Abstract
Study Objectives:
Habitual short sleep duration is associated with increased cardiovascular disease morbidity and mortality resulting from atherothrombotic events. The mechanisms responsible for this heightened cardiovascular risk are not fully understood. The capacity of the endothelium to release tissue-type plasminogen activator (t-PA), the primary activator of the fibrinolytic system, is a key endogenous defense mechanism against intravascular fibrin deposition and thrombosis. We tested the hypothesis that endothelial t-PA release is impaired in adults who sleep less than 7 h/night compared with adults who sleep between 7 and 9 h/night.
Design:
Thirty adult men were stratified based on average nightly habitual sleep duration: 15 with normal sleep duration (age: 55 ± 2 years; sleep duration: 7.6 h/night) and 15 with short sleep duration (56 ± 2 years; 6.1 h/night). Net endothelial release of t-PA was determined, in vivo, in response to intra-brachial infusions of bradykinin (12.5-50.0 ng/100 mL tissue/min) and sodium nitroprusside (1.0-4.0 μg/100 mL tissue/min).
Measurements and Results:
Net endothelial t-PA release to bradykinin was significantly lower (∼25%) in the short (from 0.4 ± 0.8 to 41.5 ± 4.3 ng/100 mL tissue/min) compared with the normal (0.4 ± 0.5 to 64.9 ± 6.7 ng/100 mL/tissue/min) sleep duration group. Furthermore, there was an inverse relation between average nightly sleep duration and peak t-PA release to bradykinin (r = 0.36, P < 0.05).
Conclusions:
Endothelial t-PA release is impaired in adults who report short habitual sleep duration. Impaired endothelial fibrinolytic function may underlie the increased atherothrombotic risk associated with chronic short sleep.
Citation:
Weil BR; Greiner JJ; Stauffer BL; DeSouza CA. Self-reported habitual short sleep duration is associated with endothelial fibrinolytic dysfunction in men: a preliminary report. SLEEP 2013;36(2):183-188.
Keywords: Sleep, endothelial function, tissue-type plasminogen activator, fibrinolysis
INTRODUCTION
Over the past century, there has been a dramatic fall in average sleep duration in the United States, from approximately 9.0 hours in 1910 to 6.8 hours in 2005.1,2 While the behavioral and cognitive consequences of this reduced sleep time are well-documented,3 it has become increasingly apparent that chronic short sleep duration also adversely affects cardiovascular health. Indeed, a number of epidemiological studies have reported that short sleep duration is associated with an increased risk of atherothrombotic vascular disease and its clinical consequences.4– For example, in a recent meta-analysis that included almost 500,000 adults, habitual sleep duration of less than 7 h/night was independently associated with an increased risk of stroke and coronary artery disease.8 Although the factors underlying the link between chronic short sleep duration and cardiovascular risk remain poorly understood, there is emerging evidence that vascular endothelial dysfunction is an important contributing mechanism. Data from our laboratory9 and others10 indicate that adults who chronically sleep less than 7 h/night exhibit endothelial vasomotor dysfunction that may contribute to the development and progression of atherosclerotic vascular disease.
In addition to contributing to the regulation of blood flow, the vascular endothelium plays a central role in the control of fibrinolysis, the primary endogenous defense mechanism against thrombosis.11 Endothelial cells are the site of synthesis and release of tissue-type plasminogen activator (t-PA), the main plasminogen activator in fibrinolysis.12 The ability of endothelial cells to release t-PA resulting in the activation of plasminogen on the surface of a developing clot is critical to the fibrinolytic process.13,14 Impaired endothelial t-PA release contributes to the initiation, progression, and severity of atherothrombotic vascular disease.15–18 Although it has been suggested that alterations in the balance between coagulation and fibrinolysis are involved in the pathogenesis of short sleep-related vascular disease,19,20 it is unclear if chronic short sleep duration is associated with a diminished capacity of the endothelium to release t-PA. If so, this would represent a novel mechanism that may underlie the increased risk of atherothrombotic events in adults with habitual short sleep.
To determine whether short sleep duration is associated with impaired endothelial t-PA release, we utilized an isolated forearm model to assess endothelial t-PA release in vivo in adults who were classified into two groups based on self-reported average nightly habitual sleep duration. We hypothesized that the capacity of the endothelium to release t-PA is lower in adults with habitual sleep duration < 7 h/night compared with adults with habitual sleep duration between 7 and 9 h/night.
