Abstract
More than 70% of adolescents report to have smoked a cigarette at least once. At the adolescent stage the brain has not completed its maturation. The prefrontal cortex (PFC), the brain area responsible for executive functions and attention performance, is one of the last brain areas to mature and is still developing during adolescence. Smoking during adolescence increases the risk of developing psychiatric disorders and cognitive impairment in later life. In addition, adolescent smokers suffer from attention deficits, which aggravate with the years of smoking. Recent studies in rodents reveal the molecular changes induced by adolescent nicotine exposure that alter the functioning of synapses in the PFC and that underlie the lasting effects on cognitive function. Here we provide an overview of these recent findings.
Nicotine exposure during adolescence alters acetylcholine and glutamate receptor signaling in the prefrontal cortex, one of the last brain areas to mature. This may help explain altered cognitive function and attention performance in adolescents who smoke.
Adolescence is a truly revolutionary time period in anyone’s life, the age of explosive development of both emotional and cognitive sides of the mind. This is the age when passions ignite, when creativity is at its peak, bold and original ideas shake old theories, friendships and first loves are found, and important breakthroughs are made. But adolescence also has a dark side. The uncontrollable emotions create a risk zone for behavioral problems, psychopathology, and addiction. To quote John Ciardi: “You don’t have to suffer to be a poet. Adolescence is enough suffering for anyone.” Indeed, adolescence also marks a period of increase in the number of suicides, accidents, homicides, mood disorders, unwanted pregnancies, anorexia, bulimia, and substance abuse, such as tobacco smoking (Resnick et al. 1997; Ozer et al. 2004).
What makes adolescence such a painful period some people are happy to survive? The answer may lie in adolescent brain development. Brain development continues throughout adolescence, although the speed and timing of maturation varies for different brain areas (Gogtay et al. 2004). Subcortical limbic structures important for emotional processing, such as hypothalamus, midbrain dopamine areas, nucleus accumbens, dorsal and ventral striatum, and amygdala, experience a major developmental boost around the onset of puberty (Sowell et al. 2003; Casey et al. 2005). Their maturation is important for social and sexual behaviors and is triggered by pubertal hormones. In contrast, development of frontal cortical areas of the brain, responsible for cognitive control over behavior, depends on age and experience and continues throughout adolescence and into adulthood (Sowell et al. 2003; Giedd 2004). Thus, during adolescence emotional drive has already become very strong, whereas cognitive self-control and adult decision-making strategies still are developing. Thereby, brain development may be responsible for characteristic adolescent traits—uncontrollable mood swings, impulsivity, risk taking, and peer-directed social interactions (Orr and Ingersoll 1995; Spear 2000; Galvan et al. 2007). Although indispensable for transition from child to independent status of adult, these traits can backfire and cause damage. Indeed, risk-taking behavior, so typical for adolescents, is associated with high rates of mortality and morbidity among young people (Grunbaum et al. 2004).
The impulsive, peer-influenced nature of adolescent choices leads to another important health risk—experimenting with drugs of abuse. Since nicotine is one of the most socially accepted drugs in our society, the first choice usually falls on tobacco smoking. According to a recent study conducted in 41 countries in Europe and North America, 19% of 15-year-olds smoke at least once a week and 30% report experimenting with cigarettes before the age of 14 (Currie et al. 2008). Serious health risks of smoking are well known: Smoking leads to millions of premature deaths worldwide and tobacco smoking has been marked as an epidemic disease (Peto et al. 1999). Nicotine is also a psychoactive and addictive substance that directly acts on brain areas involved in emotional and cognitive processing. Early exposure to nicotine during the transition from child to adult may be harmful, since it may derange the normal course of brain maturation and have lasting consequences for cognitive ability, mental health, and even personality (Brown et al. 1996; Choi et al. 1997; Richards et al. 2003; Brook et al. 2004; Deas 2006). In this review, we will highlight recent findings that start to uncover causal relations between nicotine exposure during adolescence and cognitive deficits in later life, pinpointing the underlying functional synaptic adaptations in prefrontal networks.
SENSITIVITY TO NICOTINE OF THE ADOLESCENT BRAIN
Comparing smoking behavior of adolescents to that of adults may point to an enhanced sensitivity of the adolescent brain to addictive properties of nicotine. Adolescents report symptoms of dependence even at low levels of cigarette consumption (Colby et al. 2000; Kandel and Chen 2000). The most susceptible youth lose autonomy over tobacco intake already within 1 or 2 days of first inhaling from a cigarette. Among adolescents the appearance of tobacco withdrawal symptoms and failed attempts to stop smoking can precede daily smoking dependence and appear even before consumption reaches two cigarettes per day (DiFranza et al. 2007).
The difference in sensitivity to nicotine between adolescents and adults is also reported for laboratory animals (Slotkin 2002; Adriani et al. 2003). Rats first exposed to nicotine during adolescence self-administer more nicotine than rats exposed in adulthood and these differences in self-administration at first exposure persist into later age (Levin et al. 2003). Similarly, much lower doses of nicotine or a single injection are sufficient to establish conditioned place preference in adolescent rats, but not in adult animals (Vastola et al. 2002; Belluzzi et al. 2004; Brielmaier et al. 2007). Thus, paradigms for both self-administration and conditioned place preference in rats suggest that adolescence may be a developmental stage of particular vulnerability to the effects of nicotine exposure.
The vulnerability to rewarding effects of nicotine during adolescence may be explained by adolescent brain development. Structural and functional MRI data show earlier maturation of reward systems and much slower development of prefrontal cognitive control (Spear 2000; Chambers et al. 2003; Casey et al. 2005; Ernst et al. 2005; Ernst and Fudge 2009). Compared with adults, adolescents are generally more motivated by rewards, are less averse to risks, and are more easily influenced by peers (Spear 2000; Steinberg 2005; Galvan et al. 2006). The same applies to estimation of health risks of smoking—adolescents have a more optimistic attitude regarding their smoking behavior than adults, believing that they “could smoke for a few years and then quit” if they wished (Arnett 2000). Lack of mature cognitive control in adolescents makes them also more susceptible to social pressure. The smoking behavior of parents, siblings, and friends leads to a higher risk of smoking among adolescents and this social influence decreases with age (Vink et al. 2003). Adolescents with ADHD symptoms, whose behavior is even more characterized by impulsive and risk-taking choices, are more likely to experiment with smoking and to become regular tobacco users (Tercyak et al. 2002; McClernon et al. 2008). Importantly, nicotine may also lead to higher levels of dependence by exerting neurotoxic effects in the prefrontal cortex (PFC) interfering with adolescent cognitive development, executive functioning, and inhibitory control. These effects are particularly evident under stressful or emotionally intense states and are most pronounced when smoking begins during early adolescence (DeBry and Tiffany 2008).
