Background: Stil is a conserved gene in vertebrate species that is involved in neural development and cancer cell proliferation through the Shh pathway.
Results: Lack of Stil expression leads to increased toxic susceptibility of dopaminergic cells to neurotoxin 6-hydroxydopamine.
Conclusion: Functional expression of Stil protects dopaminergic cells from toxin-induced apoptosis.
Significance: We have identified a novel function for Stil in neural protection in vertebrates.
Keywords: Cell Death, Neurons, Neurotoxin, Retina, Zebra Fish
Abstract
We previously isolated a dominant mutation, night blindness b (nbb), which causes a late onset of retinal dopaminergic cell degeneration in zebrafish. In this study, we cloned the zebrafish nbb locus. Sequencing results revealed that nbb is a homolog of the vertebrate SCL/TAL1 interrupting locus (Stil). The Stil gene has been shown to play important roles in the regulation of vertebrate embryonic neural development and human cancer cell proliferation. In this study, we demonstrate that functional expression of Stil is also required for neural survival. In zebrafish, decreased expression of Stil resulted in increased toxic susceptibility of retinal dopaminergic cells to 6-hydroxydopamine. Increases in Stil-mediated Shh signaling transduction (i.e. by knocking down the Shh repressor Sufu) prevented dopaminergic cell death induced by neurotoxic insult. The data suggest that the oncogene Stil also plays important roles in neural protection.
Introduction
During development and aging, a large number of CNS neurons undergo programmed cell death (1–3). Numerous factors (i.e. genes, growth factors and nutrients) have been identified that play important roles in the regulation of apoptosis (3, 4). Excessive neural cell death due to gene mutations or exposure to environmental toxins can lead to dysfunctions in the CNS. In humans, for example, mutations in α-synuclein, Lrrk2, or Parkin loci results in increased dopaminergic (DA)3 cell death in the substantia nigra and eventually leads to Parkinson disease (5–8). In laboratory animals, knock-out of Parkinson disease-specific genes or exposure to neurotoxins such as 6-hydroxydopmine (6-OHDA) or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine also results in excessive DA cell death and Parkinson disease-like symptoms (9–11).
Zebrafish (Danio rerio) have been used to study the genetic and cellular mechanisms of DA cell development and degeneration. A number of gene mutations that disrupt DA cell differentiation and proliferation have been identified (12, 13). Recent studies suggest that zebrafish are also suitable for studying toxin-induced DA cell degeneration. In zebrafish, exposures to neurotoxins (i.e. 6-OHDA or MPTP) or pesticides (i.e. rotenone or paraquat) led to excessive DA cell degeneration and Parkinson disease-like motor defects (14). Molecular cues that may protect DA cells from degeneration remain to be discovered.
Through a behavioral screening of the F1 generation of chemically mutagenized adult zebrafish, we isolated a dominant mutation, night blindness b (nbb), which causes a late onset of retinal dopaminergic interplexiform cell (DA-IPC) degeneration (15). In heterozygous mutants, after 4 months of age, DA-IPCs slowly but progressively degenerate. The olfacto-retinal centrifugal pathway, which originates from the olfactory bulb and terminates on retinal DA-IPCs, is also disrupted in the mutants. In the current study, we cloned the nbb locus. Sequence analysis revealed that nbb is a homolog of the vertebrate Stil gene, which was originally identified from a leukemia-associated chromosomal translocation (16, 17). In humans, Stil encodes a 143-kDa protein with no clear homology to other functional protein families or motifs. In mammals, Stil plays critical roles in the regulation of embryonic neural development through the Sonic hedgehog (Shh) signaling pathway (18, 19). Recent studies have shown that Stil also plays a role in tumor cell proliferation (20). Previously, two mutations in zebrafish Stil (hi1262Tg and cassiopeia; induced by retroviral insertion and chemical mutagenesis, respectively) have been reported to be allelic of Stil (21, 22). The roles of Stil expression in zebrafish cell development have been characterized (22).
Because nbb mutants show loss of DA cells, it is conceivable to propose that Stil is required for DA cell survival. In this research, we examine the roles of Stil in protecting retinal DA cells in zebrafish. We demonstrate that deficiency in Stil expression (i.e. in nbb mutants or in wild-type fish treated with antisense Stil morpholinos) or inhibition of Stil-mediated Shh signaling transduction (by pharmacological approaches) results in increased toxic susceptibility of DA-ICPs to 6-OHDA (a compound known to selectively destroy DA cells) (23–25). Increasing Shh signaling transduction (by knockdown of the Shh repressor Sufu) protects DA-IPCs from degeneration after exposure to neurotoxins. Together, the data suggest that, in addition to its roles in neural development and cancer cell proliferation, Stil is also required for neural protection.
EXPERIMENTAL PROCEDURES
Fish Maintenance
Zebrafish (Danio rerio) were maintained at the University of Notre Dame Animal Facility according to National Institutes of Health guidelines and published protocols (26). Zebrafish were maintained in a 14:10 light/dark cycle in circulating water at 28.5 °C. In this study, developing embryos, juvenile and adult zebrafish were used.
Cloning
Genomic DNA was isolated from wild-type, heterozygous, and homozygous nbb larvae (5 days post-fertilization) for linkage group mapping as described (27). The nbb mutation was linked to Bac 192M14 on zebrafish chromosome 22. The candidate gene approach was used to clone and sequence various genes in the linked region, including the Stil gene. Wild-type, heterozygous, and homozygous mutant cDNA was prepared using the RNA isolation reagent TRIzol (Invitrogen) and the High Capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). Primers were designed for the coding region of the Stil gene as follows: Stil (194 nt), TCATATTCACGCTCACACCCGTAC (forward), Stil (4635 nt) CACTTCATACAAGGCACTTAGTTAT (reverse). High fidelity PCR assays were performed using High Fidelity DNA Taq polymerase (Invitrogen). PCR products were cloned into the pCR4.0-TOPO vector (Invitrogen) according to manufacturer protocol. The plasmids were purified by the PureLink Quick Plasmid Miniprep kit (Invitrogen) and sequenced by Sequetech (Mountain View, CA).
