Background: Does the C-terminal cationic chondroadherin sequence bind cell surface proteoglycans to modulate cell behavior?
Results: Chondroadherin and its C-terminal domain bind tightly to heparin and select proteoglycans, eliciting signals and cell spreading.
Conclusion: Peptides binding cell surface proteoglycans provide modulation of responses to cell receptor interactions.
Significance: These findings provide insights into the role of extracellular matrix in regulating cellular activities.
Keywords: Cartilage Biology, Cell Adhesion, Cell Surface Receptor, Heparan Sulfate, Heparin, Heparin-binding Protein, Proteoglycan, Chondroadherin, Syndecan
Abstract
Chondroadherin, a leucine-rich repeat family member, contains a very C-terminal sequence CKFPTKRSKKAGRH359, now shown to bind to heparin with a KD of 13 μm. This observation led us to investigate whether chondroadherin interacts via this C-terminal heparin-binding domain with glycosaminoglycan chains of proteoglycans at the cell surface. Cells were shown to bind this heparin-binding peptide in FACS analysis, and the interaction was shown to be with glycosaminoglycans because it was abolished when sulfation was inhibited by chlorate treatment of the cells. In separate experiments, heparin and heparan sulfate inhibited the peptide interaction in a dose-dependent manner. Using a human chondrosarcoma and a murine osteoblast cell line, heparan sulfate proteoglycans were identified as the cell surface receptors involved in the binding. Different binding syndecans were identified in the two different cell lines, indicating that the same protein core of a proteoglycan may have structural and functional differences in the attached heparan sulfate chains. Upon binding to coated peptide, cells spread, demonstrating engagement of the cytoskeleton, but no focal adhesion complex was formed. The number of cells adhering via their β1 integrin receptor to collagen type II or chondroadherin was profoundly and rapidly enhanced by the addition of the heparin-binding peptide. The peptide added to the cells caused ERK phosphorylation, showing that it triggered intracellular signaling. The results show that heparan sulfate chains differ between various members of the proteoglycan families on a given cell, but also differ between the same proteoglycan on different cells with a potential for differential regulation of cellular activities.
Introduction
Cell-matrix interactions play a vital role in tissue homeostasis. The chondrocyte is the only cell type in cartilage and occupies less than 10% of the total tissue volume. The cell is surrounded by an extensive extracellular matrix, which provides for key features of mechanical stability and resistance to load. A major constituent of this matrix is the collagen network with type II representing about 95% of the collagens (1). The overall organization and function of the network also depend on associated collagenous (exemplified by collagen IX and XI) and noncollagenous proteins such as leucine-rich repeat proteins e.g. fibromodulin, PRELP, asporin, and decorin as well as other proteins including COMP (2–4) and matrilins (5). The major functions of the collagen network are to provide tensile strength and retention of the negatively charged aggrecan, the other major component of the tissue (6). Aggrecan is active in retaining water, which is important for the cartilage resistance to deformation. A distinct collagenous network, with collagen VI as its major constituent, is located closer to the cells in the territorial matrix and interacts back to the collagen II-based network as well as to aggrecan indirectly via a linker module of biglycan/decorin and a matrilin (7). Although the role of this network is not clear, its interactions indicate a function in tissue assembly and cell protection.
Matrix assembly and remodeling to adapt to new requirements are an important feature of cartilage and essential in adapting to new load requirements and in correcting effects of wear and tear, e.g. fatigue. This process is orchestrated and finely tuned by the chondrocytes.
An important element in this regulation is the ability of the cells to use a diversity of surface receptors to interact with matrix proteins or protein fragments. These receptors include integrins (8), syndecans (9), and collagens (10), such as those binding to hyaluronan (11, 12); the discoidin family (11); as well as receptors for growth factors and cytokines (13). There are also molecules at the cell surface that do not directly cause signals when binding their ligand. Examples are hyaluronan and the glycosylphosphatidylinositol-anchored glypicans, which may still have roles in the communications of the cells with their surroundings. Several matrix proteins contain both integrin-binding and glycosaminoglycan-binding domains, e.g. fibronectin, and the formation of certain signaling complexes depends on targeting more than one cell surface receptor (14). There are a number of distinct integrins, where one of some 18 α chains combines with one of eight β chains to form the specific receptor. These have different ligands and elicit different responses when occupied by their particular interaction partner (8, 15). In most cases, an interaction between a matrix protein and an integrin elicits tyrosine phosphorylation in a signaling cascade and interactions with the cytoskeleton. Downstream events are cell spreading, migration, and/or division. Another class of signaling cell surface molecules are the syndecans. This family of four transmembrane heparan sulfate proteoglycans (16–18) commonly contains heparan sulfate chains, which can bind growth factors such as basic fibroblast growth factor and present them to their receptor. These glycosaminoglycan chains also bind a variety of matrix proteins including fibronectin, laminin, tenascin, vitronectin, collagens, and thrombospondins 1 and 2 (19). Cells attach and spread on fibronectin with the formation of a complete focal adhesion complex requiring engagement of both integrin and syndecan receptors. This has particularly been studied for syndecan 4 in combination with integrin α5β1(14).
Chondroadherin belongs to the family of leucine-rich repeat proteins. There are two forms of the protein in cartilage, only one containing the basic C-terminal extension peptide (20, 21). Like other members of this family, the protein binds to triple helical collagen with high affinity (22). Chondroadherin binds cells via the α2β1 integrin. Upon binding, cells remain round, which is unlike the spreading normally observed when matrix proteins bind to an integrin (23–25).
In this study, we demonstrate that chondroadherin in solution binds to heparin structures including those of syndecans. Indeed, in the cells studied, binding appears selective for heparan sulfate among the glycosaminoglycans. The isolated chondroadherin C-terminal heparin-binding domain (hbd)4 of 13 amino acids was shown to stimulate bound chondrocytes to spread and to prominently increase attachment to integrins with the formation of focal adhesion complexes.
EXPERIMENTAL PROCEDURES
Materials and Antibodies
Collagen type II was isolated from bovine nasal cartilage by pepsin digestion (42). Alexa Fluor 488-conjugated Streptavidin was from Life Technologies.
Mouse monoclonal anti-vinculin antibodies (V9131) and phalloidin-TRITC (P1951) were from Sigma. Alexa Fluor 488 goat anti-mouse antibody, Alexa Fluor 488 donkey anti-mouse antibody (A21202), and DAPI were from Molecular Probes (Eugene, OR). FITC goat anti-rabbit antibody (111-095-045) was from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
The chondroadherin specific rabbit antibody has been described (26), and the pan-syndecan antibody as well as the specific mouse syndecan 1–4 antibodies were kind gifts from Dr. Rapraeger, University of Wisconsin-Madison. Antibodies against human syndecan 1 (sc-12765) were from Santa Cruz Biotechnology; human syndecan 2 (36-6200), human syndecan 3 (36-2400), and syndecan 4 (PAB9045) were from Abnova, San Francisco, CA.
Anti-rabbit HRP antibodies, phospho-ERK antibodies (phospho-p44/42MAP kinase), and total ERK1/ERK2 antibodies were from Cell Signaling Technology Inc. Heparitinase (heparitinase I) and chondroitinase ABC were from Seikagaku. COMPLETE®, EDTA-free protease inhibitor mixture tablets were from Roche Diagnostics GmbH. Heparin was from Sigma. Glycosaminoglycans were those obtained from the National Institutes of Health standard.
Expression and Purification of Recombinant Protein
Recombinant chondroadherin was expressed in (U293) EBNA cells (22) or in Escherichia coli M15 (pREP4) and purified as described (24). Recombinant chondroadherin expressed without the cationic most C-terminal part of 13 amino acids with the amino acids PGWAA as a C-terminal extension was generated using the primer 5′-ATGGTCCGCCCAATGCTC-3′ with a flanking HindIII site and 5′-ACGCCTTCCGCAGCTGCCCGGGCTGGGCTGCCTAG-3′ with a flanking BamHI site and expressed in EBNA cells in the same manner as described in Ref. 22.
Mass Spectrometry
Mass spectrometry (MS) was performed using a Bruker Scout 384 Reflex III matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS instrument. The mass spectrometer was used in the positive ion mode with delayed extraction and an acceleration voltage of 26 kV. Peptide samples were analyzed using the reflector detector, and 50–150 single-shot spectra were accumulated for improved signal-to-noise ratio. The ProFound software was used to identify the obtained peptides.
Synthetic Peptides
Peptides corresponding to the C-terminal hbd-CKFPTKRSKKAGRH359 and biotin-CKFPTKRSKKAGRH359 were synthesized and purified by reversed phase chromatography by Schafer-N (Copenhagen, Denmark). Biotin-CRFPTRKSRRAGKH359 was synthesized and purified by GenScript. The structures of the peptides were verified by mass spectrometry.