Taken together, most likely owing to its ongoing development, the adolescent brain is more vulnerable to the effects of nicotine than the adult brain. Adolescents progress faster to nicotine dependence than adults, find nicotine more rewarding, underestimate the risks of smoking, and are more influenced by smoking behavior in their social milieu. This may explain why one of five adolescents smokes regularly and up to 70% of adolescents have experimented with smoking (Currie et al. 2008; Sidransky 2010). Because nicotine acts directly on the pathways involved in cognitive control, development of the PFC during adolescence may be affected by nicotine exposure. What are the acute consequences of nicotine exposure for neuronal circuits in the PFC of the adolescent brain?
IMMEDIATE EFFECTS OF NICOTINE ON THE ADOLESCENT PREFRONTAL CORTICAL NETWORK
Once nicotine has entered the body, it is distributed quickly through the bloodstream and crosses the blood–brain barrier reaching the brain within 10–20 sec after inhalation (Le Houezec 2003). Once in the brain, it binds to its target, the nicotinic acetylcholine receptors (nAChR), which take part in cholinergic signaling in the PFC. Twelve genes have been identified encoding neuronal nicotinic receptors (for a review, see Le Novere et al. 2002; Millar and Gotti 2009). In the central nervous system nine α-subunits (α2–α10) and three β-type subunits (β2–β4) are expressed. These subunits assemble in different stoichiometries to form pentameric channels, and subunit compositions of nAChRs vary depending on the brain region (for a review, see Grady et al. 2002; Le Novere et al. 2002; McGehee 2002; Alkondon and Albuquerque 2004; Wonnacott et al. 2005; Mineur and Picciotto 2008; Millar and Gotti 2009). Nicotinic AChRs are cation selective channels that permit the flow of Na+, K+, and Ca2+ across the membrane, which leads to depolarizing currents and activate neurons (McGehee and Role 1995; Millar and Gotti 2009).
In the PFC, nAChR expression is found across all layers (Gioanni et al. 1999; Poorthuis et al. 2012). nAChRs can alter pyramidal neuron activity by enhancing glutamatergic inputs or by activating postsynaptic receptors directly (Poorthuis et al. 2009). Hippocampal pyramidal neurons express functional α7 nAChR (Ji et al. 2001). In motor cortex, somatosensory cortex, and visual cortex layers II–III and layer V pyramidal neurons do not contain nAChRs (Nicoll et al. 1996; Gil et al. 1997; Xiang et al. 1998; Porter et al. 1999; Gulledge et al. 2007). We find that PFC layers II–III pyramidal cells also do not contain nAChRs, and also glutamatergic inputs to these pyramidal neurons are not modulated by nAChRs. Hence, nAChRs do not augment the output of superficial pyramidal neurons in the PFC.
In contrast, in layer V pyramidal neurons, activation of presynaptic β2* nAChRs on glutamatergic inputs from the thalamus strongly enhances activity of these neurons (Gioanni et al. 1999; Lambe et al. 2003; Couey et al. 2007; Poorthuis et al. 2012). These presynaptic mechanisms are specific to layer V, as they are absent in layers II–III and moderate in layer VI. This may suggest that nAChR-mediated modulation of thalamic inputs to the PFC is specifically targeting layer V pyramidal neurons, which project to the striatum and hypothalamus (Gabbott et al. 2005). Nicotinic enhancement of thalamic inputs to the cortex also plays a role in primary sensory areas, where it enhances sensory representation in the cortical target structure (Penschuck et al. 2002; Disney et al. 2007; Kawai et al. 2007). In addition to presynaptic β2* nAChRs that can augment its activity, layer V pyramidal neurons also contain postsynaptic α7 nAChRs. In contrast to layer V, excitatory glutamatergic inputs to layer VI pyramidal neurons were mildly modulated by nAChRs. These neurons are modulated by β2* nAChRs that are responsible for the strong activation of the layer VI neuronal population (Kassam et al. 2008; Poorthuis et al. 2012). Layer VI pyramidal neurons in entorhinal cortex also have been reported to be modulated by non-α7 nAChRs, most likely containing β2 subunits (Tu et al. 2009).
In addition to direct activation of PFC pyramidal neurons by nAChRs, PFC GABAergic interneurons are also directly activated by nAChR stimulation. Interneurons form a highly diverse group of cells with distinct roles in cortical computation (Kawaguchi 1993; Markram et al. 2004). Fast-spiking cells target the perisomatic region of pyramidal neurons (Kawaguchi and Kubota 1997; Kawaguchi and Kondo 2002) and are therefore thought to be involved in regulating the activity window of pyramidal neurons. In somatosensory areas fast-spiking cells regulate feedforward inhibition of incoming thalamic inputs (Sun et al. 2006). Feedforward inhibition in the PFC plays an important role in the integration of hippocampal inputs, which enter the PFC through superficial layers (Jay and Witter 1991; Tierney et al. 2004). Fast-spiking cells in PFC layers II–III contain α7 nAChRs, as do about half of the fast-spiking cells in layer V (Poorthuis et al. 2012). nAChR activation on fast-spiking interneurons in PFC layer II/III may alter processing of hippocampal inputs.
Somatostatin-positive cells target distal dendritic regions (Kawaguchi and Kondo 2002; Silberberg and Markram 2007) and can mediate disynaptic inhibition between pyramidal neurons (Kapfer et al. 2007; Silberberg and Markram 2007). Regular-spiking and somatostatin-positive cells in PFC layers II–II and V are positive for nAChRs, suggesting that nAChRs play an important role in modulating feedback inhibition among pyramidal neurons in these layers (Poorthuis et al. 2012).
Increased inhibition through activation of nAChRs expressed by interneurons has been found in many different brain regions (Jones and Yakel 1997; Xiang et al. 1998; McQuiston and Madison 1999; Alkondon et al. 2000; Ji and Dani 2000; Mansvelder et al. 2002; Gulledge et al. 2007). When activated by nAChR stimulation, interneurons can alter activity and plasticity in pyramidal neurons (Xiang et al. 1998; Alkondon et al. 2000; Ji and Dani 2000; Ji et al. 2001; Couey et al. 2007). Increased inhibition can lead to blockade of long-term potentiation (LTP) induction in the hippocampus (Ji et al. 2001) and increase in the threshold for induction of spike-timing-dependent plasticity (STDP) (Couey et al. 2007). Similar mechanisms may play a role across PFC layers because we find that non-fast-spiking cells in all layers express nAChRs.