Protein Alignment
Protein alignment of vertebrate Stil sequences was performed using CLUSTALW (28, 29). Protein sequences used for comparison were as follows: human (Homo sapiens; GenBankTM accession no. NP_001041631.1), mouse (Mus musculus; GenBankTM accession no. NP_033211.1), frog (Xenopus laevis; GenBankTM accession no. NP_001084821.1), and zebrafish (D. rerio; GenBankTM accession no. NP_775351.1).
In Situ Hybridization
Standard PCR was performed using primers designed for the zebrafish Stil sequence (GenBankTM accession no. NM_173244.1): Stil (2429 nt), CTATGGGTCTCCTACCTGCTGA (forward) and Stil (3111 nt), TTGGTCCTGCTCTACCTTATCC (reverse). The PCR products were purified and cloned into the pCR4.0-TOPO vector (Invitrogen), linearized by NotI digestion, and reverse transcribed by the DIG RNA labeling kit (Roche Applied Science). The embryos were anesthetized and fixed in 4% paraformaldehyde at 4 °C and hybridized with either sense or antisense probes. Probes were removed by washing with gradient SSCT. The embryos were treated in 1:2000 diluted sheep anti-digoxigenin antibody (Roche Applied Science) at 4 °C overnight. Color reactions were stopped by washes with phosphate buffered saline, 0.05% Tween-20.
Quantitative RT-PCR
RNA was isolated from adult fish retinas (n = 5) or embryos (n = 20) using TRIzol (Invitrogen) for each experiment. For a 20-μl reaction, 1.0 μg of RNA was reverse transcribed by the High Capacity cDNA reverse transcription kit (Applied Biosystems). For each experiment, three independent qRT-PCR were performed with Taqman custom designed gene expression probes for Stil and Gli1 (Applied Biosystems). Quantity values were normalized by the 18 S rRNA quantity amount.
RT-PCR
To genotype the wild-type and mutant embryos, standard high fidelity RT-PCR was performed using cDNA and primers that flanked the mutant insertion at exon 8–9 junction (forward, TGTGGGCGTGGTGTTTGAGGTAC; reverse, CCGGATCTCGATGAGTCAGGGAA).
For single-cell RT-PCR, dissected wild-type retinas were digested with papain (Sigma) and briefly cultured in L-15 medium (Thermo Scientific, Rockford, IL) for 30 min as described (30). DA-IPCs were isolated by morphology and placed in a PCR reaction containing SuperScript III Reverse Transcriptase/Platinum Taq Mix (Invitrogen), RNase Out (Invitrogen), and multiplex primers for two genes: tyrosine hydroxylase (forward, TACATACGGCACGCTTCCTC and reverse, CAGTCCTGCTCCA TACGCTT) and Stil (forward, GTGCCAGCATTTGGGGGCCA and reverse, CTGAGTTCGTCCCGCCACGC). Products were analyzed by standard gel electrophoresis.
Western Blot
Total proteins were isolated from four adult retinas for each experiment. Retinas were lysed in lysis buffer containing 50 mm NaCl, 10 mm Tris (pH 8.0), 2 mm MgCl2, 1 mm DTT, 1% Trition X-100, aprotin, leupeptin, and PMSF. Proteins were electrophoresed by SDS-PAGE and transferred onto nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk in Tris buffered saline, 0.05% Tween-20 and incubated with primary antibodies: anti-STIL (1:1000 anti-rabbit custom antibody; Biomatik, Wilmington, DE), anti-Sufu (1:1000 anti-rabbit; AnaSpec, Fremont, CA), and anti-actin (1:3000 anti-rabbit; Sigma). After Tris buffered saline, 0.05% Tween-20 washes, the membrane was incubated with anti-rabbit peroxidase-conjugated secondary antibody (1:3000 for SIL and SUFU, 1:30,000 for actin; Jackson ImmunoResearch Laboratories, West Grove, PA). The membrane was washed with Tris buffered saline, 0.05% Tween-20, and the SuperSignal West Pico chemiluminescent substrate (Thermo Scientific) was used for detection.
Intraocular Injections
Before injections, the fish were anesthetized with 0.016% 3-aminobenzoic acid ethyl ester (Sigma). Injections were performed under a dissecting microscope. 6-OHDA (Sigma) was dissolved in PBS at 5 μg/μl concentration. The stock was further diluted 1:15 in PBS (0.33 μg/μl). A 0.5-μl mixture of 1:1 6-OHDA and pargyline (Sigma) was injected into the vitreous space of the eye. This dose of 6-OHDA was not toxic to wild-type retinal DA-IPCs and therefore was designated as sub-toxic. PBS was used for control (sham) injections.
Cyclopamine (Sigma) was used to inhibit Shh signaling transduction (31). It was dissolved in ethanol at 10 μm. For each injection, a 0.5-μl drug solution was injected. Control (sham) injections included only ethanol.
Antisense oligonucleotide morpholinos (anti-Stil, 5′- GTTGTTTGGACGGTTTGCACATGCC-3′; anti-Sufu, 5′- GCTGCTAGGCCGCATCTCATCCATC-3′) were synthesized (Gene Tool). A 0.5-μl solution that contained either antisense or control (5 base pairs mismatched) morpholinos was injected. After the injection, electroporation was immediately performed using a CUY21 Square Wave Electroporator (Protech International, Boerne, TX) (32).
Immunohistochemistry
Flat-mount retinas were fixed in 4% formaldehyde at 4 °C. Following PBS washes, the samples were incubated in blocking solution (2% normal goat serum, 0.2% Triton X-100, 1% dimethyl sulfoxide in PBS) and then with primary anti-tyrosine hydroxylase (TH) antibody (1:200 anti-mouse, Millipore, Billerica, MA). After PBS washes, the samples were incubated with secondary antibodies (1:500 anti-mouse Alexa Fluor 568 IgG; Invitrogen). Samples were mounted with Fluoromount G (Electron Microscopy Sciences, Hatfield, PA) and viewed under a fluorescent microscope. TH-positive cells were counted for the entire retina.
Statistical Analysis
Statistical significance was determined by Student's t test. For all statistical analyses, we set the significance level at p < 0.001.
RESULTS
Cloning of Zebrafish nbb Mutation
The night blindness b (nbb) mutation was discovered from a previous behavioral screening aimed to identify visual system mutations in zebrafish. The nbb mutation causes both dominant and recessive phenotypes. After four months of age, heterozygous mutants showed decreased visual sensitivity levels during prolonged dark adaptation and decreased numbers of retinal DA-IPCs. The homozygous mutants, however, are embryonic lethal (15).
