Abstract
The tumor-associated stroma has been shown to play a significant role in cancer formation. Paracrine signaling interactions between epithelial tumor cells and stromal cells are a key component in the transformation and proliferation of tumors in several organs. Whereas the intracellular signaling pathways regulating the expression of several pro- and antiangiogenic proteins have been well characterized in human cancer cells, the intercellular signaling that takes place between tumor cells and the surrounding tumor-associated stroma has not been as extensively studied with regard to the regulation of angiogenesis. In this chapter we define the key players in the regulation of angiogenesis and examine how their expression is regulated in the tumor-associated stroma. The resulting analysis is often seemingly paradoxical, underscoring the complexity of intercellular signaling within tumors and the need to better understand the environmental context underlying these signaling mechanisms.
Carcinoma-associated fibroblasts deposit large amounts of extracellular matrix. This creates a hypoxic environment in tumors which may lead to the induction of proangiogenic factors (e.g., VEGF).
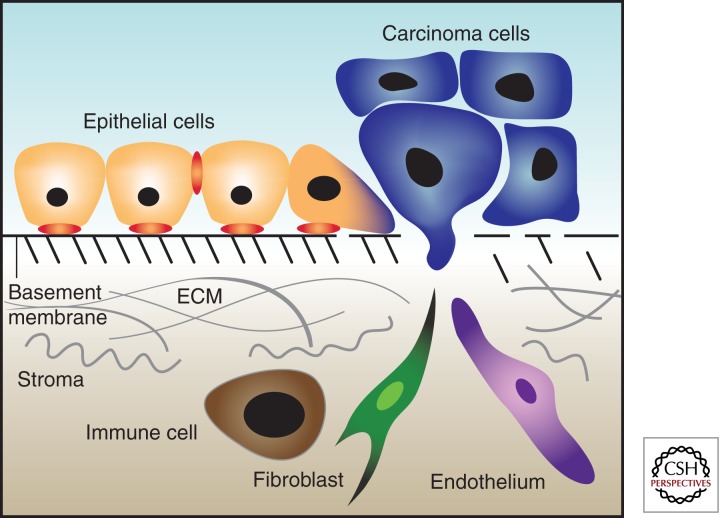
In the earliest stages of cancer, epithelial tumors, carcinomas, are physically confined within the region of the tissue from whence they arise. These early lesions (carcinomas in situ) are separated from the tissue parenchyma by the basement membrane (Hanahan and Weinberg 2000). Opposite the basement membrane are a myriad of cells consisting of fibroblasts, myofibroblasts, immune/inflammatory cells, and endothelial cells (Ronnov-Jessen et al. 1996). In addition to these cell types are the extracellular matrix proteins which they secrete and to which they, and tumor cells, attach (Ronnov-Jessen et al. 1996).
To progress to a clinically relevant and potentially lethal disease, tumor cells must also acquire the ability to escape the confines of the epithelial compartment and thus invade locally and disseminate systemically. To make this escape, tumor cells must degrade the basement membrane separating the epithelial compartment from the tissue parenchyma. The process of invading the tissue parenchyma, or being invaded by cells from the tissue parenchyma, initiates a new phase of tumor progression in which tumor growth becomes partially regulated by non-cell-autonomous processes regulated by paracrine and juxtacrine interactions with the tumor microenvironment (Chung and Davies 1996; Henshall et al. 2001; Tuxhorn et al. 2001). The tumor-associated stroma provides oxygen and nutrients via the vasculature as well as soluble and matrix-bound growth factors and enzymes that promote tumor proliferation and progression (Hanahan and Folkman 1996). The evidence for stromal cells in tumor progression suggests that they play a key role in matrix remodeling, tumor invasion, and metastatic spread (Picard et al. 1986; Grey et al. 1989; Camps et al. 1990). More importantly, epithelial cancer cells are able to alter their surrounding stromal fibroblasts to enhance tumor growth (Olumi et al. 1999). Specifically, paracrine signaling interactions between epithelial tumor cells and stromal cells have been shown to be a key component in the transformation and proliferation of tumors in several organs (Cunha et al. 1985; Donjacour and Cunha 1991; Hom et al. 1998). It has been shown, for instance, that stromal fibroblasts isolated from a prostate tumor induce tumor formation of immortal but nontransformed prostate epithelial cells when the mixture is injected orthotopically into nude mice (Olumi et al. 1999).
One process in tumor progression enhanced by tumor–stromal signaling is the induction of angiogenesis. As the endothelium does not cross the basement membrane in normal tissue architecture, in order for tumors to gain access to blood vessels they must first invade the surrounding stroma. Once the tumor cells have invaded the tissue parenchyma they must be able to transmit paracrine signals to the stromal cells to induce a proangiogenic environment. Although a myriad of pro- and antiangiogenic factors have been discovered and studied, the major effort in understanding their regulation has been in a cell-autonomous fashion. To date, the tumor cell-autonomous regulation of vascular endothelial growth factor (VEGF) (Rak et al. 1995; Damert et al. 1997; Wojta et al. 1999; Xiong et al. 2001; Akiyama et al. 2002) and Thrombospondin-1 (Tsp-1) (Rak et al. 2000; Watnick et al. 2003), two of the major positive and negative regulators of angiogenesis, have been described in detailed biochemical fashion.
The regulation of angiogenesis via signaling between epithelial tumor cells and stromal fibroblasts and endothelial cells may also be important in the establishment and proliferation of metastases. It has been well documented that tumors from various organs have distinct metastatic profiles (Chambers et al. 2002). For example, prostate cancer metastasizes preferentially to bone and liver, whereas breast cancer metastasizes to bone and lung, although in xenograft models it is possible to isolate variants of tumor cell lines that metastasize preferentially to lymph nodes (Pettaway et al. 1996). The ability of a tumor cell to survive and proliferate in a metastatic environment may ultimately rely on its ability to manipulate the angiogenicity of the stroma in this new environment.
The tumor-associated stroma, or tumor microenvironment, can grossly be categorized into two types of cells: (1) cells that are present in the normal tissue parenchyma before tumor development; and (2) cells that are recruited to the tumor-associated stroma from distal sites (i.e., the circulation or bone marrow). The first type is largely comprised of fibroblasts and endothelial cells, whereas the second type of cells is largely comprised of immune/inflammatory cells, including T- and B-cells, macrophages, neutrophils, mast cells, and other bone marrow–derived cells. In this work, the different cell types, as well as the extracellular matrix, and their contribution to tumor progression will be detailed and explained.
FIBROBLASTS
The tissue parenchyma of most organs is largely comprised of fibroblasts that, along with the extracellular matrix they secrete, make up the structural scaffolding of organs (Fig. 1). There are two distinct types of fibroblasts in normal tissue: fibroblasts and myofibroblasts. These two types of fibroblasts are distinguished by, among other markers, the expression of smooth muscle actin, which is expressed by myofibroblasts but not normal fibroblasts. Fibroblasts, although morphologically distinct, are poorly defined molecularly and were originally characterized more for what they are not: vascular, inflammatory, or epithelial cells (Tarin and Croft 1969). Fibroblasts are responsible for the synthesis and deposition of the extracellular matrix (ECM), the regulation of epithelial cell differentiation, and the regulation of inflammatory response to tissue insults (Parsonage et al. 2005; Tomasek et al. 2005). Fibroblasts are also key mediators of wound repair, where they invade the wound, synthesize and secrete ECM to anchor other cells recruited to the site of the wound, and facilitate healing wound contractions via the contractile properties of collagen (Gabbiani 2003). Fibroblasts in wound repair attain what has been termed an activated phenotype (Castor et al. 1979).
Figure 1.
Schematic of tumor microenvironment.
Activated fibroblasts proliferate at a faster rate, produce greater amounts of ECM and, as stated above, express α-smooth-muscle actin (Gabbiani 2003). Fibroblasts can be activated by growth factors released from damaged epithelial cells, such as TGF-β and basic fibroblast growth factor (bFGF), or via direct cell–cell contacts with leukocytes at the sites of wounds (Clayton et al. 1998; Choi and Tseng 2001). To accommodate the production and secretion of large amounts of ECM proteins, activated fibroblasts contain an oval-shaped euchromatic nucleus, rough endoplasmic reticulum, and prominent Golgi apparatus (Castor et al. 1979). Activated fibroblasts also produce proteases, such as matrix metalloproteases (MMPs) that degrade the ECM. These proteases aid in the turnover and reorganization of the ECM and secrete growth factors like hepatocyte growth factor (HGF) and bFGF (Rodemann and Muller 1991). Interestingly, once the wound is repaired, the number of activated fibroblasts is greatly decreased, although the overall number of fibroblasts in the area is not significantly changed (Gabbiani 2003). Thus, it is not easily discernible whether the decrease in the number of activated fibroblasts is caused by cell death or apoptosis and subsequent repopulation of normal fibroblasts from neighboring tissue or if the activated fibroblasts revert back to normal fibroblasts. However, the general consensus is that the activation is transient and once wound repair is complete, the fibroblasts revert back to a quiescent phenotype.
CARCINOMA-ASSOCIATED FIBROBLASTS
Tumor progression from the in situ stage to metastatic disease has been shown to be promoted by fibroblasts present in the tumor microenvironment (Elenbaas and Weinberg 2001). These fibroblasts found in the tumor microenvironment have been termed carcinoma-associated fibroblasts (CAFs) (Olumi et al. 1999). The molecular and genetic characterization of CAFs indicates that they are similar, if not identical, to activated fibroblasts found in the stroma of tissues undergoing wound repair, described above (Durning et al. 1984; Tsukada et al. 1987; Schor et al. 1988a,b). Specifically, they express smooth muscle actin, EGF (Normanno et al. 1995; Panico et al. 1996), HGF (Montesano et al. 1991; Seslar et al. 1993; Jin et al. 1997; Vande Woude et al. 1997; To and Tsao 1998), IGF-1 and -2 (Yee et al. 1989; Cullen et al. 1992; Ellis et al. 1994), and matrix remodeling enzymes such as MMPs (Basset et al. 1990; Wolf et al. 1993; Basset et al. 1994; Engel et al. 1994; Newell et al. 1994; Heppner et al. 1996; Chambers and Matrisian 1997; Lochter et al. 1998; Masson et al. 1998; Noel et al. 1998; McCawley and Matrisian 2000).
