Abstract
Study Objectives:
To evaluate efficacy and safety of 3.5-mg zolpidem tartrate sublingual tablets (ZST) on latency to sleep onset after middle-of-the-night (MOTN) awakenings in patients with insomnia characterized by difficulty returning to sleep after MOTN awakenings.
Design:
Multicenter randomized, double-blind, placebo-controlled, parallel-group.
Setting:
Outpatient.
Patients:
There were 295 adults (median age 43 y; 68.1% female) with primary insomnia and difficulty returning to sleep after MOTN awakenings (three or more MOTN awakenings/wk during screening).
Interventions:
After a 2-wk, single-blind placebo eligibility period, participants were randomized 1:1 to as-needed MOTN dosing with 3.5 mg ZST or placebo for 28 nights. An interactive voice response system determined if the study drug could be taken and recorded sleep/wake efficacy measures.
Results:
ZST significantly (P < 0.0001) decreased latency to sleep onset over 4 wk (baseline 68.1 min; ZST 38.2 min) compared with placebo (baseline 69.4 min; placebo 56.4 min). Ratings of morning sleepiness/alertness significantly (P = 0.0041) favored the ZST group on nights medication was taken but not on other nights. Participants in the ZST group took the study drug on 62% of nights during the 4 wk; members of the placebo group took study medication on 64% of nights. Adverse events were generally mild and at the same rate (19.3% of participants) in both groups. There were no treatment-related serious adverse events (SAEs), and one adverse event-related study discontinuation from the placebo group. Dosing/week did not increase across the study.
Conclusions:
3.5 mg ZST used as needed significantly reduced latency to return to sleep in comparison with placebo in these patients with insomnia. Sleep quality was improved, and morning sleepiness/alertness scores also improved. ZST was well tolerated. These data demonstrate the utility of a sleep-promoting agent when used as needed in the MOTN.
Clinical Trial Information:
Clinical Trials Registration: NCT00466193: “A Study of Zolpidem Tartrate Tablet in Adult Patients with Insomnia” http://www.clinicaltrials.gov/ct2/show/NCT00466193?spons=%22Transcept+Pharmaceuticals%22&spons_ex=Y&rank=2
Citation:
Roth T; Krystal A; Steinberg FJ; Singh NN; Moline M. Novel sublingual low-dose zolpidem tablet reduces latency to sleep onset following spontaneous middle-of-the-night awakening in insomnia in a randomized, double-blind, placebo-controlled, outpatient study. SLEEP 2013;36(2):189-196.
Keywords: Insomnia, middle-of-the-night awakenings, MOTN insomnia, pharmacotherapy, zolpidem
INTRODUCTION
Middle-of-the-night (MOTN) awakening has been identified as one of the most common forms of sleep disturbance.1 In a survey conducted by Ohayon et al.2 with a representative sample of 8,937 non-institutionalized individuals age 18 y or older living in Texas, New York, and California, 35.5% reported frequent MOTN awakenings on at least 3 nights per wk, and 43.0% of those individuals with nocturnal awakenings reported having great difficulty resuming sleep. Of those who had MOTN awakenings with difficulty returning to sleep, 77.5% reported associated daytime impairment, defined as having an effect on daytime functioning (mood, fatigue, daytime sleepiness, cognitive functioning).
Patients with insomnia do not typically experience sleep difficulty every night,3 yet until recently, there was no medication specifically approved for as-needed (prn) use4 at the time when the problem manifests. Currently, there are numerous hypnotic treatment options for patients who have a full night (7-8 h) of available bedtime. The ability to dose when the problem occurs has been proposed as an important therapeutic alternative to dosing prophylactically at the beginning of the night.3 Ideally a MOTN prn medication would lead to rapid onset of sleep promotion and short-enough duration of action that would not result in morning residual effects.
Because of its favorable pharmacokinetic profile as reported previously4, zolpidem tartrate at 1.75 and 3.5 mg doses was selected for development as a potential therapeutic agent for prn treatment of insomnia when MOTN awakening was followed by difficulty returning to sleep. A novel sublingual formulation of zolpidem tartrate (Intermezzo®; Purdue Pharma L.P.; ZST) was developed and was approved by the U.S. Food and Drug Administration (FDA) in November 2011.5 The sublingual tablets contain a binary buffer system that promotes in situ conversion of hydrophilic zolpidem tartrate into its lipophilic free-base form, which would be expected to penetrate the oral mucosa more easily than the hydrophilic form.
Pharmacokinetic studies showed that the sublingual formulation is more rapidly absorbed in the initial 15-20 min6 than the standard tablet, without significantly altering the total bioavailability of zolpidem. Bicarbonate carbonate buffers in the sublingual tablet are thought to work by transiently raising the pH of the saliva, which accelerates the permeation and absorption of the drug across the buccal membrane without interfering with the disposition and elimination properties of the drug. Consequently the time to peak plasma concentration (Tmax) is shortened, whereas the peak plasma concentration (Cmax) and the area under the curve (AUC) remain largely unchanged.
These findings provided the basis for suggesting that this sublingual formulation might reduce time to sleep onset at doses of zolpidem tartrate lower than those currently approved (10-12.5 mg zolpidem tartrate in adult patients, 5-6.25 mg in elderly patients). In addition, ZST hypnotic activity has been shown to last for approximately 2.5-4 h.6
A sleep laboratory study using polysomnographic measures demonstrated the efficacy of ZST (3.5 mg and 1.75 mg) compared with placebo in reducing sleep latency after a scheduled awakening in patients whose insomnia complaints were characterized by difficulty falling back to sleep after MOTN awakenings.7 The current study evaluated the safety and efficacy of ZST 3.5 mg when taken prn after a MOTN awakening with difficulty returning to sleep in a home setting.