Through linkage group mapping, we localized the nbb mutation to the zebrafish linkage group 22 (BAC 192M14) and cloned the nbb locus using the candidate gene approach method. Sequence analysis revealed that nbb is a homolog of the vertebrate Stil (SCL/TAL1 interrupting locus) (Fig. 1). STIL is highly conserved in vertebrate species, i.e. M. musculus, H. sapiens, X. laevis, and D. rerio. The D. rerio Stil homolog is 37% identical to M. musculus and H. sapiens and 38% identical to X. laevis.
FIGURE 1.
Comparison of STIL protein sequences in different vertebrate species. The online CLUSTALW tool was used to align partial sequences of STIL proteins (N terminus). Amino acids are numbered on the right side of the sequence. In nbb mutants, the predicted STIL protein is truncated after amino acid Lys-301 (arrow). Asterisk, fully conserved; colon, strongly conserved; period, weakly conserved.
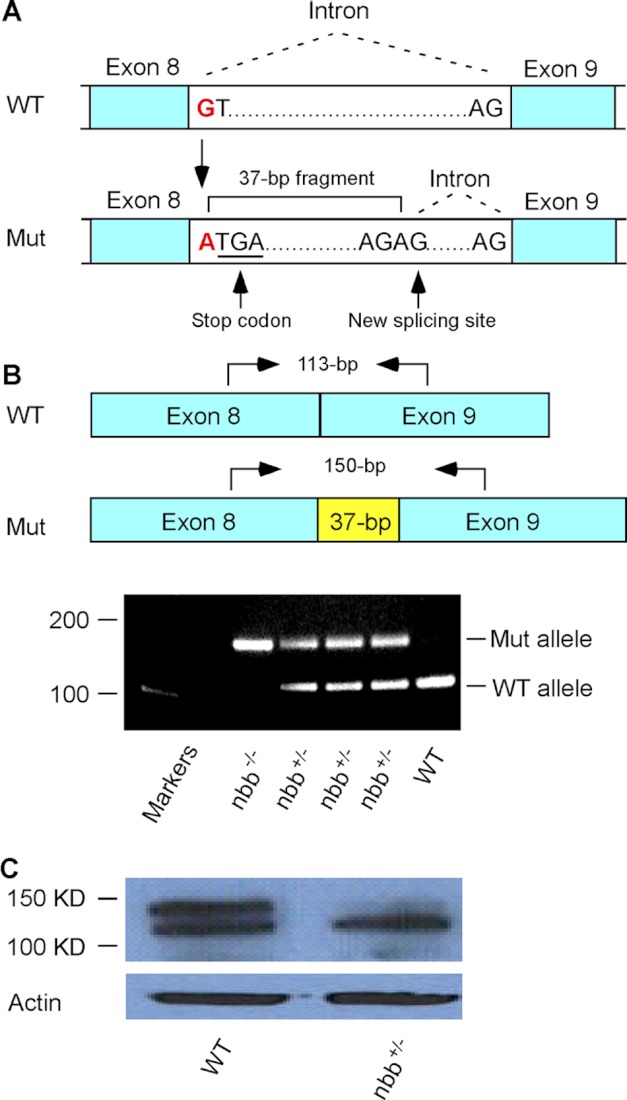
In nbb mutant fish, the Stil locus is mutated due to the substitution of nucleotide G to A at the beginning of intron 8–9 (Fig. 2A). The mutation altered the donor splice site and resulted in an insertion of a 37-bp fragment, which includes a stop codon (Fig. 2A). The insertion of the 37-bp DNA fragment can be readily amplified by PCR in both heterozygous and homozygous mutants (Fig. 2B), and the decrease in STIL protein expression can be detected by Western blot in heterozygous adult mutant retinas (Fig. 2C).
FIGURE 2.
Analysis of zebrafish nbb mutation. A, a diagram that shows the mutation at the beginning of intron 8–9. The substitution of nucleotides from G to A altered the donor splicing site, resulting in a 37-bp insertion with a premature stop codon. B, RT-PCR analysis of Stil expression in wild-type, heterozygous, and homozygous nbb mutants. In the diagram, arrows indicate RT-PCR primer sites used to amplify the region at exon 8–9 junction from cDNA. In heterozygous mutants (nbb+/−), both wild-type (113-bp) and mutant (150-bp) alleles were amplified. In homozygous mutants (nbb−/−), only the 150-bp fragment was amplified. In wild-type embryos, the 113-bp fragment was amplified. C, Western blot of retinal lysates from wild-type and heterozygous nbb mutant fish. The mutant fish showed decreased STIL protein expression. Mut, mutant.
Expression of nbb in Developing Embryos and Adult Fish
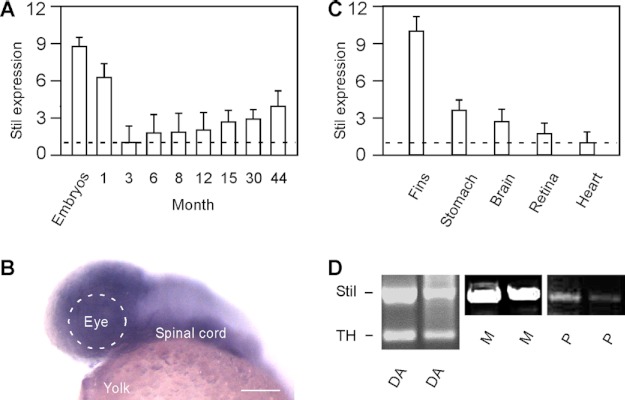
Stil is expressed in both developing embryos and adult animals. As revealed by qRT-PCR, the highest expression of Stil was detected during embryonic stages (Fig. 3A), during which time the expression was ∼9-fold higher than the expression in adult (e.g. 3 months old) (n > 30 in embryos; n = 9 in adult). Stil was also highly expressed in juveniles and young adults (1 month old). At 3 months of age, the expression of Stil mRNA sharply decreased to the lowest levels of expression (normalized to 1). After 3 months, the expression of Stil began to increase. The expression of Stil persisted and increased steadily throughout the life of the fish (up to 44 months examined; n = 9 in each adult group) (Fig. 3A).
FIGURE 3.