Furthermore, in some cancers ∼80% of the fibroblasts within the tumor stroma are thought to become activated (Sappino et al. 1988). The signaling mechanism by which CAFs are generated has not been conclusively demonstrated; however, in vitro studies have shown that TGF-β can induce CAF-like properties in normal fibroblasts (Ronnov-Jessen and Petersen 1993). Moreover, studies suggest that human carcinoma cells can convert normal fibroblasts into CAFs in a mouse xenograft model (Orimo and Weinberg 2006). Once fibroblasts become CAFs, they can be cultured in the absence of carcinoma cells and retain their CAF phenotype in culture until they undergo senescence (Orimo et al. 2005). It is interesting to note that chickens infected with Rous sarcoma virus develop invasive carcinomas when wounded, showing the oncogenecity of tumor stroma (Dolberg et al. 1985). These studies show the importance of tumor stroma, and CAFs in particular, to the process of tumor progression.
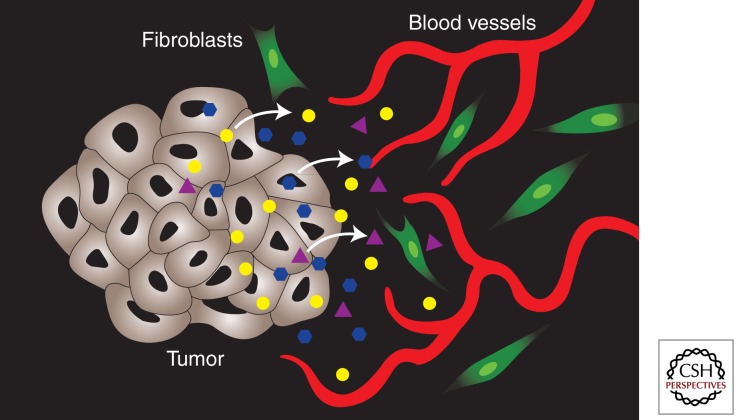
When carcinomas progress to the invasive state the basement membrane is degraded and stromal cells, including CAFs, inflammatory response cells, and newly formed capillaries, come into contact with the tumor cells (Hanahan and Weinberg 2000). CAFs in the stroma of invasive carcinoma continue depositing large amounts of ECM, including tenascin C in some cases (Chiquet-Ehrismann et al. 1986; Inaguma et al. 1988). It has been shown that in breast and bladder carcinomas expression of tenascin C correlates with increased tumor invasiveness (Mackie et al. 1987; Brunner et al. 2004). The accumulation of ECM in tumors contributes to increased interstitial fluid pressure which hinders oxygen and nutrient diffusion (Netti et al. 2000; Brown et al. 2004). Thus, CAF-mediated hypoxia could lead to the expression of HIF-1α and the induction of VEGF, thus providing a mechanism by which CAFs can promote angiogenesis in tumors (Fig. 2).
Figure 2.
Tumor–stromal paracrine signaling. Tumors secrete factors into the microenvironment that act on the stromal cells to either promote or inhibit the growth of new blood vessels (angiogenesis).
As stated above, CAFs are associated with tumor cells at most stages of cancer progression. Many studies have shown the ability of fibroblasts to promote cancer. For example, patients genetically predisposed to breast cancer contained skin fibroblasts that proliferated more rapidly in vitro (Kopelovich 1982). It has also been shown that CAFs can promote tumor growth in a mouse xenograft model whereas normal fibroblasts cannot (Olumi et al. 1999). Olumi et al. (1999) demonstrated that massive tumors grew in the grafts containing CAFs, whereas no tumors grew in grafts containing normal fibroblasts. This shows how CAFs aid in the formation of tumors, probably through induction of tumorigenic changes in epithelial cells.
Tumor progression is also mediated by CAFs. It has recently been shown that mixing human breast carcinoma cells with CAFs in a mouse xenograft gave rise to tumors that were larger and more angiogenic than when mixed with normal fibroblasts (Orimo et al. 2005). Furthermore, it was shown that the increase in tumor cell proliferation was mediated by stromal cell–derived factor 1 (SDF1) secreted by the CAFs binding to the CXCR4 receptor on tumor cells. Additionally, in this model it was shown that secreted SDF1 stimulated angiogenesis by recruitment of endothelial progenitor cells (EPCs) into the tumor. It has been previously shown that EPCs are recruited during tumor angiogenesis and differentiate into vascular endothelial cells (Lyden et al. 2001).
Another study showed the ability of CAFs to induce invasiveness in vivo with rat colon carcinoma cells that were not invasive in vitro (Dimanche-Boitrel et al. 1994). CAFs also secrete MMPs that help degrade the basement membrane and promote tumor invasion. For example, MMP3 secreted by CAFs can promote tumor cell invasiveness (Lochter et al. 1997). This is accomplished by MMP3-mediated cleavage of the extracellular domain of the adhesive protein E-cadherin on the surface of mammary epithelial cells. Cleavage of E-cadherin causes mammary epithelial cells to disperse and undergo epithelial-to-mesenchymal transition, which promotes tumor cell invasiveness.
CAFs have also been implicated in tumor metastasis, by promoting the proliferation of tumor cells at the metastatic site. For example, a hepatic metastatic cell line was shown to secrete factors that activate fibroblasts in vitro (Olaso et al. 1997). These activated fibroblasts were shown to be within the tumor stroma of the metastasis, and quiescent fibroblasts taken from the liver of mice were activated when cultured with conditioned media (CM) from the melanoma metastasis. The tumor CM induced fibroblast migration, proliferation, and production of MMP2. This suggests that CAFs help to create a niche for tumor cells at metastatic sites (Olaso and Vidal-Vanaclocha 2003).
Yet another study showed that mice deficient for the Mts1 protein, which stimulates tumor metastasis, failed to grow metastases when highly metastatic mammary carcinoma cells were grafted onto these mice (Grum-Schwensen et al. 2005). Furthermore there was a significant delay in tumor uptake as well a decrease in tumor incidence as compared to wild-type mice injected with the carcinoma cells. When the tumor cells were mixed with Mts1 competent fibroblasts and injected into the mts1 knockout mice, the ability of these tumors to metastasize was partially restored. Additionally, it has been shown that conditioned media from metastatic human breast and prostate carcinoma cell lines are able to repress the expression of Tsp-1 in fibroblasts from tissues where the carcinoma is known to metastasize (Kang et al. 2009). This shows the ability of tumors to prime metastatic sites for angiogenesis by decreasing the levels of an endogenous angiogenesis inhibitor.
It is clear that angiogenesis is an essential step in the progression and metastasis of tumors. Fibroblasts play an important role in promoting not only tumor progression but angiogenesis as well. CAFs produce growth factors like VEGF which aid in the recruitment and activation of endothelial cells within the tumor stroma. During tumor invasion, CAFs produce not only angiogenic growth factors, but also produce proteases which break down not only the basement membrane of the tumor-associated tissue but can break down the basement membrane of stromal blood vessels, an essential step in angiogenesis. Finally, during tumor metastasis CAFs are able to create permissive environments for tumor growth and angiogenesis at metastatic sites. Studies from Kalas et al. (2005) and Kang et al. (2009) have shown that tumors secrete factors that are able to repress the expression of Tsp-1 in fibroblasts. These studies underscore the importance of Tsp-1 in the induction of angiogenesis. In order for tumor cells to induce angiogenesis, the balance of angiogenic stimulators and inhibitors must be shifted toward induction of angiogenic stimulators in the tumor stroma.
CELL SIGNALING MECHANISMS AND FACTORS INFLUENCING STROMAL ANGIOGENESIS
Vascular Endothelial Growth Factor
One of the most potent proangiogenic proteins, VEGF induces endothelial cell migration and proliferation as well as vascular permeability (Senger et al. 1983; Leung et al. 1989). The regulation of VEGF in carcinoma cells has been extensively studied. Signal transduction cascades emanating from Ras and PI3 kinase lead to the increased transcription of VEGF and consequently to its secretion into the extracellular space (Rak et al. 1995). It has also been discovered that carcinoma cells secrete proteins into the extracellular space that modulate VEGF production and secretion by stromal fibroblasts in the tumor-associated stroma, such as TGF-β, platelet-derived growth factor (PDGF), and bFGF (Brogi et al. 1994; Tsai et al. 1995).
Stromal VEGF expression was first shown to be regulated by carcinoma cells using a transgenic mouse model in which GFP expression was driven by the VEGF promoter (Fukumura et al. 1998). In this model, the activation of the VEGF promoter downstream from a signal transduction cascade would result in the expression of GFP. Examination of tumor xenografts in these VEGF-GFP mice by fluorescence microscopy revealed that the fibroblasts infiltrating and immediately surrounding the carcinoma cells were bright green, indicating that the VEGF promoter had been activated. In the absence of tumors, however, the fibroblasts in normal tissues in these mice did not express GFP. These results indicated that tumors secreted a protein into the extracellular space that stimulated VEGF expression in the stroma.
The significance of the stromal expression of VEGF in tumor growth and progression was not immediately obvious from these results. It was clear that VEGF expression was being stimulated; however, it was not clear whether this stimulation was required for tumor growth, supportive of tumor growth, or merely a by-product of tumor growth. The evidence that stromally produced VEGF was important for tumor growth came from experiments testing the efficacy of the human specific anti-VEGF antibody bevacizumab (Avastin). In these experiments human tumor cells were implanted into immunocompromised mice and the resulting tumors were treated with the human-specific VEGF antibody (Kim et al. 1993). Whereas the antibody was effective at significantly slowing tumor growth, it was not 100% effective. It was postulated that perhaps a residual angiogenic stimulus was being provided by the production of murine VEGF, whose activity was not inhibited by the human-specific antibody. To test this hypothesis, human tumor xenografts were treated with human-specific VEGF antibodies as well as mFlt(1-3)-IgG, which inhibits both human and murine VEGF by acting as a decoy receptor (Gerber et al. 2000). Treatment with both VEGF therapies resulted in the complete blockade of tumor growth, thus demonstrating the requirements for tumor- and stromal-produced VEGF.
