of TNF- and IL-1-induced NF-κB activation pathways.
In the mammalian overexpression system, DUSP14 potently inhibited TNF- and IL-1-triggered NF-κB activation. In contrast, knockdown of endogenous DUSP14 potentiated TNF- and IL-1-induced NF-κB activation. These results suggest that DUSP14 negatively regulates TNF- and IL-1-triggered NF-κB signaling pathways.
Co-immunoprecipitation and immunoblot assays indicated that DUSP14 interacted with TAK1 in both overexpression and endogenous systems. TAK1 phosphorylation and activation are essential steps in TNF- and IL-1-induced NF-κB activation pathways (6–8). It has been demonstrated that phosphorylation of the conserved residue Thr-187 within the kinase activation loop of TAK1 is required for its activation (15, 16). Previous studies have suggested that phosphorylation and dephosphorylation are critically involved in regulation of several key components of TNF- or IL-1-triggered NF-κB signaling pathways, such as TAK1, IKKβ, and p65 (17, 18). Previous reports have demonstrated that protein phosphatases 6 and 2C are involved in suppression of TNF- and IL-1-induced TAK1 activation (15, 18, 22, 23). In this study, we found that overexpression of DUSP14 dephosphorylated TAK1 at Thr-187, whereas knockdown of DUSP14 potentiated this phosphorylation in unstimulated and TNF- or IL-1-stimulated cells. In addition, a phosphatase-deficient mutant of DUSP14, DUSP14(C111S), lost the ability to dephosphorylate TAK1 or inhibit TAK1-mediated NF-κB activation. Taken together, these findings suggest that DUSP14 negatively regulates TNF- or IL-1-induced NF-κB activation pathways by dephosphorylating TAK1 at Thr-187. Our study reveals a new post-translational regulatory mechanism of NF-κB activation triggered by the proinflammatory cytokines.
Acknowledgments
We thank members of our laboratory for technical help and stimulating discussions.
This work was supported by Chinese Ministry of Science and Technology Grants 2012CB910201 and 2010DFA31100, National Science Foundation of China Grants 91029302 and 30921001, and Chinese 111 Project Grant B06018.
- IKK
- IκB kinase
- TRAF
- TNF receptor-associated factor
- SeV
- Sendai virus.
REFERENCES
- 1. Siebenlist U., Franzoso G., Brown K. (1994) Structure, regulation and function of NF-κB. Annu. Rev. Cell Biol. 10, 405–455 [DOI] [PubMed] [Google Scholar]
- 2. Hayden M. S., Ghosh S. (2011) NF-κB in immunobiology. Cell Res. 21, 223–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vallabhapurapu S., Karin M. (2009) Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 [DOI] [PubMed] [Google Scholar]
- 4. Bouwmeester T., Bauch A., Ruffner H., Angrand P. O., Bergamini G., Croughton K., Cruciat C., Eberhard D., Gagneur J., Ghidelli S., Hopf C., Huhse B., Mangano R., Michon A. M., Schirle M., Schlegl J., Schwab M., Stein M. A., Bauer A., Casari G., Drewes G., Gavin A. C., Jackson D. B., Joberty G., Neubauer G., Rick J., Kuster B., Superti-Furga G. (2004) A physical and functional map of the human TNF-α/NF-κB signal transduction pathway. Nat. Cell Biol. 6, 97–105 [DOI] [PubMed] [Google Scholar]
- 5. Ninomiya-Tsuji J., Kishimoto K., Hiyama A., Inoue J., Cao Z., Matsumoto K. (1999) The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398, 252–256 [DOI] [PubMed] [Google Scholar]
- 6. Yamaguchi K., Shirakabe K., Shibuya H., Irie K., Oishi I., Ueno N., Taniguchi T., Nishida E., Matsumoto K. (1995) Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science 270, 2008–2011 [DOI] [PubMed] [Google Scholar]