The expression of Stil in developing embryos and adult zebrafish. A, qRT-PCR analysis of Stil expression at different ages. The lowest expression in 3-month-old fish was normalized to 1. The highest expression was detected in developing embryos (at 1 day post-fertilization). Data represent the means ± S.E. (n > 30 in embryos; n = 9 in each juvenile and adult stage). B, in situ hybridization against Stil mRNA at 1 day post-fertilization. Stil was highly expressed in the brain and spinal cord. Scale bar, 100 μm. C), qRT-PCR analysis of Stil expression in different tissues and organs isolated from adult zebrafish (6 months old). The lowest expression in the heart was normalized to 1. Data represent the means ± S.E. (n = 9). D, single-cell RT-PCR analysis of Stil expression in isolated DA-IPCs (DA), Müller glia cells (M), and photoreceptor cells (P). TH primers were used to confirm the DA cell type.
During development, robust Stil expression was detected in the central nervous system (Fig. 3B). In adults, Stil was expressed in all tissues and organs examined (Fig. 3C; n = 9 in each organ group). The lowest expression was found in the heart (normalized to 1). The highest expression was detected in the fin, which was ∼10-fold higher than the expression in the heart. Single-cell RT-PCR analysis demonstrated that Stil was expressed in different retinal cell types, which include DA-IPCs, Müller glia cells, and photoreceptor cells (Fig. 3D).
Stil Deficiency Increased the Susceptibility of DA-IPCs to Neurotoxins
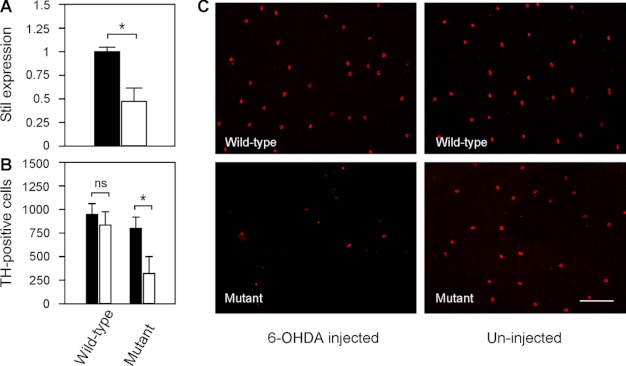
As revealed by qRT-PCR, the expression of Stil in nbb retinas decreased to 0.48 ± 0.14 in comparison with wild-type retinas (normalized to 1) (Fig. 4A; means ± S.E., n = 4). Deficiency in Stil expression resulted in increased toxic susceptibility of DA-IPCs to the neurotoxin 6-OHDA. In wild-type fish, intraocular injections of subtoxic 6-OHDA (0.33 μg/μl) produced no toxic effects on DA-IPCs. At 3 days post 6-OHDA treatment, immunolabeling of the wild-type retinas with anti-tyrosine hydroxylase antibodies (anti-TH, which specifically labels dopaminergic cells) revealed no differences in the number of DA-IPCs in 6-OHDA-treated (845 ± 146) and untreated retinas (952 ± 107) (Fig. 4, B and C; means ± S.E., n = 3 in each group). In nbb mutant retinas, by contrast, treatments with subtoxic 6-OHDA resulted in significant losses of DA-IPCs. At 3 days post 6-OHDA treatment, for example, the number of DA-IPCs in 6-OHDA treated mutant retinas was 321 ± 176. In PBS-injected mutant retinas, the number of DA-IPCs was 802 ± 114 (Fig. 4B, C; means ± S.E., n = 3 in each group).
FIGURE 4.
Toxicity of DA-IPCs to 6-OHDA in wild-type and mutant retinas. A, relative Stil mRNA expression in wild-type and nbb mutant retinas. The expression of Stil in wild-type retinas was normalized to 1 (black bar). Note the decrease of Stil expression in nbb mutant retinas (white bar). Data represent the means ± S.E. (n = 4 in each group). *, p < 0.01. B, number of anti-TH positive retinal DA-IPCs after injection of subtoxic 6-OHDA (white bars) or PBS (black bars). In wild-type fish, injection of subtoxic 6-OHDA produced no effects on DA-IPCs. In nbb retinas, the same treatment resulted in degeneration of DA-IPCs. Data represent the means ± S.E. (n = 3 in each group). ns, not significant; *, p < 0.01. C, fluorescent images of flat-mount wild-type and nbb mutant retinas labeled with anti-TH antibodies. Note the decreased number of DA-IPCs in mutant retinas after subtoxic 6-OHDA treatment. The images were taken at the nasal retina adjacent to the optic nerve. Scale bar, 100 μm.
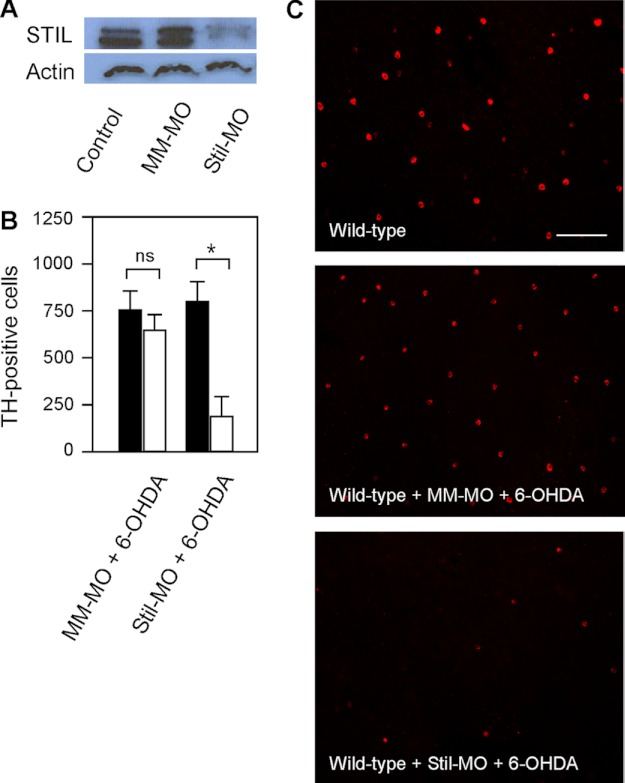
To further demonstrate the roles of Stil on DA-IPCs, we examined subtoxic 6-OHDA-treated wild-type zebrafish retinas, whereas the expression of Stil was inhibited by antisense Stil morpholinos (Stil-MO). Intraocular injections of Stil-MO efficiently diminished the expression of STIL (Fig. 5A). At 24-h post MO treatment, the retinas were injected with subtoxic 6-OHDA. In Stil-MO treated wild-type retinas, subtoxic 6-OHDA treatment caused degeneration of DA-IPCs. At 3 days post 6-OHDA treatment, the number of DA-IPCs in Stil-MO treated retinas was 193 ± 101, whereas the number of DA-IPCs in PBS-injected (control) retinas was 802 ± 102 (Fig. 5, B and C; means ± S.E., n = 5 in each group). In mismatched MO (MM-MO) treated retinas, the number of DA-IPCs was similar between 6-OHDA (755 ± 100) and PBS (650 ± 81) injected retinas (Fig. 5, B and C; means ± S.E., n = 5 in each group).
