Abstract
Study Objective:
To test the hypothesis that the reflex and corticobulbar motor excitability of jaw muscles is reduced during sleep.
Design:
Polysomnographic recordings in the electrophysiological study.
Setting:
University sleep research laboratories.
Participants and Interventions:
The reflex and corticobulbar motor excitability of jaw muscles was determined during the quiet awake state (QW) and quiet sleep (QS) in monkeys (n = 4).
Measurements and Results:
During QS sleep, compared to QW periods, both tongue stimulation-evoked jaw-opening reflex peak and root mean square amplitudes were significantly decreased with stimulations at 2-3.5 × thresholds (P < 0.001). The jaw-opening reflex latency during sleep was also significantly longer than during QW. Intracortical microstimulation (ICMS) within the cortical masticatory area induced rhythmic jaw movements at a stable threshold (≤ 60 μA) during QW; but during QS, ICMS failed to induce any rhythmic jaw movements at the maximum ICMS intensity used, although sustained jaw-opening movements were evoked at significantly increased threshold (P < 0.001) in one of the monkeys. Similarly, during QW, ICMS within face primary motor cortex induced orofacial twitches at a stable threshold (≤ 35 μA), but the ICMS thresholds were elevated during QS. Soon after the animal awoke, rhythmic jaw movements and orofacial twitches could be evoked at thresholds similar to those before QS.
Conclusions:
The results suggest that the excitability of reflex and corticobulbar-evoked activity in the jaw motor system is depressed during QS.
Citation:
Yao D; Lavigne GJ; Lee JC; Adachi K; Sessle BJ. Jaw-opening reflex and corticobulbar motor excitability changes during quiet sleep in non-human primates. SLEEP 2013;36(2):269-280.
Keywords: Jaw-opening reflex, rhythmic jaw movement, intracortical microstimulation, orofacial twitch, quiet sleep
INTRODUCTION
Sleep is a physiological state usually associated with a lower muscle tone.1–3 Limb reflexes have been investigated in human and animal sleep studies to assess alteration of motor excitability during sleep. During rapid eye movement (REM) sleep and non-REM sleep of humans, the threshold and latency of the nociceptive spinal flexion reflex are markedly increased and amplitude decreased in comparison to quiet awake (QW) state,4 although the specific effects of sleep on the reflex responses vary between studies.5–8
To evaluate alterations in excitability of the jaw motor system across awake and quiet sleep (QS) states in animal models, the jaw-opening reflex and jaw-closing reflex have been examined.9,14,15–16 The jaw-opening reflex can be triggered by low-threshold or high-threshold intraoral stimulation (e.g., of lingual nerve or tongue).9,,–16 The findings in animal studies of the effects of sleep on the amplitude of the reflex, however, are variable—some showing that the reflex excitability is reduced, others showing it is increased or unchanged during QS in comparison to QW.9,11,18
Another approach to test motor excitability is to stimulate the corticobulbar pathways that activate brainstem motoneurons supplying the jaw muscles. Intracortical microstimulation (ICMS) of the cortical masticatory area triggers rhythmic jaw movements in rabbit, cat, guinea pig, and monkey,19–29 likely through the activation of the so-called brainstem central pattern generator of mastication.30–33 Also, electrical stimulation of the face primary motor cortex (face MI) produces twitch-like movements involving face, jaw, or tongue muscles as well as rhythmic jaw movements.21,24,28,34,35 Surprisingly, very little information is available regarding the excitability of these corticobulbar pathways during sleep. In cats, the thresholds of craniofacial movements as well as limb movements or sciatic nerve firing evoked by stimulation applied to frontal cortex or motor cortex are increased during non-REM sleep compared to those during the awake state, suggesting that the cortically induced excitability of motoneurons is attenuated during sleep.36–38 Data obtained in monkeys have also revealed that the ICMS threshold to trigger lip, arm, or neck muscle twitches is increased when the animal is dozing compared to the quiet or alert state.39 However, it remains to be demonstrated whether the excitability of the corticobulbar pathways is increased or depressed in parallel with the reduction in jaw muscle tone observed during sleep.3,40,41
Transcranial magnetic stimulation has been used to assess cortical excitability during sleep in humans, but as with animal and human studies of reflexes during sleep, reports are variable as to the effect of sleep on cortical excitability.42–44 However, it is impossible to address the changes in excitability of corticobulbar projections producing jaw muscle activities during sleep in humans with transcranial magnetic stimulation, since during wakefulness, transcranial magnetic stimulation requires tonic voluntary contraction of the jaw muscles (e.g., jaw clenching), which is impossible to maintain during sleep; in addition, this approach is also reported to trigger arousal during sleep.45–47 The use of low-intensity ICMS in animals offers a potentially useful approach to assess corticobulbar excitability without triggering sleep microarousal or awakening as occurs with transcranial magnetic stimulation in humans. Therefore, an animal study was carried out to investigate the excitability of the jaw motor system during wakefulness and sleep. The aim of the study was to test the hypothesis that the reflex and corticobulbar motor excitability of jaw muscles is reduced during sleep.
METHODS
One female Macaca mulatta (6.2 kg) and 3 female Macaca fascicularis monkeys (3.5-5.9 kg) were trained to sleep in a primate chair in the late afternoon and early evening. The overhead lights in the animals' home room were automatically turned off at 17:00 and on at 05:00 every day for the duration of the training and experiments. Two of three Macaca fascicularis were used for the jaw-opening reflex study, and one of these two Macaca fascicularis, as well as the Macaca mulatta, were used for the ICMS study (see below). The University Animal Care Committee approved the surgical and experimental protocols that were in accordance with the guidelines of the American Physiological Society and the Canadian Council for Animal Care.
Training Procedures
Under unrestrained conditions, the monkeys used in this study usually slept in their home cage in a sitting position with both legs on each side of the body (≈ 80% sleeping time), one shoulder lying on the cage wall with the head on the chest. To habituate the animal to the experimental sleep environment (a shielded room in which the experiment procedures were carried out) and to sleeping in a primate chair, training was carried out in 2 blocks of ≥ 11 sessions for each animal. The first training period, over 5-7 weeks, allowed the animal to develop a more natural sleep pattern while it sat in the chair with the lights turned off for a period of 1-3 h. A black light (low emission) was added to the room to allow video monitoring of the animal's behavior. Sound interferences were attenuated by constant noise in the room (air conditioning fan at ≈ 60 dB). An interval of 3 nights was needed between training sessions, since the animal showed unstable sleep (e.g., short duration, several awakenings) if it was under the experimental conditions for more than 2 evenings in a row. To facilitate habituation, the animal was provided with food that it enjoyed (e.g., hazelnut jam, pieces of banana, pear, apple, and liquid such as grapefruit juice) before and after the sleep training periods. The first training block was carried out in the late afternoon and was considered completed when the monkey remained quiet and showed some behavioral signs of drowsiness, with the eyes closed and its head resting on its chest in the absence of body movements, for a period > 5 min.
For the second training block (6-8 sessions), after the surgery described below, the same conditions as in the first training block were used, except the animal's head was fixed (to prevent movement during experimental recordings) in an upright position for a period that was gradually increased from 1-4 h. After the animal got used to sitting in the chair with its head fixed in the upright position, stimulation experiments were initiated as described below.
Surgical Procedures
The following procedures have been described in detail in several papers from this laboratory21,28,29,34,35,48–50 and so are outlined only briefly. In the first surgical procedure, after the first training period, a headcap of dental acrylic was fixed to the animal's skull under general anesthesia (induction with ketamine HCl 20 mg/kg, 2:1 N2O/O2 and 3% isoflurane mixture and maintained with 0.5-1.0% isoflurane). The surgery was carried out under aseptic procedures. The headcap was maintained in place with stainless-steel screws and dental acrylic, and supported a stainless-steel cylinder (25 mm in diameter) that was implanted over the exposed dura covering the left or right lateral pericentral cortex. For chronic recordings, wire electrodes (36-40 gauge, single or multiple stranded, Teflon coated; Coroner Wire, Chatsworth, CA, USA) were placed: (1) intramuscularly in the anterior digastric and superficial masseter muscles bilaterally as well as the genioglossus muscle for recording electromyographic (EMG) activity; (2) subcutaneously in the front of the chest for recording electrocardiographic activity; (3) subcutaneously in the outer canthus of each eye to allow electrooculographic activity recording for active sleep recognition (as described below); (4) on stainless-steel screws fixed to the skull over the central and occipital cortical areas to permit recording of electroencephalographic (EEG) activity; and (5) into the left intercostal muscle to monitor respiratory movements. All electrodes were connected to a microplug and fixed with dental acrylic to the headcap to allow easy access for repeated sessions of recording and stimulation. A small magnet (5.0 mm diameter and 2.0 mm thick) was implanted under the chin so that jaw movements could be recorded by a motion-sensing device detecting the changes in the magnetic field. After surgery, the animal received analgesics and antibiotics for a week; a period of 2 weeks was allowed for recovery.
