Abstract
Study Objectives:
We analyzed the potential predictive factors for precocious puberty, observed in some cases of childhood narcolepsy with cataplexy (NC) and for obesity, a much more common feature of NC, through a systematic assessment of pubertal staging, body mass index (BMI), and metabolic/endocrine biochemical analyses.
Design:
Cross-sectional on consecutive recruitment.
Setting:
Hospital sleep center and pediatric unit.
Patients:
Forty-three children and adolescents with NC versus 52 age-matched obese children as controls.
Interventions:
N/A.
Measurements and Results:
Patients underwent clinical interview, polysomnographic recordings, cerebrospinal fluid hypocretin-1 measurement, and human leukocyte antigen typing. Height, weight, arterial blood pressure, and Tanner pubertal stage were evaluated. Plasma lipid and glucose profiles were analyzed. When an altered pubertal development was clinically suspected, plasma concentrations of hypothalamic-pituitary-gonadal axis hormones were determined. Children with NC showed a high prevalence of overweight/obesity (74%) and a higher occurrence of precocious puberty (17%) than obese controls (1.9%). Isolated signs of accelerated pubertal development (thelarche, pubic hair, advanced bone age) were also present (41%). Precocious puberty was significantly predicted by a younger age at first NC symptom onset but not by overweight/obesity or other factors. In addition, overweight/obesity was predicted by younger age at diagnosis; additional predictors were found for overweight/obesity (short disease duration, younger age at weight gain and lower high-density lipoprotein cholesterol), which did not include precocious puberty. NC symptoms, pubertal signs appearance, and body weight gain developed in close temporal sequence.
Conclusions:
NC occurring during prepubertal age is frequently accompanied by precocious puberty and overweight/obesity, suggesting an extended hypothalamic dysfunction. The severity of these comorbidities and the potential related risks require a multidiagnostic approach and a tailored therapeutic management.
Citation:
Poli F; Pizza F; Mignot E; Ferri R; Pagotto U; Taheri S; Finotti E; Bernardi F; Pirazzoli P; Cicognani A; Balsamo A; Nobili L; Bruni O; Plazzi G. High prevalence of precocious puberty and obesity in childhood narcolepsy with cataplexy. SLEEP 2013;36(2):175–181.
Keywords: Endocrine disorders, hypocretin, hypothalamic-pituitary axis, pubertal development, sleep
INTRODUCTION
Narcolepsy with cataplexy (NC) is a rare central nervous system hypersomnia occurring during childhood or adolescence in half of the cases.1 NC is characterized by the tetrad of excessive daytime sleepiness (EDS) with irresistible sleep attacks, cataplexy (a sudden loss of muscle tone mostly triggered by emotions), sleep paralysis, and hypnopompic and hypnagogic hallucinations, in addition to disrupted nocturnal sleep.2 NC is a chronic condition and symptoms are thought to be stable over time.3 The clinical manifestations of childhood NC can be variable,4 with frequent absence of the full classical tetrad5 leading to diagnostic delay and frequent misdiagnosis.6,7 Close to NC onset, EDS can manifest as paradoxical hyperactivity (similar to attention deficit hyperactivity disorder) or may be considered as laziness, or misinterpreted as a consequence of sleep apnea syndrome. Hallucinations can be underrecognized because of the difficulty for children to describe them and for the possible coexistence of nightmares, rapid eye movement (REM) sleep behavior disorder, sleep terrors, and confusional arousals.4,5,7 Children with NC also may not fulfill the conventional multiple sleep latency test (MSLT) cutoffs established for adults, thus challenging the diagnosis.8–10 Recently, specific motor alterations in addition to cataplexy have been described in children with NC, characterized by an acute-onset movement disorder,11 as well as abrupt NC onset after H1N1 seasonal influenza or its vaccination.12–15
Because of its specific manifestations, childhood NC requires tailored patient assessments and investigations for comorbidities that may not be observed in adults. Studies in adult NC cases indicated metabolic and endocrine alterations including excess body weight and obesity,16 high prevalence of metabolic syndrome in young adults,17 and type 2 diabetes mellitus in older individuals,18 as well as hormonal alterations, including those affecting gonadal function.19 Children with NC also appear to be overweight and/or obese,20 and an association with precocious puberty was anecdotally reported, suggesting the presence of a wider metabolic/hormonal derangement.21–24 However, the endocrine and metabolic alterations in childhood NC, which are likely to have implications for childhood development and patient care, were poorly investigated. In the current study, we systematically examined the potential metabolic and endocrine abnormalities in a well-characterized cohort of children with NC referred to our center for narcolepsy. Moreover, to assess the potential role of excess body weight, we compared children with NC with a group of age-matched obese children who did not have narcolepsy.