BASIC FIBROBLAST GROWTH FACTOR
Basic fibroblast growth factor (bFGF, FGF2), is another extremely potent proangiogenic growth factor (Shing et al. 1984; Klagsbrun et al. 1986; Folkman and Klagsbrun 1987). Interestingly, despite the presence of high-affinity cell surface receptors (Dionne et al. 1990) and the fact that bFGF stimulates endothelial cell proliferation and angiogenesis both in vivo and in vitro, it lacks a signal sequence that would otherwise direct its secretion (Abraham et al. 1986). Although the role of bFGF as a stimulator of angiogenesis is unequivocal, its paracrine regulation in stromal fibroblasts and subsequent effect on tumor angiogenesis is clouded by the fact that that it also potently stimulates tumor cell proliferation via FGFR signaling via both autocrine and paracrine signaling (Rogelj et al. 1988, 1989; Gleave et al. 1991). Nevertheless, it has been shown that bFGF expression in the stroma of lung adenocarcinoma inversely correlates with disease progression and patient survival (Guddo et al. 1999). In addition to its paracrine regulation in fibroblasts, it has been shown that stem cell factor (SCF) and TGF-β are potent stimulators of bFGF expression in inflammatory cells, including macrophages, mast cells, and neutrophils (Qu et al. 1998). The role of these cells in tumor angiogenesis will be detailed later.
Thrombospondin-1
Tsp-1 is an endogenous inhibitor of angiogenesis which functions via a bimodal approach: it binds to CD36 on the endothelial cell surface and renders the cell insensitive to both VEGF and bFGF, via an as yet undetermined mechanism. Tsp-1 also binds to and functionally inactivates MMP-9, a MMP shown to liberate VEGF from the ECM (Bergers et al. 2000; Rodriguez-Manzaneque et al. 2001). One signal transduction pathway that has been shown to induce the repression of Tsp-1 leads from PI3-kinase to the Rho GTPase to ROCK to Myc, which represses Tsp-1 in a phosphorylation-dependent manner (Watnick et al. 2003). This pathway has been shown to be active in several human breast cancer cell lines in which Tsp-1 expression was virtually silenced (Watnick et al. 2003). Furthermore, in a majority of the surveyed breast cancer cell lines the pathway previously described (Watnick et al. 2003) was shown to be responsible for the silencing. Thus, this pathway represents the first biochemical elucidation of a cell-autonomous “angiogenic switch.”
Although the expression of VEGF in the tumor-associated stroma is widely accepted to have a positive correlation with tumor progression (Fukumura et al. 1998; Brown et al. 1999; Mueller and Fusenig 2002), the role of Tsp-1 expression in the tumor-associated stroma is unclear. Tsp-1 expression by epithelial tumor cells is observed infrequently and ectopic expression of Tsp-1 is inhibitory to tumor growth (Streit et al. 1999; Watnick et al. 2003). Stromal Tsp-1, meanwhile, has been correlated with a desmoplastic response and increased invasiveness in a subset of breast cancers (Wong et al. 1992; Bertin et al. 1997; Brown et al. 1999), whereas it has been shown to be inhibitory to early-stage breast cancers (Clezardin et al. 1993). Expression of Tsp-1 by stromal fibroblasts has been shown to be inhibitory to tumor formation and growth (Filleur et al. 2001). Intriguingly, the same report showed that tumors that arose in an environment high in Tsp-1 eventually overcame the inhibitory effects of this protein by increasing their production of VEGF. Thus, the complex interrelationship between these two proteins and their relative expression levels in the tumor-associated stroma can play a key role in the induction and maintenance of the angiogenic phenotype in human tumors.
The work described above shows that VEGF expression in the stroma is a critical component in tumor-mediated angiogenesis. Conversely, Tsp-1 expression in the tumor-associated stroma can be a potent inhibitor of tumor angiogenesis and growth. The question that arises, then, is how do tumors stimulate the expression of VEGF in the stroma while concomitantly repressing the expression of Tsp-1?
TGF-β
One of the most interesting paradoxes involving tumor-derived growth factors involves the effects of TGF-β. It had been shown that TGF-β displays a potent proangiogenic activity in vivo (Roberts et al. 1986). In seemingly diametric opposition are the in vitro data that demonstrate the growth-inhibitory activity of TGF-β on cultured endothelial cells (Baird and Durkin 1986; Frater-Schroder et al. 1986). These diverse activities were resolved with the discovery that TGF-β stimulated the expression of VEGF in both fibroblasts and tumor cells, indicating that tumor-secreted TGF-β could elicit proangiogenic effects via the induction of VEGF in stromal fibroblasts (Brogi et al. 1994; Pertovaara et al. 1994). Moreover, TGF-β has been shown to have profound effects on the expression of bFGF in fibroblasts, stimulating its expression by more than sixfold (Goldsmith et al. 1991). These results suggest that at lower levels tumor-secreted TGF-β may act on tumor-associated fibroblasts to stimulate VEGF and bFGF production to stimulate angiogenesis. Conversely, when expressed at higher levels, TGF-β may act directly on endothelial cells to inhibit their proliferation and thus inhibit angiogenesis.
Adding to the paradox of TGF-β’s role in tumor angiogenesis is the fact that TGF-β stimulates the expression of Tsp-1 and, in turn, is activated by Tsp-1 (Penttinen et al. 1988; Murphy-Ullrich et al. 1992; Schultz-Cherry and Murphy-Ullrich 1993; Schultz-Cherry et al. 1994a,b). TGF-β is produced and secreted by cells in an inactive, latent form that covalently attached to the latent associated peptide. TGF-β can be activated by proteases that cleave the latent form or by undergoing a conformational change, induced by Tsp-1 binding that exposes the receptor-binding region. TGF-β expression in fibroblasts has also been shown to be induced by hypoxia (Falanga et al. 1991). Thus, the Tsp-1-mediated inhibition of angiogenesis would result in a state of hypoxia, which would then trigger the expression of TGF-β by the tumor stroma. The latent TGF-β would subsequently be activated by Tsp-1 and induce the expression of VEGF, whose expression would also be stimulated by the Tsp-1-induced hypoxia (Brogi et al. 1994). In this way a tumor could overcome the effects of Tsp-1 and induce angiogenesis. However, if the levels of Tsp-1 were so high as to inhibit the excess VEGF produced, then the tumor would remain in a state of dormancy until the balance of angiogenic activity was tipped decisively toward the positive.
Intriguingly, although TGF-β induces the expression of both VEGF and Tsp-1, it accomplishes these diverse activities through the activation of different transcription factors. TGF-β binding to its receptors TGFβR1 and R2 activates a signal transduction cascade which culminates in the activation of the smad family of transcription factors (Hoodless et al. 1996; Liu et al. 1996). The TGF-β-mediated stimulation of Tsp-1 expression is achieved via activation of Smad2, whereas stimulation of VEGF is mediated via activation of Smad3 (Nakagawa et al. 2004). Thus, depending on the contextual environment within the tumor-associated stroma, TGF-β can stimulate the expression of VEGF, Tsp-1, or both proteins.
Platelet-Derived Growth Factor
Another growth factor that seemingly defies characterization as a pro- or antiangiogenic protein is PDGF. It was shown by Goldsmith et al. (1991) that PDGF was a potent stimulator of bFGF. Specifically, treatment of lung fibroblasts with recombinant PDGF resulted in a twofold increase in bFGF expression (Goldsmith et al. 1991). In response to the observations described above that the contribution of stromal VEGF needed to be inhibited to effectively block tumor growth, it was determined that stromal VEGF expression was stimulated by tumor-derived PDGF (Dong et al. 2004). Furthermore, inhibition of PDGF activity via administration of soluble PDGFR abrogated the stimulation of VEGF in the tumor-associated stroma and inhibited tumor angiogenesis. Moreover, a second member of the PDGF family, PDGF B, had previously been shown to up-regulate VEGF expression in vascular smooth muscle cells (Brogi et al. 1994). These data indicated that PDGF expression by tumor cells was a potent inducer of VEGF expression in the tumor-associated stroma.
One conclusion that could be drawn from the above study was that PDGF expression by tumor cells promotes angiogenesis via stromal VEGF induction. As with TGF-β, however, the activities of this growth factor are not as straight forward as these results would indicate. In addition to its ability to stimulate VEGF and bFGF expression to stimulate angiogenesis, PDGF has also been shown to stimulate Tsp-1 expression (Majack et al. 1987). The stimulation of Tsp-1 by PDGF was shown to be via the Raf-MAPK pathway in an analogous fashion to the stimulation of Tsp-1 by serum (Majack et al. 1985). Intriguingly, unlike the differential activation of transcription factors by TGF-β, the PDGF-mediated stimulation of VEGF is also via the Raf-MAPK pathway (Chang et al. 2006). Thus, its effect on angiogenesis mediated by tumor-associated fibroblasts is most likely mediated by the activation of different receptor/signal transduction pathways.
Hormones and Nuclear Receptors
The results presented above indicate that two of the most potent inducers of stromal VEGF and, in turn, angiogenesis also possess the seemingly counterproductive ability to stimulate the expression of Tsp-1. These seemingly diverse events downstream from TGF-β and PDGF ligation to its receptor indicate that tumor-derived TGF-β and PDGF expression should have no net angiogenic activity, because their activity stimulates the expression of both pro- and antiangiogenic proteins. However, inhibition of PDGF activity has been shown to inhibit tumor angiogenesis despite its effects on Tsp-1 (Dong et al. 2004). Similarly, TGF-β has been shown to stimulate angiogenesis, again, despite its stimulatory effect on Tsp-1. One explanation for the observed proangiogenic activities of these two proteins is that the expression of Tsp-1 in the tumor-associated stroma is somehow suppressed by an independent signaling mechanism. The suppression of Tsp-1 would result in the net stimulation of only the proangiogenic growth factors VEGF and bFGF by these two growth factors and would account for their observed proangiogenic activity.
Two candidates for such a Tsp-1-repressing factor are the hormones estrogen and androgen. Estrogen has, in fact, been shown to repress the expression of Tsp-1 (Sengupta et al. 2004). Similarly, androgen has been shown to repress Tsp-1 expression (Colombel et al. 2005). Although these two hormones have similar effects on Tsp-1 expression, the mechanisms by which they repress Tsp-1 are different. Estrogen inhibition of Tsp-1 is dependent on both ERK1/2 and JNK activity (Sengupta et al. 2004). Furthermore, the repression of Tsp-1 expression by estrogen appears to be mediated through a combination of transcriptional repression and inhibition of protein secretion. Conversely, androgen-mediated suppression of Tsp-1 expression appears to be solely via transcriptional repression, as an androgen-responsive element has been identified in the Tsp-1 promoter (Colombel et al. 2005).
