Abstract
Study Objectives:
There are no clinical data comparing adherence and quality of life between auto-adjusting positive airway pressure (APAP) and two different flex positive airway pressure (PAP) devices (A-Flex, C-Flex) in patients with obstructive sleep apnea (OSA).
Design and Setting:
Ninety-three patients in whom OSA was newly diagnosed were randomly assigned to receive 3 mo of APAP (n = 31), APAP with C-Flex (n = 31), or APAP with A-Flex (n = 31). Objective adherence was determined after 3 mo of CPAP treatment, and the Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI), and Calgary Sleep Apnea Quality of Life Index (SAQLI) were examined at baseline and after 3 mo. After 3 mo, patients in the APAP with A-Flex group and those in the APAP with C-Flex group were crossed over and those in the APAP group were switched to A-Flex for an additional 3 mo.
Measurements and Results:
The groups were similar demographically. Treatment adherence during the first 3 mo was significantly greater in the APAP with C-Flex group (APAP with C-Flex: 5.19 ± 1.84 h/night versus APAP: 3.96 ± 1.66 h/night versus APAP with A-Flex: 4.27 ± 2.12 h/night, P = 0.04). There was a significant improvement in two of four of the SAQLI domain scores and in the ESS and PSQI in the APAP with C-Flex group. Adherence significantly improved among the poor compliers (< 4 h/night of use) in the APAP group after change to APAP with A-Flex (P = 0.01).
Conclusions:
Of these three modes of PAP delivery, adherence was greatest with APAP with C-Flex.
Clinical Trial Registration:
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00873977.
Citation:
Chihara Y; Tsuboi T; Hitomi T; Azuma M; Murase K; Toyama Y; Harada Y; Aihara K; Tanizawa K; Handa T; Yoshimura C; Oga T; Yamamoto K; Mishima M; Chin K. Flexible positive airway pressure improves treatment adherence compared with auto-adjusting PAP. SLEEP 2013;36(2):229-236.
Keywords: A-Flex, C-Flex, continuous positive airway pressure, obstructive sleep apnea, treatment adherence
INTRODUCTION
Continuous positive airway pressure (CPAP), the mainstay of treatment for moderate to severe obstructive sleep apnea (OSA), has been shown to normalize sleep architecture, reduce daytime sleepiness, enhance daily function, and decrease cardiovascular events.1–3 Although CPAP is a highly effective treatment, adherence is suboptimal. To improve patient comfort and treatment adherence, various CPAP modalities have been developed. Auto-adjusting positive airway pressure (APAP) devices continuously adjust the pressure in real time according to that required to maintain upper airway patency. It has been reported that treatment compliance with APAP was equivalent to that with fixed PAP therapy.4–6 Adherence with flexible CPAP (C-Flex; Philips Respironics, Murraysville, PA, USA), which flexes airway pressure on exhalation and inhalation on a breath-by-breath basis to reduce the work of breathing, has been reported to be significantly better compared with fixed PAP therapy in one study.7 However, other studies reported that adherence with C-Flex and fixed positive airway pressure (PAP) therapy was similar.8–11 To our knowledge, a comparison of treatment adherence of APAP with APAP with C-Flex has not been reported. Because the use of APAP for the treatment of OSA has increased, a prospective randomized study comparing APAP to APAP with C-Flex with respect to CPAP adherence is needed. In addition, a new CPAP device (A-Flex; Philips Respironics) is designed to further improve breathing comfort. Like C-Flex, A-Flex flexes pressure at the beginning of exhalation but also has a fixed 2 cm H2O pressure difference between inspiration and expiration.
The aim of this study was to compare objective adherence to CPAP between APAP, APAP with C-Flex, and APAP with A-Flex over an initial 3-mo period. We also investigated daytime sleepiness, sleep quality, and quality of life (QOL). Additionally, after 3 mo, patients in the APAP with A-Flex group and the APAP with C-Flex groups were crossed over to use the alternate mode (C-Flex or A-Flex) to compare adherence. In addition, the APAP group was crossed over to the A-Flex for these final 3 mo.
METHODS
Study Participants
Patients in whom OSA was newly diagnosed (apnea-hypopnea index [AHI] > 20) were enrolled in the current study according to the Japanese Health Insurance System. Patients were excluded if they were younger than 20 y, had a major medical or psychiatric condition that would interfere with the demands of the study and adherence to PAP, or had ever used CPAP therapy. This study was approved by the Ethics Committee of Kyoto University. All patients gave written informed consent to participate.
C-Flex and A-Flex PAP Machines
C-Flex pressure relief was developed to make CPAP therapy more comfortable by reducing pressure at the beginning of exhalation and returning to the therapeutic pressure just before inhalation (Figure 1A). The level of pressure relief varies based on the patient’s expiratory flow and which of the three C-Flex settings (1, 2, and 3) has been selected.
Figure 1.