METHODS
Study Objectives
This study was designed to evaluate the safety and efficacy of low-dose (3.5 mg) ZST versus placebo in reducing latency to sleep onset after spontaneous MOTN awakenings (LSOMOTN) in adult patients with insomnia characterized by difficulty returning to sleep after MOTN awakenings. Secondary efficacy endpoints included subjective assessments of total sleep time (TST) post-MOTN awakening (TSTMOTN), number of subsequent awakenings (NAWMOTN), wake time after sleep onset following the awakening (WASOMOTN), and other subjective sleep and wake variables.
Patient Selection and Screening
Adult males and females ages 18-64 y, inclusive, meeting diagnostic criteria for primary insomnia according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR)8 were considered for enrollment. Prospects were respondents to media advertisements and physician referrals for individuals reporting waking up in the MOTN with difficulty falling back to sleep. Study participants were required to report at least a 3-mo history of prolonged MOTN awakenings characterized by three or more such awakenings per wk; an average TST of less than 6.5 h; usual time spent in bed of 7-9 h; and usual bedtime between 21:00 and 24:00, which did not vary by more than 2 h on 5 of 7 days.
Key exclusion criteria for the study included: (1) any sleep disorder other than insomnia as determined by a sleep history, planned travel across three or more time zones during the course of the study, or regular night shift work within the past 6 mo prior to screening; (2) clinically significant ongoing medical/neurologic conditions; (3) history of psychiatric disorder (Axis I), as defined in DSM-IV-TR; (4) history of current alcohol abuse or drug addiction, or current consumption of more than 14 drinks of alcohol per wk; (5) use of herbal preparations, over-the-counter or prescription medications known to affect sleep-wake function; (6) smoking more than five cigarettes per day or inability to abstain from smoking during a MOTN awakening; (7) sensitivity to zolpidem or excipients in the formulation.
All study participants provided informed consent. The study was approved by an institutional review board as required by each site.
Study Design
This multicenter, randomized, double-blind, placebo-controlled, parallel group, outpatient study was conducted at 25 sites across the United States. After providing written informed consent, eligible participants began a 14-day single-blind screening/baseline period to confirm study entrance criteria. Study participants received a bottle containing 15 placebo sublingual tablets (Visit 1). They were instructed to call an interactive voice response system (IVRS) when they experienced a MOTN awakening of at least 10 min duration. After calling the IVRS and responding to qualification questions regarding the duration of the MOTN awakening and having at least 4 h of time remaining in bed, participants were given permission by the IVRS to take study medication. Study participants were instructed to place the tablet under the tongue and to avoid swallowing until the tablet dissolved (about 2 min).
Study participants were also instructed to call the IVRS every morning, whether or not study medication was taken during the night, to answer questions concerning their previous night’s sleep. Efficacy and safety were evaluated using participant responses recorded by the IVRS and during study visits.
To qualify for randomization, over the 2-wk screening period study participants were required to have had at least 3 MOTN awakenings/wk, with one MOTN awakening/week of ≥ 60 min, and two awakenings/wk ≥ 30 min. All three awakenings also had to be followed by at least 4 h of bedtime remaining. In addition, to qualify for randomization, participants also had to demonstrate compliance with IVRS calling and dosing instructions. Eligible participants were randomly assigned to receive either 3.5 mg ZST or matching placebo tablets in accordance with a 1:1 blocked randomization schedule.
Upon randomization into the double-blind 4-wk treatment period (Visit 2), participants received a bottle containing 15 tablets of 3.5 mg ZST or matching placebo. At the 2-wk visit (Visit 3), a second bottle of study medication was dispensed and unused medication from the previous 2 wk was collected. The final study visit (Visit 4) occurred after 28 days of treatment at the end of the treatment period or upon early discontinuation.
Efficacy and Safety Analyses
Primary Endpoint
The primary efficacy endpoint was LSOMOTN across the 4 wk of the study. Nightly LSOMOTN was the response to the IVRS diary question, “How long did it take you to fall asleep after taking your study medication?”
Secondary Endpoints
Several secondary sleep-related endpoints were assessed after a spontaneous MOTN awakening, including subjective assessments of TSTMOTN, NAWMOTN, and WASOMOTN.
Sleep quality was assessed using a nine-point scale. Each morning, regardless of whether a participant took study medication, the sleep quality rating was recorded. Participants who recorded that they took study medication responded to the following IVRS question for that day: “On a scale of 1 to 9, with 1 being extremely poor and 9 being excellent, please rate your quality of sleep after taking your study medication”. Participants who did not take study medication the previous night responded to the following IVRS question for that day: “On a scale of 1 to 9, with 1 being extremely poor and 9 being excellent, please rate your quality of sleep.” Sleep quality was averaged over the 4-wk treatment period separately for nights study medication was taken and for nights study medication was not taken.
To determine if MOTN administration of ZST resulted in next-morning residual effects, morning sleepiness/alertness was assessed using a ninepoint scale (1 = “very sleepy” to 9 = “wide awake and alert”) based on the response to the IVRS question, “On a scale of 1 to 9, with 1 being very sleepy and 9 being wide awake and alert, how sleepy do you feel this morning?”
Safety Evaluation
The safety analysis dataset included all study participants who took at least one dose of study medication during the double-blind treatment period. Safety was assessed by physical examination (including vital signs) conducted at each study visit, adverse event reports (AEs) and laboratory parameters. AEs were defined according to the Medical Dictionary for Regulatory Activities (MedDRA Version 10.1). AEs with onset (or worsening) after the start of double-blind study drug were considered treatment emergent. The frequency of treatment-emergent AEs and the frequency of events by body system were summarized by treatment period according to preferred term and system organ class.