Whereas hormone-mediated effects on tumor growth have been largely studied through their actions on hormone-responsive tumor cells, it has recently been shown that estrogen can have a systemic proangiogenic effect (Gupta et al. 2007). This study showed that estrogen receptor (ER)-positive stromal cells stimulate angiogenesis and promote tumor growth in response to estrogen even for ER-negative tumor cells. Although the regulation of VEGF and Tsp-1 was not the focus of that study, it is not unreasonable to assume that the stimulation of estrogen-mediated stimulation of angiogenesis observed could be partially mediated by a proangiogenic activity.
It has also been recently shown that another nuclear receptor family, the peroxisome proliferator-activator receptors (PPAR), can regulate the expression of VEGF and Tsp-1. Specifically, it has been shown that tumor cells injected into PPARα−/− mice failed to grow beyond a microscopic size (Kaipainen et al. 2007). It was shown that the dormant state of these tumors was the result of increased Tsp-1 expression in the host stroma. Surprisingly, it was later determined that two ligands of PPARα, fenofibrate and WY14643, also stimulated the expression of Tsp-1 (Panigrahy et al. 2008). These seemingly discordant observations with respect to Tsp-1 expression could indicate that, in the absence of PPARα, another member of the PPAR family—perhaps PPARγ, which has been shown to stimulate expression of CD36 (Han et al. 2000), a receptor for Tsp-1—may compensate and stimulate the expression of Tsp-1. In keeping with these observations, it was shown that the PPARγ agonists rosiglitazone and pioglitazone inhibit bFGF and VEGF-mediated angiogenesis in the chick chorioallantoic membrane assay (CAM) (Aljada et al. 2008).
Nonprotein Mediators of Angiogenesis
The vast majority of paracrine signaling studies focus on the roles of cytokines and growth factors. However, one largely understudied signaling mechanism, mediated by lipids and phospholipids, has recently been investigated with respect to the regulation of Tsp-1 expression. Two independent studies revealed that generation of phospholipids and the resultant downstream signal transduction cascades could potently repress the expression of Tsp-1 in stromal fibroblasts. The first showed that generation of phospholipids, specifically sphingosine 1 phosphate (S1P), mediated by platelets could repress the expression of Tsp-1 in dermal fibroblasts via activation of Gi-protein-coupled S1P receptors (Kalas et al. 2005). It has been widely noted that platelets are commonly trapped in tumors, most likely caused by the presence of high levels of heparin-sulfates (Sack et al. 1977). Thus, platelet-mediated repression of Tsp-1, and increased angiogenesis and tumor growth, may be a by-product of this seemingly random event.
The second study investigating the role of S1P in repression of Tsp-1 showed that Ras-transformed cells secrete a low molecular weight (<3 kD) molecule that represses Tsp-1 in dermal fibroblasts via an S1P-dependent mechanism (Kalas et al. 2005). These results suggest that angiogenic tumor cells secrete factors that actively repress Tsp-1 in the surrounding tumor-associated stroma. As with the activity of estrogen and androgen noted above, the secretion of factor(s) by tumor cells that suppress the expression of Tsp-1 in the tumor-associated stroma may be a critical process in the escape from dormancy.
Matrix Metalloproteases
Local invasion across the basement membrane and within the tissue microenvironment of tumors is critical for tumor growth and ultimately progression to metastasis. One critical step in tumor migration and invasion is the degradation of the ECM and the resultant remodeling. A key driver of matrix degradation and remodeling is the MMPs. For example, it has been shown that co-injection of MCF7 breast cancer cells with fibroblasts significantly accelerated tumor growth (Noel et al. 1993). When the fibroblasts were engineered to express TIMP-2 (tissue inhibitor of metalloprotease 2), an inhibitor of MMP activity, the tumor-stimulating activity was lost (Noel et al. 1998). Analogously, when a general inhibitor of MMP activity, batimastat, was administered to mice that were co-injected with MCF7 cells and fibroblasts the tumors grew at the same rate as MCF7 cells alone (Noel et al. 1998).
In addition to allowing tumor cells to migrate and invade, MMP-mediated matrix remodeling also allows endothelial cells to migrate and form the leading edge of new blood vessels. Additionally, MMPs may function to liberate angiogenic growth factors like VEGF and bFGF that would otherwise be sequestered by the ECM. Evidence for the role of MMPs in angiogenesis was generated by crossing tumor-prone RIP-TAG2 mice with different matrix protease knockout mice (Bergers et al. 2000). In this model mice develop pancreatic islet cell tumors driven by the expression of the SV40 Large T antigen from the rat insulin promoter (Hanahan 1985). Crossing the RIP-TAG mice with MMP2 knockout mice impaired tumor growth but had no effect on angiogenesis (Bergers et al. 2000). Crossing the RIP-TAG mice with urokinase knockout mice had no effect on tumor growth. However, crossing RIP-TAG mice with MMP9 knockout mice inhibited tumor growth and angiogenesis (Bergers et al. 2000). In addition to cleaving matrix proteins, it has also been shown that MMP9 can cleave TGF-β, which is normally secreted as a pro-protein covalently bound to the latent associated peptide and is thus inactive. The ability of MMP9 to convert TGF-β from the latent to active form has been shown to stimulate growth of mammary tumor model (Yu and Stamenkovic 2000).
BONE MARROW–DERIVED CELLS
In addition to fibroblasts, the tumor microenvironment is made up several other types of cells that were present before tumorigenesis or migrated as a result. Most prominent among these cells are bone marrow–derived cells: mesenchymal stem cells (MSCs), macrophages, neutrophils, mast cells, and T cells. These cells migrate in response to the growing tumor mass, often interpreted as inflammation, and by the secretion of discrete growth factors and chemokines produced by the tumor cells.
Mesenchymal Stem Cells
MSCs are bone marrow–derived cells that have been characterized by the ability to differentiate into a myriad of mesenchymal cells: fibroblasts, osteoblasts, chondrocytes, adipocytes, pericytes, and muscle cells. MSCs are an exceedingly rare cell type within the bone marrow, comprising between 0.01% and 0.001% of the mononuclear cells (Civin et al. 1996; Pittenger et al. 1999). Human MSCs are defined by the expression of cell surface markers: CD44 adhesion molecule (HCAM), CD73, CD90, CD105 (endoglin), CD106 (VCAM-1), and STRO-1 (Dennis and Charbord 2002).
MSCs have been shown to be recruited to sites of wounding or inflammation, as well as to tumors (Hall et al. 2007). MSCs are recruited to tumors by multiple different growth factors and cytokines, including VEGF, bFGF, IL-8, EGF, HGF, and PDGF as well as CCL2, CCL7, and CXCL12 (SDF-1) (Schichor et al. 2006; Birnbaum et al. 2007; Dwyer et al. 2007; Kidd et al. 2008; Spaeth et al. 2008). In melanoma, a correlation has been demonstrated between MSCs and angiogenesis (Sun et al. 2005). Furthermore, following recruitment to the tumor, MSCs have been shown to secrete VEGF to stimulate angiogenesis (Coffelt et al. 2009).
In addition to correlation and expression studies examining the role of MSCs in angiogenesis, they have also been shown to be recruited and stimulate angiogenesis in vitro as well as in murine pancreatic xenografts (Beckermann et al. 2008). Tumors in mice injected with wild-type, vector control MSCs had twice as many blood vessels as normal tumors. Conversely, tumors in mice injected with MSCs in which VEGF had been silenced by lentiviral VEGF shRNA had comparable numbers of blood vessels to normal tumors (Beckermann et al. 2008). Thus, the ability of MSCs to home to, produce, and secrete VEGF can contribute to tumor growth via enhanced angiogenesis.
Macrophages
By far the most prevalent immune/inflammatory cell type present in tumors is the tumor associate macrophage (TAM; Balkwill and Mantovani 2001). Activated macrophages (i.e., those that have been recruited to sites of inflammation) are generally categorized into two types, M1 and M2, depending on the type of inflammation (Sher et al. 2003; Mantovani et al. 2004; Balkwill et al. 2005). M1 macrophages are effector cells that are able to potently kill microorganisms as well as tumor cells and secrete high levels of proinflammatory cytokines (Balkwill et al. 2005). M2 macrophages can have different response phenotypes based on the type of signals present in the inflammation. Thus, they are able to scavenge debris and stimulate angiogenesis, as well as tissue remodeling and repair (Goerdt and Orfanos 1999; Mantovani et al. 2002; Gordon 2003; Mosser 2003; Balkwill et al. 2005). TAMs, to the extent they have been studied, are most similar to, and share many properties with, M2 macrophages.
TAMs have been shown to display a growth-promoting activity for both human and experimental tumor models (Crowther et al. 2001). TAMs are preferentially recruited to sites of hypoxia which, in nontumorous tissue generally, is symptomatic of wounded or infected tissue (Crowther et al. 2001). Hypoxia stimulates the activity of the transcription factor HIF-1 which, in turn, stimulates the expression of the proangiogenic growth factors VEGF, bFGF, TNFα, and CXCL8 (Crowther et al. 2001). VEGF and bFGF directly stimulate endothelial migration and proliferation leading to new blood vessel growth into the hypoxic region of the tumor. Additionally, hypoxia stimulates the secretion of CXCL12 (SDF1), which potentiates the activity of VEGF and bFGF on endothelial cells (Salcedo et al. 1999; Schioppa et al. 2003). In addition, SDF1 has been shown to induce angiogenesis by recruiting bone marrow–derived endothelial precursor cells to tumors (Orimo et al. 2005).
Although for most of the time the net result of macrophage activity is growth promoting on tumors, macrophages can also inhibit the growth of tumors. Specifically, it has been shown that CSF stimulates macrophage production and secretion of metalloelastase (Dong et al. 1997, 1998). Metalloelastase is an extracellular protease which, among other substrates, cleaves plasminogen into multiple fragments, one of which is the antiangiogenic protein angiostatin (O’Reilly et al. 1994). Thus, macrophage activity with respect to tumor growth is highly context dependent and can, in certain circumstances, inhibit angiogenesis and tumor growth—in keeping with the macrophage’s general role of protecting the body from disease.