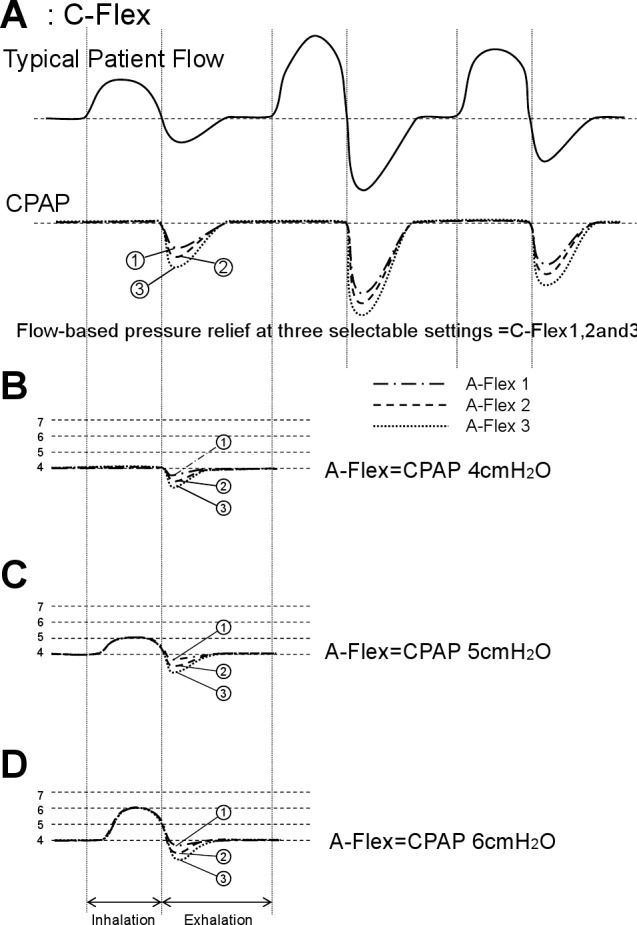
(A) C-Flex pressure relief reduced pressure at the beginning of exhalation and returned to the therapeutic pressure just before inhalation. The level of pressure relief varies based on the patient’s expiratory flow and which of the three C-Flex settings (1, 2, and 3) has been selected. (B-D) A-Flex has the same function as C-Flex during expiration. At 4 cm H2O CPAP, the A-Flex PAP machine is same as the C-Flex machine PAP (B). At 5 cm H2O CPAP, the A-Flex PAP machine can provide 1 cm H2O pressure support at the inspiration (C). From 6 or more cm H2O CPAP, A-Flex machine can make 2 cm H2O pressure supports at the inspiration (D).
Like C-Flex, A-Flex provides flow-based pressure relief at the beginning of exhalation. At 4 cm H2O CPAP, the A-Flex PAP machine is the same as the C-Flex machine PAP (Figure 1B). However, at 5 cm H2O CPAP, the A-Flex PAP machine can provide 1 cm H2O pressure support at inspiration (Figure 1C). From 6 cm or more H2O CPAP, the A-Flex machine can provide 2 cm H2O pressure support at inspiration (Figure 1D). A-Flex has the same function as C-Flex during expiration (Figure 1B-D).
Study Design
The study was a randomized single-blind (patients blinded) crossover trial of APAP versus APAP with C-Flex (set to dip level 3) versus APAP with A-Flex (set to dip level 3). The dip level is the level of pressure reduction during expiration, and dip level 3 is the greatest pressure reduction during expiration. Data were collected at baseline and after 3 and 6 mo of PAP treatment. At baseline, we recorded the patients’ demographic characteristics and polysomnographic data. In addition, subjective sleepiness, sleep quality, and health-related QOL were measured using the Epworth Sleepiness Scale (ESS),12 Pittsburgh Sleep Quality Index (PSQI),13 and Calgary Sleep Apnea Quality of Life Index (SAQLI).14,15 The SAQLI consists of five domains: daily functioning (domain A), social interactions (domain B), emotional functioning (domain C), symptoms (domain D), and treatment-related symptoms (domain E). The total SAQLI score for domains A through D was obtained before and after CPAP treatment, and the score for domain E was factored in after the patient received CPAP. CPAP titration was performed with autotitration during full-night polysomnography (PSG) attended by sleep technicians and the pressure range was between 4 and 20 cm H2O. For CPAP titration, each patient underwent full-night PSG on the allocated CPAP mode (i.e., APAP with C-Flex group underwent full-night PSG on APAP with C-Flex). The attending technicians checked for abnormal movements of the titrated PAP machine. However, they could not find any trouble during this trial.
The primary outcome was objective adherence after 3 mo of CPAP treatment. Objective adherence was downloaded from the memory card (Encore Pro Smartcard; Philips-Respironics) located in the PAP device. Secondary outcomes were ESS, PSQI, and SAQLI at 3 mo after the beginning of CPAP treatment.
Additionally, after 3 mo of PAP treatment, the APAP with A-Flex group and the APAP with C-Flex group were crossed over to the alternate mode (C-Flex or A-Flex) and the APAP group was switched to A-Flex for an additional 3 mo. The evaluations performed at 3 mo were repeated 6 mo after the start of CPAP treatment.
Polysomnography
The diagnosis of OSA was confirmed by PSG (SomnoStar Pro, Cardinal Health, Dublin, OH, USA), which was started at 22:00 and ended at 6:00 the following morning. Surface electrodes were attached using standard techniques to obtain an electrooculogram, electromyogram of the chin, and 12-lead electroencephalograph. Sleep stages were defined according to the criteria of Rechtschaffen and Kales.16 Ventilation/respiratory effort was monitored by inductive plethysmography (Respitrace QDC, Viasys Healthcare, Palm Springs, CA, USA). Airflow was monitored by a nasal pressure transducer (PTAFlite, Pro-Tech Services Inc., Mukilteo, WA, USA) and supplemented by an oronasal thermal sensor (Sleepmate Technologies, Midlothian, VA, USA). Arterial oxygen saturation (SaO2) was monitored continuously with a pulse oximeter (Adult Flex System, Nonin Medical, Plymouth, MN, USA).
Apnea was defined as the complete cessation of airflow and hypopnea as a clear decrease in airflow of 50% or more lasting for 10 sec or more accompanied by a decrease in SpO2 of at least 3% and/or associated with arousal.17 All AHI values were expressed as the number of episodes of apnea and hypopnea per h over the total sleep time. The lowest SpO2 during sleep was calculated in each patient.
Randomization
This study was randomized via blinded envelope prior to the beginning of the study.
Power Analysis
Based on a previous study,7 differences of 1 h/night of PAP adherence between each group were decided to be clinically significant, and standard deviation (SD) of 1.5 h/night was expected. The sample size was set to achieve 80% power at a 5% significance level. The calculated sample size in each group was 36 patients.