METHODS
Subjects
Thirty sedentary adult men participated in the study, 15 with normal habitual sleep duration (range: 7.0-8.1 h/night) and 15 with short habitual sleep duration (5.0-6.9 h/night). All subjects were free of hypertension (arterial blood pressure ≥ 140/90 mm Hg) and overt cardiovascular disease as assessed by medical history, physical examination, fasting blood chemistries, electrocardiograms, and blood pressure at rest and during incremental exercise performed to exhaustion. None of the subjects smoked, were taking medications (including vitamins), or performed regular physical exercise for ≥ 1 year before the start of the study. Prior to participation, all of the subjects had the research study and its potential risks and benefits explained fully before providing written informed consent. All of the procedures were performed per institutional guidelines and were approved by the Institutional Review Board of the University of Colorado (Boulder, CO).
Sleep Duration
Sleep duration was measured as a component of the Stanford Physical Activity Questionnaire21 as previously described by our laboratory.9,22 Subjects were asked the number of hours per night they had slept over the past 7 days and were asked separately about sleep duration on weeknights (Sunday-Thursday) and weekend nights (Friday, Saturday). Consistent with previous studies,23– nightly average reported sleep duration was calculated as the weighted average of weeknights and weekend values [(5 × weekday sleep duration) + (2 × weekend sleep duration)/7]. Subjects were divided into 2 groups based upon their reported sleep duration: 7 to 9 h/night = “normal sleep duration” and < 7 h/night = “short sleep duration.” These criteria were chosen based on previously published reports that indicate that habitual sleep duration < 7 h/night is associated with increased health risks, including hypertension, coronary artery disease, and stroke.4,6,7,26–28
Body Composition and Metabolic Measurements
Body mass was measured to the nearest 0.1 kg using a medical beam balance. Percent body fat was determined by dual energy X-ray absorptiometry (Lunar Corp., Madison, WI). Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Minimal waist circumference was measured according to published guidelines.29 Fasting plasma lipid, lipoprotein, glucose, and insulin concentrations were determined using standard techniques as previously described.30
Intra-Arterial Infusion Protocol
All studies were performed between 07:00 am and 10:00 am after a 12-h overnight fast in a temperature-controlled room as previously described by our laboratory.30,31 Briefly, an intravenous catheter was placed in a deep antecubital vein of the non-dominant arm. Thereafter, a 5-cm, 20-gauge catheter was inserted into the brachial artery of the same arm under local anesthesia (1% lidocaine). Heart rate and arterial blood pressure were continuously measured throughout the infusion protocol. Forearm blood flow (FBF) at rest and in response to each pharmacologic agent was measured using strain-gauge venous occlusion plethysmography (DE Hokanson, Bellevue, WA) and presented as mL/100 mL tissue/min. Following the measurement of resting blood flow for 5 min, bradykinin was infused intra-arterially at rates of 12.5, 25, and 50 ng/100 mL tissue/min and sodium nitroprusside at 1.0, 2.0, and 4.0 μg/100 mL tissue/min for 5 min at each dose as previously described.30 To avoid an order effect, the sequence of drug administration was randomized. Forearm volume was determined by the water displacement method.
Net endothelial release of t-PA antigen and PAI-1 antigen in response to bradykinin and sodium nitroprusside was calculated according to Jern et al.32 using the following equation:
where CV and CA represent the concentration in the vein and artery, respectively. For both t-PA and PAI-1, a positive difference indicated a net release and a negative difference, net uptake. Arterial and venous blood samples were collected simultaneously at baseline and at the end of each drug dose to determine t-PA and PAI-1 antigen concentrations. All samples were collected into tubes containing 0.45 M sodium citrate buffer, pH 4.3 (Stabilyte, Biopool AB, Sweden), aliquoted, and stored for analysis. Plasma concentrations of t-PA and PAI-1 antigen were determined by enzyme immunoassay. Hematocrit was measured in triplicate using the standard microhematocrit technique and corrected for trapped plasma volume within the trapped erythrocytes.33 The total amount of t-PA antigen released across the forearm in response to all 3 doses of bradykinin was calculated as the total area under each curve above baseline using a trapezoidal model. To avoid confounding effects from potential infection/inflammation-associated fibrinolytic changes, all subjects were free of recent infection/inflammation (< 2 wk), as determined by questionnaire.34
Statistical Analysis
Differences in subject baseline characteristics and area under the curve data were determined by between-groups analysis of variance (ANOVA). Group differences in FBF and endothelial t-PA and PAI-1 antigen release in response to bradykinin and sodium nitroprusside were determined by repeated-measures ANOVA. When indicated by a significant F value, a post hoc test using the Newman-Keuls method was performed to identify differences between the groups. Relations between variables of interest were assessed by means of Pearson correlation coefficient and linear regression analysis. All data are expressed as means ± SE. Statistical significance was set a priori at P < 0.05.