Heparin Binding
Intact recombinant chondroadherin (30 μg, expressed in EBNA cells) diluted in PBS (150 mm NaCl, 5 mm sodium phosphate, pH 7.4) and the chondroadherin protein without the basic most C-terminal amino acids (t-CHAD) (200 μg) as well as the hbd-CKFPTKRSKKAGRH359 (30 μg) were passed over a 1-ml heparin-Sepharose column (Amersham Biosciences), which was subsequently washed with 3 volumes of starting buffer, 5 mm sodium phosphate, 0.075 m NaCl, pH 7.4 (0.15 m NaCl in 5 mm sodium phosphate, pH 7.4, in the case of t-CHAD) and eluted with a gradient of 20 ml of 0.075–1 m NaCl (0.15–1 m in the case of t-CHAD) in 5 mm sodium phosphate, pH 7.4.
Isothermal Titration Calorimetry (ITC)
ITC was performed using a MicroCal VP-ITC microcalorimeter (MicroCal, Northampton, MA). Heparin (H0777, Sigma) was dissolved in PBS at 0.3 mm (4.5 mg/ml) calculated on an average mass of 15,000 daltons. The short hbd-KFPTKRSKKAGRH359 was dissolved at 0.1 mm in the same buffer and filled into the calorimeter cell. The heparin solution was titrated into the peptide solution by injections of 5 μl with a delay of 180 s between the injections. The effect of dilution of the heparin solution in the titration cell was controlled by a blank titration with heparin into the buffer solution. All experiments were performed at 30 °C. To abolish noise in the measurements of the sample, the heparin blank titration was subtracted before the calculation. The binding constant was evaluated by using the ITC data analysis in Origin® software supplied with the MicroCal VP-ITC instrument.
Cells and Cell Culture
The cell line (105kc), originating from a human chondrosarcoma, was a kind gift from Dr. Sven Inerot, The Sahlgrenska University Hospital, Gothenburg, Sweden. The 105kc cells were cultured in a mix of 40% Dulbecco's modified Eagle's medium, 40% minimum essential medium α, and 10% Ham's F12, supplemented with 100 nm hydrocortisone, 100 ng/ml insulin, 10% fetal bovine serum, 25 μg/ml ascorbic acid, 50 IU of penicillin, and 50 μg/ml streptomycin (Life Technologies).
Mouse osteoblasts (MC3T3-E1) were cultured in Ham's F12, 10% fetal bovine serum. Human chondrocytes (kindly provided by Dr. Recklies, Shriners hospital, Montreal, Canada) were prepared from cartilage obtained at autopsy from a 2-year-old individual. Cells were isolated as described previously (27) and stored frozen after passage 1. For the work described here, frozen chondrocytes were expanded and used at passage level 5. Cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 100 μg/ml penicillin plus 100 units/ml streptomycin.
Bovine articular chondrocytes were isolated by collagenase (CLS1; Worthington Biochemical Corp.) digestion of articular cartilage from 4–6-month-old calves as described (28). Briefly, cartilage slices were digested for 16 h with the crude collagenase in Earle's balanced salt solution (Gibco). The cells were filtered through a 100-μm nylon filter and washed three times in PBS containing 0.2% BSA (11930, SERVA Electrophoresis).
To harvest cells, culture dishes were rinsed three times with Ca2+/Mg2+-free PBS and briefly incubated with 0.5% trypsin and 1 mm EDTA. Detached cells were suspended in PBS containing 1 mg/ml trypsin inhibitor (Sigma) and then washed in PBS. Cells contain membrane-bound collagens, e.g. type XIII (29), which were removed by treatment with collagenase (CLSPA, Worthington Biochemical) at 100 units/ml in PBS for 30 min at 37 °C. Cells were then washed in PBS.
Sodium Chlorate Treatment of the Human 105kc Cells
Human 105kc cells were plated in culture dishes and allowed to adhere overnight. The cells were rinsed with PBS followed by Ham's F12 medium. The cells were incubated for 24 h in Ham's F12 medium containing either 0.13 m NaCl or 0.13 m sodium chlorate (NaClO3). The cells were detached by a brief incubation with 5 mm sodium phosphate, pH 7.4, 0.5 mm EDTA, 0.3 m NaCl and directly used in FACS analysis assay of peptide binding as described below. In parallel, cells were incubated in the same manner as above but in the presence of [35S]sulfate. Cell extracts were separated by SDS-PAGE and analyzed for the presence of incorporated sulfate by autoradiography.
Immunochemical Staining
Chamber slides (four-chamber; Lab-Tek®, Nunc Inc., Naperville, IL) were coated overnight with 5 μg/ml chondroadherin in 4 m guanidine-HCl, 50 mm sodium acetate, pH 5.8 or 33 μg/ml (20 μm) hbd-CKFPTKRSKKAGRH359 and blocked for nonspecific binding with 0.5% BSA in PBS for 3 h. Human chondrosarcoma cells (105kc, 100,000 cells/chamber) were allowed to adhere and spread for 1 h with or without the peptide hbd-CKFPTKRSKKAGRH359. Unbound cells were removed, and adherent cells were fixed in 2% paraformaldehyde in PBS. Cells were permeabilized with 0.5% Triton X-100 in PBS, and background binding was blocked with 0.1% BSA in PBS followed by incubation with an anti-vinculin antibody (1:200 in PBS, 0.1% BSA) for 1 h. After washing in PBS, sections were incubated with Alexa Fluor 488-conjugated antibodies (1:200 in PBS, 0.1% BSA) and phalloidin-TRITC (50 μg/ml) for 30 min. Washed sections were then incubated with DAPI (1:2000 in PBS), washed twice with PBS and once in Milli-Q-water, and mounted with AquaPerm mounting medium (IMMUNONTM, Thermo Fisher Scientific). All incubations were carried out at room temperature. Microscopy was carried out using a Leica DM 4000B microscope equipped with a DFC420 digital camera.
Cell Adhesion
Tissue culture 48-well or 5-cm dishes (Nunclon, Nunc, Denmark) were coated overnight with 5 or 0.04 μg/ml full-length chondroadherin in 4 m guanidine-HCl, 50 mm sodium acetate, pH 5.8. Alternatively, coating of the hbd-CKFPTKRSKKAGRH359 was at 0.16, 0.6, 2.5, 10, or 20 μm in PBS. The C-terminally truncated chondroadherin (t-CHAD) was coated overnight at 5 μg/ml in PBS. Collagen type II was diluted with PBS from a 0.5 m acetic acid stock solution immediately prior to coating at 5 or 0.04 μg/ml. Coated surfaces were blocked for nonspecific binding with 0.5% BSA in PBS for 3 h.
To test cell binding, the chondrosarcoma cells or bovine chondrocytes were suspended in PBS containing 0.1% BSA, 25 units/ml collagenase (CLSPA-grade, Worthington) and added to the wells (50 000 cells/well), in the presence (250 μg/ml, 150 μm) or absence of the synthetic peptide. Nonadherent cells were removed after 1 h, and bound cells were quantified by measuring lysosomal N-acetylglucosaminidase (30). Cell adhesion and spreading were visualized by light microscopy.
Flow Cytometric Analysis
Human chondrosarcoma cells (105kc) were detached with 0.5 mm EDTA in 5 mm sodium phosphate, pH 7.4, 0.3 m NaCl prior to incubation for 30 min on ice with the synthetic hbd-peptide biotin-CKFPTKRSKKAGRH359 or variant peptide biotin-CRFPTRKSRRAGKH359 in complex with streptavidin-Alexa Fluor 488. For the inhibition assay, National Institutes of Health reference standard glycosaminoglycans (heparan sulfate, heparin, CS-A, CS-B, CS-C, or keratan sulfate, at 100, 20, 4, 0.8 and 0.16 μg/ml) were preincubated for 30 min on ice with the peptide-streptavidin Alexa Fluor 488 complex. The glycosaminoglycan-peptide mixture was then allowed to interact with the cells for 30 min. Alternatively, antibodies recognizing the different syndecans were allowed to interact with the cells followed by either FITC-conjugated goat anti-rabbit antibody or Alexa Fluor 488-conjugated sheep anti-mouse antibody. All cells were washed in PBS, 0.5% BSA after incubations. Background staining was assessed by omitting the peptide or the primary antibody. Antibody binding to cells was analyzed using a FACSCalibur (BD Biosciences).