Statistical Analysis
Data from 25 sites across the United States were pooled regionally for analysis, resulting in 12 pooled sites with at least 16 study participants per site. The baseline value for all efficacy measures was the mean for that variable derived from nights during which the study participant took study medication during the 2-wk screening period. Differences between treatment groups were analyzed using an analysis of covariance model that included fixed effects for treatment and pooled study site and the baseline value (average of the 2-wk screening period) of the outcome variables baseline value as a covariate. The analyses were performed using all available observations from the 4-wk, double-blind treatment period. Log-transformed least square means (LS means) and 95% confidence intervals for the LS means were provided for each of the treatments.
For secondary endpoints, no logarithmic transformation was imposed. To avoid inflation of the type I error rate when testing multiple endpoints, a hierarchical testing procedure was prospectively specified. In this procedure, the three endpoints were to be tested in the following order: (1) TSTMOTN, (2) NAWMOTN, and (3) WASOMOTN. If the test of treatment difference for any endpoint was not significant at the 0.05 level, then inferential analysis of the remaining endpoints in the hierarchy would be considered exploratory.
The data from two of the secondary endpoints, WASOMOTN and NAWMOTN, were not normally distributed due to the large number of patients who had no additional awakenings after returning to sleep. Therefore, nonparametric analyses were used in the assessment of these variables using the row mean scores statistic.
RESULTS
Patient Demographics
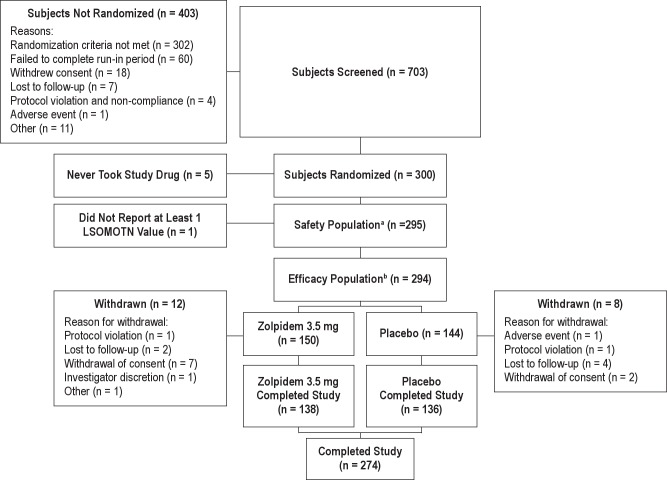
Figure 1 illustrates the disposition of patients. Seven hundred three patients were entered into the 14-day screening period. Of those screened, 300 met the inclusion criteria and were randomized to receive the study drug. Five patients did not take any study drug after randomization, resulting in 295 patients in the safety population. The efficacy population (n = 294, active 150, placebo 144) included all patients who took at least one dose of study