Statistical Analysis
Data analysis was conducted using a statistical software program (Statview, version 5.0; SAS Institute Inc., Cary, NC, USA). Data were expressed as mean ± SD or absolute numbers and percentages in each study group. The patients’ demographic characteristics, polysomnographic data, responses to three questionnaires at baseline and after 3 mo of CPAP treatment, and adherence after 3 mo of CPAP treatment were compared among the three groups using a one-way analysis of variance. When a significant difference was observed, we used the Bonferroni/Dunn method to identify where the differences were significant. For categorical variables, the χ2 test was used. Within the group, comparisons of adherence (3 mo versus 6 mo) or of results from the three questionnaires (baseline versus 3 mo, 3 mo versus 6 mo) were analyzed using a paired t test. In all analyses, P < 0.05 was considered statistically significant.
RESULTS
Baseline Assessments
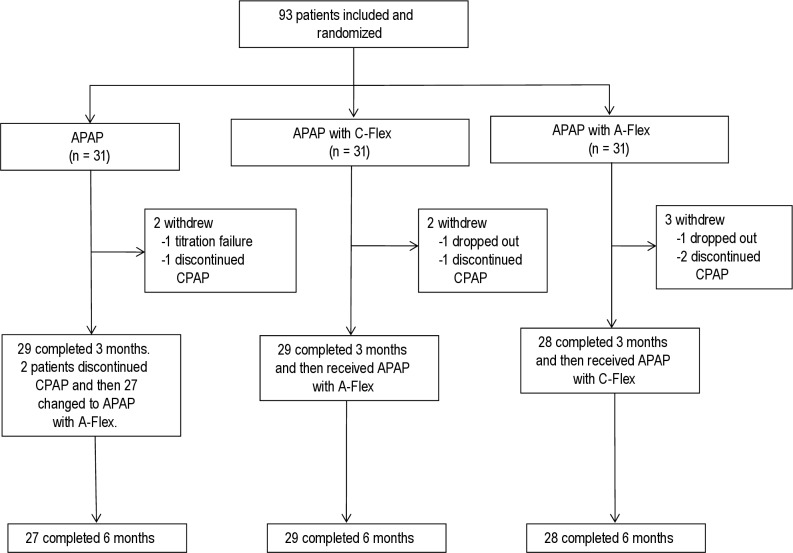
Figure 2 shows the study flow chart. Ninety-three patients in whom OSA was newly diagnosed were randomly assigned to receive 3 mo of APAP (n = 31), APAP with C-Flex (n = 31), or APAP with A-Flex (n = 31). During the first 3 mo, two patients withdrew from the APAP group (one discontinued PAP, one had Cheyne-Stokes breathing during PAP titration), two patients withdrew from the APAP with C-Flex group (one dropped out, one discontinued PAP), and three patients withdrew from the APAP with A-Flex group (one dropped out, two discontinued PAP). Data on patients who dropped out during the first 3 mo were excluded from the analysis.
Figure 2.
Flow chart of the study population. APAP, auto-adjusting positive airway pressure; CPAP, continuous positive airway pressure.
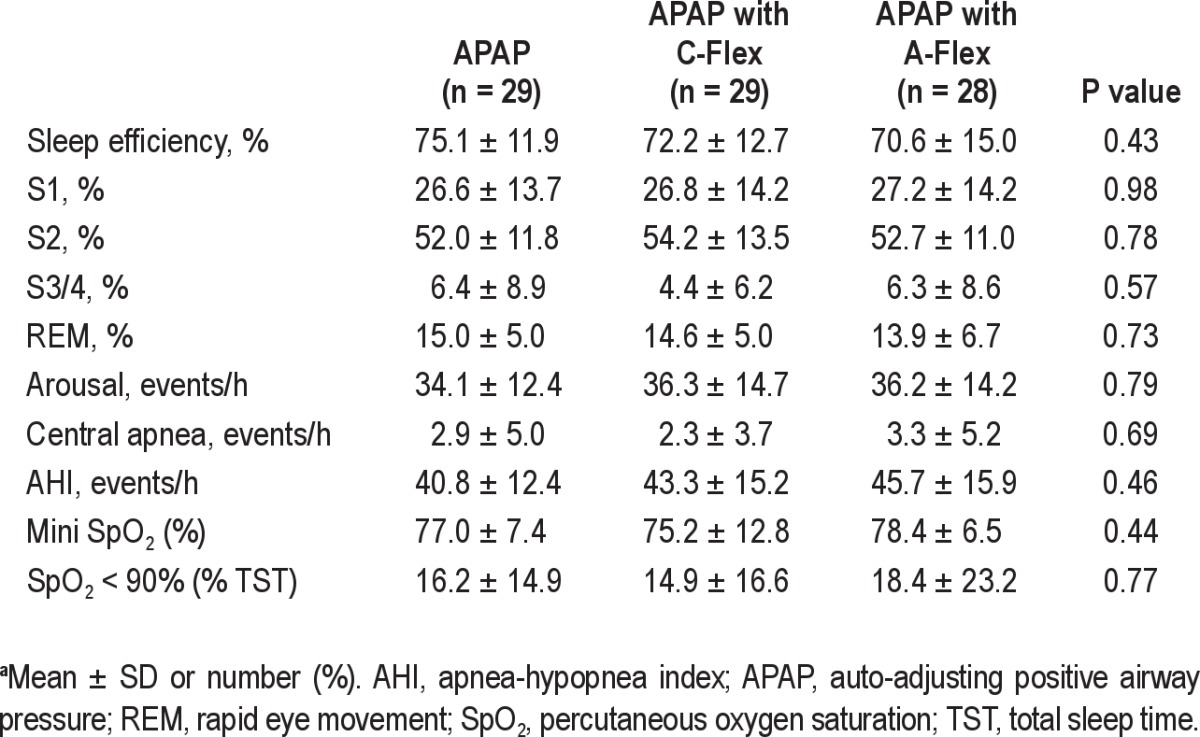
Tables 1 and 2 summarize baseline characteristics and polysomnographic data, respectively, on study participants. Patients were predominantly male, middle-aged, and had moderate to severe OSA. Neither baseline characteristics nor polysomnographic data differed among the three groups.
