Background: Mice lacking phosphatidylethanolamine N-methyltransferase (Pemt−/−) are glucose- and insulin-sensitive when fed a high fat diet.
Results: Increased plasma glucagon and hepatic gluconeogenesis occurred in Pemt−/− mice fed excess choline.
Conclusion: Supplementation of choline induces insulin intolerance in Pemt−/− mice via increased glucagon action.
Significance: The role of choline should be factored into our thinking about insulin resistance.
Keywords: Fatty Acid Oxidation, Insulin Resistance, Phosphatidylcholine, Phosphatidylethanolamine, Triacylglycerol, Glucagon Receptor, Gluconeogenesis
Abstract
Biosynthesis of hepatic choline via phosphatidylethanolamine N-methyltransferase (PEMT) plays an important role in the development of type 2 diabetes and obesity. We investigated the mechanism(s) by which choline modulates insulin sensitivity. PEMT wild-type (Pemt+/+) and knock-out (Pemt−/−) mice received either a high fat diet (HF; 60% kcal of fat) or a high fat, high choline diet (HFHC; 4 g of choline/kg of HF diet) for 1 week. Hepatic insulin signaling and glucose and lipid homeostasis were investigated. Glucose and insulin intolerance occurred in Pemt−/− mice fed the HFHC diet, but not in their Pemt−/− littermates fed the HF diet. Plasma glucagon was elevated in Pemt−/− mice fed the HFHC diet compared with Pemt−/− mice fed the HF diet, concomitant with increased hepatic expression of glucagon receptor, phosphorylated AMP-activated protein kinase (AMPK), and phosphorylated insulin receptor substrate 1 at serine 307 (IRS1-s307). Gluconeogenesis and mitochondrial oxidative stress were markedly enhanced, whereas glucose oxidation and triacylglycerol biosynthesis were diminished in Pemt−/− mice fed the HFHC diet. A glucagon receptor antagonist (2-aminobenzimidazole) attenuated choline-induced hyperglycemia and insulin intolerance and blunted up-regulation of phosphorylated AMPK and IRS1-s307. Choline induces glucose and insulin intolerance in Pemt−/− mice through modulating plasma glucagon and its action in liver.
Introduction
Phosphatidylcholine (PC)5 is made in mammalian cells via the choline pathway and the phosphatidylethanolamine N-methyltransferase (PEMT) pathway. The PEMT pathway is only quantitatively significant in liver (1). PEMT is responsible for ∼30% of hepatic PC biosynthesis, whereas the choline pathway generates the remaining 70% (2, 3). A link since choline and human diseases has attracted attention because choline deficiency was characterized in 1932 (4). Taking advantage of PEMT-deficient (Pemt−/−) mice (5), we have investigated the role of PEMT in mouse metabolism. Despite the lack of PEMT, the levels of hepatic PC and phosphatidylethanolamine in Pemt−/− mice remain unchanged when fed a chow diet (5). Pemt−/− mice develop normally and have normal bile secretion and composition (5). However, secretion of very low density lipoproteins is lower in cultured hepatocytes isolated from Pemt−/− compared with Pemt+/+ mice, demonstrating that PEMT is required for normal lipoprotein secretion (6). Recently we reported that Pemt−/− mice are protected from high fat (HF) diet-induced obesity and insulin resistance (7). This protection was eliminated when excess choline was added to the HF diet, indicating a possible link between choline and obesity/insulin resistance. Choline injection into rats increases plasma glucose, insulin, glucagon, and catecholamine (8–11). However, the mechanism(s) by which choline is linked to insulin sensitivity remains unexplained. We now report that choline causes an increase in plasma glucagon which induces glucose and insulin intolerance in Pemt−/− mice.
EXPERIMENTAL PROCEDURES
Animals
All procedures were approved by University of Alberta Institutional Animal Care Committee in accordance with guidelines of the Canadian Council on Animal Care. Male C57BL/6 Pemt+/+ and Pemt−/− mice had free access to standard chow (LabDiet 5001), HF diet (Bio-Serv F3282), or HF, high choline (HFHC) diet (2.7g of choline was added per kg of HF diet) for 1 week (7) Five mice were used in each group in the experiments. The basal choline mass in HF diet is 1.3 g/kg. We found that the protective effect of PEMT deficiency on 10-week HF-induced obesity and insulin resistance was abolished when 2.7 g/kg choline was supplemented into the HF diet (7). In the current study we used the same amount of choline supplementation. The glucagon receptor antagonist 2-aminobenzimidazole (50 mg/kg of body weight) was injected intraperitoneally every other day for 1 week into Pemt−/− mice fed the HFHC diet. Mice in the control groups received saline. Mice were fasted for 12 h before collection of blood by cardiac puncture. Tissues were stored at −80 °C until further usage.
Assessment of Lipid, Glycogen, Choline, Glucagon, and Insulin Levels
Triacylglycerol (TG) mass in liver was measured by gas-liquid chromatography (7, 12). Hepatic glycogen content was measured as described (13). Plasma insulin and glucagon were measured using commercial kits from Meso Scale Discovery. Plasma choline was quantified by AB Sciex 4000 Qtrap mass spectrometer coupled to an Agilent 1290 Liquid Chromatography system.
Insulin, Glucose, and Pyruvate Tolerance Tests
Mice were fasted for 6, 12, or 18 h, followed by intraperitoneal injection of 0.75 unit of human insulin/kg of body weight (Sigma), glucose (2 g/kg of body weight), or 2 g/kg sodium pyruvate (Sigma), respectively. Blood glucose was measured by a glucometer prior to injection and at the indicated times afterward.
Enzymatic Activity Assays
Activities of pyruvate dehydrogenase (PDH) and glycerol-3-phosphate acyltransferase (GPAT) were measured as described (14). Mitochondrial complex I/II activities were assessed by spectrophotometric methods (15).
Immunoblotting
Proteins from tissue lysates (cytosol or nuclei) were quantified using the Bradford method (Bio-Rad). Equal amounts of protein were subjected to electrophoresis and immunoblotted with rabbit polyclonal antibodies: PEPCK/PDK4/Mn-superoxide dismutase (Mn-SOD) (1:1000; Abcam); PGC-1α (1:1,000; Santa Cruz Biotechnology); total and phospho-IRS1-s307/AMPK/AKT/GSK/AS160/JNK/p38 MAPK (1:1000; Cell Signaling). Anti-tubulin (1:10,000, mouse monoclonal antibody; Sigma) or lamin A (1:1000, goat polyclonal antibody; Santa Cruz Biotechnology) was used as a loading control for cytosol and nuclear proteins, respectively.
Statistical Analysis
Data are means of five mice ± S.D. Analysis of variance was performed to compare means unless otherwise specified. A p value of < 0.05 was considered to be significant.