medication during the double-blind treatment phase of the study and who reported LSOMOTN data from at least 1 night of the double-blind treatment period. One patient took a single dose of study medication, but did not complete the morning assessment and therefore was excluded from the efficacy analysis population. Approximately 92% of the ZST group and 94% of the placebo group completed the study, with the major reason for discontinuation being withdrawal of consent (4.6% ZST; 1.4% placebo) and lost to follow-up (1.3% ZST; 2.8% placebo).
Figure 1.
Patient disposition. aThe safety population includes all randomized patients who took at least one dose of study medication during the double-blind treatment phase of the study. bThe efficacy population includes all randomized subjects who took at least 1 dose of study medication and provided at least 1 LSOMOTN value. LSOMOTN latency to sleep onset following middle-of-the-night awakening.
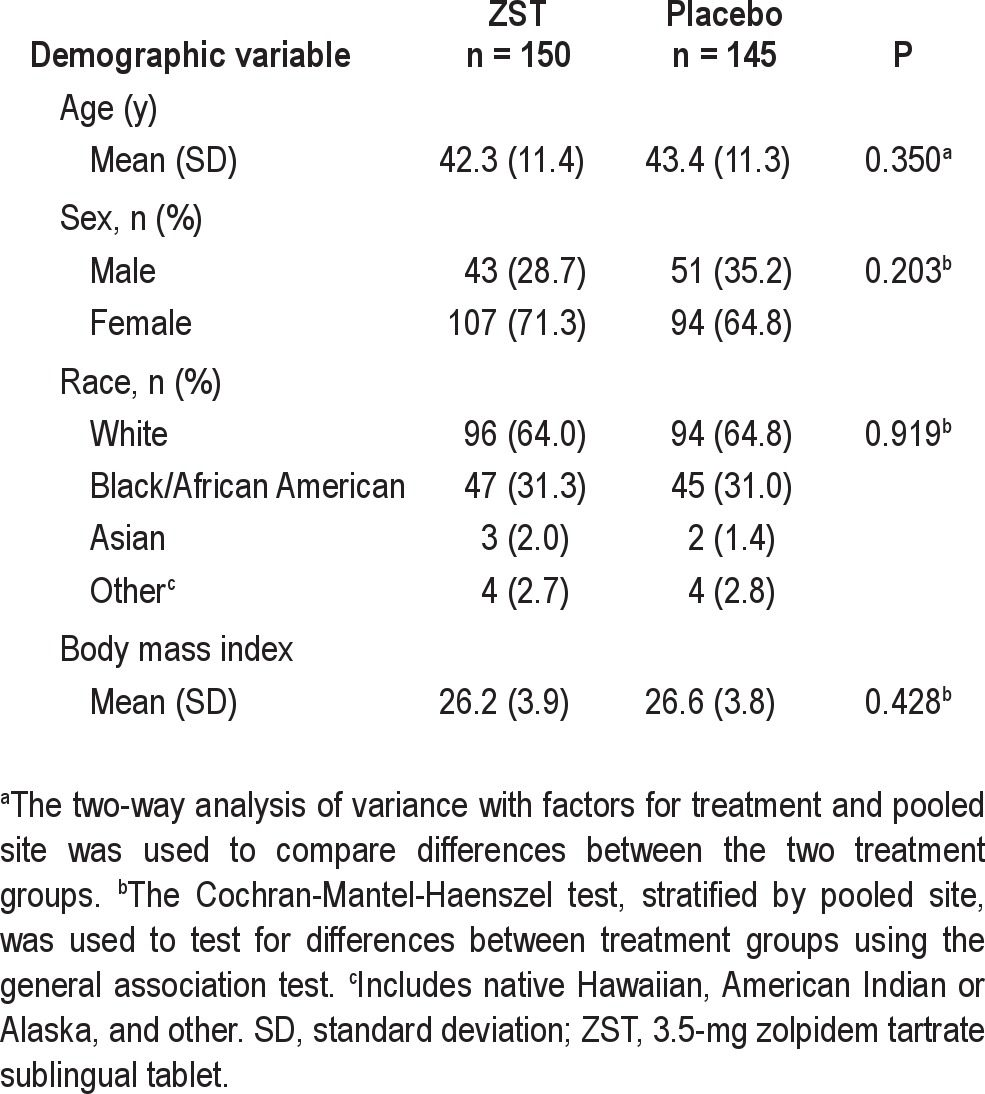
The median age of randomized patients was 43 y; 68.1 % were female; 64.4% were white, and 31.2% were African American. The demographics of the study population are shown in Table 1. No significant differences in demographics or other baseline characteristics were found between ZST and placebo treatment groups.
Table 1.
Patient demographics (safety population)
There was no difference between treatment groups in the number of study drug tablets taken during the 2 wk of the placebo run-in period. The mean ± standard error (SE) number of tablets taken by the ZST group was 9.9 ± 2.6, and 9.9 ± 2.9 tablets by the placebo group (P = 0.893). The median number of tablets in both groups was 10, equivalent to 71.4% of the nights.
Efficacy Evaluations
Baseline sleep latencies were 68.1 min in the ZST group and 69.4 min in the placebo group (Table 2). Across the 4-wk treatment period, the primary endpoint, LSOMOTN, was significantly reduced in the ZST group versus placebo (P < 0.0001). The ZST group also had significant reductions in LSOMOTN relative to the placebo group (P < 0.0001) for each of the four individual treatment weeks (Figure 2).
Table 2.
Baseline efficacy values (intent-to-treat population)
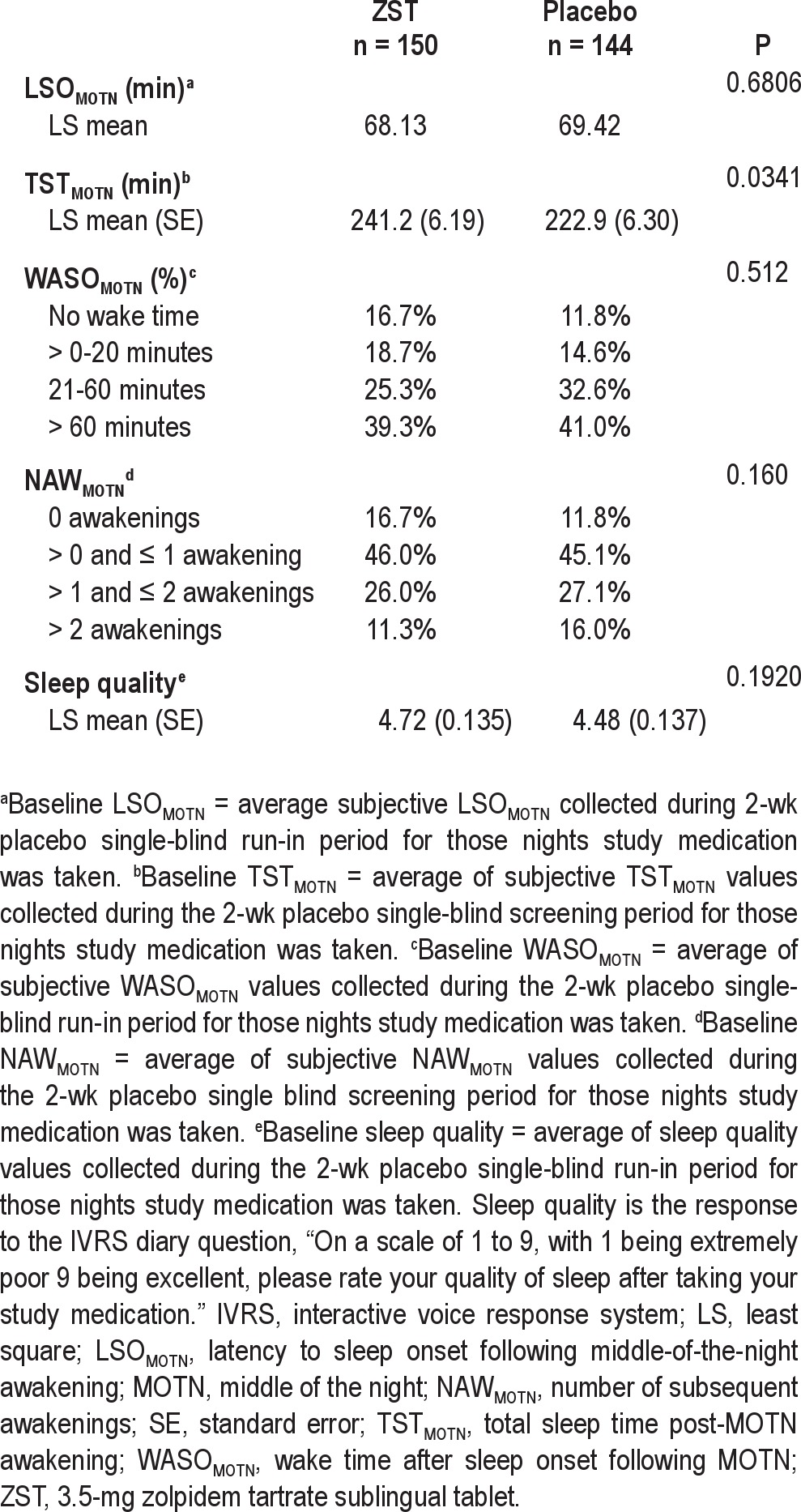
Figure 2.