Mast Cells
Mast cells are multifunctional secretory cells, characterized by numerous large electron-dense granules composed of proteoglycans, of which heparin is the major component (Norrby 2002). Mast cells descend from pluripotent bone marrow progenitor cells that express the cell surface markers CD34, c-kit, and CD13 (Kirshenbaum et al. 1999). Mast cells in circulation are progenitor-like cells that differentiate/mature after being recruited to a given tissue. One consistent characteristic of precursor mast cells is the ability to produce MMP9, which is essential for migration into different tissue types (Tanaka et al. 1999). Mast cells express a variety of proteases including chymases, tryptases, and MMPs, which are stored in secretory granules (Norrby 2002). These proteases, especially the MMPs, specifically MMP2 and 9, are vital to mast cells’ ability to promote tissue remodeling and repair (Matrisian 1990). In addition to proteases, the secretory granules of mast cells are also depots for cytokines and growth factors, including TNF-α, GM-CSF, SCF, bFGF, EGF, PDGF, VEGF, and IFN-γ, IL-3, -4, -5, -6, -8, -10, -13, -14, and chemokines, such as (MIP)-1-α, I-309, (MCP)-1, and lymphotactin (Norrby 2002). The release of proteases, cytokines, and growth factors stored in the secretory granules of macrophages can be triggered by multiple cytokines, including IL-1, IL-3, and GM-CSF, Platelet factor 4, IL-8, SCF, (MCP)-1, and MIP-1-α (Tazzyman et al. 2009). Moreover, mast cells also produce and secrete MMPs 2 and 9, which have been shown to promote angiogenesis by liberating VEGF and bFGF from the ECM (Coussens et al. 1999, 2000). Interestingly, mast cells have been shown to be recruited to tumors by the proangiogenic proteins VEGF, bFGF, and TGF-β (Gruber et al. 1994, 1995). Thus, conditions within a tumor that necessitate the growth of new blood vessels recruit mast cells, which in turn further stimulate angiogenesis.
Experimental evidence for the functional role of mast cells in angiogenesis and tumor growth was provided by an elegant murine genetic model in which Myc expression in β cells was driven via fusion to a mutant form of the ER (Soucek et al. 2007). In this model it was shown that Myc activation by systemic administration of 4-hydroxy tamoxifen, induced β-cell tumors characterized by blood vessel infiltration accompanied by mast cell recruitment. These findings indicated that mast cells are required for angiogenesis at the onset of tumorigenesis and for maintenance of angiogenesis during tumor growth and progression.
Neutrophils
Whereas macrophages, TAMS, are the most prevalent and common leukocyte present in the tumor microenvironment, neutrophils are the most abundant leukocyte in the circulation (Tazzyman et al. 2009). Leukocytes originate in the bone marrow from hematopoietic pluripotent stem cells and differentiate via a process termed myelopoiesis. Neutrophil recruitment from the bone marrow is, in part, mediated by CXCL12 (SDF-1) and its cognate receptor, CXCR4, is expressed at high levels on the cell surface of neutrophils (Suratt et al. 2004). There are two types of neutrophils present in the circulation: circulating neutrophils which, as their name suggests, are freely circulating; and marginated neutrophils, which are bound to the endothelium of small blood vessels (Tazzyman et al. 2009). The marginated pool can be mobilized into the circulating pool by the cytokines such as IL-6 (Steele et al. 1987; Suwa et al. 2000). Neutrophils from the circulating pool are those recruited to sites of inflammation and tumors (Kanwar and Cairo 1993; Friedman 2002).
Elevated levels of neutrophils have been observed in multiple human tumors, including colon, lung, myxofibrosarcoma, gastric carcinoma, and melanoma (Nielsen et al. 1996; Bellocq et al. 1998; Mentzel et al. 2001; Mhawech-Fauceglia et al. 2006). In addition to CXCL12, one of the most potent chemo-attractants of neutrophils is CXCL8, which is expressed by both tumor and stromal cells of many types of human tumors (Bellocq et al. 1998; Xie 2001). Once recruited to tumors, neutrophils are able to stimulate angiogenesis by directly secreting VEGF and by secreting MMPs, which can release angiogenic growth factors such as VEGF and bFGF from their sequestration by the ECM (Coussens and Werb 1996; Gaudry et al. 1997).
Experimentally, it has been shown that, in a genetic murine model of squamous cell skin carcinoma, the MMP9 produced and secreted by neutrophils is required for the angiogenic switch (Coussens et al. 2000). In this model, it was observed that the source of MMP9 in the skin tumors was not from the tumors themselves but from neutrophils. These results have since been recapitulated by anti-GR1-mediated neutrophil ablation in the RIP-TAG2 islet cell tumor model and in a human ovarian cancer xenograft model in MMP9 deficient mice (Huang et al. 2002; Nozawa et al. 2006). Thus, it is now firmly established that neutrophils are important and, in some cases, required for tumor angiogenesis.
Like macrophages and mast cells, neutrophils also possess antitumor activity. For example, as early as 1975 it was observed that neutrophils could kill tumor cells (Clark and Klebanoff 1975). It was originally thought that the killing was mediated exclusively by myeloperoxidase. However, it has since been shown that neutrophils can kill tumor cells by multiple different mediators, including the release of proteases, membrane perforating agents, reactive oxygen species, and cytokines such as TNFα and IL-1β (Di Carlo et al. 2001). Moreover, neutrophils can inhibit angiogenesis via two distinct mechanisms, both of which are mediated by the protease neutrophil elastase. First, neutrophil elastase can inhibit angiogenesis by degrading VEGF and bFGF (Ai et al. 2007). Second, neutrophil elastase can cleave plasminogen into angiostatin, which inhibits VEGF- and bFGF-mediated angiogenesis (Scapini et al. 2002). Thus, this is another example of a cell type which can influence angiogenesis in the opposite way depending on the contextual signals within the tumor microenvironment.
CONCLUSIONS
Angiogenesis is a complex process driven by many different growth factors and cytokines and inhibited by a diverse range of proteins. As such, the regulation of angiogenesis by the tumor microenvironment is equally, if not more, complex. The signaling molecules secreted by tumors that act on stromal cells in a paracrine fashion can often have different activities with respect to the production and secretion of pro- and antiangiogenic proteins. As such, the composition of the tumor microenvironment as well as the stage of the tumor have profound effects on determining whether the tumor microenvironment is proangiogenic and growth promoting or antiangiogenic and thus growth inhibitory. The complex signaling mechanisms provide a myriad of potential, and as yet largely untapped, targets for therapeutic intervention to inhibit tumor growth in patients. Ultimately, the strategy of targeting tumor–stromal signaling molecules may prove to be hugely successful as the accounts of genomic instability and mutation in the stroma are exceedingly rare. As such, stromal-based antiangiogenic therapy may encounter less acquired resistance than traditional therapeutic strategies.
Footnotes
Editors: Michael Klagsbrun and Patricia D’Amore
Additional Perspectives on Angiogenesis available at www.perspectivesinmedicine.org
REFERENCES
- Abraham JA, Mergia A, Whang JL, Tumolo A, Friedman J, Hjerrild KA, Gospodarowicz D, Fiddes JC 1986. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science 233: 545–548 [DOI] [PubMed] [Google Scholar]
- Ai S, Cheng XW, Inoue A, Nakamura K, Okumura K, Iguchi A, Murohara T, Kuzuya M 2007. Angiogenic activity of bFGF and VEGF suppressed by proteolytic cleavage by neutrophil elastase. Biochem Biophys Res Commun 364: 395–401 [DOI] [PubMed] [Google Scholar]
- Akiyama H, Tanaka T, Maeno T, Kanai H, Kimura Y, Kishi S, Kurabayashi M 2002. Induction of VEGF gene expression by retinoic acid through Sp1–binding sites in retinoblastoma Y79 cells. Invest Ophthalmol Vis Sci 43: 1367–1374 [PubMed] [Google Scholar]
- Aljada A, O'Connor L, Fu YY, Mousa SA 2008. PPAR γ ligands, rosiglitazone and pioglitazone, inhibit bFGF- and VEGF-mediated angiogenesis. Angiogenesis 11: 361–367 [DOI] [PubMed] [Google Scholar]
- Baird A, Durkin T 1986. Inhibition of endothelial cell proliferation by type beta-transforming growth factor: Interactions with acidic and basic fibroblast growth factors. Biochem Biophys Res Commun 138: 476–482 [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A 2001. Inflammation and cancer: Back to Virchow? Lancet 357: 539–545 [DOI] [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A 2005. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7: 211–217 [DOI] [PubMed] [Google Scholar]
- Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P 1990. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature 348: 699–704 [DOI] [PubMed] [Google Scholar]
- Basset P, Wolf C, Rouyer N, Bellocq JP, Rio MC, Chambon P 1994. Stromelysin-3 in stromal tissue as a control factor in breast cancer behavior. Cancer 74: 1045–1049 [DOI] [PubMed] [Google Scholar]
- Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner W, Diehlmann A, et al. 2008. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer 99: 622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, Mayaud C, Milleron B, Baud L, Cadranel J 1998. Neutrophil alveolitis in bronchioloalveolar carcinoma: Induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol 152: 83–92 [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. 2000a. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2: 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin N, Clezardin P, Kubiak R, Frappart L 1997. Thrombospondin-1 and -2 messenger RNA expression in normal, benign, and neoplastic human breast tissues: Correlation with prognostic factors, tumor angiogenesis, and fibroblastic desmoplasia. Cancer Res 57: 396–399 [PubMed] [Google Scholar]
- Birnbaum T, Roider J, Schankin CJ, Padovan CS, Schichor C, Goldbrunner R, Straube A 2007. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol 83: 241–247 [DOI] [PubMed] [Google Scholar]
- Brogi E, Wu T, Namiki A, Isner JM 1994. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation 90: 649–652 [DOI] [PubMed] [Google Scholar]
- Brown LF, Guidi AJ, Schnitt SJ, Van De Water L, Iruela Arispe ML, Yeo TK, Tognazzi K, Dvorak HF 1999. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res 5: 1041–1056 [PubMed] [Google Scholar]
- Brown EB, Boucher Y, Nasser S, Jain RK 2004. Measurement of macromolecular diffusion coefficients in human tumors. Microvasc Res 67: 231–236 [DOI] [PubMed] [Google Scholar]