RESULTS
Glucose and Insulin Tolerance Are Impaired in Pemt−/− Mice Fed the HFHC Diet
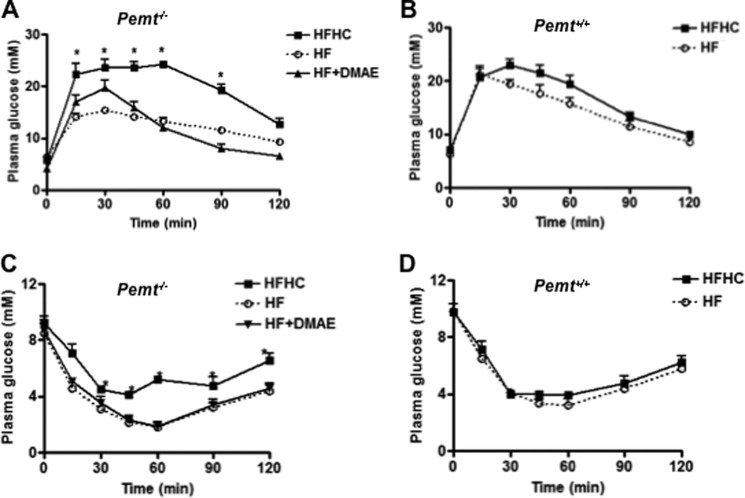
Pemt−/− mice developed glucose and insulin intolerance when fed the HFHC diet for 10 weeks (7). To explore the link between choline and impaired glucose/insulin tolerance, without development of obesity, Pemt−/− and Pemt+/+ mice were fed the HFHC or HF diet for 1 week. Body weight was not changed significantly in any of the mice (data not shown). However, systemic glucose clearance assessed by a glucose tolerance test (GTT) was significantly delayed in Pemt−/− mice fed the HFHC diet compared with littermates on the HF diet (Fig. 1A). No difference in the GTT was found in Pemt+/+ mice fed either diet (Fig. 1B). An intraperitoneal insulin tolerance test (ITT) showed that Pemt−/− mice fed the HFHC diet were less insulin-sensitive compared with their littermates fed the HF diet (Fig. 1C), whereas the diet did not alter the ITT in Pemt+/+ mice (Fig. 1D).
FIGURE 1.
Impaired glucose and insulin tolerance in Pemt−/− mice fed the HFHC diet. Pemt−/− mice were fed the HF or HFHC diet for 1 week. In one group of the mice, the additional choline in the HFHC diet was replaced by 2.7 g of dimethylethanolamine (DMAE), a choline analog. An intraperitoneal glucose tolerance test was performed in Pemt−/− mice (A) and in Pemt+/+ mice (B). An intraperitoneal insulin tolerance test was performed in Pemt−/− mice (C) and in Pemt+/+ mice (D). Data are means ± S.D. (error bars) from three independent experiments. *, p < 0.05, for HFHC compared with HF diet.
Choline-induced Hyperglycemia Is Choline-specific
Dimethylethanolamine, structurally related to choline, is converted to phosphatidyldimethylethanolamine, which can substitute for PC in membrane structure (16, 17). Despite this similarity in structure, phosphatidyldimethylethanolamine did not substitute for PC in preventing liver failure in Pemt−/− mice fed a choline-deficient diet (18). To understand whether glucose and insulin intolerance induced in Pemt−/− mice fed the HFHC diet was choline-specific, the same amount of dimethylethanolamine replaced the additional choline in the HFHC diet. After 1 week of feeding, dimethylethanolamine did not alter either the GTT (Fig. 1A) or the ITT (Fig. 1C). Thus, choline-induced glucose and insulin intolerance appear to be choline-specific.
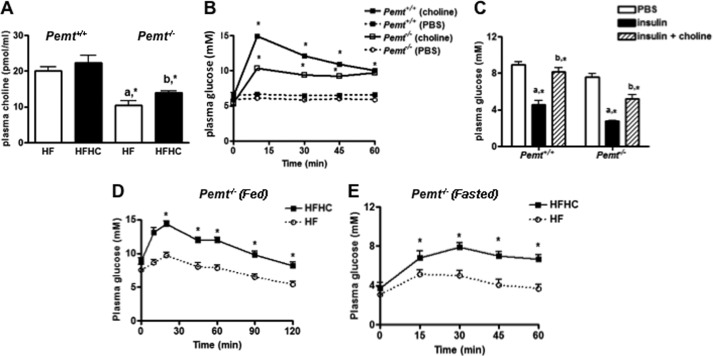
We next measured the concentration of plasma choline. Plasma choline levels were not significantly changed in Pemt+/+ mice on the HFHC diet compared with the littermates on the HF diet (Fig. 2A). In contrast, plasma choline levels were significantly elevated in Pemt−/− mice on the HFHC compared with the littermates on the HF diet (Fig. 2A). In addition, we also evaluated the effect of acute choline injection on plasma glucose in mice fed a chow diet. A dramatic increase in plasma glucose occurred 10 min after injection of choline both in Pemt+/+ and in Pemt−/− mice (Fig. 2B), and this enhancement was maintained for at least 60 min. Conversely, injection of dimethylethanolamine did not alter plasma glucose (data not shown). Furthermore, to determine whether choline-induced acute hyperglycemia was due to an antagonism of insulin action, choline was injected into mice 2 min prior to insulin. The effect of insulin on lowering plasma glucose was blunted both in Pemt+/+ and in Pemt−/− mice fed the chow diet supplemented with choline (Fig. 2C). To understand whether the choline-induced antagonism of insulin was due to altered coordination between glucagon and insulin, an intraperitoneal glucagon challenge was performed in Pemt−/− mice. Plasma glucose levels rapidly increased in Pemt−/− mice fed the HFHC diet under both fed (Fig. 2D) and fasted states (Fig. 2E). Glucose clearance was significantly delayed when compared with Pemt−/− mice fed the HF diet in both fed (Fig. 2D) and fasted state (Fig. 2E). Moreover, plasma glucagon levels were significantly elevated in Pemt−/− mice either after injection of choline or 1 week of the HFHC diet (Table 1). The ratio of glucagon to insulin was significantly higher in Pemt−/− mice but not in Pemt+/+ mice fed the HFHC diet compared with Pemt−/− littermates fed the HF diet (Table 1). Plasma insulin levels remained unchanged (Table 1). Thus, there appears to be a specific association between choline-induced hyperglycemia/insulin intolerance and elevated plasma glucagon.
FIGURE 2.
Plasma choline levels and choline-induced hyperglycemia. A, Pemt−/− or Pemt+/+ mice were fed the HF or HFHC diet for 1 week. Plasma choline was measured. a, *, p < 0.05, compared with Pemt+/+ mice fed on the same diet. b, *, p < 0.05, compared with the littermates fed the HF diet. B, choline (100 mg/kg of body weight) was injected intraperitoneally into Pemt+/+ or Pemt−/− mice fed a chow diet, and plasma glucose was measured at the indicated times.*, p < 0.05, compared with 0 min. C, 0.75 unit/kg insulin was injected into mice (intraperitoneally) 2 min before injection of choline, and plasma glucose was measured 10 min after choline injection. a, *, p < 0.05, compared with mice that received phosphate-buffered saline (PBS). b, *, p < 0.05, compared with mice receiving insulin. D and E, Pemt−/− mice were fed the HF or HFHC diet for 1 week. Plasma glucose was monitored after glucagon challenge (144 μg/kg of body weight, intraperitoneally) under fed condition (D) and after 12-h fasted condition (E). Data are means ± S.D. (error bars) from three independent experiments. *, p < 0.05, for HFHC compared with HF feeding.
TABLE 1.