RESULTS
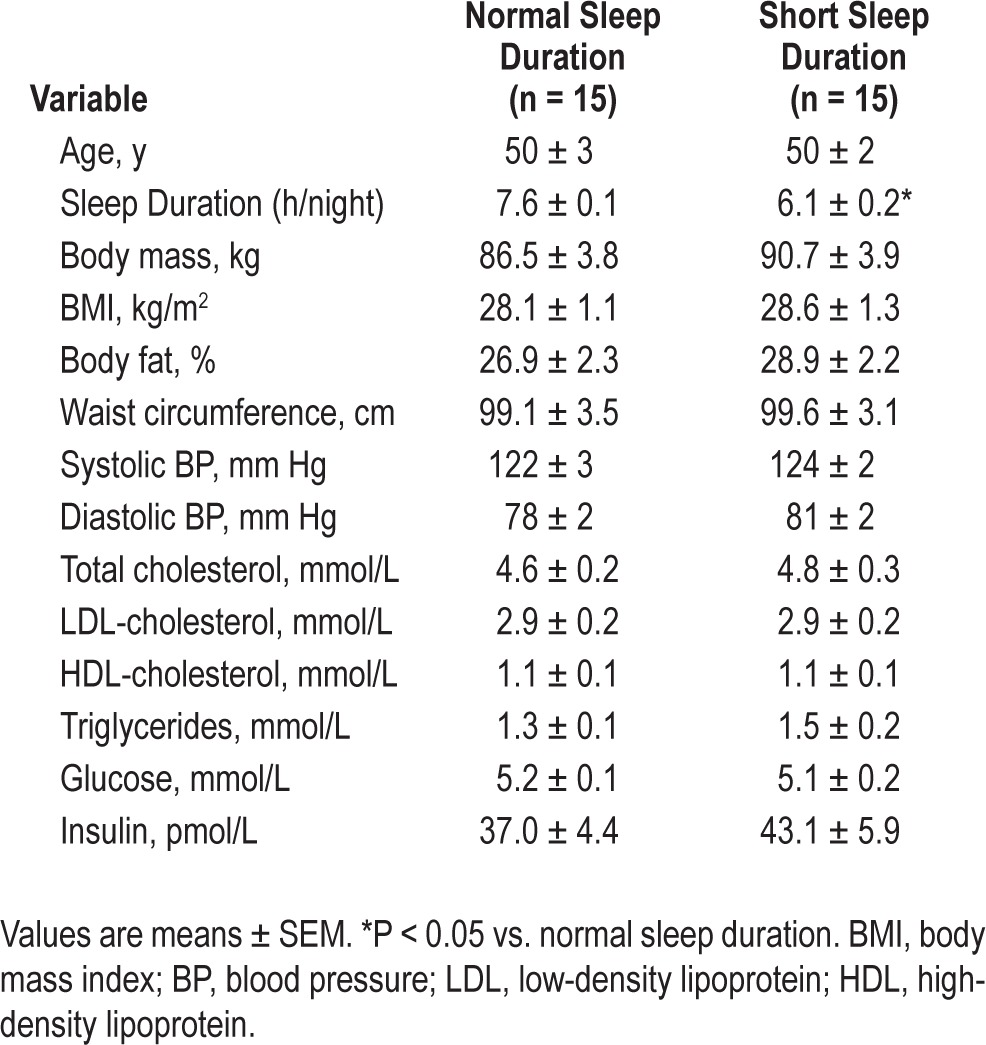
Selected subject characteristics are presented in Table 1. By design, average habitual sleep duration was significantly lower (∼20%) in the short (6.1 ± 0.2 h/night) compared with the normal (7.6 ± 0.1 h/night) sleep duration group. There were no differences in anthropometric, hemodynamic, or metabolic variables between the groups.
Table 1.
Selected subject characteristics
Forearm blood flow responses to bradykinin were not different between the normal (4.7 ± 0.3 to 16.1 ± 0.8 mL/100 mL tissue/min) and short (4.6 ± 0.2 to 14.9 ± 0.9 mL/100 mL tissue/min) sleep duration groups (Figure 1). Similarly, there were no group differences in the FBF responses to SNP (Figure 1). Forearm blood flow in the non-infused arm remained constant throughout the infusion protocols and did not differ significantly between groups.
Figure 1.