UP-REGULATION OF nAChRs AND SYNAPTIC mGluRs IN PREFRONTAL CORTEX BY NICOTINE EXPOSURE DURING ADOLESCENCE
A current hypothesis explaining why adolescents are more vulnerable to nicotine addiction is that nicotine has greater positive effects on adolescents than adults, whereas the negative effects associated with nicotine, such as withdrawal, are smaller in adolescents (O’Dell 2009). Nicotine administration during, but not following, adolescence has long-lasting effects on cognitive, addictive, and emotional behavior in rats (Adriani et al. 2003; Iniguez et al. 2008; Counotte et al. 2009, 2011). Furthermore, adolescent animals are more sensitive to nicotine-conditioned place preference than adults and show this at lower nicotine doses (Vastola et al. 2002; Belluzzi et al. 2004; Shram et al. 2006; Brielmaier et al. 2007; Kota et al. 2009). Adolescent nicotine exposure leads to acute and longer-lasting changes in nAChR binding (Abreu-Villaca et al. 2003; Doura et al. 2008) and function (Kota et al. 2009) in brain regions such as cortex and striatum. We recently found that the adolescent rodent brain is more sensitive to nicotinic receptor up-regulation in the medial PFC (mPFC) than adults (Counotte et al. 2012). Naïve rats show an age-related decrease in 3H-epibatidine labeled high-affinity nicotinic receptors in the mPFC, but not in occipital cortex. Adolescent, but not adult nicotine exposure increases 3H-Epi binding of mPFC receptors on the first day of abstinence following 10 days of nicotine injections. This is paralleled by an mPFC-specific increase in expression of nAChRs containing α4 and β2 (but not α5) subunits. The increased expression of high-affinity nAChRs in adolescents is accompanied by an increase in nicotine-stimulated GABAergic synaptic transmission in the mPFC (Counotte et al. 2012).
One of the first and most common cellular adaptations following chronic nicotine exposure is the up-regulation of nicotinic receptor levels (Dani and Bertrand 2007). Especially α4β2 type of nAChRs appears to be selectively up-regulated via posttranslational mechanisms (Miwa et al. 2011). The up-regulation of α4β2 nAChRs by chronic nicotine treatment has been replicated many times in numerous systems—transfected cell lines, neurons in culture, brain slices, and smokers’ brains (Wonnacott 1990; Fu et al. 2009; Lester et al. 2009; Marks et al. 2011; Miwa et al. 2011). Up-regulation is not accompanied by an increase in nAChR subunit mRNA (Marks et al. 1992); instead it leads to increased nAChR protein levels resulting from increased assembly and/or decreased degradation of nAChRs (Marks et al. 2011). Nicotine appears to act intracellularly as a selective pharmacological chaperone of acetylcholine receptor (Lester et al. 2009). It stabilizes nAChRs during assembly and maturation and this stabilization is most pronounced for the highest-affinity nAChR containing α4β2 subunits. Indeed, we found that specifically high-affinity nicotinic receptors containing the α4 and β2 subunits were up-regulated in the adolescent PFC shortly following nicotine exposure. This up-regulation was paralleled by a functional elevation in nicotine-stimulated GABAergic transmission, indicating that functional surface nAChRs are up-regulated as well (Counotte et al. 2012).
Given that pyramidal neurons and excitatory projections in layers II/III of the PFC do not express nAChRs (Poorthuis et al. 2012) the functional consequence of α4β2 nAChR up-regulation on interneurons in layers II/III will be an increased inhibitory transmission in superficial PFC layers. In the deep layers of the PFC, β2 subunits are expressed by both interneurons, as well as layer VI pyramidal neurons and excitatory inputs to layer V pyramidal neurons. An up-regulation of these receptors will lead to a combined increase in activation of pyramidal neurons and interneurons. It follows that during chronic nicotine exposure of the adolescent PFC, the pattern of activity in the prefrontal network may gradually shift toward activation of excitatory neurons in deep layers in the context of increased overall inhibition. This may affect plasticity and refinement of cortical connections (Couey et al. 2007), and because β2-containing nAChRs in the medial PFC control attention performance (Guillem et al. 2011), it may have functional implications for maturation and function of the prefrontal network.
In addition to an up-regulation of nAChRs, we recently found in a large-scale iTRAQ-based proteomics screen of synaptic protein levels in the PFC that metabotropic glutamatergic receptors type 2 (mGluR2) are significantly up-regulated during adolescent nicotine exposure (Fig. 1) (Counotte et al. 2011). These receptors are located presynaptically on glutamatergic synapses and their activation reduces the probability of glutamate release. Thereby, an up-regulation of mGluR2 receptor levels diminishes activity of excitatory glutamatergic synapses in the PFC. Thus, increases in functional nAChR on inhibitory neurons and increased nicotine-stimulated excitation in deep layers of the PFC may be counteracted by reduced excitatory synaptic activity mediated by increased mGluR2 activity.
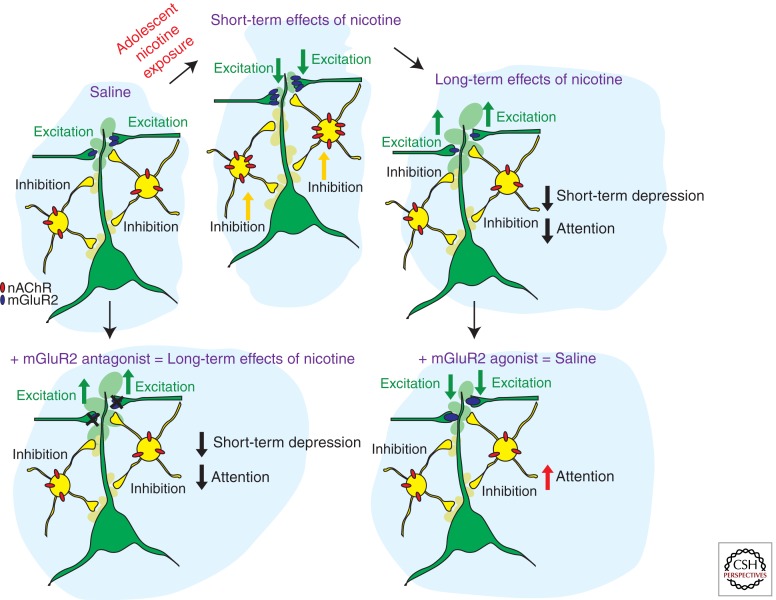
Figure 1.