METHODS
Patients
Forty-three children or adolescents (23 boys, mean age 11.78 ± 3.64 years), referred to the outpatient clinic for narcolepsy of the Department of Neurological Sciences, University of Bologna (Italy), were recruited from June 2007 to October 2011. Twenty-seven of these patients were also included in another independent study.11 Each patient underwent a one-week hospitalization to confirm NC diagnosis.2 Brain magnetic resonance imaging (MRI) was carried out to exclude secondary causes of NC.2 One boy with secondary NC (holoprosencephaly at MRI) was excluded.25 Physical examination included height and body weight measurements and pubertal development staging. The study was approved by the local ethics committee; written parental informed consent and written assent were obtained in all cases.
Narcolepsy Assessment
During the evaluation, each child was accompanied by a parent and underwent nocturnal polysomnography followed by a five nap-opportunity MSLT.2 Blood and cerebrospinal fluid samples were taken for human leukocyte antigen (HLA) DQB1*06:02 genotyping26 and hypocretin-127 (hcrt-1, Human orexin-A RIA Kit, Phoenix Pharmaceutical, Inc., Belmont, CA) determination, respectively. A self-completed Epworth Sleepiness Scale modified for children (mESS)28 was administered (pathological score > 11).
Pubertal Status Assessment
Pubertal status was assessed by means of the visual Tanner score scale.29,30 If a misalignment between chronological age and pubertal signs was clinically suspected, patients were referred to the pediatric endocrinology department. Pubertal endocrine assessment included the gonadotropin-releasing hormone (GnRH) stimulation curve—samples were collected just before GnRH administration (gonadorelin, 50 mcg intravenously at 08:00) and after 30 and 60 minutes respectively (at 08:30 and 09:00)31—X-ray of the nondominant wrist for bone/chronological age ratio, pelvic ultrasound (girls), and brain MRI of the hypothalamic-pituitary region. A prepubertal status was identified in the case of absence or mild presence of axillary/pubic hair (up to PH1 at Tanner scale), and/or thelarche (up to B1 at Tanner scale), and/or testis enlargement (up to 4 mL). Precocious puberty was diagnosed when children fulfilled the following criteria32: (1) appearance of secondary sexual characteristics before the age of 8 or 9 years in girls or boys, respectively (level 2 at Tanner scale and subsequent levels); (2) markedly elevated plasma luteinizing hormone (LH) levels, i.e., above 5 mIU/mL after GnRH stimulation (this provides the ability to distinguish true central puberty from adrenarche), compared with the normal range for the corresponding pubertal stage33; and (3) absence of brain MRI alterations.
Isolated advanced bone age at wrist X-ray and isolated signs of accelerated pubertal development, namely isolated thelarche (B2 at Tanner scale) and/or isolated presence of pubic hair (PH2 at Tanner scale), were also considered in children not fulfilling the criteria for precocious puberty.
Anthropometric/Metabolic Assessment
For each child, height and weight were measured, body mass index (BMI) was calculated, and BMI percentile was determined in comparison with the Italian growth percentile scales for boys and girls.34 Obesity was defined as BMI > 97th percentile, overweight as BMI between the 85th and 97th percentiles, and normal weight as BMI < 85th percentile.35 The age of a rapid increase in body weight (i.e., when the child started to develop adiposity, over a time span of three months), defined as “age at weight gain”, was identified using records from the children's general pediatrician. Additionally, family history of metabolic/internal diseases was collected, including diagnoses of type 2 diabetes, obesity, other nonspecified endocrinopathies, dyslipidemia, and cardiovascular disease.
Blood samples were collected in the morning after fasting from midnight onward. Analyses included: total lipoprotein, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol; triglycerides; and high sensitivity C-reactive protein (hsCRP Assay Siemens®, Tarrytown, NY). An oral glucose tolerance test with glucose and insulin measurements was performed (1.75 g glucose/kg of body weight, maximal dose of 75 g, with blood samples taken at baseline and 30, 60, 90, and 120 minutes after glucose load). The responses of glucose and insulin to the oral glucose tolerance test were analyzed by calculating the area under the curve, using the trapezoidal method.
Controls
We assessed the prevalence of precocious puberty in obese children without narcolepsy from the same geographic area of our children with NC in a group of 52 consecutive patients referred to the Obesity Outpatient Center of the Pediatric Clinic, S. Orsola-Malpighi General Hospital, Bologna, Italy. The diagnosis of obesity was based on the same criteria used for children with NC. In this way, we recruited 29 boys and 23 girls in the same age range of our patients with NC; their mean age (± standard deviation) was 11.6 ± 3.12 years, weight 71.0 ± 22.20 kg, height 153.3 ± 16.86 cm, and BMI 29.4 ± 4.74. We clinically excluded severe sleep disorders in these subjects and their mESS was 7.1 ± 1.73. Precocious puberty was diagnosed following the same criteria used in the NC group and was found in one subject.
Statistical Analysis
The nonparametric Mann-Whitney U test for unpaired data sets was used to analyze differences in the response of LH to GnRH. The binomial logistic regression was used to determine the best predictors for the presence or absence of precocious puberty in the children with NC. We first conducted a series of individual logistic regression analyses with the puberty status used as a dependent variable and each single parameter considered in this study as the predictor; after this step, we included the predictors that yielded a statistically significant result in an additional logistic regression analysis using all these significant predictors at once. Finally, the odds ratio of children with NC for developing precocious puberty versus an age-matched obese controls was computed and its statistical significance was estimated by means of the chi-square test.