Cell Signaling
Tissue culture 6-well dishes (Costar, Corning Life Science) were coated overnight with 5 μg/ml chondroadherin in 4 m guanidine-HCl, 50 mm sodium acetate, pH 5.8, or 20 μm of the hbd-CKFPTKRSKKAGRH359 in PBS. The dishes were blocked for nonspecific binding with 0.5% BSA in PBS for 1 h. To determine ERK phosphorylation, the human chondrocytes were serum-starved for 48 h prior to the experiment. To harvest cells, culture dishes were rinsed three times with Ca2+/Mg2+-free PBS, and the cells were incubated for 30 s with 0.5% trypsin, 1 mm EDTA. Detached cells were suspended in PBS containing 1 mg/ml trypsin inhibitor (Sigma) and then washed in PBS, suspended in PBS containing 0.1% BSA, added to the wells coated with chondroadherin or BSA (500,000 cells/well, 2 ml/well), and allowed to adhere for 1 h at 37 °C. All cells (bound and unbound) were collected and lysed with SDS-PAGE (150 μl) sample buffer. Aliquots (30 μl) of the cell lysates were separated by linear 10% SDS-PAGE under reducing conditions and transferred electrophoretically to nitrocellulose membranes. Blots were incubated with the phosphorylation-specific ERK antibody at dilutions recommended by the manufacturer. Following visualization using the ECL chemiluminescence system (GE Healthcare, Uppsala, Sweden), antibodies were removed from the membranes by incubation for 1 h at room temperature in a 0.2 m Tris/glycine buffer, pH 2.8 (containing 0.1% SDS and 0.1% Tween 20). The membranes were reprobed with an antibody to determine total ERK1/ERK2. Gel-Pro Analyzer® software (Media Cybernetics) was used for scanning of films representing the exposure of the blots for quantification of phosphorylation.
Coupling of Affinity Matrix
Recombinant chondroadherin (expressed in EBNA cells) was coupled to Mini-Leak agarose (Kem-En-Tec, A/S) (2.5 mg/ml agarose) according to the manufacturer's instructions. The control agarose was treated in the same manner but with no protein added. The synthetic hbd-CKFPTKRSKKAGRH359 was coupled to UltraLink agarose (Pierce, Thermo Fisher Scientific) via the N-terminal cysteine residue, and excessive binding sites were blocked according to the instructions of the manufacturer. Control matrix was treated in the same manner, omitting the peptide.
Surface Labeling
Human chondrosarcoma cells (105kc, 20 × 106/ml) suspended in PBS were incubated with EZ-Link sulfo-NHS-LC biotin (Pierce, Thermo Fisher Scientific) at a final concentration of 1 mg/ml, for 1 h at room temperature. The cells were then incubated for 10 min in 0.1 m glycine in PBS to block further reactivity of free biotin and washed three times in PBS, and the membrane fraction was isolated as described below (“Membrane Preparation for Proteoglycan Isolation”).
Membrane Preparation for Proteoglycan Isolation
Unlabeled or biotin-labeled 105kc cells were suspended and homogenized in 10 mm KCl, 1 mm EDTA, 20 mm Tris/HCl, pH 7.4 (1 ml/106 cells). Nuclei were removed by centrifugation at 1500 × g for 5 min. NaCl (0.15 m) was added to the supernatant, and the membrane fraction was collected by centrifugation at 50,000 × g for 30 min. The pellet was suspended in 8 m urea, 2% Triton-X-100 in 25 mm Tris, pH 7.4, 0.15 m NaCl (TBS) supplemented with COMPLETE® proteinase inhibitor. The lysate was cleared by centrifugation at 14,000 × g for 20 min.
The MC3T3-E1 cells were prepared in the same manner as above except that 1 m NaCl was added to the supernatant after the first centrifugation. The pellet was in this case suspended in 6 m urea, 1% Triton-X-100, 3% Tween 20, 3% Nonidet P-40, 0.5% sodium deoxycholate in 25 mm Tris, pH 7.4, 0.15 m NaCl (TBS) supplemented with COMPLETE® for 1 h in 4 °C and cleared as above.
Affinity Purification of Chondroadherin-binding Proteoglycans
The affinity matrices (1 ml) of control and chondroadherin were packed in separate mini columns (Bio-Rad) and equilibrated with 20 volumes of running buffer (6 m urea, 1% Triton-X-100 in TBS, pH 7.4), supplemented with COMPLETE®. Lysates of biotin-labeled cells in the same buffer were passed twice over the control column and then incubated overnight with the affinity matrix column with continuous end-over-end mixing at 4 °C. The affinity and control matrix columns were washed with 20 volumes of running buffer and eluted (1-ml fractions) with 0.5 m NaCl, 6 m urea in TBS, pH 7.4, supplemented with COMPLETE®. Eluted proteins were precipitated with ethanol and resuspended in heparitinase buffer (50 mm Hepes, 50 mm sodium acetate, 150 mm NaCl, pH 6.5). Half of the material was digested with heparitinase (0.3 milliunits) and chondroitinase ABC (100 milliunits) at 37 °C overnight. Undigested and digested materials were separated by a 4–12% gradient SDS-polyacrylamide gel under reducing conditions and electrotransferred to a PVDF membrane (Pall Corp.). Nonspecific binding to the membrane was blocked with 2% BSA in 10 mm Tris-HCl, 0.15 m NaCl, 2% Tween, pH 7.4 (blocking buffer). Biotin-labeled proteins were visualized after incubation with streptavidin-HRP (1:5000 in blocking buffer) by chemiluminescence detection using the ECL system.
Identification of Syndecans on Human Chondrosarcoma 105kc Cells and MC3T3-E1 Cells
To demonstrate that 105kc and MC3T3-E1 cells express syndecan molecules, they were homogenized in PBS, 1% Triton and COMPLETE® proteinase inhibitor. The lysates were cleared by centrifugation (12,000 × g, 20 min) and precipitated with 3 volumes of methanol.
The precipitates were dissolved in heparitinase buffer and digested at 37 °C as described above. Syndecans present and expressed by the 105kc or MC3T3-E1 cells were separated on a 10% SDS-polyacrylamide gel under reducing conditions and electrophoretically transferred to a PVDF membrane. The membrane was blocked for nonspecific binding with blocking buffer. Syndecans present were detected with the various syndecan antibodies (1 μg/ml) followed by the appropriate secondary HRP-tagged antibody (anti-rabbit or anti-mouse antibody) and detected by chemiluminescence using the ECL system.
Affinity Purification of Membrane Proteoglycans Binding to Synthetic hbd-CKFPTKRSKKAGRH359
The procedure for affinity purification was the same as that used for the biotin-tagged proteoglycan, except that the 105kc cells were unlabeled and the peptide was used for affinity. The cell membrane fraction of the MC3T3-E1 cells was applied to the peptide column equilibrated in 6 m urea, 1% Triton-X-100, 3% Tween 20, 3% Nonidet P-40, 0.05% sodium deoxycholate in 25 mm Tris, pH 7.4, 0.15 m NaCl (TBS) supplemented with COMPLETE® and washed with the same buffer. Proteins present were eluted with 1.5 m NaCl in the same buffer as above and precipitated with ethanol, resuspended in heparitinase buffer, and divided into two aliquots where one was digested with heparitinase at 37 °C overnight. Digested materials were separated by linear 10% SDS-polyacrylamide gels (reducing conditions) and electrotransferred to a PVDF membrane. The Western blot procedure for detecting the presence of syndecans was performed as described above.
Immunoprecipitation
Biotin-labeled cell surface material was affinity-purified using immobilized chondroadherin as described above. To change buffer, fractions were passed over a PD-10 column (Amersham Biosciences), equilibrated, and eluted with 0.1% Triton X-100 in TBS supplemented with COMPLETE®. Eluted material was incubated overnight with a pan-syndecan antibody (5 μg/ml), and the immune complex was collected using protein-A-Sepharose. The gel beads were washed three times with heparitinase buffer, resuspended in the same buffer, and digested with heparitinase at 37 °C for 16 h with two additions of 0.3 milliunits each, 2 h apart. Immunoprecipitated proteins were eluted with SDS sample buffer, separated by SDS-PAGE on 10% gels (reducing conditions), and electrotransferred to PVDF membranes. The membranes were blocked with blocking buffer, and proteins were visualized after incubation with streptavidin-HRP (1:5000 in blocking buffer) by chemiluminescence detection using the ECL system.
RESULTS
Chondroadherin Binding to Heparin
The very C-terminal part of chondroadherin (KFPTKRSKKAGRH) contains a heparin-binding consensus sequence similar to the one described by Cardin et al. (31), XBBXBX, and the corresponding synthesized peptide interacts with heparin (21). It is, however, not known whether the sequence is exposed in the intact protein. To address this, purified recombinant chondroadherin was passed over a heparin-Sepharose column followed by a wash with 5 mm sodium phosphate, 0.075 m NaCl, pH 7.4, and then eluted with a gradient from 0.075 to 1 m NaCl. We found that chondroadherin (pI 9.43) indeed binds to heparin and elutes as a broad peak with maximum at 0.6 m NaCl (Fig. 1).
FIGURE 1.