Levels of plasma insulin (I) and glucagon (G) in Pemt+/+ and Pemt−/− mice after 1 week of either high fat or high fat/ high choline feeding
In another set of experiments, choline (100 mg/kg of body weight) was injected intraperitoneally into Pemt+/+ and Pemt−/− mice fed a chow diet, and 10 min later mice were sacrificed followed by the measurement of plasma I and G. The concentration of plasma insulin and glucagon (pg/ml) was measured, and the relative ratio of G/I was then calculated.
| Glucose or insulin | Chronic choline (1 week of feeding) |
Acute choline (injection, chow diet) |
||||||
|---|---|---|---|---|---|---|---|---|
|
Pemt+/+ |
Pemt−/− |
Pemt+/+ |
Pemt−/− |
|||||
| HF | HFHC | HF | HFHC | PBS | Choline | PBS | Choline | |
| I | 108.0 ± 14.0 | 99.7 ± 22.1 | 135.9 ± 17.9 | 135.2 ± 8.5 | 138.5 ± 55.2 | 89.9 ± 42.4 | 108.3 ± 32.5 | 191.9 ± 81.0 |
| G | 6.0 ± 0.5 | 7.1 ± 0.2a | 4.7 ± 0.9 | 7.3 ± 0.4a | 8.1 ± 0.8 | 8.3 ± 0.5 | 5.4 ± 0.4 | 9.1 ± 0.2b |
| G/I | 0.57 ± 0.12 | 0.75 ± 0.19 | 0.34 ± 0.03 | 0.56 ± 0.12a | 0.69 ± 0.33 | 1.17 ± 0.43 | 0.53 ± 0.12 | 0.54 ± 0.22 |
| Increment (fold) | ||||||||
| 1.00 ± 0.1 | 1.2 ± 0.2a | 1.0 ± 0.2 | 1.64 ± 0.3a | |||||
a p < 0.05 compared with high fat diet.
b p < 0.05 compared with mice that only received PBS.
Activation of Hepatic Glucagon Receptor Contributes to Choline-induced Glucose and Insulin Intolerance in Pemt−/− Mice Fed the HFHC Diet
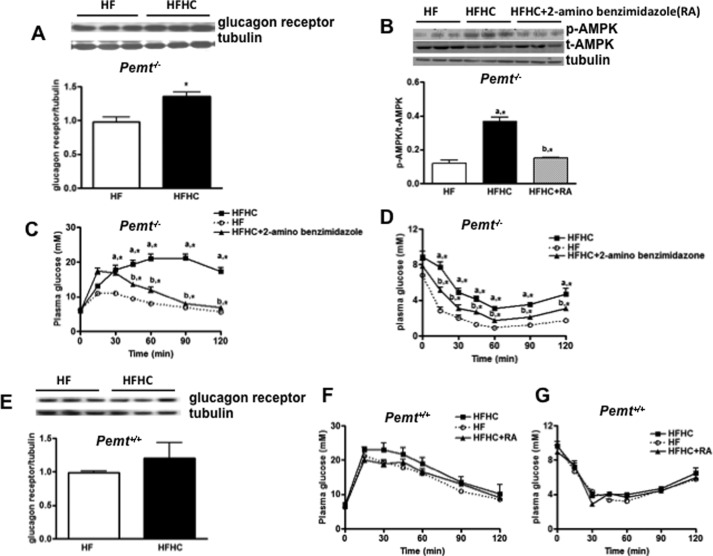
Glucagon signals through glucagon receptors, which have a crucial role in mediating hyperglycemia in diabetes (19, 20). Besides kidney, the liver expresses the most abundant glucagon receptors (19). Hence, we investigated a possible role of hepatic glucagon receptors in choline-induced glucose and insulin intolerance in Pemt−/− mice. In comparison with mice fed the HF diet, the glucagon receptor was significantly elevated in Pemt−/− mice fed the HFHC diet (Fig. 3A). Activation of the glucagon receptor induces AMPK signaling (21). Indeed, phosphorylation of AMPK (p-AMPK) was also enhanced in Pemt−/− mice on the HFHC diet (Fig. 3B). When 2-aminobenzimidazole, a glucagon receptor antagonist, was injected on alternate days for 1 week, increased p-AMPK was blunted (Fig. 3B), in conjunction with improved glucose (Fig. 3C) and insulin intolerance (Fig. 3D), in Pemt−/− mice fed the HFHC diet. In contrast, hepatic glucagon receptor (Fig. 3E), glucose (Fig. 3F), and insulin tolerance (Fig. 3G) were not altered in Pemt+/+ mice by fed the HFHC diet. These results suggest a direct involvement of hepatic glucagon receptors in choline-induced hyperglycemia and insulin intolerance.
FIGURE 3.
HFHC-induced glucose and insulin intolerance in Pemt−/− mice involves hepatic glucagon receptors and AMPK. Pemt−/− and Pemt+/+ mice were fed the HF or HFHC diet for 1 week. Representative immunoblots for the hepatic glucagon receptor in Pemt−/− mice (A) and Pemt+/+ mice (E) are shown. Glucagon receptor antagonist, 2-aminobenzimidazole (50 mg/kg of body weight), was injected intraperitoneally on alternate days into Pemt+/+ and Pemt−/− mice, followed by measurement of GTT in Pemt−/− (C) and Pemt+/+ mice (F), as well as ITT in Pemt−/− (D) and Pemt+/+ mice (G). B, representative immunoblots for hepatic phospho-AMPK and total AMPK are shown. Density values of p-AMPK were normalized to t-AMPK levels. a, *, p < 0.05, compared with HF-fed mice. b,*, p < 0.05, compared with HFHC-fed mice.
Choline Enhances Hepatic Gluconeogenesis and Blocks Hepatic Insulin Signaling in Pemt−/− Mice
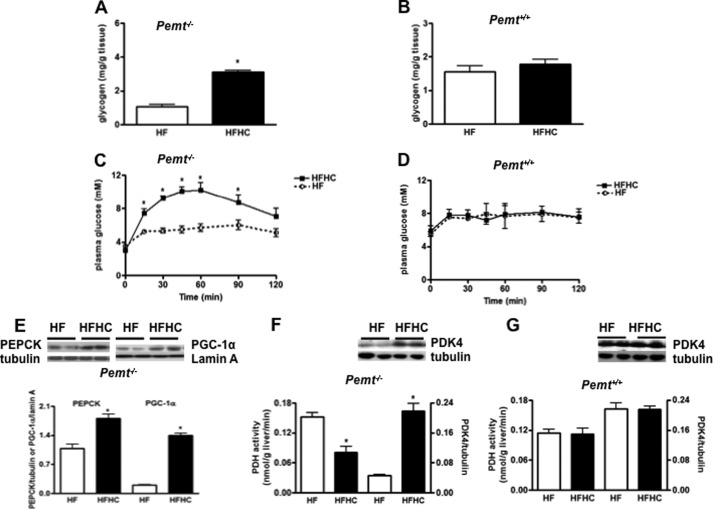
Glucagon plays a crucial role in regulating plasma glucose by increasing glycogen breakdown and promoting gluconeogenesis (19, 22). Hepatic glycogen was increased (Fig. 4A) in Pemt−/− mice, but not in Pemt+/+ mice (Fig. 4B), fed the HFHC diet compared with the HF diet, indicating that choline-induced hyperglycemia in Pemt−/− mice is not due to glycogen breakdown. In contrast, addition of choline to the HF diet increased hepatic gluconeogenesis in Pemt−/− mice (Fig. 4C) demonstrated by an intraperitoneal pyruvate tolerance test, whereas no difference was seen in Pemt+/+ mice (Fig. 4D). In agreement, the amount of PEPCK, a key enzyme involved in hepatic gluconeogenesis, was higher in Pemt−/− mice on the HFHC diet (Fig. 4E), as was the amount of nuclear PGC-1α (Fig. 4E), an upstream regulator of PEPCK. Furthermore, key enzymes involved in glucose oxidation were also examined. The activity of PDH, which converts pyruvate to acetyl-CoA, was significantly reduced in Pemt−/− mice fed the HFHC diet (Fig. 4F), which is consistent with the enhanced expression of PDK4, a negative regulator of PDH activity (Fig. 4F), in these mice. In contrast, hepatic PDH activity and PDK4 expression (Fig. 4G) were unaffected in Pemt+/+ mice fed either diet. Thus, elevated hepatic gluconeogenesis and reduced glucose oxidation contribute to choline-induced hyperglycemia in Pemt−/− mice fed the HFHC diet.
