Abstract
The β-thalassemia syndromes reflect deficient or absent β-globin synthesis usually owing to a mutation in the β-globin locus. The relative excess of α-globin results in the formation of insoluble aggregates leading to ineffective erythropoiesis and shortened red cell survival. A relatively high capacity for fetal hemoglobin synthesis is a major genetic modifier of disease severity, with polymorphisms in other genes also having a significant role. Iron overload secondary to enhanced absorption and red cell transfusions causes an increase in liver iron and in various other tissues, leading to endocrine and cardiac dysfunction. Modern chelation regimens are effective in removing iron and preserving or restoring organ function.
In β-thalassemia, the relative excess of α-globin leads to ineffective erythropoiesis and shortened red cell survival. Iron overload after red cell transfusions leads to liver, endocrine, and cardiac dysfunction.
The β-thalassemias are genetic disorders of hemoglobin synthesis characterized by deficient (β+) or absent (β0) synthesis of the β-globin subunit of hemoglobin molecule (Weatherall and Clegg 2001). The vast majority of individuals with thalassemia inherit their disorder as a Mendelian recessive. Heterozygous individuals have mild anemia and microcytosis and are categorized as having thalassemia minor or trait, and homozygous individuals have severe anemia of varying degrees and are characterized as having homozygous β-thalassemia or thalassemia major or intermedia, as discussed in detail below. Much rarer is a dominantly inherited β-thalassemia in which disease occurs in heterozygous individuals because of synthesis of a highly unstable β-globin variant (Thein 1999). Usually the disturbance is only in β-globin synthesis, but rare deletional mutations may remove one or more of the other genes on chromosome 11, resulting in forms of the disease characterized as δβ-, γδβ-, or εγδβ-thalassemia.
HETEROZYGOUS β-THALASSEMIA
The hematological features of thalassemia trait are microcytosis, hypochromia, and usually an increase in the percentage of HbA2. The hemoglobin composition is 92%–95% HbA, >3.8% HbA2, and variable amounts of HbF amounting to 0.5%–4%. In addition to microcytosis and hypochromia, there is marked variation in size and shape of red blood cells with the red cells of β0-thalassemia trait having a lower mean corpuscular volume than those of β+-thalassemia trait (Cao and Galanello 2010). Historically, the mild anemia with microcytic, hypochromic red cells characteristic of thalassemia trait has been thought to lack clinical consequences other than its association with the anemia of pregnancy (White et al. 1985). However, a recently controlled trial performed in Sri Lanka suggested that individuals with thalassemia trait may experience symptoms of anemia including headache, lethargy, fatigue, dizziness, and exercise intolerance despite having hemoglobin levels that overlap the normal range (Premawardhena et al. 2008). There was no difference in the frequency of these symptoms between the two groups that had either mild anemia or hemoglobin levels in the normal range. There was also a significant increase in frequency of infectious episodes in individuals with β-thalassemia trait. Men but not women with thalassemia trait have a reduced frequency of advanced coronary artery disease, and myocardial infarction occurs at an older age in men with thalassemia trait (Tassiopoulos et al. 2005).
HOMOZYGOUS β-THALASSEMIA
The clinical spectrum of patients with homozygous β-thalassemia is highly variable (Weatherall and Clegg 2001; Cao and Galanello 2010). Many individuals present with severe anemia early in life and remain transfusion dependent for their entire lives. Such individuals carry the diagnosis of thalassemia major. Others with homozygous β-thalassemia present with milder anemia and never require transfusion, and some have variable degrees of anemia and may require transfusion intermittently. Such individuals are designated as having thalassemia intermedia. The degree of anemia in those with thalassemia intermedia is from nearly normal levels to sufficiently severe anemia to require occasional blood transfusions. Erythroid hyperplasia leads to medullary expansion with facial deformity and osteoporosis, which may be quite severe. Extramedullary hematopoiesis results in enlargement of the liver and spleen and paraspinal and pulmonary masses of erythroid cells. Diagnostic criteria for the major versus the intermedia syndrome are rather ill defined and are largely based on the hemoglobin level without transfusion. Generally, a cutoff of 7 g/dL is used to distinguish between the two forms, but this criterion is confounded by the fact that the severity of the anemia and associated splenomegaly and defective development may vary in individual patients at different times, and the use of transfusion is partly based on socioeconomic issues as well as access to an adequate blood supply. As noted in a definitive review of this topic, the remarkable phenotypical diversity of β-thalassemias reflects the heterogeneity of mutations of the β-globin locus, the action of many secondary and tertiary modifiers, and a wide range of environmental factors (Weatherall 2001).
HEMOGLOBIN E β-THALASSEMIA
Hemoglobin E has a substitution of lysine for glutamic acid at position 26 of the β-globin chain. This hemoglobin variant is particularly common in Southeast Asia (Weatherall and Clegg 2001). Both heterozygotes and homozygotes for HbE have hypochromic and microcytic cells, and molecular studies have documented deficient accumulation of the βE mRNA. Molecular analysis indicates that the GAG change to AAG at codon 26, which is responsible for the amino acid substitution, also creates a cryptic splice donor site that slows splicing of intron 1 and results in abnormally spliced mRNA species (Orkin et al. 1982). Double heterozygosity for a β-thalassemia mutation and the βE mutation account for almost 50% of the patients with severe β-thalassemia worldwide. This genotype is particularly common in certain countries in Southeast Asia (Olivieri et al. 2010a). Because of the Asian migration to the United States, this genotype is common where Asians have settled (e.g., California). The phenotype of HbE β-thalassemia is highly variable, with many patients remaining largely free of transfusion throughout their lifetime, whereas others are started on transfusion at an early age (Olivieri et al. 2010a). For those for whom transfusions were initiated early in life for unclear reasons, discontinuation of transfusion support may be possible later in life (Olivieri et al. 2010b). Recent reviews of the clinical features of HbE β-thalassemia summarize the clinical heterogeneity and the approach to management of these patients (Olivieri et al. 2008, 2010b). This condition is discussed in more detail by Fucharoen and Weatherall (2012).
PATHOPHYSIOLOGY
Erythropoiesis in individuals with β-thalassemia reflects the consequences of excess, unpaired α-globin (Nathan and Gunn 1966; Nathan et al. 1969; Cao and Galanello 2010; Sankaran and Nathan 2010). Indeed, the degree of imbalance in the α-globin versus β + γ-globin biosynthetic ratio is the major determinate of disease severity rather than the underproduction of hemoglobin (Weatherall et al. 1965; Bank and Marks 1966; Nathan and Gunn 1966; Nathan et al. 1969; Weatherall 2001; Rund and Rachmilewitz 2005). In β-thalassemia trait there is a twofold excess in the synthesis of α-globin, which is consistent with fairly normal hematopoiesis with only mild microcytosis and hypochromia of the red cells. The α to non-α biosynthetic ratio in individuals with thalassemia intermedia is typically 3–4/1 because residual capacity for β-globin synthesis along with usually modest but variable γ-globin synthesis mitigates the consequences of excess α-globin production. Individuals with β0-thalassemia mutations have marked chain biosynthetic imbalance as the underlying basis for their severe phenotype.