Chondroadherin binding to heparin. Recombinant chondroadherin (30 μg) expressed in EBNA cells and the hbd-CKFPTKRSKKAGRH359 (30 μg), respectively, were passed over a heparin-Sepharose column (Amersham Biosciences). The column (1 ml) was washed with 5 mm sodium phosphate, 0.075 m NaCl, pH 7.4, and eluted with a gradient of 0.075–1 m NaCl (100% B) in 5 mm sodium phosphate, pH 7.4. Recombinant chondroadherin eluted with the peak at 0.6 m NaCl, whereas the peptide showed higher affinity eluting at 0.8 m NaCl (inset). The SDS-PAGE shows the preparation of chondroadherin applied to the heparin column (inset).
For comparison, chondroadherin, lacking the C-terminal heparin-binding domain (pI 9.02), was expressed in EBNA cells in the same manner as the full-length chondroadherin protein. The truncated protein is still very basic and binds to heparin but eluted from the column at a lower ionic strength of 0.5 m NaCl (supplemental Fig. 1). This demonstrates that the C-terminal domain contributes to heparin binding of this overall very basic protein.
Further proof was obtained in studies of the isolated peptide in the form of synthetic hbd-CKFPTKRSKKAGRH359. This peptide was found to elute at 0.8 m NaCl from the heparin-Sepharose column (Fig. 1, inset), confirmed by MALDI-TOF MS.
ITC measurement of binding of the hbd-CKFPTKRSKKAGRH359 peptide to heparin was performed to evaluate the interaction. This identified a KD of 13 μm, which indicates a high affinity between the two molecules (supplemental Fig. 2).
Cellular Interaction of the Heparin-binding Peptide Analyzed by FACS
Heparin-binding domains, e.g. in fibronectin, have the potential to interact with cell surface proteoglycans. FACS analysis was performed to evaluate whether the heparin-binding domain of chondroadherin had similar features. The hbd-biotin-CKFPTKRSKKAGRH359 was incubated with 105kc cells, and binding was evaluated by flow cytometry. The peptide already showed strong binding at the lowest dose tested (5 μm) (Fig. 2A). To verify that the interaction was mediated via sulfated glycosaminoglycans of cell surface proteoglycans, cells were treated with sodium chlorate. The cells were shown to not incorporate any sulfate to the cell surface proteoglycans (Fig. 2B, inset). The hbd-biotin-CKFPTKRSKKAGRH359 peptide showed little remaining affinity to cells, indicating that binding requires sulfated glycosaminoglycans at the cell surface (Fig. 2B).
FIGURE 2.
Binding of the heparin-binding peptide CKFPTKRSKKAGRH359 to cells demonstrated by FACS. A, 105kc cells were suspended in PBS, 0.5% BSA and incubated on ice in the presence of the hbd-biotin-CKFPTKRSKKAGRH359 in complex with Alexa Fluor 488-conjugated streptavidin (streptavidin-488) (thick line) or streptavidin-488 (thin line) and analyzed for binding by FACS analysis. B, 105kc cells cultured in medium with sodium chlorate present were detached and incubated on ice in the presence of the hbd-biotin-CKFPTKRSKKAGRH359 in complex with streptavidin-488 (thick line) or streptavidin-488 (thin line) and analyzed for binding by FACS analysis. Inset: autoradiography of 105kc cell cultured with or without sodium chlorate in the presence of [35S]sulfate.
To further study the specificity of the binding of the hbd-CKFPTKRSKKAGRH359 peptide, a mutated variant was designed where all arginine residues were exchanged for lysine residues and all lysine residues were exchanged for arginine residues (CRFPTRKSRRAGKH). The peptides were compared for binding to the human chondrosarcoma cells by FACS analysis. Interestingly, the original peptide showed four times higher affinity in binding to the cell surface proteoglycans (Fig. 3, A and B).
FIGURE 3.

Binding of the hbd-CKFPTKRSKKAGRH359 and the variant peptide CRFPTRKSRRAGKH to cells demonstrated by FACS. 105kc cells were suspended in PBS, 0.5% BSA and incubated on ice in the presence of the hbd-biotin-CKFPTKRSKKAGRH359 (A) or biotinylated control variant peptide CRFPTRKSRRAGKH (B) at 2.5, 5, and 10 μm in complex with streptavidin-488 and analyzed for binding by FACS.
Identification of Membrane Proteoglycans Binding to Chondroadherin and Synthetic hbd-CKFPTKRSKKAGRH359
As the heparin-binding domain was found to be exposed in the intact protein in solution and the hbd-biotin-CKFPTKRSKKAGRH359 interacts with cell surface components, we set out to identify the receptor responsible for this interaction. Human chondrosarcoma cells (105kc) were surface-labeled with biotin, and membrane proteins were isolated and affinity-purified on chondroadherin-agarose. Affinity purification was carried out at high urea concentrations to abolish protein-to-protein interactions, thereby focusing on glycosaminoglycan-to-protein interactions. Bound material was eluted with 0.5 m NaCl, separated on a 4–12% gradient SDS-polyacrylamide gel, and detected by Western blot analysis before and after a combined heparitinase and chondroitinase ABC treatment (Fig. 4). Untreated affinity-purified material migrated as a diffuse band with a molecular mass range distributed around 250 kDa. The size was reduced to a distinct band corresponding to ∼50 kDa after enzyme digestions, clearly demonstrating that the component represents a proteoglycan (Fig. 4). In a separate experiment, similar results showing one major band at 50 kDa were obtained after digestion with heparitinase only (data not shown), demonstrating that the proteoglycan carries heparan sulfate chains. The data suggest that syndecans at the cell surface represent the receptor. Indeed, the 105kc cells appear to express most, if not all, of the syndecans (Figs. 5 and 6A). The presence of the four proteoglycans was demonstrated by FACS analysis (Fig. 5) using specific antibodies. In parallel, their core proteins were identified using a pan-syndecan antibody in Western blots of cell extracts after heparitinase digestion (Fig. 6A). This experiment further verified that these syndecans carry heparan sulfate side chains by the necessity to apply digestion with heparitinase to visualize their core proteins as distinct bands.
FIGURE 4.
Chondroadherin binding to membrane proteoglycans on 105kc cells. Biotin-labeled membrane proteins (from 20 × 106 cells) were passed over a control agarose column followed by a chondroadherin-agarose column (Mini-Leak). The control (lanes 1, 2, 5, and 6) and chondroadherin (lanes 3, 4, 7, and 8) columns were washed and eluted with 10 mm EDTA containing 6 m urea (lanes 1, 3, 5, and 7) and then 0.5 m NaCl (lanes 2, 4, 6, and 8). Eluted material was precipitated with ethanol and separated on a 4–12% SDS-polyacrylamide gel, electrotransferred to a PVDF membrane, and detected by Western blotting before (lanes 5–8) or after heparitinase and chondroitinase ABC treatment (lanes 1–4). Biotin-labeled proteins were detected using streptavidin HRP and the ECL system.
FIGURE 5.
FACS analysis to determine the presence of syndecans at the human chondrosarcoma 105kc cell surface. A, 105kc cells (0.1 × 106) were suspended in PBS, 0.5% BSA, labeled with either syndecan 1 (red line) or syndecan 2 (blue line) specific antibodies, and detected by an Alexa Fluor 488-conjugated sheep anti-mouse antibody. B, 105kc cells (0.1 × 106) were suspended in PBS, 0.5% BSA, labeled with either syndecan 3 (red line) or syndecan 4 (blue line) specific antibodies, and detected by a FITC-conjugated goat anti-rabbit antibody. The control (Ctrl, black line) was without the primary antibody.
FIGURE 6.
Affinity of cell surface proteoglycans to chondroadherin and the hbd-CKFPTKRSKKAGRH359 peptide from human chondrosarcoma 105kc cells. A, membrane proteins (from 5 × 106 105kc cells) were digested with heparitinase and separated by SDS-PAGE (14–12% gel), transferred to a PVDF membrane, incubated with the pan-syndecan antibody (PAN-ab) (or syndecan 4 specific antibody (S4 ab), inset), and detected using an anti-rabbit HRP antibody and the ECL system. The arrows indicate the expected positions of the core proteins of the four purified syndecans. B, biotin-labeled membrane proteins (from 5 × 106 cells) were passed over a control agarose column followed by a chondroadherin-agarose column. Bound material was eluted with 0.5 m NaCl, and eluted proteoglycans were immunoprecipitated with a pan-syndecan antibody. Immunoprecipitated material was treated with heparitinase, separated by SDS-PAGE (10% gel), and transferred to a PVDF membrane; labeled core proteins were detected using streptavidin HRP and the ECL system. Lane 1 represents material eluted from the control column, and lane 2 represents material eluted from the chondroadherin column. C, membrane proteins (from 5 × 106 cells) were passed over a control column followed by the heparin-binding peptide (immobilized to UltraLink) column. Bound material was eluted with 0.5 m NaCl, treated with heparitinase, separated by SDS-PAGE (10% gel), and transferred to a PVDF membrane followed by detection using a pan-syndecan antibody and an anti-rabbit HRP antibody using the ECL system. Lane 1 represents material eluted from the control column, and lane 2 represents material eluted from the peptide column.
