Abstract
Study Objectives:
Black race has been associated with decreased continuous positive airway pressure (CPAP) adherence. Short sleep duration, long sleep latency, and insomnia complaints may affect CPAP adherence as they affect sleep and opportunity to use CPAP. We assessed whether self-reported sleep measures were associated with CPAP adherence and if racial variations in these sleep characteristics may explain racial differences in CPAP adherence.
Design:
Analysis of data from a randomized controlled trial (HomePAP), which investigated home versus laboratory-based diagnosis and treatment of obstructive sleep apnea.
Setting:
Seven American Academy of Sleep Medicine-accredited sleep centers in five cities in the United States.
Patients or Participants:
Enrolled subjects (n = 191) with apnea-hypopnea index ≥ 15 and sleepiness (Epworth Sleepiness Scale > 12).
Interventions:
N/A.
Measurements and Results:
Multivariable regression was used to assess if subjective sleep measures and symptoms predicted 3-mo CPAP use. Mediation analysis was used to assess if sleep measures mediated the association of race with CPAP adherence. Black participants reported shorter sleep duration and longer sleep latency at baseline than white and Hispanic participants. Shorter sleep duration and longer sleep latency predicted worse CPAP adherence. Sleep duration mediated the association of black race with lower CPAP adherence. However, insomnia symptoms were not associated with race or CPAP adherence.
Conclusions:
Among subjects with similar severity of obstructive sleep apnea and sleepiness, baseline self-reported sleep duration and latency, but not perceived insomnia, predicted CPAP adherence over 3 mo. Sleep duration explains some of the observed differences in CPAP use by race. Sleep duration and latency should be considered when evaluating poor CPAP adherence.
Clinical Trial Information:
Portable Monitoring for Diagnosis and Management of Sleep Apnea (HomePAP) URL: http://clinicaltrials.gov/show/NCT00642486. NIH clinical trials registry number: NCT00642486.
Citation:
Billings ME; Rosen CL; Wang R; Auckley D; Benca R; Foldvary-Schaefer N; Iber C; Zee P; Redline S; Kapur VK. Is the relationship between race and continuous positive airway pressure adherence mediated by sleep duration? SLEEP 2013;36(2):221-227.
Keywords: CPAP adherence, race, sleep habits
INTRODUCTION
Sleep quality and quantity are associated with health outcomes.1,2 For example, short sleepers (less than 6 h per night) have a greater likelihood of obesity, hypertension, diabetes, cardiovascular morbidity, and mortality.3–5 Short sleepers perceive their general health status6 and physical and mental health to be worse.7 People with poor quality sleep also perceive their health to be worse and experience more physical distress.8 The combination of insomnia and short sleep duration in men is associated with higher mortality.9
Habitual sleep patterns differ by race and residential socioeconomic status (SES) in large epidemiologic studies of the general population. Blacks report shorter sleep duration and longer sleep latency than whites,10,11 yet blacks report fewer insomnia symptoms.12 Residential and environmental factors may contribute to racial differences: living in poor neighborhoods is associated with shorter sleep duration.10 Neighborhood disorder (crime, noise) is associated with poor sleep quality and a lower perception of health.13 Perception of sleep quality also differs by race and SES. Among perimenopausal women, black women report worse sleep quality than white and Asian women.14 Low SES is also associated with more sleep complaints.15 Therefore, overall health perception is worse in populations suffering from poor quality sleep who live in poorer residential areas.
Obstructive sleep apnea (OSA) may be more severe among minorities and in those living in poorer neighborhoods, possibly due to environmental factors such as sleep disruption and air pollution.16 Continuous positive airway pressure (CPAP) is an efficacious treatment for OSA,17 but CPAP adherence often limits its effectiveness.18 Prior cohort studies demonstrate that black race and low SES are factors that are associated with lower CPAP adherence.19–23 Our previous analysis found a significant difference in CPAP use by race (black, non-Hispanic versus nonblack).24 Black race predicted over 1 h less use of CPAP per night over 3 mo in adjusted analyses. Though access to care, health literacy, health belief models, and cost are possible reasons for these findings, few studies have explored explanatory factors. In particular, few studies have examined the relationship of habitual sleep and insomnia to CPAP use and none have looked at its association with race or SES.
Sleep apnea and insomnia frequently coexist25 and this may affect CPAP adherence. Studies show 40-50% of patients with OSA have insomnia complaints.26,27 OSA symptoms of poor quality sleep, delayed sleep onset, fragmented sleep, and daytime sleepiness28,29 overlap considerably with insomnia symptoms. Subjects with chronic insomnia frequently have occult OSA.30,31 It is unknown why some patients with OSA suffer insomnia symptoms. It is possible that demographic, cultural, environmental, and socioeconomic factors contribute to symptom manifestations in OSA of poor quality sleep, insomnia, and sleepiness. OSA symptom manifestation may affect motivation as well as tolerance and subsequent adherence to CPAP therapy.