Sleep Recording and Scoring
Sleep-related electrophysiological activity (e.g., EMG, electrooculography, EEG, electrocardiography) was recorded at 128 Hz and scored with epochs of 10 sec instead of the standard 30 sec,51 since sleep efficiency is low when monkeys are in the restrained sleep position52,53 and subjected to repetitive stimuli6 (also see Discussion). A commercial software was used for sleep recording and scoring (Natus [formerly Stellate Harmonie], USA). All electrophysiological signals were filtered and amplified. EMG activities were filtered for high frequency/low pass at 3 KHz and low frequency/high pass at 30 Hz, electrooculographic activities at 1 KHz and 0.1 Hz, respectively; EEG activities at 500 Hz and 0.5 Hz, respectively; and electrocardiographic activities at 5 KHz and 0.5 Hz, respectively. The amplification was 1,000 × for all these signals except for electrooculographic activity (100 ×). At the same time, all the electrophysiological activities were also recorded with Spike2 software (CED, Cambridge, UK) and a second computer as the sampling frequencies of EMG activities were much higher (masseter and genioglossus at 2,400 Hz, and anterior digastric at 7,140 Hz) to make the analysis more accurate. The QW state was characterized by fast EEG activities with alpha-beta dominance and/or desynchronized EEG trace, high muscle tone, eyes open, and some eye and body movements. Based on human standard scoring criteria51,54 and scoring in previous primate sleep studies,53,55 non-REM sleep stages were scored over 10-sec epochs due to the animal's short sleep episodes, as described above. Sleep stages were divided into: (1) quiet sleep stage 1 (QS S1, reduction in alpha dominance, slower EEG activity for ≈ 50% of the 10-sec scoring epoch, eyes closed with indication of rolling eyes on electrooculographic traces); (2) quiet sleep stage 2 (QS S2, slower EEG activities for most of scoring section of 10 sec, presence of theta EEG activity with appearance of K-complexes56 and EEG spindles, no electrooculographic activity); and (3) deep sleep stage 3 (≈ 20% of scoring epoch of 10 sec with large delta EEG activity, low EMG activity and no electrooculographic activity). The total sleep time, duration of each sleep stage, transition of sleep stages (from deep QS to light QS or light QS to QW), number of arousals (visual scoring > 3 sec changes in EEG/alpha intrusion and EMG tone), and awakenings (increase in EEG activity with eyes open for 5-30 sec) were determined. We excluded data of 3 nights due to unstable sleep (e.g., inability to sleep as result of noise outside) and to loss of the EEG signal (n = 1).
Evaluation of Jaw-Opening Reflex
The jaw-opening reflex was elicited while the animal was awake or asleep, by stimulating (pulse duration 0.2 msec) the genioglossus muscle through 2 chronically implanted electrodes in order to activate afferent fibers in the tongue. The low-intensity stimulation used in the experiments varied from 15-700 μA between animals and between nights. The jaw-opening reflex was recorded at 7,140 Hz on a second computer with another commercial software (Spike2, CED, Cambridge, UK) to allow a higher resolution analysis. None of the stimulations evoked any behavioral reaction (e.g., voluntary movement, vocalization, increase in heart rate, apparent discomfort or pain). The animal's behavior and motor activity were monitored with the electrophysiological recordings and the use of an infra-red video camera. The stimulations were triggered by a remote control outside the experimental room to avoid disturbing the animal. Stimuli were delivered at 0.5-1 Hz to evoke the jaw-opening reflex in blocks of ≈30-35, and a minimum of 60 sec was allowed between blocks (to prevent habituation). The stimulus intensity-reflex response curve was first determined in the QW state at 1-8 × threshold; threshold was defined as the lowest stimulus intensity evoking a jaw-opening reflex response in > 80% of stimulations.57 During sleep, no stimulations were delivered in the first 20 min to avoid sleep instability or awakenings. Stimulations were given in QS sleep stages 1 or 2 that were scored on-line in real time, as described above. During sleep, the stimulations were repeated at 1, 2, and 3.5 × the QW state threshold; this range was selected since it only activates low-threshold afferents,58 and since studies in humans and in our pilot study in two monkeys showed that sleep arousal could be triggered at stimulus intensities > 1 mA.6–8 Thus, to avoid waking the animal during sleep, none of the stimulus intensities was > 3.5 × threshold (i.e., not > 700 μA). Moreover, periods of 1-5 min were needed between stimulation blocks to preserve sleep continuity. Immediately after the animal awoke from sleep, the 3 stimulation intensities were repeated to elicit the jaw-opening reflex. For each stimulation intensity, one block of stimuli was delivered during QW before QS, and QW after QS, once the reflex threshold was determined. During QS, more than 2 blocks of stimuli (i.e., 3-5 blocks) for each stimulation intensity was delivered since the animals showed frequent sleep stage shifts and woke frequently (see below).The reflex latency and amplitude data before, during, and after sleep were analyzed over 7 awake and 5 sleep experimental sessions (including after sleep) in the 2 Macaca fascicularis monkeys (see below).
The jaw-opening reflex signal was first digitized and rectified, and it was scored only if a clear increase in the EMG amplitude was present, as described below (e.g., > 2 SD above baseline activity before stimulation). The reflex was also scored only if its latency was ≤ 25 msec. A jaw-opening reflex occurring with other body movements was excluded from the analysis. We also excluded the first 5 reflex responses from the analysis because of some initial variation in reflex amplitude. The anterior digastric EMG signal was rectified and then smoothed,11 and the following parameters of the jaw-opening reflex were measured: (1) the mean baseline EMG activity in the 25 msec before lingual stimulation, by using the root mean square (RMS) function of the Spike2 software; (2) reflex latency, defined as the time between the electrical stimulation onset and the point at which the reflex response exceeded 2 SD above the mean baseline EMG activity before stimulation58 (e.g., Table 1); (3) reflex amplitude in the time period between the reflex onset (> 2 SD) and offset (< 2 SD), using the RMS and maximum peak amplitude function of the Spike2 software. All analyses were made by one technician who was blind to the study objectives.
Table 1.
Latencies (msec) of jaw-opening reflex during QW and during sleep stages 1 or 2 according to stimulation intensity
ICMS
After the monkey was trained, face MI was identified and mapped for evoked orofacial twitch-like movements by systematically applying ICMS (≤ 30 μA, 35-msec train of 12 cathodal pulses, each pulse 0.2 msec, 333 Hz [short-train stimulus]) at 250-μm intervals to a depth of 8-10 mm during daily transdural microelectrode penetrations of the MI, as previously detailed.24,28,29,35,49 A longer pulse ICMS train (3-sec train, 0.2-ms pluses at 50 Hz, ' 60 μA [continuous stimulus]) was also systematically applied to evoke rhythmic jaw movements within the cortical masticatory area in one of the two Macaca fascicularis and in the Macaca mulatta.28,34,35 Once the mapping was completed, ICMS thresholds for evoking rhythmic jaw movements and twitch-like movements were determined during QW before sleep, different sleep stages, and QW after sleep. The ICMS data before, during, and after sleep were analyzed over 6 nights in 2 Macaca fascicularis monkeys and 7 nights in the Macaca mulatta monkey (see below).
ICMS-induced jaw movements > 5 mm of opening were aligned and averaged at the start of ICMS. Jaw movements > 5 mm of opening and > 2 continuous cycles were considered to be rhythmic jaw movements. As there was no difference in ICMS threshold between QS stage 1 and stage 2 in the experiments, ICMS-induced jaw movements in both stage 1 and 2 were collectively analyzed.
To test the change in orofacial muscle EMG activities during sleep (without ICMS), the area under the curve (AUC) and RMS amplitude of anterior digastric, genioglossus, and masseter EMG activities and heart rate for the 10 sec during QW before sleep was calculated and compared with those during QS and QW after sleep. Since there were no significant differences of the data of anterior digastric, genioglossus, and masseter EMG activities and heart rate data during stage 1 and 2 of QS, the EMG data of the 3 muscles and the heart rate data during QS were not divided into stage 1 and stage 2 for this analysis.
To assess whether ICMS at the highest intensities used in our experiments woke the animal, the EMG activities of anterior digastric, masseter, and genioglossus before and after ICMS during QS, and the AUC and RMS amplitude of the EMG activities of these muscles within 3 sec before and after ICMS were analyzed. The heart rate (Hz) for 3 sec before, during, and after ICMS during stage 1 and 2 of QS was also analyzed.
Histology
After all electrophysiological experiments were completed, electrolytic lesions (DC, 10 μA, 10 s) were placed at selected intracortical sites. Then, the animal was first anesthetized with ketamine (100 mg/kg) along with atropine and xylazine (50 mg/kg), and a lethal dose of T61 was given (i.v.), and then animal was perfused through the heart with heparin-saline followed by 10% buffered formalin. Histological reconstruction of the cortex was carried out following the procedures, as previously described.48 Stimulation sites were reconstructed according to the intracortical depth of ICMS sites, the location of electrolytic lesions, and the coordinates of microelectrode tracks. The loci of the implanted wire electrodes were checked and confirmed.