Schematic representation of the short-term and long-term adaptations in prefrontal cortex (PFC) neuronal networks caused by nicotine exposure during adolescence. The upper panels show the sequence of adaptations in nAChR and mGluR2 protein levels and the resulting changes in inhibition and excitation and attention behavior from control conditions (saline) to nicotine exposure during adolescence (short-term effects of nicotine) and 5 weeks following nicotine exposure (long-term effects of nicotine). The lower panels show the effects of mGluR2 agonists and antagonists in saline and nicotine-exposed animals. Applying mGluR2 antagonists to the adult medial PFC reduces mGluR2 function and short-term depression of glutamatergic synapses and reduces attention performance of the animal. Providing mGluR2 agonists to the medial PFC of adult rats that were exposed to nicotine during adolescence increases mGluR2 function at glutamatergic synapses and improves attention performance.
LONG-TERM CONSEQUENCES OF NICOTINE EXPOSURE DURING ADOLESCENCE
Several studies indicate that smoking during adolescence is associated with disturbances in working memory and attention as well as reduced PFC activation (Jacobsen et al. 2005, 2007; Musso et al. 2007). Although these studies focus on the short-term effects of adolescent smoking on cognition, they show that impaired cognitive processing in PFC already takes place during this age. Importantly, the history of smoking duration in years is correlated with the extent of diminished PFC activity, indicating a progression of deleterious effects of nicotine which may last into later life (Musso et al. 2007). Smoking is a prospective risk factor for impaired cognitive function in later life; heavy smoking predicts incident cognitive impairment and decline (Cervilla et al. 2000; Richards et al. 2003) and middle-aged smokers have a lower psychomotor speed and cognitive flexibility compared to never smokers (Kalmijn et al. 2002). Several studies have shown that adolescent tobacco use is associated with later risk of developing mental and behavioral problems such as major depressive disorder, agoraphobia, panic disorder, addiction to other substances, antisocial personality disorder, or academic problems (Brown et al. 1996; Brook et al. 1998, 2002; Johnson et al. 2000; McGee et al. 2000; Ellickson et al. 2001).
Animal studies have shown that exposure during adolescence induces stronger changes in gene expression in the PFC than during other periods of development and adulthood (Schochet et al. 2005, 2008; Polesskaya et al. 2007). The adolescent PFC shows maximal nicotine response in gene regulation involved in vesicle release, signal transduction, cytoskeleton dynamics, and transcription, suggesting the role of chronic nicotine exposure in initiating long-term structural and functional adaptations (Polesskaya et al. 2007). The activity of specific early response genes (arc and c-fos) used as a marker for the functional activation of neurons was found to be elevated in adolescent PFC after nicotine exposure (Leslie et al. 2004; Schochet et al. 2005).
The expression of key molecules involved in plasticity is also altered in the PFC by adolescent nicotine exposure. Acute nicotine induces increases in the expression of the dendritically targeted dendrin mRNA in PFC of adolescent but not adult animals. Dendrin is an important component of cytoskeletal modifications at the synapse and therefore can lead to unique plasticity changes in the adolescent PFC (Schochet et al. 2008). Lasting synaptic adaptations involve activation of intracellular signaling pathway and such enzymes as extracellular regulated protein kinase (ERK) and cAMP response element binding protein (CREB). Specifically in the PFC, increases in phosphorylation of both these enzymes were found after repeated nicotine exposure (Brunzell et al. 2003). Also changes in macromolecular constituents indicative of cell loss (reduced DNA) and altered cell size (protein/DNA ratio) can be seen in cortical regions of rodents after adolescent nicotine treatment (Trauth et al. 2000).
Although these findings only describe direct changes after nicotine exposure, altered expression of genes involved in neuroplasticity can lead to structural changes in PFC neurons that last into adulthood. Indeed, repeated nicotine exposure also changes the structure of neurons in medial PFC: it increases both dendritic length and spine density (Brown and Kolb 2001). Long-term changes were observed in dendritic morphology of specific subpopulations of pyramidal neurons and these structural changes depended on the age of drug exposure (Bergstrom et al. 2008).
Also on the behavioral level, nicotine during adolescence leads to persisting deficits. Adolescent, but not adult, nicotine treatment reduces accuracy of correct stimulus detection in a visuospatial attentional task, with an increase in premature and time-out responding. This suggests impaired attention and lack of impulsive control, which is part of normal adolescent maturation (Counotte et al. 2009). Similar nicotine-induced deficits have been found in a serial pattern learning paradigm (Fountain et al. 2008).
Taken together, these studies in rodents show that nicotine exposure during adolescence induces significant changes in gene expression and neuronal morphology in PFC. Thus, nicotine does not only change cholinergic signaling by altering nicotinic receptor levels in the adolescent PFC, but can also lead to secondary adaptations involving structural and functional changes in cognition. What are the changes that underlie the changes in cognitive performance?
LASTING SYNAPTIC ADAPTATIONS IN THE PFC THAT AFFECT COGNITIVE PERFORMANCE IN LATER LIFE
In adult rodents that were exposed to nicotine during adolescence only a handful of proteins show long-term adaptations following adolescent nicotine exposure that persisted into later life. Nicotinic AChR levels in the PFC returned to baseline 5 weeks following adolescent nicotine exposure (Counotte et al. 2012). In contrast, mGluR2 levels show a strong down-regulation at this time (Counotte et al. 2011). Reduced mGluR2 function in medial PFC synapses resulted in impaired attention performance. Stimulating mGluR2s with specific agonists improved attention performance in animals that were exposed to nicotine during adolescence (Counotte et al. 2011). Interestingly, the association between changes in mGluR2 signaling and nicotine exposure is not limited to the PFC. Also in other brain areas involved in reward processing such as ventral tegmental area (VTA) and the nucleus accumbens (NAcc) lasting adaptations in mGluR2 function follow nicotine exposure and were found to affect rewarding properties of nicotine (Helton et al. 1997; Kenny et al. 2003; Kenny and Markou 2004; Liechti et al. 2007). In these brain areas, activation of mGlu2/3 receptors decreases nicotine self-administration (Liechti et al. 2007), and they play an important role in the development of drug dependence and the expression of the negative affective state observed during withdrawal (Kenny and Markou 2004). However, the role of group II mGlu receptors in withdrawal appears complex and most likely depends on changes in multiple brain areas.