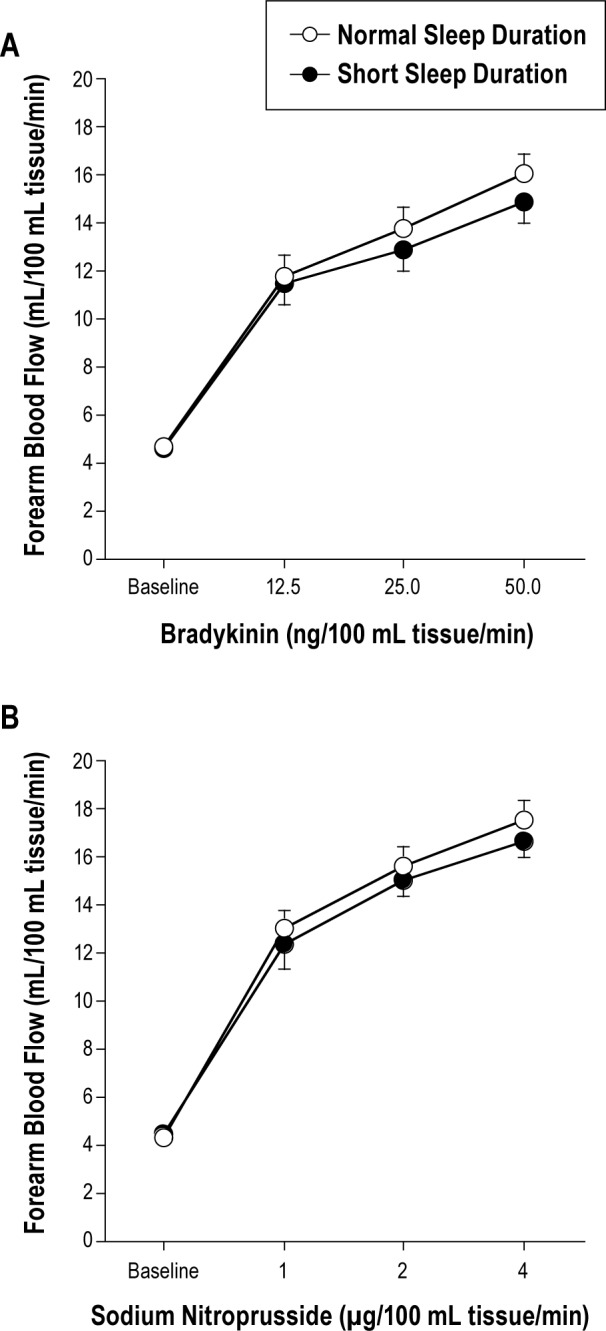
Forearm blood flow responses to bradykinin (A) and sodium nitroprusside (B) in the normal and short sleep duration groups. Values are mean ± SEM.
Basal endothelial t-PA antigen release was not significantly different between groups. However, compared with the normal sleep duration group, the capacity of the endothelium to release t-PA in response to bradykinin was significantly blunted in the short sleep duration group (Figure 2). Net release of t-PA antigen was ∼25% lower (P < 0.05) in the short (from 0.4 ± 0.8 to 41.5 ± 4.3 ng/100 mL tissue/min) compared with the normal (from 0.4 ± 0.5 to 64.9 ± 6.7 ng/100 mL tissue/min) sleep duration group. As a result, the total amount of t-PA antigen released (area under the bradykinin curve) was markedly lower (∼30%; P < 0.05) in adults with short (220 ± 26 ng/100 mL tissue) compared with normal (299 ± 25 ng/100 mL tissue) sleep duration (Figure 2). There was a significant positive relation between average nightly sleep duration and peak t-PA release to bradykinin (r = 0.36; Figure 3). Sodium nitroprusside did not significantly affect t-PA release in either the normal (from −0.6 ± 0.8 to 5.4 ± 3.3 ng/100 mL tissue/min) or short (from −1.6 ± 0.8 to 1.0 ± 3.2 ng/100 mL tissue/min) sleep duration groups. Neither bradykinin nor sodium nitroprusside elicited significant changes in PAI-1 antigen release in either group (data not shown).
Figure 2.