Statistical Analysis
Jaw-opening reflex data were pooled across animals and nights, but pooling could not be done for the ICMS data because of a “ceiling” effect in one animal, where the ICMS threshold for evoking jaw movements was above the ICMS intensity limit set for the study (in order to avoid cortical damage by high ICMS electrical current). For the ICMS data analysis, each animal acted as its own control where its baseline (before sleep) data was normalized, and sleep-related changes in that monkey were compared to baseline values. One-way repeated measures ANOVA with post hoc Tukey test, Student's t-test, and paired t-test were also used where appropriate. Normality test (Shapiro-Wilk) and equal variance test were performed before one-way repeated measures ANOVA was done. Normality test (Shapiro-Wilk) was also performed before Student's t-test and paired t-test. Specifically for the jaw-opening reflex, the variability in latency or amplitude between animals and evenings was calculated as standard deviation divided by mean. Other values are expressed as mean ± SEM; P < 0.05 was considered significant.
RESULTS
Sleep Parameters
Over the 5 nights used in the analysis of the jaw-opening reflex data, the total cumulative sleep duration was 139 min in one monkey and 182 min in another monkey. The corresponding times for the ICMS data analysis were 255 min (6 nights) and 329 min (7 nights). The number of sleep awakenings (e.g., increase in EEG activity with eyes open for 5-30 sec) was frequent, at a rate of 15-25/h of sleep, and frequent sleep stage shifts (from stage 3 to 2, stage 2 to 1, or to wakefulness) were observed every 66-72 sec. The duration of sleep stage 2 represented 32% to 56% of the total sleep duration, while stage 3 represented approximately 2%; no REM episode lasted more than 10 sec (data not shown).
Jaw-Opening Reflex during Wakefulness and Sleep
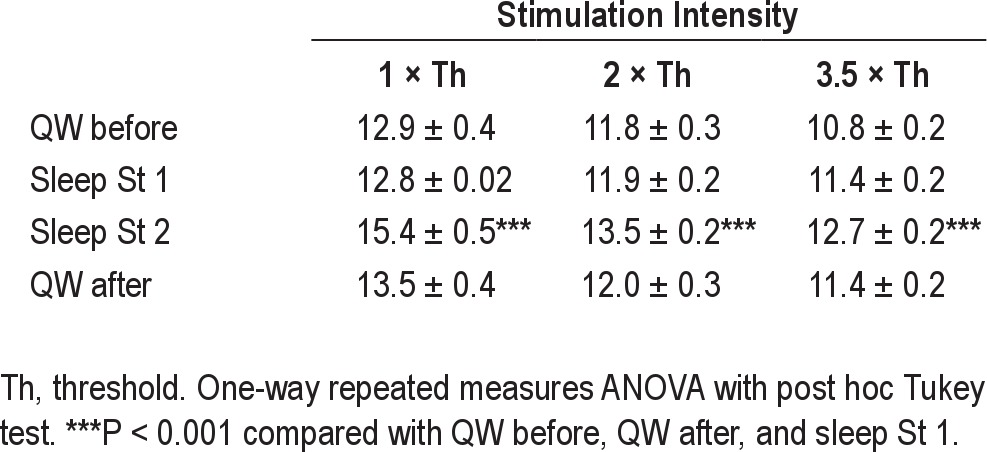
In general, the latency of the jaw-opening reflex decreased significantly with increases in lingual stimulus intensity (F5,1 = 45.6; P < 0.001) during QW before sleep. The latency ranged from an average of 12.9 msec (at 1 × threshold) to an average of 10.8 msec (at 3.5 × threshold) (Table 1). The amplitude of the reflex increased linearly (P < 0.05) with the rise in stimulus intensity; the correlation coefficients (“r”) were 0.89 and 0.94 for RMS and amplitude, respectively. As shown in Table 2, the RMS and peak amplitude of the jaw-opening reflex became greater with increasing lingual stimulus intensity. The reflex latency and amplitude were nonetheless consistent within the same QW session when the stimulus intensity was kept constant (e.g., Figure 1). When the ratio of the reflex amplitude at 8 × stimulus threshold to the amplitude at 1 × stimulus threshold was calculated during QW, it was found that the RMS increased by a factor of 5.8 (r = 0.89; F5,1 = 48.2, P < 0.01) when stimulus intensity was increased from 1 × threshold to 8 × stimulus threshold. The ratio of the peak amplitude of the reflex also increased, in this case by a factor of 7 (r = 0.94; F5,1 = 36.5, P < 0.001), when stimulus intensity was increased from 1 × to 8 × threshold (data not shown). The animals were always calm during the lingual stimulations.
Table 2.
Amplitudes of jaw-opening reflex during QW and during sleep stages 1 or 2
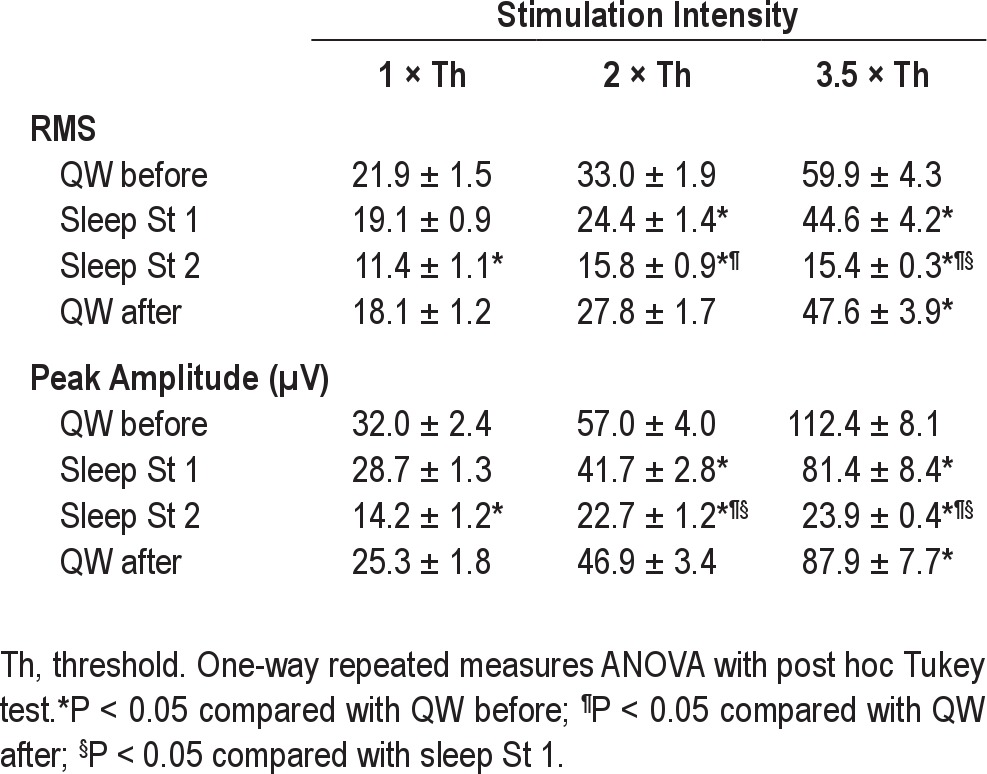
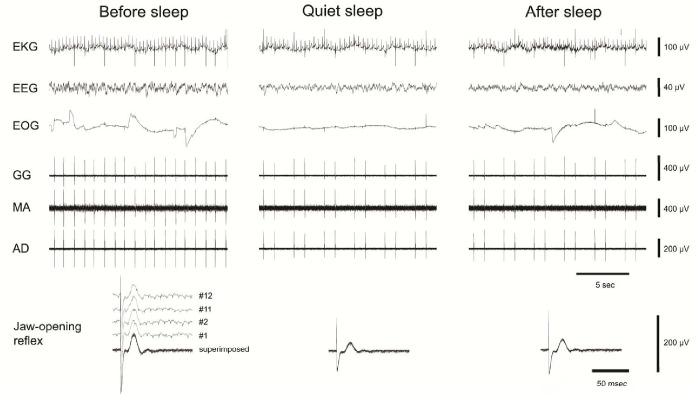
Figure 1.
Examples of jaw-opening reflexes evoked by electrical lingual stimulation. Top panels show single raw data traces of electrocardiographic (EKG), electroencephalographic (EEG), electrooculographic (EOG) activities, and EMG activities of genioglossus (GG), masseter (MA), and anterior digastric (AD) muscles before, during, and after quiet sleep (stage 2) when lingual stimulation was applied to evoke the jaw-opening reflex. The bottom panels show EMG activity of the AD at a time base different from that of the top EMG activities to illustrate the evoked jaw-opening reflex more clearly. The superimposed jaw-opening reflexes (12 trials for each) before, during and after quiet sleep (stage 2) as well as the 1st, 2nd, 11th, and 12th single traces of the jaw-opening reflex before sleep illustrate the consistency of the reflex with repeated stimulation at the same stimulus intensity. Notes the decrease of the jaw-opening reflex during QS.