RESULTS
Demographic and Clinical Characteristics
Forty-two patients (22 boys, mean age 11.42 ± 3.59 years) entered the study (Table 1). All patients presented with EDS (mESS mean score of 17 ± 7),28 confirmed by MSLT results (mean MSLT of 3.73 ± 2.76 min with 4.24 ± 0.94 sleep-onset rapid eye movement periods, SOREMPs). HLA DQB1*06:02 allele was present in 39 of 42 children (93%),26 and mean cerebrospinal fluid hcrt-1 concentration was in the low/undetectable range (27.93 ± 29.91 pg/mL), also in the three HLA DQB1*06:02 negative patients.2 Mean age at onset of the first symptom (either EDS or cataplexy) was 8.75 ± 2.88 y, the mean disease duration was 2.22 ± 2.34 years, and the mean age at weight gain was 8.78 ± 3.09 years.
Table 1.
Demographic and sleep characteristics of the group of children with narcolepsya

Children with NC presented with a mean BMI of 22.19 ± 4.39 kg/m2, and its comparison with the Italian growth percentile scales showed that 31 children (74%) satisfied pediatric criteria for excess body weight or obesity.20,35 The children's general pediatric records and their parents' history revealed a body weight increase temporally close to NC onset in 25 children (60%). Investigation for metabolic or cardiovascular disease in patients' relatives disclosed type 2 diabetes (42%), obesity (39%), other nonspecified endocrinopathies (42%), dyslipidemia (50%), myocardial infarction (42%), stroke (28%), and arterial hypertension (92%).
Pubertal and Anthropometric/Metabolic Characteristics
Physical examination led to the clinical suspicion of misalignment between chronological age and pubertal signs in 22 of 42 children (52%) who therefore underwent the pubertal assessment (Figure 1). A diagnosis of precocious puberty was made in 7 of 22 children (32%, 4 girls and 3 boys, mean age 8.47 ± 0.87 years; Figure 2 shows the curve of the LH level after 50 mcg GnRH stimulation). Precocious puberty was excluded in the remaining children, who presented only advanced bone age at wrist X-ray and/or isolated signs of accelerated pubertal development (both in 6 of 22, 27%, with possible overlap). Overall, 9 of 22 patients (41%) displayed accelerated pubertal development. On the basis of chronological age and presence of precocious puberty, we defined three groups of patients with NC: children with precocious puberty (7 of 42, 17%), children still at risk for developing precocious puberty (9 of 42, 21%), and children not at risk for developing precocious puberty (26 of 42, 62%). Isolated signs of accelerated pubertal development were equally distributed between children still at risk and children no longer at risk (Figure 1).
Figure 1.
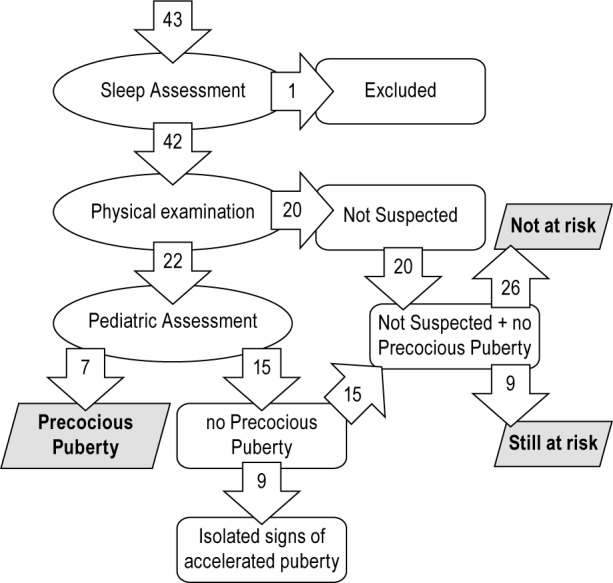
Flowchart of pubertal assessment. Precocious Puberty: Children with precocious puberty. Still at Risk: Children without precocious puberty but still at risk because of their young age (below precocious puberty cutoff age: 8 y for girls; 9 y old for boys). Not at Risk: Children without precocious puberty and no more at risk because of their older age (above precocious puberty cutoff age: 8 y for girls; 9 y for boys).
Figure 2.