FIGURE 5.
Effects of STIL knockdown on DA-IPCs in wild-type retinas. A, Western blot of retinal lysates probed with anti-STIL antibodies. STIL was detected in wild-type and mismatched MO-treated (MM-MO) retinas, but not in Stil-MO-treated retinas (SM-MO). B, number of anti-TH positive DA-IPCs in MM-MO- and Stil-MO-treated wild-type retinas that received subtoxic 6-OHDA (white bars) or PBS (black bars) injections. Note the decrease in the number of DA-IPCs in Stil-MO-injected retinas after 6-OHDA treatment. Data represent the means ± S.E. (n = 5 in each group). ns, not significant; *, p < 0.01. C, fluorescent images of 6-OHDA-treated flat-mount retinas labeled with anti-TH antibodies. Note the decreased number of DA-IPCs in Stil-MO-treated retinas after 6-OHDA treatment. The images were taken at the nasal retina adjacent to the optic nerve. Scale bar, 100 μm.
Inhibition of Shh Signal Transduction Increased the Susceptibility of DA-IPCs to 6-OHDA
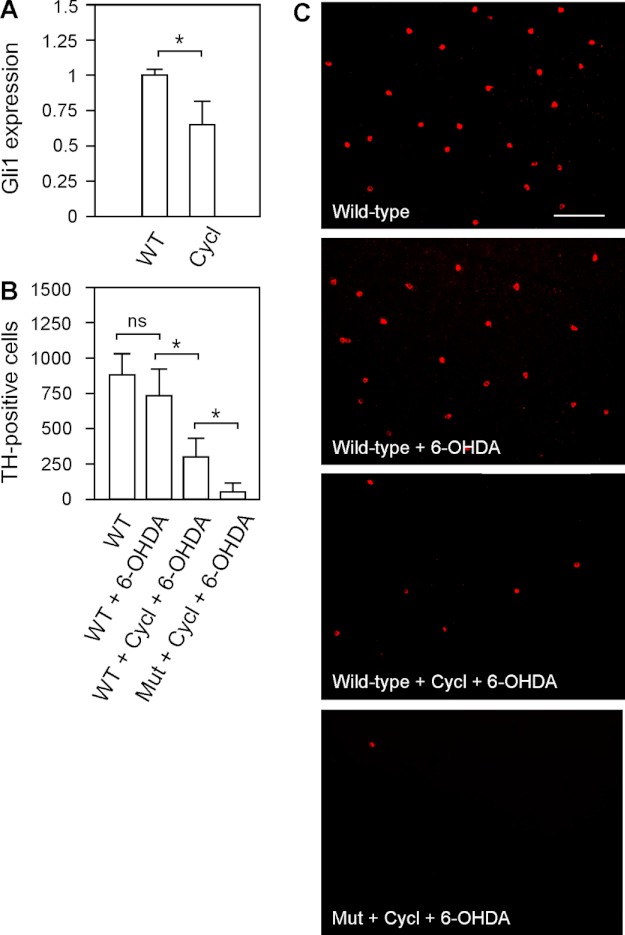
Previous studies have shown that Stil functions in the Shh signal transduction pathway (18, 19). To demonstrate the involvement of Stil-mediated Shh signaling transduction in zebrafish DA-IPCs, we examined the survival of DA-IPCs (in response to 6-OHDA treatment) in wild-type and nbb mutant zebrafish in which the Shh pathway was pharmacologically blocked by cyclopamine (31). The application of cyclopamine (by intraocular injection, 1 μm) efficiently inhibited Shh signaling transduction, as revealed by significant decreases in the transcription of one of the Shh-targeted genes, Gli1. In response to cyclopamine treatment, for example, the expression of Gli1 decreased to 0.65 ± 0.17 in comparison to untreated retinas (normalized to 1) (Fig. 6A; means ± S.E., n = 3 in each group).
FIGURE 6.
Effects of 6-OHDA on DA-IPCs in wild-type retinas treated with Shh signaling antagonist cyclopamine. A, RT-PCR analysis of Gli1 expression in control and cyclopamine-treated retinas. The injection of cyclopamine resulted in decreased Shh-targeted Gli1 gene expression. Data represent the means ± S.E. (n = 3 in each group). *, p < 0.01. B, number of anti-TH positive DA-IPCs in control, 6-OHDA-treated, and cyclopamine (Cycl)- and 6-OHDA-co-treated wild-type and heterozygous mutant retinas. Note the decrease in the number of DA-IPCs in co-treated retinas. Data represent the means ± S.E. (n = 9 in each group). ns, not significant; *, p < 0.01. C, fluorescent images of flat-mount control and treated retinas labeled with anti-TH antibodies. Note the decreased number of DA-IPCs in cyclopamine and 6-OHDA co-treated retinas. The images were taken at the nasal retina adjacent to the optic nerve. Scale bar, 100 μm. Mut, mutant.
After 24 h of cyclopamine treatment, subtoxic 6-OHDA was injected into the eyes. Three days later, the retinas were isolated and labeled with anti-TH antibodies. Immunolabeling experiments revealed that in cyclopamine-treated retinas, the toxic susceptibility of DA-IPCs to 6-OHDA increased. For example, in cyclopamine and 6-OHDA co-injected wild-type retinas, the number of DA-IPCs significantly decreased (301 ± 142) in comparison with the number of DA-IPCs in retinas which only received 6-OHDA injections (741 ± 190). In nbb retinas, co-treatment with cyclopamine and 6-OHDA resulted in a greater decrease in the number of DA-IPCs (52 ± 56) in comparison with wild-type retinas with the same treatment (Fig. 6, B and C; means ± S.E., n = 9 in each group).