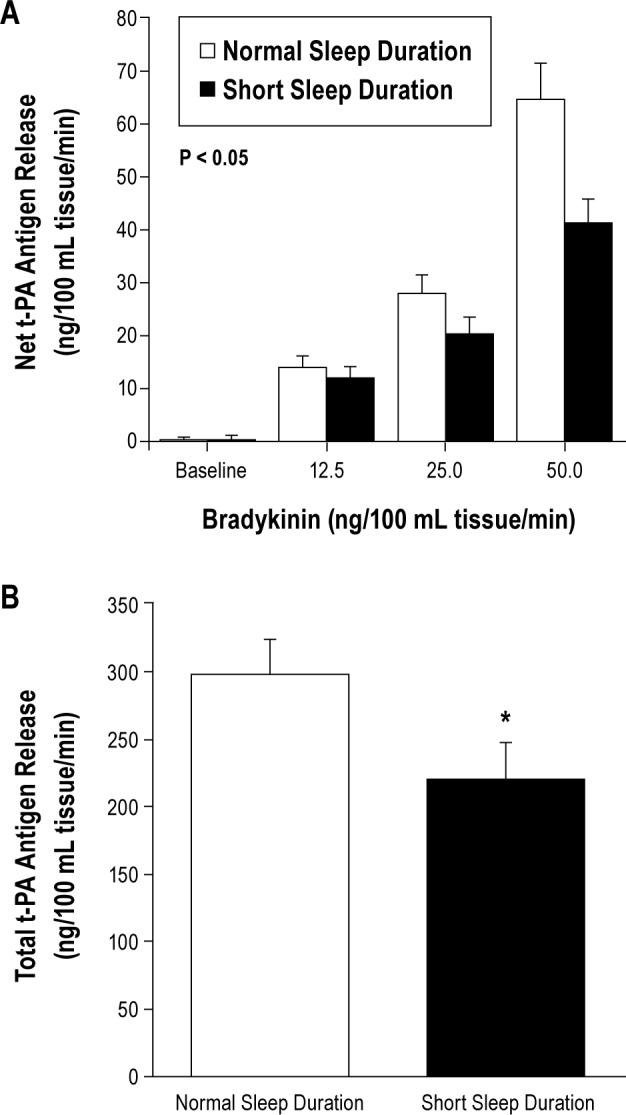
Net release (A) and total amount (area under the curve; B) of tissue-type plasminogen activator (t-PA) antigen released across the forearm in response to bradykinin in the normal and short sleep duration groups. Values are mean ± SEM; *P < 0.05 vs. normal sleep duration.
Figure 3.
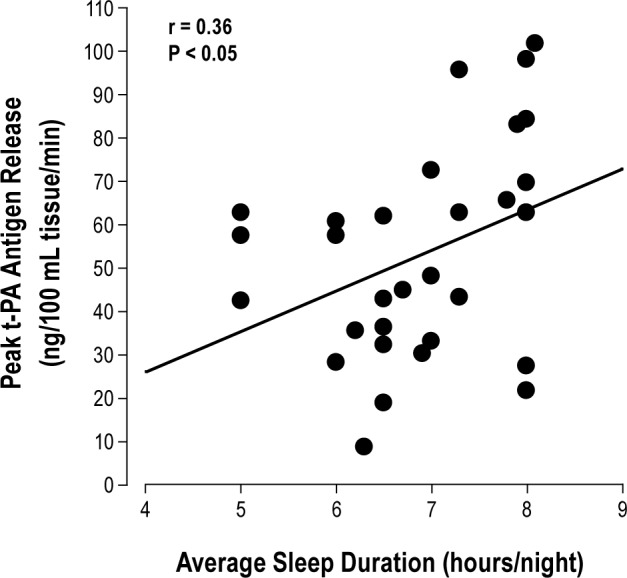
Correlation between peak t-PA release to bradykinin and average nightly sleep duration in the overall study population.
DISCUSSION
The novel finding of the present study is that the capacity of the endothelium to release t-PA is impaired in middle-aged, healthy men with short daily sleep duration compared with those with normal daily sleep duration. Consistent with our hypothesis, we observed significantly lower t-PA release to bradykinin in men who report sleeping less than 7 hours/night compared with men who report sleeping 7-9 hours/night. Furthermore, a modest, but significant, positive correlation was observed between mean nightly sleep duration and endothelial t-PA release in the overall study population. To our knowledge, this is the first study to examine the influence of habitual sleep duration on endothelial fibrinolytic function.