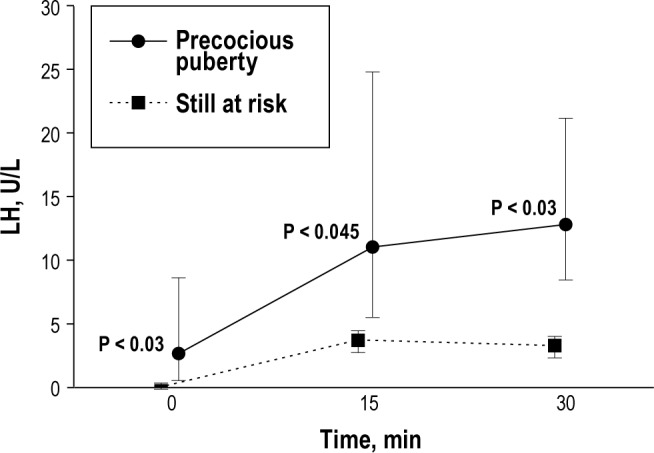
Curve of the luteinizing hormone (LH) level after 50 mcg gonadotropin-releasing hormone stimulation in children with narcolepsy with cataplexy and precocious puberty and those still at risk. Black-filled squares and circles represent median values; whiskers represent 95% confidence intervals.
However, for the statistical analysis, we clustered patients in two groups: one with precocious puberty (7 of 42, 17%) and the other without precocious puberty (the remaining 35 subjects, 83%). Table 2 shows the details of the binomial logistic regression analysis run for several general, obesity- and sleep-related parameters, using the precocious puberty status as a dependent variable. Only age at diagnosis and age at onset of the first symptom (EDS or cataplexy) predicted the appearance of precocious puberty. When these two variables were used together for the final logistic regression, they still yielded a significant predictive value for precocious puberty (R2 = 0.138, P < 0.02).
Table 2.
Logistic regression analysis of possible predictive factors for the occurrence of precocious puberty in patients with narcolepsya
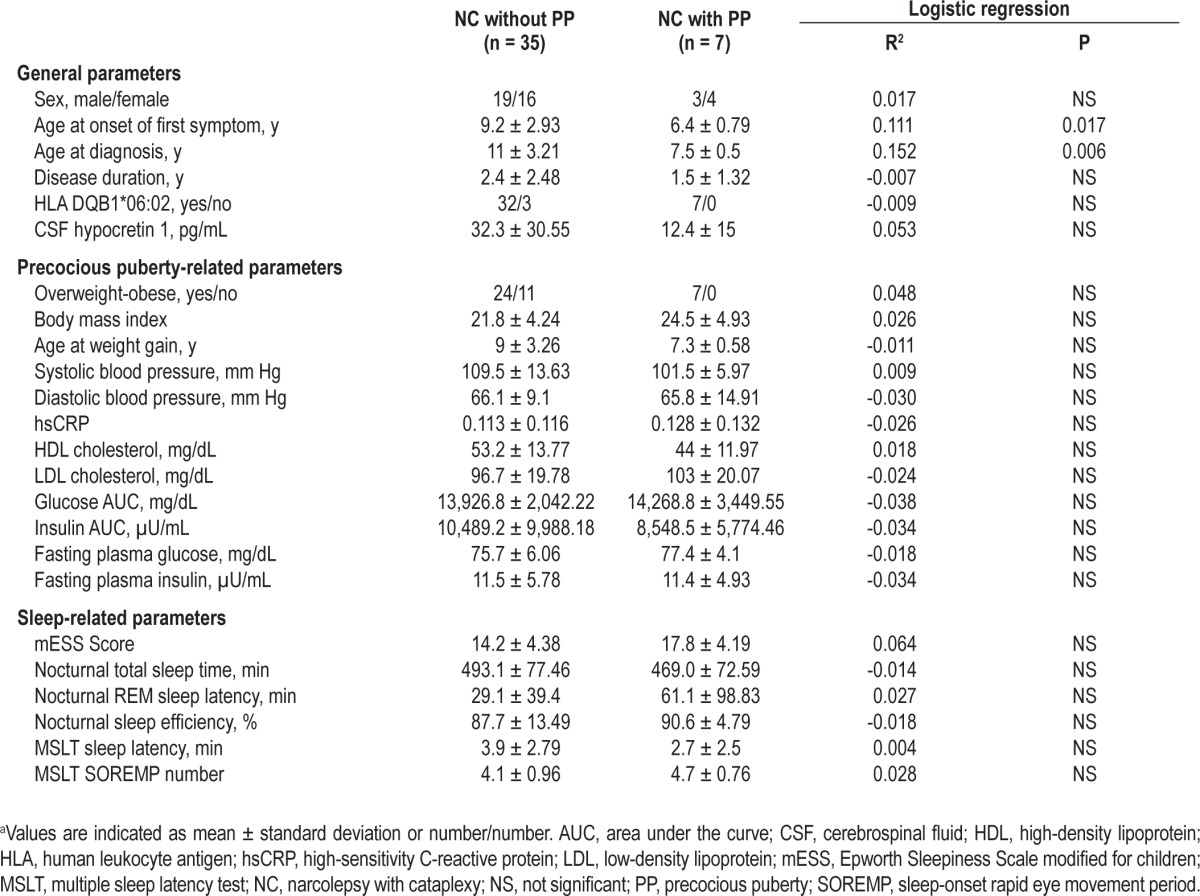
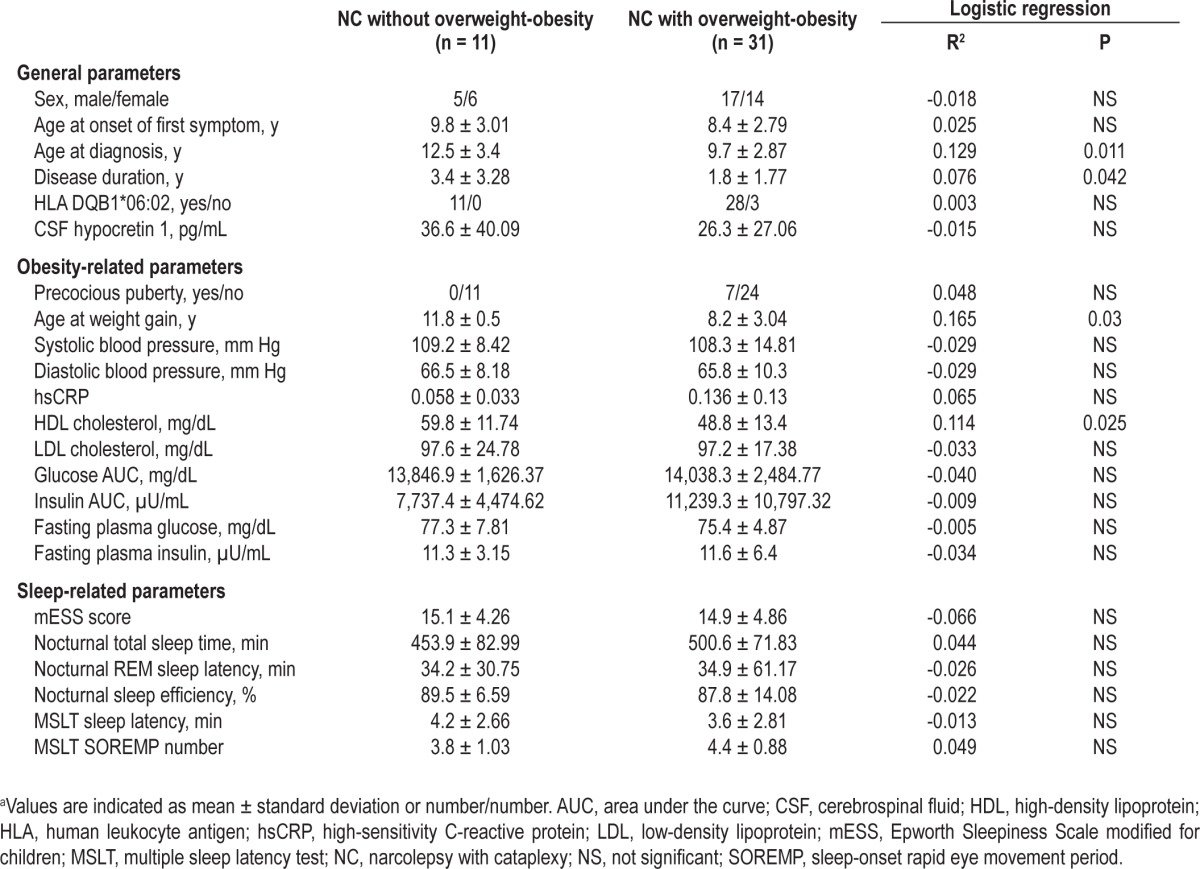
Table 3 shows the binomial logistic regression analysis of possible predictive factors for the occurrence of obesity in patients with NC. Similar to the analysis reported previously, a younger age at diagnosis of NC was predictive for obesity. In addition, disease duration was shorter in obese children. Among the expected obesity-related factors, a younger age at weight gain and a lower HDL cholesterol level were also predictive for obesity. Including these four predictive variables together for the final logistic regression, they still yielded a significant and robust predictive value for obesity (R2 = 0.397, P < 0.015).
Table 3.
Logistic regression analysis of possible predictive factors for the occurrence of overweight-obesity in patients with narcolepsya
Finally, Table 4 reports the comparison between the prevalence of precocious puberty in patients with NC (16.7%) versus an age-matched obese controls (1.9%). The odds ratio of children with NC for developing precocious puberty versus an age-matched obese controls was estimated to be 10.2 and was statistically significant (chi-square test and 95% confidence interval nonoverlapping with zero).
Table 4.
Comparison between the prevalence of precocious puberty in patients with narcolepsy and in age-matched obese control subjectsa

DISCUSSION
This study, through a systematic assessment of precocious puberty, proved the association between childhood NC and precocious puberty, which was previously only anecdotally
reported.21–24 The systematic physical examination and the endocrine/metabolic assessment of consecutive children with NC revealed a precocious puberty prevalence of 17%, which strikingly increases up to 32% when considering children still at a critical age for precocious puberty (i.e., excluding those already too old at observation time). This prevalence is nearly 1,000 times higher than that reported in the general population (0.015%).32 We also found a doubled prevalence of excess body weight/obesity as compared with the general Italian pediatric population (74% versus 36%),36 confirming previous observations,20 and we showed lower levels of HDL cholesterol in obese children with NC.