It was hypothesized that habitual sleep may differ by race and residential SES in patients with OSA and potentially serve as confounders or mediators of the association of race with CPAP use. Data from the HomePAP trial were used to explore the contribution of self-reported sleep measures, duration and latency, to CPAP adherence. Whether insomnia symptoms differed by demographics and were predictive of CPAP adherence was also evaluated. The goal was to identify explanatory factors for the observed differences in CPAP adherence by race.
METHODS
A secondary analysis was performed on data derived from the HomePAP study. As described elsewhere,24,32 the HomePAP study was a randomized trial comparing unattended home-based studies to laboratory-based testing for the diagnosis and treatment of sleep apnea in individuals with moderate to severe OSA. The primary outcome for the study was CPAP adherence over the first 3 mo.
Participants
The subjects included in this analysis were HomePAP study subjects from seven American Academy of Sleep Medicine-accredited sleep centers in five cities in the United States (Seattle, WA, Chicago, IL, Madison, WI, Minneapolis, MN, and Cleveland, OH; Cleveland included three sites). Subjects had a high probability of moderate to severe sleep apnea based on a clinical algorithm including neck circumference, hypertension, habitual snoring, witnessed apneas, choking, or gasping. Additionally, all subjects had a minimum Epworth Sleepiness Scale (ESS) score of 12. Exclusion criteria included advanced chronic lung disease, heart failure, narcotic use or heavy alcohol use, psychiatric disorders other than mild depression, narcolepsy, severe restless legs syndrome, and severe insomnia. The study protocol and procedures were approved by the human subjects department and internal review boards at each participating institution.
Procedure
Eligible subjects completed questionnaires assessing demographic data, medical co-morbidities, medication use, and baseline sleepiness (ESS). Subjects selecting “black/African-American” and who were not Hispanic were classified as black. Black subjects were compared with all others (nonblack) in our analysis. Level of education (> / ≤ high school degree or equivalent), employment status (employed versus unemployed), and ZIP code data were also collected. Residential SES status (lowest quartile ZIP code SES versus other) was derived from 2000 US census data using subjects' ZIP code at enrollment.33 As previously described,24 we created a summary ZIP code SES Z-score using multiple SES variables as in prior studies20,34,35
Upon enrollment subjects completed a sleep habits questionnaire adapted from the Sleep Heart Health Study Sleep Habits Questionnaire (SHHS SHQ).36 It included an evaluation of sleep duration, latency, and insomnia symptoms including difficulty staying asleep, getting back to sleep, and falling asleep. The SHHS SHQ has been previously validated with good reliability.37 Subjects endorsing having trouble falling asleep “frequently” or “always” were characterized as having sleep-onset insomnia symptoms. Subjects characterized their typical time to fall asleep (sleep latency) in intervals (< 5 min, 6-30 min, 31-59 min, 1-2 h, more than 2 h). Sleep latency was dichotomized to ≤ and > 30 min for analyses. Subjects detailed their typical nightly sleep duration on weekends and on weekdays, responding to the question “how much time are you actually asleep” in h and min. The SHQ did not specify a period of recall. Average sleep duration per night at baseline was derived from weighted averages of weekday and weekend estimates of actual nocturnal sleep times. Subjects were asked napping frequency but not duration of naps, so daytime sleep was not included in our measure of sleep duration. Sleep duration was dichotomized into short sleepers (< 6 h) versus others (6 h or more).
Subjects were randomized to either home or laboratory-based diagnostic/titration studies. Subjects with an apnea-hypopnea index (AHI) greater than or equal to 15 qualified for continuation in the study and CPAP titration. CPAP machines and supplies were provided at no cost to the subjects. Enrolled subjects were followed up in clinic at 1 and 3 mo; their CPAP use data were downloaded. Trained staff addressed factors limiting adherence. At the 3-mo follow-up subjects completed questions regarding typical sleep patterns (self-reported sleep duration) but not regarding sleep latency.
Analysis
Average sleep duration per night at baseline and 3 mo, baseline sleep latency, and sleep-onset insomnia complaints (difficulty falling asleep) were compared by race (black versus nonblack), education, and ZIP code SES using the Mann-Whitney and chi-squared tests.
Average nightly CPAP use over 3 mo was compared by baseline sleep latency (≤ / > 30 min), sleep duration (< / ≥ 6 h) and sleep-onset insomnia complaints. The associations of baseline insomnia complaints, self-reported sleep duration, and sleep latency with CPAP adherence were evaluated using multivariable linear regression. The association of race, education, and ZIP code SES and reported sleep latency and duration was tested with logistic and linear regression.
Sleep duration and latency were assessed as mediators of the observed association of race with CPAP use, evaluating for mediation using methodology described by Baron and Kenny.38,39 The association of 1. race with sleep duration and latency and 2. sleep duration and latency with CPAP adherence were determined with linear regression. The mediated effect is in effect the regression coefficient for the association of black race with sleep duration multiplied by the regression coefficient for the association of sleep duration with CPAP adherence adjusted for race (Figure 1). Point estimate and 95% confidence intervals for mediated effects were calculated using resampling methods as described by MacKinnon et al.40 AHI expressed as quartiles, and study arm (home versus laboratory based) were controlled for in all models because these were previously identified as predictors of CPAP use in the HomePAP population.24
Figure 1.