Following synthesis, α-globin interacts with its molecular chaperone, α-hemoglobin stabilizing protein (AHSP), to form a protein complex before it is released to interact with β-globin in forming the hemoglobin tetramer (Yu et al. 2007; Weiss and dos Santos 2009). AHSP facilitates folding of α-globin and prevents the formation of misfolded aggregates. α-Globin mutations that impair interaction with AHSP are associated with microcytosis and anemia in humans (Yu et al. 2009). Loss of AHSP has also been shown to impair erythropoiesis in a mouse model of β-thalassemia (Kong et al 2004). Evidence suggests that AHSP levels may influence the phenotype of β-thalassemia (Lai et al. 2006).
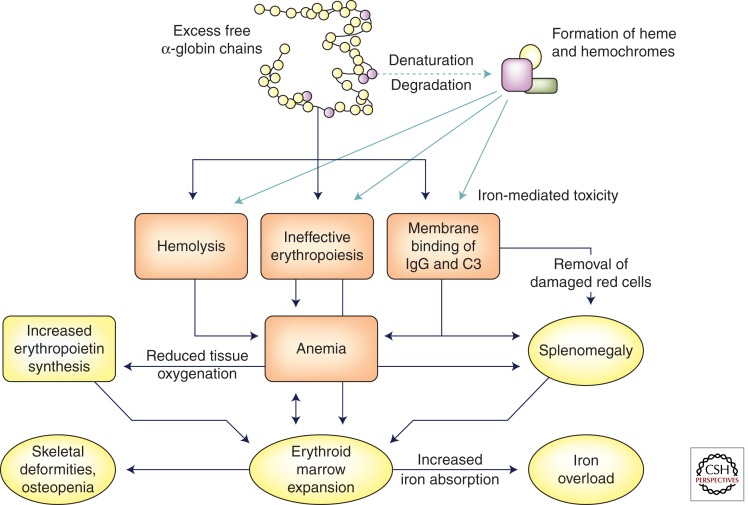
Once the capacity of AHSP is exceeded, α-globin forms molecular aggregates, which precipitate, forming inclusions that damage the cell membrane and the membranes of intracellular organelles (Fig. 1). Aggregated α-chains also trigger the formation of reactive oxygen species, which further damage the protein and lipid constituents of cell membranes. As well as heme and iron, one of the most toxic products of unpaired α-chains is hemichromes, which bind to the membrane and promote clustering of band 3, one of its major constituents (Rund and Rachmilewitz 2005). The formation of α-chain inclusions occurs early during erythropoiesis and peaks in the polychromatophilic erythroblasts, leading to cellular apoptosis (Mathias et al. 2000). Thus, anemia in the severe β-thalassemias reflects both ineffective erythropoiesis as well as shortened red cell survival as a consequence of α-globin inclusions (Nathan and Gunn 1966; Nathan et al. 1969). The pathophysiology of β-thalassemia has been compared with other disorders such as Parkinson’s disease and Huntington’s disease, which are caused by accumulations of unstable, aggregation-prone proteins (Khandros and Weiss 2010; Khandros et al. 2011). Almost all cells have some capacity to detoxify and remove damaging proteins via multiple biochemical pathways termed protein quality control (PQC). The ubiquitin–proteasome system (UPS) and the lysosome-autophagy pathways that function in PQC are thought to participate in the degradation of α-globin, but the capacities of these pathways are exceeded in the erythroid cells of individuals with the severe forms of β-thalassemia.
Figure 1.
Pathophysiology of β-thalassemia. Because of the imbalance in chain synthesis, an excess of freed α-globin chains accumulates within erythroid cells. Aggregation, denaturation, and degradation of these chains leads to the formation of insoluble precipitates as well as hemichromes, which damage cell membranes. Membrane damage leads to ineffective erythropoiesis within the bone marrow, hemolysis of red cells within the circulation, and binding of immunoglobulin and complement components to red cell membranes, triggering loss of red cells in the spleen. The resulting anemia leads to diminished tissue oxygenation, an increase in erythropoietin levels, and further stimulation of the bone marrow. Bone marrow expansion causes skeletal deformities and osteopenia. Substances released from degenerating red cells increase iron absorption, which contributes to iron overload.
GENETIC MODIFIERS
The thalassemias are heterogeneous at the molecular level, with more than 200 disease-causing mutations having been identified (Weatherall 2001; Rund and Rachmilewitz 2005; Cao and Galanello 2010; Thein 2013). Alleles characterized by a mild phenotype typically include promoter mutations, although splicing and frameshift mutations have also been discovered in individuals homozygous for β-thalassemia but having a mild phenotype. Coinheritance of an α-thalassemia gene is another mechanism that mitigates the phenotype of homozygous β-thalassemia (Kan and Nathan 1970; Wainscoat et al. 1983; Cao et al. 1994). Individuals doubly heterozygous for α- and β-thalassemia have microcytosis but essentially normal circulating hemoglobin concentrations. Individuals homozygous for β-thalassemia who inherit a chromosome having a single α-globin gene deletion may have a milder phenotype, whereas deletion of both α-globin genes on one chromosome is typically associated with thalassemia intermedia. Another mechanism for thalassemia intermedia is heterozygous β-thalassemia inherited along with either triplicated α-globin gene arrangement or with homozygosity for a triplicated globin gene arrangement (Premawardhena et al. 2005).
Fetal hemoglobin is a much more common and major modifier of disease severity in individuals with β-thalassemia. In normal individuals, HbF synthesis occurs in a minority of erythroid cells. Only 5%–8% of red cells, called F cells, contain HbF, amounting to 5%–20% of the total hemoglobin in those cells (Boyer et al. 1975). In the context of a severe deficiency of β-globin synthesis, even the low levels of γ-globin in F cells reduce the relative excess of α-globin and provide a potent selective survival advantage for cells making HbF in the context of the ineffective erythropoiesis characteristic of the most severe forms of β-thalassemia. This selective survival is thought to account for the elevated levels of fetal hemoglobin percentage in a significant majority of patients who are homozygous for β-thalassemia (Gabuzda et al. 1962; Weatherall 2001). Certain β-globin promoter mutations are associated with increased γ-chain synthesis from the same chromosome (Cao and Galanella 2010). The number of F cells in normal individuals, and presumably those with β-thalassemia, is under genetic control and varies among individuals (Galarneau et al. 2010). Presumably having F-cell numbers at the higher range of normal is associated with a milder clinical phenotype. Study of a large pedigree has shown that coinheritance of an HPFH gene is associated with higher mean corpuscular hemoglobin, mean corpuscular volume, and HbA2 levels (Garner et al. 2003). A recent study in untransfused patients with thalassemia intermedia has documented an inverse correlation between HbF levels and the frequencies of morbidities reflecting disease severity (Musallam et al. 2012). Hydroxyurea has been reported to increase HbF in some untransfused patients with severe β-thalassemia and to reduce the transfusion requirement in a minority of patients on regular transfusions (Karimi et al. 2011). A favorable response to hydroxyurea may be more frequent in thalassemic individuals with certain haplotypes (Italia et al. 2009).