Increase of Shh Signaling Transduction Protected DA-IPCs from Toxic Insult
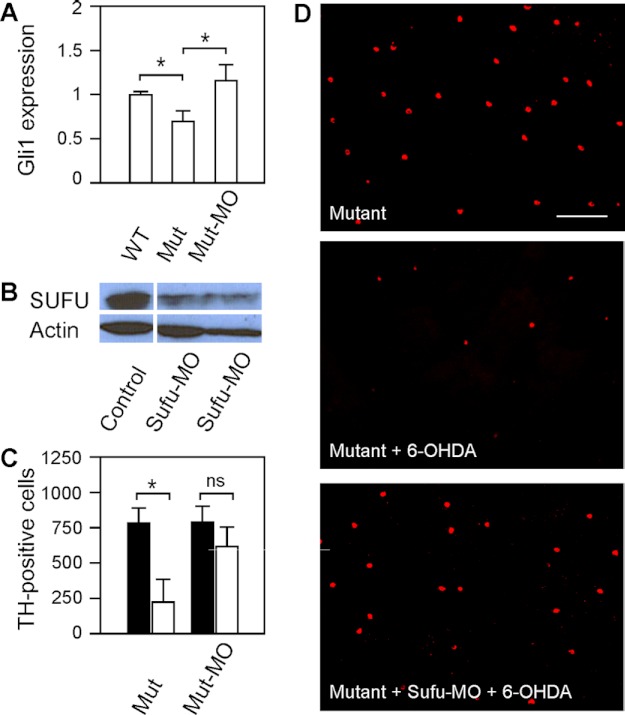
In nbb retinas, the Shh signal transduction pathway was down-regulated, as revealed by decreased levels of Shh-targeted Gli1 transcription. In nbb retinas, the expression of Gli1 decreased to 0.69 ± 0.11 in comparison with wild-type retinas (normalized to 1) (Fig. 7A; means ± S.E., n = 3 in each group). To further demonstrate the roles of Shh signaling in DA-IPCs (i.e. cell survival after 6-OHDA insult), we examined DA-IPCs in mutant retinas in which the Shh signaling transduction was up-regulated. This was achieved by inhibiting Sufu expression in mutant retinas. In the Shh pathway, Sufu functions as an inhibitor by binding to Gli1, thereby repressing Gli1-mediated downstream events, including transcription of Gli1 (17). We blocked Sufu translation by intraocular injections of anti-Sufu MOs (Fig. 7B). After the Sufu MO treatment, the expression of Gli1 increased to 1.17 ± 0.19 in comparison with wild-type retinas (Fig. 7A; means ± S.E., n = 3 in each group).
FIGURE 7.
Effects of 6-OHDA on DA-IPCs in mutant retinas after Shh up-regulation by Sufu knockdown. A, relative Gli1 mRNA expression in wild-type, nbb mutant, and Sufu-MO-treated nbb mutant retinas. The expression of Gli1 in wild-type retinas was normalized to 1. The expression of Gli1 decreased in mutant retinas but increased when the expression of Sufu was inhibited. Data represent the means ± S.E. (n = 3 in each group). *, p < 0.01. B, Western blot of retinal lysates probed with anti-SUFU antibodies. Note the decrease of Sufu expression in Sufu-MO treated retinas. C, number of anti-TH positive DA-IPCs in untreated and Sufu-MO-treated nbb retinas in response to subtoxic 6-OHDA (white bars) or PBS (black bars) injections. In nbb mutants, subtoxic 6-OHDA treatment resulted in significant losses of DA-IPCs. Treatment with Sufu-MO protected DA-IPCs from degeneration after exposure to 6-OHDA. Data represent the means ± S.E. (n = 8 in each group). *, p < 0.01; ns, not significant. (D) Fluorescent images of flat-mount nbb retinas labeled with anti-TH antibodies. Note the decreased number of DA-IPCs in 6-OHDA-treated mutant retinas and the rescue after Sufu-MO treatments. The images were taken at the nasal retina adjacent to the optic nerve. Scale bar, 100 μm. Mut, mutant.
Twenty-four hours after Sufu-MO treatments, subtoxic 6-OHDA was injected into the mutant eyes. Three days later, the retinas were labeled with anti-TH antibodies. Increase of Shh signaling transduction (by Sufu-MO treatment) protected mutant DA-IPCs from 6-OHDA induced degeneration. In Sufu-MO treated nbb retinas, treatment with subtoxic 6-OHDA did not cause degeneration of DA-IPCs. For example, the immunolabeling with anti-TH antibodies revealed no significant differences in the number of DA-IPCs in Sufu-MO and 6-OHDA co-treated mutant retinas (621 ± 145) in comparison with the number of DA-IPCs in Sufu-MO and PBS co-injected mutant retinas (800 ± 102) (Fig. 7, C and D; means ± S.E., n = 8 in each group).
DISCUSSION
The zebrafish nbb mutant provides a tool for studying the genetic and cellular mechanisms of CNS development and neural survival. The nbb mutation was discovered from a previous screening for visual system defect (15). Although the homozygous mutants are embryonic lethal, heterozygous mutants are viable with no obvious gross morphological defects but show a late onset of dopaminergic cell degeneration (15). In this research, we cloned the nbb locus. Sequence analysis of the nbb locus revealed that nbb is a homolog of the mammalian Stil gene. Previously, two zebrafish mutations in the Stil gene (hi1262Tg and cassiopeia) have been reported (21, 22). In cassiopeia mutants, the fish showed defects in mitosis (22). Complementation tests revealed that the nbb and cassiopeia mutations are allelic.4 In this research, we examined the roles of Stil expression in the survival of adult retinal DA-IPCs.
Deficiency in Stil expression results in increased toxic susceptibility of DA-IPCs to neurotoxic insult. This is evident in nbb and Stil-MO-treated retinas, in which the expression of Stil was reduced or completely inhibited. The roles of Stil in DA-IPCs are likely mediated by the Shh signal transduction pathway. Stil has been demonstrated to function downstream in the Shh pathway (18, 19, 33). Normally, Sufu inhibits Shh signaling by binding to Gli1, whereas Stil promotes Shh signaling transduction by binding to Sufu when the pathway is activated, thereby derepressing Gli1 (18). In nbb retinas, inhibition of Sufu translation (by Sufu-MO) increased Gli1 expression and increased the resistance of DA-IPCs to neurotoxins. By contrast, inhibition of Shh-signaling (by cyclopamine treatment) resulted in increased toxic susceptibility of DA-IPCs to neurotoxins.