The jaw-opening reflex in the QW after sleep was always of a lower RMS and peak amplitude than the QW before sleep (Table 2), but a significant difference between the QW before and after sleep was observed only at 3.5 × stimulation threshold for both RMS and peak amplitude responses. During sleep stage 1, the linear increase in the amplitude of the jaw-opening reflex between 1-3.5 × threshold was similar to that during QW before and after sleep (RMS increased by a factor of 2.5; peak amplitude increased by a factor of 2.9; P < 0.05; see Table 2). On the other hand, during sleep stage 2, no significant change in the reflex amplitude for the 1-3.5 × threshold stimulations was observed. A significant reduction in the reflex amplitude (RMS and peak amplitude) occurred during sleep stage 2 compared to QW before (P < 0.001) and after (P < 0.05) sleep (Figure 1, Table 2). Compared with values in QW before sleep, reductions were noted in RMS values of the reflex (P < 0.05): (1) at 1 × threshold in stage 2 only (47.9%); (2) at 2 × threshold in stages 1 and 2 (26% and 52.1%, respectively); (3) at 3.5 × threshold in both stages 1 and 2 (25.5% and 74.3%, respectively). At 3.5 ×, the RMS value of the reflex in stage 2 was also significantly lower than in stage 1. Compared with values in QW before sleep, reductions were also noted in peak amplitude of the reflex (P < 0.05): (1) at 1 × threshold in stage 2 only (55.6%); (2) at 2 × in both stages 1 and 2 (26.7% and 60%, respectively); (3) at 3.5 × threshold in both stages 1 and 2 (27.1% and 78.7%, respectively). There was also a significant reduction in reflex amplitude during stage 2 in comparison to stage 1, at both 2 × and 3.5 × threshold (see Table 2). Compared with values in QW after sleep, the RMS and peak amplitude values of the reflex in stage 2 were smaller at both at 2 × and 3.5 × threshold (P < 0.05).
During sleep stage 2, in comparison to QW before sleep, sleep stage 1, and QW after sleep, the latency of the jaw-opening reflex was significantly longer for all stimulation intensities (P < 0.001; see Table 1). The response was 16% later at 1 × threshold, 12.5% at 2 ×, and 15% at 3.5 × in comparison to that during QW before sleep. The latencies during QW before and after sleep and sleep stage 1 were not significantly different.
Variability in Jaw-Opening Reflex Latency and Amplitude between Animals and Evenings
The variability (% = SD/mean value) in the jaw-opening reflex latency between animals and between evenings during QW was higher at 1 × threshold (19.1%) than at 3.5 × threshold (11.6%). The variability between animals and evenings in the reflex amplitude during QW was higher at 1 × threshold (34.5% for RMS and 30.9% for peak amplitude) than at 3.5 × threshold (15.9% for RMS and 19.4% for peak amplitude). During sleep, the variability in the reflex responses at 3.5 × threshold was much lower: 8.1% for RMS and 7.8% for peak amplitude. These data suggest that during sleep, the reflex was more reproducible than during QW at 3.5 × threshold and was more variable at low stimulation intensities (e.g., at 1 × threshold, the reflex was highly variable between animals, with RMS ranges of 6.7-33 units, and peak amplitude ranges of 9-48 μV).
Cortical Excitability during Wakefulness and Sleep
Based upon the histological verification, the ICMS sites from which rhythmic jaw movements were induced during QW (Figures 2 and 3) were located in the deep part of the left cortical masticatory area 21,34,35,51 in the Macaca fascicularis monkey (Figure 3) and in the principal part of the left cortical masticatory area21,34,35,48 in the Macaca mulatta monkey. The ICMS thresholds for evoking rhythmic jaw movements were stable over repeated trials during QW before sleep and were ≤ 60 μA for rhythmic jaw movements and < 40 μA for sustained tetanic-like jaw-opening movements (lasting 3-14 sec) that could sometimes be evoked. The latencies for rhythmic jaw movements during QW before sleep were 0.997 ± 0.253 sec (Figure 2).
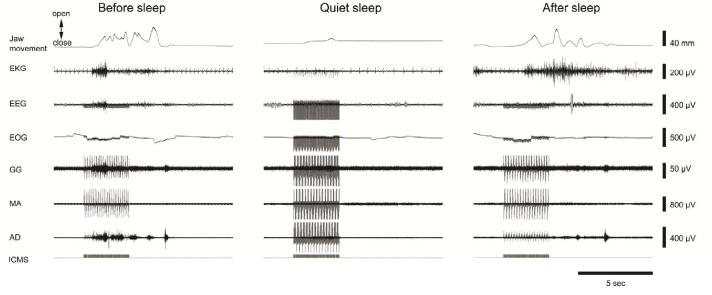
Figure 2.
An example of changes in intracortical stimulation (ICMS) within the cortical masticatory area induced jaw movements during quiet sleep (QS). Single raw data traces of jaw movements, electrocardiographic (EKG), electroencephalographic (EEG), electrooculographic (EOG) activities, and EMG activities of genioglossus (GG), masseter (MA), and anterior digastric (AD) muscles; and ICMS before, during, and after quiet sleep are illustrated. Before sleep, rhythmic jaw movements were induced by ICMS at a threshold of 40 μA. During QS, only sustained jaw movements were induced by ICMS at a threshold of 400 μA. After sleep, rhythmic jaw movements were induced again by ICMS at a threshold of 40 μA.
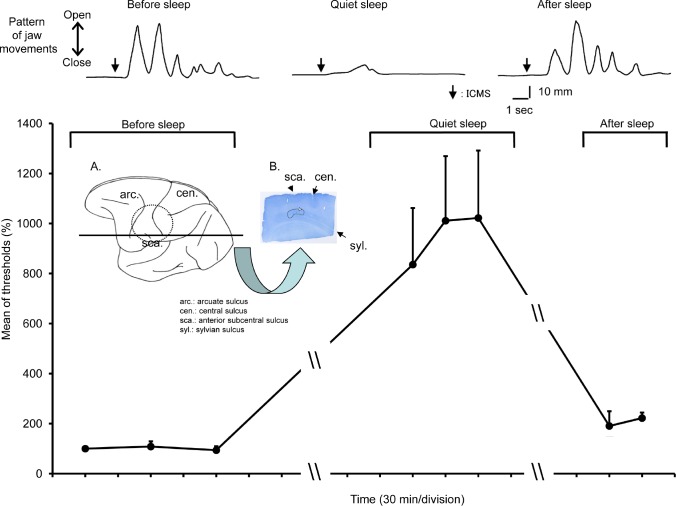
Figure 3.
A plot of the ICMS threshold for inducing jaw movements in a Macaca fascicularis monkey. The ICMS threshold for inducing rhythmic jaw movements (RJMs) during quiet sleep (QS) was significantly higher (one-way repeated measures ANOVA, F7,3 = 16.7, P < 0.001) in comparison to before sleep and after sleep. Examples are shown in the top traces. Insert: Location of chamber and explored cortical area. A. The chamber was located as shown by the broken line, and rostrocaudal histological sections were taken from the plane marked by the horizontal line. B. Location of electrolytic lesion as shown by the white arrow on the rostrocaudal section (the black line showed the approximately area received ICMS). The penetrations, in which ICMS induced RJMs during quiet awake state (QW), were applied at ± 1 mm mediolateral area of this lesion. The penetrations, in which ICMS induced sustained tetanic-like jaw-opening movements during quiet wakefulness (QW) was at 2 mm posterior and 2.5-2.75 mm medial area of this lesion.
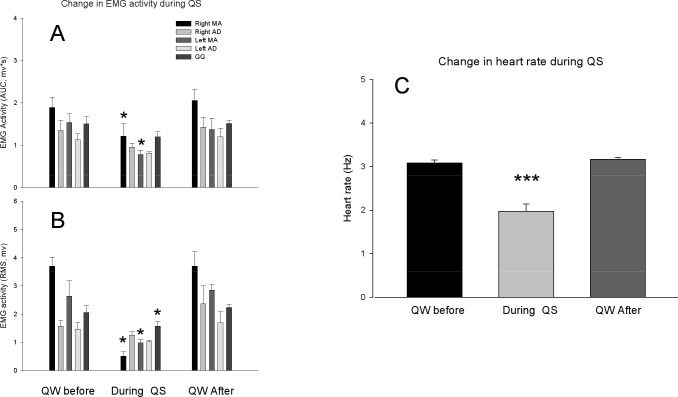
During QS in the Macaca fascicularis monkey, ICMS up to 400 μA failed to induce any rhythmic jaw movements (Figures 2 and 3). Nevertheless, sustained jaw-opening movements could be evoked, but at a threshold (150-400 μA) that was significantly (F7,3 = 16.7, P < 0.001, one-way repeated measures ANOVA) higher than the rhythmic jaw movement threshold during the QW before and after sleep (Figures 2 and 3). In the Macaca mulatta monkey, ICMS up to 700 μA failed to induce any rhythmic jaw movements or sustained jaw-opening movements, although rhythmic jaw movements could be evoked at the thresholds similar to those before sleep once the animal was awake. There was no significant change (P > 0.05, paired t-test) in heart rate, anterior digastric, genioglossus, and masseter muscle activities during QS immediately after ICMS compared with those before ICMS when no movements were evoked (Figure 4). There was no significant change in anterior digastric EMG activities during QS compared with QW, although heart rate and masseter EMG activities were significantly decreased compared with those during awake conditions (Figure 5).