FIGURE 4.
Impaired hepatic glucose homeostasis in Pemt−/− mice fed the HFHC diet. Pemt+/+ or Pemt−/− mice were fed the HF or HFHC diet for 1 week. A and B, hepatic glycogen content in Pemt−/− (A) and Pemt+/+ (B) mice. C and D, a pyruvate tolerance test performed by intraperitoneal pyruvate injection (2 g/kg of body weight) in 18-h fasted Pemt−/− (C) and Pemt+/+ mice (D). E, representative immunoblots for the expression of PEPCK, nuclear PGC-1α in Pemt−/− mice. F and G, hepatic PDH activity and representative blots for hepatic protein expression of PDK4 in Pemt−/− (F) and in Pemt+/+ mice (G). Data are mean ± S.D. (error bars) from three independent experiments. *, p < 0.05 compared with littermates on HF diet.
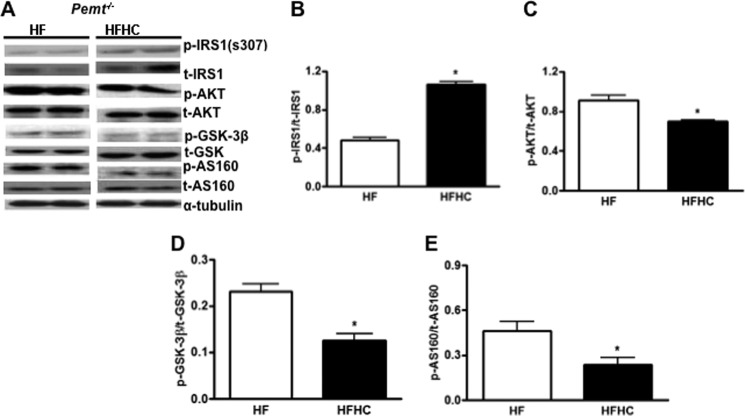
Next, we determined whether choline impaired the insulin signaling pathway in Pemt−/− mice fed the HFHC diet. Phosphorylation of IRS1-s307 was increased in Pemt−/− mice fed the HFHC diet compared with littermates fed the HF diet (Fig. 5, A and B). As a result, insulin-stimulated levels of phospho-AKT (Fig. 5, A and C), phospho-GSK (Fig. 5, A and D), and phospho-AS160 (Fig. 5, A and E), the downstream targets of IRS1, were significantly attenuated in Pemt−/− mice fed the HFHC diet compared with the HF diet. Apparently, excess choline impairs hepatic insulin signaling, which contributes to insulin intolerance in Pemt−/− mice.
FIGURE 5.
Impaired hepatic insulin signaling in Pemt−/− mice fed the HFHC diet. Pemt−/− mice were fed the HF or HFHC diet for 1 week and then fasted overnight. Insulin was injected intraperitoneally (1 unit/kg of body weight) into mice, and tissues were harvested 5 min later. A, representative immunoblots for the expression of insulin-stimulated phospho-IRS1-s307, phospho-AKT, phospho-GSK-3β, phospho-AS160, relative to the corresponding total (t) proteins. B–E, protein amounts quantified by densitometry of the immunoblots in A. Data are mean ± S.D. (error bars). *, p < 0.05 compared with HF-fed mice.
Fatty Liver Is Ameliorated in Pemt−/− Mice Fed the HFHC Diet
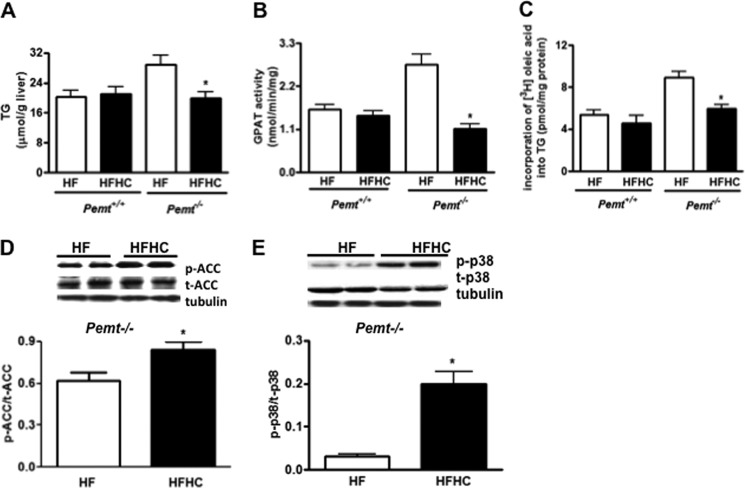
Fatty liver is often associated with insulin resistance (23). The HF diet induces fatty liver in Pemt−/− mice (7). We, therefore, explored whether choline-induced insulin resistance in Pemt−/− mice might be associated with fatty liver. However, hepatic TG content was significantly lower in Pemt−/− mice fed the HFHC diet compared with the HF diet (Fig. 6A), whereas no difference was observed in hepatic TG in Pemt+/+ mice (Fig. 6A). Because addition of choline to the HF diet ameliorates fatty liver in Pemt−/− mice fed the HF diet, hepatic TG may not be a causal factor for choline-induced hepatic insulin resistance.
FIGURE 6.
Excess choline modulates intracellular fatty acid utilization in Pemt−/− mice fed the HF diet. Pemt−/− mice and Pemt+/+ mice were fed the HF or HFHC diet for 1 week. A, hepatic TG in 12-h fasted Pemt−/− and Pemt+/+. B, hepatic GPAT activity in both Pemt−/− and Pemt+/+ mice. C, incorporation of [3H]oleic acid into hepatic TG in both Pemt−/− and Pemt+/+ mice assessed 1 h after intraperitoneal injection of [3H]oleic acid (2 μCi/g). D and E, representative immunoblots of phospho-ACC, total ACC, and α-tubulin. D, density values of p-ACC normalized to t-ACC levels. Representative immunoblots for the expression of phospho-p38 MAPK, total MAPK and α-tubulin. E, density values of p-p38 normalized to t-p38 levels. Data are means ± S.D. (error bars). *, p < 0.05, for HFHC diet compared with HF feeding.
To understand how excess choline reduces hepatic TG levels in Pemt−/− mice, we investigated TG biosynthesis and lipogenesis. The expression of CD36, an important regulator of plasma fatty acid uptake (24), was unaltered (data not shown), suggesting that lower hepatic TG may not be due to altered fatty acid uptake. The activity of hepatic GPAT, a key enzyme for TG biosynthesis, was significantly lower in Pemt−/− mice fed the HFHC compared with the HF diet (Fig. 6B), whereas no difference was observed in Pemt+/+ mice (Fig. 6B). A choline-induced decrease in TG biosynthesis was further demonstrated by in vivo experiments; there was diminished incorporation of [3H]oleic acid into hepatic TG in Pemt−/− mice (Fig. 6C), but not in Pemt+/+ mice, fed the HFHC diet (Fig. 6C). In addition, hepatic acetyl-CoA carboxylase (ACC), a key enzyme for lipogenesis and a downstream target of AMPK, was examined. The phosphorylation/inactivation of ACC was enhanced in Pemt−/− mice fed the HFHC diet (Fig. 6D). Of interest, phospho-p38 MAPK, which has been shown to inhibit hepatic lipogenesis in glucagon-exposed primary hepatocytes (25), was strikingly increased in Pemt−/− mice fed the HFHC diet (Fig. 6E). Thus, choline appears to ameliorate fatty liver in Pemt−/− mice fed the HF diet by reducing TG biosynthesis.