To identify the ligand, affinity purification was repeated as above by using the chondroadherin column, and the eluted material was immunoprecipitated using the pan-syndecan-antibody. The antibody primarily precipitated a protein of about 50 kDa after heparitinase digestion, whereas no distinct protein was detected corresponding to this size from the control column (Fig. 6B).
In further support of the binding to the C-terminal peptide, a heparitinase digest of material affinity-purified on the immobilized hbd-CKFPTKRSKKAGRH359 peptide likewise revealed a prominent component of about 50 kDa (Fig. 6C), whereas no material was found in the eluate from the control column. Proteins eluted from the affinity columns were separated by SDS-PAGE followed by Western blotting. An attempt to identify the proteoglycan receptors was performed using specific antibodies to the four human syndecans. None of the specific antibodies showed reaction with the material eluted from the columns. The antibodies also failed to recognize the syndecans in total extracts from the cells, although the pan-syndecan antibody clearly identified proteins corresponding to the size of the four syndecan receptors. Only the antibody to mouse syndecan 4 was found to react with a protein of the expected size in Western blotting (Fig. 6A).
In a further attempt to elucidate the specificity, mouse osteoblast cells MC3T3-E1 were used in affinity purification over the hbd-CKFPTKRSKKAGRH359 immobilized via its cysteine. Mouse specific antibodies directed to syndecans 1 and 4 identified both receptors in total extracts from the cells (Fig. 7A). Interestingly, only syndecan 4 was detected in affinity-purified proteoglycans from the cells, indicating selective binding of one of the cell surface proteoglycans (Fig. 7B). The syndecan 4 receptor identified on the MC3T3-E1 cells is of a different size when compared with the receptor isolated from human 105kc cells, possibly representing a differently spliced receptor on these cells. The data further support the results from the chlorate treatment to demonstrate that the peptide interacts with glycosaminoglycan chains with a specific sulfation pattern rather than with specificity for the core protein carrying the particular glycosaminoglycan.
FIGURE 7.
Affinity of proteoglycans from MC3T3-E1 cells to the hbd-CKFPTKRSKKAGRH359 and their immunodetection. A, protein extracts from 5 × 106 MC3T3-E1 cells, undigested (−) or digested with heparitinase (+), were separated by SDS-PAGE (10% gel), transferred to a PVDF membrane, incubated with the specific syndecan antibodies, and detected using an anti-mouse HRP antibody and the ECL system. S1–S4, syndecan 1–4. B, proteins extracted from 5 × 106 MC3T3-E1 cells were passed over a control (ctrl) column and then over the heparin-binding peptide (pept) (immobilized to UltraLink) column. Bound material was eluted with 1.5 m NaCl. Eluted material was divided into two aliquots, and one was treated with heparitinase. Undigested (−) and digested (+) material was separated by SDS-PAGE (10% gel) and transferred to a PVDF membrane. The membrane was probed using syndecan 1 and 4 specific antibodies followed by an anti-rabbit HRP antibody using the ECL system.
Signaling Elicited by Binding of the Heparin Ligand Peptide
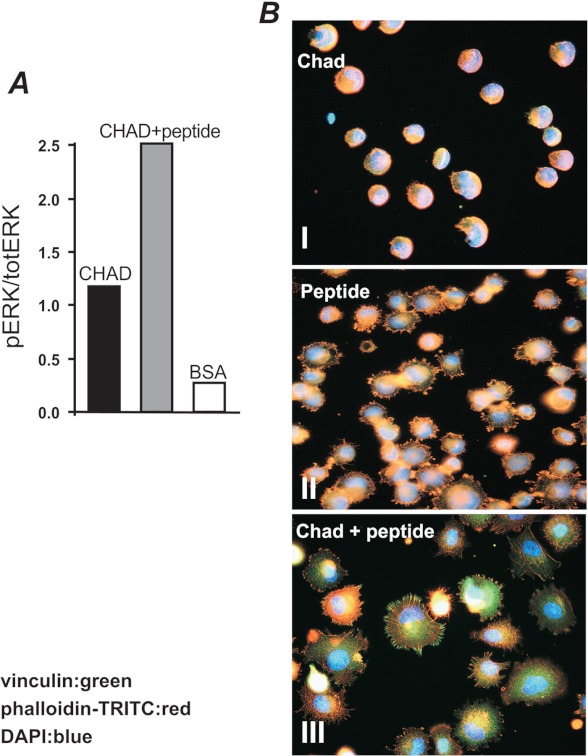
To determine whether the interaction elicits intracellular signals, intact chondroadherin and the isolated heparin-binding domain CKFPTKRSKKAGRH359 were coated separately onto cell culture plates. Surface coated with albumin only was used as a control. All cells, both bound and unbound, were collected, lysed, and analyzed by Western blot using antibodies specific for phospho-ERK and total ERK (Figs. 8A and 9A), respectively. A representative example of the gels scanned is shown in Fig. 8C. Indeed, the binding via the cell surface syndecan to the chondroadherin heparin-binding domain increased ERK phosphorylation 5-fold when compared with the control (Fig. 8A). One consequence of the binding was a novel observation of a dose-dependent cell spreading on a heparin sulfate ligand (Fig. 8B).
FIGURE 8.
Signaling elicited by binding of heparin ligand peptide. A, tissue culture 6-well dishes (Costar) were coated overnight with 20 μm peptide. The dishes were blocked for nonspecific binding with 0.5% BSA. To determine ERK phosphorylation, human chondrocytes were serum-starved for 48 h prior to the experiment. Cells were added to the wells (500 000/well), coated with the peptide or onto wells blocked with BSA only, and allowed to adhere for 1 h. All cells (bound and unbound) were lysed and analyzed by linear (10%) SDS-polyacrylamide gel and Western blotting. Blots were developed with the phosphorylation-specific antibody followed by visualization with chemiluminescence detection using the ECL system (Amersham Biosciences). The membranes were reprobed with an antibody to determine total ERK1/ERK2. Gel-Pro Analyzer® was used for scanning of blots for quantification of phosphorylations. pERK, phospho-ERK; totERK, total ERK. B, tissue culture dishes were coated with 0.16, 0.6, 2.5, or 10 μm peptide and blocked for nonspecific binding with BSA. The cells were suspended in PBS containing 0.1% BSA and 25 units/ml collagenase (CLSPA, Worthington), added to the wells, and allowed to adhere for 1 h at 37 °C. Nonadherent cells were removed by washing. Spreading was visualized by light microscopy. C, representative blots are shown to demonstrate the level of specificity in the experiments.
FIGURE 9.
Activation of signaling pathways and rearrangement of cytoskeletal elements upon exposure of cells to the hbd-CKFPTKRSKKAGRH359. A, tyrosine phosphorylation of signaling pathways in cells bound to chondroadherin upon the addition of the hbd-CKFPTKRSKKAGRH359. Tissue culture 6-well dishes (Costar) were coated overnight with 5 μg/ml chondroadherin. The dishes were blocked for nonspecific binding with 0.5% BSA. To determine ERK phosphorylation, primary human chondrocytes were serum-starved and analyzed as described in the legend for Fig. 8. pERK, phospho-ERK; totERK, total ERK. B, immunochemical staining of adhesion complexes. Chamber slides were coated with 5 μg/ml CHAD (top and bottom panels) or 33 μg/ml (20 μm) of the hbd-CKFPTKRSKKAGRH359 peptide (middle panel) and blocked for nonspecific binding with BSA (0.5%). The cells (105kc) were allowed to adhere for 1 h in the absence (top and middle panel) or in the presence (bottom panel) of the peptide (150 μm, 250 μg/ml), and adhesion complexes were identified using antibodies against phalloidin (red) and vinculin (green).
We have previously demonstrated that cell adhesion to intact chondroadherin or its integrin-binding sequence does not induce spreading (23). This led us to investigate whether the heparin-binding domain identified is masked when the protein is passively coated to cell culture plastic. In support, chondroadherin coated onto cell culture plates as described failed to bind biotin-tagged heparin, indicating that the heparin-binding domain indeed is masked when chondroadherin binds to the plastic surface (data not shown). Adhesion to chondroadherin induced tyrosine phosphorylation of ERK at a level some four times over that of the control (Fig. 9A). As expected, this binding did not induce any cell spreading (Fig. 9B, top panel). Interestingly, when the heparin-binding peptide was added in solution to cells attaching via the α2β1-binding domain of chondroadherin, spreading was induced and formation of focal adhesion complex was demonstrated by the typical organization of vinculin and actin (Fig. 9B, bottom panel). For comparison, cells demonstrate spreading when binding to this heparin-binding peptide, but no formation of focal adhesion complex (Fig. 9B, middle panel), indicating that engagement of both integrin and syndecan receptors is required for the formation of focal adhesion complexes. The addition of the heparin-binding peptide resulted in a further increased ERK phosphorylation when compared with the chondroadherin integrin binding alone (Fig. 9A).