Although the sequence of events linking mGluR2 adaptations to nAChR activation is unknown, it seems that the reasons for its up- and down-regulation pattern after adolescent nicotine exposure may lie in its function. Metabotropic GluR2 receptors are located on presynaptic glutamatergic terminals where they are activated by glutamate spillover to inhibit glutamate release (Mateo and Porter 2007). It was shown that activation of mGluR2s can also regulate release of other neurotransmitters: it can inhibit GABA release via a presynaptic mechanism (Bradley et al. 2000; Pilc et al. 2008). Given the inhibitory role of mGluR2 in neurotransmitter release, its function seems to counteract that of nAChR, which enhances both excitatory and inhibitory synaptic transmission (Lambe et al. 2003; Couey et al. 2007; Poorthuis et al. 2012). The short-term effects of adolescent nicotine exposure most likely involve enhanced levels of inhibition in prefrontal network. Accordingly, we found an initial and transient up-regulation of inhibitory mGluR2 receptor directly following nicotine exposure during adolescence (Counotte et al. 2011), which would contribute to the same effect.
In general, factors that lead to enhanced excitation can cause alterations in mGluR2 transmission and cause cognitive deficits (Melendez et al. 2004; Pozzi et al. 2011). Enhanced glutamate release in PFC was found to be associated with attention deficit and loss of impulse control (Pozzi et al. 2011). MGluR2 agonists are effective in improving cognitive deficits if enhanced glutamate release is caused by NMDA receptor antagonists (Pozzi et al. 2011). Furthermore, the important role of prefrontal mGluR2 signaling in cognition is stressed by its link to brain disorders such as depression and schizophrenia. Activation of this receptor has even been proposed as a novel treatment approach for these disorders (Gupta et al. 2005; Palucha and Pilc 2005; Pilc et al. 2008; Conn et al. 2009). Thus, mGluR2 signaling seems to be a good candidate for shaping cognitive behavior and its impairment leads to disturbances in cognitive function.
At the level of synapse function, alterations in mGluR2 levels affect short-term synaptic plasticity in later life. Short-term depression (STD) is reduced in adult animals as a result of nicotine exposure during adolescence (Counotte et al. 2011). In control animals, blocking mGluR2 signaling with mGluR2 antagonists also results in reduced STD. Reduced mGluR2 signaling after nicotine exposure has a similar effect on STD as mGluR2 block by antagonist (Fig. 1). Thereby, mGluR2 may act as an inhibitory feedback mechanism in conditions of excessive excitation and high glutamate release, as occurs when a neuron fires a train of action potentials. Especially at high-frequency stimulation the effect of mGluR2 on STD was most prominent at excitatory synapses on layer V pyramidal neurons in the PFC. The lasting reduction of mGluR2 levels and function after adolescent nicotine exposure leads to reduced inhibitory feedback on pyramidal cells and reduces the regulatory role of this receptor in short-term plasticity. Most likely, activation of mGluR2s affects presynaptic calcium channel function as was found in the calyx of Held, by direct electrophysiological recordings from presynaptic terminals (Takahashi et al. 1996). Agonists of mGluRs suppressed high voltage-activated P/Q-type calcium channels in the presynaptic terminal, thereby inhibiting transmitter release (Takahashi et al. 1996). Because presynaptic Ca2+ dynamics play a key role in short-term plasticity (Zucker and Regehr 2002), decrease in Ca2+ current may explain mGluR-dependent modulation of STD.
STD may equip the synapse with low-pass filtering properties, by which the synapse will pass on the first of stimulus in a train of stimuli unaltered, whereas the rest are attenuated. In this manner it shapes the information transfer by synaptic networks and gives rise to sensory and behavioral phenomena (Zucker 1989). For example, in the somatosensory cortex of rat, in vivo whole-cell recordings in cortical neurons during whisker deflection directly showed that synaptic depression of thalamic input to the cortex contributes to rapid adaptation of sensory responses (Chung et al. 2002). Selective attention, the ability of an organism to filter out relevant information in the face of distractors, can build on just such a synaptic process. Layer V pyramidal neurons in PFC handle diverse incoming information from mediodorsal thalamus and from local neurons, and these connections are important in mediating executive functions such as, for example, working memory (Floresco et al. 1999). STD on this level may represent a higher level of sensory adaptation that can be expressed as decreased levels of attention and responsiveness. Reduced short-term plasticity after nicotine exposure compromises the ability of prefrontal neurons to efficiently filter out irrelevant information.
CONCLUDING REMARKS
The prefrontal cortex, the brain area responsible for executive functions and attention performance, is one of the last brain areas to mature and is still in the process of developing during adolescence. This places the adolescent brain in a vulnerable state of imbalance, susceptible to the influence of psychoactive substances such as nicotine. In prefrontal networks nicotine modulates information processing on multiple levels by activating and desensitizing nicotine receptors on different cell types and in this way affects cognition. The adolescent brain is particularly sensitive to the effects of nicotine. Studies in human subjects indicate that smoking during adolescence increases the risk of developing psychiatric disorders and cognitive impairment in later life. In addition, adolescent smokers suffer from attention deficits, which aggravate with the years of smoking.
From studies in the rodent brain it is becoming clear that on the short-term, adolescent, but not adult, nicotine exposure increases the expression of nAChRs containing α4 and β2 subunits in the medial PFC, which leads to an increase in nicotine-induced GABAergic synaptic transmission. In addition, mGluR2 levels on presynaptic glutamatergic terminals in the PFC are increased, causing a reduction in glutamatergic synapse strength (Fig. 1). Changes in nAChR levels are reversible: In the adult rodent brain, weeks after nicotine levels have subsided, nAChR levels in the PFC return to baseline levels. In contrast, at this stage, mGluR2 levels have reduced significantly below baseline levels, thereby altering mGluR2 signaling during short-term plasticity and hampering attention performance. This reduction in mGluR2 signaling underlies the reduced attention performance observed in animals after nicotine exposure during adolescence (Counotte et al. 2011).
New questions and opportunities arise from these recent findings. The long-term adaptations involving mGluR2s can have profound implications for network functioning and affect more complex levels of information processing. A consequence of increased glutamatergic transmission in adult PFC caused by reduced mGluR2 function could be the impairment of other types of plasticity than STD, such as mechanisms of long-term plasticity. Changes in inhibitory tonus and excitatory transmission following adolescent nicotine exposure may have different short- and long-term effects on long-term plasticity.