In 1983, Chisholm et al.21 described a 5.5-y-old girl with NC, thelarche (B2 at Tanner stage), and advanced bone age, and suggested that the very early onset of NC could account for the precocious puberty. Subsequently, other studies22,23 along with Plazzi et al.,24 reported the same association in seven hcrt-1 deficient children with NC who also were concomitantly overweight/obese and/or presented with other neuroendocrine dysfunctions, such as corticotropic deficiency, polycystic ovarian syndrome, and insulin resistance.
In the current study, adopting rigorous criteria for the diagnosis of precocious puberty and considering isolated signs of accelerated pubertal growth as a separate category,32 we found a large prevalence of true precocious puberty in pediatric NC. Beyond a clear-cut precocious puberty, several children, among those who underwent the pubertal assessment, presented isolated signs of accelerated pubertal development (9 of 22, 41%). Therefore, the routine clinical examination of children for the detection of puberty revealed a high sensitivity of this simple clinical approach (Figure 1) and the utility of an extended pediatric evaluation in such patients. Children with precocious puberty were more frequently obese rather than overweight.
The findings depict a subtype of childhood NC with a complex phenotype characterized by obesity and pubertal alterations. The close temporal link between the onset of NC, weight gain, and pubertal alterations further suggests a tight association between these three conditions. The association between obesity and precocious puberty has already been reported in the general population,37 but it is not confirmed evidence.38 It also remains unclear whether pubertal changes are the cause or the results of the increased body fat.39 Moreover, overweight/obese children with NC have dysmetabolic features (lower HDL),35,36 independent of familial predisposition, suggesting other determinants for the increased BMI.
We have carefully analyzed the interdependencies between NC, precocious puberty, and obesity in our group of patients and we found that obesity was not a predictive factor for precocious puberty in our children; conversely, precocious puberty did not predict obesity in the same group. This independency is further reinforced by the comparison with a control group of obese children.
A younger age at onset of the first NC symptom is a predictor of precocious puberty, suggesting that age at onset of NC is crucial for this comorbidity. Moreover, a younger age at onset of weight gain is a predictor of childhood obesity. Therefore, the younger the child, the more pubertal timing and body weight are likely to be affected in close temporal link with NC onset.
In addition to studies reporting specific features of childhood NC encompassing sleepiness manifestations, personality traits, severe motor abnormalities,4–11 and laboratory sleep characteristics, the current work further depicts the pediatric NC phenotype, which now includes precocious puberty and dysmetabolic obesity.
Whether this complex childhood NC phenotype is solely explained by the hcrt-1 deficiency at a young age is unclear. An association between hypothalamic dysfunction, sleepiness, and accelerated puberty has been described.40 Studies in rats suggest a direct action of hypocretin neurons on GnRH secretion, crucial in the pubertal timing determination.41,42 Therefore, this action may be affected in hypocretin-deficient subjects, leading to alteration in the pubertal timing with acceleration of puberty up to a definite precocious puberty. In favor of this hypothesis, other studies found maximal levels of plasma gonadotropins and plasma hcrt-1 in the first year of life, when cerebrospinal hcrt-1 also peaks, and just before the puberty.43,44
We acknowledge that our study design does not allow us to identify the mechanisms leading to comorbid NC, obesity, and precocious puberty in young children with NC. These three conditions could represent the wide spectrum of a common origin, at least in a subgroup of vulnerable subjects. Nevertheless, we believe that the reported findings strongly suggest the need for a new clinical multidiagnostic assessment of these children, who should refer either to a sleep medicine center or to a pediatric department. Larger groups of subjects and longitudinal studies will be needed to deepen and confirm these preliminary observations. Our findings also indicate the opportunity of reverse translational studies aiming to further define the role of hcrt-1 in the regulation of the hypothalamic-pituitary-gonadal axis across pubertal development.
In conclusion, we report for the first time a high comorbidity between NC, precocious puberty, and obesity during childhood. NC highly affects quality of life,45 precocious puberty causes psychological and auxological impairments,32 and childhood obesity is linked to adult obesity and future excess mortality.46 Early disease recognition and a multidiagnostic clinical assessment are therefore mandatory.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Plazzi has consulted for UCB Pharma and Jazz Pharmaceuticals. Prof. Mignot has consulted for Jazz Pharmaceuticals, the Federal Trade Commission, Roche, Cephalon, Merck, Arena, Novo Nordish, and GSK; he serves on the advisory board of GSK and Lily. He also was paid as an expert witness for Fred Langer, LLC and has reviewed grants for the RLS Foundation, Klarman, and the AASM. Dr. Taheri is funded by the UK National Institute of Health Research CLAHRC program and has received research support from Novo Norgyk (Lilly) Allersad. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are indebted to all the participants of the study and to the Italian Association of Narcolepsy and Hypersomnia Patients (Associazione Italiana Narcolettici e Ipersonni - AIN), and to Dr. Monia Gennari and Dr. Martina Zanotti from the Endocrinology Unit, Department of Clinical Medicine, S. Orsola-Malpighi General Hospital, Bologna, Italy. Work for this study was performed at: Department of Neurological Sciences, University of Bologna - IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy; Pediatric Clinic, S. Orsola-Malpighi General Hospital Bologna, Italy.