Cell Adhesion to Chondroadherin Is Enhanced by hbd-CKFPTKRSKKAGRH359
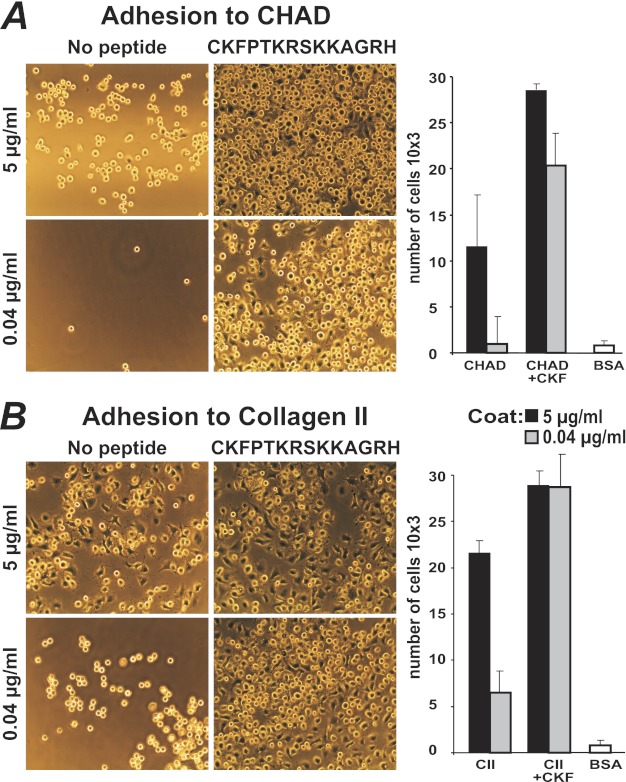
Wells were coated with either chondroadherin or collagen type II at 0.04 and 5 μg/ml, and remaining sites were blocked with BSA. 105kc cells were allowed to adhere in the presence of the hbd-CKFPTKRSKKAGRH359 to determine whether binding and spreading were modulated. Adhesion and spreading to collagen type II were pronounced at high coating concentrations (5 μg/ml) and were marginally increased by the peptide (Fig. 10B). However, using lower coating concentrations of collagen type II, we found that both the number of cells adhering and their degree of spreading markedly increased in the presence of the peptide (Fig. 10B, histograms). Adhesion to chondroadherin at any of the coating concentrations increased the number of cells adhering in the presence of the peptide (Fig. 10A). Cells plated on chondroadherin lacking the C-terminal heparin-binding domain showed the same behavior as when plated on full-length chondroadherin both with and without the heparin-binding peptide present (supplemental Fig. 3), confirming that the sequence is not exposed upon binding to plastic. Bovine primary chondrocytes were used to confirm that the peptide likewise increases binding of primary cells. Indeed, adhesion similarly increased when cells were pretreated with the heparin-binding peptide CKFPTKRSKKAGRH359 (Fig. 11).
FIGURE 10.
Adhesion of human chondrosarcoma 105kc cells to human chondroadherin and collagen type II in the presence of the hbd-CKFPTKRSKKAGRH359. 48-well tissue culture dishes were coated with chondroadherin, expressed in E. coli (A) or collagen type II (CII) (B) at 0.04 or 5 μg/ml PBS, and blocked for nonspecific binding with BSA. The cells were suspended in PBS, containing 0.1% BSA plus 25 units/ml collagenase, added to the wells in the absence or presence of synthetic peptide (150 μm, 250 μg/ml), and allowed to adhere for 1 h at 37 °C (see histograms). Nonadherent cells were removed by washing. Spreading was visualized by light microscopy, and adhesion was determined by analyses of lysosomal N-acetylglucosaminidase. Adhesion is expressed as number of cells, where the data represent the average of two wells. Independent experiments showed similar results. CKF, CKFPTKRSKKAGRH359.
FIGURE 11.
Adhesion of primary bovine chondrocytes to human chondroadherin in the presence of the hbd-CKFPTKRSKKAGRH359. Tissue culture dishes were coated with chondroadherin (5 μg/ml) expressed in E. coli and blocked for nonspecific binding with BSA. The cells were suspended in PBS containing 0.1% BSA and 25 units/ml collagenase, added to the wells in the absence or presence of the synthetic hbd-CKFPTKRSKKAGRH359 (150 μm, 250 μg/ml) and allowed to adhere for 1 h at 37 °C. Nonadherent cells were removed by washing. Adhesion was determined by analyzing lysosomal N-acetylglucosaminidase. Adhesion is expressed as percentages of cells, and the data represent the average of two wells. Independent experiments showed similar results.
Effect of Glycosaminoglycans on Cell Interaction of hbd-CKFPTKRSKKAGRH359
The influence of glycosaminoglycans on the binding of the synthetic hbd-CKFPTKRSKKAGRH359 was studied to define the degree of specificity. The synthetic hbd-CKFPTKRSKKAGRH359 labeled with streptavidin Alexa Fluor 488 was incubated with heparin, heparan sulfate, CS-A, CS-B, CS-C, or keratan sulfate at different concentrations followed by analysis of the interactions with the 105kc cells by FACS. Binding of the peptide to the cells decreased in a dose-dependent manner in the presence of heparin and heparan sulfate (Fig. 12, A and B), indicating a binding to the peptide via heparan sulfates at the cell surface. Very high concentrations of CS-B (dermatan sulfate) did result in a similar degree of inhibition of peptide binding to cells, whereas CS variants and keratan sulfate showed no inhibition. This inhibition by dermatan sulfate may be due to the fact that this glycosaminoglycan contains stretches of highly sulfated domains in analogy with the highly sulfated domains in heparan sulfate.
FIGURE 12.

The effect of glycosaminoglycans on cell interaction of the hbd-CKFPTKRSKKAGRH359 demonstrated by FACS. The hbd-biotin CKFPTKRSKKAGRH359 in complex with streptavidin-488 was preincubated with heparan sulfate (HS), heparin, Chondroitin sulfate A (CS-A), dermatan sulfate (DS), chondroitin sulfate C (CS-C), or keratan sulfate (KS) at different concentrations (100, 20, 4, 0.8, and 0.16 μg/ml PBS, 0.5% BSA). The human chondrosarcoma cells were incubated with the preincubated mixtures on ice and washed twice prior to analysis by FACS. A, FACS analysis chromatogram of heparan sulfate and CS-C. B, FACS analysis of all glycosaminoglycans (GAG) presented as fluorescence intensity.
DISCUSSION
Cell adhesion to chondroadherin has been shown to be mediated via the integrin α2β1 (23, 24). Upon binding, the cells do not spread, unlike effects observed when they adhere to other matrix proteins such as collagen or fibronectin. One difference is that many, if not most, of the integrin-binding proteins also contain an additional domain with the capacity to bind heparan sulfate present on the cell surface, for example, as a part of syndecans. It has been shown that cells adhering to the isolated integrin-binding domain of fibronectin fail to form focal contacts, but the addition of the isolated heparin-binding domain of the protein induced spreading and focal contact formation (32).
Earlier work has shown that spreading of cells bound to chondroadherin can be induced upon phorbol 12-myristate 13-acetate stimulation (23). A synthetic peptide of the very C-terminal part of chondroadherin contains a cluster of basic amino acids having heparin-binding properties (21). This led us to investigate whether the chondroadherin protein has the potential to bind to heparin and whether this domain may elicit cell spreading.
In initial experiments, we found that the bacterially expressed chondroadherin, when passively bound to a plastic surface, did not appear to present the heparin-binding structure because biotinylated heparin showed no binding (data not shown). In another set of experiments, chondroadherin was passively coated onto a polystyrene surface of an Attana® quartz microbalance and shown not to bind heparin, whereas the coated isolated peptide representing the heparin-binding domain showed binding to the glycosaminoglycan. At the same time, in an isothermal titration calorimetry experiment, the equilibrium constant in the interaction between heparin and the short hbd-KFPTKRSKKAGRH359 peptide was measured and had a high KD of 13 μm, and we could also show that chondroadherin in solution can bind to heparin. The mammalian expressed protein eluted in a broad peak from the heparin column, whereas the peptide peak was sharp. The difference could result from the presence of forms with the heparin-binding domain cleaved off. In analogy with other small leucine-rich repeat proteoglycans (SLRPs) (33), when a proportion of the chondroadherin molecules dimerizes, complexes with different contents of the heparin-binding domain are formed with most likely different chromatographic properties, resulting in the broad peak from the heparin column.