Several large observational studies have reported an association between short habitual sleep duration and increased cardiovascular morbidity and mortality resulting from atherothrombotic events.4–8 Recent data from over 30,000 participants in the National Health Interview Survey indicate that, compared with sleep duration of 7 hours/night, short sleep duration is associated with an increased risk of myocardial infarction and stroke.35 Although the mechanisms underlying the link between chronic short sleep duration and thrombotic risk are unclear, it has been suggested that abnormalities in the balance between coagulation and fibrinolysis may play a role.19 This notion is largely supported by data demonstrating elevated plasma levels of pro-thrombotic markers, such as von Willebrand factor and soluble tissue factor antigen, in adults with short or disrupted habitual sleep patterns.19,20 However, plasma concentrations of these proteins provide an indirect and non-specific assessment of coagulation and fibrinolytic balance.32,36 The present results significantly extend these findings by demonstrating that short habitual sleep duration is associated with an impaired capacity of the endothelium to acutely release t-PA, the primary determinant of endogenous fibrinolytic activity.11 Disruption of endothelial t-PA release is considered an important antecedent to atherothrombotic disease. For example, mice deficient in t-PA exhibit enhanced macrovascular fibrin deposition and accelerated atherogenesis,37 and impaired endothelial t-PA release predicts the future risk of atherothrombotic events in humans with coronary artery disease.18 Therefore, a reduction in endothelial fibrinolytic potential may play an important role in the pathogenesis of atherothrombotic vascular disease in adults who habitually sleep fewer than 7 hours/night. Previous studies have demonstrated alterations in endothelial vasomotor function with acute and chronic sleep restriction. In a study population similar to the present study, we observed enhanced endothelin-1-mediated vasoconstrictor tone in adults who reported habitually sleeping fewer than 7 hours/night.9 Also, Sauvet et al.10 recently reported that acute sleep deprivation results in impaired microvascular endothelium-dependent vasodilation in healthy men. Interestingly, the forearm blood flow responses to bradykinin were not different between groups in the present study. Bradykinin-induced vasodilation is primarily mediated by endothelial hyperpolarizing factor and not nitric oxide. As such, it appears that habitual short sleep duration does not compromise this vasodilator pathway. Moreover, considering there were no significant group differences in the FBF responses to either bradykinin or sodium nitroprusside, it is important to emphasize that the sleep-related deficit observed in endothelial t-PA release is not a blood flow related phenomenon.
The mechanisms responsible for the impaired endothelial t-PA release in adults with habitual short sleep duration are unclear. Chronic sleep restriction has been linked to metabolic changes that promote the development of obesity and the metabolic syndrome, such as alterations in plasma leptin concentrations and reduced glucose tolerance.38–41 As a result, it has been suggested that a large portion of the cardiovascular risk associated with short sleep duration is attributable to increased adiposity,42 a condition itself that is associated with impaired endothelial t-PA release.30 However, in the present study, there were no differences in body mass, body mass index, body fat percentage, or waist circumference between groups, suggesting that short sleep duration is associated with endothelial fibrinolytic dysfunction independent of adiposity. Also, it is possible that short sleep-related impairments in endothelial t-PA release are mediated by dysfunction of vascular repair processes that restore endothelial function after injury.43 This is unlikely, however, in light of the recent finding that short sleep duration is not associated with a reduction in the number or function of endothelial progenitor cells, an integral component of endogenous vascular repair processes.22 Other potential mechanisms include inflammation, oxidative stress, and sympathetic nervous system activation; each of these factors are associated with chronic short sleep duration39,44,45 and are known to adversely influence endothelial fibrinolytic function.46–49 In the present study, plasma concentrations of C-reactive protein (normal sleep: 1.2 ± 0.6 mg/L vs. short sleep: 1.3 ± 0.4 mg/L) and oxidized LDL (normal sleep: 49.9 ± 4.0 U/L vs. short sleep: 56.4 ± 4.9 U/L) were not different within a subset of individuals in the normal (n = 12) and short (n = 8) sleep duration groups. Although this argues against their potential role in fibrinolytic dysfunction with chronic short sleep, it is possible that other inflammatory and/or oxidative mediators may be involved. We did not assess sympathetic nervous system activity, which can be elevated with sleep deprivation50,51; thus, future studies will be necessary to elucidate the possible contribution of sympathetic activation to habitual short sleep-related impairments in endothelial t-PA release.
There are three experimental considerations regarding the present study that should be mentioned. Firstly, to minimize the potentially confounding effects of lifestyle behaviors besides habitual sleep duration, we studied nonsmoking adults who were similar in age and not taking vitamins or medications. Nevertheless, we cannot completely exclude the possibility that other behavioral differences or genetic factors may have influenced our results. Secondly, we acknowledge that assessment of habitual sleep duration by self-report has the potential to introduce bias and experimental error. However, previous studies have reported a strong correlation between self-reported sleep duration and that obtained objectively through actigraphic monitoring.52,53 Moreover, self-reported sleep duration has been used to assess habitual sleep duration in several previous studies examining the relation between sleep duration and indices of cardiometabolic risk.23–25 Finally, it is important to emphasize that the present study involved only men. We have previously reported a marked difference in the capacity of the endothelium to release t-PA between middle-aged men and women, and it is possible that these gender differences may exist with chronic sleep restriction.54 Consequently, the present results should be viewed within the context of the study population.