Table 1.
Baseline characteristics of study participantsa
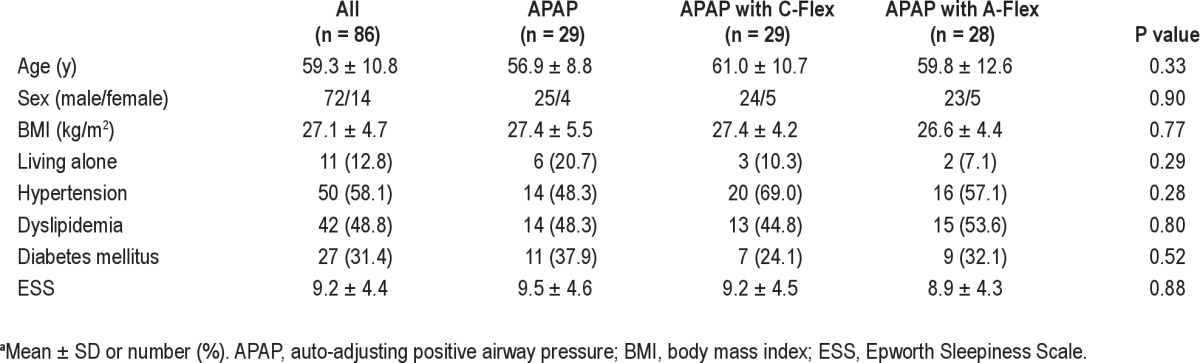
Table 2.
Baseline polysomnographic data on study participantsa
Effects of CPAP Treatment on PSG Variables
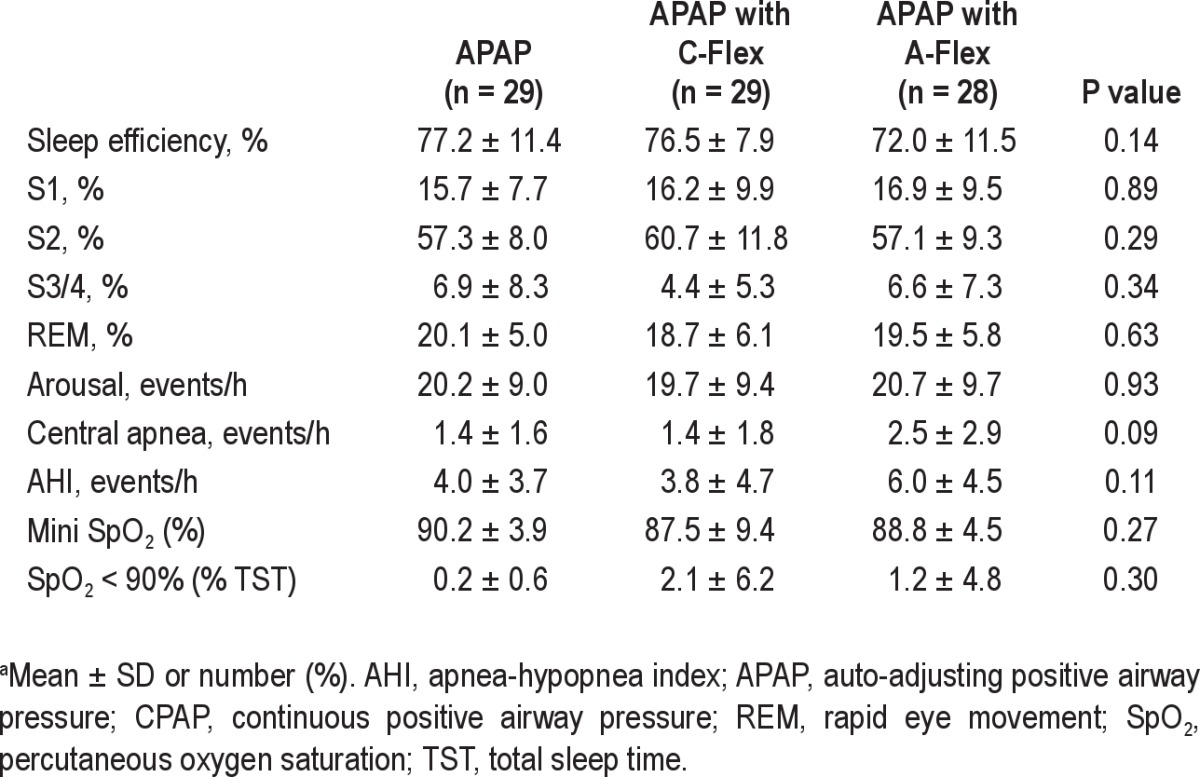
Table 3 shows the polysomnographic data on the PAP night. Compared with the baseline PSG, significant changes in polysomnographic variables such as the AHI and arousal index were noted in all three groups. There were no significant differences among the three groups in the polysomnographic data on CPAP titration. Also, the number of patients who used hypnotic agents during CPAP titration did not differ significantly among the three groups (APAP with C-Flex: 7 of 29, 24.4% versus APAP: 11 of 29, 37.9% versus APAP with A-Flex: 9 of 28, 32.1%; P = 0.52).
Table 3.
Polysomnographic data on study participants upon CPAP titrationa
After 3 mo of treatment, the 90th percentile PAP, leak, and mean AHI were downloaded and recorded from the memory card located in the PAP device. There were no significant differences among the three groups in terms of residual AHI (APAP with C-Flex: 3.4 ± 2.2 events/h versus APAP: 3.5 ± 2.0 h/night versus APAP with A-Flex: 4.6 ± 2.9 h/night; P = 0.11), 90th percentile PAP (APAP with C-Flex: 8.4 ± 1.7 cm H2O versus APAP: 8.2 ± 1.3 cm H2O versus APAP with A-Flex: 8.8 ± 1.7 cm H2O; P = 0.32), or 90th percentile leak (APAP with C-Flex: 45.7 ± 7.0 L/min versus APAP: 49.7 ± 13.1 L/min versus APAP with A-Flex: 46.5 ± 11.5 L/min; P = 0.34).