The very C-terminal CKFPTKRSKKAGRH359 peptide sequence interacts with heparin and mediates cell binding that distinctly depends on sulfated proteoglycans at the cell surface because preventing sulfation abolishes binding. This peptide, as well as the intact chondroadherin molecule, was used to selectively purify cell surface ligands by affinity chromatography under conditions of high urea concentration to prevent nonionic interactions. We demonstrated using a pan-syndecan antibody that primarily one of the syndecans present on human 105kc cells bound to the heparin-binding domain. Judging by the size, syndecan 3 but not syndecan 4 is a possible candidate. In further experiments, using a battery of commercial syndecan antibodies, we could not identify which of the four syndecans was involved in binding to the peptide because of lack of specificity in Western blots of the available antibodies to the human syndecans. In another set of experiments with MC3T3-E1 cells using antibodies to mouse syndecans, we could also show selective binding to syndecans to the chondroadherin heparin-binding peptide. These cells were shown to present syndecans 1 and 4, whereas only syndecan 4 was bound and affinity-purified. It is of particular interest that of two or more syndecans, only one of the syndecans on the cell appears to bind to the chondroadherin sequence. In further support, FACS analysis with specific antibodies verified the presence of all four syndecans at the cell surface on the human chondrosarcoma cells (Fig. 5). Together the data provide strong evidence for a preferred binding of the peptide to the glycosaminoglycan chains of only one of the syndecans on these cells. This indicates that the heparan sulfate (or other glycosaminoglycans) chains of the four syndecans on a cell are not necessarily identical. We could in a separate set of experiments show that the binding was specific to heparan sulfate because only heparin and heparan sulfate efficiently reduced binding of the peptide to the cell surface proteoglycan (Fig. 12). Because binding is mediated via the heparan sulfate chains, it appears that their structure differs between the various syndecans on the cell lines studied (34). The results provide a focus on aspects governing synthesis of these glycosaminoglycan side chains and how the structure of these oligomers of repeat disaccharides differs. An important factor is sulfation, where alterations could be coupled to different levels of epimerization of the uronic acid. Mechanisms to form different heparan sulfate chains may involve timing of the production of the particular syndecan or possibly different compartmentalization of the synthetic process. The observations point at a mechanism whereby in a unique way subtle specificities for cellular interactions can be created. This offers specific cellular options for sensing events in their surrounding matrix.
Integrins are the most extensively characterized adhesion receptor family. The syndecans are now also recognized as important cell surface adhesion co-receptors that can actively participate in adhesion and signaling (18, 35, 36). The hbd-CKFPTKRSKKAGRH359 was shown to profoundly stimulate adhesion, spreading, and migration upon integrin-mediated binding to chondroadherin as well as on collagen in vitro. Similar effects on cell adhesion have been shown after phorbol 12-myristate 13-acetate treatment (14, 23, 37, 38). Phorbol 12-myristate 13-acetate is known to activate protein kinase C signaling and thereby stimulate adhesion and spreading in a process involving syndecan 4 and integrins (14). We now show that adhesion to the hbd-CKFPTKRSKKAGRH359 alone will promote cells to adhere firmly and interestingly to also spread. In this case, with the 105kc, one of the syndecan proteoglycans involved is different from syndecan 4, which has previously been implicated in rearrangement of the cytoskeleton (14). This active peptide sequence in chondroadherin is rather short when compared with other heparan sulfate-binding domains investigated. However, in a study of smaller segments of the large heparin-binding domain of fibronectin, it was shown that a short peptide was sufficient to generate the effects (14). It is clear that cell binding via syndecan to the heparin-binding domain of chondroadherin leads to potent activation of intracellular signaling documented as tyrosine phosphorylation of ERK. We could further show that binding also induced cell spreading (Figs. 8B and 9B), but for formation of the focal adhesion complex, additional signaling events via integrin binding were required, whereas binding to the integrin alone neither induced cell spreading nor induced formation of the focal adhesion complex (Figs. 9B and 10). The combined action of these signaling events presents interesting possibilities for fine tuning of cellular responses in relation to the microenvironment and interactions at the cell surface.
The nature of the signals elicited by the binding of the peptide to the heparan sulfate chains of syndecan is not clear. The very rapidly induced increased cell binding and cell spreading by the peptide indicate that protein synthesis is not involved. The short peptide (corresponding to an extended length of some 40 Å) will bind to a small segment of the heparan sulfate chain only (corresponding to 4–5 disaccharides). We consider it unlikely that a conformational change is mediated via the flexible glycosaminoglycan chain to the core protein and then to the cytosolic side of the proteoglycan. It is possible that the peptide will influence interactions and network formation at the cell surface, which should represent a very rapid process, similar to the effects we observe. Indeed, interactions between the syndecan core protein and integrin mediated by synstatin has been shown to modulate cells behavior (39). In previous work, it has been suggested that the much larger fibronectin-derived heparan sulfate-binding domain elicits clustering of the syndecan (14). Ensuing interactions with the core proteins have been proposed to lead to the conformational changes. We now find that this very short peptide binds tightly to heparin and promotes all the cellular reactions, although its dimension makes it an unlikely candidate to cluster heparan sulfate chains.
Mesenchymal cells use syndecans to adhere to ADAM 12 and β1 integrins to induce spreading (36, 40). The study by Iba et al. (40) describes a novel finding in cell adhesion whereby a syndecan is the primary receptor, but requires β1 integrins for cell spreading and stress fiber formation. This is in contrast to the more extensively studied adhesion to fibronectin, where β1 integrins are the primary receptor but syndecans are also required for full spreading and cytoskeletal reorganization (14). We now show that this process can be induced by a short heparan sulfate-binding peptide alone.
The presence of the peptide in the tissue may be regulated by the cells. Chondroadherin exists in two forms in cartilage, where one form represents a cleavage product that lacks the last nine amino acids (20), constituting most of the peptide that we have now studied. Whether this cleavage is regulated, and if so how is not known, but different cartilages appear to contain different relative proportions (41). Another issue is whether the peptide can be released to stimulate the cells or whether this is accomplished by a longer fragment or the full-length protein containing the peptide sequence.
It is relevant to speculate that the peptide fragment generated after cleavage plays an important role in the tissue through feedback regulation to the cell. The presented experiments show several novel findings. It appears that the detailed structure of the syndecan side chains and their interactions vary between the family members even on a homogeneous cell line. The specific interacting domain of the glycosaminoglycans is likely to represent only a few disaccharide repeats where the chondroadherin peptide would represent a stretch of some 40 Å, provided that it is extended, which matches the expected length of the glycosaminoglycan-binding domain. A peptide longer than the presently studied one will extend over a longer sequence of disaccharides with an ensuing contribution by more, some weak, interactions. An effect will be binding expanded to include a larger number of variants of the disaccharide repeat structure. This is likely to lead to decreased specificity for the binding. Signals elicited by the interaction of the heparan sulfate chain are clearly sufficient to induce changes of the cytoskeleton and enhance the presentation and/or activity of the integrins.
Supplementary Material
Acknowledgments
We appreciate the assistance from Attana AB in testing binding of heparin to chondroadherin and peptide immobilized onto a polystyrene surface. We are most grateful to Alan Rapraeger for making the pan-syndecan antibody and mouse syndecan specific antibodies available and Annneliese Recklies for providing the human chondrocytes.
This work was supported by funding from The Swedish Research Council, the King Gustaf V:s 80-year Fund, the Österlund Foundation, the Kock Foundation, the European Commission 7th Framework Program for Research, NanoDiaRa, and Anamar Medical.

This article contains supplemental Figs. 1–3.
- hbd
- heparin-binding domain
- TRITC
- tetramethylrhodamine isothiocyanate
- EBNA
- Epstein-Barr virus nuclear antigen
- ITC
- isothermal titration calorimetry
- CHAD
- chondroadherin
- CS
- chondroitin sulfate.