In conclusion, the results of the present study indicate that the capacity of the endothelium to release t-PA is impaired in adults who report habitually obtaining fewer than 7 hours of sleep per night. These findings extend previous studies demonstrating endothelial dysfunction with chronic sleep restriction and identify a novel mechanism that may contribute to the elevated atherothrombotic risk associated with habitual short sleep duration.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all of the subjects who participated in the study, as well as Yoli Casas for her administrative assistance. Funding Support: National Institutes of Health Awards HL088891, HL077450, HL076434, RR00051, and 1 UL1 RR025780, as well as American Heart Association award 0840167N.
REFERENCES
- 1.Egan BM. Sleep and hypertension: burning the candle at both ends really is hazardous to your health. Hypertension. 2006;47:816–7. doi: 10.1161/01.HYP.0000217363.62667.95. [DOI] [PubMed] [Google Scholar]
- 2.Sleep in America Poll. Washington: National Sleep Foundation; 2009. [Google Scholar]
- 3.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–28. [PMC free article] [PubMed] [Google Scholar]
- 4.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 5.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi AI, Giles WH, Croft JB, Bliwise DL. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10-year follow-up from NHANES I. Neurology. 1997;48:904–11. doi: 10.1212/wnl.48.4.904. [DOI] [PubMed] [Google Scholar]
- 8.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 9.Weil BR, Mestek ML, Westby CM, et al. Short sleep duration is associated with enhanced endothelin-1 vasoconstrictor tone. Can J Physiol Pharmacol. 2010;88:777–81. doi: 10.1139/Y10-046. [DOI] [PubMed] [Google Scholar]
- 10.Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol. 2010;108:68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- 11.Oliver JJ, Webb DJ, Newby DE. Stimulated tissue plasminogen activator release as a marker of endothelial function in humans. Arterioscler Thromb Vasc Biol. 2005;25:2470–9. doi: 10.1161/01.ATV.0000189309.05924.88. [DOI] [PubMed] [Google Scholar]
- 12.Lijnen HR, Collen D. Endothelium in hemostasis and thrombosis. Prog Cardiovasc Dis. 1997;39:343–50. doi: 10.1016/s0033-0620(97)80032-1. [DOI] [PubMed] [Google Scholar]
- 13.Brommer EJ. The level of extrinsic plasminogen activator (t-PA) during clotting as a determinant of the rate of fibrinolysis; inefficiency of activators added afterwards. Thromb Res. 1984;34:109–15. doi: 10.1016/0049-3848(84)90067-7. [DOI] [PubMed] [Google Scholar]
- 14.Fox KA, Robison AK, Knabb RM, Rosamond TL, Sobel BE, Bergmann SR. Prevention of coronary thrombosis with subthrombolytic doses of tissue-type plasminogen activator. Circulation. 1985;72:1346–54. doi: 10.1161/01.cir.72.6.1346. [DOI] [PubMed] [Google Scholar]
- 15.Cushman M, Lemaitre RN, Kuller LH, et al. Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:493–8. doi: 10.1161/01.atv.19.3.493. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Hennekens CH, Stampfer MJ, Manson JE, Vaughan DE. Prospective study of endogenous tissue plasminogen activator and risk of stroke. Lancet. 1994;343:940–3. doi: 10.1016/s0140-6736(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Vaughan DE, Stampfer MJ, Manson JE, Hennekens CH. Endogenous tissue-type plasminogen activator and risk of myocardial infarction. Lancet. 1993;341:1165–8. doi: 10.1016/0140-6736(93)90998-v. [DOI] [PubMed] [Google Scholar]
- 18.Robinson SD, Ludlam CA, Boon NA, Newby DE. Endothelial fibrinolytic capacity predicts future adverse cardiovascular events in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 2007;27:1651–6. doi: 10.1161/ATVBAHA.107.143248. [DOI] [PubMed] [Google Scholar]
- 19.Miller MA, Kandala NB, Kumari M, Marmot MG, Cappuccio FP. Relationships between sleep duration and von Willebrand factor, factor VII, and fibrinogen: Whitehall II study. Arterioscler Thromb Vasc Biol. 2010;30:2032–8. doi: 10.1161/ATVBAHA.110.206987. [DOI] [PubMed] [Google Scholar]
- 20.von Kanel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest. 2007;131:733–9. doi: 10.1378/chest.06-2006. [DOI] [PubMed] [Google Scholar]
- 21.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 22.Weil BR, MacEneaney OJ, Stauffer BL, DeSouza CA. Habitual short sleep duration and circulating endothelial progenitor cells. J Cardiovasc Dis Res. 2011;2:110–14. doi: 10.4103/0975-3583.83039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–43. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–74. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 25.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 27.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 28.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 29.Lohman TG, Roche AF, Mortorelli R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 30.Van Guilder GP, Hoetzer GL, Smith DT, et al. Endothelial t-PA release is impaired in overweight and obese adults but can be improved with regular aerobic exercise. Am J Physiol Endocrinol Metab. 2005;289:E807–13. doi: 10.1152/ajpendo.00072.2005. [DOI] [PubMed] [Google Scholar]