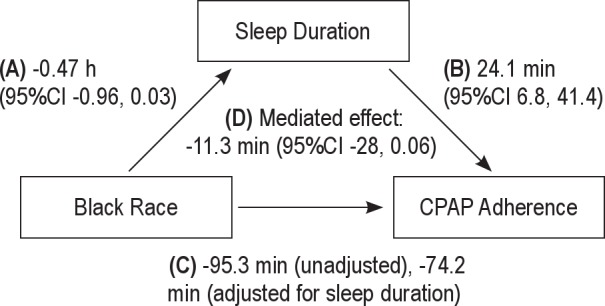
The mediated effect of sleep duration on the association of black race with continuous positive airway pressure (CPAP) adherence. The regression coefficients and their 95% confidence intervals (CI) are displayed. (A) Black race is associated with 0.47 h less sleep per night. (B) Each h of sleep duration is associated with 24 min more CPAP use. (C) The association of black race with CPAP adherence with and without adjustment for sleep duration. (D) The mediated effect of sleep duration on the association of black race with CPAP adherence is 11.3 min. The shorter sleep duration among black subjects accounts for 11.3 min of the reduced CPAP use.
As a sensitivity analysis, a new outcome of CPAP adherence adjusted for sleep opportunity was derived by dividing CPAP use by self-reported sleep duration at 3 mo. Linear regression analyses were used to assess whether baseline sleep latency and race were predictors of percent of sleep duration using CPAP over 3 mo.
RESULTS
Of the 373 randomized and screened subjects, 191 were eligible for full study enrollment with an AHI ≥ 15 and included in analysis. Subjects were predominantly obese (87% body mass index > 30); 62% were white, 22% black, 9% Hispanic, and 6% other; 22% had only a high school education. The subjects reported an average nightly sleep duration of 6.3 h (standard deviation ± 1.4 h) at baseline. Sixty-six subjects (37%) reported an average of less than 6 h of sleep and 40 subjects (22%) reported that it typically took longer than 30 min to fall asleep. Baseline reported sleep duration was significantly shorter in those with greater than 30 min sleep latency (Table 1). Almost one-fourth of eligible subjects (24%, n = 45) had trouble falling asleep “frequently” to “always”. After 3 mo of CPAP intervention, average reported sleep duration increased significantly to a mean of 6.9 h (± 1.7) per night, P = 0.0002.