- 7. Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., Akira S. (2005) Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 8. Takaesu G., Surabhi R. M., Park K. J., Ninomiya-Tsuji J., Matsumoto K., Gaynor R. B. (2003) TAK1 is critical for IκB kinase-mediated activation of the NF-κB pathway. J. Mol. Biol. 326, 105–115 [DOI] [PubMed] [Google Scholar]
- 9. Shibuya H., Yamaguchi K., Shirakabe K., Tonegawa A., Gotoh Y., Ueno N., Irie K., Nishida E., Matsumoto K. (1996) TAB1: an activator of the TAK1 MAPKKK in TGF-β signal transduction. Science 272, 1179–1182 [DOI] [PubMed] [Google Scholar]
- 10. Sakurai H., Miyoshi H., Mizukami J., Sugita T. (2000) Phosphorylation-dependent activation of TAK1 mitogen-activated protein kinase kinase kinase by TAB1. FEBS Lett. 474, 141–145 [DOI] [PubMed] [Google Scholar]
- 11. Ono K., Ohtomo T., Sato S., Sugamata Y., Suzuki M., Hisamoto N., Ninomiya-Tsuji J., Tsuchiya M., Matsumoto K. (2001) An evolutionarily conserved motif in the TAB1 C-terminal region is necessary for interaction with and activation of TAK1 MAPKKK. J. Biol. Chem. 276, 24396–24400 [DOI] [PubMed] [Google Scholar]
- 12. Takaesu G., Kishida S., Hiyama A., Yamaguchi K., Shibuya H., Irie K., Ninomiya-Tsuji J., Matsumoto K. (2000) TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol. Cell 5, 649–658 [DOI] [PubMed] [Google Scholar]
- 13. Jiang Z., Ninomiya-Tsuji J., Qian Y., Matsumoto K., Li X. (2002) Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol. Cell. Biol. 22, 7158–7167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheung P. C., Nebreda A. R., Cohen P. (2004) TAB3, a new binding partner of the protein kinase TAK1. Biochem. J. 378, 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kishimoto K., Matsumoto K., Ninomiya-Tsuji J. (2000) TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J. Biol. Chem. 275, 7359–7364 [DOI] [PubMed] [Google Scholar]
- 16. Singhirunnusorn P., Suzuki S., Kawasaki N., Saiki I., Sakurai H. (2005) Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-β-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding proteins TAB1 and TAB2. J. Biol. Chem. 280, 7359–7368 [DOI] [PubMed] [Google Scholar]
- 17. Karin M., Ben-Neriah Y. (2000) Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18, 621–663 [DOI] [PubMed] [Google Scholar]
- 18. Naumann M., Scheidereit C. (1994) Activation of NF-κB in vivo is regulated by multiple phosphorylations. EMBO J. 13, 4597–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patterson K. I., Brummer T., O'Brien P. M., Daly R. J. (2009) Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem. J. 418, 475–489 [DOI] [PubMed] [Google Scholar]
- 20. Lountos G. T., Tropea J. E., Cherry S., Waugh D. S. (2009) Overproduction, purification and structure determination of human dual-specificity phosphatase 14. Acta Crystallogr. D Biol. Crystallogr. 65, 1013–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Q., Yan J., Mao A. P., Li C., Ran Y., Shu H.-B., Wang Y. Y. (2011) Tripartite motif 8 (TRIM8) modulates TNFα- and IL-1β-triggered NF-κB activation by targeting TAK1 for K63-linked polyubiquitination. Proc. Natl. Acad. Sci. U.S.A. 108, 19341–19346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kajino T., Ren H., Iemura S., Natsume T., Stefansson B., Brautigan D. L., Matsumoto K., Ninomiya-Tsuji J. (2006) Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway. J. Biol. Chem. 281, 39891–39896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanada M., Ninomiya-Tsuji J., Komaki K., Ohnishi M., Katsura K., Kanamaru R., Matsumoto K., Tamura S. (2001) Regulation of the TAK1 signaling pathway by protein phosphatase 2C. J. Biol. Chem. 276, 5753–5759 [DOI] [PubMed] [Google Scholar]