REFERENCES
- 1. Eyre D., Wu J. J., Woods P. (1992) Cartilage specific collagens. Structural studies. in Articular Cartilage and Osteoarthritis. (Kuettner K. E., Schleyerbach R., Peyron J. G., Hascall V. C. eds) pp. 118–131, Raven Press, New York [Google Scholar]
- 2. Hedbom E., Heinegård D. (1993) Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J. Biol. Chem. 268, 27307–27312 [PubMed] [Google Scholar]
- 3. Rosenberg K., Olsson H., Mörgelin M., Heinegård D. (1998) Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J. Biol. Chem. 273, 20397–20403 [DOI] [PubMed] [Google Scholar]
- 4. Vogel K. G., Paulsson M., Heinegård D. (1984) Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem. J. 223, 587–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deák F., Wagener R., Kiss I., Paulsson M. (1999) The matrilins: a novel family of oligomeric extracellular matrix proteins. Matrix Biol. 18, 55–64 [DOI] [PubMed] [Google Scholar]
- 6. Sandell L. J., Heinegård D., Herring T. (2007) Cell biology, biochemistry, and molecular biology of articular cartilage in osteoarthritis. in Osteoarthritis (Moskowitz R. W., Altman R., Buckwalter J., Goldberg V. M., Hochberg M., eds) pp. 73–106, Wolters Kluwer/Lippincott, Philadelphia [Google Scholar]
- 7. Wiberg C., Klatt A. R., Wagener R., Paulsson M., Bateman J. F., Heinegård D., Mörgelin M. (2003) Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J. Biol. Chem. 278, 37698–37704 [DOI] [PubMed] [Google Scholar]
- 8. Hynes R. O. (1992) Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25 [DOI] [PubMed] [Google Scholar]
- 9. Woods A., Oh E. S., Couchman J. R. (1998) Syndecan proteoglycans and cell adhesion. Matrix Biol. 17, 477–483 [DOI] [PubMed] [Google Scholar]
- 10. Franzke C. W., Bruckner P., Bruckner-Tuderman L. (2005) Collagenous transmembrane proteins: recent insights into biology and pathology. J. Biol. Chem. 280, 4005–4008 [DOI] [PubMed] [Google Scholar]
- 11. Leitinger B. (2003) Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2: Identification of collagen binding sites in DDR2. J. Biol. Chem. 278, 16761–16769 [DOI] [PubMed] [Google Scholar]
- 12. Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. (1990) CD44 is the principal cell surface receptor for hyaluronate. Cell 61, 1303–1313 [DOI] [PubMed] [Google Scholar]
- 13. de Caestecker M. (2004) The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev. 15, 1–11 [DOI] [PubMed] [Google Scholar]
- 14. Woods A., Longley R. L., Tumova S., Couchman J. R. (2000) Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch. Biochem. Biophys. 374, 66–72 [DOI] [PubMed] [Google Scholar]
- 15. Ruoslahti E. (1991) Integrins. J. Clin. Invest. 87, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Humphries M. J., Mostafavi-Pour Z., Morgan M. R., Deakin N. O., Messent A. J., Bass M. D. (2005) Integrin-syndecan cooperation governs the assembly of signalling complexes during cell spreading. Novartis Found. Symp. 269, 178–188; discussion 188–192, 223–230 [PubMed] [Google Scholar]
- 17. Morgan M. R., Humphries M. J., Bass M. D. (2007) Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 8, 957–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rapraeger A. C. (2000) Syndecan-regulated receptor signaling. J. Cell Biol. 149, 995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernfield M., Götte M., Park P. W., Reizes O., Fitzgerald M. L., Lincecum J., Zako M. (1999) Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 [DOI] [PubMed] [Google Scholar]
- 20. Neame P. J., Sommarin Y., Boynton R. E., Heinegård D. (1994) The structure of a 38-kDa leucine-rich protein (chondroadherin) isolated from bovine cartilage. J. Biol. Chem. 269, 21547–21554 [PubMed] [Google Scholar]
- 21. Tillgren V., Önnerfjord P., Haglund L., Heinegård D. (2009) The tyrosine sulfate-rich domains of the LRR proteins fibromodulin and osteoadherin bind motifs of basic clusters in a variety of heparin-binding proteins including bioactive factors. J. Biol. Chem. 284, 28543–28553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Månsson B., Wenglén C., Mörgelin M., Saxne T., Heinegård D. (2001) Association of chondroadherin with collagen type II. J. Biol. Chem. 276, 32883–32888 [DOI] [PubMed] [Google Scholar]
- 23. Camper L., Heinegârd D., Lundgren-Åkerlund E. (1997) Integrin α2β1 is a receptor for the cartilage matrix protein chondroadherin. J. Cell Biol. 138, 1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haglund L., Tillgren V., Addis L., Wenglén C., Recklies A., Heinegård D. (2011) Identification and characterization of the integrin α2β1 binding motif in chondroadherin mediating cell attachment. J. Biol. Chem. 286, 3925–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sommarin Y., Larsson T., Heinegård D. (1989) Chondrocyte-matrix interactions. Attachment to proteins isolated from cartilage. Exp. Cell Res. 184, 181–192 [DOI] [PubMed] [Google Scholar]
- 26. Larsson T., Sommarin Y., Paulsson M., Antonsson P., Hedbom E., Wendel M., Heinegård D. (1991) Cartilage matrix proteins: a basic 36-kDa protein with a restricted distribution to cartilage and bone. J. Biol. Chem. 266, 20428–20433 [PubMed] [Google Scholar]
- 27. Recklies A. D., Golds E. E. (1992) Induction of synthesis and release of interleukin-8 from human articular chondrocytes and cartilage explants. Arthritis Rheum. 35, 1510–1519 [DOI] [PubMed] [Google Scholar]
- 28. Sommarin Y., Heinegård D. (1983) Specific interaction between cartilage proteoglycans and hyaluronic acid at the chondrocyte cell surface. Biochem. J. 214, 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Latvanlehto A., Snellman A., Tu H., Pihlajaniemi T. (2003) Type XIII collagen and some other transmembrane collagens contain two separate coiled-coil motifs, which may function as independent oligomerization domains. J. Biol. Chem. 278, 37590–37599 [DOI] [PubMed] [Google Scholar]
- 30. Landegren U. (1984) Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J. Immunol. Methods 67, 379–388 [DOI] [PubMed] [Google Scholar]
- 31. Cardin A. D., Weintraub H. J. (1989) Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9, 21–32 [DOI] [PubMed] [Google Scholar]
- 32. Yoneda J., Saiki I., Igarashi Y., Kobayashi H., Fujii H., Ishizaki Y., Kimizuka F., Kato I., Azuma I. (1995) Role of the heparin-binding domain of chimeric peptides derived from fibronectin in cell spreading and motility. Exp. Cell Res. 217, 169–179 [DOI] [PubMed] [Google Scholar]
- 33. Scott P. G., McEwan P. A., Dodd C. M., Bergmann E. M., Bishop P. N., Bella J. (2004) Crystal structure of the dimeric protein core of decorin, the archetypal small leucine-rich repeat proteoglycan. Proc. Natl. Acad. Sci. U.S.A. 101, 15633–15638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zako M., Dong J., Goldberger O., Bernfield M., Gallagher J. T., Deakin J. A. (2003) Syndecan-1 and -4 synthesized simultaneously by mouse mammary gland epithelial cells bear heparan sulfate chains that are apparently structurally indistinguishable. J. Biol. Chem. 278, 13561–13569 [DOI] [PubMed] [Google Scholar]
- 35. Couchman J. R., Chen L., Woods A. (2001) Syndecans and cell adhesion. Int. Rev. Cytol. 207, 113–150 [DOI] [PubMed] [Google Scholar]
- 36. Thodeti C. K., Albrechtsen R., Grauslund M., Asmar M., Larsson C., Takada Y., Mercurio A. M., Couchman J. R., Wewer U. M. (2003) ADAM12/syndecan-4 signaling promotes β1 integrin-dependent cell spreading through protein kinase Cα and RhoA. J. Biol. Chem. 278, 9576–9584 [DOI] [PubMed] [Google Scholar]
- 37. Danilov Y. N., Juliano R. L. (1989) Phorbol ester modulation of integrin-mediated cell adhesion: a postreceptor event. J. Cell Biol. 108, 1925–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilcox-Adelman S. A., Denhez F., Goetinck P. F. (2002) Syndecan-4 modulates focal adhesion kinase phosphorylation. J. Biol. Chem. 277, 32970–32977 [DOI] [PubMed] [Google Scholar]
- 39. Beauvais D. M., Ell B. J., McWhorter A. R., Rapraeger A. C. (2009) Syndecan-1 regulates αvβ3 and αvβ5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J. Exp. Med. 206, 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iba K., Albrechtsen R., Gilpin B., Fröhlich C., Loechel F., Zolkiewska A., Ishiguro K., Kojima T., Liu W., Langford J. K., Sanderson R. D., Brakebusch C., Fässler R., Wewer U. M. (2000) The cysteine-rich domain of human ADAM 12 supports cell adhesion through syndecans and triggers signaling events that lead to β1 integrin-dependent cell spreading. J. Cell Biol. 149, 1143–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heinegård D., Pimentel R. (1992) Cartilage matrix proteins. in Articular Cartilage and Osteoarthritis (Kuettner K. E., Schleyerbach R., Peyron J.G., Hascall V. C., eds) pp, 95–111, Raven Press, New York [Google Scholar]
- 42. Miller E. J. (1972) Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry 11, 4903–4909 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.