Table 1.
Baseline characteristics of HomePAP subjects eligible for CPAP by sleep latency and sleep duration, unadjusted
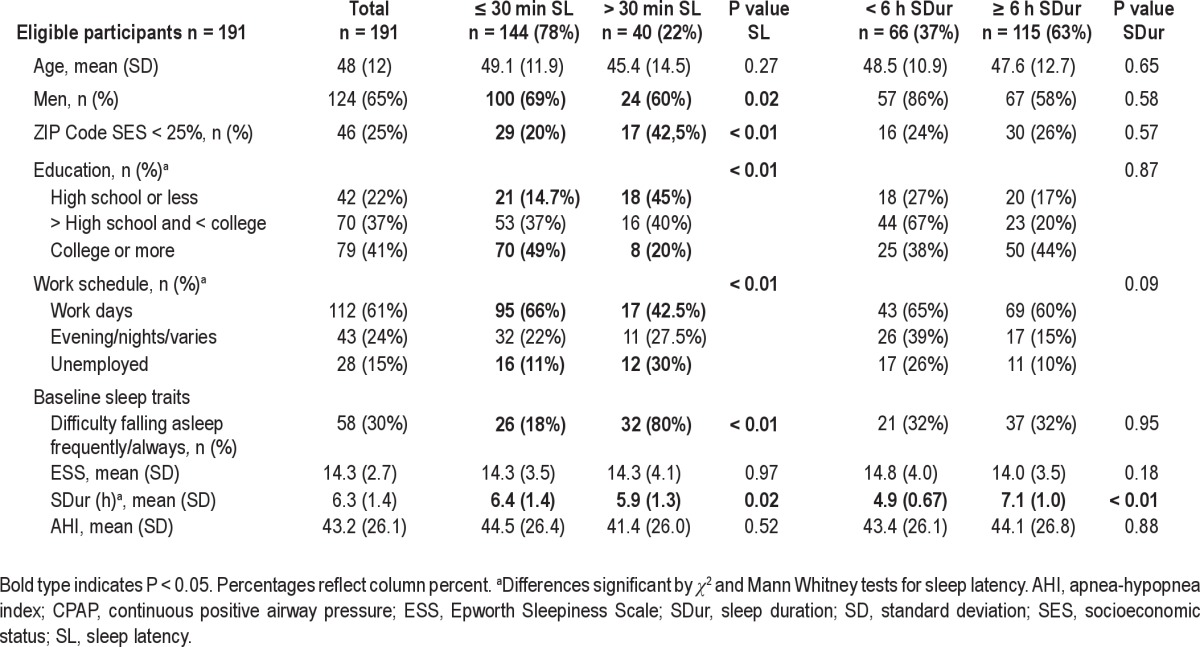
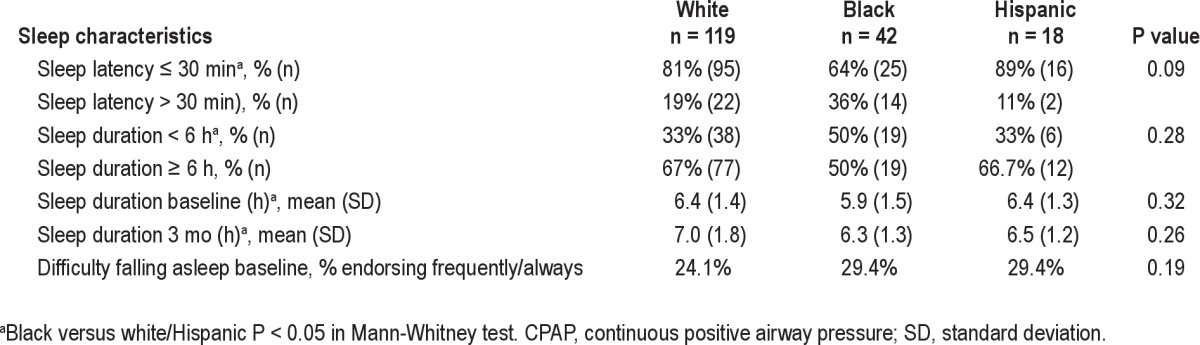
Reported sleep measures at baseline differed by demographic factors. Black subjects reported significantly shorter baseline sleep duration than whites and Hispanics, P = 0.04 (Table 2). After adjusting for education and ZIP code SES, black subjects' sleep duration was an average 43 min less than that of other groups, (P = 0.01). Long sleep latency (> 30 min) was also more common among black subjects, as well as among women, subjects with only a high school education or less, and those residing in the lowest quartile SES ZIP codes (Table 1). High school education or less was associated with 31% greater odds of long sleep latency after adjusting for race and ZIP code SES, (P = 0.002). Sleep-onset insomnia complaints did not correlate with reported long sleep latency.
Table 2.
Baseline sleep characteristics of HomePAP subjects eligible for CPAP by race
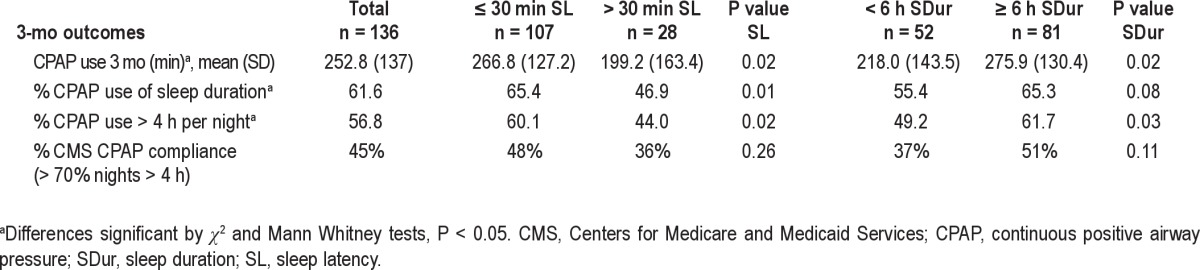
Those with long sleep latency at baseline used CPAP less frequently over 3 mo (Table 3). Specifically, CPAP use averaged 67 min less in those with long sleep latency at baseline compared with those with shorter sleep latency (P = 0.02); a significantly lower proportion of those with long sleep latency used CPAP for more than 4 h per night (P = 0.02). Of note, CPAP use did not differ by baseline insomnia symptoms.
Table 3.