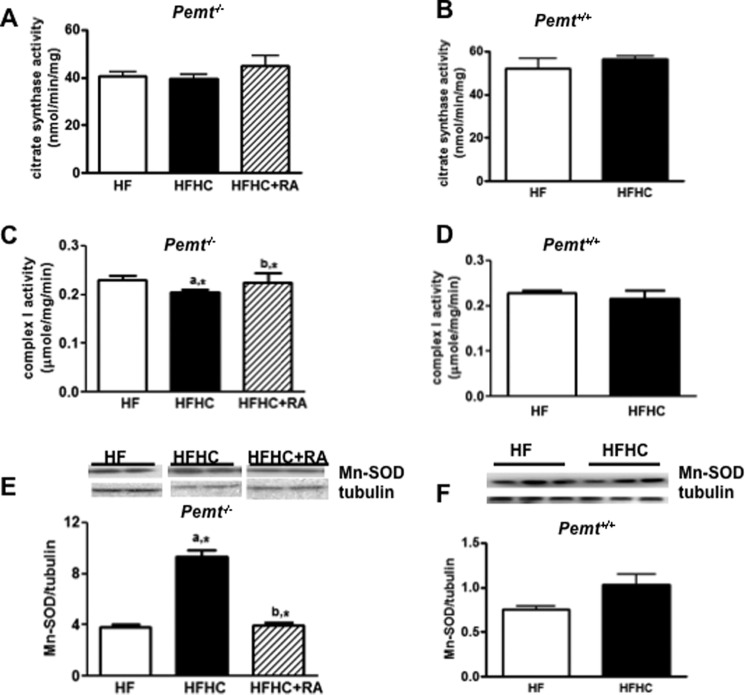
Mitochondrial Oxidative Stress Induced by HFHC Is Improved by an Antagonist of the Glucagon Receptor in Pemt−/− Mice
Aberrant mitochondrial function contributes to insulin resistance (26). However, activity of citrate synthase, an enzyme of the tricarboxylic acid cycle, was unaffected in Pemt−/− mice (Fig. 7A) and in Pemt+/+ mice (Fig. 7B) by either diet. We then examined whether the mitochondrial electron transport chain and/or antioxidative capacity was altered by the HFHC diet. Mitochondrial complex II activity was not altered in Pemt−/− and Pemt+/+ mice by addition of choline to either diet (data not shown). However, the activity of mitochondrial complex I, a major source of reactive oxygen species in mitochondria (27), was slightly lower in Pemt−/− mice (Fig. 7C), but not in Pemt+/+ mice (Fig. 7D) fed the HFHC diet. In contrast, the amount of Mn-SOD was up-regulated in Pemt−/− mice (Fig. 7E), but not in Pemt+/+ mice (Fig. 7F), by the HFHC diet, suggesting a counteractive response to increased oxidative stress in Pemt−/− mice. Furthermore, the reduction in complex I activity in Pemt−/− mice by the HFHC diet was prevented by the glucagon receptor antagonist, 2-aminobenzimidazole (Fig. 7C); 2-aminobenzimidazole also decreased the amount of Mn-SOD (Fig. 7E).
FIGURE 7.
Hepatic mitochondrial oxidative stress and impaired mitochondrial respiratory chain capacity in Pemt−/− mice fed the HFHC diet. Pemt−/− mice and Pemt+/+ mice were fed the HF or HFHC diet for 1 week. One group of Pemt−/− mice on the HFHC diet were injected intraperitoneally with saline or glucagon receptor antagonist, 2-aminobenzimidazole (RA), every other day (50 mg/kg of body weight). A and B, hepatic activity of citrate synthase in Pemt−/− mice (A) and in Pemt+/+ mice (B). C and D, mitochondrial complex I activity in Pemt−/− mice (C) and in Pemt+/+ mice (D). Representative immunoblots show expression of Mn-SOD. E and F, density values of Mn-SOD normalized to tubulin in Pemt−/− mice (E) and in Pemt+/+ mice (F). Protein amounts were quantified by densitometry of the immunoblots. Data are the mean ± S.D. (error bars). a, *, p < 0.05, compared with HF-fed mice. b,* p, < 0.05, compared with HFHC-fed mice.
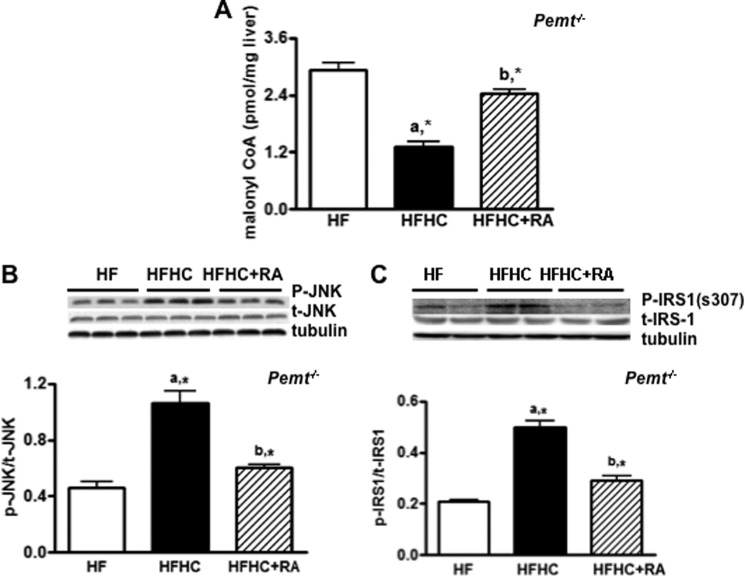
Aberrant mitochondrial fatty acids uptake is a factor associated with mitochondrial oxidative stress (28, 29). It is well known that malonyl-CoA strongly inhibits mitochondrial fatty acid uptake; we, therefore, investigated whether increased mitochondrial oxidative stress occurred in conjunction with decreased malonyl-CoA levels in Pemt−/− mice fed the HFHC diet. Malonyl-CoA levels were, indeed, reduced in Pemt−/− mice fed the HFHC compared with the littermates on the HF diet (Fig. 8A). Of interest, 2-amino-benzimidazole restored the levels of malonyl-CoA in the Pemt−/− mice fed the HFHC diet (Fig. 8A). In addition, the phosphorylation of c-Jun N-terminal kinase, which links mitochondrial production of reactive oxygen species with insulin resistance (30), was also blunted by 2-aminobenzimidazole in Pemt−/− mice fed the HFHC diet (Fig. 8B), as was the phosphorylation of IRS1-s307 (Fig. 8C), a downstream target of c-Jun N-terminal kinase (31). Apparently, choline-induced mitochondria fatty acid uptake and oxidative stress are associated with impairment of hepatic insulin signaling in Pemt−/− mice.
FIGURE 8.