The involvement of Stil in retinal cell proliferation is also evident. In wild-type retinas treated with 6-OHDA (at toxic concentration of 5 μg/μl), Stil expression increased, as well as PCNA (proliferating cell nuclear antigen) expression.5 The increased Stil expression was most evident at 6 days post 6-OHDA injections, during which time, degeneration of DA-IPCs had occurred and proliferation of inner retinal cells became robust. PCNA expression also peaked at 6 days post 6-OHDA injections, suggesting that the increase of Stil expression is required for augmenting cell proliferation. In nbb retinas treated with 6-OHDA, the expression of Stil only slightly increased, and the expression of PCNA was not robust as seen in 6-OHDA treated wild-type retinas, suggesting that functional expression of Stil is necessary for DA-IPC regeneration after degeneration.
In zebrafish, most tissues grow throughout the life of the animal, and virtually all cell types can be regenerated after injury (34–39). The high expression level of Stil in juvenile and adult fish may well explain this continual growth. In adult fish, the overall growth rate is reduced, but proliferation continues at a slower pace (i.e. fins and retinas), thus explaining the observed reduced Stil expression (lowest level). The observed small increases in Stil expression in older animals (e.g. after 12 months of age) may be required for cell proliferation to replace lost cells or repair injured tissues. Together, the data support the roles of functional expression of Stil in cell proliferation.
Based on previous findings (the roles of Stil expression in cell proliferation) and our observations (the roles of Stil expression on DA-IPC neural protection), we conclude that when the expression of Stil becomes deficient, two consequences will occur. First, cell survival in response to toxic insult or other stress factors decreases, i.e. DA-IPCs become more sensitive or less resistant to neurotoxins. Second, after neural degeneration, the rate of cell proliferation (regeneration) becomes less efficient and possibly requires a longer time frame. Regeneration may also remain incomplete after injury. In nbb retinas, for example, we observed decreased numbers of DA-IPCs starting at 4 months of age, although the decrease of DA-IPCs becomes greater after 12 months of age (15). This may be due to a combination of DA cell degeneration and lack of cell proliferation.
Acknowledgments
We thank the Zebrafish Center and the Freimann Animal Facility at the University of Notre Dame for maintaining the zebrafish colonies and Debi Smith for fish care and husbandry.
This work was supported in part by National Institutes of Health Grant R01EY013147, National Natural Science Foundation of China Grants 30970941 and 81171066, and Tianjin Science and Technology Committee Grant 12JCZDJC24000.
A. Carr, O. Li, L. Sun, and L. Li, unpublished results.
J. Li, A. Carr, E. Lee, L. Li, unpublished results.
- DA
- dopaminergic
- DA-IPC
- dopaminergic interplexiform cell
- 6-OHDA
- 6-hydroxydopamine
- Shh
- Sonic hedgehog
- Sufu
- suppressor of fused
- qRT-PCR
- quantitative RT-PCR
- TH
- tyrosine hydroxylase
- MO
- morpholino(s).
REFERENCES
- 1. Fuchs Y., Steller H. (2011) Programmed cell death in animal development and disease. Cell 147, 742–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosello A., Warnes G., Meier U. (2012) Cell death pathways and autophagy in the central nervous system and its involvement in neurodegeneration, immunity and central nervous system infection: to die or not to die–that is the question. Clin. Exp. Immunol. 168, 52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parsadanian A. S., Cheng Y., Keller-Peck C. R., Holtzman D. M., Snider W. D. (1998) Bcl-XL is antiapoptotic regulator for postnatal CNS neurons. J. Neurosci. 18, 1009–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arbour N., Vanderluit J. L., Le Grand J. N., Jahani-Asl A., Ruzhynsky V. A., Cheung E. C., Kelly M. A., MacKenzie A. E., Park D. S., Opferman J. T., Slack R. S. (2008) Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J. Neurosci. 28, 6068–6078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scott D. A., Tabarean I., Tang Y., Cartier A., Masliah E., Roy S. (2010) A pathologic cascade leading to synaptic dysfunction in α-synuclein-induced neurodegeneration. J. Neurosci. 30, 8083–8095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nguyen H. N., Byers B., Cord B., Shcheglovitov A., Byrne J., Gujar P., Kee K., Schüle B., Dolmetsch R. E., Langston W., Palmer T. D., Pera R. R. (2011) Lrrk2 mutant ipsc-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8, 267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mizuno Y., Hattori N., Mori H., Suzuki T., Tanaka K. (2001) Parkin and Parkinson's disease. Curr. Opin. Neurol. 14, 477–482 [DOI] [PubMed] [Google Scholar]
- 8. Lo Bianco C., Ridet J. L., Schneider B. L., Deglon N., Aebischer P. (2002) α-Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 99, 10813–10818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spencer B., Potkar R., Trejo M., Rockenstein E., Patrick C., Gindi R., Adame A., Wyss-Coray T., Masliah E. (2009) Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in α-synuclein models of Parkinson's and Lewy body diseases. J. Neurosci. 29, 13578–13588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bezard E., Imbert C., Deloire X., Bioulac B., Gross C. (1997) A chronic MPTP model reproducing the slow evolution of Parkinson's disease: Evolution of motor symptoms in the monkey. Brain Res. 766, 107–112 [DOI] [PubMed] [Google Scholar]
- 11. Schober A. (2004) Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 318, 215–224 [DOI] [PubMed] [Google Scholar]
- 12. Guo S., Wilson S. W., Cooke S., Chitnis A. B., Driever W., Rosenthal A. (1999) Mutations in the zebrafish unmask shared regulatory pathways controlling the development of catecholaminergic neurons. Dev. Biol. 208, 473–487 [DOI] [PubMed] [Google Scholar]