Primary Outcome: Adherence Over 3 Mo
Figure 3 shows a comparison among the three groups with regard to adherence to CPAP treatment over a 3-mo period. Median adherence was significantly greater in the APAP with C-Flex group, especially compared with the APAP group (APAP with C-Flex, n = 29: 5.19 ± 1.84 h/night versus APAP, n = 292 9: 3.96 ± 1.66 h/night versus APAP with A-Flex, n = 28: 4.27 ± 2.12 h/night; P = 0.04). In the post hoc analysis, adherence to APAP with C-Flex was significantly greater in comparison with APAP (P = 0.01). In addition, there were significant differences among the groups in percentage of days PAP was used (APAP with C-Flex: 91.9 ± 10.6% versus APAP: 79.4 ± 21.8% versus APAP with A-Flex: 82.5 ± 22.9%; P = 0.04) but not in percentage of days PAP used > 4 h (APAP with C-Flex: 66.6 ± 29.8% versus APAP: 54.8 ± 27.9% versus APAP with A-Flex: 57.6 ± 32.7%; P = 0.30). Post hoc testing revealed that the percentage of days PAP was used in the APAP with C-Flex group was significantly higher than in the APAP group (P = 0.02).
Figure 3.
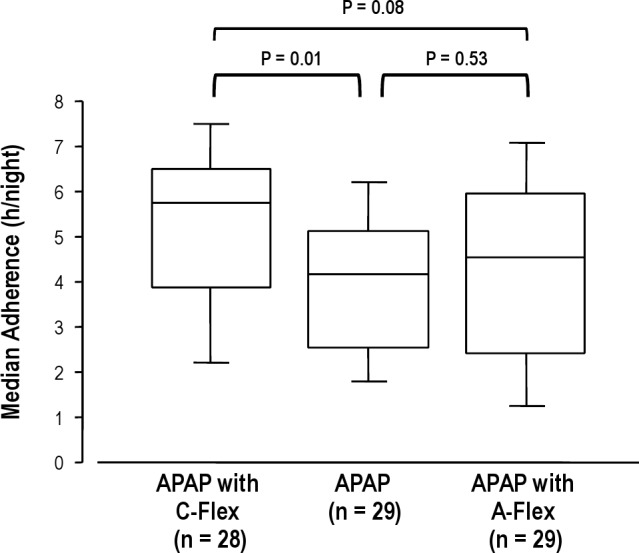
CPAP adherence with APAP with C-Flex, APAP, and APAP with A-Flex after 3 mo of treatment. Data are shown as a bow-whisker plot with median and 25th percentile of CPAP adherence. APAP, auto-adjusting positive airway pressure.
Secondary Outcomes: ESS, PSQI, and SAQLI After 3 Mo of CPAP Treatment
Table 4 details the effect of CPAP treatment on ESS, PSQI, and SAQLI in the three groups. For the entire group, ESS (P = 0.004) and domain A (P = 0.04), domain C (P = 0.006), and domain D (P < 0.0001) in the SAQLI questionnaire were significantly improved after 3 mo of PAP. Differences in responses to these three questionnaires were not statistically significant among the three groups at baseline, but after 3 mo of PAP treatment differences were noted. ESS and PSQI scores were significantly improved using APAP with C-Flex (ESS, 9.2 ± 4.5 to 7.3 ± 3.8, P = 0.01; PSQI, 7.2 ± 3.6 to 6.1 ± 2.8, P = 0.04), whereas significant improvements with APAP and APAP with A-Flex were not observed. In the SAQLI questionnaire, two domains were significantly improved and the other two domains trended toward improvement in the APAP with C-Flex group. On the other hand, the APAP and APAP with A-Flex groups had a significant improvement only in one domain of the SAQLI. However, as to the changes in the values for ESS, PSQI, and SAQLI among the three groups between before and after 3 mo of CPAP, the APAP with C-Flex group tended to have greater improvement in PSQI (P = 0.08) than the other two groups, whereas differences in the ESS and SAQLI were not significantly different among the three groups (Table 5).
Table 4.
Analysis of health-related quality of life and sleep related questionnairesa
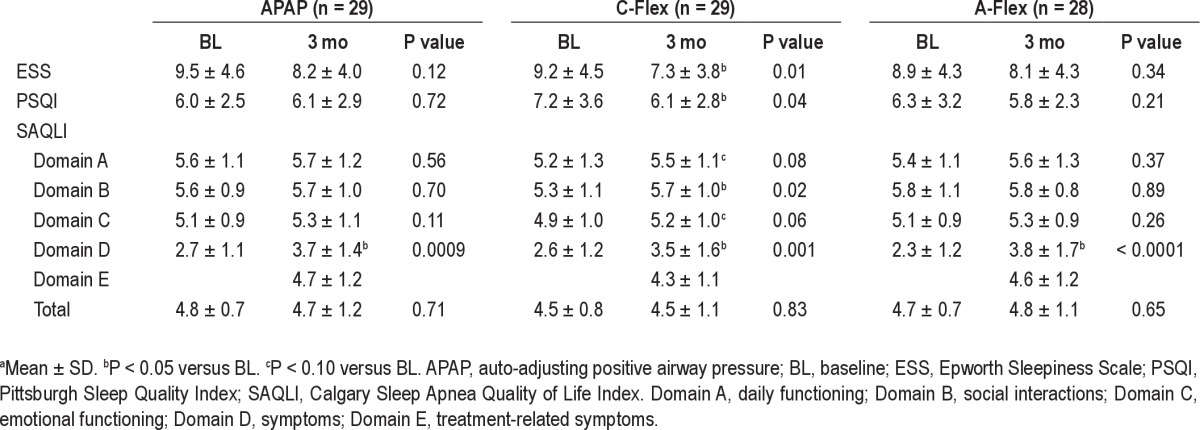
Table 5.