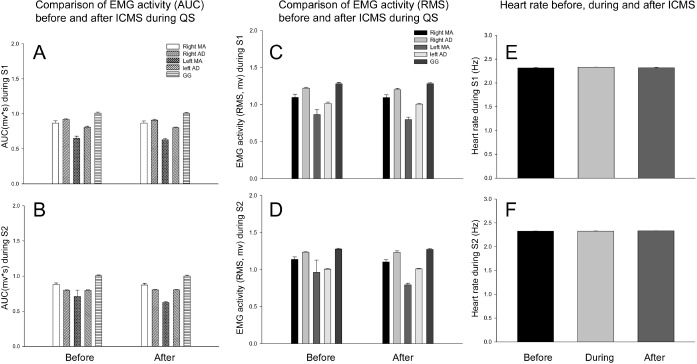
Figure 4.
The effects of ICMS (continuous stimulation) on background EMG activities and heart rate during QS. There was no significant difference in EMG activities of anterior digastric (AD), masseter (MA), and genioglossus (GG) muscles between those immediately before or after ICMS during QS (P > 0.05, paired t-test). The EMG activities during ICMS could not be analyzed due to ICMS artifact contamination. There was also no significant difference in heart rate among those immediately before, during, immediately after ICMS during QS (P > 0.05, one-way repeated measures ANOVA). There was no significant difference in EMG activity and heart rate between stage 1 and stage 2 of QS (P > 0.05, Student's t-test).
Figure 5.
The comparisons of the background orofacial muscle EMG activities (A, B) and heart rate (C) during QW before, QS and QW after QS. The area under the curve (AUC) and root mean square (RMS) amplitudes of masseter (MA) muscles and heart rate were significantly decreased (one-way repeated measures ANOVA, *P < 0.05, ***P < 0.01). The RMS amplitudes but not AUC of genioglossus (GG) muscle also significantly decreased (P < 0.05). Neither RMS amplitude nor AUC of anterior digastric (AD) muscles was significantly changed (P > 0.05).
An analogous sleep-related decrease in excitability was found when orofacial muscle twitches were evoked from histologically verified sites in the precentral gyrus of the left MI in the Macaca mulatta and the right MI in the Macaca fascicularis monkey. During QW, ICMS within face MI induced orofacial twitches at a stable threshold (≤ 35 μA), but during QS, thresholds for ICMS to evoke orofacial twitches were markedly elevated (up to 140 μA); soon after the animal awoke, orofacial twitches were evoked at ICMS thresholds similar to those before QS.
DISCUSSION
The present study has documented that during QS in comparison to QW there is a clear decrease in jaw-opening reflex amplitude and a prolongation of the reflex latency, as well as an increase in ICMS thresholds for evoking rhythmic jaw movements and orofacial twitches in macaque monkeys. These findings suggest that the excitability of the jaw motor system is reduced during light non-REM sleep.
Study Limitations and Advantages
Consistent with previous observations in monkeys under unrestrained conditions,59 the monkeys in the present study usually slept in their home cage in a sitting position with both legs on each side of the body (≈ 80% sleeping time), one shoulder lying on their cage wall with the head on the chest. The animals were trained to sleep in a primate chair with the head restrained in an upright position. Although we could study the jaw-opening reflex excitability during both the awake state and sleep, we had difficulties in training the animals to sleep for long periods without movement or awakenings. A restrained position was essential to prevent body movements that would have otherwise contaminated the quality of the recorded signals. We recognize that sleeping in a primate chair is not ideal to study “natural” sleep patterns; we noted that the sleep periods were brief and we observed several sleep stage shifts. Previous studies also have reported that the sleep of monkeys in a chair is associated with the presence of several sleep awakenings, numerous sleep stages shifts, short duration of slow wave deep sleep and of active-REM sleep, plus low and variable sleep efficiency (i.e., the total time the animal sleeps over the total time in darkness) between animals (≈55%; ideal is > 90%).52,53 The number of animals (n = 4) used in the current experiments was small, but for each animal we were able to collect data from the same animal over several sessions before and after sleep.
Among the advantages of such an experimental model is that the experimental and environmental conditions can be well controlled (e.g., movement artifacts, wake and sleep pattern, diet). Monkey experiments also permit the implantation of electrodes for chronic recordings and repetitive stimulations to study muscle reflexes or cortical-evoked activity. Although the study of reflexes and of corticobulbar excitability could be tested in humans by noninvasive methods (e.g., transcranial magnetic stimulation) to assess the integrity of afferent and efferent sensorimotor pathways and/or to assess the excitability of the jaw motor system, human studies have some limitations60–62 such as the external (i.e., skin or intradermal) fixation of stimulating or recording EMG and EEG electrodes that does not allow repetitive and long-term recordings. Another strength is the use of both jaw-opening reflex and ICMS to test for motor excitability in this animal model. In addition to testing jaw-opening reflex excitability during QW and QS, this model also allowed us to assess corticobulbar excitability. Furthermore, the stimuli used in this animal model did not evoke microarousals and did not disturb sleep, as indicated by insignificant changes by ICMS in the background anterior digastric, genioglossus, and masseter EMG activities as well as heart rate (see Figure 4).
Jaw-Opening Reflex Excitability during Sleep
The jaw-opening reflex has been extensively used to assess excitability of the jaw motor system during the QW state and also during sleep in animals.9,11,14,17,63–65 This reflex has many similarities with the spinal limb flexion reflex and can be evoked following innocuous (low-threshold) or noxious (high-threshold) stimulations.4,17 The jaw-opening reflex can be typically induced in the anterior digastric or lateral pterygoid muscles by orofacial stimulation in the lightly anesthetized or awake cat, monkey, or rabbit, with a latency of 8-15 msec.14,63,65–69 The present experiment used a similar approach to earlier studies in cats and rats to evoke the jaw-opening reflex;15,63 it was triggered by tongue tissue stimulation and was recorded in the anterior digastric muscle, and had a response latency of ≈12 msec. Importantly for this protocol, the reflex was elicited without any other behavioral reactions (e.g., vocalization or movement) or changes in heart rate, suggesting low or no stress for the animal. A previous monkey study also showed that it is possible to study the jaw-opening reflex in an awake and calm animal trained to perceived changes in intensity of low-threshold tooth pulp stimulation (40-200 μA; 1-3 × threshold).66
The present study showed a significant prolongation in jaw-opening reflex latency and a reduction in reflex amplitude during sleep, suggesting a decrease in excitability of the jaw-opening motor system, in accordance with previous studies' interpretation of changes in jaw-opening reflex latency and amplitude.11,17,37,64,65,69,70–72 These findings contrast with some earlier reports in the monkey, rabbit, and cat of the jaw-opening reflex evoked by direct stimulation of the inferior alveolar or the lingual nerves during sleep.9,11 Several factors could explain the difference in findings. Inoue et al.11 reported no change in the jaw-opening reflex amplitude from QW to non-REM sleep, but this might be explained by the low stimulation intensity used to evoke the reflex (set at 1.5 × threshold). The present experiment showed that when the stimulation intensity was set below 2 × threshold for the jaw-opening reflex, it was more difficult to discriminate changes in the reflex. Chase9 reported a facilitation (e.g., increase in amplitude) of the jaw-opening reflex during QS of cats, but notably in that study during QW, the reflex amplitude was large (≈ 800 μV) compared to the previous findings during QW before sleep in rabbits (amplitude of 76-305 μV)11 or the present study in monkeys (amplitude of 18.1-126.7 μV). These differences suggest that Chase9 probably evoked the jaw-opening reflex at a much higher stimulus intensity which might have triggered sleep microarousals or awakening as observed in several studies with reflex stimulation during sleep (see below). We avoided triggering sleep arousal responses in the present study, as noted above. Our data nonetheless are consistent with other findings from chronic recordings of cat jaw-opener and jaw-closer motoneurons that their activity is reduced in non-REM sleep and almost completely suppressed in REM sleep.73,74 The present jaw-opening reflex data are also consistent with previous human findings suggesting that during QS (i.e., non-REM sleep) there is a reduction in the excitability of polysynaptic reflexes, involving these in limb and tongue muscles, produced by low-intensity stimulation which did not induce any sleep arousal or awakening.4,18,75,76 The jaw-closing reflex has also been reported to be inhibited during QS (and REM) sleep in cats77; the amplitude of the monosynaptic H reflex in limb muscles has also been reported to be smaller during QS (i.e., non-REM sleep) of both humans and animals.38,78–80 However, Inoue et al.11 reported that the masseteric monosynaptic reflex induced by stimulation of jaw-closing muscle spindle afferents does not show any significant changes during QS compared with that during the QW in the rabbit. The difference in findings might be explained by some difference in jaw-closing muscle activity in different species during QS. The tonic masseter activities in the rabbit did not change significantly during QS compared with that during the QW,11 in contrast to the current study showing that tonic masseter activities were smaller during QS in the monkey (Figure 5).