Another interesting question would be whether mGluR2 signaling is involved in a broader spectrum of attention impairments with different etiology. If change in mGluR2 signaling is a common underlying mechanism for attention malfunction it would make it a suitable pharmacological target for therapy.
ACKNOWLEDGMENTS
H.D.M. received funding from the FP7 program SynSys, the European Research Council (ERC), NWO (917.76.360), the NeuroBasic consortium, VU University board, and Neuroscience Campus Amsterdam (NCA).
Footnotes
Editors: R. Christopher Pierce and Paul J. Kenny
Additional Perspectives on Addiction available at www.perspectivesinmedicine.org
REFERENCES
- Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA 2003. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: Critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology 28: 1935–1949 [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV 2003. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci 23: 4712–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX 2004. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res 145: 109–120 [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX 2000. Nicotinic receptor activation in human cerebral cortical interneurons: A mechanism for inhibition and disinhibition of neuronal networks. J Neurosci 20: 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ 2000. Optimistic bias in adolescent and adult smokers and nonsmokers. Addict Behav 25: 625–632 [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM 2004. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 174: 389–395 [DOI] [PubMed] [Google Scholar]
- Bergstrom HC, McDonald CG, French HT, Smith RF 2008. Continuous nicotine administration produces selective, age-dependent structural alteration of pyramidal neurons from prelimbic cortex. Synapse 62: 31–39 [DOI] [PubMed] [Google Scholar]
- Bradley SR, Marino MJ, Wittmann M, Rouse ST, Awad H, Levey AI, Conn PJ 2000. Activation of group II metabotropic glutamate receptors inhibits synaptic excitation of the substantia Nigra pars reticulata. J Neurosci 20: 3085–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF 2007. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol Teratol 29: 74–80 [DOI] [PubMed] [Google Scholar]
- Brook JS, Cohen P, Brook DW 1998. Longitudinal study of co-occurring psychiatric disorders and substance use. J Am Acad Child Adolesc Psychiatry 37: 322–330 [DOI] [PubMed] [Google Scholar]
- Brook DW, Brook JS, Zhang C, Cohen P, Whiteman M 2002. Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Arch Gen Psychiatry 59: 1039–1044 [DOI] [PubMed] [Google Scholar]
- Brook JS, Schuster E, Zhang C 2004. Cigarette smoking and depressive symptoms: A longitudinal study of adolescents and young adults. Psychol Rep 95: 159–166 [DOI] [PubMed] [Google Scholar]
- Brown RW, Kolb B 2001. Nicotine sensitization increases dendritic length and spine density in the nucleus accumbens and cingulate cortex. Brain Res 899: 94–100 [DOI] [PubMed] [Google Scholar]
- Brown RA, Lewinsohn PM, Seeley JR, Wagner EF 1996. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. J Am Acad Child Psychiatry 35: 1602–1610 [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Russell DS, Picciotto MR 2003. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem 84: 1431–1441 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S 2005. Imaging the developing brain: What have we learned about cognitive development? Trends Cogn Sci 9: 104–110 [DOI] [PubMed] [Google Scholar]
- Cervilla JA, Prince M, Mann A 2000. Smoking, drinking, and incident cognitive impairment: A cohort community based study included in the Gospel Oak project. J Neurol Neurosurg Psychiatry 68: 622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN 2003. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am J Psychiatry 160: 1041–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Patten CA, Gillin JC, Kaplan RM, Pierce JP 1997. Cigarette smoking predicts development of depressive symptoms among U.S. adolescents. Ann Behav Med 19: 42–50 [DOI] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB 2002. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron 34: 437–446 [DOI] [PubMed] [Google Scholar]
- Colby SM, Tiffany ST, Shiffman S, Niaura RS 2000. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Depend 59: S83–S95 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK 2009. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci 30: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couey JJ, Meredith RM, Spijker S, Poorthuis R, Smit AB, Brussaard AB, Mansvelder HD 2007. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron 54: 73–87 [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T 2009. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology 34: 299–306 [DOI] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Schoffelmeer ANM, Smit AB, Mansvelder HD, Pattij T, et al. 2011. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat Neurosci 14: 417–419 [DOI] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Moretti M, Smoluch MT, Irth H, Clementi F, Schoffelmeer ANM, Mansvelder HD, Smit AB, Gotti C, et al. 2012. Adolescent nicotine exposure transiently increases high-affinity nicotinic receptors and modulates inhibitory synaptic transmission in rat medial prefrontal cortex. FASEB J 10.1096/fj.11-198994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie C, Gabhainn SN, Godeau E, Roberts C, Smith R, Currie D, Pickett W, Richter M, Morgan A, Barnekow V 2008. Inequalities in young people’s health: HBSC international report from the 2005/06 Survey. In Health policy for children and adolescents, 5th ed. (ed. Currie C). WHO Regional Office for Europe, Copenhagen [Google Scholar]
- Dani JA, Bertrand D 2007. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47: 699–729 [DOI] [PubMed] [Google Scholar]
- Deas D 2006. Adolescent substance abuse and psychiatric comorbidities. J Clin Psychiatry 67: 18–23 [PubMed] [Google Scholar]
- DeBry SC, Tiffany ST 2008. Tobacco-induced neurotoxicity of adolescent cognitive development (TINACD): A proposed model for the development of impulsivity in nicotine dependence. Nicotine Tob Res 10: 11–25 [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, O’Loughlin J, Pbert L, Ockene JK, McNeill AD, Hazelton J, Friedman K, Dussault G, et al. 2007. Symptoms of tobacco dependence after brief intermittent use: The development and assessment of nicotine dependence in youth-2 study. Arch Pediat Adol Med 161: 704–710 [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ 2007. Gain modulation by nicotine in macaque v1. Neuron 56: 701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC 2008. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res 1215: 40–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellickson PL, Tucker JS, Klein DJ 2001. High-risk behaviors associated with early smoking: Results from a 5-year follow-up. J Adolesc Health 28: 465–473 [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL 2009. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev 33: 367–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS 2005. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage 25: 1279–1291 [DOI] [PubMed] [Google Scholar]
- Floresco SB, Braaksma DN, Phillips AG 1999. Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci 19: 11061–11071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD, Kelley BM, Willey AR, Nolley EP 2008. Adolescent exposure to nicotine impairs adult serial pattern learning in rats. Exp Brain Res 187: 651–656 [DOI] [PubMed] [Google Scholar]