CPAP use at 3-mo follow-up by baseline sleep latency and duration
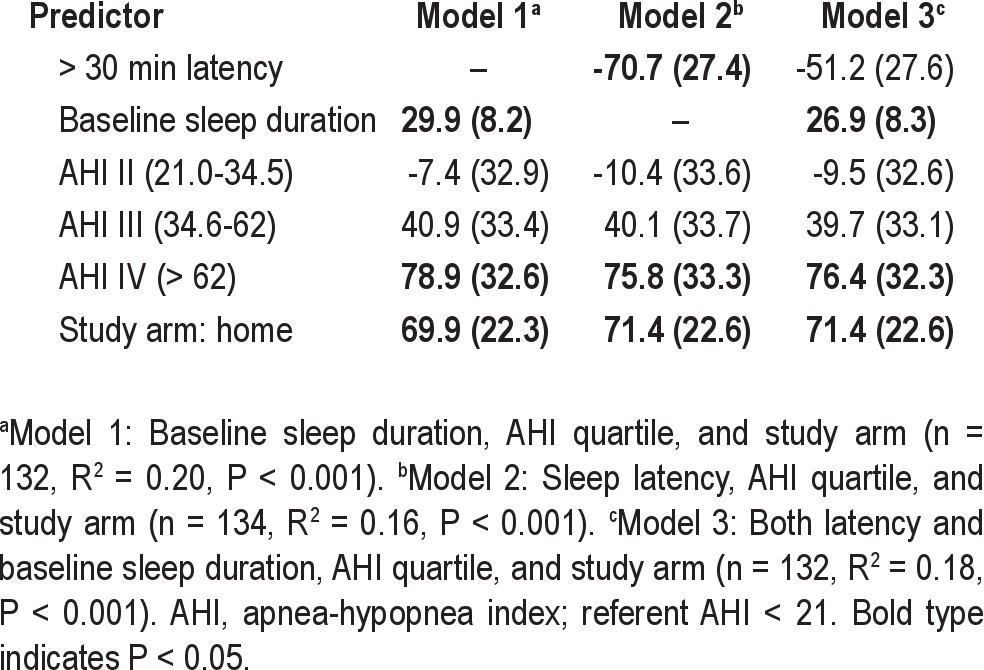
After controlling for AHI and study arm, baseline sleep duration and sleep latency were each predictive of 3-mo CPAP use in separate linear regression models (Table 4). One h longer baseline sleep duration predicted 30 min more nightly CPAP use over 3 mo. Long sleep latency predicted 70 min less CPAP use per night. Insomnia symptoms did not predict CPAP use nor influence the observed association between CPAP use with race, subjective sleep latency, and duration.
Table 4.
Baseline sleep latency and duration as predictors of 3-mo CPAP use reported as β (standard error) in average min/day unadjusted for race
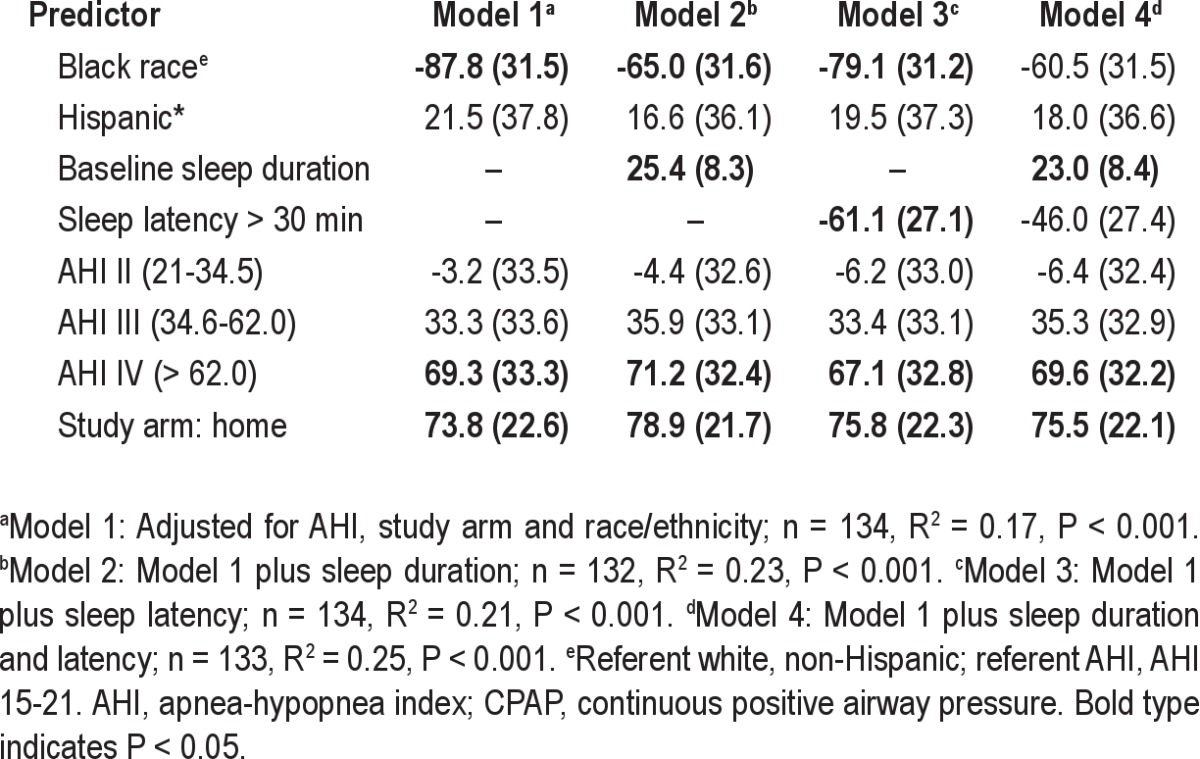
There was some evidence that baseline sleep duration was a mediator of the association between race and CPAP use over 3 mo. Table 5 shows a series of regression models that demonstrate the attenuation of the black race parameter estimate by including sleep duration. The mediated effect of sleep duration on the association of black race with CPAP use was −11.3 min, 95% confidence interval (-28.0, 0.06), P = 0.05 in formal mediation analysis (Figure 1). In contrast, sleep latency did not appear to mediate the association of black race with CPAP use.
Table 5.