Glucagon receptor antagonist improves hepatic insulin signaling and restores the level of hepatic malonyl-CoA in Pemt−/− mice fed the HFHC diet. Pemt−/− mice were fed the HF or HFHC diet for 1 week. One group of mice fed the HFHC diet were injected intraperitoneally (50 mg/kg of body weight) with 2-aminobenzimidazole (RA) as indicated. A, amount of hepatic malonyl-CoA. B, representative immunoblots and densitometry of phospho-c-Jun N-terminal kinase (JNK) normalized to total JNK. C, representative immunoblots and densitometry of phospho-IRS1-s307. Data are means ± S.D. (error bars). a, *, p < 0.05, for mice fed HFHC diet compared with the HF diet. b, *, p < 0.05, for mice fed the HFHC + RA diet compared with mice fed the HFHC diet.
DISCUSSION
The novel findings are: (i) Addition of choline to the HF diet induced glucose and insulin intolerance in Pemt−/− mice despite alleviating fatty liver. (ii) The induction was choline-specific, and increased plasma glucagon thereby activating hepatic glucagon receptors/AMPK axis to modulate gluconeogenesis, glucose oxidation and lipid metabolism. (iii) Mitochondrial stress in Pemt−/− mice caused by excess choline is associated with hepatic insulin resistance; (iv) Antagonism of the glucagon receptor improved the choline-induced hyperglycemia and insulin resistance in Pemt−/− mice.
Specificity of Choline on Enhancing Plasma Glucose Levels
Pemt−/− mice depend completely on dietary choline to meet the daily choline requirement (5, 32). In comparison with Pemt+/+ mice fed the HF diet, basal plasma choline levels are significantly reduced in Pemt−/− mice (Fig. 2A). It could be that the reduced level of basal plasma choline in Pemt−/− mice reflects greater hepatic uptake of choline for PC biosynthesis (33, 34), and therefore increased hepatic PC content in Pemt−/− mice, but not in Pemt+/+ mice, after chronic choline supplementation (7). Of interest, HFHC feeding does not change plasma choline levels in Pemt+/+ mice, but significantly enhanced plasma choline levels in Pemt−/− mice. Plasma glucagon levels are elevated in both types of mice by the HFHC diet, but the increment is about 64% in Pemt−/− mice, compared with only 20% in Pemt+/+ mice (Table 1). It is currently unknown whether the availability of choline to the pancreas is greater in Pemt−/− mice than in Pemt+/+ mice, thereby resulting in a different stimulation of glucagon secretion. The difference in the enhanced magnitude of plasma glucagon may contribute to the different response in the expression of hepatic glucagon receptors that is up-regulated only in Pemt−/− mice, but not in Pemt+/+ mice. In addition, HFHC-induced increase in the ratio of glucagon to insulin seen in Pemt−/− mice is not observed in Pemt+/+ mice. Apparently, not only plasma glucagon but also the balance between plasma insulin and glucagon associates with choline-induced insulin resistance. It may partially explain why insulin resistance does not develop in Pemt+/+ mice fed the HFHC diet.
To this point, choline injection affects neither plasma glucagon nor the ratio of glucagon to insulin in Pemt+/+ mice. But plasma glucose levels are markedly increased (Fig. 2B), indicative of alternative mechanisms. To our knowledge, the mechanisms that mediate the cholinergic regulation of insulin or glucagon release involve mainly three separate autonomic systems: the parasympathetic and sympathetic pathways innervating β- and α-cells, and the adrenal medulla, releasing epinephrine (9). Choline injection in normal rats increases plasma epinephrine concentrations (8). It has been shown that epinephrine markedly stimulates glycogen breakdown in muscle and, to a lesser extent in the liver that is more responsive to glucagon (35). Thus, it is possible that elevated plasma glucose by choline injection in Pemt+/+ mice is an effect of choline via epinephrine-induced glycogen breakdown in muscle or liver. If so, one might postulate that choline acts primarily on α-cells in Pemt−/− mice, but on adrenal medulla in Pemt+/+mice. This could also be true in the chronic condition, as choline-induced amplification of plasma glucagon is greater in Pemt−/− compared with Pemt+/+ mice.
Dissociation of Fatty Liver from Choline-induced Hepatic Insulin Resistance in Pemt−/− Mice
Despite the fatty liver in Pemt−/− mice, HF fed-Pemt−/− mice are insulin-sensitive. Excess choline does not improve hepatic TG secretion, yet reduces hepatic TG content and provokes insulin resistance. On the molecular level, supplementation of choline to Pemt−/− mice raises plasma glucagon, which initiates hepatic signaling by activating hepatic AMPK (21, 36), to mobilize fatty acids from TG stores toward mitochondrial uptake (21, 37).
Glucagon suppresses lipogenesis in part via decreased expression of sterol regulatory element-binding protein-1c (SREBP-1c) (25, 38) through the p38 MAPK-dependent inhibitory effect on the SREBP-1c promoter (25). In line with this, we observed increased phosphorylation of p38 MAPK and reduced activities of ACC and GPAT, direct downstream targets of SREBP-1c (39), in livers of Pemt−/− mice fed the HFHC diet. In addition, it has been demonstrated that genetic elimination or antagonism of the glucagon receptor lowers fasting glucose, improves the GTT and pancreatic β-cell function in mice (22, 40), humans (41), and other rodent models (42). Consistently, antagonism of the glucagon receptor in Pemt−/− mice fed the HFHC diet improved insulin sensitivity and enhanced hepatic TG, which occurred in conjunction with inactivating AMPK and increasing hepatic malonyl-CoA levels.
As an endogenous inhibitor of carnitine palmitoyltransferase-1, malonyl-CoA strongly inhibits the rate of mitochondrial fatty acid uptake (37). Supplementation of choline lowered hepatic malonyl-CoA by 55% in Pemt−/− mice compared with littermates fed the HF diet. This could cause enhanced mitochondrial fatty acid uptake and lead to mitochondrial stress (28, 29, 43). This notion is supported by our observations, in which reduction in the activity of mitochondrial complex I, and elevated expression of Mn-SOD expression is evident in Pemt−/− mice fed the HFHC diet. The role of glucagon in this process is implied because antagonism of the glucagon receptor restored complex I activity and Mn-SOD expression in conjunction with decreased phosphorylation of JNK and IRS1-s307. Of interest, antagonism of the glucagon receptor also prevented the decrease in malonyl-CoA in Pemt−/− mice fed the HFHC diet. Hence, mitochondrial stress that contributes to the choline-induced glucose and insulin intolerance in Pemt−/− mice fed the HF diet.
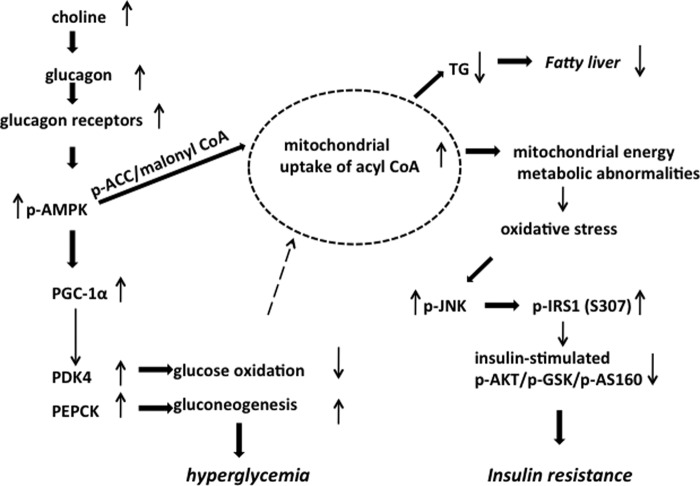
Proposed Mechanism Underlying the Excess Choline-induced Hepatic Glucose and Insulin Intolerance (Fig. 9)
FIGURE 9.