- Fu XW, Lindstrom J, Spindel ER 2009. Nicotine activates and up-regulates nicotinic acetylcholine receptors in bronchial epithelial cells. Am J Resp Cell Mol Biol 41: 93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ 2005. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492: 145–177 [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ 2006. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci 26: 6885–6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ 2007. Risk-taking and the adolescent brain: Who is at risk? Dev Sci 10: F8–F14 [DOI] [PubMed] [Google Scholar]
- Giedd JN 2004. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci 1021: 77–85 [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y 1997. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron 19: 679–686 [DOI] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PB, Lepouse C, Thierry AM, Vidal C 1999. Nicotinic receptors in the rat prefrontal cortex: Increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. Eur J Neurosci 11: 18–30 [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF III, Herman DH, Clasen LS, Toga AW, et al. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci 101: 8174–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC 2002. Characterization of nicotinic agonist-induced [H-3] dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther 301: 651–660 [DOI] [PubMed] [Google Scholar]
- Grunbaum JA, Kann L, Kinchen S, Ross J, Hawkins J, Lowry R, Harris WA, McManus T, Chyen D, Collins J 2004. Youth risk behavior surveillance—United States, 2003. MMWR Surveill Summ 53: 1–96 [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, Spijker S, Mansvelder HD 2011. Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Science 333: 888–891 [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ 2007. Heterogeneity of phasic cholinergic signaling in neocortical neurons. J Neurophysiol 97: 2215–2229 [DOI] [PubMed] [Google Scholar]
- Gupta DS, McCullumsmith RE, Beneyto M, Haroutunian V, Davis KL, Meador-Woodruff JH 2005. Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse 57: 123–131 [DOI] [PubMed] [Google Scholar]
- Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ 1997. LY354740: A metabotropic glutamate receptor agonist which ameliorates symptoms of nicotine withdrawal in rats. Neuropharmacology 36: 1511–1516 [DOI] [PubMed] [Google Scholar]
- Iniguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Manojlovic Z, Bolanos-Guzman CA 2008. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology 34: 1609–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR 2005. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry 57: 56–66 [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR 2007. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl) 193: 557–566 [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP 1991. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 313: 574–586 [DOI] [PubMed] [Google Scholar]
- Ji D, Dani JA 2000. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol 83: 2682–2690 [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA 2001. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron 31: 131–141 [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS 2000. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA 284: 2348–2351 [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL 1997. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol 504: 603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ 2002. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. Am J Epidem 156: 936–944 [DOI] [PubMed] [Google Scholar]
- Kandel DB, Chen K 2000. Extent of smoking and nicotine dependence in the United States: 1991–1993. Nicotine Tob Res 2: 263–274 [DOI] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M 2007. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci 10: 743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassam SM, Herman PM, Goodfellow NM, Alves NC, Lambe EK 2008. Developmental excitation of corticothalamic neurons by nicotinic acetylcholine receptors. J Neurosci 28: 8756–8764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y 1993. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol 69: 416–431 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S 2002. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol 31: 277–287 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y 1997. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7: 476–486 [DOI] [PubMed] [Google Scholar]
- Kawai H, Lazar R, Metherate R 2007. Nicotinic control of axon excitability regulates thalamocortical transmission. Nat Neurosci 10: 1168–1175 [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A 2004. The ups and downs of addiction: Role of metabotropic glutamate receptors. Trends Pharmacol Sci 25: 265–272 [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A 2003. Group II metabotropic and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther 306: 1068–1076 [DOI] [PubMed] [Google Scholar]
- Kota D, Robinson SE, Imad Damaj M 2009. Enhanced nicotine reward in adulthood after exposure to nicotine during early adolescence in mice. Biochem Pharmacol 78: 873–879 [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK 2003. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology 28: 216–225 [DOI] [PubMed] [Google Scholar]
- Le Houezec J 2003. Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: A review. Int J Tuberc Lung Dis 7: 811–819 [PubMed] [Google Scholar]
- Le Novere N, Corringer PJ, Changeux JP 2002. The diversity of subunit composition in nAChRs: Evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol 53: 447–456 [DOI] [PubMed] [Google Scholar]
- Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzia JD 2004. Adolescent development of forebrain stimulant responsiveness: Insights from animal studies. Ann NY Acad Sci 1021: 148–159 [DOI] [PubMed] [Google Scholar]
- Lester HA, Xiao C, Srinivasan R, Son CD, Miwa J, Pantoja R, Banghart MR, Dougherty DA, Goate AM, Wang JC 2009. Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. AAPS J 11: 167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS 2003. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 169: 141–149 [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A 2007. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci 27: 9077–9085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS 2002. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33: 905–919 [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C 2004. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807 [DOI] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC 1992. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci 12: 2765–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, McClure-Begley TD, Whiteaker P, Salminen O, Brown RW, Cooper J, Collins AC, Lindstrom JM 2011. Increased nicotinic acetylcholine receptor protein underlies chronic nicotine-induced up-regulation of nicotinic agonist binding sites in mouse brain. J Pharmacol Exp Ther 337: 187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Z, Porter JT 2007. Group II metabotropic glutamate receptors inhibit glutamate release at thalamocortical synapses in the developing somatosensory cortex. Neuroscience 146: 1062–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Fuemmeler BF, Kollins SH, Kail ME, Ashley-Koch AE 2008. Interactions between genotype and retrospective ADHD symptoms predict lifetime smoking risk in a sample of young adults. Nicotine Tob Res 10: 117–127 [DOI] [PubMed] [Google Scholar]
- McGee R, Williams S, Poulton R, Moffitt T 2000. A longitudinal study of cannabis use and mental health from adolescence to early adulthood. Addiction (Abingdon, England) 95: 491–503 [DOI] [PubMed] [Google Scholar]