Changes in values of ESS, PSQI, and SAQRI among the three groups from before and after 3 mo of CPAPa
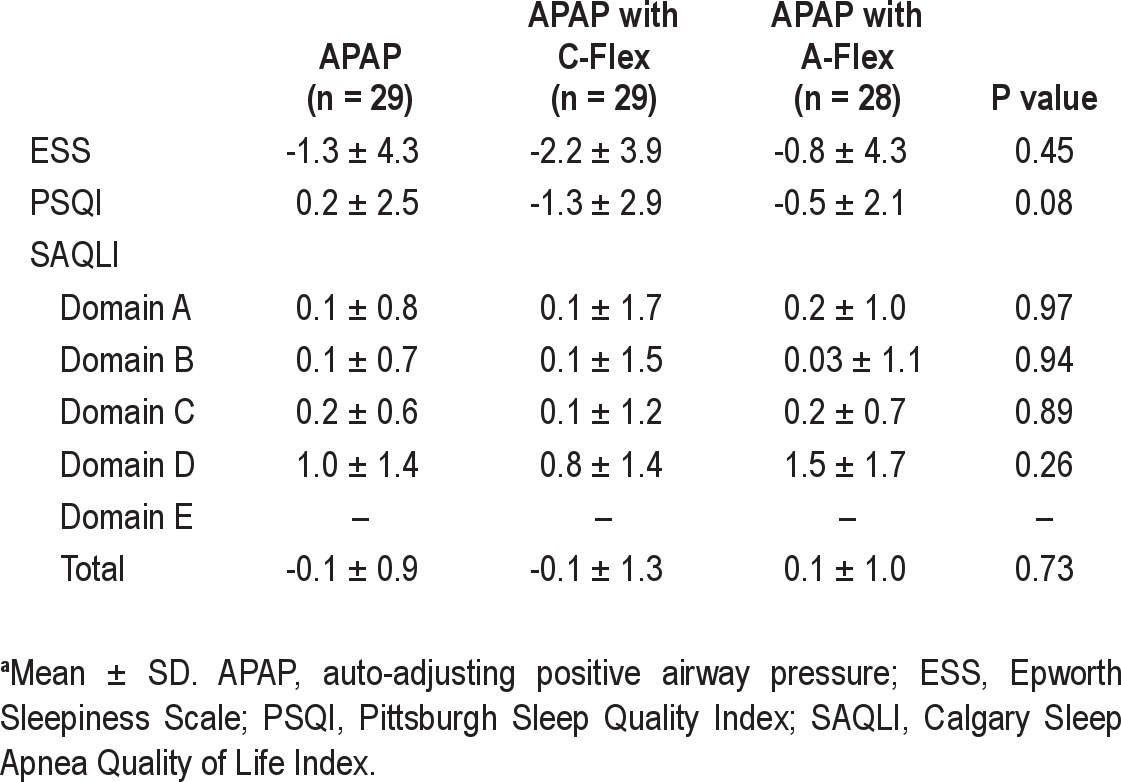
Crossover in CPAP Mode
After 3 mo, two patients discontinued PAP treatment in the APAP group. The change in the CPAP mode did not have a significant effect on PAP adherence in any group (from APAP to APAP with A-Flex, n = 27, from 4.15 ± 1.51 h/night to 4.12 ± 1.16 h/night, P = 0.89; from APAP with C-Flex to APAP with A-Flex, n = 29, from 5.19 ± 1.84 h/night to 4.95 ± 1.94 h/night, P = 0.17; from APAP with A-Flex to APAP with C-Flex, n = 28, from 4.27 ± 2.12 h/night to 4.15 ± 1.99 h/night, P = 0.56). Although the group of patients moving from APAP to APAP with A-Flex tended to show improvement in domain B of the SAQLI (P = 0.07), ESS, PSQI, and SAQLI scores did not significantly change in any group (results not shown).
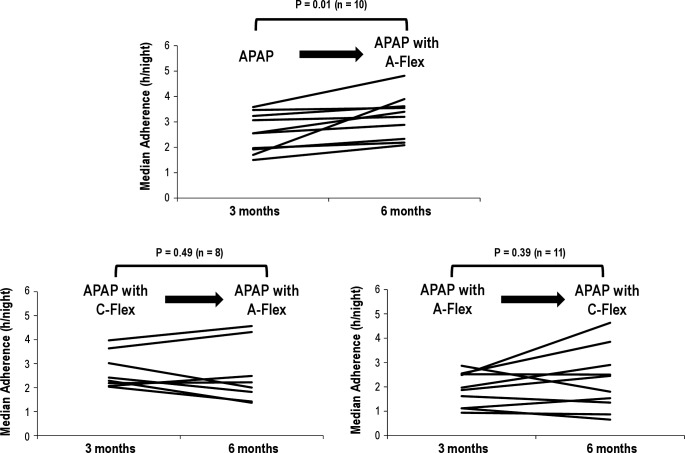
In a subgroup analysis of poor compliers (< 4 h/night of PAP use), there was a significant increase in PAP adherence in the patients moving from APAP to APAP with A-Flex (n = 10, from 2.55 ± 0.76 h/night to 3.20 ± 0.85 h/night, P = 0.01) (Figure 4). In contrast, adherence did not change between 3 and 6 mo in the other groups.
Figure 4.
Analysis of poor compliers (< 4 h/night of CPAP use) between 3 and 6 mo. Individual data are presented.
DISCUSSION
The current study is the first to show a significant superiority of APAP with C-Flex on treatment adherence during the first 3 mo of use compared with APAP. Also, only patients using APAP with C-Flex had a significant improvement in subjective sleepiness and sleep quality. These results suggest that APAP with C-Flex may be a superior CPAP modality for the initial treatment of moderate to severe OSA.