Proposed mechanism underlying the excess choline-induced hepatic glucose and insulin intolerance in Pemt−/− mice. An increase in choline leads to an increase in plasma glucagon and increase in the expression of hepatic glucagon receptors. The glucagon causes an increase in phospho-AMPK that leads to an increase in PGC-1α. This leads to an increase in PDK4 which inhibits pyruvate dehydrogenase and causes a decrease in glucose oxidation. In parallel, PGC-1α promotes an increase in PEPCK that leads to an increase in gluconeogenesis. As a result of an increase in p-AMPK, the phosphorylation of ACC is increased, leading to a decrease in ACC activity and in the levels of malonyl-CoA. Lower malonyl-CoA promotes an increase in fatty acid uptake in mitochondria. Fewer fatty acids are available for TG biosynthesis, and therefore there is a decrease in fatty liver. The abnormal increase in mitochondrial fatty acids uptake leads to oxidative stress causing an increase in phospho-JNK. As a result, there is an increase in phospho-IRS1. Insulin stimulation causes a decrease in phospho-AKT, phospho-GSK, and phospho-AS160 resulting in insulin resistance in the Pemt−/− mice fed the HFHC diet.
Our data indicate that choline induces hyperglycemia via increasing plasma glucagon. Consequently, hepatic gluconeogenesis is enhanced by sequential activation of the glucagon receptor/AMPK/PGC-1α/PEPCK pathway. Concurrently, the increased expression of PGC-1α increases the expression of PDK4, thereby inhibiting PDH activity and impairing glucose oxidation. Furthermore, increased gluconeogenesis decreases the availability of acyl-CoA and glycerol 3-phosphate for TG biosynthesis so that fatty liver is prevented. However, enhanced mitochondrial fatty acid uptake causes oxidative stress so that phospho-c-Jun N-terminal kinase and phosphorylation of IRS1-s-307 are increased. As a result, insulin-mediated signal transduction is blocked (30).
In conclusion, increased provision of choline to Pemt−/− mice fed the HF diet improves fatty liver, but promotes hepatic insulin resistance via increased plasma glucagon. Polymorphisms have been identified in the human PEMT gene (44). Patients with mutations that attenuate PEMT activity are likely to have increased dietary requirements for choline because PEMT is an endogenous source of choline, particularly during pregnancy (45). On the other hand, too much dietary choline supplement may exacerbate glucose and insulin intolerance in humans with obesity or type 2 diabetes. A glucagon receptor antagonist might, therefore, be useful for treatment of type 2 diabetes.
Acknowledgments
We thank Dr. Jelske van der Veen and Susanne Lingrell of the University of Alberta for assistance with the plasma glucagon assay and Dr. Jean Vance of the University of Alberta for helpful comments on the manuscript.
This research was supported Canadian Institutes of Health Research Grant MOP 89793 and Genome Canada.
- PC
- phosphatidylcholine
- ACC
- acetyl-CoA carboxylase
- AMPK
- AMP-activated protein kinase
- GPAT
- glycerolphosphate acyltransferase
- GSK
- glycogen synthase kinase
- GTT
- glucose tolerance test
- HF
- high fat
- HFHC
- high fat, high choline
- IRS1-s307
- insulin receptor substrate 1 at serine 307
- ITT
- insulin tolerance test
- PGC-1α
- peroxisome proliferator-activated receptor-γ co-activator 1α
- PDH
- pyruvate dehydrogenase
- PDK4
- pyruvate dehydrogenase kinase 4
- PEMT
- phosphatidylethanolamine N-methyltransferase
- PEPCK
- phosphoenolpyruvate carboxykinase
- SOD
- superoxide dismutase
- SREBP
- sterol response element-binding protein
- TG
- triacylgycerol.
REFERENCES
- 1. Vance D. E., Ridgway N. D. (1988) The methylation of phosphatidylethanolamine. Prog. Lipid Res. 27, 61–79 [DOI] [PubMed] [Google Scholar]
- 2. Sundler R., Akesson B. (1975) Biosynthesis of phosphatidylethanolamines and phosphatidylcholines from ethanolamine and choline in rat liver. Biochem. J. 146, 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeLong C. J., Shen Y. J., Thomas M. J., Cui Z. (1999) Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J. Biol. Chem. 274, 29683–29688 [DOI] [PubMed] [Google Scholar]
- 4. Best C. H., Huntsman M. E. (1932) The effects of the components of lecithine upon deposition of fat in the liver. J. Physiol. 75, 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walkey C. J., Donohue L. R., Bronson R., Agellon L. B., Vance D. E. (1997) Disruption of the murine gene encoding phosphatidylethanolamine N-methyltransferase. Proc. Natl. Acad. Sci. U.S.A. 94, 12880–12885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noga A. A., Zhao Y., Vance D. E. (2002) An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J. Biol. Chem. 277, 42358–42365 [DOI] [PubMed] [Google Scholar]
- 7. Jacobs R. L., Zhao Y., Koonen D. P., Sletten T., Su B., Lingrell S., Cao G., Peake D. A., Kuo M. S., Proctor S. D., Kennedy B. P., Dyck J. R., Vance D. E. (2010) Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J. Biol. Chem. 285, 22403–22413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cansev M., Ilcol Y. O., Yilmaz M. S., Hamurtekin E., Ulus I. H. (2008) Peripheral administration of CDP-choline, phosphocholine or choline increases plasma adrenaline and noradrenaline concentrations. Auton. Autacoid. Pharmacol. 28, 41–58 [DOI] [PubMed] [Google Scholar]
- 9. Cansev M., Ilcol Y. O., Yilmaz M. S., Hamurtekin E., Ulus I. H. (2008) Choline, CDP-choline or phosphocholine increases plasma glucagon in rats: involvement of the peripheral autonomic nervous system. Eur. J. Pharmacol. 589, 315–322 [DOI] [PubMed] [Google Scholar]
- 10. Ilcol Y. O., Cansev M., Yilmaz M. S., Hamurtekin E., Ulus I. H. (2008) Peripheral administration of CDP-choline and its cholinergic metabolites increases serum insulin: muscarinic and nicotinic acetylcholine receptors are both involved in their actions. Neurosci. Lett. 431, 71–76 [DOI] [PubMed] [Google Scholar]
- 11. Ilcol Y. O., Gurun M. S., Taga Y., Ulus I. H. (2002) Intraperitoneal administration of choline increases serum glucose in rat: involvement of the sympathoadrenal system. Horm. Metab. Res. 34, 341–347 [DOI] [PubMed] [Google Scholar]
- 12. Wu G., Sher R. B., Cox G. A., Vance D. E. (2009) Understanding the muscular dystrophy caused by deletion of choline kinase β in mice. Biochim. Biophys. Acta 1791, 347–356 [DOI] [PubMed] [Google Scholar]
- 13. Lo S., Russell J. C., Taylor A. W. (1970) Determination of glycogen in small tissue samples. J. Appl. Physiol. 28, 234–236 [DOI] [PubMed] [Google Scholar]
- 14. Zhang L., Ussher J. R., Oka T., Cadete V. J., Wagg C., Lopaschuk G. D. (2011) Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc. Res. 89, 148–156 [DOI] [PubMed] [Google Scholar]
- 15. Janssen A. J., Trijbels F. J., Sengers R. C., Smeitink J. A., van den Heuvel L. P., Wintjes L. T., Stoltenborg-Hogenkamp B. J., Rodenburg R. J. (2007) Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin. Chem. 53, 729–734 [DOI] [PubMed] [Google Scholar]