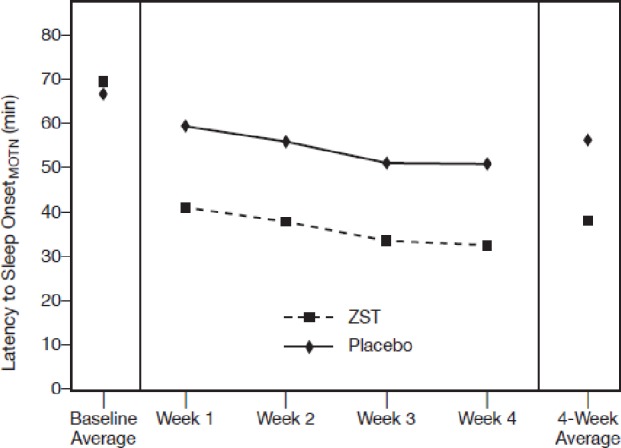
LSOMOTN during the double-blind treatment period. Analysis of covariance model with log-transformed mean LSOMOTN as response; treatment and pooled site as fixed effects and baseline log-transformed average LSOMOTN as covariate. P-value shown is for treatment. All values < 0.0001 ZST compared to placebo. LSOMOTN, latency to sleep onset following middle-of-the-night awakening; ZST, 3.5-mg zolpidem tartrate sublingual tablet.
The baseline TSTMOTN in the ZST and placebo groups was 241.2 and 222.9 min, respectively, a significant difference (Table 2). After treatment, an increase in TSTMOTN was noted in both groups. The TSTMOTN was sustained over the course of the study in the ZST group (Figure 3). Although the ZST group slept longer post-MOTN awakening (264.1 ± 4.25; LS mean ± SE) than the placebo-treated group (255.0 ± 4.33), the difference between groups was not statistically significant across the 4-wk treatment period. Statistically significant improvement in TSTMOTN was seen for wk 1 (P = 0.0107) and 2 (P = 0.0469) in the ZST group as compared with placebo. The TSTMOTN in the placebo group gradually increased during the course of the study, leading to no difference between groups at wk 3 and 4.
Figure 3.
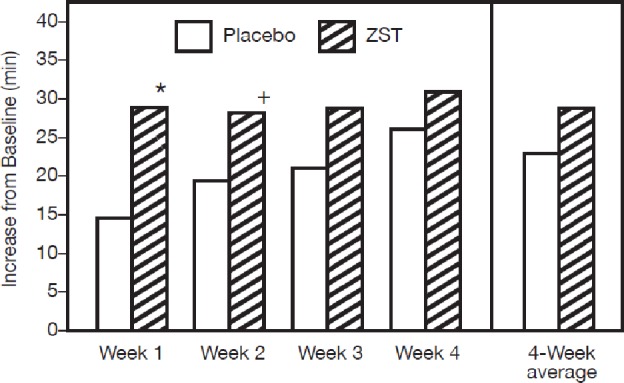
Change from baseline in TSTMOTN. Baseline TSTMOTN was derived from the average of values collected during the 2-wk placebo run-in period for those nights study medication was taken. Analysis of covariance model with mean TSTMOTN as the response; treatment and pooled site as fixed effects and average TSTMOTN at baseline as a covariate. *P < 0.02; +P < 0.05. TSTMOTN, subjective total sleep time following middle-of-the-night awakening; ZST 3.5-mg zolpidem tartrate sublingual tablet.
Because many patients did not wake up again during the night after dosing, sleep maintenance data were not normally distributed, necessitating categorical analyses on NAWMOTN and WASOMOTN. When NAWMOTN was analyzed by subcategory (no awakenings, one to two awakenings, and more than two awakenings per night), statistically significant improvement was seen in NAWMOTN in the ZST group in comparison with the placebo group across all 4 wk of the double-blind treatment period (P < 0.001) (Figure 4B), and for wk 1 (P = 0.027), wk 2 (P = 0.011), and wk 3 (P = 0.007). The difference was not statistically significant for wk 4 (P = 0.064). The treatment effect was evident, however, as the ZST group had a higher percentage of patients reporting zero awakenings than did the placebo group during each of the 4 wk of double-blind treatment.
Figure 4.