ABBREVIATIONS
- BMI
body mass index
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EDS
excessive daytime sleepiness
- GABA
gamma-amino-butyric-acid
- GnRH
gonadotropin-releasing-hormone
- hcrt-1
hypocretin-1
- HDL
high density lipoprotein
- HLA
human leukocyte antigen
- hsCRP
high sensitivity C-reactive protein
- LDL
low density lipoprotein
- LH
luteinizing hormone
- mESS
Epworth sleepiness scale modified for children
- MRI
magnetic resonance imaging
- MSLT
multiple sleep latency test
- NC
narcolepsy with cataplexy
- OGTT
oral glucose tolerance test
- PSG
polysomnography
- SOREMPs
sleep onset rapid eye movement periods
Footnotes
A commentary on this article appears in this issue on page 161.
REFERENCES
- 1.Yoss RE, Daly DD. Narcolepsy in children. Pediatrics. 1960;25:1025–33. [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine. diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders, [Google Scholar]
- 3.Ohayon MM, Ferini-Strambi L, Plazzi G, Smirne S, Castronovo V. How age influences the expression of narcolepsy. J Psychosom Res. 2005;59:399–405. doi: 10.1016/j.jpsychores.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 4.Nevsimalova S. Narcolepsy in childhood. Sleep Med Rev. 2009;13:169–80. doi: 10.1016/j.smrv.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 5.McKenna L, McNicholas F. Childhood onset narcolepsy-a case report. Eur Child Adolesc Psychiatry. 2003;12:43–7. doi: 10.1007/s00787-003-0292-8. [DOI] [PubMed] [Google Scholar]
- 6.Kotagal S, Goulding P. The laboratory assessment of daytime sleepiness in childhood. J Clin Neurophysiol. 1996;13:208–18. doi: 10.1097/00004691-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Guilleminault C, Pelayo R. Narcolepsy in prepubertal children. Ann Neurol. 1998;43:135–42. doi: 10.1002/ana.410430125. [DOI] [PubMed] [Google Scholar]
- 8.Guilleminault C, Pelayo R. Narcolepsy in children: a practical guide to its diagnosis, treatment and follow-up. Paediatr Drugs. 2000;2:1–9. doi: 10.2165/00148581-200002010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Kotagal S, Hartse KM, Walsh JK. Characteristics of narcolepsy in preteenaged children. Paediatrics. 1990;85:205–9. [PubMed] [Google Scholar]
- 10.Stores G. Practitioner review: assessment and treatment of sleep disorders in children and adolescents. J Child Psychol Psychiatry. 1996;37:907–25. doi: 10.1111/j.1469-7610.1996.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 11.Plazzi G, Pizza F, Palaia V, et al. Complex movement disorders at disease onset in childhood narcolepsy with cataplexy. Brain. 2011;134:3480–92. doi: 10.1093/brain/awr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han F, Lin L, Warby SC, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in china. Ann Neurol. 2011;70:410–7. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 13.Dauvilliers Y, Montplaisir J, Cochen V, et al. Post-H1N1 narcolepsy-cataplexy. Sleep. 2010;33:1428–30. doi: 10.1093/sleep/33.11.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardage C, Persson I, Ortqvist A, Bergman U, Ludvigsson JF, Granath F. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ. 2011;343:d5956. doi: 10.1136/bmj.d5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haba-Rubio J, Rossetti AO, Tafti M, Heinzer R. Narcolepsy with cataplexy associated with H1N1 vaccination. Rev Neurol. 2011;167:563–6. doi: 10.1016/j.neurol.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Schuld A, Hebebrand J, Geller F, Pollmächer T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355:1274–5. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- 17.Poli F, Plazzi G, Di Dalmazi, et al. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep. 2009;32:1491–7. doi: 10.1093/sleep/32.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda Y, Doi Y, Ninomiya R, Ninomiya C. Increased frequency of non-insulin-dependent diabetes mellitus among narcoleptic patients. Sleep. 1986;9:254–9. doi: 10.1093/sleep/9.1.254. [DOI] [PubMed] [Google Scholar]
- 19.Kok SW, Roelfsema F, Overeem S, et al. Pulsatile LH release is diminished, whereas FSH secretion is normal, in hypocretin-deficient narcoleptic men. Am J Physiol Endocrinol Metab. 2004;287:E630–6. doi: 10.1152/ajpendo.00060.2004. [DOI] [PubMed] [Google Scholar]
- 20.Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med. 2004;5:147–50. doi: 10.1016/j.sleep.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Chisholm RC, Brook CJ, Harrison GF, Lyon L, Zukaitis D. Prepubescent narcolepsy in a six year old girl. Sleep Res. 1985;15:113. [Google Scholar]