- McGehee DS 2002. Nicotinic receptors and hippocampal synaptic plasticity … it’s all in the timing. Trends Neurosci 25: 171. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW 1995. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol 57: 521–546 [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV 1999. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci 19: 2887–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Gregory ML, Bardo MT, Kalivas PW 2004. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology 29: 1980–1987 [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C 2009. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56: 237–246 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR 2008. Genetics of nicotinic acetylcholine receptors: Relevance to nicotine addiction. Biochem Pharmacol 75: 323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa JM, Freedman R, Lester HA 2011. Neural systems governed by nicotinic acetylcholine receptors: Emerging hypotheses. Neuron 70: 20–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso F, Bettermann F, Vucurevic G, Stoeter P, Konrad A, Winterer G 2007. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology (Berl) 191: 159–169 [DOI] [PubMed] [Google Scholar]
- Nicoll A, Kim HG, Connors BW 1996. Laminar origins of inhibitory synaptic inputs to pyramidal neurons of the rat neocortex. J Physiol 497: 109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE 2009. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology 56: 263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr DP, Ingersoll GM 1995. The contribution of level of cognitive complexity and pubertal timing to behavioral risk in young adolescents. Pediatrics 95: 528–533 [PubMed] [Google Scholar]
- Ozer EM, Adams SH, Gardner LR, Mailloux DE, Wibbelsman CJ, Irwin CE Jr 2004. Provider self-efficacy and the screening of adolescents for risky health behaviors. J Adolesc Health 35: 101–107 [DOI] [PubMed] [Google Scholar]
- Palucha A, Pilc A 2005. The involvement of glutamate in the pathophysiology of depression. Drug News Perspect 18: 262–268 [DOI] [PubMed] [Google Scholar]
- Penschuck S, Chen-Bee CH, Prakash N, Frostig RD 2002. In vivo modulation of a cortical functional sensory representation shortly after topical cholinergic agent application. J Comp Neurol 452: 38–50 [DOI] [PubMed] [Google Scholar]
- Peto R, Chen ZM, Boreham J 1999. Tobacco—The growing epidemic. Nat Med 5: 15–17 [DOI] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM 2008. Mood disorders: Regulation by metabotropic glutamate receptors. Biochem Pharmacol 75: 997–1006 [DOI] [PubMed] [Google Scholar]
- Polesskaya OO, Fryxell KJ, Merchant AD, Locklear LL, Ker KF, McDonald CG, Eppolito AK, Smith LN, Wheeler TL, Smith RF 2007. Nicotine causes age-dependent changes in gene expression in the adolescent female rat brain. Neurotoxicol Teratol 29: 126–140 [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Goriounova NA, Couey JJ, Mansvelder HD 2009. Nicotinic actions on neuronal networks for cognition: General principles and long-term consequences. Biochem Pharmacol 78: 668–676 [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Bloem B, Schak B, Wester J, de Kock CPJ, Mansvelder HD 2012. Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb Cortex 10.1093/cercor/bhr390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Tsuzuki K, Lambolez B, Rossier J, Audinat E 1999. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J Neurosci 19: 5228–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi L, Baviera M, Sacchetti G, Calcagno E, Balducci C, Invernizzi RW, Carli M 2011. Attention deficit induced by blockade of N-methyl D-aspartate receptors in the prefrontal cortex is associated with enhanced glutamate release and cAMP response element binding protein phosphorylation: Role of metabotropic glutamate receptors 2/3. Neuroscience 176: 336–348 [DOI] [PubMed] [Google Scholar]
- Resnick MD, Bearman PS, Blum RW, Bauman KE, Harris KM, Jones J, Tabor J, Beuhring T, Sieving RE, Shew M, et al. 1997. Protecting adolescents from harm. Findings from the National Longitudinal Study on Adolescent Health. JAMA 278: 823–832 [DOI] [PubMed] [Google Scholar]
- Richards M, Jarvis MJ, Thompson N, Wadsworth ME 2003. Cigarette smoking and cognitive decline in midlife: Evidence from a prospective birth cohort study. Am J Public Health 93: 994–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochet TL, Kelley AE, Landry CF 2005. Differential expression of arc mRNA and other plasticity-related genes induced by nicotine in adolescent rat forebrain. Neuroscience 135: 285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochet TL, Bremer QZ, Brownfield MS, Kelley AE, Landry CF 2008. The dendritically targeted protein Dendrin is induced by acute nicotine in cortical regions of adolescent rat brain. Eur J Neurosci 28: 1967–1979 [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD 2006. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 186: 201–208 [DOI] [PubMed] [Google Scholar]
- Sidransky MD 2010. How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease: A report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta: [PubMed] [Google Scholar]
- Silberberg G, Markram H 2007. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53: 735–746 [DOI] [PubMed] [Google Scholar]
- Slotkin TA 2002. Nicotine and the adolescent brain: Insights from an animal model. Neurotoxicol Teratol 24: 369–384 [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW 2003. Mapping cortical change across the human life span. Nat Neurosci 6: 309–315 [DOI] [PubMed] [Google Scholar]
- Spear LP 2000. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24: 417–463 [DOI] [PubMed] [Google Scholar]
- Steinberg L 2005. Cognitive and affective development in adolescence. Trends Cogn Sci 9: 69–74 [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA 2006. Barrel cortex microcircuits: Thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci 26: 1219–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K 1996. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science (New York, NY) 274: 594–597 [DOI] [PubMed] [Google Scholar]
- Tercyak KP, Lerman C, Audrain J 2002. Association of attention-deficit/hyperactivity disorder symptoms with levels of cigarette smoking in a community sample of adolescents. J Am Acad Child Adolesc Psychiatry 41: 799–805 [DOI] [PubMed] [Google Scholar]
- Tierney PL, Degenetais E, Thierry AM, Glowinski J, Gioanni Y 2004. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur J Neurosci 20: 514–524 [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA 2000. An animal model of adolescent nicotine exposure: Effects on gene expression and macromolecular constituents in rat brain regions. Brain Res 867: 29–39 [DOI] [PubMed] [Google Scholar]
- Tu B, Gu Z, Shen JX, Lamb PW, Yakel JL 2009. Characterization of a nicotine-sensitive neuronal population in rat entorhinal cortex. J Neurosci 29: 10436–10448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP 2002. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav 77: 107–114 [DOI] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, Boomsma DI 2003. The association of current smoking behavior with the smoking behavior of parents, siblings, friends and spouses. Addiction (Abingdon, England) 98: 923–931 [DOI] [PubMed] [Google Scholar]
- Wonnacott S 1990. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci 11: 216–219 [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJK 2005. Nicotine: From molecular mechanisms to behaviour. Curr Opin Pharmacol 5: 53. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA 1998. Cholinergic switching within neocortical inhibitory networks. Science (New York, NY) 281: 985–988 [DOI] [PubMed] [Google Scholar]
- Zucker RS 1989. Short-term synaptic plasticity. Ann Rev Neurosci 12: 13–31 [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG 2002. Short-term synaptic plasticity. Ann Rev Physiol 64: 355–405 [DOI] [PubMed] [Google Scholar]