Predictors of CPAP use at 3 mo β (standard error) in average min/day including race/ethnicity
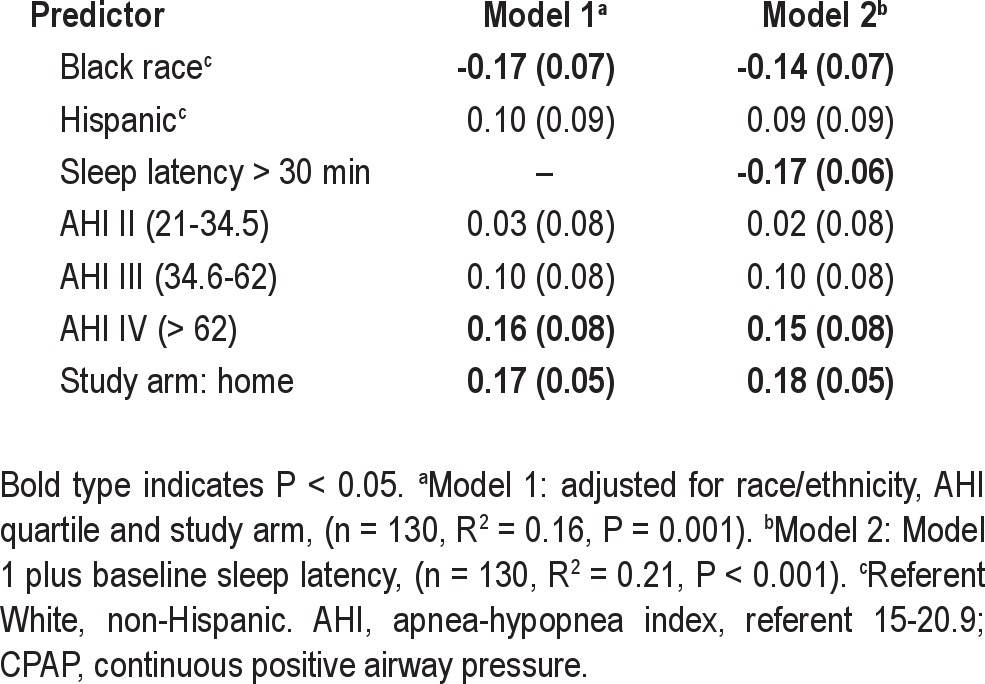
CPAP use as a proportion of reported sleep duration at 3 mo was significantly lower in blacks than nonblacks (47% versus 65%, P < 0.01). In linear regression, black race and longer sleep latency remained significant predictors of the proportion of sleep duration with CPAP use (Table 6).
Table 6.
Predictors of percent of sleep duration at 3 mo using CPAP, β (standard error) in percentage
DISCUSSION
There were significant differences in CPAP adherence by black race and baseline sleep duration and latency in a racially diverse patient sample provided with comparable access to CPAP and sleep apnea management. Among the HomePAP study participants, self-reported longer sleep latency and shorter sleep duration at baseline were associated with black race and less CPAP use over 3 mo. Through the use of statistical methods for assessing mediation, it was demonstrated that the association between black race and CPAP adherence was partially mediated by the shorter sleep duration in blacks in comparison with other racial/ethnic groups. Furthermore, the shorter sleep duration among blacks compared with other groups was associated with longer sleep latency. Thus, these findings point to an as-yet underrecognized target for improving CPAP adherence: sleep duration and latency.
To our knowledge a strong association between long sleep latency and lower CPAP use, particularly in minority and low SES populations, has not been previously highlighted. Only one-third of subjects with > 30 min sleep latency had CPAP adherence that met Medicare requirements. After adjustment, long sleep latency predicted 1 h less CPAP use nightly. Those who usually took longer to fall asleep may have had more frustration with CPAP. The facemask and air pressure, which characterize CPAP, may have been a greater nuisance to those lying awake longer. This may explain in part the reported benefit of eszopiclone on CPAP adherence in severe OSA.41 Perhaps its effects on sleep duration and latency mediate the observed greater CPAP adherence of eszopiclone users. Long sleep latency and short sleep duration may also be markers of underlying poor health or mood disorders that were not fully captured by our study measures, and these health attributes may contribute to adherence.
OSA may exacerbate insomnia and comorbid insomnia may hinder CPAP adherence.26 Insomnia symptoms have been found to be predictive of CPAP adherence in one observational study42 but not in another.43 In our study, subjects reporting long sleep latency did not necessarily perceive trouble sleeping and those who reported difficulties initiating sleep did not have more difficulty adhering to CPAP. This contrasts with the poor CPAP adherence among those who reported long sleep latency. The lack of association between perceived difficulty falling asleep and long sleep latency in our study may be related to our selection of sleepy subjects (ESS > 12); this may have reduced the representation of individuals with the hyper-arousal insomnia phenotype. However, it is also possible that other reporting biases may have influenced our observations.