- 22.Perriol MP, Cartigny M, Lamblin MD, et al. Childhood-onset narcolepsy, obesity and puberty in four consecutive children: a close temporal link. J Pediatr Endocrinol Metab. 2010;23:257–65. doi: 10.1515/jpem.2010.23.3.257. [DOI] [PubMed] [Google Scholar]
- 23.Peraita-Adrados R, García-Peñas JJ, Ruiz-Falcó L, et al. Clinical, polysomnographic and laboratory characteristics of narcolepsy-cataplexy in a sample of children and adolescents. Sleep Med. 2011;12:24–7. doi: 10.1016/j.sleep.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Plazzi G, Parmeggiani A, Mignot E, et al. Narcolepsy-cataplexy associated with precocious puberty. Neurology. 2006;66:1577–9. doi: 10.1212/01.wnl.0000216142.21375.71. [DOI] [PubMed] [Google Scholar]
- 25.Plazzi G, Tonon C, Rubboli G, et al. Narcolepsy with cataplexy associated with holoprosencephaly misdiagnosed as epileptic drop attacks. Mov Disord. 2010;25:788–90. doi: 10.1002/mds.23008. [DOI] [PubMed] [Google Scholar]
- 26.Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997;20:1012–20. [PubMed] [Google Scholar]
- 27.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 28.Murali H, Kotagal S. Off-label treatment of severe childhood narcolepsy-cataplexy with sodium oxybate. Sleep. 2006;29:1025–9. doi: 10.1093/sleep/29.8.1025. [DOI] [PubMed] [Google Scholar]
- 29.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee PA. Laboratory monitoring of children with precocious puberty. Arch Pediatr Adolesc Med. 1994;148:369–76. doi: 10.1001/archpedi.1994.02170040035006. [DOI] [PubMed] [Google Scholar]
- 32.Partsch CJ, Sippell WG. Treatment of central precocious puberty. Best Pract Res Clin Endocrinol Metab. 2002;16:165–89. doi: 10.1053/beem.2002.0188. [DOI] [PubMed] [Google Scholar]
- 33.Sathasivam A, Garibaldi L, Shapiro S, Godbold J, Rapaport R. Leuprolide stimulation testing for the evaluation of early female sexual maturation. Clin Endocrinol (Oxf) 2010;73:375–81. doi: 10.1111/j.1365-2265.2010.03796.x. [DOI] [PubMed] [Google Scholar]
- 34.Cacciari E, Milani S, Balsamo A, et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr) J Endocrinol Invest. 2006;29:581–93. doi: 10.1007/BF03344156. [DOI] [PubMed] [Google Scholar]
- 35.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 36.Giordano P, Del Vecchio GC, Cecinati V, et al. Metabolic, inflammatory, endothelial and haemostatic markers in a group of Italian obese children and adolescents. Eur J Pediatr. 2011;170:845–50. doi: 10.1007/s00431-010-1356-7. [DOI] [PubMed] [Google Scholar]
- 37.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140:399–410. doi: 10.1530/REP-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cesario SK, Hughes LA. Precocious puberty: a comprehensive review of literature. J Obstet Gynecol Neonatal Nurs. 2007;36:263–74. doi: 10.1111/j.1552-6909.2007.00145.x. [DOI] [PubMed] [Google Scholar]
- 39.Walvoord EC. The timing of puberty: is it changing? Does it matter? J Adolesc Health. 2010;47:433–9. doi: 10.1016/j.jadohealth.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 40.Boris NW, Hagino OR, Steiner GP. Case study: hypersomnolence and precocious puberty in a child with pica and chronic lead intoxication. J Am Acad Child Adolesc Psychiatry. 1996;35:1050–4. doi: 10.1097/00004583-199608000-00016. [DOI] [PubMed] [Google Scholar]
- 41.López M, Nogueiras R, Tena-Sempere M, Diéguez C. Orexins (hypocretins) actions on the GHRH/somatostatin-GH axis. Acta Physiol (Oxf) 2010;198:325–34. doi: 10.1111/j.1748-1716.2009.02042.x. [DOI] [PubMed] [Google Scholar]
- 42.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–76. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 43.Tomasik PJ, Spodaryk M, Sztefko K. Plasma concentrations of orexins in children. Ann Nutr Metab. 2004;48:215–20. doi: 10.1159/000080453. [DOI] [PubMed] [Google Scholar]
- 44.Aran A, Shors I, Lin L, Mignot E, Schimmel MS. CSF levels of hypocretin-1 (orexin-A) peak during early infancy in humans. Sleep. 2012;35:187–91. doi: 10.5665/sleep.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vignatelli L, D'Alessandro R, Mosconi P, et al. Health-related quality of life in Italian patients with narcolepsy: the SF-36 health survey. Sleep Med. 2004;5:467–75. doi: 10.1016/j.sleep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–93. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]