- 13. Rink E., Guo S. (2004) The too few mutant selectively affects subgroups of monoaminergic neurons in the zebrafish forebrain. Neuroscience 127, 147–154 [DOI] [PubMed] [Google Scholar]
- 14. Bretaud S., Lee S., Guo S. (2004) Sensitivity of zebrafish to environmental toxins implicated in Parkinson's disease. Neurotoxicol. Teratol. 26, 857–864 [DOI] [PubMed] [Google Scholar]
- 15. Li L., Dowling J. E. (2000) Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish. J. Neurosci. 20, 1883–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aplan P. D., Lombardi D. P., Ginsberg A. M., Cossman J., Bertness V. L., Kirsch I. R. (1990) Disruption of the human scl locus by 'illegitimate' v-(d)-j recombinase activity. Science 250, 1426–1429 [DOI] [PubMed] [Google Scholar]
- 17. Aplan P. D., Lombardi D. P., Kirsch I. R. (1991) Structural characterization of sil, a gene frequently disrupted in T-cell acute lymphoblastic leukemia. Mol. Cell. Biol. 11, 5462–5469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kasai K., Inaguma S., Yoneyama A., Yoshikawa K., Ikeda H. (2008) Scl/tal1 interrupting locus derepresses gli1 from the negative control of suppressor-of-fused in pancreatic cancer cell. Cancer Res. 68, 7723–7729 [DOI] [PubMed] [Google Scholar]
- 19. Izraeli S., Lowe L. A., Bertness V. L., Campaner S., Hahn H., Kirsch I. R., Kuehn M. R. (2001) Genetic evidence that sil is required for the sonic hedgehog response pathway. Genesis 31, 72–77 [DOI] [PubMed] [Google Scholar]
- 20. Erez A., Castiel A., Trakhtenbrot L., Perelman M., Rosenthal E., Goldstein I., Stettner N., Harmelin A., Eldar-Finkelman H., Campaner S., Kirsch I., Izraeli S. (2007) The sil gene is essential for mitotic entry and survival of cancer cells. Cancer Res. 67, 4022–4027 [DOI] [PubMed] [Google Scholar]
- 21. Golling G., Amsterdam A., Sun Z., Antonelli M., Maldonado E., Chen W., Burgess S., Haldi M., Artzt K., Farrington S., Lin S. Y., Nissen R. M., Hopkins N. (2002) Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat. Genet. 31, 135–140 [DOI] [PubMed] [Google Scholar]
- 22. Pfaff K. L., Straub C. T., Chiang K., Bear D. M., Zhou Y., Zon L. I. (2007) The zebra fish cassiopeia mutant reveals that sil is required for mitotic spindle organization. Mol. Cell. Biol. 27, 5887–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin Z. S., Yazulla S. (1994) Depletion of retinal dopamine increases brightness perception in goldfish. Vis. Neurosci. 11, 683–693 [DOI] [PubMed] [Google Scholar]
- 24. Li L., Dowling J. (2000) Effects of dopamine depletion on visual sensitivity of zebrafish. J. Neurosci. 20, 1893–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anichtchik O. V., Kaslin J., Peitsaro N., Scheinin M., Panula P. (2004) Neurochemical and behavioural changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Neurochem. 88, 443–453 [DOI] [PubMed] [Google Scholar]
- 26. Westerfield M. (2007) The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th Ed., University of Oregon, Eugene, OR [Google Scholar]
- 27. Kelly P. D., Chu F., Woods I. G., Ngo-Hazelett P., Cardozo T., Huang H., Kimm F., Liao L., Yan Y. L., Zhou Y., Johnson S. L., Abagyan R., Schier A. F., Postlethwait J. H., Talbot W. S. (2000) Genetic linkage mapping of zebrafish genes and ESTs. Genome Res. 10, 558–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou Y., Zon L. I. (2011) The zon laboratory guide to positional cloning in zebrafish, Methods Cell Biol. 104, 287–309 [DOI] [PubMed] [Google Scholar]
- 29. Shimoda N., Knapik E. W., Ziniti J., Sim C., Yamada E., Kaplan S., Jackson D., de Sauvage F., Jacob H., Fishman M. C. (1999) Zebrafish genetic map with 2000 microsatellite markers. Genomics 58, 219–232 [DOI] [PubMed] [Google Scholar]
- 30. Wang X., Huang L., Li Y., Li X., Li P., Ray J., Li L. (2011) Characterization of gfp-tagged gnrh-containing terminalis neurons in transgenic zebrafish. J. Cell. Physiol. 226, 608–615 [DOI] [PubMed] [Google Scholar]
- 31. Büttner A., Busch W., Klüver N., Giannis A., Scholz S. (2012) Transcriptional responses of zebrafish embryos exposed to potential sonic hedgehog pathway interfering compounds deviate from expression profiles of cyclopamine. Reprod. Toxicol. 33, 254–263 [DOI] [PubMed] [Google Scholar]
- 32. Thummel R., Kassen S. C., Montgomery J. E., Enright J. M., Hyde D. R. (2008) Inhibition of müller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev. Neurobiol. 68, 392–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Izraeli S., Lowe L. A., Bertness V. L., Good D. J., Dorward D. W., Kirsch I. R., Kuehn M. R. (1999) The sil gene is required for mouse embryonic axial development and left-right specification. Nature 399, 691–694 [DOI] [PubMed] [Google Scholar]
- 34. Kishimoto N., Shimizu K., Sawamoto K. (2012) Neuronal regeneration in a zebrafish model of adult brain injury. Dis. Model Mech. 5, 200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chablais F., Jazwinska A. (2012) The regenerative capacity of the zebrafish heart is dependent on tgfβ signaling. Development 139, 1921–1930 [DOI] [PubMed] [Google Scholar]
- 36. Shao J., Chen D., Ye Q., Cui J., Li Y., Li L. (2011) Tissue regeneration after injury in adult zebrafish: The regenerative potential of the caudal fin. Dev. Dyn. 240, 1271–1277 [DOI] [PubMed] [Google Scholar]
- 37. Powell C., Elsaeidi F., Goldman D. (2012) Injury-dependent müller glia and ganglion cell reprogramming during tissue regeneration requires apobec2a and apobec2b. J. Neurosci. 32, 1096–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Becker C. G., Lieberoth B. C., Morellini F., Feldner J., Becker T., Schachner M. (2004) L1.1 is involved in spinal cord regeneration in adult zebrafish. J. Neurosci. 24, 7837–7842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thummel R., Kassen S. C., Enright J. M., Nelson C. M., Montgomery J. E., Hyde D. R. (2008) Characterization of müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp. Eye Res. 87, 433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]