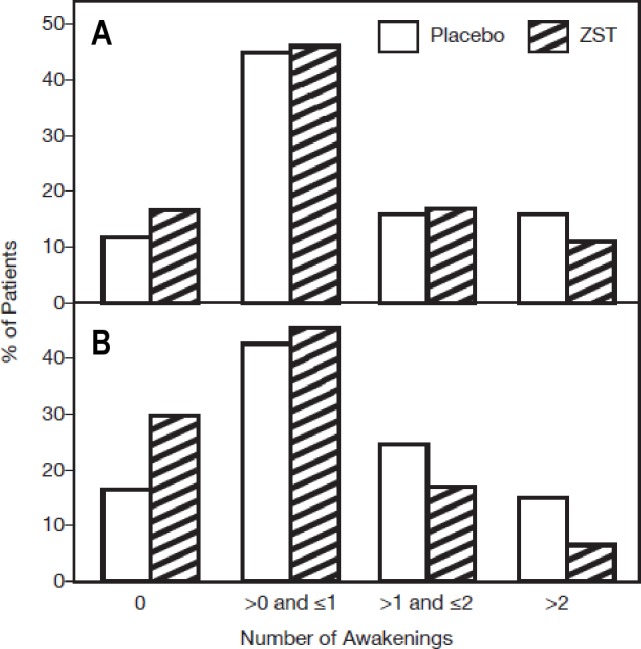
NAWMOTN during the run-in and double-blind treatment period. Continuous values obtained by averaging were then categorized as shown. The row mean score statistic was employed for categorized mean NAWMOTN. (A) Baseline NAWMOTN is the average of values collected during the 2-wk placebo single-blind screening period for those nights study medication was taken. No statistical difference between groups. (B) Four-wk treatment average. P < 0.001. NAWMOTN, subjective number of awakenings following middle-of-the-night awakening; ZST, 3.5-mg zolpidem tartrate sublingual tablet.
Similarly, when WASOMOTN was analyzed by subcategory (no min, 1-20 min, 21-60 min, > 60 min), statistically significant improvement was seen in the ZST group as compared with placebo across all 4 wk of the double-blind treatment (P = 0.006), and for wk 1 (P = 0.021), wk 2 (P = 0.005), and wk 3 (P = 0.042). The difference was not statistically significant at wk 4 (P = 0.063).
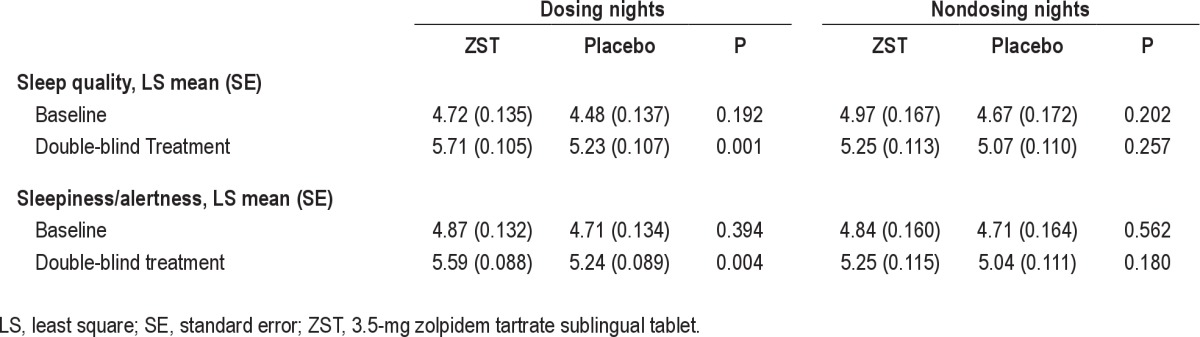
To assess the patient’s overall evaluation of sleep throughout the 4-wk treatment period, sleep quality was assessed after dosing nights as well as nondosing nights. For dosing nights, the ZST group reported improved ratings of sleep quality compared with the placebo group. The between-group differences were statistically significant for every treatment wk and throughout the double-blind treatment period (ZST 5.71 ± 0.105; placebo 5.23 ± 0.107; P = 0.0011).
Safety Results
The ZST was well tolerated and no new safety issues were identified. The observed safety profile was consistent with the known safety profile of zolpidem tartrate. The most commonly reported treatment-emergent AEs were headache (2.7%), nausea (1.3%) and fatigue (1.3%) in the zolpidem tartrate group, and nasopharyngitis (3.4%), somnolence (1.4%), headache (1.4%), back pain (1.4%), and abdominal pain (1.4%) reported by those in the placebo group. Only one patient discontinued from the trial due to AEs, and was from the placebo group reporting abdominal pain and headache. No serious AEs were reported during the double-blind treatment period. No deaths occurred during the study.
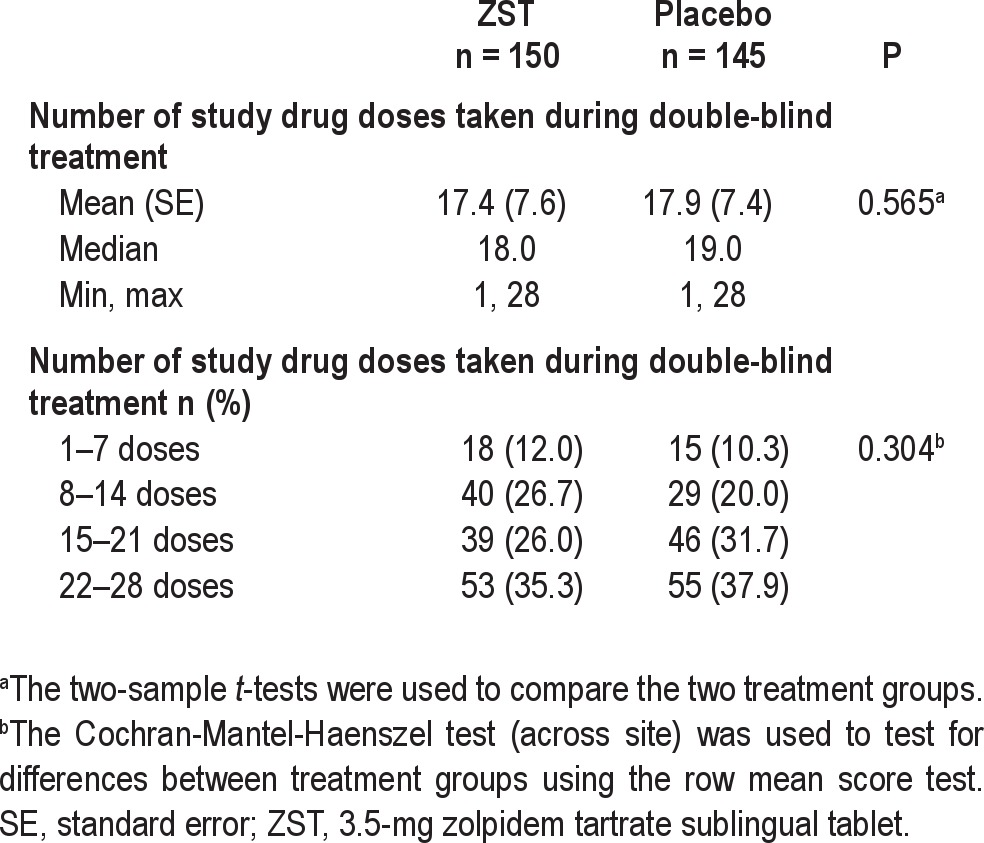
There was no increase in weekly usage of ZST or placebo over the course of the study (Figure 5) or differences between treatment groups. Patients in the ZST group took the study drug on 62% of nights during the 4-wk period; placebo patients took study medication on 64% of the nights (Table 3).