Hereditary persistence of HbF (HPFH) was initially observed more than 50 years ago (Weatherall and Clegg 2001). Recent molecular studies have shown that HPFH may result from deletion mutations or point mutations within the β-globin gene cluster. Deletion mutations involving the δ- and β-globin genes may cause the phenotype of δβ-thalassemia, characterized by microcytosis and heterocellular distribution of HbF, or be associated with increased production of HbF in a pancellular distribution with morphologically normal red cells, the phenotype of HPFH. Recent characterization of several deletional mutations in individuals with either the HPFH phenotype or the phenotype of δβ-thalassemia has identified a functional element within a 3.5-kb region between the Aγ- and δ-globin genes that, when present, results in relative silencing of the γ-globin genes and the γβ-thalassemia phenotype and, when absent, allows higher levels of HbF expression and the HPFH phenotype (Sankaran et al. 2011). This region includes sites for binding of the transcription factor BCL11A. Alternatively, augmentation of HbF production may reflect a single nucleotide polymorphism (SNP) in one of the γ-globin gene promoters or elsewhere in the globin locus, which leads to overexpression of the cognate gene. Among the most common is an SNP at −158 bp of the Gγ gene promoter that creates an XMNI site polymorphism. Detailed linkage analysis has shown that this SNP is not the actual causal mechanism of HPFH, but, rather, augmentation of Gγ gene expression occurs as a consequence of an SNP in linkage disequilibrium with the aforementioned Xmn-1 site located midway between Aγ- and δ-globin genes, which may affect BCL11A binding (Galarneau et al. 2010). Fetal hemoglobin is normal in normal subjects and in β-thalassemia heterozygotes with this polymorphism, but the variant leads to increased production during hematopoietic stress such as occurs in homozygous β-thalassemia (Labie et al. 1985). Another HPFH SNP, a substitution at −96 bp of the Aγ promoter, has been found linked to a nonsense mutation in the β-globin gene in individuals with Sardinian β-thalassemia (Pirastu and Kan 1984). Homozygotes for this combination of mutations are clinically normal other than having predominantly HbF in their red cells.
In addition to SNPs within the β-globin locus, genome-wide association studies (GWAS) have identified additional loci that can contribute significantly to the variation in HbF levels (Garner et al. 2000; Uda et al. 2008; Galarneau et al. 2010). An SNP in the BCL11A gene has been shown to contribute to the level of HbF expression. As discussed by Sankaran and Orkin (2013), functional studies have shown that BCL11A is a significant component of the silencing mechanism that turns off the γ-globin genes (Sankaran et al. 2009). Suppression of BCL11A levels in cultured thalassemic erythroid cells markedly increases HbF production (Wilber et al. 2011a). Experimental evidence shows correction of sickle cell disease in a mouse model by suppression of Bcllla (Xu et al. 2011). BCL11A binds several points in the region between the Aγ- and δ-globin genes including within the critical region that differentiates HPFH from δβ-thalassemia deletions (Sankaran et al. 2011). The other locus that has been implicated in modulating HbF levels is MYB (Galarneau et al. 2010). An SNP in what is thought to be a regulatory region near the gene is associated with differences in the level of MYB expression. Individuals with lower levels of MYB have higher HbF levels than individuals with higher MYB levels. In another study, a 3-bp deletion in an intragenic region near the MYB gene with enhancer-like activity was associated with higher HbF levels (Farrell et al. 2011). Variants in both the BCL11A and MYB loci have been shown to modify disease severity in individuals homozygous for Sardinian β0-thalassemia (Galanello et al. 2009). Other transcription factors, including KLF1, have been implicated in regulation of HbF levels (for review, see Wilber et al. 2011b). KLF1 may mediate its effects on HbF through a dual mechanism acting both on the β-globin and BCL11A promoters.
Individuals with thalassemia have hyperbilirubinemia secondary to ongoing hemolysis and ineffective erythropoiesis. Iron loading occurs because of increased iron absorption as well as the administration of blood transfusions. Osteoporosis is common, potentially secondary to hypogonadism and other endocrine abnormalities as well as expansion of marrow cavities. Increased susceptibility to infection is also characteristic of the severe forms of β-thalassemia. Genetic polymorphisms in various genes involved with these clinical features have been described as acting as tertiary modifiers of the phenotype of thalassemia (Weatherall 2001).
IRON OVERLOAD
Patients with the more severe forms of β-thalassemia, both intermedia and major, have increased tissue deposition of iron. Senescence of transfused red cells in patients with transfusion-dependent β-thalassemia results in iron deposition within the reticuloendothelial system. As iron overload progresses, deposited iron also appears within the hepatic parenchyma, various endocrine tissues, and, more slowly, in the myocardium.
In normal humans, iron homeostasis is achieved by controlling absorption (see Ganz and Nemeth 2012). Only ∼1 mg is lost from the body each day, largely through shedding of the epithelial cells from the intestine, urinary tract, skin, and other mucosal organs. Each milliliter of transfused blood contains ∼1 mg of iron, thus receipt of a unit of packed red cells typically results in the deposition of 200 mg of iron ultimately in the tissues following red cell senescence. Lacking excretory mechanisms, individuals with thalassemia who receive blood transfusion inevitably experience significant iron overload.
In individuals with thalassemia intermedia, increased tissue iron occurs as a consequence of occasional transfusions but mainly reflects increased iron absorption (Ginzburg et al. 2011). This paradoxical increase in iron absorption despite systemic iron overload probably reflects release of erythroid factors during the cellular apoptosis associated with ineffective erythropoiesis that inhibit hepcidin production by the liver. Hepcidin is a 25-amino-acid peptide hormone that negatively regulates iron flow into the plasma by inhibiting the absorption of dietary iron, the release of iron from macrophages, and the release of stored iron from hepatocytes (Ganz and Nemeth 2011). Ferric iron exits from cells via the iron exporter ferroportin, a multipass transmembrane protein. Ferroportin is abundant in duodenal enterocytes, in splenic and hepatic macrophages, and in hepatocytes, all cells known to export iron (Ganz and Nemeth 2011). Hepcidin inhibits iron efflux by binding ferroportin and triggering endocytosis of both molecules with subsequent lysosomal degradation (Nemeth et al. 2004). Factors released from apoptotic erythroblasts include growth differentiation factor 15 (GDF15), a member of the transforming growth factor-β superfamily (Tanno et al. 2007). Serum from thalassemia patients suppressed the production of hepcidin by primary human hepatocytes, and depletion of GDF15 prevented the hepcidin suppression. A second molecule, named twisted gastrulation (TWSGI), was also shown to be highly expressed in erythroblasts and also able to inhibit hepcidin expression in mouse and human erythroid cells (Tanno et al. 2009). The roles of GDF15 and TWSGI remain to be fully defined. The end result of suppression of hepcidin expression is to enhance iron absorption from the intestine and to allow iron release from macrophages. The normal and pathological regulation of hepcidin synthesis has recently been reviewed (Nemeth 2010). Synthetic mini-hepcidins with a longer half-life have been shown to reduce iron absorption in mice after oral administration and have been proposed as potential therapeutic agents in individuals with severe β-thalassemia (Preza et al. 2011).