It is also to be noted that during REM sleep (or active sleep), the jaw-opening reflex amplitude was largely reduced or suppressed in rabbits and cats.9,11 This is not surprising since REM sleep is characterized by an hypotonia of several submental and neck muscles, as first observed half a century ago.81 Indeed, the reduction in muscle tone during REM is a recognized criterion to score the REM sleep stage in parallel with the presence of rapid eye movements and desynchronized EEG activity.51,54
Corticobulbar Excitability during Sleep
This study also found an increase in the ICMS threshold in face MI for evoking orofacial twitch-like movements during QS. This is consistent with earlier reports of an increase in the threshold of frontal or MI-evoked head and limb movements or sciatic nerve activity during QS compared to wakefulness, suggesting that the cortically induced motor excitability is attenuated during QS.36,38 It is also consistent with findings that the ICMS threshold to trigger orofacial, arm, or neck muscle twitches in monkeys is markedly increased when the animal is dozing compared to the quiet and alert state.39 Our data are also consistent with the decreased motor excitability during sleep in humans, as assessed by transcranial magnetic stimulation. During QS, cortical pyramidal neuron excitability progressively diminishes and the efficiency of the intracortical GABA-ergic network increases82 and cortical activations become more local and stereotypical, indicating a significant impairment of the functioning of the intracortical network.44 As a result, the amplitudes of motor responses induced by transcranial magnetic stimulation of the motor cortex decrease during QS sleep.42,82
We also found an increase in the threshold of ICMS for producing rhythmic jaw movements and tetanic jaw movements during QS, indicating that during QS there is reduced excitability of the jaw motor system which is consistent with our finding of a reduction of jaw-opening reflex excitability during QS. In animals such as the rabbit, cat, guinea pig, and monkey, electrical stimulation of the cortical masticatory area or adjacent face MI when they are awake will trigger rhythmic jaw movements.19–29,35,49 Such stimulation is believed to excite brainstem motoneurons, as well as neurons located in the so-called central pattern generator of mastication that in turn activate trigeminal motoneurons supplying the jaw-opening or jaw-closing muscles.30–33,83,84 The masticatory central pattern generator receives both excitatory and inhibitory projections from structures (e.g., sensorimotor cortex, thalamus, the basal ganglia) involved in the genesis of sleep and the associated reduction in muscle tone (e.g., hypotonia in non-REM sleep and nearly atonia in REM sleep).
During the drowsy state or QS in monkeys, a dichotomous reaction has been reported; the evoked response to visual stimulation was reduced or abolished while the activity in the visual cortex remained.85 In the present study, the absence of evoked rhythmic jaw movements and orofacial twitches by stimulating the monkey's cortical masticatory area or face MI during QS is consistent with a marked reduction in the motor cortical network during sleep. The closest parallel related to such a finding comes from studies using transcranial magnetic stimulation in humans, although transcranial magnetic stimulation may induce a different type of response than ICMS which stimulates a more restricted region of cortex. During QW, transcranial magnetic stimulation of the pre-motor cortical area tends to trigger a widespread response on both cortical hemispheres; during sleep, the evoked response is restricted to the stimulated brain hemisphere.86 This is further supported by the observation that application of transcranial magnetic stimulation to the large central and parietal cortical areas (C3 and P3) in humans produces a reduction in the amplitude and increased response latency of the motor potential recorded at biceps and triceps muscles during sleep in comparison to the awake state.82 It is unknown whether decreased excitability of the jaw motor system is due to a general depression of neuronal excitability or networks during sleep or to other inhibitory mechanisms.87–89 The sleep-related suppression of the ICMS-evoked motor activities and of the jaw-opening reflex could have resulted from several mechanisms74,87–89,90 involving presynaptic and/or postsynaptic inhibition of jaw motoneurons themselves, suppression of cortical or subcortical networks influencing the excitability of the jaw motor system, or even conceivably suppression of peripheral neuromuscular transmission in the jaw muscles. It is also unclear whether the jaw-opening reflex suppression is responsible for or contributes to the suppression of the ICMS-evoked activities and whether changes in neural structures influence each other independently or in combination. The EMG techniques used in the present study to record motor activities do not allow for such delineations, and future studies using other techniques are needed to define the mechanisms involving the dominant sleep-related suppression.
CONCLUSIONS
These results indicate that the primate jaw-opening reflex evoked by low-intensity intraoral stimulation as well as ICMS-induced rhythmic jaw movements and orofacial twitches are markedly reduced during QS, suggesting that the excitability of reflex and corticobulbar-evoked activity in the jaw motor system is depressed during QS.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Lavigne has been invited to be a speaker at a congress funded by UCB, Belgium and he is also a consultant for Pfizer, Canada (lectures to Pfizer staff on sleep – pain). Dr. Sessle is a member of a grant review committee for Pfizer, Canada, and of an advisory committee for Lilly, Canada. The other authors have indicated no financial conflicts of interest in relation to this paper.
ACKNOWLEDGMENTS
The authors thank Dr. Richard Horner for reviewing earlier drafts of the manuscript and for advice throughout the study. We are also thankful to Ms. Susan Carter, Ms. Sylvie Rompré, and Mr. Ken MacLeod for technical assistance. This research was supported by CIHR Grant MOP-4918. B.J.S. and G.J.L. are holders of Canada Research Chairs. Dr. Adachi's current address is: Division of Pharmacology, Meikai University School of Dentistry, Saitama, Japan.
REFERENCES
- 1.Brooks PL, Peever JH. GABAergic and glycinergic control of upper airway motoneurons in rapid eye movement sleep. Adv Exp Med Biol. 2010;669:259–62. doi: 10.1007/978-1-4419-5692-7_52. [DOI] [PubMed] [Google Scholar]
- 2.Cygan F, Oudiette D, Leclair-Visonneau L, Leu-Semenescu S, Arnulf I. Night-to-night variability of muscle tone, movements, and vocalizations in patients with REM sleep behavior disorder. J Clin Sleep Med. 2010;6:551–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson A, Kales A, Lehmann D, and Hoedemaker FS. Muscle tonus inhuman subjects during sleep. Exp Neurol. 1964;10:418–24. doi: 10.1016/0014-4886(64)90033-0. [DOI] [PubMed] [Google Scholar]
- 4.Sandrini G, Milanov I, Rossi B, et al. Effects of sleep on spinal nociceptive reflexes in humans. Sleep. 2001;24:13–7. doi: 10.1093/sleep/24.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Bara-Jimenez W, Aksu M, Graham B, Sato S, Hallett M. Periodic limb movements in sleep. State-dependent excitability of the spinal flexor reflex. Neurology. 2000;4:1609–15. doi: 10.1212/wnl.54.8.1609. [DOI] [PubMed] [Google Scholar]
- 6.Decker MJ, Haaga J, Arnold JL, Atzberger D, Strohl KP. Functional electrical stimulation and respiration during sleep. J Appl Physiol. 1993;75:1053–61. doi: 10.1152/jappl.1993.75.3.1053. [DOI] [PubMed] [Google Scholar]
- 7.Eisele DW, Smith PL, Alam DS, Schwartz AR. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1997;123:57–61. doi: 10.1001/archotol.1997.01900010067009. [DOI] [PubMed] [Google Scholar]
- 8.Guilleminault C, Bowman B. The effect of electrical stimulation on obstructive sleep apnea syndrome. Chest. 1995;107:67–73. doi: 10.1378/chest.107.1.67. [DOI] [PubMed] [Google Scholar]
- 9.Chase MH. The digastric reflex in the kitten and adult cat: paradoxical amplitude fluctuations during sleep and wakefulness. Arch Ital Biol. 1970;108:403–22. [PubMed] [Google Scholar]
- 10.Inoue M, Nozawa-Inoue K, Miyaoka Y, Yamada Y. Changes in jaw reflexes by stimulation of the hypothalamus in anesthetized rabbits. Neurosci Res. 2001;41:61–5. doi: 10.1016/s0168-0102(01)00260-7. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Yamamura K, Nakajima T, Yamada Y. Changes in reflex responses of the masseter and digastric muscles during sleep in freely behaving rabbits. Neurosci Res. 1999;34:37–44. doi: 10.1016/s0168-0102(99)00031-0. [DOI] [PubMed] [Google Scholar]