Figure 5.
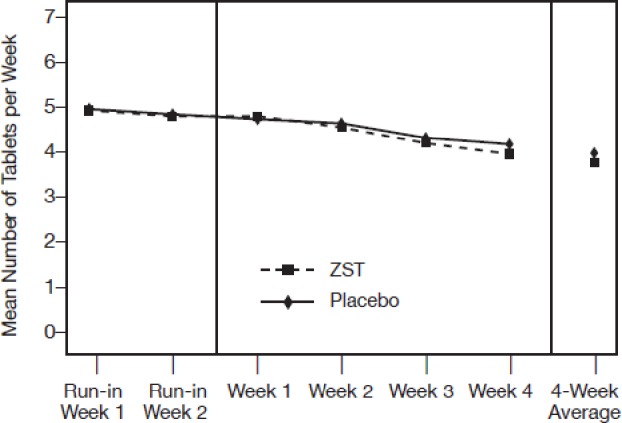
Mean number of study medication tablets consumed per wk during the single-blind and double-blind periods. There was no statistical difference between groups at either phase.
Table 3.
Study medication consumption during the double-blind treatment period
Morning sleepiness/alertness also significantly improved compared with placebo at every time point after nights during which study medication was taken (Table 4; ZST 5.59 ± 0.088; placebo 5.24 ± 0.089; P = 0.0041). On nondosing nights, no statistically significant differences were noted between drug and placebo for either sleep quality or morning sleepiness/alertness.
Table 4.
Daytime assessments after dosing and nondosing nights
DISCUSSION AND CONCLUSIONS
In this study, LSOMOTN was significantly reduced in the ZST group compared with the placebo group. Patients taking ZST 3.5 mg experienced a 30-min reduction in LSOMOTN from baseline values and an approximately 18-min faster sleep onset compared with patients in the placebo group (P < 0.0001). The improvement in LSOMOTN was sustained during the entire 4-wk study. In addition, patients in the ZST group reported better sleep quality and were less sleepy in the morning after dosing than patients in the placebo group.
The results of this study parallel the findings of the ZST 3.5 and 1.75 mg treatment groups in the earlier study incorporating polysomnography (PSG)7. The current study demonstrates that the therapeutic benefits of ZST are seen not only with PSG assessment of MOTN awakenings induced in the laboratory setting, but also when self-reported measures of sleep are used and when the medication is used prn to treat MOTN awakenings occurring naturally in patients with insomnia at home. Both studies showed a robust effect on latency to return to sleep after a nocturnal awakening.
Another parallel between the PSG study and the current patient-reported results with ZST is a significant difference between active and placebo groups on ratings of sleep quality and alertness upon arising. In various pharmacologic studies, sleep quality sometimes but not always shows a drug effect, but alertness on arising rarely separates drug from placebo.9 The significant treatment differences observed on both of these domains in both studies raises the possibility that the more proximal a medication is dosed relative to the evaluation of sleep, the greater the perceived benefit. In other words, patients with insomnia may prefer improving sleep in the later part of the night relative to the first part of the night. This hypothesis would need to be tested in future studies.