To date, it has not been shown that PAP adherence can be improved by using different pressure applications of PAP. A meta-analysis of a number of published studies comparing APAP with fixed CPAP revealed that there was no difference between the two modalities regarding subjective sleepiness and adherence to PAP.4,18 The difficulty of exhaling against a fixed positive pressure is a common complaint when using CPAP, so C-Flex was developed, which reduces pressure at the beginning of exhalation and increases pressure to a level determined to be therapeutic for the latter part of exhalation. Aloia et al.7 demonstrated that use of a C-Flex device provided a significant improvement in CPAP adherence (1.7 h/night of additional use after a 12-wk follow-up period compared with a fixed PAP therapy). However, this study was not a randomized trial. Thereafter, Marshall et al.19 reported that in a 4-wk randomized study there was a trend toward greater CPAP adherence with C-Flex compared with fixed PAP therapy (C-Flex, 4.7 ± 2.9 h/night versus fixed PAP, 3.0 ± 2.1 h/night), but the difference was not statistically significant because of the small sample size. A meta-analysis also found that C-Flex does not provide any significant benefit over fixed PAP in terms of treatment adherence.20
In the current study, we confirmed that APAP with C-Flex was associated with significantly greater CPAP adherence than APAP. Many studies suggested that APAP reduces the mean PAP requirements, which are thought to influence CPAP adherence.4–6 C-Flex is designed to flexibly deliver pressure on a breath-by-breath basis by adjusting pressure within exhalation, which may be a more important variable for treatment adherence than the overall PAP level. This C-Flex technology may be responsible for the significant superiority of treatment adherence with APAP with C-Flex compared with APAP. Our study also showed that adherence in the APAP with A-Flex group did not improve after changing to the use of APAP with C-Flex. CPAP adherence within the first 3 mo has been described as a strong predictor of long-term CPAP use.21
CPAP adherence is a key issue in OSA, as greater improvements in daytime sleepiness, blood pressure, and QOL occur with greater CPAP use.22–25 A number of different variables independent of the CPAP mode can influence CPAP adherence. Older age, the use of lipid-lowering medications, the use of sedative/hypnotic agents on CPAP titration, a higher AHI, and greater daytime sleepiness were all factors shown to increase CPAP adherence.21,26–30 In contrast, a lower body mass index (BMI) (≤ 30 kg/m2) and psychosocial factors such as living alone have been shown to be associated with poor CPAP adherence.31,32 In the current study, there was no significant difference in these variables among the three groups. Also, all study patients received the same education on CPAP treatment. In addition, no humidification was used in any participant and their interfaces for CPAP treatment did not change during the study period. Therefore, we think that the CPAP mode was the main driver of the results of our study.
Pépin et al.10 reported that after changing the CPAP mode from a fixed PAP to C-Flex, CPAP adherence was significantly improved in patients with low adherence (< 4 h/night of CPAP use) during the initial 3 mo, whereas this improvement did not occur in patients who were already using CPAP for > 4 h/night. These results suggest that C-Flex is an effective modality for improving adherence. Compared with patients in previous reports,8–11 our patients had relatively low CPAP adherence (mean ± SD: 4.5 ± 1.9 h/night). Several reasons could account for this finding. As described previously, our study population had a low BMI (27.1 ± 4.7) and mild to moderate daytime sleepiness (mean ESS:,9.2 ± 4.4). It has already been described that a lower BMI and less daytime sleepiness were associated with poor CPAP adherence.21,31 In addition, poor sleep quality and QOL were only minimally abnormal in our patients compared with previous reports.20,32,33 Improving sleep-related QOL after CPAP in patients already having comparatively high sleep quality and QOL would seem to be difficult. Wells et al.34 demonstrated that patients who experienced greater improvements in daily functioning had higher levels of CPAP adherence. Therefore, the absence of improvement in subjective symptoms makes it unlikely that high levels of CPAP adherence would be achieved. Taken together, our patients had several traits that could be associated with poor CPAP compliance. This might explain why C-Flex had a significant effect on CPAP adherence in the current study.
We also demonstrated that the use of APAP with A-Flex significantly improved some QOL factors and that CPAP adherence in the APAP with A-Flex group was not greater than in the APAP group. On the other hand, a significant improvement in CPAP adherence was observed in the subgroup of poor compliers who were moved from APAP to APAP with A-Flex. This result suggests that A-Flex may be an alternative CPAP mode for improving adherence in patients with poor compliance. However, further studies using randomized prospective designs are needed to confirm this result. In addition, a future study that investigates the change in PAP adherence after a switch from APAP to APAP with C-Flex is warranted.
This study had some limitations. First, our study population was Asian, and their BMI was comparatively low. Therefore, it might be difficult to apply the results of the current study to all patients with OSA. Second, this was not a double-blind but a single-blind (patients blinded) randomized study. The investigators might be influenced in their assessments by knowing the treatment received. However, the same education on PAP therapy and follow-up methods after randomization were conducted for all patients. Thus, we think this possible limitation had a minimal effect on the study outcomes.
In conclusion, this prospective, randomized study demonstrated that APAP with C-Flex led to significantly greater adherence than APAP. APAP with C-Flex is a potentially superior CPAP mode among the three tested. Although we did not observe significant changes in PAP adherence after the switch from APAP to APAP with C-Flex, A-Flex seemed to be an effective CPAP approach in patients with poor adherence to APAP.
ACKNOWLEDGMENTS
The authors thank Dr. Takegami for her kind comments and suggestions for statistical analysis, along with Mses. Toki, Tamura, and Kimura for their support of this clinical research.Work for this study was performed at the Sleep unit of Kyoto University Hospital. This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (nos. 20590921 and 22590860), Respiratory Failure Research Group and Health Science Research Grants (Comprehensive Research on Life-Style Related Diseases Including Cardiovascular Diseases and Diabetes Mellitus) from the Ministry of Health, Labor and Welfare of Japan, and the Japan Vascular Disease Research Foundation.