Iron released from cells is bound to transferrin and transported to the bone marrow and other tissues, where iron is taken up by the transferrin receptors. Less than 1% of the total body iron is found in blood at any one time, although up to 25 mg may circulate as transferrin-bound iron throughout the 24-h cycle. The expanded erythropoiesis in individuals with β-thalassemia results in a dramatic increase in plasma iron turnover of 10-fold to 15-fold over normal (Hershko et al. 2005). Normally, the transferrin saturation is maintained in a relatively low range of ∼10%–50%. Iron is stored in tissue in the form of ferritin, a multicomponent protein shell that internalizes iron, thereby protecting cellular constituents from potential oxidative damage. Intracellular ferritin iron is in equilibrium with the cytosolic soluble iron pool. Hemosiderin is an insoluble aggregate of iron that forms in lysosomes when ferritin is degraded (Wu et al. 2010; Kim et al. 2011). Both ferritin and hemosiderin accumulate in the cells of individuals with severe β-thalassemia. Accumulation of iron in reticuloendothelial cells is relatively harmless, whereas accumulation of iron in parenchymal tissues may damage critical cells in the heart, endocrine glands, and liver (Hershko et al. 2005).
As a consequence of iron overload in patients with thalassemia either from blood transfusion or excessive absorption or a combination of the two, transferrin saturation increases to 75%–100% and non-transferrin-bound iron (NTBI) is found in the blood (Hershko et al. 1978). NTBI is very heterogeneous and exists in various forms including in complex with citrate and proteins (Evans et al. 2008). Strategies have been devised to detect labile plasma iron (LPI), a fraction of the NTBI pool that is metabolically active in interacting with membrane constituents, leading to membrane damage via the formation of reactive oxygen species (ROS) (Pootrakul et al. 2004; Hershko 2010). Rather than using the transferrin receptor, NTBI enters cells by various cellular channels in forms that have the potential to damage cells (Oudit et al. 2003; Liuzzi et al. 2006). The liver and myocardium clear NTBI at a rate 200-fold that of transferrin-bound iron (Oudit et al. 2003, 2006). A portion of the NTBI is accessible to iron chelators directly (Breuer et al. 2001), whereas additional iron may be chelated in the presence of iron-mobilizing agents (Esposito et al. 2003). Chelators administered to patients with thalassemia have the potential to reduce NTBI (Porter et al. 1996). Intravenous administration is more effective at reducing NTBI than subcutaneous administration. The reduction and reemergence of NTBI have rather complex kinetics (Porter et al. 1996), although these studies support the use of continuous rather than intermittent chelation in high-risk patients. As discussed in detail below, cardiac myocytes are a critical target for iron-induced ROS. Using an in vitro model of neonatal rat cardiomyocytes, the oral chelators deferasirox and deferiprone were able to effectively access and reduce the intracellular labile iron compartments, whereas desferrioxamine removed iron mainly by eliminating the LPI in plasma (Glickstein et al. 2006). These in vitro studies suggested that the rapid accessibility of oral chelators to intracellular labile iron compartments may render them potentially efficacious for protection and reversal of cardiac damage induced by iron overload (Glickstein et al. 2006).
CLINICAL MANIFESTATIONS
Anemia
Classically, individuals with severe β-thalassemia have presented with variable but often very severe degrees of anemia, expansion of the bone marrow spaces secondary to erythroid hyperplasia, hepatosplenomegaly, and extramedullary hematopoiesis in the chest and abdomen. The external appearance is characterized by pallor and slight jaundice, frontal bossing and other abnormalities of the facies secondary to marrow expansion, and abdominal enlargement due to hepatosplenomegaly. Usually, these manifestations are absent or minimally present in patients with thalassemia major if transfusion therapy is initiated early during the first year of life provided that the hemoglobin levels are maintained at 9–10 g/dL. Where transfusion is readily available, the classical physical findings of thalassemia are now more commonly found in patients with thalassemia intermedia, particularly those who are at the severe end of the phenotypical spectrum. In such patients, the physical manifestations can be suppressed in part with subsequent transfusion. Transfusion practices for thalassemia intermedia vary considerably. Individuals with thalassemia intermedia in developed countries are likely to be transfused, whereas transfusion is used more sparingly in countries where resources are limited (Rachmilewitz and Giardina 2011; Taher et al. 2011). The natural history of thalassemia intermedia is highly variable, although the complications of severe anemia are common (Borgna-Pignatti et al. 2010). Splenectomy may be required because of very large spleen size, with increasing anemia or increasing transfusion requirement with or without a decrease in neutrophil or platelet count.
Iron Overload
The clinical manifestations of iron overload have come to dominate the clinical phenotype of individuals with severe β-thalassemia. Cardiac dysfunction is the main clinical problem that may lead to early death. Endocrine abnormalities, particularly hypogonadism, low growth hormone, hypothyroidism, and diabetes mellitus, are also significant problems. Iron deposition of the liver may be substantial, although functional abnormalities are usually mild unless iron overload is very severe. Fortunately chelation therapy prevents and, when given in intensive combination therapy, may reverse the complications of iron overload (Farmaki et al. 2010).
Noninvasive Measurement of Tissue Iron
Hepatic biopsy and measurement of liver iron concentration long remained the gold standard for estimating the degree of iron overload in patients with severe β-thalassemia. Magnetic susceptibility imaging was the first noninvasive quantitative technique, which established a correlation between liver iron concentration by biopsy and this noninvasive measurement (Brittenham et al. 1982). The instrumentation required for this technique is available in only a few institutions, and therefore this method has not been widely applied. Only when liver iron concentration could be reliably estimated by magnetic resonance imaging (MRI) with clinically available devices that could be standardized from institution to institution, did noninvasive measurement of liver iron supplant hepatic biopsy as the gold standard (Gandon et al. 2004; St Pierre et al. 2005).
MRI has also been applied to estimate cardiac iron. The T2* parameter and its reciprocal 1/R2* have been shown to correlate with the degree of iron overload in the liver and, by extrapolation, to the concentration of iron in the myocardium (Wood 2011). Limited autopsy studies support a direct correlation between chemically determined myocardial iron and MRI measurements (Carpenter et al. 2011). MRI has also been applied to the evaluation of iron deposition in the pituitary gland and the pancreas. Availability of these noninvasive techniques has been useful in determining the relative rate of mobilization of iron from various tissues during chelation therapy. Iron is mobilized more readily from the liver and much more slowly from the heart and endocrine tissues (Wood 2011).
Cardiac Manifestations
The institution of regular transfusions for patients with thalassemia major prevents the overt consequences of anemia. However, iron accumulation progresses with iron deposition in the myocardium and toxic NTBI in the plasma, becoming problematic during the late teenage years or early twenties. Cardiac abnormalities include arrhythmias, both atrial and ventricular, and/or congestive heart failure (Engle 1964). Sudden death often occurred, whereas other patients died of progressive congestive heart failure. Death by the mid twenties was the norm.