- Brunner A, Mayerl C, Tzankov A, Verdorfer I, Tschorner I, Rogatsch H, Mikuz G 2004. Prognostic significance of tenascin-C expression in superficial and invasive bladder cancer. J Clin Pathol 57: 927–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps JL, Chang SM, Hsu TC, Freeman MR, Hong SJ, Zhau HE, von Eschenbach AC, Chung LW 1990. Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci 87: 75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castor CW, Wilson SM, Heiss PR, Seidman JC 1979. Activation of lung connective tissue cells in vitro. Am Rev Res Dis 120: 101–106 [DOI] [PubMed] [Google Scholar]
- Chambers AF, Matrisian LM 1997. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 89: 1260–1270 [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC 2002. Dissemination and growth of cancer cells in metastatic sites. 2: 563–572 [DOI] [PubMed] [Google Scholar]
- Chang HJ, Park JS, Kim MH, Hong MH, Kim KM, Kim SM, Shin BA, Ahn BW, Jung YD 2006. Extracellular signal-regulated kinases and AP-1 mediate the up-regulation of vascular endothelial growth factor by PDGF in human vascular smooth muscle cells. Int J Oncol 28: 135–141 [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T 1986. Tenascin: An extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell 47: 131–139 [DOI] [PubMed] [Google Scholar]
- Choi TH, Tseng SC 2001. In vivo and in vitro demonstration of epithelial cell-induced myofibroblast differentiation of keratocytes and an inhibitory effect by amniotic membrane. Cornea 20: 197–204 [DOI] [PubMed] [Google Scholar]
- Chung LW, Davies R 1996. Prostate epithelial differentiation is dictated by its surrounding stroma. Mol Biol Rep 23: 13–19 [DOI] [PubMed] [Google Scholar]
- Civin CI, Trischmann T, Kadan NS, Davis J, Noga S, Cohen K, Duffy B, Groenewegen I, Wiley J, Law P, et al. 1996. Highly purified CD34–positive cells reconstitute hematopoiesis. J Clin Oncol 14: 2224–2233 [DOI] [PubMed] [Google Scholar]
- Clark RA, Klebanoff SJ 1975. Neutrophil-mediated tumor cell cytotoxicity: Role of the peroxidase system. J Exp Med 141: 1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Evans RA, Pettit E, Hallett M, Williams JD, Steadman R 1998. Cellular activation through the ligation of intercellular adhesion molecule-1. J Cell Sci 111: 443–453 [DOI] [PubMed] [Google Scholar]
- Clezardin P, Frappart L, Clerget M, Pechoux C, Delmas PD 1993. Expression of thrombospondin (TSP1) and its receptors (CD36 and CD51) in normal, hyperplastic, and neoplastic human breast. Cancer Research 53: 1421–1430 [PubMed] [Google Scholar]
- Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, Tomchuck SL, Honer zu Bentrup K, Danka ES, Henkle SL, et al. 2009. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci 106: 3806–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel M, Filleur S, Fournier P, Merle C, Guglielmi J, Courtin A, Degeorges A, Serre CM, Bouvier R, Clezardin P, et al. 2005. Androgens repress the expression of the angiogenesis inhibitor thrombospondin-1 in normal and neoplastic prostate. Cancer Res 65: 300–308 [PubMed] [Google Scholar]
- Coussens LM, Werb Z 1996. Matrix metalloproteinases and the development of cancer. Chem Biol 3: 895–904 [DOI] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z 2000. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D 1999. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev 13: 1382–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther M, Brown NJ, Bishop ET, Lewis CE 2001. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol 70: 478–490 [PubMed] [Google Scholar]
- Cullen KJ, Allison A, Martire I, Ellis M, Singer C 1992. Insulin-like growth factor expression in breast cancer epithelium and stroma. Breast Cancer Res Treat 22: 21–29 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Bigsby RM, Cooke PS, Sugimura Y 1985. Stromal-epithelial interactions in adult organs. Cell Differ 17: 137–148 [DOI] [PubMed] [Google Scholar]
- Damert A, Ikeda E, Risau W 1997. Activator-protein-1 binding potentiates the hypoxia-inducible factor-1–mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J 327: 419–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis JE, Charbord P 2002. Origin and differentiation of human and murine stroma. Stem Cells 20: 205–214 [DOI] [PubMed] [Google Scholar]
- Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P 2001. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood 97: 339–345 [DOI] [PubMed] [Google Scholar]
- Dimanche-Boitrel MT, Vakaet L Jr, Pujuguet P, Chauffert B, Martin MS, Hammann A, Van Roy F, Mareel M, Martin F 1994. In vivo and in vitro invasiveness of a rat colon-cancer cell line maintaining E-cadherin expression: An enhancing role of tumor-associated myofibroblasts. Int J Cancer 56: 512–521 [DOI] [PubMed] [Google Scholar]
- Dionne CA, Crumley G, Bellot F, Kaplow JM, Searfoss G, Ruta M, Burgess WH, Jaye M, Schlessinger J 1990. Cloning and expression of two distinct high-affinity receptors cross-reacting with acidic and basic fibroblast growth factors. EMBO J 9: 2685–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolberg DS, Hollingsworth R, Hertle M, Bissell MJ 1985. Wounding and its role in RSV-mediated tumor formation. Science 230: 676–678 [DOI] [PubMed] [Google Scholar]
- Dong J, Grunstein J, Tejada M, Peale F, Frantz G, Liang WC, Bai W, Yu L, Kowalski J, Liang X, et al. 2004. VEGF-null cells require PDGFR α signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J 23: 2800–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Kumar R, Yang X, Fidler IJ 1997. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell 88: 801–810 [DOI] [PubMed] [Google Scholar]
- Dong Z, Yoneda J, Kumar R, Fidler IJ 1998. Angiostatin-mediated suppression of cancer metastases by primary neoplasms engineered to produce granulocyte/macrophage colony-stimulating factor. J Exp Med 188: 755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR 1991. Stromal regulation of epithelial function. Cancer Treat Res 53: 335–364 [DOI] [PubMed] [Google Scholar]
- Durning P, Schor SL, Sellwood RA 1984. Fibroblasts from patients with breast cancer show abnormal migratory behaviour in vitro. Lancet 2: 890–892 [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O'Brien T, Kerin MJ 2007. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res 13: 5020–5027 [DOI] [PubMed] [Google Scholar]
- Elenbaas B, Weinberg RA 2001. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res 264: 169–184 [DOI] [PubMed] [Google Scholar]
- Ellis MJ, Singer C, Hornby A, Rasmussen A, Cullen KJ 1994. Insulin-like growth factor mediated stromal-epithelial interactions in human breast cancer. Breast Cancer Res Treat 31: 249–261 [DOI] [PubMed] [Google Scholar]
- Engel G, Heselmeyer K, Auer G, Backdahl M, Eriksson E, Linder S 1994. Correlation between stromelysin-3 mRNA level and outcome of human breast cancer. Int J Cancer 58: 830–835 [DOI] [PubMed] [Google Scholar]
- Falanga V, Qian SW, Danielpour D, Katz MH, Roberts AB, Sporn MB 1991. Hypoxia upregulates the synthesis of TGF-beta 1 by human dermal fibroblasts. J Invest Dermatol 97: 634–637 [DOI] [PubMed] [Google Scholar]
- Filleur S, Volpert OV, Degeorges A, Voland C, Reiher F, Clezardin P, Bouck N, Cabon F 2001. In vivo mechanisms by which tumors producing thrombospondin 1 bypass its inhibitory effects. Genes Dev 15: 1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M 1987. Angiogenic factors. Science 235: 442–447 [DOI] [PubMed] [Google Scholar]
- Frater-Schroder M, Muller G, Birchmeier W, Bohlen P 1986. Transforming growth factor-β inhibits endothelial cell proliferation. Biochem Biophys Res Commun 137: 295–302 [DOI] [PubMed] [Google Scholar]
- Friedman AD 2002. Transcriptional regulation of granulocyte and monocyte development. Oncogene 21: 3377–3390 [DOI] [PubMed] [Google Scholar]
- Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, et al. 1998. Tumor induction of VEGF promoter activity in stromal cells. Cell 94: 715–725 [DOI] [PubMed] [Google Scholar]
- Gabbiani G 2003. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200: 500–503 [DOI] [PubMed] [Google Scholar]
- Gaudry M, Bregerie O, Andrieu V, El Benna J, Pocidalo MA, Hakim J 1997. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood 90: 4153–4161 [PubMed] [Google Scholar]
- Gerber HP, Kowalski J, Sherman D, Eberhard DA, Ferrara N 2000. Complete inhibition of rhabdomyosarcoma xenograft growth and neovascularization requires blockade of both tumor and host vascular endothelial growth factor. Cancer Res 60: 6253–6258 [PubMed] [Google Scholar]
- Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW 1991. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res 51: 3753–3761 [PubMed] [Google Scholar]
- Goerdt S, Orfanos CE 1999. Other functions, other genes: Alternative activation of antigen-presenting cells. Immunity 10: 137–142 [DOI] [PubMed] [Google Scholar]
- Goldsmith KT, Gammon RB, Garver RI Jr 1991. Modulation of bFGF in lung fibroblasts by TGF-β and PDGF. Am J Physiol 261: L378–L385 [DOI] [PubMed] [Google Scholar]
- Gordon S 2003. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35 [DOI] [PubMed] [Google Scholar]
- Grey AM, Schor AM, Rushton G, Ellis I, Schor SL 1989. Purification of the migration stimulating factor produced by fetal and breast cancer patient fibroblasts. Proc Natl Acad Sci 86: 2438–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber BL, Marchese MJ, Kew RR 1994. Transforming growth factor-β 1 mediates mast cell chemotaxis. J Immunol 152: 5860–5867 [PubMed] [Google Scholar]
- Gruber BL, Marchese MJ, Kew R 1995. Angiogenic factors stimulate mast-cell migration. Blood 86: 2488–2493 [PubMed] [Google Scholar]
- Grum-Schwensen B, Klingelhofer J, Berg CH, El-Naaman C, Grigorian M, Lukanidin E, Ambartsumian N 2005. Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene. Cancer Res 65: 3772–3780 [DOI] [PubMed] [Google Scholar]
- Guddo F, Fontanini G, Reina C, Vignola AM, Angeletti A, Bonsignore G 1999. The expression of basic fibroblast growth factor (bFGF) in tumor-associated stromal cells and vessels is inversely correlated with non-small cell lung cancer progression. Hum Pathol 30: 788–794 [DOI] [PubMed] [Google Scholar]
- Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, Kuperwasser C 2007. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res 67: 2062–2071 [DOI] [PubMed] [Google Scholar]
- Hall B, Andreeff M, Marini F 2007. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol 180: 263–283 [DOI] [PubMed] [Google Scholar]
- Han J, Hajjar DP, Tauras JM, Feng J, Gotto AM Jr, Nicholson AC 2000. Transforming growth factor-beta1 (TGF-β1) and TGF-β2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-gamma. J Biol Chem 275: 1241–1246 [DOI] [PubMed] [Google Scholar]