DISCLOSURE STATEMENT
This was not an industry supported study. Drs. Hitomi, Yoshimura, Oga, and Chin belong to departments that have received support from Philips-Respironics, Teijin Pharma, and Fukuda Denshi. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 2.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–5. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 3.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–13. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 4.Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep. 2004;27:249–53. doi: 10.1093/sleep/27.2.249. [DOI] [PubMed] [Google Scholar]
- 5.Nolan GM, Doherty LS, Mc Nicholas WT. Auto-adjusting versus fixed positive pressure therapy in mild to moderate obstructive sleep apnoea. Sleep. 2007;30:189–94. doi: 10.1093/sleep/30.2.189. [DOI] [PubMed] [Google Scholar]
- 6.Hukins C. Comparative study of autotitrating and fixed-pressure CPAP in the home: a randomized, single-blind crossover trial. Sleep. 2004;27:1512–7. doi: 10.1093/sleep/27.8.1512. [DOI] [PubMed] [Google Scholar]
- 7.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005;127:2085–93. doi: 10.1378/chest.127.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilius G, Happel A, Domanski U, Ruhle KH. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest. 2006;130:1018–24. doi: 10.1378/chest.130.4.1018. [DOI] [PubMed] [Google Scholar]
- 9.Dolan DC, Okonkwo R, Gfullner F, Hansbrough JR, Strobel RJ, Rosenthal L. Longitudinal comparison study of pressure relief (C-Flex) vs. CPAP in OSA patients. Sleep Breath. 2009;13:73–7. doi: 10.1007/s11325-008-0199-1. [DOI] [PubMed] [Google Scholar]
- 10.Pépin JL, Muir JF, Gentina T, et al. Pressure reduction during exhalation in sleep apnea patients treated by continuous positive airway pressure. Chest. 2009;136:490–7. doi: 10.1378/chest.08-2646. [DOI] [PubMed] [Google Scholar]
- 11.Bakker J, Campbell A, Neill A. Randomized controlled trial comparing flexible and continuous positive airway pressure delivery: effects on compliance, objective and subjective sleepiness and vigilance. Sleep. 2010;33:523–9. doi: 10.1093/sleep/33.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 13.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 14.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med. 1998;158:494–503. doi: 10.1164/ajrccm.158.2.9712036. [DOI] [PubMed] [Google Scholar]
- 15.Flemons WW, Reimer MA. Measurement properties of the calgary sleep apnea quality of life index. Am J Respir Crit Care Med. 2002;165:159–64. doi: 10.1164/ajrccm.165.2.2010008. [DOI] [PubMed] [Google Scholar]
- 16.Rechtschaffen A, Kales A. Washington, DC: National Institutes of Health; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, Illinois: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 18.Smith I, Lasserson TJ. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD003531.pub3. CD003531. [DOI] [PubMed] [Google Scholar]
- 19.Marshall NS, Neill AM, Campbell AJ. Randomised trial of compliance with flexible (C-Flex) and standard continuous positive airway pressure for severe obstructive sleep apnea. Sleep Breath. 2008;12:393–6. doi: 10.1007/s11325-008-0189-3. [DOI] [PubMed] [Google Scholar]
- 20.Bakker JP, Marshall NS. Flexible pressure delivery modification of continuous positive airway pressure for obstructive sleep apnea does not improve compliance with therapy: systematic review and meta-analysis. Chest. 2011;139:1322–30. doi: 10.1378/chest.10-2379. [DOI] [PubMed] [Google Scholar]
- 21.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 22.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–8. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 23.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stradling JR, Davies RJ. Is more NCPAP better? Sleep. 2000;23:S150–3. [PubMed] [Google Scholar]
- 25.Zimmerman ME, Arnedt JT, Stanchina M, Millman RP, Aloia MS. Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest. 2006;130:1772–8. doi: 10.1378/chest.130.6.1772. [DOI] [PubMed] [Google Scholar]
- 26.Collen J, Lettieri C, Kelly W, Roop S. Clinical and polysomnographic predictors of short-term continuous positive airway pressure compliance. Chest. 2009;135:704–9. doi: 10.1378/chest.08-2182. [DOI] [PubMed] [Google Scholar]
- 27.Platt AB, Kuna ST, Field SH, et al. Adherence to sleep apnea therapy and use of lipid-lowering drugs: a study of the healthy-user effect. Chest. 2010;137:102–8. doi: 10.1378/chest.09-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest. 2009;136:1263–8. doi: 10.1378/chest.09-0811. [DOI] [PubMed] [Google Scholar]
- 29.Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA. CPAP promotion and prognosis-The Army Sleep Apnea Program Trial: effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151:696–702. doi: 10.7326/0003-4819-151-10-200911170-00006. [DOI] [PubMed] [Google Scholar]
- 30.Drake CL, Day R, Hudgel D, et al. Sleep during titration predicts continuous positive airway pressure compliance. Sleep. 2003;26:308–11. doi: 10.1093/sleep/26.3.308. [DOI] [PubMed] [Google Scholar]
- 31.Pelletier-Fleury N, Rakotonanahary D, Fleury B. The age and other factors in the evaluation of compliance with nasal continuous positive airway pressure for obstructive sleep apnea syndrome: a Cox’s proportional hazard analysis. Sleep Med. 2001;2:225–32. doi: 10.1016/s1389-9457(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 32.Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27:134–8. doi: 10.1093/sleep/27.1.134. [DOI] [PubMed] [Google Scholar]
- 33.Siccoli MM, Pepperell JC, Kohler M, Craig SE, Davies RJ, Stradling JR. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2008;31:1551–8. doi: 10.1093/sleep/31.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells RD, Freedland KE, Carney RM, Duntley SP, Stepanski EJ. Adherence, reports of benefits, and depression among patients treated with continuous positive airway pressure. Psychosom Med. 2007;69:449–54. doi: 10.1097/psy.0b013e318068b2f7. [DOI] [PubMed] [Google Scholar]