This dismal clinical course was modified with the introduction of regular chelation with desferrioxamine, particularly when it was given by continuous subcutaneous infusion over a significant portion of each 24-h period (Propper et al. 1976, 1977; Pippard et al. 1978; Wolfe et al. 1985; Olivieri et al. 1994; Brittenham et al. 1993). Not unexpectedly, compliance with the cumbersome subcutaneous administration via a small infusion pump varied widely among participants, providing an unintended opportunity to compare well-chelated versus poorly chelated individuals (Brittenham et al. 1994; Olivieri et al. 1994). The dramatic reduction in cardiac mortality in well-chelated groups was among the first unequivocal evidence that chelation therapy could modify the clinical phenotype with respect to the cardiac manifestations of iron overload. Subsequent large studies have strongly supported the use of chronic chelation to prevent cardiac death (Borgna-Pignatti et al. 2006; Wood 2011).
Improvement in established cardiac disease was first achieved with continuous interfusion of desferrioxamine (Anderson et al. 2004). Iron was mobilized more readily from the liver than the heart as established by MRI T2* measurements. Some evidence suggests that mobilization of iron from the myocardium is more efficient with the low-molecular-weight chelator deferiprone (Brittenham et al. 2003), and that desferrioxamine and deferiprone administration together are more effective than desferrioxamine alone in reversing cardiac dysfunction as shown in a randomized trial (Tanner et al. 2007). Deferasirox has also been shown to reduce and prevent cardiac iron overload (Pennell et al. 2010, 2011).
Pulmonary hypertension is an increasingly recognized complication in patients with severe β-thalassemia (Morris and Vichinsky 2010). Advancing age and history of splenectomy are the major risk factors that have been identified. Among the pathophysiological factors are red cell membrane pathology, coagulation abnormalities, platelet activation, oxidative stress, and chronic hemolysis with release of thrombogenic vesicles (Morris and Vichinsky 2010). Although the incidence of pulmonary hypertension is more common in patients with thalassemia intermedia, a recent cross-sectional study showed that pulmonary hypertension may occur in both children and adults with age, splenectomy, hepatitis C infection, and smoking as significant univariate risk factors (Morris et al. 2011). When present, pulmonary hypertension may contribute to the severity of cardiovascular symptomatology and complicate heart failure due to left ventricular dysfunction.
Endocrine Abnormalities
Growth retardation secondary in part to growth hormone deficiency and hypogonadism are typically the initial manifestations of iron overload in β-thalassemic patients (Chatterjee and Bajoria 2010). In unchelated patients, failure to develop secondary sex characteristics during the teenage years was very common. Regular chelation therapy has reduced the incidence of hypogonadism, although hormone replacement is often required (Wood 2011). Well-chelated young men with thalassemia are fertile, although potentially requiring hormonal administration, and women with severe β-thalassemia may achieve motherhood either with or without obstetrical intervention. Other endocrine complications include impaired glucose tolerance and diabetes (Noetzli et al. 2011), hypothyroidism, hypoparathyroidism, and growth hormone deficiency (Borgna-Pignatti et al. 2004a).
Hepatic Manifestations
Progressive iron deposition is characteristic in patients with severe β-thalassemia. Invasive liver biopsies and now non-invasive methodologies (see below) have established the correlation between liver iron concentration and histological and functional abnormalities. Iron accumulation to the point of 7 mg/g liver (dry weight) seems well tolerated, but as the liver iron concentration increases with regular transfusion and no or inadequate chelation, fibrosis in the periportal areas and ultimately frank cirrhosis occur. Liver functional abnormalities remain mild until iron overload is severe. Hepatitis C infection was common before the development of effective screening and potentially accelerated the development of cirrhosis and increased the risk for development of hepatocellular carcinoma (Borgna-Pignotti et al. 2004a; Mancuso 2010). Chelation therapy prevents iron accumulation, although the increased risk of hepatocellular carcinoma as a consequence of hepatic iron overload is not completely eliminated.
Other Complications
Osteoporosis often occurs in patients with thalassemia, reflecting marrow expansion, endocrine deficiencies, iron toxicity, and the potential toxicity of chelators (Terpos and Voskaridou 2010). Cortical thinning and subclinical fractures as well as problematic clinical fractures may occur, the latter with minimal trauma. A hypercoagulable state, which increases the risk for thromboembolism, has also been described in patients with thalassemia secondary to platelet activation, red cell membrane damage, and endothelial cell activation (Cappellini et al. 2010). Another clinical manifestation is the occurrence of chronic skin ulceration about the ankles secondary to chronic anemia. All of these complications are more common in untransfused patients with thalassemia intermedia than in frequently transfused patients with thalassemia intermedia or patients with thalassemia major who receive regular transfusions (Borgna-Pignatti et al. 2010). Nutritional deficiencies are common in individuals with severe β-thalassemia and may contribute to other complications such as osteoporosis and diabetes mellitus (Fung 2010). The role of inhibitors of osteoclast function in thalassemia is not firmly established, but small trials suggest that such treatment may be useful (Leung et al. 2009).
Footnotes
Editors: David Weatherall, Alan N. Schechter, and David G. Nathan
Additional Perspectives on Hemoglobin and Its Diseases available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, Porter JB, Walker JM, Pennell DJ 2004. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: A prospective study using T2* cardiovascular magnetic resonance. Br J Haematol 127: 348–355 [DOI] [PubMed] [Google Scholar]
- Bank A, Marks PA 1966. Excess α chain synthesis relative to β chain synthesis in thalassaemia major and minor. Nature 212: 1198–1200 [DOI] [PubMed] [Google Scholar]
- Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, Romeo MA, Forni GL, Gamberini MR, Ghilardi R, et al. 2004. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 89: 1187–1193 [PubMed] [Google Scholar]
- Borgna-Pignatti C, Cappellini MD, De Stefano P, Del Vecchio GC, Fomi GL, Gamberini MR, Ghilard R, Piga A, Romeo MA, Zhao H, et al. 2006. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood 107: 3733–3737 [DOI] [PubMed] [Google Scholar]