- 12.Jadidi F, Castrillon E, Svensson P. Effect of conditioning electrical stimuli on temporalis electromyographic activity during sleep. J Oral Rehabil. 2008;35:171–83. doi: 10.1111/j.1365-2842.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura Y, Fujimoto J. A study on the jaw opening reflex. Med J of Osaka Univ. 1958;9:377–87. [Google Scholar]
- 14.Matthews B. Mastication. In: Lavelle CLB, editor. Applied physiology of the mouth. Bristol: Johns Wright and Sons; 1975. pp. 199–242. [Google Scholar]
- 15.Tal M, Sharav Y, Devor M. Modulation of the jaw opening reflex by peripheral electrical stimulation. Exp Neurol. 1981;74:907–19. doi: 10.1016/0014-4886(81)90262-4. [DOI] [PubMed] [Google Scholar]
- 16.Thexton AJ. Jaw opening and jaw closing reflexes in the cat. Brain Res. 1974;66:425–33. [PubMed] [Google Scholar]
- 17.Lund JP. Mastication and its control by the brainstem. Crit Rev Oral Biol Med. 1991;2:33–64. doi: 10.1177/10454411910020010401. [DOI] [PubMed] [Google Scholar]
- 18.Baldissera F, Broggi G, Mancia M. Monosynaptic and polysynaptic spinal reflexes during physiological sleep and wakefulness. Arch Ital Biol. 1966;104:112–33. [PubMed] [Google Scholar]
- 19.Bremer F. Physiologie nerveuse de la mastication chez le chat et lapin. Réflexes de la mastication. Réponses masticatrices corticales et centre cortical du goût. Arch Int Physiol. 1923;21:308–52. [Google Scholar]
- 20.Goldberg LJ, Tal M. Intracellular recording in trigeminal motoneurons of the anesthetized guinea pig during rhythmic jaw movements. Exp Neurol. 1978;58:102–11. doi: 10.1016/0014-4886(78)90125-5. [DOI] [PubMed] [Google Scholar]
- 21.Huang CS, Hiraba H, Sessle BJ. Input-output relationships of the primary face motor cortex in the monkey (Macaca fascicularis) J Neurophysiol. 1989;61:350–62. doi: 10.1152/jn.1989.61.2.350. [DOI] [PubMed] [Google Scholar]
- 22.Lund JP, Lamarre Y. Activity of neurons in the lower precentral cortex during voluntary and rhythmical jaw movements in the monkey. Exp Brain Res. 1974;19:282–99. doi: 10.1007/BF00233235. [DOI] [PubMed] [Google Scholar]
- 23.Magoun HM, Ranson SW, Fisher C. Corticofugal pathways for mastication, lapping and other motor function in the cats. Arch Neurol Psychiat. 1933;30:292–308. [Google Scholar]
- 24.Murray GM, Sessle BJ. Functional properties of single neurons in the face primary motor cortex of the primate. I. Input and output features of tongue motor cortex. J Neurophysiol. 1992;67:747–58. doi: 10.1152/jn.1992.67.3.747. [DOI] [PubMed] [Google Scholar]
- 25.Murray GM, Sessle BJ. Functional properties of single neurons in the face primary motor cortex of the primate. II. Relations with trained orofacial motor behavior. J Neurophysiol. 1992;67:759–74. doi: 10.1152/jn.1992.67.3.759. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura Y, Kubo Y, Nozaki S, Takatori M. Cortically induced masticatory rhythm and its modification by tonic peripheral inputs in immobilized cats. Bull Tokyo Med Dent Univ. 1976;23:101–7. [PubMed] [Google Scholar]
- 27.Vogt C, Vogt O. Die vergleichend-architektonische und die vergleichend-reizphysiologische Felderung der Grosshirnrinde unter besonderer Berucksichtigung der menschlichen. Naturwissenschaften. 1926;14:1190–4. [Google Scholar]
- 28.Yao D, Yamamura K, Narita N, Martin RE, Murray GM, Sessle BJ. Neuronal activity patterns in primate primary motor cortex related to trained or semiautomatic jaw and tongue movements. J Neurophysiol. 2002;87:2531–41. doi: 10.1152/jn.2002.87.5.2531. [DOI] [PubMed] [Google Scholar]
- 29.Yao D, Yamamura K, Narita N, Murray GM, Sessle BJ. Effects of reversible cold block of face primary somatosensory cortex on orofacial movements and related face primary motor cortex neuronal activity. Somatosens Mot Res. 2002;19:261–71. doi: 10.1080/0899022021000037728. [DOI] [PubMed] [Google Scholar]
- 30.Lund JP, Kolta A, Westberg KG, Scott G. Brainstem mechanisms underlying feeding behaviors. Cur Opin Neurobiol. 1998;8:718–24. doi: 10.1016/s0959-4388(98)80113-x. [DOI] [PubMed] [Google Scholar]
- 31.Lund JP, Scott G, Kolta A, Westberg KG. Role of cortical inputs and brainstem interneuron populations in patterning mastication. In: Nakamura Y, Sessle BJ, editors. Neurobiology of mastication - from molecular to systems approach. Amsterdam: Elsevier; 1999. pp. 504–14. [Google Scholar]
- 32.Nakamura Y, Katakura N. Generation of masticatory rhythm in the brainstem. Neurosci Res. 1995;23:1–19. [PubMed] [Google Scholar]
- 33.Nakamura Y, Katakura N, Nakajima M. Generation of rhythmical food ingestive activities of the trigeminal, facial, and hypoglossal motoneurons in in vitro CNS preparations isolated from rats and mice. J Med Dent Sci. 1999;46:63–73. [PubMed] [Google Scholar]
- 34.Huang CS, Hiraba H, Murray GM, Sessle BJ. Topographical distribution and functional properties of cortically induced rhythmical jaw movements in the monkey (Macaca fascicularis) J Neurophysiol. 1989;61:635–50. doi: 10.1152/jn.1989.61.3.635. [DOI] [PubMed] [Google Scholar]
- 35.Martin RE, Kempainen P, Masuda Y, Yao D, Murray GM, Sessle BJ. Features of cortically evoked swallowing in the awake primate (Macaca fascicularis) J Neurophysiol. 1999;82:1529–41. doi: 10.1152/jn.1999.82.3.1529. [DOI] [PubMed] [Google Scholar]
- 36.Baldissera F, Ettore G, Infuso L, Mancia M, Pagni CA. Comparative study of the responses evoked by stimulation of the cortico-spinal pathways during sleep and wakefulness in humans and in animals. Rev Neurol (Paris) 1966;115:82–4. [PubMed] [Google Scholar]
- 37.Chase MH, McGinty DJ. Modulation of spontaneous and reflex activity of the jaw musculature by orbital cortical stimulation in the freely-moving cat. Brain Res. 1970;19:117–26. doi: 10.1016/0006-8993(70)90241-6. [DOI] [PubMed] [Google Scholar]
- 38.Hodes R, Suzuki JI. Comparative thresholds of cortex, vestibular system and reticular formation in wakefulness, sleep and rapid eye movement periods. Electroencephalogr Clin Neurophysiol. 1965;18:239–48. doi: 10.1016/0013-4694(65)90090-8. [DOI] [PubMed] [Google Scholar]
- 39.Sessle BJ, Wiesendanger M. Structural and functional definition of the motor cortex in the monkey (Macaca fascicularis) J Physiol. 1982;323:245–65. doi: 10.1113/jphysiol.1982.sp014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bliwise D, Coleman B, Bergmann B, Wincor MZ, Pivik RT, Rechtschaffen A. Facial muscle tonus during REM and NREM sleep. Psychophysiology. 1974;11:497–508. doi: 10.1111/j.1469-8986.1974.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 41.Okura K, Kato T, Montplaisir JY, Sessle BJ, Lavigne GJ. Quantitative analysis of surface EMG activity of cranial and leg muscles across sleep stages in human. Clin Neurophysiol. 2006;117:269–78. doi: 10.1016/j.clinph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Grosse P, Khatami R, Salih F, Kühn A, Meyer BU. Corticospinal excitability in human sleep as assessed by transcranial magnetic stimulation. Neurology. 2002;59:1988–91. doi: 10.1212/01.wnl.0000038762.11894.da. [DOI] [PubMed] [Google Scholar]
- 43.Hess CW, Mills KR, Murray NM, Schriefer TN. Excitability of the human motor cortex is enhanced during REM sleep. Neurosci Lett. 1987;82:47–52. doi: 10.1016/0304-3940(87)90169-8. [DOI] [PubMed] [Google Scholar]
- 44.Massimini M, Ferrarelli F, Murphy M, et al. Cortical reactivity and effective connectivity during REM sleep in humans. Cogn Neurosci. 2010;1:176–83. doi: 10.1080/17588921003731578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cruccu G, Berardelli A, Inghilleri M, Manfredi M. Functional organization of the trigeminal motor system in man. A neurophysiological study. Brain. 1989;112:1333–50. doi: 10.1093/brain/112.5.1333. [DOI] [PubMed] [Google Scholar]
- 46.Näsi T, Mäki H, Kotilahti K, Nissilä I, Haapalahti P, Ilmoniemi RJ. Magnetic-stimulation-related physiological artefacts in hemodynamic near-infrared spectroscopy signals. PLoS One. 2011;6:e24002. doi: 10.1371/journal.pone.0024002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith RC, Gouin PR, Minkley P, et al. Periodic limb movement disorder is associated with normal motor conduction latencies when studied by central magnetic stimulation - successful use of a new technique. Sleep. 1992;15:312–8. [PubMed] [Google Scholar]
- 48.Huang CS, Sirisko MA, Hiraba H, Murray GM, Sessle BJ. Organization of the primate face motor cortex as revealed by intracortical microstimulation and electrophysiological identification of afferent inputs and corticobulbar projections. J Neurophysiol. 1988;59:796–818. doi: 10.1152/jn.1988.59.3.796. [DOI] [PubMed] [Google Scholar]