- 31.Hoetzer GL, Stauffer BL, Irmiger HM, Ng M, Smith DT, DeSouza CA. Acute and chronic effects of oestrogen on endothelial tissue-type plasminogen activator release in postmenopausal women. J Physiol. 2003;551:721–8. doi: 10.1113/jphysiol.2003.044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jern S, Wall U, Bergbrant A, Selin-Sjogren L, Jern C. Endothelium-dependent vasodilation and tissue-type plasminogen activator release in borderline hypertension. Arterioscler Thromb Vasc Biol. 1997;17:3376–83. doi: 10.1161/01.atv.17.12.3376. [DOI] [PubMed] [Google Scholar]
- 33.Chaplin H, Jr., Mollison PL. Correction for plasma trapped in the red cell column of the hematocrit. Blood. 1952;7:1227–38. [PubMed] [Google Scholar]
- 34.Macko RF, Ameriso SF, Gruber A, et al. Impairments of the protein C system and fibrinolysis in infection-associated stroke. Stroke. 1996;27:2005–11. doi: 10.1161/01.str.27.11.2005. [DOI] [PubMed] [Google Scholar]
- 35.Sabanayagam C, Shankar A. Sleep duration and cardiovascular disease: results from the National Health Interview Survey. Sleep. 2010;33:1037–42. doi: 10.1093/sleep/33.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith DT, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. Effects of ageing and regular aerobic exercise on endothelial fibrinolytic capacity in humans. J Physiol. 2003;546(Pt 1):289–98. doi: 10.1113/jphysiol.2002.027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christie PD, Edelberg JM, Picard MH, et al. A murine model of myocardial microvascular thrombosis. J Clin Invest. 1999;104:533–9. doi: 10.1172/JCI7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shigeta H, Shigeta M, Nakazawa A, Nakamura N, Yoshikawa T. Lifestyle, obesity, and insulin resistance. Diabetes Care. 2001;24:608. doi: 10.2337/diacare.24.3.608. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 40.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 41.Hayes AL, Xu F, Babineau D, Patel SR. Sleep duration and circulating adipokine levels. Sleep. 2011;34:147–52. doi: 10.1093/sleep/34.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 43.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;82:671–7. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 44.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 45.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 46.Devaraj S, Singh U, Jialal I. The evolving role of C-reactive protein in atherothrombosis. Clin Chem. 2009;55:229–38. doi: 10.1373/clinchem.2008.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh U, Devaraj S, Jialal I. C-reactive protein decreases tissue plasminogen activator activity in human aortic endothelial cells: evidence that C-reactive protein is a procoagulant. Arterioscler Thromb Vasc Biol. 2005;25:2216–21. doi: 10.1161/01.ATV.0000183718.62409.ea. [DOI] [PubMed] [Google Scholar]
- 48.Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. Acute and chronic effects of vitamin C on endothelial fibrinolytic function in overweight and obese adult humans. J Physiol. 2008;586:3525–35. doi: 10.1113/jphysiol.2008.151555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miskin R, Abramovitz R. Enhancement of PAI-1 mRNA in cardiovascular cells after kainate injection is mediated through the sympathetic nervous system. J Mol Cell Cardiol. 2005;38:715–22. doi: 10.1016/j.yjmcc.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 50.Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease-a review of the recent literature. Curr Cardiol Rev. 2010;6:54–61. doi: 10.2174/157340310790231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong X, Hilton HJ, Gates GJ, et al. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol. 2005;98:2024–32. doi: 10.1152/japplphysiol.00620.2004. [DOI] [PubMed] [Google Scholar]
- 52.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8:175–83. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 53.Hauri PJ, Wisbey J. Wrist actigraphy in insomnia. Sleep. 1992;15:293–301. doi: 10.1093/sleep/15.4.293. [DOI] [PubMed] [Google Scholar]
- 54.Stauffer BL, Hoetzer GL, Van Guilder GP, Smith DT, Desouza CA. Gender differences in endothelial tissue-type plasminogen activator release in middle-aged adults. J Am Coll Cardiol. 2005;45:1547–9. doi: 10.1016/j.jacc.2005.02.025. [DOI] [PubMed] [Google Scholar]