- Borgna-Pignatti C, Marsella M, Zanforrin N 2010. The natural history of thalassemia intermedia. Ann NY Acad Sci 1202: 214–220 [DOI] [PubMed] [Google Scholar]
- Boyer Sh, Belding TK, Margolet L, Noyes AN 1975. Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science 188: 361–363 [DOI] [PubMed] [Google Scholar]
- Breuer W, Ermers MJ, Pootrakul P, Abramov A, Hershko C, Cabantichik ZI 2001. Desferrioxamine-chelatable iron, a component of serum non-transferrin-bound iron, used for assessing chelation therapy. Blood 197: 792–798 [DOI] [PubMed] [Google Scholar]
- Brittenham GM, Farrell DE, Harris JW, Feldman ES, Danish EH, Muir WA, Tripp JH, Bellon EM 1982. Magnetic-susceptibility measurement of human iron stores. N Engl J Med 307: 1671–1675 [DOI] [PubMed] [Google Scholar]
- Brittenham GM, Cohen AR, McLaren Ce, Martin MB, Griffith PM, Nienhuis AW, Young NS, Allen CJ, Farrell DE, Harris JW 1993. Hepatic iron stores and plasma ferritin concentration in patients with sickle cell anemia and thalassemia major. Am J Hematol 42: 81–85 [DOI] [PubMed] [Google Scholar]
- Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen CJ, Farrell DE, Harris JW 1994. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med 1331: 567–573 [DOI] [PubMed] [Google Scholar]
- Brittenham GM, Nathan DG, Olivieri NF, Pippard MJ, Weatherall DJ 2003. Deferiprone versus desferrioxamine in thalassaemia and T2* validation and utility. Lancet 361: 183. [DOI] [PubMed] [Google Scholar]
- Cao A, Galanello R 2010. β-Thalassemia. Genet Med 12: 61–76 [DOI] [PubMed] [Google Scholar]
- Cao A, Galanello R, Rosatelli MC 1994. Genotype–phenotype correlations in β-thalassemias. Blood Rev 8: 1–12 [DOI] [PubMed] [Google Scholar]
- Cappellini MD, Motta I, Musallam KM, Taher AT 2010. Redefining thalassemia as a hypercoagulable state. Ann NY Acad Sci 1202: 231–236 [DOI] [PubMed] [Google Scholar]
- Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, Sheppard MN, Porter JB, Walker JM, Wood JC, et al. 2011. On T2* magnetic resonance and cardiac iron. Circulation 123: 1519–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee R, Bajoria R 2010. Critical appraisal of growth retardation and pubertal disturbances in thalassemia. Ann NY Acad Sci 1202: 100–114 [DOI] [PubMed] [Google Scholar]
- Engle MA 1964. Cardiac involvement in Cooley’s anemia. Ann NY Acad Sci 119: 694–702 [DOI] [PubMed] [Google Scholar]
- Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI 2003. Labile plasma iron in iron overload: Redox activity and susceptibility to chelation. Blood 102: 2670–2677 [DOI] [PubMed] [Google Scholar]
- Evans RW, Rafique R, Zarea A, Rapisarda C, Cammack R, Evans PJ, Porter JB, Hider RC 2008. Nature of non-transferrin-bound iron: Studies on iron citrate complexes and thalassemic sera. J Biol Inorg Chem 13: 57–74 [DOI] [PubMed] [Google Scholar]
- Farmaki T, Tzoumari I, Pappa C, Chouliaras G, Berdoukas V 2010. Normalisation of total body iron load with very intensive combined chelation reverses cardiac and endocrine complications of thalassaemia major. Br J Haematol 148: 466–475 [DOI] [PubMed] [Google Scholar]
- Farrell JJ, Sherva RM, Chen Z, Luo H, Chu BF, Ha SY, Li CK, Lee ACW, Li RCH, Li CK, et al. 2011. A 3-bp deletion in the HBSIl-MYB intergenic region of chromosome 6q23 is associated with HbF expression. Blood 117: 4935–4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Fucharoen S, Weatherall DJ 2012. The hemoglobin E thalassemias. Cold Spring Harb Perspect Med 2: a011734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung EB 2010. Nutritional deficiencies in patients with thalassemia. Ann NY Acad Sci 1202: 188–196 [DOI] [PubMed] [Google Scholar]
- Gabuzda TG, Nathan DG, Gardner FH 1962. Comparative metabolism of haemoglobins A and F in thalassaemia. Nature 196: 781–782 [DOI] [PubMed] [Google Scholar]
- Galanello R, Sanna S, Perseu L, Sollaino MC, Satta S, Lai ME, Barella S, Uda M, Usala G, Abecasis GR, et al. 2009. Amelioration of Sardianian β0 thalassemia by genetic modifiers. Blood 114: 3935–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarneau G, Palmer CD, Sankaran VG, Orkin SH, Hirschhorn JN, Lettre G 2010. Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet 42: 1049–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandon Y, Olivie D, Guyader D, Aube C, Oberti F, Sebille V, Deugnier Y 2004. Non-invasive assessment of hepatic iron stores by MRI. Lancet 363: 357–362 [DOI] [PubMed] [Google Scholar]
- Ganz T, Nemeth E 2011. Hepcidin and disorders of iron metabolism. Annu Rev Med 62: 347–360 [DOI] [PubMed] [Google Scholar]
- *.Ganz T, Nemeth E 2012. Iron metabolism: Interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med 2: a011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C, Tatu T, Reittie JE, Littlewood T, Darley J, Cervino S, Farrall M, Kelly P, Spector TD, Thein SL 2000. Genetic influences on F cells and other hematologic variables: A twin heritability study. Blood 95: 342–346 [PubMed] [Google Scholar]
- Garner C, Dew TK, Sherwood R, Rees D, Thein SL 2003. Heterocellular hereditary persistence of fetal haemoglobin affects the haematological parameters of β-thalassemia trail. Br J Haematol 123: 353–358 [DOI] [PubMed] [Google Scholar]
- Ginzburg Y, Rivella S 2011. β-Thalassemia: A model for elucidating the dynamic regulation of ineffective erythropoiesis and iron metabolism. Blood 118: 4321–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein H, Ben El R, Link G, Breuer W, Konijn AM, Hershko C, Nick H, Cabantchik ZI 2006. Action of chelators in iron-loaded cardiac cells: Accessibility to intracellular labile iron and functional consequences. Blood 108: 3195–3203 [DOI] [PubMed] [Google Scholar]
- Hershko C 2010. Pathogenesis and management of iron toxicity in thalassemia. Ann NY Acad Sci 1202: 1–9 [DOI] [PubMed] [Google Scholar]
- Hershko C, Graham G, Bates GW, Rachmilewitz EA 1978. Non-specific serum iron in thalassaemia: An abnormal serum iron fraction of potential toxicity. Br J Haematol 40: 255–263 [DOI] [PubMed] [Google Scholar]
- Hershko C, Link G, Konijn AM, Cabantchik ZI 2005. Objectives and mechanism of iron chelation therapy. Ann NY Acad Sci 1054: 124–135 [DOI] [PubMed] [Google Scholar]
- Italia KY, Jijina FJ, Merchant R, Panjwani S, Nadkarni AH, Sawant PM, Nair SB, Ghosh K, Colah RB 2009. Response to hydroxyurea in β thalassemia major and intermedia: Experience in western India. Clin Chim Acta 407: 10–15 [DOI] [PubMed] [Google Scholar]
- Kan YW, Nathan DG 1970. Mild thalassemia: The result of interactions of α and β thalassemia genes. J Clin Invest 49: 635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Haghpanah S, Farhadi A, Yavarian M 2011. Genotype–phenotype relationship of patients with β-thalassemia taking hydroxyurea: A 13-year experience in Iran. Int J Hematol 95: 51–56 [DOI] [PubMed] [Google Scholar]