- Hanahan D 1985. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 315: 115–122 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J 1996. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86: 353–364 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA 2000. The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Henshall SM, Quinn DI, Lee CS, Head DR, Golovsky D, Brenner PC, Delprado W, Stricker PD, Grygiel JJ, Sutherland RL 2001. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res 61: 423–427 [PubMed] [Google Scholar]
- Heppner KJ, Matrisian LM, Jensen RA, Rodgers WH 1996. Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol 149: 273–282 [PMC free article] [PubMed] [Google Scholar]
- Hom YK, Young P, Wiesen JF, Miettinen PJ, Derynck R, Werb Z, Cunha GR 1998. Uterine and vaginal organ growth requires epidermal growth factor receptor signaling from stroma. Endocrinology 139: 913–921 [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Haerry T, Abdollah S, Stapleton M, O'Connor MB, Attisano L, Wrana JL 1996. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell 85: 489–500 [DOI] [PubMed] [Google Scholar]
- Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, Fidler IJ 2002. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst 94: 1134–1142 [DOI] [PubMed] [Google Scholar]
- Inaguma Y, Kusakabe M, Mackie EJ, Pearson CA, Chiquet-Ehrismann R, Sakakura T 1988. Epithelial induction of stromal tenascin in the mouse mammary gland: From embryogenesis to carcinogenesis. Dev Biol 128: 245–255 [DOI] [PubMed] [Google Scholar]
- Jin L, Fuchs A, Schnitt SJ, Yao Y, Joseph A, Lamszus K, Park M, Goldberg ID, Rosen EM 1997. Expression of scatter factor and c-met receptor in benign and malignant breast tissue. Cancer 79: 749–760 [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Kieran MW, Huang S, Butterfield C, Bielenberg D, Mostoslavsky G, Mulligan R, Folkman J, Panigrahy D 2007. PPARα deficiency in inflammatory cells suppresses tumor growth. PLoS ONE 2: e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalas W, Yu JL, Milsom C, Rosenfeld J, Benezra R, Bornstein P, Rak J 2005. Oncogenes and angiogenesis: Down-regulation of thrombospondin-1 in normal fibroblasts exposed to factors from cancer cells harboring mutant ras. Cancer Res 65: 8878–8886 [DOI] [PubMed] [Google Scholar]
- Kang SY, Halvorsen OJ, Gravdal K, Bhattacharya N, Lee JM, Liu NW, Johnston BT, Johnston AB, Haukaas SA, Aamodt K, et al. 2009. Prosaposin inhibits tumor metastasis via paracrine and endocrine stimulation of stromal p53 and Tsp-1. Proc Natl Acad Sci 106: 12115–12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar VS, Cairo MS 1993. Neonatal neutrophil maturation, kinetics, and function. In The neutrophil (ed. Abramson JS, Wheeler JG), pp. 1–16 Oxford University Press, New York [Google Scholar]
- Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B, Marini FC 2008. The (in)auspicious role of mesenchymal stromal cells in cancer: Be it friend or foe. Cytotherapy 10: 657–667 [DOI] [PubMed] [Google Scholar]
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N 1993. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362: 841–844 [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD 1999. Demonstration that human mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13). Blood 94: 2333–2342 [PubMed] [Google Scholar]
- Klagsbrun M, Sasse J, Sullivan R, Smith JA 1986. Human tumor cells synthesize an endothelial cell growth factor that is structurally related to basic fibroblast growth factor. Proc Natl Acad Sci 83: 2448–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelovich L 1982. Genetic predisposition to cancer in man: In vitro studies. Int Rev Cytol 77: 63–88 [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306–1309 [DOI] [PubMed] [Google Scholar]
- Liu F, Hata A, Baker JC, Doody J, Carcamo J, Harland RM, Massague J 1996. A human Mad protein acting as a BMP-regulated transcriptional activator. Nature 381: 620–623 [DOI] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ 1997. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139: 1861–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Sternlicht MD, Werb Z, Bissell MJ 1998. The significance of matrix metalloproteinases during early stages of tumor progression. Ann NY Acad Sci 857: 180–193 [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. 2001. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7: 1194–1201 [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Chiquet-Ehrismann R, Pearson CA, Inaguma Y, Taya K, Kawarada Y, Sakakura T 1987. Tenascin is a stromal marker for epithelial malignancy in the mammary gland. Proc Natl Acad Sci 84: 4621–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack RA, Cook SC, Bornstein P 1985. Platelet-derived growth factor and heparin-like glycosaminoglycans regulate thrombospondin synthesis and deposition in the matrix by smooth muscle cells. J Cell Biol 101: 1059–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack RA, Mildbrandt J, Dixit VM 1987. Induction of thrombospondin messenger RNA levels occurs as an immediate primary response to platelet-derived growth factor. J Biol Chem 262: 8821–8825 [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A 2002. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549–555 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686 [DOI] [PubMed] [Google Scholar]
- Masson R, Lefebvre O, Noel A, Fahime ME, Chenard MP, Wendling C, Kebers F, LeMeur M, Dierich A, Foidart JM, et al. 1998. In vivo evidence that the stromelysin-3 metalloproteinase contributes in a paracrine manner to epithelial cell malignancy. J Cell Biol 140: 1535–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian LM 1990. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet 6: 121–125 [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM 2000. Matrix metalloproteinases: Multifunctional contributors to tumor progression. Mol Med Today 6: 149–156 [DOI] [PubMed] [Google Scholar]
- Mentzel T, Brown LF, Dvorak HF, Kuhnen C, Stiller KJ, Katenkamp D, Fletcher CD 2001. The association between tumour progression and vascularity in myxofibrosarcoma and myxoid/round cell liposarcoma. Virchows Arch 438: 13–22 [DOI] [PubMed] [Google Scholar]
- Mhawech-Fauceglia P, Kaya G, Sauter G, McKee T, Donze O, Schwaller J, Huard B 2006. The source of APRIL up-regulation in human solid tumor lesions. J Leukoc Biol 80: 697–704 [DOI] [PubMed] [Google Scholar]
- Montesano R, Schaller G, Orci L 1991. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell 66: 697–711 [DOI] [PubMed] [Google Scholar]
- Mosser DM 2003. The many faces of macrophage activation. J Leukoc Biol 73: 209–212 [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE 2002. Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differentiation 70: 486–497 [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Schultz-Cherry S, Hook M 1992. Transforming growth factor-β complexes with thrombospondin. Mol Biol Cell 3: 181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Li JH, Garcia G, Mu W, Piek E, Bottinger EP, Chen Y, Zhu HJ, Kang DH, Schreiner GF, et al. 2004. TGF-β induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int 66: 605–613 [DOI] [PubMed] [Google Scholar]
- Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK 2000. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res 60: 2497–2503 [PubMed] [Google Scholar]
- Newell KJ, Witty JP, Rodgers WH, Matrisian LM 1994. Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Mol Carcinog 10: 199–206 [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Timshel S, Kjeldsen L, Sehested M, Pyke C, Borregaard N, Dano K 1996. 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int J Cancer 65: 57–62 [DOI] [PubMed] [Google Scholar]
- Noel A, De Pauw-Gillet MC, Purnell G, Nusgens B, Lapiere CM, Foidart JM 1993. Enhancement of tumorigenicity of human breast adenocarcinoma cells in nude mice by matrigel and fibroblasts. Br J Cancer 68: 909–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel A, Hajitou A, L'Hoir C, Maquoi E, Baramova E, Lewalle JM, Remacle A, Kebers F, Brown P, Calberg-Bacq CM, et al. 1998. Inhibition of stromal matrix metalloproteases: Effects on breast-tumor promotion by fibroblasts. Int J Cancer 76: 267–273 [DOI] [PubMed] [Google Scholar]
- Normanno N, Kim N, Wen D, Smith K, Harris AL, Plowman G, Colletta G, Ciardiello F, Salomon DS 1995. Expression of messenger RNA for amphiregulin, heregulin, and cripto-1, three new members of the epidermal growth factor family, in human breast carcinomas. Breast Cancer Res Treat 35: 293–297 [DOI] [PubMed] [Google Scholar]
- Norrby K 2002. Mast cells and angiogenesis. APMIS 110: 355–371 [DOI] [PubMed] [Google Scholar]
- Nozawa H, Chiu C, Hanahan D 2006. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci 103: 12493–12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J 1994. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79: 315–328 [DOI] [PubMed] [Google Scholar]
- Olaso E, Santisteban A, Bidaurrazaga J, Gressner AM, Rosenbaum J, Vidal-Vanaclocha F 1997. Tumor-dependent activation of rodent hepatic stellate cells during experimental melanoma metastasis. Hepatology Baltimore, Md 26: 634–642 [DOI] [PubMed] [Google Scholar]
- Olaso E, Vidal-Vanaclocha F 2003. Use of tumor-activated hepatic stellate cell as a target for the preclinical testing of anti-angiogenic drugs against hepatic tumor development. Methods Mol Med 85: 79–86 [DOI] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR 1999. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59: 5002–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Weinberg RA 2006. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle 5: 1597–1601 [DOI] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA 2005. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121: 335–348 [DOI] [PubMed] [Google Scholar]
- Panico L, D'Antonio A, Salvatore G, Mezza E, Tortora G, De Laurentiis M, De Placido S, Giordano T, Merino M, Salomon DS, et al. 1996. Differential immunohistochemical detection of transforming growth factor α, amphiregulin and CRIPTO in human normal and malignant breast tissues. Int J Cancer 65: 51–56 [DOI] [PubMed] [Google Scholar]
- Panigrahy D, Kaipainen A, Huang S, Butterfield CE, Barnes CM, Fannon M, Laforme AM, Chaponis DM, Folkman J, Kieran MW 2008. PPARα agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci 105: 985–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, Salmon M, Buckley CD 2005. A stromal address code defined by fibroblasts. Trends Immunol 26: 150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen RP, Kobayashi S, Bornstein P 1988. Transforming growth factor β increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci 85: 1105–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K 1994. Vascular endothelial growth factor is induced in response to transforming growth factor-β in fibroblastic and epithelial cells. J Biol Chem 269: 6271–6274 [PubMed] [Google Scholar]
- Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, Fidler IJ 1996. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res 2: 1627–1636 [PubMed] [Google Scholar]
- Picard O, Rolland Y, Poupon MF 1986. Fibroblast-dependent tumorigenicity of cells in nude mice: Implication for implantation of metastases. Cancer Res 46: 3290–3294 [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147 [DOI] [PubMed] [Google Scholar]
- Qu Z, Huang X, Ahmadi P, Stenberg P, Liebler JM, Le AC, Planck SR, Rosenbaum JT 1998. Synthesis of basic fibroblast growth factor by murine mast cells. Regulation by transforming growth factor β, tumor necrosis factor α, and stem cell factor. Int Arch Allergy Immunol 115: 47–54 [DOI] [PubMed] [Google Scholar]
- Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel RS 1995. Mutant ras oncogenes upregulate VEGF/VPF expression: Implications for induction and inhibition of tumor angiogenesis. Cancer Research 55: 4575–4580 [PubMed] [Google Scholar]
- Rak J, Mitsuhashi Y, Sheehan C, Tamir A, Viloria-Petit A, Filmus J, Mansour SJ, Ahn NG, Kerbel RS 2000. Oncogenes and tumor angiogenesis: Differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res 60: 490–498 [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, et al. 1986. Transforming growth factor type β: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci 83: 4167–4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodemann HP, Muller GA 1991. Characterization of human renal fibroblasts in health and disease: II. In vitro growth, differentiation, and collagen synthesis of fibroblasts from kidneys with interstitial fibrosis. Am J Kidney Dis 17: 684–686 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML 2001. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci 98: 12485–12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogelj S, Weinberg RA, Fanning P, Klagsbrun M 1988. Basic fibroblast growth factor fused to a signal peptide transforms cells. Nature 331: 173–175 [DOI] [PubMed] [Google Scholar]
- Rogelj S, Weinberg RA, Fanning P, Klagsbrun M 1989. Characterization of tumors produced by signal peptide-basic fibroblast growth factor-transformed cells. J Cell Biochem 39: 13–23 [DOI] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW 1993. Induction of alpha-smooth muscle actin by transforming growth factor-β 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest 68: 696–707 [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW, Bissell MJ 1996. Cellular changes involved in conversion of normal to malignant breast: Importance of the stromal reaction. Physiol Rev 76: 69–125 [DOI] [PubMed] [Google Scholar]
- Sack GH Jr, Levin J, Bell WR 1977. Trousseau's syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: Clinical, pathophysiologic, and therapeutic features. Medicine (Balt) 56: 1–37 [PubMed] [Google Scholar]
- Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ 1999. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1α. Am J Pathol 154: 1125–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino AP, Skalli O, Jackson B, Schurch W, Gabbiani G 1988. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer 41: 707–712 [DOI] [PubMed] [Google Scholar]
- Scapini P, Nesi L, Morini M, Tanghetti E, Belleri M, Noonan D, Presta M, Albini A, Cassatella MA 2002. Generation of biologically active angiostatin kringle 1–3 by activated human neutrophils. J Immunol 168: 5798–5804 [DOI] [PubMed] [Google Scholar]
- Schichor C, Birnbaum T, Etminan N, Schnell O, Grau S, Miebach S, Aboody K, Padovan C, Straube A, Tonn JC, et al. 2006. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC). Exp Neurol 199: 301–310 [DOI] [PubMed] [Google Scholar]
- Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, et al. 2003. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med 198: 1391–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor SL, Schor AM, Grey AM, Rushton G 1988a. Foetal and cancer patient fibroblasts produce an autocrine migration-stimulating factor not made by normal adult cells. J Cell Sci 90: 391–399 [DOI] [PubMed] [Google Scholar]
- Schor SL, Schor AM, Rushton G 1988b. Fibroblasts from cancer patients display a mixture of both foetal and adult-like phenotypic characteristics. J Cell Sci 90: 401–407 [DOI] [PubMed] [Google Scholar]
- Schultz Cherry S, Murphy Ullrich JE 1993. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol 122: 923–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz Cherry S, Lawler J, Murphy Ullrich JE 1994a. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-beta. J Biol Chem 269: 26783–26788 [PubMed] [Google Scholar]
- Schultz Cherry S, Ribeiro S, Gentry L, Murphy Ullrich JE 1994b. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-beta in a chemically defined system. J Biol Chem 269: 26775–26782 [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF 1983. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219: 983–985 [DOI] [PubMed] [Google Scholar]
- Sengupta K, Banerjee S, Saxena NK, Banerjee SK 2004. Thombospondin-1 disrupts estrogen-induced endothelial cell proliferation and migration and its expression is suppressed by estradiol. Mol Cancer Res 2: 150–158 [PubMed] [Google Scholar]
- Seslar SP, Nakamura T, Byers SW 1993. Regulation of fibroblast hepatocyte growth factor/scatter factor expression by human breast carcinoma cell lines and peptide growth factors. Cancer Res 53: 1233–1238 [PubMed] [Google Scholar]
- Sher A, Pearce E, Kaye P 2003. Shaping the immune response to parasites: Role of dendritic cells. Curr Opin Immunol 15: 421–429 [DOI] [PubMed] [Google Scholar]
- Shing Y, Folkman J, Sullivan R, Butterfield C, Murray J, Klagsbrun M 1984. Heparin affinity: Purification of a tumor-derived capillary endothelial cell growth factor. Science 223: 1296–1299 [DOI] [PubMed] [Google Scholar]
- Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI 2007. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med 13: 1211–1218 [DOI] [PubMed] [Google Scholar]
- Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F 2008. Inflammation and tumor microenvironments: Defining the migratory itinerary of mesenchymal stem cells. Gene Ther 15: 730–738 [DOI] [PubMed] [Google Scholar]
- Steele RW, Steele CR, Pilkington NS Jr, Charlton RK 1987. Functional capacity of marginated and bone marrow reserve granulocytes. Infect Immun 55: 2359–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M 1999. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol 155: 441–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Zhang S, Ni C, Zhang D, Liu Y, Zhang W, Zhao X, Zhao C, Shi M 2005. Correlation between melanoma angiogenesis and the mesenchymal stem cells and endothelial progenitor cells derived from bone marrow. Stem Cells Dev 14: 292–298 [DOI] [PubMed] [Google Scholar]
- Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS 2004. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood 104: 565–571 [DOI] [PubMed] [Google Scholar]
- Suwa T, Hogg JC, English D, Van Eeden SF 2000. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol 279: H2954–2960 [DOI] [PubMed] [Google Scholar]
- Tanaka A, Arai K, Kitamura Y, Matsuda H 1999. Matrix metalloproteinase-9 production, a newly identified function of mast cell progenitors, is downregulated by c-kit receptor activation. Blood 94: 2390–2395 [PubMed] [Google Scholar]
- Tarin D, Croft CB 1969. Ultrastructural features of wound healing in mouse skin. Journal of anatomy 105: 189–190 [PubMed] [Google Scholar]
- Tazzyman S, Lewis CE, Murdoch C 2009. Neutrophils: Key mediators of tumour angiogenesis. Int J Exp Pathol 90: 222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To CT, Tsao MS 1998. The roles of hepatocyte growth factor/scatter factor and met receptor in human cancers (Review). Oncol Rep 5: 1013–1024 [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, McRae J, Owens GK, Haaksma CJ 2005. Regulation of α-smooth muscle actin expression in granulation tissue myofibroblasts is dependent on the intronic CArG element and the transforming growth factor-beta1 control element. Am J Pathol 166: 1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JC, Goldman CK, Gillespie GY 1995. Vascular endothelial growth factor in human glioma cell lines: Induced secretion by EGF, PDGF-BB, bFGF. J Neurosurg 82: 864–873 [DOI] [PubMed] [Google Scholar]
- Tsukada T, McNutt MA, Ross R, Gown AM 1987. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol 127: 389–402 [PMC free article] [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Rowley DR 2001. Reactive stroma in prostate cancer progression. J Urol 166: 2472–2483 [PubMed] [Google Scholar]
- Vande Woude GF, Jeffers M, Cortner J, Alvord G, Tsarfaty I, Resau J 1997. Met-HGF/SF: Tumorigenesis, invasion and metastasis. Ciba Found Symp 212: 119–130; discussion 130–112, 148–154 [DOI] [PubMed] [Google Scholar]
- Watnick R, Cheng YN, Rangarajan A, Ince T, Weinberg RA 2003. Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell 3: 219–231 [DOI] [PubMed] [Google Scholar]
- Wojta J, Kaun C, Breuss JM, Koshelnick Y, Beckmann R, Hattey E, Mildner M, Weninger W, Nakamura T, Tschachler E, et al. 1999. Hepatocyte growth factor increases expression of vascular endothelial growth factor and plasminogen activator inhibitor-1 in human keratinocytes and the vascular endothelial growth factor receptor flk-1 in human endothelial cells. Lab Invest 79: 427–438 [PubMed] [Google Scholar]
- Wolf C, Rouyer N, Lutz Y, Adida C, Loriot M, Bellocq JP, Chambon P, Basset P 1993. Stromelysin 3 belongs to a subgroup of proteinases expressed in breast carcinoma fibroblastic cells and possibly implicated in tumor progression. Proc Natl Acad Sci 90: 1843–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Purdie AT, Han P 1992. Thrombospondin and other possible related matrix proteins in malignant and benign breast disease. An immunohistochemical study. Am J Path 140: 1473–1482 [PMC free article] [PubMed] [Google Scholar]
- Xie K 2001. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev 12: 375–391 [DOI] [PubMed] [Google Scholar]
- Xiong S, Grijalva R, Zhang L, Nguyen NT, Pisters PW, Pollock RE, Yu D 2001. Up-regulation of vascular endothelial growth factor in breast cancer cells by the heregulin-β1–activated p38 signaling pathway enhances endothelial cell migration. Cancer Res 61: 1727–1732 [PubMed] [Google Scholar]
- Yee D, Paik S, Lebovic GS, Marcus RR, Favoni RE, Cullen KJ, Lippman ME, Rosen N 1989. Analysis of insulin-like growth factor I gene expression in malignancy: Evidence for a paracrine role in human breast cancer. Mol Endocrinol 3: 509–517 [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I 2000. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176 [PMC free article] [PubMed] [Google Scholar]