As previously noted, we observed distinct differences in sleep patterns among subgroups of our sample despite their overall similarity in OSA severity and sleepiness level. Long sleep latency appeared to be more prevalent in those from disadvantaged backgrounds with less education, unemployment, and residing in lower SES ZIP codes. This is consistent with large epidemiologic studies of sleep and SES: longer sleep latency was seen in those with lower employment grade (lower SES) among Japanese men.44 Black subjects also had significantly shorter baseline sleep duration, a finding that is consistent with observational epidemiological studies of the general U.S. population.10,45,46 These sleep measures may be influenced by cultural patterns, health behaviors, neighborhood factors, and household characteristics.46 Long sleep latency may be a consequence of higher life stressors, psychological distress, depression, and neighborhood disorder13,47 more common in low SES groups and minorities.
Sleep duration differed by race and explained approximately 12% of the observed differences in CPAP adherence by race. The current use of absolute duration of CPAP use, rather than percent of sleep duration with CPAP use, as a measure of adequate adherence may discriminate against minorities and low SES populations, whose sleep duration is shorter. However, when evaluating an alternative metric of CPAP adherence–percent CPAP use of typical sleep duration–which accounts for sleep opportunity, subjects of black race still had 18% less CPAP use per night of sleep. Thus, the reported differences in subjective sleep patterns do not explain all of the observed differences in CPAP use by race, and unexplained determinants of CPAP adherence remain.
This secondary data analysis has several important limitations. Although objective measures of CPAP adherence were analyzed, self-reported measures of sleep latency and duration were used. These may not accurately reflect objective sleep habits. However, self-reported habitual sleep latency and duration may not differ significantly from objectively measured latency and duration.48 We did not assess reported sleep latency after initiation of CPAP, which may better correlate with usage patterns. Also, the dataset is limited in its ability to dissect the effects of race and SES because most subjects living in the lowest SES ZIP codes were black and adjustment for both race and residential SES was not feasible. Finally, the loss to follow-up was greater among the subjects in lowest SES ZIP codes and black subjects.
In conclusion, our findings highlight the need to understand sleep health disparities and consider habitual sleep latency and duration when addressing CPAP adherence, particularly in those residing in poor neighborhoods and in racial/ethnic minorities. Baseline differences in sleep latency and sleep duration predicted large differences in CPAP use. Subjective sleep duration and latency differed by race, education, and ZIP code SES in subjects with OSA with similar levels of sleepiness and mediated some of the observed differences in CPAP use by race. Interventions to increase sleep duration and reduce sleep latency may improve CPAP adherence, especially in groups such as racial minorities who have a high prevalence of short sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Auckley has received research support from Teva and equipment for research from ResMed. Dr. Benca has consulted for Sanofi-Aventis and Merck. Dr. Iber has consulted for Apnex Medical. Dr. Foldvary-Schaefer has received equipment for research from CleveMed and research support from UCB Pharma, Teva, CleveMed, and Lundbeck, Inc. She has also participated in speaking engagements for Jazz Pharmaceuticals and UCB Pharma. Dr. Zee has consulted for Sanofi-Aventis, Merck, Philips-Respironics and Purdue. Dr. Redline has received research support from Philips-Respironics and ResMed Foundation. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This material is based upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Health Services Research and Development (HSR&D). Dr. Billings was supported by a HSR&D MD Postdoctoral fellowship: TPM# 61-038. The HomePAP study was supported by a grant from the American Sleep Medicine Foundation 38-PM-07 Grant: Portable Monitoring for the Diagnosis and Management of OSA.
The study's contents are solely the responsibility of the authors. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the United States Government, or funding agencies. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A commentary on this article appears in this issue on page 163.
REFERENCES
- 1.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31:1087–96. [PMC free article] [PubMed] [Google Scholar]
- 3.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steptoe A, Peacey V, Wardle J. Sleep duration and health in young adults. Arch Intern Med. 2006;166:1689–92. doi: 10.1001/archinte.166.16.1689. [DOI] [PubMed] [Google Scholar]
- 7.Stranges S, Dorn JM, Shipley MJ, et al. Correlates of short and long sleep duration: a cross-cultural comparison between the United Kingdom and the United States: the Whitehall II Study and the Western New York Health Study. Am J Epidemiol. 2008;168:1353–64. doi: 10.1093/aje/kwn337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore PJ, Adler NE, Williams DR, Jackson JS. Socioeconomic status and health: the role of sleep. Psychosom Med. 2002;64:337–44. doi: 10.1097/00006842-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger PM, Onge JMS, Chang VW. Race/ethnic differences in adult mortality: the role of perceived stress and health behaviors. Social Sci Med. 2011;73:1312–22. doi: 10.1016/j.socscimed.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med. 2010;8:246–59. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- 13.Hale L, Hill TD, Burdette AM. Does sleep quality mediate the association between neighborhood disorder and self-rated physical health? Prev Med. 2010;51:275–8. doi: 10.1016/j.ypmed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32:73–82. [PMC free article] [PubMed] [Google Scholar]