- 49.Martin RE, Murray GM, Kemppainen P, Masuda Y, Sessle BJ. Functional properties of neurons in the primate tongue primary motor cortex during swallowing. J Neurophysiol. 1997;78:1516–30. doi: 10.1152/jn.1997.78.3.1516. [DOI] [PubMed] [Google Scholar]
- 50.Narita N, Yamamura K, Yao D, Martin RE, Sessle BJ. Effects of functional disruption of lateral pericentral cerebral cortex on primate swallowing. Brain Res. 1999;824:140–5. doi: 10.1016/s0006-8993(99)01151-8. [DOI] [PubMed] [Google Scholar]
- 51.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring techniques for sleep stages of human subjects. Los Angeles: Brain Research Institute, ; 1968. [Google Scholar]
- 52.Almirall H, Pigarev I, de la Calzada MD, Pigareva M, Herrero MT, Sagales T. Nocturnal sleep structure and temperature slope in MPTP treated monkeys. J Neural Transm. 1999;106:1125–34. doi: 10.1007/s007020050228. [DOI] [PubMed] [Google Scholar]
- 53.Benca RM, Obermeyer WH, Shelton SE, Droster J, Kalin NH. Effects of amygdala lesions on sleep in rhesus monkeys. Brain Res. 2000;879:130–8. doi: 10.1016/s0006-8993(00)02761-x. [DOI] [PubMed] [Google Scholar]
- 54.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The American Academy of Sleep Medicine Manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 55.De Gennaro L, Ferrara M, Bertini M. The spontaneous K-complex during stage 2 sleep: is it the `forerunner' of delta waves? Neurosci Lett. 2000;291:41–3. doi: 10.1016/s0304-3940(00)01366-5. [DOI] [PubMed] [Google Scholar]
- 56.Bara-Jimenez W, Aksu M, Graham B, Sato S, Hallett M. Periodic limb movements in sleep. State-dependent excitability of the spinal flexor reflex. Neurology. 2000;54:1609–15. doi: 10.1212/wnl.54.8.1609. [DOI] [PubMed] [Google Scholar]
- 57.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–79. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turker KS. Reflex control of human jaw muscles. Crit Rev Oral Biol Med. 2002;13:85–104. doi: 10.1177/154411130201300109. [DOI] [PubMed] [Google Scholar]
- 59.Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- 60.Cruccu G, Deuschl G. The clinical use of brainstem reflexes and hand-muscle reflexes. Clin Neurophysiol. 2000;111:371–87. doi: 10.1016/s1388-2457(99)00291-6. [DOI] [PubMed] [Google Scholar]
- 61.Jaberzadeh S, Sakuma S, Zoghi M, Miles TS, Nordstrom MA. Focal transcranial magnetic stimulation of motor cortex evokes bilateral and symmetrical silent periods in human masseter muscles. Clin Neurophysiol. 2008;119:693–703. doi: 10.1016/j.clinph.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Sowman PF, Flavel SC, McShane CL, Miles TS, Nordstrom MA. Transcranial magnetic stimulation reduces masseter motoneuron pool excitability throughout the cortical silent period. Clin Neurophysiol. 2008;119:1119–29. doi: 10.1016/j.clinph.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 63.Ono T, Ishiwata Y, Kuroda T, Nakamura Y. Suppression of jaw-opening and trigemino-hypoglossal reflexes during swallowing in the cat. J Dent Res. 1999;78:1720–6. doi: 10.1177/00220345990780110901. [DOI] [PubMed] [Google Scholar]
- 64.Satoh Y, Ishizuka K, Oskutyte D, Murakami T. Role of the parvicellular reticular formation in facilitating the jaw-opening reflex in rats by stimulation of the red nucleus. Brain Res. 2006;1083:145–50. doi: 10.1016/j.brainres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Fukuhara T, Tsujimura T, Kajii Y, Yamamura K, Inoue M. Effects of electrical stimulation of the superior laryngeal nerve on the jaw-opening reflex. Brain Res. 2011;1391:44–53. doi: 10.1016/j.brainres.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 66.Iwata K, Tsuboi Y, Sumino R. Primary somatosensory cortical neuronal activity during monkey's detection of perceived change in tooth-pulp stimulus intensity. J Neurophysiol. 1998;79:1717–25. doi: 10.1152/jn.1998.79.4.1717. [DOI] [PubMed] [Google Scholar]
- 67.Lavigne GJ, Kim JS, Valiquette C, Lund JP. Evidence that periodontal pressoreceptors provide positive feedback to jaw closing muscles during mastication. J Neurophysiol. 1987;58:342–58. doi: 10.1152/jn.1987.58.2.342. [DOI] [PubMed] [Google Scholar]
- 68.Mostafeezur R, Yamamura K, Kurose M, Yamada Y. Mastication-induced modulation of the jaw-opening reflex during different periods of mastication in awake rabbits. Brain Res. 2009;1254:28–37. doi: 10.1016/j.brainres.2008.11.084. [DOI] [PubMed] [Google Scholar]
- 69.Sessle BJ, Gurza SC. Jaw movement-related activity and reflexly induced changes in the lateral pterygoid muscle of the monkey Macaca fascicularis. Arch Oral Biol. 1982;27:167–73. doi: 10.1016/0003-9969(82)90138-8. [DOI] [PubMed] [Google Scholar]
- 70.Chiang CY, Dostrovsky JO, Sessle BJ. Periaqueductal gray matter and nucleus raphe magnus involvement in anterior pretectal nucleus-induced inhibition of jaw-opening reflex in rats. Brain Res. 1991;544:71–8. doi: 10.1016/0006-8993(91)90886-z. [DOI] [PubMed] [Google Scholar]
- 71.Dubner R, Sessle BJ, Storey AT. The neural basis of oral and facial function. New York: Plenum Press, ; 1978. [Google Scholar]
- 72.Sessle BJ. Modulation of alpha and gamma trigeminal motoneurons by various peripheral stimuli. Exp Neurol. 1977;54:323–39. doi: 10.1016/0014-4886(77)90273-4. [DOI] [PubMed] [Google Scholar]
- 73.Chase MH, Morales FR. Control of motoneurons during sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine 3rd ed. Philadelphia: W.B. Saunders; 2000. pp. 155–68. [Google Scholar]
- 74.Pedroarena C, Castillo P, Chase MH, Morales FR. The control of jaw-opener motoneurons during active sleep. Brain Res. 1994;653:31–38. doi: 10.1016/0006-8993(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 75.Vecchierini-Blineau MF, Guihneuc P. Lower limb cutaneous polysynaptic reflexes in the child, according to age and state of waking or sleeping. J Neurol Neurosurg Psychiatry. 1982;45:531–8. doi: 10.1136/jnnp.45.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horner RL, Innes JA, Morrell MJ, Shea SA, Guz A. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. J Physiol. 1994;476:141–51. [PMC free article] [PubMed] [Google Scholar]
- 77.Chase MH. Brain stem somatic reflex activity in neonatal kittens during sleep and wakefulness. Physiol Behav. 1971;7:165–72. doi: 10.1016/0031-9384(71)90278-2. [DOI] [PubMed] [Google Scholar]
- 78.Kubota K, Tanaka R, Tsuzuki N. Muscle spindle activity and natural sleep in the cat. Jpn J Physiol. 1967;17:613–26. doi: 10.2170/jjphysiol.17.613. [DOI] [PubMed] [Google Scholar]
- 79.Mercier L, Pivik RT. Spinal motoneuronal excitability during wakefulness and non-REM sleep in hyperkinesis. J Clin Neuropsychol. 1983;5:321–36. doi: 10.1080/01688638308401180. [DOI] [PubMed] [Google Scholar]
- 80.Shimizu A, Yamada Y, Yamamoto J, Fujiki A, Kaneko Z. Pathways of descending influence on H reflex during sleep. Electroenceph Clin Neurophysiol. 1966;20:337–47. doi: 10.1016/0013-4694(66)90002-2. [DOI] [PubMed] [Google Scholar]
- 81.Jouvet M, Michel F, Courjon J. Sur un stade d'activité électrique cérébrale rapide au cours du sommeil physiologique. C R Seances Soc Biol Fil. 1959;153:1024–8. [Google Scholar]
- 82.Avesani M, Formaggio E, Fuggetta G, Fiaschi A, Manganotti P. Corticospinal excitability in human subjects during nonrapid eye movement sleep: single and paired-pulse transcranial magnetic stimulation study. Exp Brain Res. 2008;187:17–23. doi: 10.1007/s00221-008-1274-3. [DOI] [PubMed] [Google Scholar]
- 83.Lund JP, Kolta A. Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia. 2006;21:167–74. doi: 10.1007/s00455-006-9027-6. [DOI] [PubMed] [Google Scholar]
- 84.Lund JP. Chew before you swallow. Prog Brain Res. 2011;188:219–28. doi: 10.1016/B978-0-444-53825-3.00020-6. [DOI] [PubMed] [Google Scholar]
- 85.Pigarev IN, Nothdurft HC, Kastner S. Evidence for asynchronous development of sleep in cortical areas. Neuroreport. 1997;8:2557–60. doi: 10.1097/00001756-199707280-00027. [DOI] [PubMed] [Google Scholar]
- 86.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 87.Kaufmann C, Wehrle R, Wetter TC, et al. Brain activation and hypothalamic functional connectivity during human non-rapid eye movement sleep: an EEG/fMRI study. Brain. 2006;129:655–67. doi: 10.1093/brain/awh686. [DOI] [PubMed] [Google Scholar]
- 88.Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–9. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magnin M, Rey M, Bastuji H, Guillemant P, Mauguière F, Garcia-Larrea L. Thalamic deactivation at sleep onset precedes that of the cerebral cortex in humans. Proc Natl Acad Sci. 2010;107:3829–33. doi: 10.1073/pnas.0909710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chase MH, Chandler SH, Nakamura Y. Intracellular determination of membrane potential of trigeminal motoneurons during sleep and wakefulness. J Neurophysiol. 1980;44:349–58. doi: 10.1152/jn.1980.44.2.349. [DOI] [PubMed] [Google Scholar]