- Khandros E, Weiss MJ 2010. Protein quality control during erythropoiesis and hemoglobin synthesis. Hematol Oncol Clin N Am 24: 1071–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandros E, Thom CS, D’Souza J, Weiss MJ 2011. Integrated protein quality control pathways degrade free α globin in β-thalassemia. Blood 119: 5265–5275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Jensen JH, Wu EX, Feng L, Au WY, Cheung JS, Ha SY, Sheth SS, Brittenham GM 2011. Rapid monitoring of iron-chelating therapy in thalassemia major by a new carciovascular MR measure: The reduced transverse relaxation rate. NRM Biomed 24: 771–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Zhou S, Kihm A, Katein AM, Yu X, Gell DA, Mackay JP, Adachi K, Foster-Brown L, Louden CS, et al. 2004. Loss of α-hemoglobin-stabilizing protein impairs erythropoiesis and exacerbates β-thalassemia. J Clin Invest 114: 1457–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labie D, Pagnier J, Lapoumeroulie C, Rauabhi F, Dunda-Belkhodja O, Chardin P, Beldjord C, Wajcman H, Fabry ME, Nagel RL 1985. Common haplotype dependency of high G γ-globin gene expression and high HbF levels in β-thalassemia and sickle cell anemia patients. Proc Natl Acad Sci 82: 2111–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MI, Jiang J, Silver N, Best S, Menzel S, Mijovic A, Colella S, Ragoussi J, Garncer C, Weiss MJ, et al. 2006. α-haemoglobin stabilizing protein is a quantitative trait gene that modifies the phenotype of β-thalassaemia. Br J Haematol 133: 675–682 [DOI] [PubMed] [Google Scholar]
- Leung TF, Chu Y, Lee Y, Cheng FW, Leung WK, Shing MM, Li CK 2009. Long-term effects of pamidronate in thalassemic patients with severe bone mineral density deficits. Hemoglobin 33: 361–369 [DOI] [PubMed] [Google Scholar]
- Liuzzi JP, Avdemir F, Nam H, Knutson MD, Cousins RJ 2006. Zip 14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci 103: 13612–13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso A 2010. Hepatocellular carcinoma in thalassemia: A critical review. World J Hepatol 2: 171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias LA, Fisher TC, Zeng L, Meiselman HJ, Weinberg KI, Hiti AL, Malik P 2000. Ineffective erythropoiesis in β-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp Hematol 28: 1343–1353 [DOI] [PubMed] [Google Scholar]
- Morris CR, Vichinsky EP 2010. Pulmonary hypertension in thalassemia. Ann NY Acad Sci 1202: 205–213 [DOI] [PubMed] [Google Scholar]
- Morris CR, Kim HY, Trachtenberg F, Wood J, Quinn CT, Sweeters N, Kwiatkowski JL, Thompson AA, Giardina PJ, Boudreaux J, et al. 2011. Risk factors and mortality associated with an elevated tricuspid regurgitant jet velocity measured by Doppler-echocardiography in thalassemia: A thalassemia clinical research network report. Blood 18: 3794–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musallam KM, Sankaran VG, Cappellini MD, Duca L, Nathan DG, Taher AT 2012. Fetal hemoglobin levels and morbidity in untransfused patients with β-thalassemia intermedia. Blood 119: 364–367 [DOI] [PubMed] [Google Scholar]
- Nathan DG, Gunn RB 1966. Thalassemia: The consequences of unbalanced hemoglobin synthesis. Am J Med 4: 815–830 [DOI] [PubMed] [Google Scholar]
- Nathan DG, Stossel TB, Gunn RB, Zarkowsky HS, Laforet MT 1969. Influence of hemoglobin precipitation on erythrocyte metabolism in α and β thalassemia. J Clin Invest 48: 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E 2010. Hepcidin in β-thalassemia. Ann NY Acad Sci 1202: 31–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J 2004. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2051–2053 [DOI] [PubMed] [Google Scholar]
- Noetzli LJ, Mittelman SD, Watanabe RM, Coates TD, Wood JC 2011. Pancreatic iron and glucose dysregulation in thalassemia major. Am J Hematol 10.1002/ajh.22223 [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, Martin M, Koren G, Cohen AR 1994. Survival in medically treated patients with homozygous β-thalassemia. N Engl J Med 331: 574–578 [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Muraca GM, O’Donnell A, Premawardhena A, Fisher C, Weatherall DJ 2008. Studies in haemoglobin E β-thalassaemia. Br J Haematol 141: 388–397 [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Pakbaz Z, Vichinsky E 2010a. HbE/β-thalassemia: Basis of marked clinical diversity. Hematol Oncol Clin North Am 24: 1055–1070 [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Thayalsuthan V, O’Donnell A, Premawardhena A, Rigobon C, Muraca G, Fisher C, Weatherall DJ 2010b. Emerging insights in the management of hemoglobin E β thalassemia. Ann NY Acad Sci 1202: 155–157 [DOI] [PubMed] [Google Scholar]
- Orkin SH, Kazzazian HH Jr, Antonarakis SE, Oster H, Goff SC, Sexton JP 1982. Abnormal RNA processing due to the exon mutation of β E-globin gene. Nature 300: 768–769 [DOI] [PubMed] [Google Scholar]
- Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, Yazdanpanah M, Wilson GJ, Schwartz A, Liu PP, et al. 2003. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med 9: 1187–1194 [DOI] [PubMed] [Google Scholar]
- Oudit GY, Trivieri MG, Khaper N, Liu PP, Backx PH 2006. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J Mol Med (Berl) 84: 349–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell DJ, Porter JB, Cappellini MD, El-Beshlawy A, Chan LL, Aydinok Y, Elalfy MS, Sutcharitchan P, Li CK, Ibrahim H, et al. 2010. Efficacy of deferasirox in reducing and preventing cardiac iron overload in β-thalassemia. Blood 115: 2364–2371 [DOI] [PubMed] [Google Scholar]
- Pennell DJ, Porter JB, Cappellini MD, Chan LL, El-Beshlawy A, Aydinok Y, Ibrahim H, Li CK, Viprakasit V, Elalfy MS, et al. 2011. Continued improvement in myocardial T2* over two years of deferasirox therapy in thalassemia major patients with cardiac iron overload. Haematologica 96: 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippard MJ, Callender ST, Weatherall DJ 1978. Intensive iron-chelation therapy with desferrioxamine in iron loading patients. Clin Sci Mol Med 54: 99–106 [DOI] [PubMed] [Google Scholar]
- Pirastu M, Kan YW 1984. Multiple mutations produce δβ0 thalassemia in Sardinia. Science 223: 929–930 [DOI] [PubMed] [Google Scholar]
- Pootrakul P, Breuer W, Sametband M, Sirankapracha P, Hershko C, Cabantchik ZI 2004. Labile plasma iron (LPI) as an indicator of chelatable plasma redox activity in iron-overloaded β-thalassemia/HbE patients treated with an oral chelator. Blood 104: 1504–1510 [DOI] [PubMed] [Google Scholar]
- Porter JB, Abeysinghe RD, Marshall L, Hider RC, Singh S 1996. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood 88: 705–713 [PubMed] [Google Scholar]