- 15.Grandner MA, Patel NP, Gehrman PR, et al. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med. 2010;11:470–8. doi: 10.1016/j.sleep.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanobetti A, Redline S, Schwartz J, et al. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban areas. Am J Respir Crit Care Med. 2010;182:819–25. doi: 10.1164/rccm.200912-1797OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siccoli MM, Pepperell JC, Kohler M, Craig SE, Davies RJ, Stradling JR. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2008;31:1551–8. doi: 10.1093/sleep/31.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joo MJ, Herdegen JJ. Sleep apnea in an urban public hospital: assessment of severity and treatment adherence. J Clin Sleep Med. 2007;3:285–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Platt AB, Field SH, Asch DA, et al. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32:799–806. doi: 10.1093/sleep/32.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg H, Fleischman J, Gouda HE, et al. Disparities in obstructive sleep apnea and its management between a minority-serving institution and a voluntary hospital. Sleep Breath. 2004;8:185–92. doi: 10.1007/s11325-004-0185-1. [DOI] [PubMed] [Google Scholar]
- 22.Simon-Tuval T, Reuveni H, Greenberg-Dotan S, Oksenberg A, Tal A, Tarasiuk A. Low socioeconomic status is a risk factor for CPAP acceptance among adult OSAS patients requiring treatment. Sleep. 2009;32:545–52. doi: 10.1093/sleep/32.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakker JP, O'Keeffe KM, Neill AM, Campbell AJ. Ethnic disparities in CPAP adherence in New Zealand: effects of socioeconomic status, health literacy and self-efficacy. Sleep. 2011;34:1595–603. doi: 10.5665/sleep.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billings ME, Auckley D, Benca R, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. 2011;34:1653–8. doi: 10.5665/sleep.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krakow B. An emerging interdisciplinary sleep medicine perspective on the high prevalence of co-morbid sleep-disordered breathing and insomnia. Sleep Med. 2004;5:431–3. doi: 10.1016/j.sleep.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Luyster FS, Buysse DJ, Strollo PJ., Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6:196–204. [PMC free article] [PubMed] [Google Scholar]
- 27.Lavie P. Insomnia and sleep-disordered breathing. Sleep Med. 2007;4:S21–5. doi: 10.1016/S1389-9457(08)70005-4. [DOI] [PubMed] [Google Scholar]
- 28.Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep Med Rev. 2003;7:335–49. doi: 10.1053/smrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 30.Guilleminault C, Palombini L, Poyares D, Chowdhuri S. Chronic insomnia, postmenopausal women, and sleep disordered breathing: part 1. Frequency of sleep disordered breathing in a cohort. J Psychosom Res. 2002;53:611–5. doi: 10.1016/s0022-3999(02)00445-2. [DOI] [PubMed] [Google Scholar]
- 31.Lichstein KL, Riedel BW, Lester KW, Aguillard RN. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67:405–10. doi: 10.1037//0022-006x.67.3.405. [DOI] [PubMed] [Google Scholar]
- 32.Rosen CL, Auckley D, Benca R, et al. A multi-site randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35:657–67. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.USA Quick Facts. US Census Bureau. 2011. [Accessed March 25]. http://quickfacts.census.gov/qfd/states/00000.html.
- 34.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149:342–7. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 35.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–78. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 36.Sleep Habits Questionaire. 2012. [Accessed September 10]. http://www.jhucct.com/shhs/details/manual/forms/sh/shhshq2.pdf.
- 37.Baldwin CM, Choi M, McClain DB, Celaya A, Quan SF. Spanish translation and cross-language validation of a sleep habits questionnaire for use in clinical and research settings. J Clin Sleep Med. 2012;8:137–46. doi: 10.5664/jcsm.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prev Sci. 2009;10:87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 40.MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav Res Methods. 2007;39:384–9. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151:696–702. doi: 10.7326/0003-4819-151-10-200911170-00006. [DOI] [PubMed] [Google Scholar]
- 42.Wickwire EM, Smith MT, Birnbaum S, Collop NA. Sleep maintenance insomnia complaints predict poor CPAP adherence: a clinical case series. Sleep Med. 2010;11:772–6. doi: 10.1016/j.sleep.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen XL, Chaskalovic J, Rakotonanahary D, Fleury B. Insomnia symptoms and CPAP compliance in OSAS patients: a descriptive study using Data Mining methods. Sleep Med. 2010;11:777–84. doi: 10.1016/j.sleep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Sekine M, Chandola T, Martikainen P, McGeoghegan D, Marmot M, Kagamimori S. Explaining social inequalities in health by sleep: the Japanese civil servants study. J Public Health (Oxf) 2006;28:63–70. doi: 10.1093/pubmed/fdi067. [DOI] [PubMed] [Google Scholar]
- 45.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 46.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169:1052–63. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman EM, Love GD, Rosenkranz MA, et al. Socioeconomic status predicts objective and subjective sleep quality in aging women. Psychosom Med. 2007;69:682–91. doi: 10.1097/PSY.0b013e31814ceada. [DOI] [PubMed] [Google Scholar]
- 48.Silva GE, Goodwin JL, Sherrill DL, et al. Relationship between reported and measured sleep times: the sleep heart health study (SHHS) J Clin Sleep Med. 2007;3:622–30. [PMC free article] [PubMed] [Google Scholar]