The results on TSTMOTN may have been affected by the significant difference in TSTMOTN at baseline. Further, it is important to note that patients were not enrolled in the study based on TST, but rather MOTN awakening with difficulty returning to sleep. Another factor may be differences between PSG and self-reported assessments of sleep difficulties after MOTN awakenings. Despite the loss of significance, patients rated themselves as less sleepy in the morning, with improved sleep quality. It should be noted, however, that as a result of the statistical analysis plan, because TSTMOTN was not statistically significant between groups, the other secondary outcome measures were considered exploratory. Like the previous ZST study performed in the laboratory, self-reported TST separated from placebo. However, although this was significant in wk 1 and 2, ZST did not separate from placebo on wk 3 and 4. This loss of efficacy over time reflects a change in the placebo group toward improved sleep rather than a loss of efficacy in the ZST group. This improvement in the placebo group is often seen in longer trials and is thought to be reflective of a placebo response or an improvement in sleep associated with protocol requirement consistent with good sleep hygiene practices (e.g., afternoon avoidance of alcohol and caffeine, regularization of time in bed and sleep and rise times, and the prohibition of napping).10,11
The final finding of note relates to the rate of consumption of a sleep medication across time in a prn dosing strategy. In the absence of data, there have been different hypotheses proposed. First, the reinforcing properties of these medications, or the negative consequence of not taking them will lead individuals to increase the rate of self-administration across time. Alternatively, it has been hypothesized that as patients become aware of the availability of a medication to help them sleep, they will feel reassured and will sleep better, thereby decreasing the rate of taking medication. In fact, neither of these hypotheses was borne out. Patients did not judge their sleep to get better without medication. Sleep quality was different between drug and placebo groups on medication nights, but not on nights when medication was not taken. Also on average, patients took these medications approximately 62% of nights, a percentage that did not change across the 4 weeks of the trial. The frequency and pattern of use across time seen in the current study is similar to that observed with a MOTN prn study carried out with indiplon.12
In summary, the results of this study validate the outcomes of the sleep laboratory study7 that used a scheduled MOTN awakening design. In both studies, there was a significant benefit in reducing time to sleep onset after awakening in the MOTN and improvement in reported sleep quality, with no evidence of residual effects on the morning after dosing. In fact, patients taking 3.5 mg sublingual zolpidem, in both studies reported a significant decrease in morning sleepiness relative to those taking placebo. The results of this study are supportive of ZST being a safe and effective treatment option for insomnia characterized by awakening in the MOTN with difficulty returning to sleep.
ACKNOWLEDGMENTS
The authors acknowledge the support of Alan Lankford, PhD; Russell Rosenberg, PhD; Martin Scharf, PhD; David Seiden, MD; and the Intermezzo study group.
DISCLOSURE STATEMENT
This manuscript reports the results of a multicenter clinical trial. Research was funded by Transcept Pharmaceuticals. Poststudy support was also provided by Purdue Pharma L.P. At the time that this study was conducted, the use of Intermezzo was in development and not a product approved by the US Food and Drug Administration (FDA). Intermezzo has subsequently been approved by the FDA for use as needed for the treatment of insomnia when a middle-of-the-night awakening is followed by difficulty returning to sleep. Dr. Roth has received grant support from Apnex and Merck. He has also served as a consultant for Abbott, Cephalon, Eisai, Lilly, Intec, Merck, Proctor and Pfizer, Sepracor, Shire, Somaxon, Somnus, Steady Sleep Rx, Takeda, and Transcept. Dr. Krystal has received grant/research support from the NIH, Sanofi-Aventis, Cephalon, GlaxoSmithKline, Merck, Neurocrine, Pfizer, Sunovion/Sepracor, Somaxon, Takeda, Transcept, Phillips-Respironics, Neurogen, Evotec, Astellas, Abbott, Neuronetics, Neosynch and Brainaway. He has also served as a consultant for Abbott, Actelion, Arena, Astellas, Axiom, AstraZeneca, BMA, Cephalon, Eisai, Lilly, GlaxoSmithKline, Jazz Pharmaceuticals, Johnson and Johnson, King, Merck, Neurocrine, Neurogen, Neuronetics, Novartis, Organon, McNeil-Janssen, Pfizer, Respironics, Roche, Sanofi-Aventis, Somnus, Sunovion/Sepracor, Somaxon Takeda, Transcept, and Kingsdown Inc. Dr. Steinberg is a consultant of Transcept Pharmaceuticals, Inc. Dr. Singh is an employee of Transcept Pharmaceuticals, Inc. Dr. Moline is a current employee of Purdue Pharma L.P. She is a former employee of Eisai Inc.
REFERENCES
- 1.Ohayon MM, Roth T. What are the contributing factors for insomnia in the general population? J Psychosom Res. 2001;51:745–55. doi: 10.1016/s0022-3999(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM, Krystal A, Roehrs TA, Roth T, Vitiello MV. Using difficulty resuming sleep to define nocturnal awakenings. Sleep Med. 2010;11:236–41. doi: 10.1016/j.sleep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estivill E. Behaviour of insomniacs and implication for their management. Sleep Med Rev. 2002;6:S3–6. doi: 10.1016/s1087-0792(02)80001-6. [DOI] [PubMed] [Google Scholar]
- 4.Roth T, Mayleben D, Corser BC, Singh N. Daytime pharmacodynamic and pharmacokinetic evaluation of low-dose transmucosal zolpidem. Hum Psychopharmacol. 2008;23:13–20. doi: 10.1002/hup.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. [Accessed August 18, 2012]. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
- 6.Roth T, Krystal AD, Maguire Y, Singh N, Maytom M. Pharmacokinetics of the sublingual zolpidem tartrate 3.5 mg lozenge compared to the oral zolpidem tartrate 10 mg tablet. Sleep. 2008;31:A235. [Google Scholar]
- 7.Roth T, Hull SG, Lankford DA, Rosenberg R, Scharf MB. Low-dose sublingual zolpidem tartrate is associated with dose-related improvement in sleep onset and duration in insomnia characterized by middle-of-the-night (MOTN) awakenings. Sleep. 2008;31:1277–84. [PMC free article] [PubMed] [Google Scholar]
- 8.Washington, DC: American Psychiatric Association; 2000. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. [Google Scholar]
- 9.Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto U. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. Br Med J. 2005;19:1169–76. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34:1433–42. doi: 10.5665/SLEEP.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh JK, Fry J, Erwin CW, Scharf M, Roth T, Vogel GW. Efficacy and tolerability of 14-day administration of zaleplon 5 mg and 10 mg for the treatment of primary insomnia. Clin Drug Invest. 1998;16:347–54. [Google Scholar]
- 12.Roth T, Zammit GK, Scharf MB, Farber R. Efficacy and safety of as-needed, post bedtime dosing with indiplon in insomnia patients with chronic difficulty maintaining sleep. Sleep. 2007;30:1731–8. doi: 10.1093/sleep/30.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]