- Premawardhena A, Fisher CA, Olivieri NF, de Silva S, Sloane-Stanley J, Wood WG, Weatherall DJ 2005. A novel molecular basis for β thalassemia intermedia poses new questions about its pathophysiology. Blood 106: 3251–3255 [DOI] [PubMed] [Google Scholar]
- Premawardhena A, Arambepola M, Katugaha N, Weatherall DJ 2008. Is the β thalassaemia trait of clinical importance? Br J Haematol 141: 407–410 [DOI] [PubMed] [Google Scholar]
- Preza GC, Ruchala P, Pinon R, Ramos E, Qiao B, Perallta MA, Sharma S, Waring G, Ganz T, Nameth E 2011. Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for treatment of iron overload. J Clin Invest 121: 4880–4888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper RD, Shurin SB, Nathan DG 1976. Reassessment of the use of desferrioxamine B in iron overload. N Engl J Med 294: 1421–1423 [DOI] [PubMed] [Google Scholar]
- Propper RD, Cooper B, Rufo RR, Nienhuis AW, Anderson WF, Burn HF, Rosenthal A, Nathan DG 1977. Continuous subcutaneous administration of deferoxamine in patients with iron overload. N Engl J Med 25: 418–423 [DOI] [PubMed] [Google Scholar]
- Rachmilewitz EA, Giardina PJ 2011. How I treat thalassemia. Blood 118: 3479–3488 [DOI] [PubMed] [Google Scholar]
- Rund D, Rachmilewitz E 2005. β-Thalassemia. N Engl J Med 353: 1135–1146 [DOI] [PubMed] [Google Scholar]
- Sankaran VG, Nathan DG 2010. Thalassemia: An overview of 50 years of clinical research. Hematol Oncol N Am 24: 1005–1020 [DOI] [PubMed] [Google Scholar]
- *.Sankaran VG, Orkin SH 2013. The switch from fetal to adult hemoglobin. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran VG, Xu J, Ragoczy T, Ippolito GC, Walkley CR, Maika SD, Fujiwara Y, Ito M, Groudine M, Bender MA, et al. 2009. Developmental and species-divergent globin switching are driven by BCL11A. Nature 460: 1093–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran VG, Xu J, Byron R, Greisman HA, Fisher C, Weatherall DJ, Sabath DE, Groudine M, Orkin SH, Premawardhena A, et al. 2011. A functional element necessary for fetal hemoglobin silencing. N Engl J Med 365: 807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pierre TG, Clark PR, Chua-Anusom W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R 2005. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105: 855–861 [DOI] [PubMed] [Google Scholar]
- Taher AT, Musallam KM, Cappellini MD, Weatherall DJ 2011. Optimal management of β thalassaemia intermedia. Br J Haematol 152: 512–523 [DOI] [PubMed] [Google Scholar]
- Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, Roughton M, Assomull R, Nair SV, Walker JM, et al. 2007. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation 115: 1876–1884 [DOI] [PubMed] [Google Scholar]
- Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang RH, et al. 2007. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med 13: 1096–1101 [DOI] [PubMed] [Google Scholar]
- Tanno T, Porayette P, Sripichai O, Noh SJ, Byrnes C, Bhupatiraju A, Lee YT, Goodnough JB, Harandi O, Ganz T, et al. 2009. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood 114: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassiopoulos S, Deftereos S, Konstantopoulos K, Farmakis D, Tsironi M, Kyriakdis M, Aessopos A 2005. Does heterozygous β-thalassemia confer a protection against coronary artery disease? Ann NY Acad Sci 1054: 467–470 [DOI] [PubMed] [Google Scholar]
- Terpos E, Voskaridou E 2010. Treatment options for thalassemia patients with osteoporosis. Ann NY Acad Sci 1202: 237–243 [DOI] [PubMed] [Google Scholar]
- Thein SL 1999. Is it dominantly inherited β thalassemia or just a β-chain variant that is highly unstable? Br J Haematol 107: 12–21 [DOI] [PubMed] [Google Scholar]
- *.Thein SL 2013. Molecular basis of β thalassemia. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uda M, Galanello R, Sanna S, Lettre G, Sankaran VG, Chen W, Usala G, Busonero F, Maschio A, Albai G, et al. 2008. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of phenotype of β-thalassemia. Proc Natl Acad Sci 105: 1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainscoat JS, Kanavakis E, Wood WG, Letsky EA, Huehns ER, Marsh GW, Higgs DR, Clegg JB, Weatherall DJ 1983. Thalassaemia intermedia in Cyprus: The interaction of α and β thalassaemia. Br J Haematol 53: 411–416 [DOI] [PubMed] [Google Scholar]
- Weatherall DJ 2001. Phenotype–genotype relationships in monogenic disease: Lessons from the thalassemias. Nat Rev Genet 2: 245–255 [DOI] [PubMed] [Google Scholar]
- Weatherall DJ, Clegg JB 2001. The thalassemia syndromes, 4th ed. Blackwell Science, Oxford [Google Scholar]
- Weatherall DJ, Clegg JB, Naughton MA 1965. Globin synthesis in thalassemia: An in vitro study. Nature 208: 1061–1065 [DOI] [PubMed] [Google Scholar]
- Weiss MJ, dos Santos CO 2009. Chaperoning erythropoiesis. Blood 113: 2136–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Richards R, Byrne M, Buchanan T, White YS, Jelenski G 1985. Thalassemia trait and pregnancy. J Clin Pathol 38: 810–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber A, Hargrove PW, Kim YS, Riberdy JM, Sankaran VG, Papanikolaou E, Georgomanoli M, Anagnou NP, Orkin SH, Nienhuis AW, et al. 2011a. Therapeutic levels of fetal hemoglobin in erythroid progeny of β-thalassemic CD34+ cells after lentiviral vector–mediated gene transfer. Blood 117: 2817–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber A, Nienhuis AW, Persons DA 2011b. Transcriptional regulation of fetal to adult hemoglobin switching: New therapeutic opportunities. Blood 117: 3945–3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe L, Olivieri N, Sallan D, Colan S, Rose V, Propper R, Freedman MH, Nathan DG 1985. Prevention of cardiac disease by subcutaneous deferoxamine in patients with thalassemia major. N Engl J Med 312: 1600–1603 [DOI] [PubMed] [Google Scholar]
- Wood JC 2011. Impact of iron assessment by MRI. Hematology Am Soc Hematol Educ Program 2011: 443–450 [DOI] [PubMed] [Google Scholar]
- Wu EX, Kim D, Tosti CL, Tang H, Jensen JH, Cheung JS, Feng L, Au WY, Ha SY, Sheth SS, et al. 2010. Magnetic resonance assessment of iron overload by separate measurement of tissue ferritin and hemosiderin iron. Ann NY Acad Sci 1202: 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Peng C, Sankaran VG, Shao Z, Esrick EB, Chong BG, Ippolito GC, Fujiwara Y, Ebert BL, Tucker PW, et al. 2011. Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science 334: 993–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Kong Y, Dore LC, Abdulmalik O, Katein AM, Zhou S, Choi JK, Gell D, Mackay JP, Gow AJ, et al. 2007. An erythroid chaperone that facilitates folding of α-globin subunits for hemoglobin synthesis. J Clin Invest 117: 1856–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Mollan TL, Butler A, Gow AJ, Olson JS, Weiss MJ 2009. Analysis of human α globin gene mutations that impair binding to the α hemoglobin stabilizing protein. Blood 113: 5961–5969 [DOI] [PMC free article] [PubMed] [Google Scholar]