- 16. Glaser M., Ferguson K. A., Vagelos P. R. (1974) Manipulation of the phospholipid composition of tissue culture cells. Proc. Natl. Acad. Sci. U.S.A. 71, 4072–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silvius J. R., Brown P. M., O'Leary T. J. (1986) Role of head group structure in the phase behavior of amino phospholipids. 1. Hydrated and dehydrated lamellar phases of saturated phosphatidylethanolamine analogues. Biochemistry 25, 4249–4258 [DOI] [PubMed] [Google Scholar]
- 18. Waite K. A., Vance D. E. (2004) Dimethylethanolamine does not prevent liver failure in phosphatidylethanolamine N-methyltransferase-deficient mice fed a choline-deficient diet. Biochim. Biophys. Acta 1636, 175–182 [DOI] [PubMed] [Google Scholar]
- 19. Dobbs R., Sakurai H., Sasaki H., Faloona G., Valverde I., Baetens D., Orci L., Unger R. (1975) Glucagon: role in the hyperglycemia of diabetes mellitus. Science 187, 544–547 [DOI] [PubMed] [Google Scholar]
- 20. Unger R. H., Cherrington A. D. (2012) Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J. Clin. Invest. 122, 4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Longuet C., Sinclair E. M., Maida A., Baggio L. L., Maziarz M., Charron M. J., Drucker D. J. (2008) The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 8, 359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liang Y., Osborne M. C., Monia B. P., Bhanot S., Gaarde W. A., Reed C., She P., Jetton T. L., Demarest K. T. (2004) Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 53, 410–417 [DOI] [PubMed] [Google Scholar]
- 23. Grønbaek H., Thomsen K. L., Rungby J., Schmitz O., Vilstrup H. (2008) Role of nonalcoholic fatty liver disease in the development of insulin resistance and diabetes. Expert Rev. Gastroenterol. Hepatol. 2, 705–711 [DOI] [PubMed] [Google Scholar]
- 24. Coburn C. T., Knapp F. F., Jr., Febbraio M., Beets A. L., Silverstein R. L., Abumrad N. A. (2000) Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275, 32523–32529 [DOI] [PubMed] [Google Scholar]
- 25. Xiong Y., Collins Q. F., An J., Lupo E., Jr., Liu H. Y., Liu D., Robidoux J., Liu Z., Cao W. (2007) p38 mitogen-activated protein kinase plays an inhibitory role in hepatic lipogenesis. J. Biol. Chem. 282, 4975–4982 [DOI] [PubMed] [Google Scholar]
- 26. Kim J. A., Wei Y., Sowers J. R. (2008) Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 102, 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirst J., King M. S., Pryde K. R. (2008) The production of reactive oxygen species by complex I. Biochem. Soc. Trans. 36, 976–980 [DOI] [PubMed] [Google Scholar]
- 28. Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., Lopaschuk G. D., Muoio D. M. (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7, 45–56 [DOI] [PubMed] [Google Scholar]
- 29. Koek G. H., Liedorp P. R., Bast A. (2011) The role of oxidative stress in non-alcoholic steatohepatitis. Clin. Chim. Acta 412, 1297–1305 [DOI] [PubMed] [Google Scholar]
- 30. Nakatani Y., Kaneto H., Kawamori D., Hatazaki M., Miyatsuka T., Matsuoka T. A., Kajimoto Y., Matsuhisa M., Yamasaki Y., Hori M. (2004) Modulation of the JNK pathway in liver affects insulin resistance status. J. Biol. Chem. 279, 45803–45809 [DOI] [PubMed] [Google Scholar]
- 31. Gao D., Nong S., Huang X., Lu Y., Zhao H., Lin Y., Man Y., Wang S., Yang J., Li J. (2010) The effects of palmitate on hepatic insulin resistance are mediated by NADPH oxidase 3-derived reactive oxygen species through JNK and p38 MAPK pathways. J. Biol. Chem. 285, 29965–29973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu X., Song J., Mar M. H., Edwards L. J., Zeisel S. H. (2003) Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem. J. 370, 987–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watkins S. M., Zhu X., Zeisel S. H. (2003) Phosphatidylethanolamine N-methyltransferase activity and dietary choline regulate liver-plasma lipid flux and essential fatty acid metabolism in mice. J. Nutr. 133, 3386–3391 [DOI] [PubMed] [Google Scholar]
- 34. Ling J., Chaba T., Zhu L. F., Jacobs R. L., Vance D. E. (2012) Hepatic ratio of phosphatidylcholine to phosphatidylethanolamine predicts survival after partial hepatectomy in mice. Hepatology 55, 1094–1102 [DOI] [PubMed] [Google Scholar]
- 35. Roach P. J., Depaoli-Roach A. A., Hurley T. D., Tagliabracci V. S. (2012) Glycogen and its metabolism: some new developments and old themes. Biochem. J. 441, 763–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kimball S. R., Siegfried B. A., Jefferson L. S. (2004) Glucagon represses signaling through the mammalian target of rapamycin in rat liver by activating AMP-activated protein kinase. J. Biol. Chem. 279, 54103–54109 [DOI] [PubMed] [Google Scholar]
- 37. Prip-Buus C., Pegorier J. P., Duee P. H., Kohl C., Girard J. (1990) Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit: perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes. Biochem. J. 269, 409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Foretz M., Pacot C., Dugail I., Lemarchand P., Guichard C., Le Lièpvre X., Berthelier-Lubrano C., Spiegelman B., Kim J. B., Ferré P., Foufelle F. (1999) ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol. Cell. Biol. 19, 3760–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eberlé D., Hegarty B., Bossard P., Ferré P., Foufelle F. (2004) SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86, 839–848 [DOI] [PubMed] [Google Scholar]
- 40. Gelling R. W., Du X. Q., Dichmann D. S., Romer J., Huang H., Cui L., Obici S., Tang B., Holst J. J., Fledelius C., Johansen P. B., Rossetti L., Jelicks L. A., Serup P., Nishimura E., Charron M. J. (2003) Lower blood glucose, hyperglucagonemia, and pancreatic α-cell hyperplasia in glucagon receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 100, 1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petersen K. F., Sullivan J. T. (2001) Effects of a novel glucagon receptor antagonist (Bay 27-9955) on glucagon-stimulated glucose production in humans. Diabetologia 44, 2018–2024 [DOI] [PubMed] [Google Scholar]
- 42. Gu W., Winters K. A., Motani A. S., Komorowski R., Zhang Y., Liu Q., Wu X., Rulifson I. C., Sivits G., Jr., Graham M., Yan H., Wang P., Moore S., Meng T., Lindberg R. A., Véniant M. M. (2010) Glucagon receptor antagonist-mediated improvements in glycemic control are dependent on functional pancreatic GLP-1 receptor. Am. J. Physiol. Endocrinol. Metab. 299, E624–632 [DOI] [PubMed] [Google Scholar]
- 43. Lopaschuk G. D., Ussher J. R., Folmes C. D., Jaswal J. S., Stanley W. C. (2010) Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90, 207–258 [DOI] [PubMed] [Google Scholar]
- 44. Saito S., Iida A., Sekine A., Miura Y., Sakamoto T., Ogawa C., Kawauchi S., Higuchi S., Nakamura Y. (2001) Identification of 197 genetic variations in six human methyltranferase genes in the Japanese population. J. Hum. Genet. 46, 529–537 [DOI] [PubMed] [Google Scholar]
- 45. Albright C. D., Tsai A. Y., Friedrich C. B., Mar M. H., Zeisel S. H. (1999) Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res. Dev. Brain Res. 113, 13–20 [DOI] [PubMed] [Google Scholar]